Abstract
Background:
Osteoarthritis (OA) in the temporomandibular joint (TMJ) in the TMJ (TMJ-OA) is difficult to treat, and new alternative treatments are needed. Recently, adipose-derived stem cells (ASCs) have been introduced as a promising cell source because of their anti-inflammatory effects. However, the cost and availability of these cells limited broader applications of stem cell therapy. Thus, Thus, stromal vascular fraction (SVF) containing sufficient amount of ASCs at low cost can be an alternative. In this study, we aimed to demonstrate the use of uncultured, optimally isolated SVF for the treatment of TMJ-OA.
Methods:
First, we optimized the method of isolation to harvest high-quality SVFs with a large yield of ASCs. Then, we analyzed the quantity of ASCs in the SVF and performed characterization of stem cell homology. Subsequently, to evaluate the anti-inflammatory effect of high-quality SVF, an in vitro study was performed to assess the expression patterns of inflammatory cytokines including prostaglandin E2 (PGE2), IL-6, and CXCL8/IL-8, COX2, TNF, IFN, CCL2/MCP-1 and CCL5/RANTES in co-culture with synoviocytes derived from the synovial fluid in the TMJ-OA patients.
Results:
The SVF containing approximately 32% ASCs was isolated via the our optimized isolation method. The SVF significantly down-regulated certain inflammatory cytokines such as PGE2, CXCL8/IL-8 in TMJ-OA tissue-derived synoviocytes.
Conclusion:
Although further study is needed, our study suggests that transplantation of adipose tissue-derived SVF cells might be a feasible and a novel therapeutic option for TMJ-OA in the future.
Keywords: Adipose-derived stem cells, Temporomandibular joint disorder, Anti-inflammation, Osteoarthritis, SVF
Introduction
Temporomandibular joint (TMJ) disorders (TMDs) represent a heterogeneous cluster of diseases characterized by pain in the TMJ or its surrounding tissues, functional limitations of the mandible, or clicking in the TMJ during motion [1]. TMDs are common and often limited to the adult population. In epidemiologic studies, up to 75% of adults show at least one sign of joint dysfunction upon examination, and as many as one third have at least one symptom. Five percent of adults with TMD symptoms require treatment, and some develop chronic or debilitating symptoms.
Osteoarthritis (OA) and rheumatoid arthritis (RA) of the TMJ are severe, debilitating disorders that can paralyze the entire masticatory system [2]. OA is a chronic disease condition of the “whole joint” characterized by the slow progressive destruction of articular cartilage accompanied by changes to the synovium and subchondral bone, degeneration of ligaments and menisci, and hypertrophy of the joint capsule, and it is mediated by chronic inflammation of the joint cavity [3]. In vivo and in vitro studies indicate that pro-inflammatory cytokines (interleukin-6, tumor necrosis factor-alpha) and chemokines (CXCL8/IL-8, COX-2) are produced by synoviocytes and chondrocytes in the synovial fluid of OA joints and contribute to the gradual degradation of the joint [4–6]. OA in the TMJ (TMJ-OA) is known as one of the most devastating OA conditions because it can cause serious discomfort during chewing, speaking, and swallowing. Unfortunately, existing drug therapies for OA provide, at best, symptomatic relief from pain and fail to prevent cartilage damage and subsequent destruction of other joint tissues [7]. Recently, the application of mesenchymal stem cells (MSCs) from diverse tissues have been tried clinically or preclinically as innovative treatments of OA [8]. Recently, it has been shown that intraarticular injections of adipose-derived stem cells (ASCs) in OA mouse and rabbit models have anti-inflammatory and chondroprotective effects [9, 10]. ASCs were proved as a promising alternative source that share many properties with MSCs [11–13]. In order to utilize cultured cells for clinical purposes, however, Food and Drug Administration (FDA)-approved facilities and techniques are required, which may be huge obstacles for clinicians who would like to use the cells for a clinical application in terms of cost and feasibility.
