Abstract
Carcinoid tumors are being seen with increasing frequency by surgeons and have become the most common type of tumors of the small bowel. These tumors produce a variety of hormones, which leads to many unique characteristics in terms of symptoms and presentation. Our knowledge of the natural history and treatment of these tumors continues to evolve, and this article will summarize these advances.
Keywords: Neuroendocrine, Small bowel, Carcinoid, Lymphadenectomy, Cytoreduction
History
Carcinoid tumors, now more specifically referred to as small bowel neuroendocrine tumors (SBNETs), were previously rare tumors which were recognized as distinct entities just over 100 years ago. They were likely to have first been described in 1888 when Lubarsch noted multiple small tumors in the ileum of 2 patients at autopsy [1]. Two years later, Ransom reported a patient who had diarrhea and shortness of breath who was found to have an ileal tumor and liver metastases [2]. Oberndorfer described 6 patients with these tumors in 1907, which resembled adenocarcinomas, but displayed more benign behavior and had not metastasized [3]. Later in his career, he conceded that these tumors actually do metastasize [4]. Masson demonstrated the enterochromaffin cells in the small bowel by silver staining and that carcinoid tumors arose in these cells in 1928 [5]. In 1953, Lembeck found that these tumors secrete serotonin [6], and a year later, Thorson et al. described a series of patients with these tumors and liver metastases who had diarrhea, flushing, asthma, and right-sided valvular disease [7].
The first large series of these patients was described by Moertel, who reported upon 203 patients in 1961, with an incidence of 0.65% on autopsy. He also noted that these tumors occurred more frequently moving distally along the small bowel, and that about 50% of tumors 1–2 cm in size metastasize [8]. His classic paper “An odyssey in the land of small tumors” from 1987 updated this series and described 183 patients who had had surgical resection [9]. Although 80% of patients were disease free at 5 years, in long-term follow-up, out to 25 years, only 23% had not recurred. Moertel also was an early adopter of somatostatin analogues for symptom relief and chemotherapy for metastatic disease.
Incidence
Our understanding of the biology and natural history of these tumors has gradually improved since these early days. These tumors were previously considered very rare, but their incidence has increased markedly between 1973 and 2012, from 2 cases to 12 cases per million [10]. There are several potential explanations for this trend. One is that in the early days of national databases (like the Surveillance, Epidemiology, and End Results [SEER] and the National Cancer Database [NCDB]), many SBNETs were considered to be benign and were not captured. Gradually, with the increasing recognition that these tumors were malignant and prone to metastasis, they advanced from being the second most common small bowel tumor in 1995 [11] to passing adenocarcinoma as the leading histology in the year 2000 [12]. Another major contributing factor to the growing incidence is the widespread use of CT scanning to evaluate patients with abdominal pain. CT scans may show liver metastases or suspiciously enlarged mesenteric nodes that lead to further work-up and diagnosis. In addition to these causes, environmental influences as well as and changes related to our modern lifestyles may be contributing to this increased incidence.
Diagnosis
History, Physical, and Biomarkers
These tumors secrete hormones, such as serotonin, histamine, and tachykinins, which are normally degraded within the liver [13]. When liver metastases are present, these compounds are not metabolized and pass directly into the systemic circulation [14]. This leads to carcinoid syndrome, where patients may manifest flushing, diarrhea, abdominal cramping, right-sided cardiac valvular disease, and wheezing [15]. Patients with earlier stage disease may be asymptomatic, but as tumors grow larger, they may cause narrowing of the bowel leading to abdominal pain and eventually small bowel obstruction. Elevation of serotonin leads to diarrhea, but perhaps not flushing [13]. The diagnosis can be made biochemically by the finding of elevated 5-HIAA in the urine [14], and chromogranin A, serotonin, and pancreastatin may have value in follow-up of patients [16–18].
