Abstract
Evidence from current studies show that squamous cell carcinomas at oral and oropharyngeal sites are distinct and unique, with their own separate etiopathogenesis, treatment, and prognosis. The aim of this work is to correlate p16 immunohistochemical expression with histomorphological features suggestive of HPV infection in oral and oropharyngeal squamous cell carcinoma. A total of 50 consecutive biopsy cases of oral squamous cell carcinoma (OSCC) and 50 consecutive biopsy cases of oropharyngeal squamous cell carcinoma (OPSCC) were evaluated for features suggestive of HPV infection like focal basaloid appearance, nests, and lobules of tumor cells with pushing borders, absence of stromal reaction, central necrosis, focal lymphoepithelial morphology, presence of koilocytes, and non-keratinizing or hybrid morphology. Immunostaining was performed using p16 monoclonal antibody (clone mouse 16P04). Only cases showing a moderate (2+) to high intensity (3+) staining in more than 75% cells were taken as p16 immunopositive. The histological features were correlated with p16 immunopositivity. A total of 18/50 (36%) cases of oral squamous cell carcinoma and 27/50 (54%) cases of oropharyngeal squamous cell carcinoma were p16 immunopositive. On statistical analysis, only nests/lobules with pushing borders were found to have a significant correlation with p16 immunopositivity (P value = 0.0012) for OSCC cases. For OPSCC cases, four histological features namely nests and lobules with pushing borders (P value = 0.0001), focal basaloid appearance (P value = 0.0041), lymphoepithelial morphology (P value = 0.0029), and non-keratinizing/hybrid morphology (P value = 0.0141) had a significant correlation with p16 immunopositivity. Histomorphological features are more helpful in predicting p16 immunopositivity in OPSCC than OSCC.
Keywords: p16 immunohistochemistry, Oral squamous cell carcinoma, Oropharyngeal squamous cell carcinoma, Histomorphological features
Introduction
Anatomically, the oral cavity and oropharynx border each other but do not overlap and hence are separate regions. Oral cavity includes subsites like the labial mucosa, buccal mucosa, floor of mouth, alveolar ridge and gingiva, anterior two-thirds of the tongue (anterior to the circumvallate papillae), hard palate, and retromolar trigone. The oropharynx includes the soft palate, base (or posterior one-third) of tongue, palatine tonsils, palatoglossal folds, vallecula, and posterior pharyngeal wall. Distinct anatomic borders that separate the two sites are
from above, the junction of the hard and soft palate, and
from below, the circumvallate papillae [1].
Evidence from current studies shows that tumors at these two different sites are distinct and unique, with their own separate etiopathogenesis, treatment, and prognosis [2]. Oral cavity squamous cell carcinoma (OSCC) is the most common carcinoma in the HNSCC. However, there has been a dramatic rise in the incidence of oropharyngeal squamous cell carcinoma (OP-SCC) [3]. Overall, the number of HNSCC is showing a decreasing trend worldwide, probably because of the decrease in uses of tobacco and alcohol [3], but epidemiological studies over the last 20 years have revealed a steady growth in the overall incidence of young patients (under 45 years) [3] having HNSCC, especially oral/oropharyngeal squamous cell carcinomas (OSCC/OPSCC). Shiboski CH et al. conducted a study that showed an increase in the incidence of OPSCC particularly in the base of the tongue and tonsillar subsites by 2–3% annually for the time period of 1973–2001 and by 5.22% annually from 2000 to 2004 in the USA [4]. A study by Annertz K et al. suggested that due to this increase, the annual number of HPV-associated OPSCC in the USA can soon overtake the incidence of invasive cervical cancer cases by 2020 [5].
Based on accumulating evidence from epidemiologic, clinicopathologic, histomorphological, and molecular studies, HPV is now considered as a major etiologic factor for a subset of HNSCC [2].
The prevalence of high-risk HPV DNA in OSCC and OPSCC shows regional geographic differences. If we discuss the prevalence separately, for OPSCC, highest prevalence was reported (approximately 60%) in North America; intermediate (approximately 36 to 45%) in Asia, Oceania, and Europe; and low (approximately 15%) in South and Central America [6–8]. However, for OSCC, high-risk HPV DNA prevalence has been reported to be highest in Asia (25% for HPV-16) [8].
