Abstract
Background:
Glaucoma, a characteristic type of optic nerve degeneration in the posterior pole of the eye, is a common cause of irreversible vision loss and the second leading cause of blindness worldwide. As an optic neuropathy, glaucoma is identified by increasing degeneration of retinal ganglion cells (RGCs), with consequential vision loss. Current treatments only postpone the development of retinal degeneration, and there are as yet no treatments available for this disability. Recent studies have shown that replacing lost or damaged RGCs with healthy RGCs or RGC precursors, supported by appropriately designed bio-material scaffolds, could facilitate the development and enhancement of connections to ganglion cells and optic nerve axons. The consequence may be an improved retinal regeneration. This technique could also offer the possibility for retinal regeneration in treating other forms of optic nerve ailments through RGC replacement.
Methods:
In this brief review, we describe the innovations and recent developments in retinal regenerative medicine such as retinal organoids and gene therapy which are specific to glaucoma treatment and focus on the selection of appropriate bio-engineering principles, biomaterials and cell therapies that are presently employed in this growing research area.
Results:
Identification of optimal sources of cells, improving cell survival, functional integration upon transplantation, and developing techniques to deliver cells into the retinal space without provoking immune responses are the main challenges in retinal cell replacement therapies.
Conclusion:
The restoration of visual function in glaucoma patients by the RGC replacement therapies requires appropriate protocols and biotechnology methods. Tissue-engineered scaffolds, the generation of retinal organoids, and gene therapy may help to overcome some of the challenges in the generation of clinically safe RGCs.
Keywords: Glaucoma, Biomaterials, Tissue engineering, Cell therapy, Retinal ganglion cells
Introduction
Retinal degenerative disorders are broadly categorised as diseases that can cause blindness [1]. Apoptosis of retinal neurons is the most common outcome of diseases that leading to retinal degeneration, examples of which include the loss of photoreceptors in the retinitis pigmentosa, age-related macular degeneration, and the loss of retinal ganglion cells (RGCs) in glaucoma [2]. This study focuses on the last of these common diseases. Glaucoma, a frequent cause of irreversible vision loss and the second leading cause of blindness (11% of all blindness cases), is affected over 76 million people worldwide in 2020, is expected to increase to over 111.8 million people by 2040 [3, 4]. Therefore, if left unresolved, the disease will continue to be a serious public health problem. Glaucoma occurs mainly in the over-50 age group as the incidence (and burden) of glaucoma is increased significantly with age [5].
Glaucoma
Due to the fact that an in-depth discussion and analysis of the pathogenesis of glaucoma is beyond the scope of this study, a summary of the glaucoma: clinical types, risk factors, pathogenesis, and diagnosis are outlined in the sections below.
This disease is broadly sub-divided into open-angle glaucoma (OAG) and angle-closure glaucoma (ACG), also known as acute or narrow-angle glaucoma; the division is based on the status of the internal drainage and the anatomy of the anterior chamber, both of which result in characteristic optic nerve degeneration in the posterior eye [6, 7]. OAG and ACG can be further sub-divided into primary and secondary glaucomas; primary glaucoma develops due to an unknown cause, whereas secondary glaucoma develops from a known cause such as an eye injury, cataract, tumour, or diabetes. OAG accounts for the majority of glaucoma cases (approximately 80% in the United States) although, ACG is also responsible for a significant number of patients with blindness. Primary and secondary OAGs and ACGs can be further characterised based on their inciting factors [5] using two theories of development, mechanical and ischemic [8]. Ischemic optic neuropathy, is identified by the growing degeneration of RGC through oxidative damage, uncontrolled immune activity and/or dysfunction of glial cells [9]. RGCs receive visual information from photoreceptors via bipolar and retina amacrine cells to collectively transmit image-forming and non-image forming visual information via the visual pathway to the brain, as shown in Fig. 1 [10]. Clinically, glaucoma is the result of RGC axonal degeneration and characteristic optic nerve head cupping [11–13]. It has been decisively established that degeneration of optic nerve head (ONH) plays an important role in initial axonal damage in the onset of glaucoma [12]. As RGCs cannot regenerate independently, degeneration of neurons surrounding RGCs followed by RGC apoptosis generally results in reduced visual acuity and/or permanent visual loss [6].
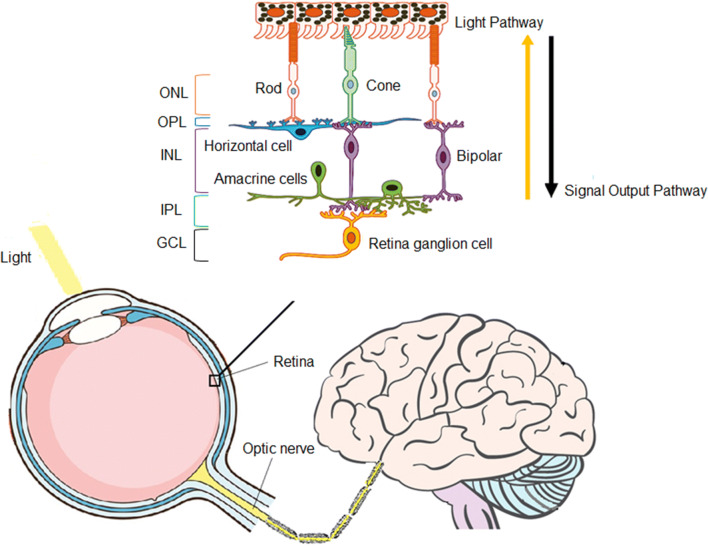
Fig. 1.
The schematics of sensory (visual) input into the bran showing RGCs receiving visual information from photoreceptors via bi-polar and retina amacrine cells to collectively transmit image forming and non-image forming visual information via the visual pathway to the brain [51] (copyright licensed provided). ONL: Outer nuclear layer, OPL: Outer plexiform layer, INL: Inner nuclear layer, IPL: Inner plexiform layer, GCL: Ganglion cell layer
The exact biological basis of glaucoma and its contributing factors are yet to be conclusively established; however, as the disease is multi-factorial, chronic and progressive, it is believed that both environmental and genetic causes play key roles in its development [14]. Vision loss in patients with OAG can be a severe and chronic, or insidious process. In both instances, it occurs mainly due to the loss of ganglion cells, is related to a combination of several concomitant factors, such as the increased intraocular pressure, advanced aging (> 50), increased cup-to-disc ratio, thinner central corneas, and family history, and is often attributed to the presence of certain systemic diseases, such as diabetes and hypertension [15]. On the other hand, hyperopia (i.e., smaller eye), advanced age, female gender and Asian ethnicity are known to be the major risk factors for ACG [16, 17] (Table 1).
Table 1.
Risk factors for glaucomas
| Major risk factors | |
|---|---|
| Primary open-angle glaucoma | Primary angle-closure glaucoma (aka. acute, narrow-angle glaucoma) |
| Elevated IOP | Advanced age |
| Advanced age | Female gender |
| Increased cup-to-disc ratio | Asian ethnicity |
| Thinner central cornea | Hyperopia |
| Race/ethnicity | Shallow anterior chamber |
| Family history | Short axial length of the eye in hyperopia |
| Diabetes | Steep corneal curvature |
| Low ocular perfusion pressure | Shallow limbal chamber depth |
| Myopia | Anteriorly positioned lenses |
Although the pathogenesis of glaucoma is not fully understood, histologic, biochemical, and genetic analysis support the evidence that elevated intraocular pressure (IOP) is related to the balance of aqueous humour production by the ciliary body and its drainage by the trabecular meshwork (conventional outflow) and uveoscleral pathway (secondary outflow) (Fig. 2) [18]. Although glaucoma occurrence is typically associated with elevated levels of IOPs, RGC apoptosis still proceeds in 25–50% of patients with advanced stage glaucoma, even after successful treatment to lower IOP. A remarkable number of patients with high IOP report no symptoms and are not aware that they have the disease [11, 18, 19].
Fig. 2.
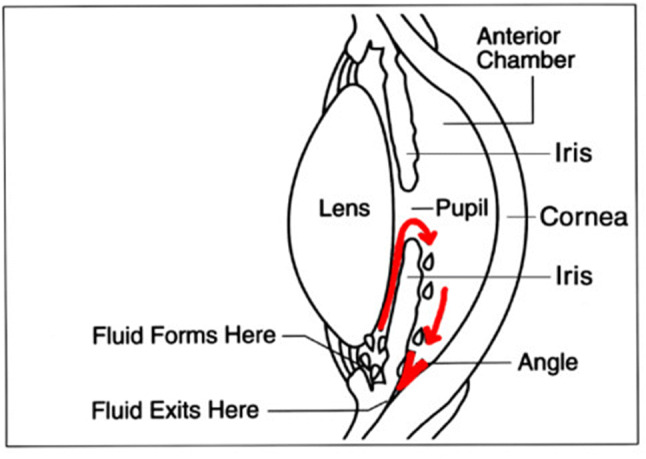
The schematics of drainage pathways of aqueous humour production in healthy eye (Creative commons licence). Image info: “Cause of Glaucoma” by National Eye Institute is licensed under CC BY 2.0
Diagnosis at an early stage of this disease is critical for preventing vision loss as retina degeneration is irreversible. However, the early detection of the condition is difficult owing to the lack of symptoms, and in addition, the glaucoma onset and the rate of glaucoma pathogenesis varies for different types of the disease among patients [20]. As such, patients identified as having glaucoma risk factors should be referred to an ophthalmologist for an examination [5, 21]. Ophthalmologists are able to monitor changes in the optic nerve by conducting specific examinations These tests provide additional input towards establishing the extent of neural (aka. neuroretinal) rim and sectoral retinal nerve fiber layer (RNFL) thinning as evidences of glaucoma onset [7, 22].
Notably, quality of life is significantly reduced in patients with glaucoma, even in those displaying early stages of the disease [23, 24]. Despite the fact that much research into the causes and treatment of glaucoma is being carried out, the exact causes of glaucoma are yet to be fully understood. With some experts seeing the disease as more of a neurological condition [25–28] than an eye disorder, it is clear that further studies into the causes and possible treatments of the disease are justified.