Radiology
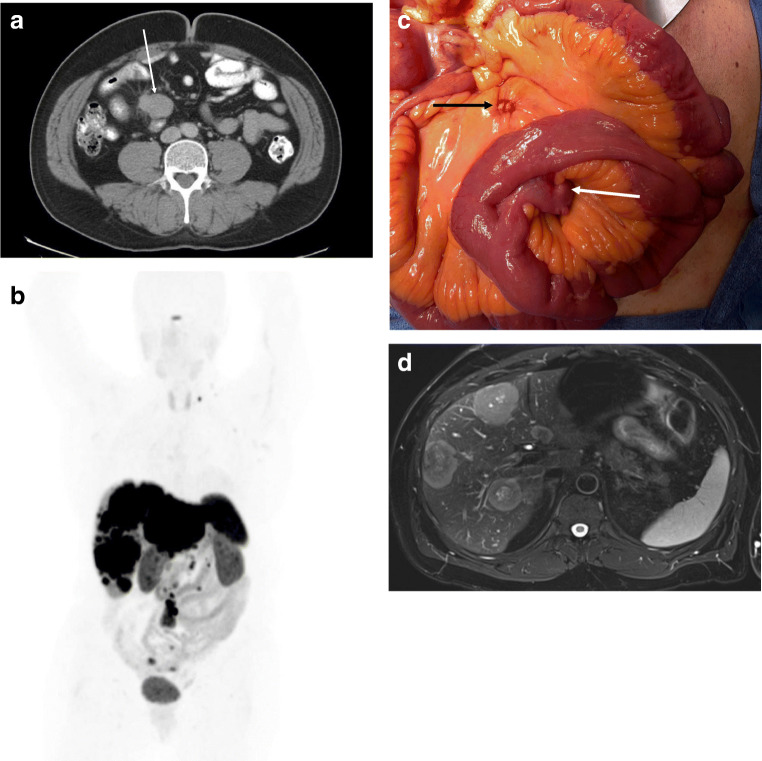
Many patients are diagnosed after finding enlarged mesenteric nodes or liver metastases on CT scan [19, 20]. Primary SBNETs may occasionally be seen on CT as a mass or possibly the site of obstruction, but in general, these tumors are small and not found. Enlarged mesenteric nodes, often with calcification, are the most frequent manifestation (Fig. 1a). MRI is better for characterizing liver lesions and is especially useful when cytoreductive surgery is planned. Octreoscan has been replaced by 68Ga-DOTAPET/CT for functional imaging, which takes advantage of the abundance of somatostatin receptors on tumors [21–23]. This imaging is useful for determining that a mass seen on CT is a NET, identifying occult primaries, and determining the extent of metastases (Fig. 1b).
Fig. 1.
a CT scan showing a mesenteric mass in patient with a SBNET. b68Ga-DOTAPET scan showing widespread metastases, with approximately 60% liver replacement by tumor, with uptake in a supraclavicular node, mesenteric nodes, and 3 small bowel lesions. c SBNET within Meckel’s diverticulum (white arrow) with adjacent calcified lymph nodes (black arrow). d MRI showing liver metastases in the same patient
Pathology
Biopsy of a liver metastasis or mesenteric mass can be helpful to make the diagnosis. Neuroendocrine tumors express synaptophysin and chromogranin by immunohistochemistry, and those of small bowel origin will also be positive for CDX2 and negative or weak for PAX6 and Islet1 (which are more specific for pancreatic NETs) [24, 25]. Determining the grade of the tumor is important using either the Ki-67 stain or mitotic rate, as this has important implications for prognosis [26].
Treatment
Exploration
Patients presenting with small bowel obstruction and suspicion of a SBNET by CT, or the characteristic mesenteric mass (often with calcifications), should be explored with or without biopsy proving the presence of a NET. Exploration is ideally carried out through a midline incision, where the abdominal cavity is surveyed for the presence of peritoneal nodules (most commonly found in the pelvis, under the diaphragm, or on the mesentery), liver lesions, enlarged mesenteric nodes, and ovarian masses. Next, the small bowel is run from the ligament of Treitz to the ileocecal valve, carefully palpating the entire length between thumb and forefinger [27]. Tumors can range from the size of a grain of rice to over 2 cm and are often subtle, firm masses within the wall of the bowel. The primary tumors may be fairly small, yet be accompanied by very large nodal and/or liver metastases (Fig. 1c and d). We measure the length of the bowel and record and mark the sites of each lesion found so as to determine the most sensible plan for resection. The average small bowel length is 5–600 cm, and resection of 100+ cm may be necessary to perform a single resection and anastomosis when multiple lesions (which occurs in 55% of patients) or large mesenteric nodes are present [28]. It is important to understand that if exploration is to be carried out laparoscopically, the incision that will be made for extracorporeal anastomosis should also be used to carefully palpate the entire length of the small bowel. Relying upon visualization of tumors through the camera or use of laparoscopic graspers for palpation will undoubtedly miss smaller tumors.