After infection with HPV, p16 is expressed abundantly because of the interaction between pRb and E7 viral protein [9]. Integration of the HPV genome into the host is a common feature of cervical cancer and indicates a poorer clinical outcome. But the integration of HPV genome in OSCC and OPSCC and its relation to the clinical outcome does not follow the same pattern. Study by Deng Z et al. identified 39% of these tumors with integrated DNA and the remaining 61% with episomal viral genomes [9, 10]
Given the importance of identifying HPV-related HNSCC, investigators have debated the merits and demerits of different techniques of HPV detection. However, as Westra WH highlighted [11], these HNSCCs have distinct morphologic features which allow categorization of HPV status to begin with hematoxylin and eosin features. Knowledge about these features can help the histopathologist to be alert about the presence of HPV and guide further testing for HPV.
It has been speculated that the reticulated epithelium lining the tonsillar crypts provides an immune privileged site for HPV. Basaloid appearance of the epithelium, dense lymphoid infiltrate, and small nests and lobules of the epithelial cells are all features of this reticulated epithelium. All these features of reticulated epithelium should theoretically be retained in the HPV-related HNSCC since most of the HPV-related HNSCC arise from the tonsillar crypts. [11]
Koilocytes have been one of the first histopathological features studied in HNSCC. Though the initial studies showed a high correlation of koilocytes with HPV detection, later studies have disputed this claim [12, 13].
Aim
To correlate p16 expression with histomorphological features suggestive of HPV infection in oral and oropharyngeal squamous cell carcinoma.
Material and Methods
A total of 100 biopsy cases were taken
Oral squamous cell carcinoma—50 consecutive cases.
Oropharyngeal squamous cell carcinoma—50 consecutive cases.
The patient information, clinical detail, and all relevant details of the patients were taken from histopathological requisition forms and patient records. All tumor samples were evaluated for the histological type and grade and features suggestive of HPV infection:
Focal basaloid appearance
Nests and lobules of tumor cells with pushing borders
Absence of stromal reaction
Central necrosis
Focal lymphoepithelial morphology
Koilocytes
Non-keratinizing or hybrid morphology. Non-keratinizing morphology was defined as a tumor composed of sheets and nests of oval to spindle cells with hyperchromatic nuclei and minimal or no keratinization (less than 10% of tumor area). The hybrid type was defined as having two components-a non-keratinizing component and a keratinizing component (> 10% areas showing keratinization) [11, 12, 14]
Blocks from oral and oropharyngeal squamous cell carcinoma biopsy cases were routinely processed for p16 immunostaining. Immunostaining was performed using p16 monoclonal antibody (Bio SB clone mouse 16P04, prediluted, ready to use). A positive control of known p16-positive high-grade cervical dysplasia was run with each batch of test slides. A negative control, wherein the primary antibody was omitted, was also put with each batch of the test slides to maintain the validity of immunostaining procedure. The sections were then evaluated in terms of the percentage of cells showing positivity, intensity of staining and the location of immune-positivity in the cells, i.e., cytoplasmic or nuclear. Staining scores were established by semiquantitative optical analysis, staining intensity from 1+ to 3+ (1+ weak, 2+ moderate and 3+ strong).
Staining was graded in a quartile manner for its extent as follows: 0—Negative, 1+—1 to 25% of cells positive, 2+—26 to 50 % of cells positive, 3+—51 to 75% of cells positive, 4+—> 75% cells positive. For the purpose of analysis, the cases were classified in a binary manner according to recent AJCC criteria as either positive (both nuclear and cytoplasmic) (grade 4 or > 75% with intensity 2+/3+) or negative [15].
The histological features predictive of p16 immunopositivity were correlated with the proportion of cases showing p16 immunopositivity by Fischer’s exact test (two tailed).
Consent
Informed patient consent was taken.
Ethical Clearance
Clearance was taken from the Institutional Ethics Committee—Human Research.
Result
A total of 100 biopsy cases, 50—oral and 50—oropharyngeal squamous cell carcinoma, were included in the study. The important demographic and other data pertaining to the cases are summarized in Table 1. The p16 immunopositivity was graded according to percentage of cells and intensity of staining (Table 2; Figs. 1 and 2).
Table 1.
