Abstract
Context
The therapeutic role of D2 lymphadenectomy in the management of gastric cancer is an ongoing controversy.
Aims
To examine the morbidity and oncological outcomes of D2 lymph node dissection for gastric cancer patients treated in a stand-alone cancer center in rural India and to compare it with international data.
Settings and Design
Retrospective study on patients treated for gastric cancer from June 2009 to December 2014.
Methods and Material
All patients underwent subtotal or total gastrectomy with modified D2 lymph node dissection preserving spleen and pancreas. The Clavien-Dindo model was used to stratify the severity of morbidity.
Statistical analysis
Descriptive statistics was used for data exploration. Chi-square test was used to compare the association of various factors with survival. Kaplan-Meier method was used to calculate the survival rates (RFS and DFS). Log-rank test was used to compare the survival of different subgroups.
Results
Fifty-four patients (41 males and 13 females) were included in the study. Four (7.4%) patients had significant postoperative morbidity. The 5-year OS and DFS respectively were 34.9% and 37.6%. Female sex was associated with poorer survival. Lymph node ratio of more than 0.2 and advanced stage at presentation showed strong tendency towards lower OS and DFS.
Conclusions
An R0 resection with D2 lymphadenectomy for gastric cancer carries acceptable morbidity and mortality in Indian patients with survival rates comparable with the western studies. Lymph node ratio more than 0.2 and female gender and advanced stage were associated with poorer oncological outcomes.
Keywords: D2 lymphadenectomy, Stomach cancer, Gastrectomy, Lymph node ratio
Introduction
Stomach cancer is the fourth largest killer among all cancers worldwide [1]. It is the fifth most common malignancy in the world, preceded by cancers of the lung, breast, colorectum, and prostate. More than 70% of cases occur in developing countries. The highest estimated mortality rates are found in Eastern Asia (24 per 100,000 among men and 9.8 per 100,000 in women) while the lowest rates are seen in Northern America (2.8 and 1.5, respectively among men and women) [2].
In India, the incidence of gastric cancer is approximately 34,000 per year, with a male preponderance (male-to-female ratio, 2:1). As per a recent survey of cancer-related mortality in India, gastric carcinoma was the second most common cause among men and women alike [3] with the highest incidence reported from the North East [3].
Radical gastrectomy with systematic regional lymphadenectomy remains the primary modality of treatment for non-metastatic gastric cancer. East Asian surgeons, especially Japanese and Korean surgeons, routinely perform D2 lymph node dissection. However, the western counterparts have not accepted D2 lymphadenectomy as standard procedure and continue to perform D1 dissection. Some trials from the west have shown mixed results of morbidity and survival [4–8]. The higher morbidity and uncertain oncological benefit had been bone of contention for accepting D2 lymphadenectomy as a standard surgical procedure in several centres in the west.
In this retrospective study, we examined the oncological outcome and morbidity associated with gastrectomy with D2 lymph node dissection in a tertiary cancer centre in South India.
Materials and Methods
This is a retrospective study of patients with stomach cancer who were treated with radical gastrectomy and D2 lymph node dissection in a tertiary cancer centre from June 2009 to December 2014. All patients with operable gastric cancer were included. Patients with metastatic disease and those who were not fit for radical surgery were excluded.
Surgery and Adjuvant Treatment
Distal subtotal gastrectomy, total gastrectomy and proximal gastrectomy were the procedures performed for gastric cancer. An open approach with midline incision was used. All patients underwent standard D2 lymph node dissection with clearance of nodal stations required as per the location of the tumour within the stomach. For distal tumours, nodal stations 1, 3, 4d, 5, 6, 7, 8a, 9, 11 and 12a were cleared. For proximal cancer stations 1, 2, 3,4sa, 4sb, 4d, 5, 6, 7, 8a, 9, 10, 11 and 12a, nodes were removed. Elective splenectomy was avoided.
The reconstruction was done in all patients using Roux-en-Y loop of the jejunum. Gastrojejunal anastomosis was done using lineal cutter stapler in antecolic, side to side fashion. Circular stapler of size 25 mm was used for performing esophagojejunal anastomosis in patients with total gastrectomy. Feeding jejunostomy was routinely performed. Duodenal stump was oversewn over the staple line regularly.
Adjuvant combination chemotherapy with epirubicin (50 mg/M2), oxaliplatin (130 mg/M2) and capecitabine (1000 mg/M2 twice daily for 14 days) was administered within 4 weeks in patients with >T2 or N+ disease. Adjuvant radiotherapy was additionally given in the case of margin positivity.