Lymphadenectomy
The length of bowel resection is also determined by the extent of nodal involvement, and the more proximally involved nodes extend up the mesentery, the greater likelihood of removing the vascular supply to a longer segment of bowel. In cases of distal ileal tumors, which are the most common site, ileocolic resection is recommended, removing the nodes up to the takeoff of the ileocolic artery and vein. For more proximal ileal (or the much less common jejunal) tumors, then removing the mesenteric nodes up to the takeoff of the segmental vessel to that portion of the bowel is recommended while preserving adjacent vessels wherever possible. If the nodes travel higher than this, then skeletonizing the SMA or SMV may be necessary; this may not be possible when nodes are heavily calcified and engulf these main trunks, and in these cases, it may be better to divide the nodes just distal to the segmental vessel takeoff. One can use an energy device or sequential clamping and tying to divide the mesentery, and staying along the edge of the mass as one comes to the proximal nodes is advised to avoid taking collateral vessels wherever possible. Beyond the most proximal nodes, it is best to try to identify and preserve the adjacent segmental vessels to maintain viability of the bowel that remains prior to division and suture ligation of the feeding segmental artery and vein. Other commonly involved nodal areas that may be seen on the preoperative CT scan are left periaortic, aortocaval, portocaval, and peripancreatic/duodenal nodes. The benefits or removing these nodes is unclear, and this adds further complexity to these procedures. We try to remove these nodes when we can, but the decision to proceed with this needs to be made within the context of what other areas of disease will be left behind (such as liver and bone metastases, more proximal mesenteric nodes, mediastinal or supraclavicular nodes), and how their removal might impact upon palliation and/or survival of the patient [29].
Peritoneal Disease
Tumors that grow through the serosa may seed the peritoneum with cells, which can end up anywhere, but most commonly in dependent areas. These tumor nodules may implant on the bowel wall, and lesions on the rectosigmoid colon may eventually lead to colonic obstruction. Other nodules may lead to kinking of the small bowel and obstruction, while others may not lead to problems. We recommend removing larger (> 5 mm) peritoneal lesions when possible to avoid future complications, and the use cautery or argon beam to treat smaller lesions. Heated intraperitoneal chemotherapy in these patients has not been shown to be of benefit [30].
Hepatic Cytoreduction
The liver is the most common site of distant metastasis from SBNETS. These tumors are often multiple, bilobar, and the extent of disease may not be appreciated unless a good-quality arterial and venous phase CT is performed, or an MRI with Eovist. If the lesions are small and diffuse, occupy more than 50% of the hepatic parenchyma, or number greater than 20–30, effective cytoreduction may not be possible [29, 31]. Otherwise, multiple lesions can be effectively treated by resection, including wedge resections and enucleations, as well as other parenchymal sparing techniques, such as microwave or radiofrequency ablation or electroporation. It used to be advocated that if 90% of the lesions could not be removed, then these procedures should not be done [32–34], but more recent studies have shown that achieving a threshold of 70% cytoreduction also likely provides a survival benefit [31, 35, 36]. It is important to remember that whatever one does in terms of cytoreduction, that recurrence is the rule rather than the exception. In one large series, 94% of patients had recurred within 5 years and 99% by 10 years [37], likely because there are many other smaller lesions present that cannot be appreciated at the time of cytoreduction [38].
Cholecystectomy
When patients have nodal or liver metastases at the time of exploration, one should carefully consider removing the gallbladder. There are several reasons for this, one of which is that patients who will be receiving long-term treatment with somatostatin analogues will eventually develop gallstones. Not all patients with gallstones will develop biliary colic or acute cholecystitis, but one study showed that up to 22% of NET patients needed cholecystectomy later when it was not performed at the initial operation [39]. Patients who undergo hepatic embolization may also be at risk for gallbladder necrosis following that procedure and cholecystectomy can avoid this problem [29].
Survival and Adjuvant Treatment
Surveys of national databases have shown a median survival of 170 months for patients with localized SBNETs, 145 months for regional disease, and 70 months for those with metastatic disease [10]. With aggressive hepatic cytoreduction of liver metastases, median survival may be improved to well over 100 months [35, 36]. Adjuvant therapy may not be necessary for patients with localized or low-volume regional disease, while it should be entertained in those with advanced disease or metastases. Somatostatin analogues have been shown to improve progression-free survival (PFS), but not overall survival [40, 41]. The mTOR inhibitor Everolimus has also been shown to modestly improve PFS (11 months vs. 4 months with placebo) [42]. Peptide receptor radiotherapy (PRRT) is also an option for those with advanced or metastatic disease. In the NETTER1 trial, the PFS of patients given 177Lu-DOTATATE was not reached vs. 8.4 months in those receiving high-dose monthly Sandostatin [43]. After surgery, we generally place patients with metastatic disease on somatostatin analogues and use PRRT or everolimus when they develop progressive disease.