Summary of the cases included in the study
| S. no | Parameter | Value |
|---|---|---|
| 1. | Total number of OSCC biopsy cases | 50 |
| 2 | Total number of OPSCC biopsy cases | 50 |
| 3 | Average age (range) of the OSCC patients in years | 50 (26–75) |
| 4 | Average age (range) of the OPSCC patients in years | 54.5 (32–75) |
| 5 | Male: Female distribution amongst OSCC cases | 45:5 |
| 6 | Male: Female distribution amongst OPSCC cases | 46:4 |
| 7 | Distribution of OSCC cases by site—buccal mucosa: Lateral border of tongue: hard palate: retromolar trigone | 27:19:3:1 |
| 8 | Distribution of OPSCC cases by site—soft palate:tonsil: base of tongue | 4:13:33 |
| 9 | OSCC patients having history of tobacco smoking | 46/50 |
| 10 | OSCC patients having history of tobacco/betel/areca nut chewing | 43/50 |
| 11 | OPSCC patients having history of tobacco smoking | 48/50 |
| 12 | OPSCC patients having history of tobacco/betel/areca nut chewing | 45/50 |
Table 2.
p16 immunopositivity according to percentage of cells (grade) and intensity
| Percentage of cells (Grade) | Intensity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 0 | 1 | 2 | 3 | 4 | Total | 0 | 1 | 2 | 3 | |
| OSCC | 50 | 19 | 2 | 8 | 3 | 18 | 50 | 20 | 10 | 18 | 2 |
| OPSCC | 50 | 15 | 1 | 4 | 3 | 27 | 50 | 19 | 3 | 14 | 14 |
| OSCC | 50 | No. of cases taken as p16 immunopositive-18 | |||||||||
| OPSCC | 50 | No. of cases taken as p16 immunopositive-27 | |||||||||
Figures in italics shows positivity by AJCC criteria
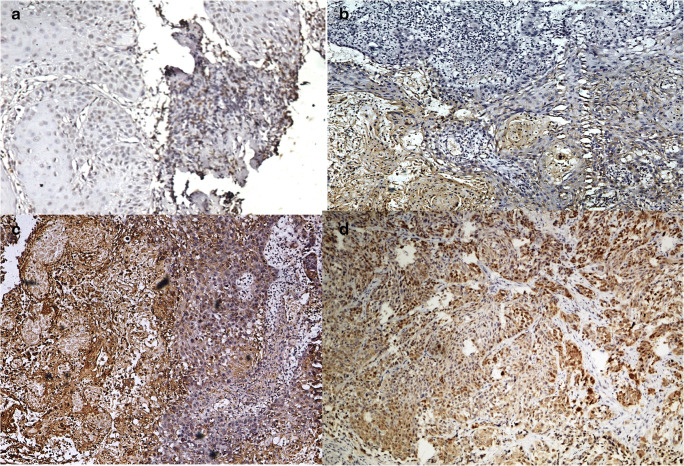
Fig. 1.
Different grades of p16 immunohistochemistry based on the percentage of immunopositive cells. a (200×) Grade 1: 0 to 25% immunopositive cells. b (200×) Grade 2: 25 to 50% immunopositive cells. c (200×) Grade 3: 50 to 75% immunopositive cells. d (200×) Grade 4: 75 to 100% immunopositive cells. For the purpose of analysis only, grade 4 cases with intensity of 2/3+ were considered positive for p16
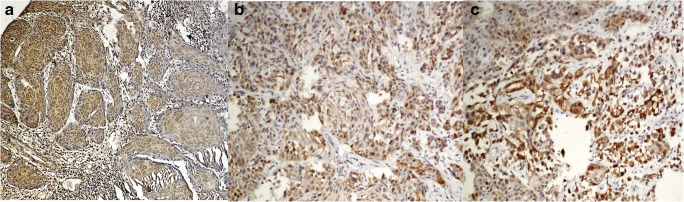
Fig. 2.
Different intensities of p16 immunopositivity. a (200×) 1+ intensity score. b (200×) 2+ intensity score. c (200×) 3+ intensity score
Hence, according to the recent AJCC criteria for p16 immunopositivity, 18/50 cases of OSCC and 27/50 cases of OPSCC were p16 positive (more than 75% cells with both nuclear and cytoplasmic staining with intensity of 2+ or 3+) (Table 2).