The stromal vascular fraction (SVF) in adipose tissue can be freshly obtained in large quantities from the patient’s own adipose tissue with minimal manipulation and contains a significant amount of adipose-derived stem cells (ASCs). Moreover, SVF cells also contain other heterogenous cell populations, including stromal cells and endothelial cells, which are well known to accelerate tissue regeneration when compare to use a single stem cell [14]. The “niche” graft is to known to improve viability of grafted cells in a new circumstance in the transplanted site. Therefore, clinical application of SVF instead of the cultured ASCs might have many merits as long as its clinical efficacies are proved. The amount of ASCs in the SVF might be a critical factor affecting its therapeutic effect on a disease condition. The conventional method of separating SVF can only produce SVF containing less than 2% ASCs, and the potential therapeutic effect might be low [14]. Therefore, it is necessary to develop a new method to increase yield of ASCs in the SVF.
Therefore, in this study, we modified the conventional method to harvest SVF with more content of ASCs. And we evaluated the anti-inflammatory effect, the most important therapeutic effect of TMJ-OA, using uncultured SVF and synoviocytes from the TMJ-OA patients tissues.
Materials and methods
Enhanced SVF separation and culture of ASCs
This study was approved by the institutional review board (IRB) of Asan Medical Center (S2013-0409-0001). According to the guidelines, patients agreed with the purposes of this study and provided informed written consent. Human subcutaneous adipose tissue samples were acquired from liposuction on the abdomen of TMJ-OA patients and used in this study.
In this study, we applied a modified technique to increase the content of ASCs in the SVF. Briefly, the adipose tissue obtained by liposuction was mixed with the same volume of phosphate buffered saline (PBS), centrifuged at 300 to 600×g for 5–10 min, and 0.07–0.08% collagenase was added to the upper adipose tissue layer and reacted at 25–30 °C for 30 to 40 min. After the reacted adipose tissue was further isolated using 18–25 gauge needles, and the same volume of PBS was added and centrifuged at 600–840×g for 3–10 min to obtain a pellet layer. The pellet layer was passed through a 60–80 µm mesh filter to remove the residue, and fine impurities and RBC were finally removed onto Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA). The obtained fraction was washed three times in Hanks’ balanced salt solution (HBSS) to obtain SVF.
The obtained SVF was cultured at 37 °C/5% CO2 in ASC control medium (alpha-modified Eagle’s media (αMEM), 10% fetal bovine serum (FBS), 100 units/ml of penicillin, 100 µg/ml of streptomycin). The cell population was maintained over 3–5 days until confluence, which was defined as passage 1. ASCs were cultured and expanded in control medium and used for the characterizing experiments at passages 3 through 5.
Flow cytometry characterization of SVF and ASCs
Freshly isolated SVF and ASCs of passage 3 were cultured in a control medium for 48 h prior to analysis and incubated with monoclonal PE-conjugated antibodies for CD34 and CD73 (both BD Pharmingen, San Diego, CA, USA) or with FITC-conjugated antibodies for CD45 and CD90 (Chemicon, Temecula, CA, USA) and CD105 (BD Pharmingen, San Diego, CA, USA) for 30 min at 20–25 °C. As a control, cells were stained with isotype control IgG. Cells were subsequently washed with PBS, fixed with 4% formaldehyde, and analyzed on a FACScan flow cytometer (Beckton Dickson, San Jose, CA, USA) using CELLQuest Pro software.
In vitro multi-lineage differentiation of ASCs
In vitro multi-lineage differentiation of ASCs was induced in control medium supplemented with one of the following three formulas as described previously [15–17]: (1) adipogenic medium: 10 mg/ml insulin, 1 mM dexamethasone, 0.5 mM isobutyl-1methyl-3-xanthine and 100 mM indomethacin in control medium; (2) osteogenic medium: 10 mM beta-glycerophosphate, medium; (3) chondrogenic medium: 10 ng/ml TGFβ1, 11 mg/ml sodium pyruvate, and 50 mg/ml ascorbate-2-phosphate. All reagents were purchased from Sigma—Aldrich. For differentiation, cells were grown to confluence before induction, and medium was changed every 3—4 days. After 15 days, cells were fixed for appropriate differentiation-specific staining: Oil Red O for adipogenesis, Alizarin Red S for osteogenesis and Alcian blue for chondrogenesis.