Treatments for glaucoma
As previously mentioned, the best characterised and most modifiable risk factor of glaucoma at this time, is increased IOP, which can be reduced by topical and oral medications, laser treatment, and other forms of surgery [1, 5]. These treatments concentrate on the lowering of IOP through various strategies, often in tandem, and include minimizing the production of aqueous humour, and/or improving drainage through the trabecular meshwork (TM), and/or enhancing uveoscleral outflow [29]. The pharmacologically active agents that control IOP are most commonly administered in the form of eye drops, and consist of mainly five categories, namely, (a) β-blockers, (b) carbonic anhydrase inhibitors, (c) prostaglandin analogues, (d) sympathomimetic drugs, and (e) parasympathomimetic drugs. Furthermore, in some cases a combination of these therapies is applied to effectively reduce IOP [7]. Although, these methods are efficient in gradually reducing the loss of visual field, the progress of the disease cannot be stopped solely by IOP reduction [6, 30]. Therefore, RGC and optic nerve damage can be observed and can also progress in patients, without increased IOP which known as the normal-tension glaucoma (NTG) with intraocular pressure of less than 22 mmHg [31]. Thus, apart from the reduction of IOP, there is a need to develop additional therapeutic strategies to treat glaucoma [1].
One of these potential approaches is neuroprotection to slow down the progression of glaucoma, which is steadily gaining popularity as an effective management/treatment modality of glaucoma [7, 32]. Neuroprotection refers to the measures to salvage, recover, protect and re-generate the nervous system, its cells, structure, and physiologic function from apoptosis arising from insult or progressive neuro-degenerative diseases [22]. Applicable to glaucoma, neuroprotection treatments are (normally) independent of IOP reduction, and accelerate biochemical pathways that prevent neuronal injury and/or block others that lead to neuronal apoptosis [11]. Various earlier investigations provided evidence for successfully applying neuroprotective approaches in animal models; these interventions, however, were found to be treatments suitable for limited applications in the early stages of the disease in humans, whereas most recent clinical trials have failed to establish strong evidence on the effectiveness of neuroprotective approaches. [33–36]. Doozandeh et al. [11] reported that compounds such as glutamate antagonists, ginkgo biloba extract, neurotrophic factors, antioxidants, calcium channel blockers, brimonidine, glaucoma medications displaying blood regulatory effects, and nitric oxide synthase inhibitors (with potential neuroprotective effects) in pre-clinical studies. However, only the few agents (e.g., brimonidine, memantine) that displayed strong neuroprotective impacts in experimental studies have been promoted to clinical trials. However, recent research on the memantine concluded the negative outcome of the memantine in clinical trials with respect to neuroprotection in glaucoma in their patient population [37, 38]. In addition, there are currently no accurate methods to evaluate neuroprotection efficacy [6] and thus, beyond neuroprotection, there is a strong need to develop methods to assist individuals whose vision loss is due to considerable loss of RGCs [36, 39, 40].
Cell transplantation therapies for the treatment of glaucoma
The cell transplantation strategies in the eye aim at both neuroprotection and cell replacement [36]. In some cases, neuroprotective therapies can protect cells before apoptosis occurs. However, applying neurotrophic factors for many patients with an advanced stage of a disease cannot be a viable solution as they have already lost their vision. Therefore, cell replacement therapy can be a promising treatment for advanced stages of the disease. Recent discoveries in ocular regeneration provide the possibility of applying cell-based approaches in future years to restore vision in glaucoma patients [2, 39–41]. Recently a group of studies focused on the development of transplantation of trabecular meshwork cells in an attempt to promote normal aqueous humour filtration, which leads to normal IOP levels and possibly halts the progression of the disease [36, 42]. Evidence supports that the RGC is the initial site for events leading to glaucoma [19], and as such, RGC repair and replacement have been investigated to an extent to identify an essential target for visual function restoration for effective treatment of the disease [43]. Currently, a number of research groups are studying approaches to deliver RGCs to the surface of the retina in order to regenerate the damaged ganglion cell layer. Because the RGC is incapable of self-renewal, replacement of diseased RGC with healthy cells has always been an ultimate goal [44, 45]. Allogeneic transplantation of RGCs can contribute to the improvement of retinal function in irreversible forms of blindness (Fig. 3). This has been considered in research studies that the isolation of RGCs from the retinas of recently diseased persons for transplantation into patients can be an approach in cell replacement therapies [41, 46]. However, RGC transplantation therapy requires a more abundant and possibly a more robust source of healthy RGCs to make it a feasible treatment option [47].
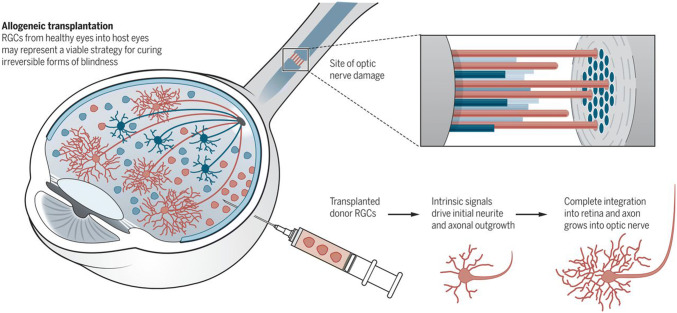
Fig. 3.
Allogeneic RGCs transplantation from the healthy retinas into patients offers a possibility of improvement of retinal function in glaucoma sufferers [46] (copyright licensed provided)
In the developing mammalian eye, RGCs are the first cells to arise from retinal progenitor cells (RPCs), a multipotent cell type that differentiates to the six major neuronal cell types [48, 49]. Therefore, RGC differentiation from stem cells offers a promising area for research and, in this sense, the introduction of stem cell-derived RGCs constitutes a new approach where an abundant, under-control source of RGC can be accessible for replacement therapy [50]. In addition, it is likely that derivation of extensive numbers of RGCs from stem cells can result in a commercially viable supply of RGCs for transplantation therapy, thus preventing genetic defects inherent in the use of autologous RGC [47]. Furthermore, a promising modality in restoring vision is intraocular transplantation of stem cells, which have the ability of RGC-specific protein expression and the development of RGC morphology features [51].
Stem cell-based therapy
The progress of stem cell-derived RGCs can introduce stem cell-based therapies as a potential approach for the restoration of vision in patients who have already lost vision from glaucoma (Fig. 4) [1, 52]. Stem cells are commonly defined as undifferentiated cells with the ability of self-renewal, proliferating and reproducing the same multipotent stem cells indefinitely in their undifferentiated state [47, 53, 54], and are capable of producing one or more differentiated cell types [55]. Below, a brief summary of the most common uses of stem cells employed in retinal cell replacement therapy is provided.
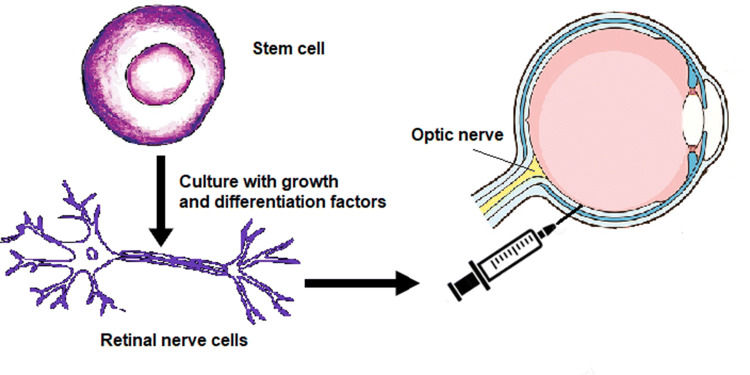
Fig. 4.
RGCs obtained, through an appropriate protocol, from stem cells help regain lost vision and compensate for areas in the eye damaged by glaucoma
Types of stem cells
Embryonic stem cells (ESCs), induced pluripotent stem cells (iPSC), and adult stem cells are three categories of stem cells, based on their origin [20]. Research has considered these stem cell types as potential sources for retinal transplantation to determine an appropriate donor cell type to rescue the degenerating retina (Table 2) [55–79].
Table 2.
Most common use of stem cells in regenerative medicine
| Stem cells | Advantages | Disadvantages | Selected references | Example of stem cells differentiation into RGCs |
|---|---|---|---|---|
| Embryonic stem cells | The immense potential of differentiation into any of the cell types | Potential risk of tumour formation and ethical arguments | [1, 60–63, 68, 69, 82–85, 150, 162] | Driving ganglion and amacrine cells from hESCs by using a combination of noggin, dkk1 and IGF-1 [157]; the differentiation mouse and hESCs into RGCs [158]; the differentiation of hESCs and iPSC cells into functional RGCs by applying a stepwise chemical protocol [159] |
| Induced pluripotent stem cells | The immense potential of differentiation into any of the cell types, no risk of immune rejection; can be directly generated from any adult tissue | Tumorigenic contamination; possible immunogenicity; ethical issues | [45, 71, 72, 74, 88, 89, 100] | The presence of retina ganglion precursor by a synthetic xeno-free culture substrate for iPSCs induction [160]; the development of RGCs and photoreceptors precursors from mouse iPSCs [161] |
| Adult stem/progenitor cells | A promising resource for autologous transplant, avoiding tumorigenesis and immunosuppression | Poor expansion, survival ability and functional (synaptic) integration of donor cells | [56, 64–67, 70] | Functional differentiation of RGCs from multipotent progenitor cells [162] |
Embryonic stem cells
Being pluripotent, ESCs have the capability to proliferate indefinitely by following the natural developmental process cycle. Moreover, ESCs have the capacity to differentiate into any cell types of all three germ layers (ectoderm, mesoderm, and endoderm) [47, 80, 81]. Recent research findings have identified modified culture conditions for ESC differentiation to achieve a particular cell type [1, 71, 82, 83]. Successful production of human RGCs from human embryonic stem cells (hESCs) has been reported [45, 84, 85]. Despite the immense potential of hESCs, the potential risk of tumour formation and existing ethical arguments surrounding the use of the human embryo are the common concerns that limit their use at present [40, 86]. However, the development of iPSCs has resulted in reduced use of hESCs [87].