The relation between different histomorphological features and p16 immunopositivity is summarized in Table 3, and the histologic features are depicted in Figs. 3 and 4. On statistical analysis, only nests/lobules with pushing borders were found to have a significant correlation with p16 immunopositivity (P value = 0.0012) for OSCC cases. For OPSCC cases, four histological features namely nests and lobules with pushing borders (P value = 0.0001), focal basaloid appearance (P value = 0.0041), lymphoepithelial morphology (P value = 0.0029), and non-keratinizing /hybrid morphology (P value = 0.0141) had a significant correlation with p16 immunopositivity.
Table 3.
Distribution of p16 positive cases of OSCC and OPSCC with different histomorphological features associated with HPV
| Histological features | Number of cases with the feature (OSCC) | Cases positive for p16 with the feature (OSCC) | P value | Number of cases with the feature (OPSCC) | Cases positive for p16 with the feature (OPSCC) | P value |
|---|---|---|---|---|---|---|
| Nest/lobules with pushing borders | 26 | 15 | 0.0012 | 34 | 26 | 0.0001 |
| Focal basaloid appearance | 32 | 11 | 0.7678 | 25 | 19 | 0.0041 |
| Absence of stromal reaction | 9 | 5 | 0.2534 | 11 | 5 | 0.733 |
| Central necrosis | 11 | 6 | 0.1718 | 4 | 2 | 1.000 |
| Focal lymphoepithelial morphology | 4 | 3 | 0.1267 | 12 | 11 | 0.0029 |
| Presence of koilocytes | 21 | 10 | 0.2324 | 19 | 13 | 0.1478 |
| Non-keratinizing or hybrid morphology | 28 | 13 | 0.1373 | 39 | 25 | 0.0141 |
Figures in italics shows significant P value of less than 0.05
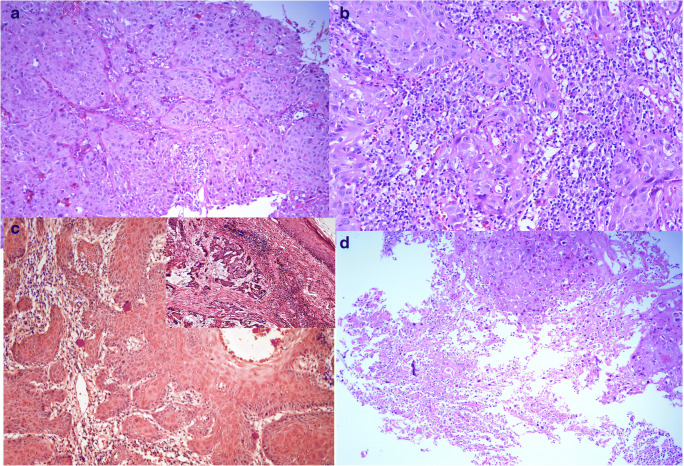
Fig. 3.
Histomorphological features suggestive of HPV-associated carcinoma. a (Hematoxylin and eosin, 200×) nests and lobules of tumor cells with pushing borders. b (Hematoxylin and eosin, 200×) lymphoepithelial morphology. c (Hematoxylin and eosin, 200×) absence of stromal reaction. c (Inset) (hematoxylin and eosin, 200×) focal basaloid appearance. d (Hematoxylin and eosin, 200×) central necrosis
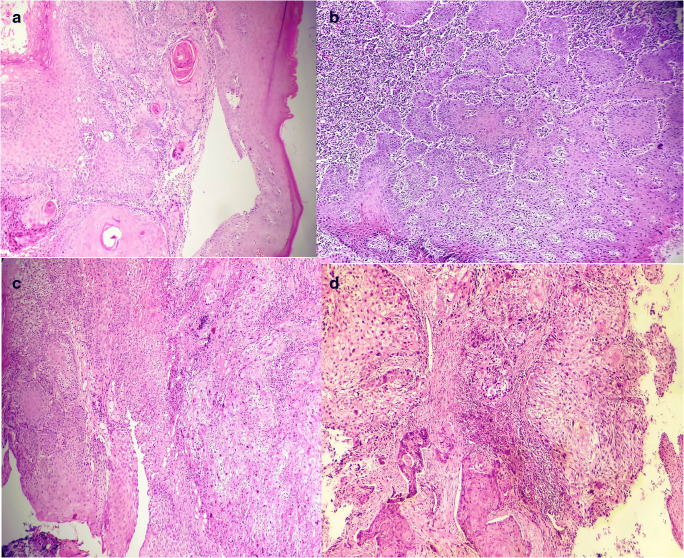
Fig. 4.