The Future
We have discussed the history and current management of SBNETS, but what does the future hold for continued progress for patients with these tumors? Radioguided surgery using 68Ga-DOTATATE may allow for improved detection of primary and metastatic SBNETs [44, 45]. Genetic studies of SBNETs have shown frequent deletions in various chromosomal regions [46, 47], but few recurring mutations, except in the gene CDKN1B [48–50]. One family has been found with a germline mutation in the inositol polyphosphate multikinase gene, but this has not been found in other families [51], and therefore genetic testing for the predisposition to these tumors is not yet possible. Our current knowledge of genetics thus far has not yielded many new therapies, but progress has been made with taking advantage of gene expression profiles of SBNETs. These can be used to determine the site of unknown primaries [52, 53] and for follow-up and recurrence [54]. Knowledge of gene expression in primaries and metastases will also likely yield new targets for therapy [55], and the development of organoid and spheroid models of SBNETs will allow for more efficient drug testing [56]. Our approach to SBNETs has become increasingly sophisticated over the past century, but much work remains for us to translate our knowledge into improved therapies and survival for these patients.
Acknowledgments
Thanks to the Society of Surgical Oncology for their relationship with the IASO.
Funding Information
Thanks to the NIH Iowa SPORE grant P50CA174521-01 for sponsoring our research.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lubarsch O. Ueber dem primaren Krebs des Ileum nebst Bemerkungen uber das gleichzeitige Vorkommen von Krebs und Tuberculose. Virchows Arch Pathol Anat. 1888;111:280–317. doi: 10.1007/BF01966242. [DOI] [Google Scholar]
- 2.Ransom WB. A case of primary carcinoma of the ileum. Lancet. 1890;2:1020–1023. doi: 10.1016/S0140-6736(00)64173-9. [DOI] [Google Scholar]
- 3.Oberndorfer S. Karzinoide tumoren des dunndarms. Frankf Z Pathol. 1907;1:426–432. [Google Scholar]
- 4.Oberndorfer S (1929) Karzinoide. In: Handbook of Pathological Anatomy. Verlag von Julius Springer, Berlin, Germany
- 5.Masson P. Carcinoids (argentaffin-cell tumors) and nerve hyperplasia of the appendicular mucosa. Am J Path. 1928;4:181–211. [PMC free article] [PubMed] [Google Scholar]
- 6.Lembeck F. 5-hydroxytryptamine in a carcinoid tumour. Nature. 1953;172:910–911. doi: 10.1038/172910a0. [DOI] [Google Scholar]
- 7.Thorson A, Biorck G, Bjorkman G, Waldenstrom J. Malignant carcinoid of the small intestine with metastases to the liver, valvular disease of the right side of the heart (pulmonary stenosis and tricuspid regurgitation without septal defects), peripheral vasomotor symptoms, bronchoconstriction, and an unusual type of cyanosis. Am Heart J. 1954;47(47):795–817. doi: 10.1016/0002-8703(54)90152-0. [DOI] [PubMed] [Google Scholar]
- 8.Moertel CG, Sauer WG, Dockerty MB, Baggenstoss AH. Life history of the carcinoid tumor of the small intestine. Cancer. 1961;14:901–912. doi: 10.1002/1097-0142(196109/10)14:5<901::aid-cncr2820140502>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Moertel CG. Karnofsky memorial lecture. An odyssey in the land of small tumors. J Clin Oncol. 1987;5(10):1502–1522. doi: 10.1200/JCO.1987.5.10.1502. [DOI] [PubMed] [Google Scholar]
- 10.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe JR, Karnell LH, Menck HR, Scott-Conner C. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985-1995. Cancer. 1999;86(12):2693–2706. doi: 10.1002/(SICI)1097-0142(19991215)86:12<2693::AID-CNCR14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 12.Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249(1):63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 13.Creutzfeldt W. Carcinoid tumors: development of our knowledge. World J Surg. 1996;20(2):126–131. doi: 10.1007/s002689900020. [DOI] [PubMed] [Google Scholar]