Induced pluripotent stem cells
Similar to ESCs, iPSCs are able to differentiate into any other cell type in the body and even under the same differentiation conditions. In other words, iPSCs share the same self-renewal and pluripotency characteristics [47] as ESCs. The derivation of iPSCs from somatic cells has introduced them as a promising treatment in regenerative medicine with, notionally, no risk of immune rejection [88]. Since iPSCs can, theoretically, be directly generated from any adult tissue, each patient could have their own iPSCs, for example, through the collection of skin biopsies. [47, 89–91]. In developing glaucoma treatments, recent studies demonstrated that human iPSCs can be differentiated into RGCs [39]. For example, Li et al. [45], induced human iPSCs to form a three-dimensional (3D) retina [78]. Then, they generated RGCs from a human iPSC-neural retina. Moreover, Tanaka et al. [92], generated self-induced RGCs with functional axons from human iPSCs. Additionally, it was recently reported that there has been successful production of human RGCs from human pluripotent stem cells [85, 93–95] and human Tenon’s capsule fibroblast-derived iPSCs [96]. In the future, regenerating the optic nerve and visual pathway may be possible via the utilization of various stem cells, consequently restoring sight in glaucoma patients [8].
Fetal and adult stem cells (or progenitor cells)
Progenitor cells are considered a promising resource for transplant, with the ability to avoid tumorigenesis and immunosuppression [2, 66, 97]. An example of this is RPCs that are able to differentiate into retinal neurons such as RGCs in a complex pathway affected by numerous intrinsic and extrinsic factors [98]. However, poor expansion, survival ability and functional (synaptic) integration of donor cells limit the clinical application of RPCs, and appropriate cell delivery techniques are needed to overcome RPC limitations [99, 100].
Cell supporting bio-material substrates
It is known that the shape, adhesion, surface confirmation, migration functions and, ultimately, the fate of cells are governed by the properties of the cell-supporting substrates. The latter are normally limited to the surface topography of the substrate, its mechanical stiffness, and the substrate’s bio-active properties (i.e., the ability of the substrate to signal peptides and proteins in a cell). The earlier works by Harrison [101] in 1912 on the spider webs showed the importance of substrate organisation and topography, on successful cell migration and morphogenesis. Harrison’s findings were later complimented by the research of Weiss [102] which showed that cells move and migrate by contact guidance. Curtis and Varde [103] were among the first researchers who successfully employed topographical features of (bio-)material substrates to decisively guide the cell behaviour. Over the past two decades much research has been performed indicating that cellular functions are greatly influenced and, in some cases, significantly improved by the substrates that are able to mimic the extra-cellular topographic features of the cells and, including the RGC cells and the RGC supporting bio-material substrates.
Roles of material substrates
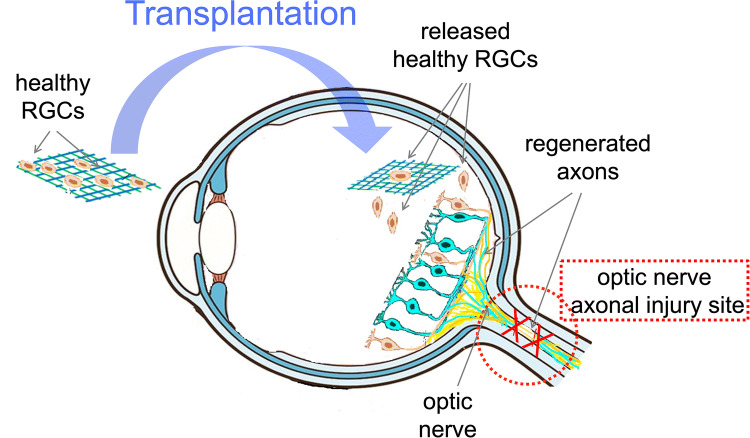
Although cell transplants are able to restore some functional vision in rodent models, the (a) low cell survival and integration at the transplant site as well as, (b) difficulties in maintaining injected cells in a targeted area, are the main challenges in this new treatment approach [2]. The survival of transplanted cells and their functionality are key to the successful and efficient cell transplantation within the transplanted environment [104]. It has been documented that the use of 2D or 3D tissue-engineered scaffolds can be an efficient strategy to overcome the limitations of cell transplantation as cell suspension has a lower immune benefit than substrates delivered as a whole structure. In addition, full differentiation and proper integrity of the underlying supporting material are the other advantages of the use of scaffolds. In other words, tissue-engineered scaffolds can provide physical support vehicles for cell delivery, survival and integration [2, 55, 105–107]. Thus, the use of scaffolds as a cell delivery vehicle demonstrates a potential for compelling success in cell transplant therapies for the treatment of glaucoma and other retinal degenerative diseases [44]. This is due to the fact that these scaffolds are capable of tailoring to the natural micro-environment surrounding neural tissues, and as a result, they help to restore lost axonal connections and the replacement of RGCs (Fig. 5) [108, 109].
Fig. 5.
The schematic diagram of an artificial scaffold, acting as a cell support/delivery vehicle designed to mimic the natural micro-environment, facilitating axonal repair and helping to restore lost axonal connections and replacing lost/damaged RGCs
Substrate properties
Both natural and synthetic polymeric biomaterials have been utilized as cell delivery substrates for various retinal cells [55]. Natural polymers maintain various positive features reflective of naturally occurring tissues and membranes, such as inherent bioactivity, and have been applied broadly as cell delivery scaffolds. However, variability of mechanical properties, have relatively poor environmental stability, cell mediated immune responses, and risk of infection are the main obstacles that currently limit the use of natural scaffold materials. Although synthetic polymer-based scaffolds allow to control properties such as mechanical strength and stiffness, 3D structure, fracture toughness, bio-degradability characteristics, and distribution of biological molecules across the scaffold by physicochemical means, they display several other drawbacks, among which the minimal cell attachment [104]. However, the ability to control the molecular composition of the polymers and with it, their physicochemical and, especially, surface properties results in a wider use of synthetic polymers compared to their natural counterparts as tissue-engineered scaffolds. Bio-compatibility is the major property of the scaffold material that should be met to avoid any toxic, injurious or immunological response [110]. In addition, tissue-engineered scaffolds are required to be extremely thin (few micrometres) and, owing to the size of the retina, implantable and flexible in order to prevent the surrounding tissue damage and, in addition, are mechanically strong in order to endure the inevitable surgical handling. Moreover, the appropriate cell attachment onto the scaffold is the result of sufficient scaffold signals and, therefore any engineered (bio)material scaffold must meet the specified attachment requirements [100, 111].
A number of bio-polymer materials, mainly containing the ester functional group, have been investigated as templates for stem cells derived cell monolayers in current ongoing preclinical research and trials [2, 9, 112, 113] (Table 3), namely poly(lactic acid) (PLA) [114, 115], poly(lactic-co-glycolic acid) (PLGA) [84, 108, 116, 117], poly(caprolactone) (PCL) [84, 85, 108, 118–123] and poly(glycerol sebacate) (PGS) [124]. These biopolymers have the advantage of being able to maintain differentiated cells, feasible for controlling RGC cell morphology and function. PLA, PCL, PLGA and PGS are known to degrade through hydrolysis, a process that proceeds through chemical or enzymatic pathways, with the ester groups of the main polyester (PE) backbone cleaved (in the presence of water), thus leading to the reduction of molecular weight [82, 105, 111, 121]. In PLA and PLGA, chains terminated by hydroxyl (–OH) groups (i.e., PLA, PLGA) appear to be more stable compared to those terminating with carboxylic acids that display an autocatalytic action [115–120] as in PCL. Degradation through hydrolysis is widely utilised for biomedical applications that explore the role of the biopolymer molecular structure, their radical interactions, pH, temperature and enzymatic activity to support various types of cells. An appropriate cell-carrier scaffold for glaucoma treatment is required to display the following attributes: (1) the enhanced capability of improving RGC migration, (2) sending local dendrites into the inner plexiform layer, (3) elongating axons into the optic nerve head and, (4) regenerating axons long distances in the injured optic nerve [44]. In addition, these bio-polymer scaffolds for glaucoma treatment should be able to direct the radial growth of the ganglion cells towards the optic nerve head to the optic chiasm and finally the brain [2, 125]. As such, research is directed to modification of tissue-engineered scaffolds to achieve appropriate growth of RGCs towards the optic nerve head. For example, Kador et al. [44], created an electrospun scaffold of PLA that mimics the radial axon paths of the nerve fibre layer of the rodent retina; the researchers explained how the fabricated scaffold retained electrophysiological properties of RGCs, and also increased RGC survival. Therefore, PLA-derived scaffolds which have shown an increase the axon growth of RGCs, resulting in the regeneration of natural long-distance arrangement of the CNS. Recently, Li et al. [45], produced PLGA-based scaffold as a substrate for human RGC and reported that the RGCs had the ability of integrating with the scaffolds and exhibiting their morphological character. This engineered PLGA/RGC-scaffold was found capable of mimicking the bundles of the RNFL. Also, the PLGA/RGC-scaffold presented dendritic arbours, extended axons, neurite networks and electrophysiological characteristics.
Table 3.