a Keratinizing squamous cell carcinoma (hematoxylin and eosin, 200×). b Non-keratinizing squamous cell carcinoma (hematoxylin and eosin, 200×). c Hybrid morphology-both keratinizing and non-keratinizing (hematoxylin and eosin, 200×). d Oral squamous cell carcinoma with koilocytes (hematoxylin and eosin, 200×)
Discussion
In India, different studies have given varying prevalence for HPV-associated HNSCC. Different studies have found a prevalence of 33.6% in the Eastern region, 48% in South India, 15% in West India, 27.5% in Central India, and 28% in Northeast India amongst OSCC [16–20]. According to western studies, prevalence was found to be between 28 and 68% in OPSCC cases [21]. Another study by Bahl et al. reported 22.8% HPV positivity [21], Murthy et al. reported 20% [22], and Sannigrahi et al. reported 15% positivity amongst OPSCC cases [23]. The overall p16 immunopositivity of 36% for OSCC and 54% for OPSCC in our study is in concordance with previous published data.
p16 overexpression may also be caused due to functional pRb disturbances, hence is not always due to HPV infection. Pannone et al. found 100% sensitivity of p16 immunostaining and no false negative for HPV-associated OSCC cases [24], but with a specificity rate of only 74% and 26% false positive (p16 positive; HPV negative) [24].
Shain AF et al. evaluated the performance of different p16 clones, comparing them with the widely used E6H4 (CINtec) in detecting high-grade squamous intraepithelial lesion .p16 clones 16P04 and JC8 performed equally well or even better than E6H4 [25]. Another study by Shelton J et al. compared the performance characteristics of E6H4, JC8 and G175-405 and found that E6H4 was best in terms of lowest inter observer variability. The other issue is cost per patient with the E6H4 clone being the most expensive while the other alternatives are cheaper [26]. We used 16P04 clone of the p16 antibody as a reasonable alternative to E6H4 clone in our study.
Previous studies on morphology of HPV-related HNSCC have concentrated on the degree of keratinization of HNSCC as a predictor of p16 immunopositivity. The non-keratinizing squamous cell carcinomas and hybrid type carcinomas having both keratinizing and non-keratinizing areas have a higher rate of p16 immunopositivity than keratinizing squamous cell carcinoma. [27] Another study showed that multinucleation and anaplasia in tumor cells is associated with a worse clinical outcome despite having HPV-related HNSCC morphology. [14]
The relationship of koilocytes with p16 immunopositivity has been controversial with some studies supporting and some studies refuting it [12, 13]. In our study, the correlation of koilocytes with p16 positivity did not reach statistical significance. According to Westra WH, HPV-related HNSCC does not follow the progression through different stages of dysplasia to carcinoma. The transition between HPV-related carcinoma and surrounding normal epithelium is more abrupt than the non-HPV-related HNSCC. This explains a fairly large number of cases not showing koilocytes (which are a marker for early dysplasia) and yet are immunopositive for p16.
According to a study by Zaferio ME et al., up to one-third cases of OSCC are p16 positive but when PCR is done on these cases; only 28% are positive for HPV, and with ISH (in situ hybridization), the positivity further decreases to only 1–10% [28, 29]. In a large number of OSCC cases, p16 immunopositivity is possibly due to non-HPV pathway. This may be an explanation for the absence of correlation of histologic predictors with p16 immunopositivity in OSCC in our study. For OPSCC, majority of p16 immunopositive cases follow the HPV pathway. It has been speculated that most of the HPV-related HNSCC arise from the tonsillar crypts [11]. This is possibly an explanation for the far better correlation noted between the histologic predictors and p16 immunopositivity in OPSCC cases in our study. Hence, based on our findings, we suggest that p16 immunopositivity in OPSCC is a better surrogate for HPV-related HNSCC as compared to OSCC. In OPSCC cases with classic morphology of HPV-related HNSCC, p16 immunopositivity may be considered sufficient evidence of HPV infection. However, in OSCC cases and in OPSCC cases with non-classical morphology, further testing is warranted to prove HPV association. A non-HPV-related pathway may be the cause of p16 immunopositivity in such cases.