- 14.Sjoerdsma A. Serotonin. N Engl J Med. 1959;261(5):231–237. doi: 10.1056/NEJM195907302610505. [DOI] [PubMed] [Google Scholar]
- 15.Kaehler HJ, Heilmeyer L. Clinical aspects and pathophysiology of carcinoid and carcinoid syndrome with special reference to the pharmacology of 5-hydroxytryptamine. Ergeb Inn Med Kinderheilkd. 1961;16:292–559. [PubMed] [Google Scholar]
- 16.Sherman SK, Maxwell JE, O’Dorisio MS, O’Dorisio TM, Howe JR. Pancreastatin predicts survival in neuroendocrine tumors. Ann Surg Oncol. 2014;21(9):2971–2980. doi: 10.1245/s10434-014-3728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maxwell JE, O’Dorisio TM, Howe JR. Biochemical diagnosis and preoperative imaging of gastroenteropancreatic neuroendocrine tumors. Surg Oncol Clin N Am. 2016;25(1):171–194. doi: 10.1016/j.soc.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott AT, Howe JR. Management of small bowel neuroendocrine tumors. J Oncol Pract. 2018;14(8):471–482. doi: 10.1200/JOP.18.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxwell JE, Howe JR. Imaging in neuroendocrine tumors: an update for the clinician. Int J Endocr Oncol. 2015;2(2):159–168. doi: 10.2217/ije.14.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keck KJ, Maxwell JE, Menda Y, Bellizzi A, Dillon J, O’Dorisio TM, Howe JR. Identification of primary tumors in patients presenting with metastatic gastroenteropancreatic neuroendocrine tumors. Surgery. 2017;161(1):272–279. doi: 10.1016/j.surg.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maxwell JE, Sherman SK, Menda Y, Wang D, O’Dorisio TM, Howe JR. Limitations of somatostatin scintigraphy in primary small bowel neuroendocrine tumors. J Surg Res. 2014;190(2):548–553. doi: 10.1016/j.jss.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadowski SM, Neychev V, Millo C, Shih J, Nilubol N, Herscovitch P, Pacak K, Marx SJ, Kebebew E. Prospective study of 68Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites. J Clin Oncol. 2016;34(6):588–596. doi: 10.1200/JCO.2015.64.0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hope TA, Bergsland EK, Bozkurt MF, Graham M, Heaney AP, Herrmann K, Howe JR, Kulke MH, Kunz PL, Mailman J, May L, Metz DC, Millo C, O’Dorisio S, Reidy-Lagunes DL, Soulen MC, Strosberg JR. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J Nucl Med. 2018;59(1):66–74. doi: 10.2967/jnumed.117.202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellizzi AM. Assigning site of origin in metastatic neuroendocrine neoplasms: a clinically significant application of diagnostic immunohistochemistry. Adv Anat Pathol. 2013;20(5):285–314. doi: 10.1097/PAP.0b013e3182a2dc67. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell JE, Sherman SK, Stashek KM, O’Dorisio TM, Bellizzi AM, Howe JR. A practical method to determine the site of unknown primary in metastatic neuroendocrine tumors. Surgery. 2014;156(6):1359–1365. doi: 10.1016/j.surg.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keck KJ, Choi A, Maxwell JE, Li G, O’Dorisio TM, Breheny P, Bellizzi AM, Howe JR. Increased grade in neuroendocrine tumor metastases negatively impacts survival. Ann Surg Oncol. 2017;24(8):2206–2212. doi: 10.1245/s10434-017-5899-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howe JR. Small bowel resection and lymphadenectomy for jejunoileal neuroendocrine tumors. In: Howe JR, editor. Endocrine and neuroendocrine surgery. Berlin: Springer; 2017. pp. 301–316. [Google Scholar]