Most common synthetic polymers used in tissue-engineered scaffolds in cell transplant therapies for the treatment of glaucoma
| Polymers | Chemical structure | Bio-degradation | Features | Selected references |
|---|---|---|---|---|
| PLA | 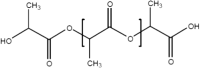 |
12–24 months–(depending on crystallinity) | PE backbone containing unsubstituted, unreactive methyl groups; displays a variable degree of crystallinity; chemically stable; mechanically robust; highly permiable; a radical scavenger; degrades via bulk erosion | [2, 44, 114, 115, 163] |
| PLGA | 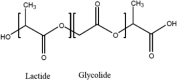 |
1–6 months | PE backbone similar to PLA containing extended co-polymer mixture (i.e., lactide and glycolide); degradation rate can be tailored depending on the molecular weight and co-polymer ratio(s); degrades via bulk erosion | [2, 45, 84, 108, 116, 117, 163] |
| PCL | 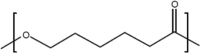 |
Over 24 months | Simple unsubstituted PE backbone; a long 6-pento-methyline bridge offers an enhanced flexibility and an ability to be modified using various physico-chemical processing steps; partially crystalline; chemically stable and mechanically robust; degrades via bulk erosion | [84, 85, 108, 118, 120–123, 163, 164] |
| PGS | 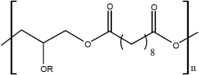 |
1–2 months | PE backbone, di-carbon acid and di-ethyl flexible chain offer similar to PCL processability, substituted glycerine moiety allows fine tuning of physical and chemical properties via -OR modification; highly customisable; degrades into glycerol and sebacic acid via surface erosion | [2, 124] |
In addition to the development of core supporting biomaterials, surface modification techniques are also vital in making cell transplantation a clinical success and moreover, the controlled cell differentiation is necessary to achieve functional retinal regeneration. An appropriate bio-polymer scaffold should also be able to promote cell differentiation among cells delivered to the retina; however, it is only achievable at the upper-most surface area of the scaffold at the nodal points of cellular attachment. One of the approaches is to enhance cell attachment to the bio-polymer carrier scaffold by means of changing its uppermost physicochemical surface properties, namely, by modifying [55, 111, 113, 126] the hydrophilic/hydrophobic properties, surface topology (including surface roughness), pH level and surface adhesive properties via careful modification of surface energy by means of attachments of oxyl, carboxyl, hydroxyl and/or aromatic hydrocarbon groups [127–129]. Additionally, protein modification of the scaffold surface is currently one of the most accessible methods for controlled surface modification [55, 111, 113, 126]. In addition to changing the surface chemistry of supporting material scaffolds, the surface topography plays an important role and can also be effectively modified to enhance cell differentiation, and as such, topographic changes at micro- and nano-scale have been found to induce strong cell re-orientation [130]. Finally, carefully selected surface topography in biomaterials scaffolds can effectively address cell morphologic adaptations and changes in protein expression levels [113]. Specific surface modifications to biomaterial scaffolds were also found to aid the survival of transplanted cells by mimicking their natural environment and, consequently, enhancing bio-compatibility and strengthening phenotype expression compared to pure (i.e., un-modified) scaffolds [47].
Major challenges to RGC replacement
The major challenges for clinical application of cell-based therapies in glaucoma patients are: (a) finding low-cost, reliable, and robust sources of RGC cells such as increasing stem cell differentiation into RGC-like cells, (b) increasing integration, survival rates, synaptogenesis and function of the transplanted cells upon implantation (e.g., promotion of RGCs axon re-growth through the ONH), (c) establishing safe, highly reproducible and reliable methods to deliver stem cell tissue engineered scaffolds into the retinal space, (d) reducing the formation of abnormal cell architectures in vivo and, consequently, reducing the risk of immune rejection, and (e) developing approaches for evaluating the RGC replacement therapies [8, 47, 131, 132].
Sources of cells
Identification of optimal donor cell sources is one of the difficulties in cell replacement therapies and, as discussed earlier, it has been proven that stem cells are highly promising as donor cells [36]. However, stem cell differentiation is a complicated and gradual process, and as such, the differentiation of stem cells into RGC-like cells offers a real challenge in stem-cell based therapy for the treatment of glaucoma when development of neurons in culture is considered [1]. To illustrate this fact, the following factors should be taken into consideration: more than forty (40) different RGC sub-types have been identified [133, 134], there is a lack of unique morphological appearance distinguishing RGCs from other types of neurons, and an absence of definitive markers which are able to distinguish in vitro generated RGCs due to not having specific signature characteristics different from other neurons. In addition, there is a lack of known RNAs or proteins that are completely expressed in RGCs and there are no specific electrophysiological properties for identifying RGCs from other known neurons. Therefore, RGC formation is a process that requires strong and sustained effort in order to be understood fully to enable the delivery of efficient RGC differentiation protocols in the future [1, 90].
Cell survival and functionality
Cell transplantation therapies have been broadly applied in optic pathways such as photoreceptors replacement [47, 135, 136], and as the result of this work, a high level of integration and restoration can be observed in the functions of photoreceptors. However, cell transplantation for the ganglion cell layer is more challenging than photoreceptor replacement in the sub-retinal space, which is a virtual cavity easily separated from the underlying retinal pigmented epithelium. The inner retina has an anatomical barrier preventing the access to the RNFL and RGC layer, which is the internal limiting membrane (ILM). The latter contains the innermost part of the Muller cells. In order for RGC implanted cells to integrate into the host retina, the ILM will have to be surgically removed, which is, as of today, a very challenging and risky surgical manoeuvre [36, 39]. In addition to receiving visual information, the transplanted RGCs need to survive, integrate and grow neurites in the host environment. Ones neurites are established the axons of the transplanted RGCs need to be directed from the optic nerve head to the brain [36, 41, 44, 57]. It must be noted that a variety of proteins are also known to have inhibitory and/or suppressing effects on axon regeneration [46] and therefore, in order to guide RGCs axons to their targets, various neurotrophic factors and guidance cues should also be applied [46, 114]. For instance, Cordeiro et al. suggested that focusing on the development of specific axonal guidance molecules such as Ephrins can be an important area of future glaucoma research [137]. EphrinB1, EphrinB2, and EphB2 axon guidance molecules were investigated by Du et al. [138] in earlier glaucoma pathogenesis studies and have been identified as having important roles in axon guidance. Kador et al. [114], applied Netrin-1 as a guidance factor to guide RGC axons toward the optic nerve head in vivo claiming that Netrin-1 can be used in cell transplant therapies for the treatment of glaucoma.
Another stream of research in the field of axonal guidance is focused on the analysis of computer-generated models of vision. Carreras et al. created an algorithm to simulate optic pathways and also described the conditions that guide axons extending from the retina to the ONH [139].
The most recent approach to guide RGCs to the optic nerve combined electro-spun cell transplantation scaffolds capable of RGC neurite guidance with thermal inkjet 3D cell printing techniques demonstrated the ability of controlling RGCs position on the actual scaffold surface [115].
Evidently, research efforts in regeneration therapy towards the RGC regeneration require to improve current transplantation approaches in order to boost the number of RGCs, whose axons could be guided towards the ONH and, consequently, fill in the full distance back into the brain.
The cells delivery methods
Employing carefully designed and fabricated biomaterials scaffolds to support RGCs is a common solution that allows to overcome cell delivery challenges such as low cell survival, integration, growth and localisation of injected cells in a targeted area. However, the concept of applying biomaterials for RGC replacement therapies need to be further improved to include new tissue-engineering solutions which could offer enhanced RGC survival, improved RGC differentiation, and greatly improved synaptogenesis and axonogenesis functions of transplanted cells within host tissue environment [100].
Host tissue rejection
Current stem cell-based cellular therapeutic treatments for glaucoma are mainly focused on improving survival and differentiation of transplanted cells within the host tissue environment. However, patients that have already extensive RGCs cell apoptosis also require specific treatments that offer some capacity for surviving RGC to function in the existing environment. The latter solution is yet to be developed, trialled and implemented [36].
Evaluation of cell replacement therapies
In cell replacement therapies, it is important to visualize the transplanted cells and study their functional contributions. Aiming to assess the outcomes of RGC replacement therapies, strategies should be developed for monitoring RGC responses and connectivity at the cellular level. Assessing and quantifying the number of RGCs and estimating their functional distribution across the retina is an important research task that requires definitive solutions in the nearest future, as much as the development of reliable means to access, examine, quantify and qualify improvements following RGCs replacement therapy treatments [132].
Future trends in retinal regenerative medicine for the treatment of glaucoma
Generation of sufficient sources of cells, improving survival and functional integration of transplanted RGCs upon implantation and improving techniques to deliver cells into the retinal space without provoking immune responses are the major challenges in the replacement of retinal cells.
Gene therapy
In order to obtain RGC-like cells in cell differentiation, gene therapy (GT), that introduces normal genes into cells in place of missing or defective cells in order to correct genetic disorders, has been considered a possible approach. With regards to application of GT to the treatment of glaucoma, genes or factors introduced into the host retina for reprogramming can indeed offer a potential solution for optimized targeting of each of major sub-classes of RGCs during differentiation protocols. However, major challenges with application of GT lies in the development of proper gene application vectors that do not provoke immune response, and in precise timing of such delivery factors [132].
Organoids
Recent progress in regenerative medicine resulted in the generation of 3D organic tissues (organoids) as a promising technical and biological solution [100]. Organoids, organ-like structures, can be developed to simulate various organs, presenting close proximity to the in vivo morphology owing to their 3D structure and, as such, are used broadly as valuable resources in application to disease modelling, drug testing and in cell transplantation therapies [140–144].
Consequently, development of retinal organoids signify a great promise in regenerative medicine in the way of treating retinal degeneration as retinal organoids can be expanded and differentiated in vitro from a small number of donor cells [140]. Application of ESCs and iPSCs have demonstrated the ability to differentiate into 3D retinal organoids [145–147], and thus, application of retinal organoids could become a major pathway in transplantation approaches in the near future [100, 148] as recent research efforts have successfully shown [149–153]. To define the contribution of specific signal pathways towards RGC differentiation, Dorgau et al. [150] considered the effect of Laminin γ3 in differentiation of RGCs in hPSC-derived retinal organoids. Other researchers explored RGCs derived from 3D retinal organoids as RGC transplantation media [154, 155]. Overall, retinal organoids do hold a promise and, 1 day could provide solutions that overcome existing technical and biological difficulties (such as a lack of donor tissue) in RGC replacement therapies, as well as in vision restoration efforts [52, 100]. However, comprehensive, safe and efficient protocols for handling of organoids are yet to be developed for producing RGCs in large quantities in a human clinic [52].
Dendritic arbours
Dendritic arbours are functional units for collecting information in all neurons [156]. Research has proven that RGC dendritic abnormalities are triggered by axonal injury in glaucoma, viz., damage to RGC axons results in structural alterations in RGC dendritic arbours [12]. Therefore, RGC dendritic arbour alterations should also be considered as a potential therapeutic target as well [164–168].
Summary and conclusion
Loss of RGCs resulting in retinal degeneration is a major cause of permanent vision impairment, affecting millions of individuals worldwide. Glaucoma is a common retinal disease resulting in vision impairment or blindness. A limitation of current therapies is that available treatments can only postpone the development of retinal degeneration, and there are no treatments at present that are able to restore permanent vision loss. Recent research findings in RGC replacement therapy have shown that solutions offered by regenerative medicine can be highly promising in this area. The evidence shows that stem cells hold the potential to overcome the limitation for RGC sources in RGC replacement therapies.