The Clavien-Dindo model was used to express postoperative morbidity. Significant morbidity was defined as any grade above IIIA. Procedure-related mortality was defined as death within 1 month of the surgical procedure.
Histopathological reports were analysed carefully and pertinent pathological variables were collected.
Follow-up
Patients were followed at intervals of 3 months until 2 years, six monthly until 5 years and yearly thereafter. At each visit, a detailed history and clinical examination was performed. Body weight was documented. Complete blood count and serum biochemical evaluation were obtained whenever clinically indicated. Imaging and/or endoscopy was done based on symptomatology or clinical suspicion of tumour recurrence.
Data Collection and Statistical Analysis
Data regarding demographic profile, comorbidities, details of surgery and follow-up was retrieved from case records. Descriptive statistical analysis was used for data exploration. Chi-square test was used to compare the association of various factors with survival. Kaplan-Meier method was used to calculate the survival rates (overall survival [OS] and disease-free survival [DFS]). Log-rank test was used to compare the survival of different subgroups under study. OS was defined as the period from date of surgery until death due to any cause or last follow-up (whichever was earlier). DFS was defined as the period from the date of surgery to the development of recurrent disease or last follow-up. Lymph node ratio (LNR) was calculated by dividing the number of lymph nodes positive for metastasis with the total number of lymph nodes dissected. A p value of < 0.05 was considered significant.
Results
The total number of patients included was 54. The patient profile and histological variables are mentioned in Table 1. The numbers of patients who had undergone distal subtotal, total and proximal gastrectomy were 37 (68%), 15 (28%) and 2 (4%) respectively. The average lymph node yield was 25.1 and a median of 23.
Table 1.
Demographics and tumor characteristics
| VARIABLES | NUMBER | PERCENTAGE | |
|---|---|---|---|
| Gender | Males | 41 | 76 |
| Females | 13 | 24 | |
| Age groups | < 60 years | 27 | 50 |
| 60-70 years | 20 | 37.03 | |
| > 70 years | 07 | 12.96 | |
| Comorbidities | Diabetes | 05 | 9.25 |
| Hypertension | 03 | 5.55 | |
| Pulmonary | 01 | 1.85 | |
| Multiple | 05 | 9.25 | |
| Others | 03 | 5.55 | |
| Stage | IA | 5 | 9.25 |
| IB | 8 | 14.81 | |
| IIA | 8 | 14.81 | |
| IIB | 11 | 20.37 | |
| IIIA | 10 | 18.52 | |
| IIIB | 8 | 14.81 | |
| IIIC | 4 | 7.41 | |
| Tumour location | Pylorus and distal body | 39 | 72.22 |
| Proximal body, fundus,cardia | 11 | 20.37 | |
| Linitisplastica | 4 | 7.40 | |
| pT category | T1 | 05 | 9.25 |
| T2 | 20 | 37.03 | |
| T3 | 19 | 35.18 | |
| T4 | 10 | 18.51 | |
| pN category | N0 | 21 | 38.89 |
| N1 | 8 | 14.81 | |
| N2 | 11 | 20.37 | |
| N3 | 14 | 25.93 | |
| Histology | WDAC-MDAC | 27 | 50 |
| PDAC | 18 | 33.33 | |
| Mucin secreting adenocarcinoma | 4 | 7.41 | |
| Signet ring cell type | 5 | 9.26 | |
| Invasion | Lymphovascular | 13 | 24.07 |
| Perineural | 15 | 27.77 | |
| Both | 10 | 18.51 | |
| No invasion | 37 | 68.51 | |
Margin positivity was seen in five patients (9.26%): two patients with tumour of the distal stomach had positive distal margin from sub-mucosal spread in the duodenal end; two patients had linitis plastica and one patient with proximal gastric cancer had deposits of tumour at the resected margins which could not be improved even after re-resection. Adjuvant radiation therapy was offered to these patients.
Postoperative 30-Day Morbidity and Mortality
Four (7.4%) patients had significant postoperative morbidity. The details are depicted in Table 2.
Table 2.