- 28.Keck KJ, Maxwell JE, Utria AF, Bellizzi AM, Dillon JS, O’Dorisio TM, Howe JR. The distal predilection of small bowel neuroendocrine tumors. Ann Surg Oncol. 2018;25(11):3207–3213. doi: 10.1245/s10434-018-6676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang YZ, Law CH, Liu EH, Kim MK, Menda Y, Morse BG, Bergsland EK, Strosberg JR, Nakakura EK, Pommier RF. The surgical management of small bowel neuroendocrine tumors: consensus guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46(6):715–731. doi: 10.1097/MPA.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elias D, David A, Sourrouille I, Honore C, Goere D, Dumont F, Stoclin A, Baudin E. Neuroendocrine carcinomas: optimal surgery of peritoneal metastases (and associated intra-abdominal metastases) Surgery. 2014;155(1):5–12. doi: 10.1016/j.surg.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell JE, Sherman SK, O’Dorisio TM, Bellizzi AM, Howe JR. Liver-directed surgery of neuroendocrine metastases: what is the optimal strategy? Surgery. 2016;159(1):320–333. doi: 10.1016/j.surg.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster JH, Berman MM. Solid liver tumors. Major Probl Clin Surg. 1977;22:1–342. [PubMed] [Google Scholar]
- 33.Foster JH, Lundy J. Liver metastases. Curr Probl Surg. 1981;18(3):157–202. doi: 10.1016/S0011-3840(81)80009-3. [DOI] [PubMed] [Google Scholar]
- 34.McEntee GP, Nagorney DM, Kvols LK, Moertel CG, Grant CS. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery. 1990;108(6):1091–1096. [PubMed] [Google Scholar]
- 35.Graff-Baker AN, Sauer DA, Pommier SJ, Pommier RF. Expanded criteria for carcinoid liver debulking: maintaining survival and increasing the number of eligible patients. Surgery. 2014;156(6):1369–1376. doi: 10.1016/j.surg.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Scott AT, Breheny PJ, Keck KJ, Bellizzi AM, Dillon JS, O’Dorisio TM, Howe JR. Effective cytoreduction can be achieved in patients with numerous neuroendocrine tumor liver metastases (NETLMs) Surgery. 2019;165(1):166–175. doi: 10.1016/j.surg.2018.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC, Celinksi SA, Kooby DA, Staley CA, Stokes JB, Chu CK, Ferrero A, Schulick RD, Choti MA, Mentha G, Strub J, Bauer TW, Adams RB, Aldrighetti L, Capussotti L, Pawlik TM. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17(12):3129–3136. doi: 10.1245/s10434-010-1154-5. [DOI] [PubMed] [Google Scholar]
- 38.Elias D, Lefevre JH, Duvillard P, Goere D, Dromain C, Dumont F, Baudin E. Hepatic metastases from neuroendocrine tumors with a “thin slice” pathological examination: they are many more than you think. Ann Surg. 2010;251(2):307–310. doi: 10.1097/SLA.0b013e3181bdf8cf. [DOI] [PubMed] [Google Scholar]
- 39.Norlen O, Hessman O, Stalberg P, Akerstrom G, Hellman P. Prophylactic cholecystectomy in midgut carcinoid patients. World J Surg. 2010;34(6):1361–1367. doi: 10.1007/s00268-010-0428-1. [DOI] [PubMed] [Google Scholar]
- 40.Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Blaker M, Harder J, Arnold C, Gress T, Arnold R, Group PS Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 41.Caplin ME, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P, Investigators C. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 42.Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Delle Fave G, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME, Rad001 in Advanced Neuroendocrine Tumours FTSG Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strosberg J. NETTER-1 phase III: progression-free survival, radiographic response, and preliminary overall survival results in patients with midgut neuroendocrine tumors treated with 177-Lu-Dotatate. J Clin Oncol. 2016;34(4S):194. doi: 10.1200/jco.2016.34.4_suppl.194. [DOI] [Google Scholar]
- 44.El Lakis M, Gianakou A, Nockel P, Wiseman D, Tirosh A, Quezado MA, Patel D, Nilubol N, Pacak K, Sadowski SM, Kebebew E. Radioguided surgery with gallium 68 dotatate for patients with neuroendocrine tumors. JAMA Surg. 2019;154(1):40–45. doi: 10.1001/jamasurg.2018.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howe JR. Radioguided surgery with gallium for neuroendocrine tumors. JAMA Surg. 2019;154(1):45–46. doi: 10.1001/jamasurg.2018.3480. [DOI] [PubMed] [Google Scholar]