Maintaining and re-establishing connections of damaged neurons in the visual pathway is a major goal for regenerative medicine strategies. To achieve this goal, therapy has to involve homogenous stem cell derived RGCs, firstly, integrating and building connections into host retina, then, transferring visual information into the optic nerve by their healthy axons. To date, the main challenges of RGC replacement therapy include establishing the donor cell sources, the delivery and integration of regenerative materials to the eye, reducing the risk of transplant rejection, reducing tumorigenicity of induced pluripotent stem cells in the long-term, ensuring that the guidance of axon regeneration forms robust connections, and monitoring the replaced RGC at the cellular level. The development of tissue-engineered scaffolds and the generation of retinal organoids may help to overcome some of these challenges by improving the delivery, integration and survival of transplanted cells [106]. Gene therapy is another modality that can be employed to develop highly efficient RGC differentiation protocols in the near future. To achieve efficient and reproducible generation of stable RGCs, these methods require appropriate protocols, biotechnology methods and therapies to derive and generate clinically safe RGCs with guidable axons and functional RGC dendritic arbours that will lead to full restoration of visual function.
Acknowledgement
S.B. acknowledges the Royan Institute for Biotechnology for the visiting doctoral training support.
Author contributions
SB designed and conceptualised the study, carried out the search and collection of review data, carried out data analysis, drafted the manuscript; AÖ and YGA critically revised the manuscript; MR designed, conceptualised and coordinated the study, drafted and critically revised the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Compliance with ethical standards
Conflict of interest
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support of this work that could have influenced its outcome.
Ethical statement
The authors confirm that material presented in this publication is exempt from formal institutional review and/or national ethical committee approval.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sluch VM, Zack DJ. Stem cells, retinal ganglion cells and glaucoma. Dev Ophthalmol. 2014;53:111–121. doi: 10.1159/000358409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kador KE, Goldberg JL. Scaffolds and stem cells: delivery of cell transplants for retinal degenerations. Expert Rev Ophthalmol. 2012;7:459–470. doi: 10.1586/eop.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Mantravadi AV, Vadhar N. Glaucoma, primary care–clinics in office. Practice. 2015;42:437–449. doi: 10.1016/j.pop.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Donegan RK, Lieberman RL. Discovery of molecular therapeutics for glaucoma: challenges, successes, and promising directions. J Med Chem. 2016;59:788–809. doi: 10.1021/acs.jmedchem.5b00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian K, Shibata-Germanos S, Pahlitzsch M, Cordeiro MF. Current perspective of neuroprotection and glaucoma. Clin Ophthalmol. 2015;9:2109–2118. doi: 10.2147/OPTH.S80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahlmann-Noor AH, Vijay S, Limb GA, Khaw PT. Strategies for optic nerve rescue and regeneration in glaucoma and other optic neuropathies. Drug Discov Today. 2010;15:287–299. doi: 10.1016/j.drudis.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Young MJ. Stem cells in the mammalian eye: a tool for retinal repair. APMIS. 2005;113:845–857. doi: 10.1111/j.1600-0463.2005.apm_334.x. [DOI] [PubMed] [Google Scholar]
- 10.Remington LA. Visual system. In: Remington LA, Goodwin D, editors. Clinical anatomy and physiology of the visual system. 3. St. Louis: Butterworth-Heinemann, Elsvier; 2012. pp. 1–9. [Google Scholar]
- 11.Doozandeh A, Yazdani S. Neuroprotection in glaucoma. J Ophthalmic Vis Res. 2016;11:209–220. doi: 10.4103/2008-322X.183923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agostinone J, Di Polo A. Retinal ganglion cell dendrite pathology and synapse loss. Prog Brain Res. 2015;220:199–216. doi: 10.1016/bs.pbr.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Mancino R, Cesareo M, Martucci A, Di Carlo E, Ciuffoletti E, Giannini C, et al. Neurodegenerative process linking the eye and the brain. 2019;26:3754–63. [DOI] [PubMed]
- 14.Huang G, Li F, Zhao X, Ma Y, Li Y, Lin M, et al. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem Rev. 2017;117:12764–12850. doi: 10.1021/acs.chemrev.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon MO, Kass MA. The ocular hypertension treatment study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–583. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 16.Foster PJ, Alsbirk PH, Baasanhu J, Munkhbayar D, Uranchimeg D, Johnson GJ. Anterior chamber depth in mongolians: variation with age, sex, and method of measurement. Am J Ophthalmol. 1997;124:53–60. doi: 10.1016/s0002-9394(14)71644-7. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Iwase A, Araie M, Suzuki Y, Abe H, Shirato S, et al. The tajimi study report 2: prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology. 2005;112:1661–1669. doi: 10.1016/j.ophtha.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retinal Eye Res. 2012;31:702–719. doi: 10.1016/j.preteyeres.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nuzzi R, Tridico F. Glaucoma: biological trabecular and neuroretinal pathology with perspectives of therapy innovation and preventive diagnosis. Front Neurosci. 2017;11:494. doi: 10.3389/fnins.2017.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 22.Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119:979–986. doi: 10.1016/j.ophtha.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramulu P. Glaucoma and disability: which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol. 2009;20:92–98. doi: 10.1097/ICU.0b013e32832401a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP, Los Angeles Latino Eye Study Group Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143:1013–1023. doi: 10.1016/j.ajo.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurwitz JH, Glynn RJ, Monane M, Everitt DE, Gilden D, Smith N, et al. Treatment for glaucoma: adherence by the elderly. Am J Public Health. 1993;83:711–716. doi: 10.2105/ajph.83.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamal D, Hitchings R. Normal tension glaucoma—a practical approach. Br J Ophthalmol. 1998;82:835–840. doi: 10.1136/bjo.82.7.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: just bystander or target for therapy? Prog Retinal Eye Res. 2015;45:30–57. doi: 10.1016/j.preteyeres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Whitmore AV, Libby RT, John SW. Glaucoma: thinking in new ways—a rôle for autonomous axonal self-destruction and other compartmentalised processes? Prog Retinal Eye Res. 2005;24:639–662. doi: 10.1016/j.preteyeres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Dautriche CN, Xie Y, Sharfstein ST. Walking through trabecular meshwork biology: toward engineering design of outflow physiology. Biotechnol Adv. 2014;32:971–983. doi: 10.1016/j.biotechadv.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Solomon AS, Kimron M, Holdengreber V, Nizan A, Yaakobowicz M, Harness E, et al. Up-regulation of semaphorin expression in retina of glaucomatous rabbits. Graefes Arch Clin Exp Ophthalmol. 2003;241:673–681. doi: 10.1007/s00417-003-0684-y. [DOI] [PubMed] [Google Scholar]
- 31.Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE, de Jong PT. The prevalence of primary open-angle glaucoma in a population-based study in the Netherlands: the Rotterdam Study. Ophthalmology. 1994;101:1851–1855. doi: 10.1016/s0161-6420(94)31090-6. [DOI] [PubMed] [Google Scholar]
- 32.Khatib TZ, Martin KR. Protecting retinal ganglion cells. Eye (Lond) 2017;31:218–224. doi: 10.1038/eye.2016.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danesh-Meyer HV, Levin LA. Neuroprotection: extrapolating from neurologic diseases to the eye. Am J Ophthalmol. 2009;148:186–191.e2. doi: 10.1016/j.ajo.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Kountouras J, Zavos C, Deretzi G, Polyzos SA, Gavalas E, Tsiaousi E, et al. Neuroprotection in glaucoma: is there a future role? Exp Eye Res. 2011;92:436–438. doi: 10.1016/j.exer.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Pearson C, Martin K. Stem cell approaches to glaucoma: from aqueous outflow modulation to retinal neuroprotection. Prog Brain Res. 2015;220:241–256. doi: 10.1016/bs.pbr.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz SD, Nagiel A, Lanza R. Cellular therapies for retinal disease: a strategic approach. Berlin: Springer; 2017. [Google Scholar]
- 37.Adams CM, Stacy R, Rangaswamy N, Bigelow C, Grosskreutz CL, Prasanna G. Glaucoma—next generation therapeutics: impossible to possible. Pharm Res. 2019;36:25. doi: 10.1007/s11095-018-2557-4. [DOI] [PubMed] [Google Scholar]
- 38.Weinreb RN, Liebmann JM, Cioffi GA, Goldberg I, Brandt JD, Johnson CA, et al. Oral memantine for the treatment of glaucoma: design and results of 2 randomized, placebo-controlled, phase 3 studies. Ophthalmology. 2018;125:1874–1885. doi: 10.1016/j.ophtha.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Chamling X, Sluch VM, Zack DJ. The potential of human stem cells for the study and treatment of glaucomahuman stem cell for treatment of glaucoma. Invest Ophthalmol Vis Sci. 2016;57:ORSFi1–ORSFi6. doi: 10.1167/iovs.15-18590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sluch VM, Zack DJ. Stem cells, retinal ganglion cells and glaucoma. Dev Ophthalmol. 2014;53:111–121. doi: 10.1159/000358409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venugopalan P, Wang Y, Nguyen T, Huang A, Muller KJ, Goldberg JL. Transplanted neurons integrate into adult retinas and respond to light. Nat Commun. 2016;7:10472. doi: 10.1038/ncomms10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams R, Lace R, Kennedy S, Doherty K, Levis H. Biomaterials for regenerative medicine approaches for the anterior segment of the eye. Adv Healthc Mater. 2018;7:e1701328. doi: 10.1002/adhm.201701328. [DOI] [PubMed] [Google Scholar]
- 43.Yucel YH, Gupta N. A framework to explore the visual brain in glaucoma with lessons from models and man. Exp Eye Res. 2018;7:e1701328. doi: 10.1016/j.exer.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Kador KE, Montero RB, Venugopalan P, Hertz J, Zindell AN, Valenzuela DA, et al. Tissue engineering the retinal ganglion cell nerve fiber layer. Biomaterials. 2013;34:4242–4250. doi: 10.1016/j.biomaterials.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li K, Zhong X, Yang S, Luo Z, Li K, Liu Y, et al. HiPSC-derived retinal ganglion cells grow dendritic arbors and functional axons on a tissue-engineered scaffold. Acta Biomater. 2017;54:117–127. doi: 10.1016/j.actbio.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 46.Laha B, Stafford BK, Huberman AD. Regenerating optic pathways from the eye to the brain. Science. 2017;356:1031–1034. doi: 10.1126/science.aal5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nazari H, Zhang L, Zhu D, Chader GJ, Falabella P, Stefanini F. Stem cell based therapies for age-related macular degeneration: the promises and the challenges. Prog Retinal Eye Res. 2015;48:1–39. doi: 10.1016/j.preteyeres.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Davis DM, Dyer MA. Retinal progenitor cells, differentiation, and barriers to cell cycle reentry. Curr Top Dev Biol. 2010;93:175–188. doi: 10.1016/B978-0-12-385044-7.00006-0. [DOI] [PubMed] [Google Scholar]
- 49.Cepko C. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat Rev Neurosci. 2014;15:615–627. doi: 10.1038/nrn3767. [DOI] [PubMed] [Google Scholar]
- 50.Divya MS, Rasheed VA, Schmidt T, Lalitha S, Hattar S, James J. Intraocular injection of ES cell-derived neural progenitors improve visual function in retinal ganglion cell-depleted mouse models. Front Cell Neurosci. 2017;11:295. doi: 10.3389/fncel.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dahlmann-Noor A, Vijay S, Jayaram H, Limb A, Khaw PT. Current approaches and future prospects for stem cell rescue and regeneration of the retina and optic nerve. Can J Ophthalmol. 2010;45:333–341. doi: 10.3129/i10-077. [DOI] [PubMed] [Google Scholar]
- 52.Miltner AM, La Torre A. Retinal ganglion cell replacement: current status and challenges ahead. Dev Dyn. 2019;248:118–128. doi: 10.1002/dvdy.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pillai RG. Stem cells for ocular tissue engineering and regeneration. Curr Top Med Chem. 2011;11:1606–1620. doi: 10.2174/156802611796117559. [DOI] [PubMed] [Google Scholar]
- 54.Silva GA, Silva NF, Fortunato TM. Stem cell and tissue engineering therapies for ocular regeneration. Curr Stem Cell Res Ther. 2011;6:255–272. doi: 10.2174/157488811796575369. [DOI] [PubMed] [Google Scholar]
- 55.Treharne AJ, Grossel MC, Lotery AJ, Thomson HA. The chemistry of retinal transplantation: the influence of polymer scaffold properties on retinal cell adhesion and control. Br J Ophthalmol. 2011;95:768–773. doi: 10.1136/bjo.2010.184002. [DOI] [PubMed] [Google Scholar]
- 56.Blenkinsop TA, Corneo B, Temple S, Stern JH. Ophthalmologic stem cell transplantation therapies. Regen Med. 2012;7:32–39. doi: 10.2217/rme.12.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalton PD, Harvey AR, Oudega M, Plant GW. Tissue engineering of the nervous system. In: van Blitterswijk CA, de Boer J, editors. Tissue engineering: 2nd ed. Elsevier, Academic Press; 2014. p. 583–625.