The current guidelines call for p16 immunostaining in all cases of OPSCC. Our study found a fairly high percentage of p16 immunopositive cases amongst OSCC (36%) in addition to the expected high percentage amongst the OPSCC cases. This points towards a possible role in the etiopathogenesis of OSCC cases as well. However, further studies with HPV-PCR and ISH are needed to confirm this.
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (Institutional Ethics Committee—Human Research approval vide Letter IEC-HR-2017/32/106) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. This study was supported by Intramural Research Grant from the college.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma--an update. CA Cancer J Clin. 2015;65(5):401–421. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- 2.Deschler DG, Richmon JD, Khariwala SS, Ferris RL, Wang MB. The “new” head and neck cancer patient-young, nonsmoker, nondrinker, and HPV positive: evaluation. Otolaryngol Head Neck Surg. 2014;151:375–380. doi: 10.1177/0194599814538605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturgis EM, Ang KK. The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? J Natl Compr Cancer Netw. 2011;9(6):665–673. doi: 10.6004/jnccn.2011.0055. [DOI] [PubMed] [Google Scholar]
- 4.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 5.Annertz K, Anderson H, Biorklund A, Moller T, Kantola S, Mork J, et al. Incidence and survival of squamous cell carcinoma of the tongue in Scandinavia, with special reference to young adults. Int J Cancer. 2002;101:95–99. doi: 10.1002/ijc.10577. [DOI] [PubMed] [Google Scholar]
- 6.Martel C, Ferlay J, Franceschi S. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 7.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 8.Ndiaye C, Mena M, Alemany L, Arbyn M, Castellsagué X, Laporte L, Bosch FX, de Sanjosé S, Trottier H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319–1331. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 9.Deng Z, Hasegawa M, Aoki K, Matayoshi S, Kiyuna A, Yamashita Y, Uehara T, Agena S, Maeda H, Xie M, Suzuki M. A comprehensive evaluation of human papillomavirus positive status and p16INK4a overexpression as a prognostic biomarker in head and neck squamous cell carcinoma. Int J Oncol. 2014;45(1):67–76. doi: 10.3892/ijo.2014.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olthof NC, Speel EM, Kolligs J, Haesevoets A, Henfling M, Ramaekers FCS, et al. Comprehensive analysis of HPV16 integration in OSCC reveals no significant impact of physical status on viral oncogene and virally disrupted human gene expression. PLoS One. 2014;9:e88718. doi: 10.1371/journal.pone.0088718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westra WH. The morphologic profile of HPV-related head and neck squamous carcinoma: implications for diagnosis, prognosis, and clinical management. Head Neck Pathol. 2012;6:S48–S54. doi: 10.1007/s12105-012-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Qahtani K, Brousseau V, Paczesny D, Domanowski G, Hamid Q, Hier M, et al. Koilocytosis in oral squamous cell carcinoma: what does it mean? J Otolaryngol. 2007;36(1):26–31. doi: 10.2310/7070.2006.0049. [DOI] [PubMed] [Google Scholar]
- 13.Miyahara GI, Simonato LE, Mattar NJ, Camilo DJ, Jr, Biasoli ER. Correlation between koilocytes and human papillomavirus detection by PCR in oral and oropharynx squamous cell carcinoma biopsies. Mem Inst Oswaldo Cruz. 2011;106(2):166–169. doi: 10.1590/s0074-02762011000200008. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JS, Jr, Scantlebury JB, Luo J, Thorstad WL. Tumor cell anaplasia and multinucleation are predictors of disease recurrence in oropharyngeal squamous cell carcinoma, including among just the human papillomavirus-related cancers. Am J Surg Pathol. 2012;36(7):1036–1046. doi: 10.1097/PAS.0b013e3182583678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, Loomis AM, Shah JP. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 16.Nagpal JK, Patnaik S, Das BR. Prevalence of high-risk human papilloma virus types and its association with P53 codon 72 polymorphism in tobacco addicted oral squamous cell carcinoma (OSCC) patients of Eastern India. Int J Cancer. 2002;97:649–653. doi: 10.1002/ijc.10112. [DOI] [PubMed] [Google Scholar]