- 46.Kulke MH, Freed E, Chiang DY, Philips J, Zahrieh D, Glickman JN, Shivdasani RA. High-resolution analysis of genetic alterations in small bowel carcinoid tumors reveals areas of recurrent amplification and loss. Genes Chromosomes Cancer. 2008;47(7):591–603. doi: 10.1002/gcc.20561. [DOI] [PubMed] [Google Scholar]
- 47.Banck MS, Kanwar R, Kulkarni AA, Boora GK, Metge F, Kipp BR, Zhang L, Thorland EC, Minn KT, Tentu R, Eckloff BW, Wieben ED, Wu Y, Cunningham JM, Nagorney DM, Gilbert JA, Ames MM, Beutler AS. The genomic landscape of small intestine neuroendocrine tumors. J Clin Invest. 2013;123(6):2502–2508. doi: 10.1172/JCI67963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francis JM, Kiezun A, Ramos AH, Serra S, Pedamallu CS, Qian ZR, Banck MS, Kanwar R, Kulkarni AA, Karpathakis A, Manzo V, Contractor T, Philips J, Nickerson E, Pho N, Hooshmand SM, Brais LK, Lawrence MS, Pugh T, McKenna A, Sivachenko A, Cibulskis K, Carter SL, Ojesina AI, Freeman S, Jones RT, Voet D, Saksena G, Auclair D, Onofrio R, Shefler E, Sougnez C, Grimsby J, Green L, Lennon N, Meyer T, Caplin M, Chung DC, Beutler AS, Ogino S, Thirlwell C, Shivdasani R, Asa SL, Harris CR, Getz G, Kulke M, Meyerson M. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat Genet. 2013;45(12):1483–1486. doi: 10.1038/ng.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crona J, Gustavsson T, Norlen O, Edfeldt K, Akerstrom T, Westin G, Hellman P, Bjorklund P, Stalberg P. Somatic mutations and genetic heterogeneity at the CDKN1B locus in small intestinal neuroendocrine tumors. Ann Surg Oncol. 2015;22(Suppl 3):S1428–S1435. doi: 10.1245/s10434-014-4351-9. [DOI] [PubMed] [Google Scholar]
- 50.Maxwell JE, Sherman SK, Li G, Choi AB, Bellizzi AM, O’Dorisio TM, Howe JR. Somatic alterations of CDKN1B are associated with small bowel neuroendocrine tumors. Cancer Genet. 2015;208(11):564–570. doi: 10.1016/j.cancergen.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sei Y, Zhao X, Forbes J, Szymczak S, Li Q, Trivedi A, Voellinger M, Joy G, Feng J, Whatley M, Jones MS, Harper UL, Marx SJ, Venkatesan AM, Chandrasekharappa SC, Raffeld M, Quezado MM, Louie A, Chen CC, Lim RM, Agarwala R, Schaffer AA, Hughes MS, Bailey-Wilson JE, Wank SA. A hereditary form of small intestinal carcinoid associated with a germline mutation in inositol polyphosphate multikinase. Gastroenterology. 2015;149(1):67–78. doi: 10.1053/j.gastro.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kerr SE, Schnabel CA, Sullivan PS, Zhang Y, Huang VJ, Erlander MG, Brachtel EF, Dry SM. A 92-gene cancer classifier predicts the site of origin for neuroendocrine tumors. Mod Pathol. 2014;27(1):44–54. doi: 10.1038/modpathol.2013.105. [DOI] [PubMed] [Google Scholar]
- 53.Sherman SK, Maxwell JE, Carr JC, Wang D, Bellizzi AM, Sue O’Dorisio M, O’Dorisio TM, Howe JR. Gene expression accurately distinguishes liver metastases of small bowel and pancreas neuroendocrine tumors. Clin Exp Metastasis. 2014;31(8):935–944. doi: 10.1007/s10585-014-9681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Modlin IM, Kidd M, Bodei L, Drozdov I, Aslanian H. The clinical utility of a novel blood-based multi-transcriptome assay for the diagnosis of neuroendocrine tumors of the gastrointestinal tract. Am J Gastroenterol. 2015;110(8):1223–1232. doi: 10.1038/ajg.2015.160. [DOI] [PubMed] [Google Scholar]
- 55.Keck KJ, Breheny P, Braun TA, Darbro B, Li G, Dillon JS, Bellizzi AM, O’Dorisio TM, Howe JR. Changes in gene expression in small bowel neuroendocrine tumors associated with progression to metastases. Surgery. 2018;163(1):232–239. doi: 10.1016/j.surg.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ear PH, Li G, Wu M, Abusada E, Bellizzi AM, Howe JR (2019) Establishment and characterization of small bowel neuroendocrine tumor spheroids. J Vis Exp 152. 10.3791/60303 [DOI] [PMC free article] [PubMed]