- 58.MacHalińska A, Baumert B, Kuprjanowicz L, Wiszniewska B, Karczewicz D, MacHaliński B. Potential application of adult stem cells in retinal repair-challenge for regenerative medicine. Curr Eye Res. 2009;34:748–760. doi: 10.1080/02713680903050592. [DOI] [PubMed] [Google Scholar]
- 59.Mason SL, Stewart RM, Kearns VR, Williams RL, Sheridan CM. Ocular epithelial transplantation: current uses and future potential. Regen Med. 2011;6:767–782. doi: 10.2217/rme.11.94. [DOI] [PubMed] [Google Scholar]
- 60.Aoki H, Hara A, Nakagawa S, Motohashi T, Hirano M, Takahashi Y, et al. Embryonic stem cells that differentiate into RPE cell precursors in vitro develop into RPE cell monolayers in vivo. Exp Eye Res. 2006;82:265–274. doi: 10.1016/j.exer.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 61.Nistor G, Seiler MJ, Yan F, Ferguson D, Keirstead HS. Three-dimensional early retinal progenitor 3D tissue constructs derived from human embryonic stem cells. J Neurosci Methods. 2010;190:63–70. doi: 10.1016/j.jneumeth.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 62.Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 63.Peng S, Gan G, Qiu C, Zhong M, An H, Adelman RA, et al. Engineering a blood-retinal barrier with human embryonic stem cell-derived retinal pigment epithelium: transcriptome and functional analysis. Stem Cells Transl Med. 2013;2:534–544. doi: 10.5966/sctm.2012-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jasty S, Suriyanarayanan S, Krishnakumar S. Influence of self-assembling peptide nanofibre scaffolds on retinal differentiation potential of stem/progenitor cells derived from ciliary pigment epithelial cells. J Tissue Eng Regen Med. 2017;11:509–518. doi: 10.1002/term.1947. [DOI] [PubMed] [Google Scholar]
- 65.Kundu J, Michaelson A, Talbot K, Baranov P, Young MJ, Carrier RL. Decellularized retinal matrix: natural platforms for human retinal progenitor cell culture. Acta Biomater. 2016;31:61–70. doi: 10.1016/j.actbio.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 66.Lawley E, Baranov P, Young M. Hybrid vitronectin-mimicking polycaprolactone scaffolds for human retinal progenitor cell differentiation and transplantation. J Biomater Appl. 2015;29:894–902. doi: 10.1177/0885328214547751. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Wang R, Zarembinski TI, Doty N, Jiang C, Regatieri C, et al. The application of hyaluronic acid hydrogels to retinal progenitor cell transplantation. Tissue Eng Part A. 2013;19:135–142. doi: 10.1089/ten.TEA.2012.0209. [DOI] [PubMed] [Google Scholar]
- 68.Sorkio A, Haimi S, Verdoold V, Juuti-Uusitalo K, Grijpma D, Skottman H. Poly(trimethylene carbonate) as an elastic biodegradable film for human embryonic stem cell-derived retinal pigment epithelial cells. J Tissue Eng Regen Med. 2017;11:3134–3144. doi: 10.1002/term.2221. [DOI] [PubMed] [Google Scholar]
- 69.Subrizi A, Hiidenmaa H, Ilmarinen T, Nymark S, Dubruel P, Uusitalo H, et al. Generation of hESC-derived retinal pigment epithelium on biopolymer coated polyimide membranes. Biomaterials. 2012;33:8047–8054. doi: 10.1016/j.biomaterials.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 70.Yip HK. Retinal stem cells and regeneration of vision system. Anat Rec (Hoboken) 2014;297:137–160. doi: 10.1002/ar.22800. [DOI] [PubMed] [Google Scholar]
- 71.Hunt NC, Hallam D, Karimi A, Mellough CB, Chen J, Steel DHW, et al. 3D culture of human pluripotent stem cells in RGD-alginate hydrogel improves retinal tissue development. Acta Biomater. 2017;49:329–343. doi: 10.1016/j.actbio.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 72.Parvini M, Satarian L, Parivar K, Javan M, Tondar M, Ahmad S, et al. Human pluripotent stem cell-derived retinal pigmented epithelium in retinal treatment: from bench to bedside. Mol Neurobiol. 2014;50:597–612. doi: 10.1007/s12035-014-8684-y. [DOI] [PubMed] [Google Scholar]
- 73.Roozafzoon R, Lashay A, Vasei M, Ai J, Khoshzaban A, Keshel SH, et al. Dental pulp stem cells differentiation into retinal ganglion-like cells in a three dimensional network. Biochem Biophys Res Commun. 2015;457:154–160. doi: 10.1016/j.bbrc.2014.12.069. [DOI] [PubMed] [Google Scholar]
- 74.Song MJ, Bharti K. Looking into the future: using induced pluripotent stem cells to build two and three dimensional ocular tissue for cell therapy and disease modeling. Brain Res. 2016;1638:2–14. doi: 10.1016/j.brainres.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Worthington KS, Green BJ, Rethwisch M, Wiley LA, Tucker BA, Guymon CA, et al. Neuronal differentiation of induced pluripotent stem cells on surfactant templated chitosan hydrogels. Biomacromolecules. 2016;17:1684–1695. doi: 10.1021/acs.biomac.6b00098. [DOI] [PubMed] [Google Scholar]
- 76.Yam GH, Peh GS, Singhal S, Goh BT, Mehta JS. Dental stem cells: a future asset of ocular cell therapy. Expert Rev Mol Med. 2015;17:e20. doi: 10.1017/erm.2015.16. [DOI] [PubMed] [Google Scholar]
- 77.Yun C, Oh J, Lee B, Lee JM, Ariunaa T, Huh K. Generation of retinal progenitor cells from human induced pluripotent stem cell-derived spherical neural mass. Tissue Eng Regen Med. 2017;14:39–47. doi: 10.1007/s13770-016-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pearson RA. Advances in repairing the degenerate retina by rod photoreceptor transplantation. Biotechnol Adv. 2014;32:485–491. doi: 10.1016/j.biotechadv.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2009;2:198–210. doi: 10.1016/j.scr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Enzmann V, Yolcu E, Kaplan HJ, Ildstad ST. Stem cells as tools in regenerative therapy for retinal degeneration. Arch Ophthalmol. 2009;127:563–571. doi: 10.1001/archophthalmol.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aoki H, Hara A, Niwa M, Motohashi T, Suzuki T, Kunisada T. Transplantation of cells from eye-like structures differentiated from embryonic stem cells in vitro and in vivo regeneration of retinal ganglion-like cells. Graefes Arch Clin Exp Ophthalmol. 2008;246:255–265. doi: 10.1007/s00417-007-0710-6. [DOI] [PubMed] [Google Scholar]
- 83.Hsiung J, Zhu D, Hinton DR. Polarized human embryonic stem cell-derived retinal pigment epithelial cell monolayers have higher resistance to oxidative stress-induced cell death than nonpolarized cultures. Stem Cells Transl Med. 2015;4:10–20. doi: 10.5966/sctm.2014-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levenberg S, Burdick JA, Kraehenbuehl T, Langer R. Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue Eng. 2005;11:506–512. doi: 10.1089/ten.2005.11.506. [DOI] [PubMed] [Google Scholar]
- 85.Sluch VM, Davis CH, Ranganathan V, Kerr JM, Krick K, Martin R, et al. Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci Rep. 2015;5:16595. doi: 10.1038/srep16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li JY, Christophersen NS, Hall V, Soulet D, Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31:146–153. doi: 10.1016/j.tins.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 87.Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10:678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Jin ZB, Okamoto S, Mandai M, Takahashi M. Induced pluripotent stem cells for retinal degenerative diseases: a new perspective on the challenges. J Genet. 2009;88:417–424. doi: 10.1007/s12041-009-0063-5. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 90.Green DI, Ou Y. Towards the development of a human glaucoma disease-in-a-dish model using stem cells. Expert Rev Ophthalmol. 2015;10:267–280. [Google Scholar]
- 91.Gill KP, Hewitt AW, Davidson KC, Pébay A, Wong RC. Methods of retinal ganglion cell differentiation from pluripotent stem cells. Transl Vis Sci Technol. 2014;3:7. doi: 10.1167/tvst.3.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tanaka T, Yokoi T, Tamalu F, Watanabe S, Nishina S, Azuma N. Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Sci Rep. 2015;5:8344. doi: 10.1038/srep08344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ohlemacher SK, Sridhar A, Xiao Y, Hochstetler AE, Sarfarazi M, Cummins TR, et al. Stepwise differentiation of retinal ganglion cells from human pluripotent stem cells enables analysis of glaucomatous neurodegeneration. Stem Cells. 2016;34:1553–1562. doi: 10.1002/stem.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen J, Riazifar H, Guan MX, Huang T. Modeling autosomal dominant optic atrophy using induced pluripotent stem cells and identifying potential therapeutic targets. Stem Cell Res Ther. 2016;7:2. doi: 10.1186/s13287-015-0264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sridhar A, Ohlemacher SK, Langer KB, Meyer JS. Robust differentiation of mRNA-reprogrammed human induced pluripotent stem cells toward a retinal lineage. Stem Cells Transl Med. 2016;5:417–426. doi: 10.5966/sctm.2015-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deng F, Chen M, Liu Y, Hu H, Xiong Y, Xu C, et al. Stage-specific differentiation of iPSCs toward retinal ganglion cell lineage. Mol Vis. 2016;22:536–547. [PMC free article] [PubMed] [Google Scholar]