Postoperative morbidity and thirty day mortality
| CLAVIEN-DINDO | NUMBER (n=8) | DETAILS |
|---|---|---|
| IIIA | 4 | Wound infection requiring drainage and dressing |
| IIIB | 1 | Burst abdomen, re-suturing under GA |
| IV | 1 | Internal bleeding from left gastric vein. Re-exploration and successful control of haemorrhage. |
| V | 2 |
- Duodenal stump leak with sepsis. Patient died of multi-organ failure. - Severe resistant pulmonary infection in the postoperative period leading to septicemia and death. |
Recurrences
Twenty-six (48.15%) patients were diagnosed with recurrent disease: twelve had peritoneal recurrences while 14 had recurrences in the liver, lung, adrenals, brain, umbilicus, subcutaneous sites or multiple sites.
Survival and Factors Predicting Cumulative Long-term Survival
The estimated mean OS was 52.88 ± 6.75 months (95% CI = 39.65–66.10 months) and estimated mean DFS was 53.43 ± 7.01 months (95% CI = 39.69–67.16 months). The corresponding 5-year OS was 34.9% and DFS was 37.6%. The median follow-up was 48.3 months.
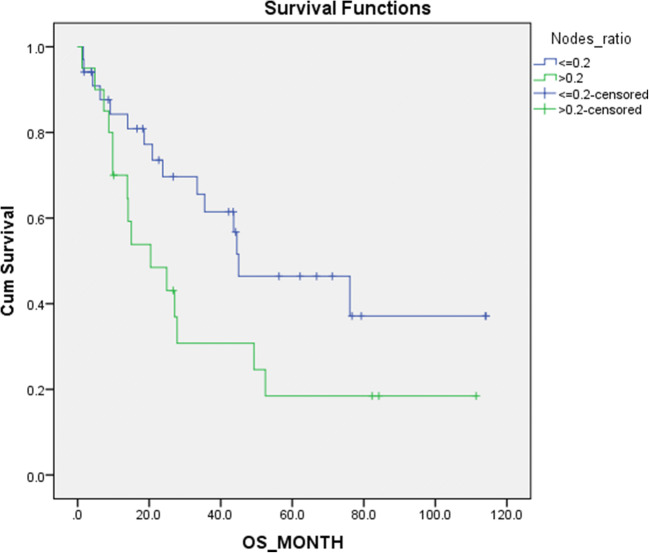
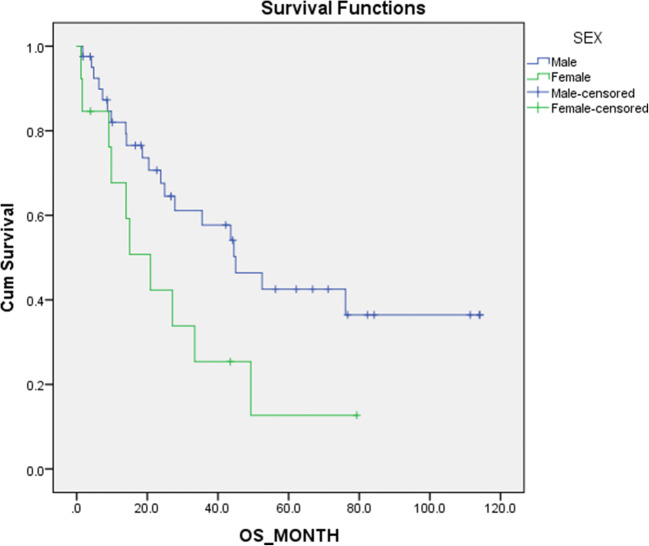
The influence of age, gender, disease stage, site of disease and LNR were analysed for the influence on OS and DFS. Our findings are shown in Table 3. It was noted that patients with LNR above 0.2 had a significantly worse DFS (p = 0.037) and tendency towards lower OS (p = 0.058) (Fig. 1). Female patients also had a significantly worse oncological outcome (Fig. 2). Stage III disease showed a lower OS and DFS, but statistical significance could not be achieved. Likewise, the site of disease did not show statistically significant effect on survival.
Table 3.