- 17.Elango KJ, Suresh A, Erode EM, Subhadradevi L, Ravindran HK, Iyer SK, Iyer SK, Kuriakose MA. Role of human papilloma virus in oral tongue squamous cell carcinoma. Asian Pac J Cancer Prev. 2011;12:889–896. [PubMed] [Google Scholar]
- 18.D’Costa J, Saranath D, Dedhia P, Sanghvi V, Mehta AR. Detection of HPV-16 genome in human oral cancers and potentially malignant lesions from India. Oral Oncol. 1998;34:413–420. doi: 10.1016/s1368-8375(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 19.Gheit T, Vaccarella S, Schmitt M, Pawlita M, Franceschi S, Sankaranarayanan R, Sylla BS, Tommasino M, Gangane N. Prevalence of human papillomavirus types in cervical and oral cancers in central India. Vaccine. 2009;27:636–639. doi: 10.1016/j.vaccine.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R, Rai AK, Das D, Das R, Kumar RS, Sarma A, et al. Alcohol and tobacco increases risk of high risk HPV infection in head and neck cancer patients: study from north-east region of India. PLoS One. 2015;10:e0140700. doi: 10.1371/journal.pone.0140700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahl A, Kumar P, Dar L, Mohanti BK, Sharma A, Thakar A, Karthikeyan V, Sikka K, Singh C, Poo K, Lodha J. Prevalence and trends of human papillomavirus in oropharyngeal cancer in a predominantly north Indian population. Head Neck. 2014;36(4):505–510. doi: 10.1002/hed.23317. [DOI] [PubMed] [Google Scholar]
- 22.Murthy V, Swain M, Teni T, Pawar S, Kalkar P, Patil A, Chande A, Ghonge S, Laskar SG, Gupta T, Budrukkar A, Agrawal J. Human papillomavirus/p16 positive head and neck cancer in India: prevalence, clinical impact, and influence of tobacco use. Indian J Cancer. 2016;53(3):387–393. doi: 10.4103/0019-509X.200668. [DOI] [PubMed] [Google Scholar]
- 23.Sannigrahi MK, Singh V, Sharma R, Panda NK, Radotra BD, Khullar M. Detection of active human papilloma virus-16 in head and neck cancers of Asian North Indian patients. Oral Dis. 2016;22(1):62–68. doi: 10.1111/odi.12382. [DOI] [PubMed] [Google Scholar]
- 24.Pannone G, Rodolico V, Santoro A. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 immunohistochemistry, consensus PCR HPV-DNA, and in situ hybridization. Infect Agent Cancer. 2012;7:1–14. doi: 10.1186/1750-9378-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shain AF, Wilbur DC, Stoler MH, Quade BJ, Kong CS. Test characteristics of specific p16 clones in the detection of high-grade squamous intraepithelial lesions (HSIL) Int J Gynecol Pathol. 2018;37(1):82–87. doi: 10.1097/PGP.0000000000000391. [DOI] [PubMed] [Google Scholar]
- 26.Shelton J, Purgina BM, Cipriani NA, Dupont WD, Plummer D, Lewis JS., Jr p16 immunohistochemistry in oropharyngeal squamous cell carcinoma: a comparison of antibody clones using patient outcomes and high-risk human papillomavirus RNA status. Mod Pathol. 2017;30(9):1194–1203. doi: 10.1038/modpathol.2017.31. [DOI] [PubMed] [Google Scholar]
- 27.Mendelsohn AH, Lai CK, Shintaku IP, Elashoff DA, Dubinett SM, Abemayor E, et al. Histopathologic findings of HPV and p16 positive HNSCC. Laryngoscope. 2010;120(9):1788–1794. doi: 10.1002/lary.21044. [DOI] [PubMed] [Google Scholar]
- 28.Zafereo ME, Xu L, Dahlstrom KR, Viamonte CA, El-Naggar AK, Wei Q, et al. Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral Oncol. 2016;56:47–53. doi: 10.1016/j.oraloncology.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuschenbach M, Kansy K, Garbe K, Vinokurova S, Flechtenmacher C, Toth C, et al. Lack of evidence of human papillomavirus-induced squamous cell carcinomas of the oral cavity in southern Germany. Oral Oncol. 2013;49(9):937–942. doi: 10.1016/j.oraloncology.2013.03.451. [DOI] [PubMed] [Google Scholar]