- 97.Aftab U, Jiang C, Tucker B, Kim JY, Klassen H, Miljan E, et al. Growth kinetics and transplantation of human retinal progenitor cells. Exp Eye Res. 2009;89:301–310. doi: 10.1016/j.exer.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 98.Wu N, Wang Y, Yang L, Cho KS. Signaling networks of retinal ganglion cell formation and the potential application of stem cell-based therapy in retinal degenerative diseases. Hum Gene Ther. 2016;27:609–620. doi: 10.1089/hum.2016.083. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y, Zhang D, Shen B, Zhang Y, Gu P. Stem/progenitor cells and biodegradable scaffolds in the treatment of retinal degenerative diseases. Curr Stem Cell Res Ther. 2018;13:160–173. doi: 10.2174/1574888X13666171227230736. [DOI] [PubMed] [Google Scholar]
- 100.Singh R, Cuzzani O, Binette F, Sternberg H, West MD, Nasonkin IO. Pluripotent stem cells for retinal tissue engineering: current status and future prospects. Stem Cell Rev Rep. 2018;14:463–483. doi: 10.1007/s12015-018-9802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harrison RG. The cultivation of tissues in extraneous media as a method of morpho-genetic study. Anat Rec (Hoboken) 1912;6:181–193. [Google Scholar]
- 102.Weiss P. The problem of specificity in growth and development. Yale J Biol Med. 1947;19:235–278. [PMC free article] [PubMed] [Google Scholar]
- 103.Curtis ASG, Varde M. Control of cell behavior: topological factors fot retinal repair. J Natl Cancer Inst. 1964;33:15–26. [PubMed] [Google Scholar]
- 104.West EL, Pearson RA, MacLaren RE, Sowden JC, Ali RR. Cell transplantation strategies for retinal repair. Prog Brain Res. 2009;175:3–21. doi: 10.1016/S0079-6123(09)17501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tomita M, Lavik E, Klassen H, Zahir T, Langer R, Young MJ. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells. 2005;23:1579–1588. doi: 10.1634/stemcells.2005-0111. [DOI] [PubMed] [Google Scholar]
- 106.Gater R, Khoshnaw N, Nguyen D, El Haj AJ, Yang Y. OCT as a convenient tool to assess the quality and application of organotypic retinal samples. In: Tuchin VV, Larin KV, Leahy MJ, Wang RK, editors. Dynamics and fluctuations in biomedical photonics Xiii. SPIE BiOS; 2016. p. 97071C.
- 107.Dutta RC, Dey M, Dutta AK, Basu B. Competent processing techniques for scaffolds in tissue engineering. Biotechnol Adv. 2017;35:240–250. doi: 10.1016/j.biotechadv.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 108.Farrell K, Kothapalli CR, Axonal regeneration, biomimetic polymeric substrates for. 2014.
- 109.Zhang N, Yan H, Wen X. Tissue-engineering approaches for axonal guidance. Brain Res Rev. 2005;49:48–64. doi: 10.1016/j.brainresrev.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 110.Young MJ, Borrás T, Walter M, Ritch R. Tissue bioengineering: potential applications to glaucoma. Arch Ophthalmol. 2005;123:1725–1731. doi: 10.1001/archopht.123.12.1725. [DOI] [PubMed] [Google Scholar]
- 111.Yoon DM, Fisher JP. Polymeric scaffolds for tissue engineering applications. In: Fisher JP, editor. Tissue Engineering. 2. Boca Raton: Taylor & Francis; 2007. [Google Scholar]
- 112.Trese M, Regatieri CV, Young MJ. Advances in retinal tissue engineering. Materials (Basel) 2012;5:108–120. doi: 10.3390/ma5010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yao J, Tao SL, Young MJ. Synthetic polymer scaffolds for stem cell transplantation in retinal tissue engineering. Polymers (Basel). 2011;3:899–914. [Google Scholar]
- 114.Kador KE, Alsehli HS, Zindell AN, Lau LW, Andreopoulos FM, Watson BD, et al. Retinal ganglion cell polarization using immobilized guidance cues on a tissue-engineered scaffold. Acta Biomater. 2014;10:4939–4946. doi: 10.1016/j.actbio.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kador KE, Grogan SP, Dorthé EW, Venugopalan P, Malek MF, Goldberg JL, et al. Control of retinal ganglion cell positioning and neurite growth: combining 3D printing with radial electrospun scaffolds. Tissue Eng Part A. 2016;22:286–294. doi: 10.1089/ten.tea.2015.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Warnke PH, Alamein M, Skabo S, Stephens S, Bourke R, Heiner P, et al. Primordium of an artificial Bruch’s membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers. Acta Biomater. 2013;9:9414–9422. doi: 10.1016/j.actbio.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 117.Hotaling NA, Khristov V, Wan Q, Sharma R, Jha BS, Lotfi M. Nanofiber scaffold-based tissue-engineered retinal pigment epithelium to treat degenerative eye diseases. J Ocul Pharmacol Ther. 2016;32:272–285. doi: 10.1089/jop.2015.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sharifi F, Patel BB, Dzuilko AK, Montazami R, Sakaguchi DS, Hashemi N. Polycaprolactone microfibrous scaffolds to navigate neural stem cells. Biomacromolecules. 2016;17:3287–3297. doi: 10.1021/acs.biomac.6b01028. [DOI] [PubMed] [Google Scholar]
- 119.Sepahvandi A, Eskandari M, Mortarzadeh F. Fabrication and characterization of SrAl2O4: Eu(2+)Dy(3+)/CS-PCL electrospun nanocomposite scaffold for retinal tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2016;66:306–314. doi: 10.1016/j.msec.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 120.Yao J, Ko CW, Baranov PY, Regatieri CV, Redenti S, Tucker BA, et al. Enhanced differentiation and delivery of mouse retinal progenitor cells using a micropatterned biodegradable thin-film polycaprolactone scaffold. Tissue Eng Part A. 2015;21:1247–1260. doi: 10.1089/ten.tea.2013.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Redenti S, Tao S, Yang J, Gu P, Klassen H, Saigal S, et al. Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly(e-caprolactone) nanowire scaffold. J Ocul Biol Dis Infor. 2008;1:19–29. doi: 10.1007/s12177-008-9005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steedman MR, Tao SL, Klassen H, Desai TA. Enhanced differentiation of retinal progenitor cells using microfabricated topographical cues. Biomed Microdevices. 2010;12:363–369. doi: 10.1007/s10544-009-9392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xiang P, Wu KC, Zhu Y, Xiang L, Li C, Chen DL, et al. A novel Bruch’s membrane-mimetic electrospun substrate scaffold for human retinal pigment epithelium cells. Biomaterials. 2014;35:9777–9788. doi: 10.1016/j.biomaterials.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 124.Redenti S, Neeley WL, Rompani S, Saigal S, Yang J, Klassen H, et al. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials. 2009;30:3405–3414. doi: 10.1016/j.biomaterials.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, et al. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci U S A. 2012;109:9149–9154. doi: 10.1073/pnas.1119449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang D, Ni N, Chen J, Yao Q, Shen B, Zhang Y, et al. Electrospun SF/PLCL nanofibrous membrane: a potential scaffold for retinal progenitor cell proliferation and differentiation. Sci Rep. 2015;5:14326. doi: 10.1038/srep14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bolbasov EN, Rybachuk M, Golovkin AS, Antonova LV, Shesterikov EV, Malchikhina AI, et al. Surface modification of poly(l-lactide) and polycaprolactone bioresorbable polymers using RF plasma discharge with sputter deposition of a hydroxyapatite target. Mater Lett. 2014;132:281–284. [Google Scholar]
- 128.Kearns V, Mistry A, Mason S, Krishna Y, Sheridan C, Short R, et al. Plasma polymer coatings to aid retinal pigment epithelial growth for transplantation in the treatment of age related macular degeneration. J Mater Sci Mater Med. 2012;23:2013–2021. doi: 10.1007/s10856-012-4675-6. [DOI] [PubMed] [Google Scholar]
- 129.Petlin DG, Tverdokhlebov SI, Anissimov YG. Plasma treatment as an efficient tool for controlled drug release from polymeric materials: a review. J Control Release. 2017;266:57–74. doi: 10.1016/j.jconrel.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 130.Liu ZP, Yu N, Holz FG, Yang F, Stanzel BV. Enhancement of retinal pigment epithelial culture characteristics and subretinal space tolerance of scaffolds with 200 nm fiber topography. Biomaterials. 2014;35:2837–2850. doi: 10.1016/j.biomaterials.2013.12.069. [DOI] [PubMed] [Google Scholar]
- 131.Kundu J, Michaelson A, Baranov P, Young MJ, Carrier RL. Approaches to cell delivery: substrates and scaffolds for cell therapy. Dev Ophthalmol. 2014;53:143–154. doi: 10.1159/000357369. [DOI] [PubMed] [Google Scholar]