Various factors influencing survival
| VARIABLE | SUBGROUPS | MEAN OS (MONTHS) | p VALUE | MEAN DFS (MONTHS) | p VALUE |
|---|---|---|---|---|---|
| Age | <=50 (n=8) | 42.00 +/-9.87 | 0.982 | 39.03+/-10.53 | 0.896 |
| >50 (n=46) | 53.22+/-7.43 | 53.95+/-7.71 | |||
| Gender | Male (n=41) | 59.96+/-7.94 | 0.040 | 61.26+/-8.18 | 0.020 |
| Female(n=13) | 27.48+/-7.21 | 24.33+/-7.31 | |||
| Site of disease | Distal (n=37) | 50.26+/-7.47 | 0.406 | 51.30+/-7.80 | 0.460 |
| Proximal (n=17) | 62.85+/-14.73 | 63.20+/-14.65 | |||
| Stage | Early stage (I&II)(n=32) | 60.24+/-9.24 | 0.229 | 62.36+/-9.61 | 0.170 |
| Advanced stage (Stage III) (n=22) | 37.16+/-6.67 | 35.86+/-6.79 | |||
| Lymph node ratio | <=0.2 (n=32) | 62.56+/-8.964 | 0.058 | 64.54+/-9.31 | 0.037 |
| >0.2 (n=22) | 37.04+/-8.96 | 35.41+/-9.07 | |||
| Adjuvant chemotherapy | Received (n=28) | 53.07+/-9.75 | 0.937 | 55.21+/-10.33 | 0.786 |
| Not received (n=26) | 52.39+/-9.27 | 52.01+/-9.45 |
Fig. 1.
Cumulative survival
Fig. 2.
Comparison of survival against gender
Discussion
Radical gastrectomy with D2 lymphadenectomy has been the standard of surgical management in Japan and South Korea for decades. Several Japanese and Korean retrospective studies have demonstrated low morbidity and high survival rates with D2 lymphadenectomy [9–11].
Two large-scale RCTs performed in the west to assess the role of D2 lymphadenectomy in the management of gastric cancers were the Dutch Gastric Cancer trial [4, 12–14] and the Medical Research Council Gastric Cancer Surgical trial [5, 15]. Both trials showed higher morbidity and mortality with D2 lymph node dissection and failed to show a clear survival advantage in D2 lymphadenectomy over D1 lymphadenectomy. However, both trials have been criticised for lack of standardisation of the procedure and inadequate surgeon experience. High rate of surgical morbidity and mortality overshadowed possible advantage from D2 dissection.
Despite the lack of definite survival advantage with randomised trials, several nonrandomised studies from the west have shown acceptable morbidity and survival benefit in D2 lymphadenectomy [16–18]. Fifteen-year follow-up results of Dutch Gastric Cancer trial also concluded that there is an improvement in DFS and cancer-related death following D2 lymphadenectomy compared with D1 dissection. They recommended spleen-preserving D2 lymphadenectomy as standard surgical procedure for gastric cancer [14]. A recent Italian paper also showed possible benefit with D2 dissection in patients with high nodal burden even though statistically significant difference in survival could not be demonstrated [19]. A 2015 Cochrane review showed improved disease-specific survival with D2 lymphadenectomy. However, the therapeutic effect was reduced by increased mortality associated with D2 dissection [20]. A meta-analysis from Australia, based on 6 RCTs with three large studies from the western world, showed no difference in 5-year survival for D2 compared with D1 lymphadenectomy [21].
We have adopted D2 lymphadenectomy as standard surgical procedure for stomach cancer in our institute. All the gastric cancer surgeries were performed by single specialised unit and the techniques were standardised. In our series, significant postoperative morbidity (Clavien-Dindo IIIB or more) was encountered in 4 (7.4%) patients and there were two deaths (3.7%). Table 4 compares the morbidity and mortality of the major international trials on D2 lymphadenectomy with our study.
Table 4.
Comparison of morbidity and mortality of major published studies
| STUDY | COUNTRY | n ( D1/ D2) | MORBIDITY (%) (D1/D2 ) | MORTALITY (%) (D1/D2 ) |
|---|---|---|---|---|
| Dutch study (1989–1993) (5,12) | Netherlands | 380 / 331 | 25 / 43 | 4 / 10 |
| MRC trial (1987–1994) (5) | UK | 200 / 200 | 28 / 46 | 6.5 / 13 |
| Italian study (1999–2002) (18) | Italy | NA/ 191 | NA/ 20.9 | NA/ 3.1 |
| Taiwanese trial(1993–1999) (27) | Taiwan | D1/D3 110/111 | D1/D3 7.3/17.1 | D1/D3 0/0 |
| Shrikhande SV et al (2002-2006) (28) | India | NA/ 159 | NA/4.4 | NA/1.25 |
| Present study (2009 -2014) | India | NA/ 54 | NA/ 7.4 | NA/ 3.7 |
It has been suggested that the reason for difference in survival after treatment for gastric cancer between the eastern patients and their western counterparts is multifactorial. Higher patient age, higher body mass index, higher stage of the disease, more of proximal tumours for the western patients and stage migration with more nodal examination in the east are some of the factors which are pointed out [22]. In the presence of confounding factors like the difference in adjuvant practices and difference in tumour biology between the east and west, it is difficult to commend on the real benefit of D2 lymphadenectomy.