- 132.Gamm DM, Wong R, The AGI Workshop Panelists Report on the national eye institute audacious goals initiative: photoreceptor regeneration and integration workshop. Transl Vis Sci Technol. 2015;4:2. doi: 10.1167/tvst.4.6.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laboissonniere LA, Goetz JJ, Martin GM, Bi R, Lund TJ, Ellson L, et al. Molecular signatures of retinal ganglion cells revealed through single cell profiling. Sci Rep. 2019;9:15778. doi: 10.1038/s41598-019-52215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rheaume BA, Jereen A, Bolisetty M, Sajid MS, Yang Y, Renna K, et al. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat Commun. 2018;9:2759. doi: 10.1038/s41467-018-05134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barak Y, Heroman WJ, Tezel TH. The past, present, and future of exudative age-related macular degeneration treatment. Middle East Afr J Ophthalmol. 2012;19:43–51. doi: 10.4103/0974-9233.92115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hynes SR, Lavik EB. A tissue-engineered approach towards retinal repair: scaffolds for cell transplantation to the subretinal space. Graefes Arch Clin Exp Ophthalmol. 2010;248:763–778. doi: 10.1007/s00417-009-1263-7. [DOI] [PubMed] [Google Scholar]
- 137.Cordeiro MF, Erskine L. Back to basics—ephrins, axonal guidance, neuroprotection and glaucoma. Br J Ophthalmol. 2007;91:1106. doi: 10.1136/bjo.2007.116368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Du J, Tram T, Sretavan DW. Axon guidance molecules upregulated at the optic nerve head of DBA/2 J glaucomatous mice alter RGC intra-axonal calcium levels in vitro. Invest Ophthalmol Vis Sci. 2005;46:1288. [Google Scholar]
- 139.Carreras FJ, Medina J, Ruiz-Lozano M, Carreras I, Castro JL. Virtual tissue engineering and optic pathways: plotting the course of the axons in the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2014;55:3107–3119. doi: 10.1167/iovs.13-13387. [DOI] [PubMed] [Google Scholar]
- 140.Bartfeld S, Clevers H. Stem cell-derived organoids and their application for medical research and patient treatment. J Mol Med (Berl) 2017;95:729–738. doi: 10.1007/s00109-017-1531-7. [DOI] [PubMed] [Google Scholar]
- 141.Achberger K, Haderspeck JC, Kleger A, Liebau S. Stem cell-based retina models. Adv Drug Deliv Rev. 2019;140:33–50. doi: 10.1016/j.addr.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 142.Lowe A, Harris R, Bhansali P, Cvekl A, Liu W. Intercellular adhesion-dependent cell survival and ROCK-regulated actomyosin-driven forces mediate self-formation of a retinal organoid. Stem Cell Reports. 2016;6:743–756. doi: 10.1016/j.stemcr.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mazerik JN, Becker S, Sieving PA. 3-D retina organoids: building platforms for therapies of the future. Cell Med. 2018;10:1–6. doi: 10.1177/2155179018773758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lou YR, Leung AW. Next generation organoids for biomedical research and applications. Biotechnol Adv. 2018;36:132–149. doi: 10.1016/j.biotechadv.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 145.Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 146.Kaewkhaw R, Swaroop M, Homma K, Nakamura J, Brooks M, Kaya KD, et al. Treatment paradigms for retinal and macular diseases using 3-D retina cultures derived from human reporter pluripotent stem cell lines. Invest Ophthalmol Vis Sci. 2016;57:ORSFl1–ORSFl11. doi: 10.1167/iovs.15-17639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Luo Z, Zhong X, Li K, Xie B, Liu Y, Ye M, et al. An optimized system for effective derivation of three-dimensional retinal tissue via wnt signaling regulation. Stem Cells. 2018;36:1709–1722. doi: 10.1002/stem.2890. [DOI] [PubMed] [Google Scholar]
- 148.Browne AW, Arnesano C, Harutyunyan N, Khuu T, Martinez JC, Pollack HA, et al. Structural and functional characterization of human stem-cell-derived retinal organoids by live imaging. Invest Ophthalmol Vis Sci. 2017;58:3311–3318. doi: 10.1167/iovs.16-20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ito SI, Onishi A, Takahashi M. Chemically-induced photoreceptor degeneration and protection in mouse iPSC-derived three-dimensional retinal organoids. Stem Cell Res. 2017;24:94–101. doi: 10.1016/j.scr.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 150.Felemban M, Dorgau B, Hunt NC, Hallam D, Zerti D, Bauer R, et al. Extracellular matrix component expression in human pluripotent stem cell-derived retinal organoids recapitulates retinogenesis in vivo and reveals an important role for IMPG1 and CD44 in the development of photoreceptors and interphotoreceptor matrix. Acta Biomater. 2018;74:207–221. doi: 10.1016/j.actbio.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 151.DiStefano T, Chen HY, Panebianco C, Kaya KD, Brooks MJ, Gieser L, et al. Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell Reports. 2018;10:300–313. doi: 10.1016/j.stemcr.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen HY, Kaya KD, Dong L, Swaroop A. Three-dimensional retinal organoids from mouse pluripotent stem cells mimic in vivo development with enhanced stratification and rod photoreceptor differentiation. Mol Vis. 2016;22:1077–1094. [PMC free article] [PubMed] [Google Scholar]
- 153.Lakowski J, Welby E, Budinger D, Di Marco F, Di Foggia V, Bainbridge JWB. Isolation of human photoreceptor precursors via a cell surface marker panel from stem cell-derived retinal organoids and fetal retinae. Stem Cells. 2018;36:709–722. doi: 10.1002/stem.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kobayashi W, Onishi A, Tu HY, Takihara Y, Matsumura M, Tsujimoto K, et al. Culture systems of dissociated mouse and human pluripotent stem cell-derived retinal ganglion cells purified by two-step immunopanning. Invest Ophthalmol Vis Sci. 2018;59:776–787. doi: 10.1167/iovs.17-22406. [DOI] [PubMed] [Google Scholar]
- 155.Reichman S, Slembrouck A, Gagliardi G, Chaffiol A, Terray A, Nanteau C. Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in xeno-free and feeder-free conditions. Stem Cells. 2017;35:1176–1188. doi: 10.1002/stem.2586. [DOI] [PubMed] [Google Scholar]
- 156.Calkins DJ, Horner PJ. The cell and molecular biology of glaucoma: axonopathy and the brain. Invest Ophthalmol Vis Sci. 2012;53:2482–2484. doi: 10.1167/iovs.12-9483i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maekawa Y, Onishi A, Matsushita K, Koide N, Mandai M, Suzuma K, et al. Optimized culture system to induce neurite outgrowth from retinal ganglion cells in three-dimensional retinal aggregates differentiated from mouse and human embryonic stem cells. Curr Eye Res. 2016;41:558–568. doi: 10.3109/02713683.2015.1038359. [DOI] [PubMed] [Google Scholar]
- 159.Riazifar H, Jia Y, Chen J, Lynch G, Huang T. Chemically induced specification of retinal ganglion cells from human embryonic and induced pluripotent stem cells. Stem Cells Transl Med. 2014;3:424–432. doi: 10.5966/sctm.2013-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tucker BA, Anfinson KR, Mullins RF, Stone EM, Young MJ. Use of a synthetic xeno-free culture substrate for induced pluripotent stem cell induction and retinal differentiation. Stem Cells Transl Med. 2013;2:16–24. doi: 10.5966/sctm.2012-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xie BB, Zhang XM, Hashimoto T, Tien AH, Chen A, Ge J, et al. Differentiation of retinal ganglion cells and photoreceptor precursors from mouse induced pluripotent stem cells carrying an Atoh7/Math5 lineage reporter. PLoS One. 2014;9:e112175. doi: 10.1371/journal.pone.0112175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tamalu F, Chiba C, Ishida A, Saito T. Functional differentiation of ganglion cells from multipotent progenitor cells in sliced retina of adult goldfish. J Comp Neurol. 2000;419:297–305. doi: 10.1002/(sici)1096-9861(20000410)419:3<297::aid-cne3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 163.Pitt CG, Gu ZW. Modification of the rates of chain cleavage of poly(ϵ-caprolactone) and related polyesters in the solid state. J Control Release. 1987;4:283–292. [Google Scholar]
- 164.Della Santina L, Inman DM, Lupien CB, Horner PJ, Wong RO. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J Neurosci. 2013;33:17444–17457. doi: 10.1523/JNEUROSCI.5461-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.El-Danaf RN, Huberman AD. Characteristic patterns of dendritic remodeling in early-stage glaucoma: evidence from genetically identified retinal ganglion cell types. J Neurosci. 2015;35:2329–2343. doi: 10.1523/JNEUROSCI.1419-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Drummond ES, Rodger J, Penrose M, Robertson D, Hu Y, Harvey AR. Effects of intravitreal injection of a Rho-GTPase inhibitor (BA-210), or CNTF combined with an analogue of cAMP, on the dendritic morphology of regenerating retinal ganglion cells. Restor Neurol Neurosci. 2014;32:391–402. doi: 10.3233/RNN-130360. [DOI] [PubMed] [Google Scholar]
- 167.Lorenzetto E, Ettorre M, Pontelli V, Bolomini-Vittori M, Bolognin S, Zorzan S, et al. Rac1 selective activation improves retina ganglion cell survival and regeneration. PLoS One. 2013;8:e64350. doi: 10.1371/journal.pone.0064350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morquette B, Morquette P, Agostinone J, Feinstein E, McKinney R, Kolta A. REDD2-mediated inhibition of mTOR promotes dendrite retraction induced by axonal injury. Cell Death Differ. 2015;22:612–625. doi: 10.1038/cdd.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]