Lymph node ratio (LNR) has been found to be an independent prognostic factor for gastric cancers and gastro-oesophageal junction tumours [23, 24]. Female patients were found to have worse survival.
Advanced stage (stage III) was associated with worse survival compared with those with stages I and II even though statistical significance could not be achieved. We believe that this was due to small number of patients. Age and site of disease in the stomach were not found to have significant correlation with survival.
Overall survival rate of the patients in our study is comparable with that of western patients [25, 26], but inferior to their Japanese and Korean counterparts. The probable reasons for reduced survival include advanced tumour with higher node positivity and lower rate of completion of adjuvant treatment.
Conclusion
R0 resection with gastrectomy and D2 lymphadenectomy for gastric cancer is feasible in a stand-alone cancer centre in rural India. The low morbidity and mortality associated with D2 dissection along with potential survival benefit justify accepting it as the surgical standard of care in experienced hands. Lymph node positivity ratio and female sex are found to have significant adverse influence on survival in our series.
Authors’ Contribution
Nizamudheen Mangalasseri Pareekutty: concept, study design, write up
Sachin Kadam: data collection, write up
Basavaraj Ankalkoti: data collection, contributed to study
Satheesan Balasubramanian: Concept, supervised the project, editing
Bindu Anilkumar: statistical analysis
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Statement by all authors
We hereby affirm that the manuscript is the original work of the above-mentioned authors and has been read and approved by all. The authorship criteria as mentioned above have been met. We hereby agree to transfer the publication right to the Journal.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nizamudheen Mangalasseri Pareekutty, Email: drnizamudheen@gmail.com.
Sachin Kadam, Email: kool_sachin555@yahoo.com.
Basavaraj Ankalkoti, Email: basuhvr@yahoo.co.
Satheesan Balasubramanian, Email: gabas9@rediffmail.com.
Bindu Anilkumar, Email: binduanilkumarmcc@gmail.com.
References
- 1.World Health Organization. Cancer: Fact Sheet No 297. WHO. Available at http://www.who.int/mediacentre/factsheets/fs297/en/
- 2.Globocon 2012 (IARC), Section of cancer surveillance (28/12/2016) Stomach cancer estimated incidence, mortality and prevalence worldwide in 2012
- 3.Indian Council of Medical Research. Consensus document for management of gastric cancer 2014, chapter1; pp.1 [DOI] [PMC free article] [PubMed]
- 4.Bonenkamp J, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, van Elk P, Obertop H, Gouma DJ, Taat CW. Randomized comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345(8952):745–748. doi: 10.1016/S0140-6736(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 5.Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, Cook P. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. Lancet. 1996;347(9007):995–999. doi: 10.1016/S0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]
- 6.Kavaliauskas P, Maziukas R, Samalavicius NE, Kuliavas J, Lunevicius R. Subtotal gastrectomy with conventional D2 lymphadenectomy for carcinoma of the distal gastric portion: a retrospective cohort study on clinical outcome. Ann Med Surg (Lond) 2016;6:36–41. doi: 10.1016/j.amsu.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roviello F, Marrelli D, Morgagni P, de Manzoni G, di Leo A, Vindigni C, Saragoni L, Tomezzoli A, Kurihara H, Italian Research Group for Gastric Cancer Survival benefit of extended D2 lymphadenectomy in gastric cancer with involvement of second level lymph nodes: a longitudinal multicenter study. Ann Surg Oncol. 2002;9(9):894–900. doi: 10.1007/BF02557527. [DOI] [PubMed] [Google Scholar]
- 8.Biffi R, Chiappa A, Luca F, et al. Extended lymph node dissection without routine spleno-pancreatectomy for treatment of gastric cancer: low morbidity and mortality rates in a single centre series of 250 patients. J Surg Oncol. 2006;93(5):394–400. doi: 10.1002/jso.20495. [DOI] [PubMed] [Google Scholar]
- 9.Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg. 1987;11:418–425. doi: 10.1007/BF01655804. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi M, Miyazakit I. Prognostic significance and surgical management of lymph node metastasis in gastric cancer. Br J Surg. 1996;83:156–161. doi: 10.1002/bjs.1800830205. [DOI] [PubMed] [Google Scholar]
- 11.Park DJ, Lee HJ, Kim HH, et al. Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg. 2005;92:1099–1102. doi: 10.1002/bjs.4952. [DOI] [PubMed] [Google Scholar]
- 12.Hartgritik HH, Van de Velde CJH, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch Gastric Cancer Group trial. J Clin Oncol. 2004;22(11):2069–2077. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Bunt AMG, Hermans J, Boon MC, van de Velde C, Sasako M, Fleuren GJ, Bruijn JA. Evaluation of the extent of lymphadenectomy in a randomized trial of Western- versus Japanese-type surgery in gastric cancer. J Clin Oncol. 1994;12(2):417–422. doi: 10.1200/JCO.1994.12.2.417. [DOI] [PubMed] [Google Scholar]
- 14.Songun I, Putter H, Kranenbarg EMK, Sasako M, Van de Velde CJH. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11(5):439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 15.Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 dissections for gastric cancer: long-term results of the MRC randomized surgical trial surgical cooperative group. Br J Cancer. 1999;79:1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reis E, Kama NA, Doganay M, Atli M, Dolapci M. Long-term survival is improved by an extended lymph node dissection in potentially curable gastric cancer. Hepatogastroenterology. 2002;49:1167–1171. [PubMed] [Google Scholar]
- 17.Brennan MF. Current status of surgery for gastric cancer: a review. Gastric Cancer. 2005;8:64–70. doi: 10.1007/s10120-005-0319-6. [DOI] [PubMed] [Google Scholar]
- 18.Degiuli M, Sasako M, Calgaro M, Garino M, Rebecchi F, Mineccia M, Scaglione D, Andreone D, Ponti A, Calvo F, Italian Gastric Cancer Study Group Morbidity and mortality after D1 and D2 gastrectomy for cancer: interim analysis of the Italian Gastric Cancer Study Group (IGCSG) randomised surgical trial. Eur J Surg Oncol. 2004;30:303–308. doi: 10.1016/j.ejso.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Degiuli M, Sasako M, Ponti A, Vendrame A, Tomatis M, Mazza C, Borasi A, Capussotti L, Fronda G, Morino M, Italian Gastric Cancer Study Group Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg. 2014;101(2):23–31. doi: 10.1002/bjs.9345. [DOI] [PubMed] [Google Scholar]
- 20.Mocellin S, McCulloch P, Kazi H, Gama-Rodrigues JJ, Yuan Y, Nitti D (2015) Extent of lymph node dissection for adenocarcinoma of the stomach. Cochrane Database Syst Rev 8. Art. No.:CD001964. 10.1002/14651858.CD001964.pub4 [DOI] [PMC free article] [PubMed]
- 21.Memon MA, Subramanya MS, Khan S, Hossain MB, Osland E, Memon B. Meta-analysis of D1 versus D2 gastrectomy for gastric adenocarcinoma. Ann Surg. 2011;253:900–911. doi: 10.1097/SLA.0b013e318212bff6. [DOI] [PubMed] [Google Scholar]
- 22.Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640–646. doi: 10.1097/SLA.0b013e3181d3d29b. [DOI] [PubMed] [Google Scholar]
- 23.Komatsu S, Ichikawa D, Nishimura M, Kosuga T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H, Otsuji E. Evaluation of prognostic value and stage migration effect using positive lymph node ratio in gastric cancer. Eur J Surg Oncol. 2017;43:203–209. doi: 10.1016/j.ejso.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Melis M, Masi A, Pinna A, Cohen S, Hatzaras I, Berman R, Pachter LH, Newman E. Does lymph node ratio affect prognosis in gastroesophageal cancer? Am J Surg. 2015;210:443–450. doi: 10.1016/j.amjsurg.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 25.De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE-5 a population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 26.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. SEER cancer statistics review, 1975-2014, National Cancer Institute. Bethesda, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017
- 27.Wu CW, Hsiung CA, Lo SS, Hsieh MC, Shia LT, Whang-Peng J. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg. 2004;91(3):283–287. doi: 10.1002/bjs.4433. [DOI] [PubMed] [Google Scholar]
- 28.Shrikhande SV, Shukla PJ, Qureshi S, Siddachari R, Upasani V, Ramadwar M, Kakade AC, Hawaldar R. D2 lymphadenectomy for gastric cancer in Tata Memorial Hospital: Indian data can now be incorporated in future international trials. Dig Surg. 2006;23:192–197. doi: 10.1159/000094537. [DOI] [PubMed] [Google Scholar]