Isolation and culture of synoviocytes
Synoviocytes were isolated from synovial tissue as previously reported [15, 16]. Briefly, synovial tissue specimens were cut into small pieces. The synovium was minced into fragments and cultured in 100-mm culture dishes containing RPMI 1640 (Life Technologies, Calsbad, CA, USA) with 10% FBS, 100 units/ml of penicillin G sodium, and 100 µg/ml streptomycin sulfate (Life Technologies). After 1–3 days of incubation, tissue was harvested, and single cells were collected by vigorous pipetting. Cell suspensions were washed once, and viable cells were cultured in 10 ml Optimem-1 (Life Technologies) supplemented with 15% FBS and 50 g/ml gentamicin in 100-mm culture dishes. After 7 days of culture, residual tissue fragments were removed, and the synoviocytes were maintained in culture. Cells of passage 1–3 were used for the subsequent experiments.
Cell co-cultures
There were 3 experimental groups: Group 1, synoviocytes co-cultured with ASCs; Group 2, synoviocytes co-cultured with traditional SVF (tSVF: contained ≤ 2% ASCs); Group 3, synoviocytes co-cultured with advanced SVF (aSVF: contained ≥ 30% ASCs), and the control was the monolayer culture condition for each cell type. Co-cultures were performed by seeding synoviocytes in the lower chamber of a 100-mm culture plate and ASCs, tSVF, and aSVF in Transwells (0.4 μm pore size; Corning) in F12/DMEM (Life Technologies) serum-free medium with 100 units/ml of penicillin G sodium and 100 µg/ml streptomycin sulfate. On day 7, both monocultures and co-cultures were harvested, in addition to their supernatant, and were analyzed.
Conditioned medium concentration
The conditioned medium (CM) of group 3 and the control groups was collected after day 7 of culture, centrifuged at 300×g for 5 min, and filtered using a 0.22 µm syringe filter. The CM of all groups was concentrated by centrifugation using a 3 k nominal molecular weight limit (NMWL) regenerated cellulose and polyethersulfone membrane in a stirred ultrafiltration cell (Millipore Co. MA) at 4 °C. All CM was collected and then concentrated by a factor of 250.
Cytokine quantification
The levels of prostaglandin E2 (PGE2), IL-6, and CXCL8/IL-8, COX2, TNF, IFN, CCL2/MCP-1 and CCL5/RANTES were measured in concentrated CM via enzyme-linked immunosorbent assay(ELISA) kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Briefly, the samples and standards were diluted appropriately in the respective assay diluent and then incubated at room temperature in wells precoated with the specific capturing antibodies. After washing, the respective detection antibody conjugate was added and then incubated at room temperature before washing again. The addition of substrate solution then resulted in color development. After the addition of a stop solution, the color intensity was measured in an ELISA plate reader at the appropriate wavelength. All samples and standards were measured in duplicate.
Statistical analysis
Statistical analysis was performed using mainly nonparametric tests since the data were not normally distributed and had a strongly asymmetric distribution (Friedman’s nonparametric analysis of variance and Dunn’s post hoc test for paired data, Kruskal–Wallis test and Dunn’s post hoc test for unpaired data, and Mann–Whitney U test for unpaired two-group data). Parametric tests were also used for data with normal and symmetric distributions. Depending on the distribution, values were expressed as the median and interquartile range, as the median and range (minimum–maximum), or as the mean SD. CSS Statistica Statistical Software (StatSoft) was used for analysis. P values less than 0.05 were considered significant.
Results
Isolation of SVF cells
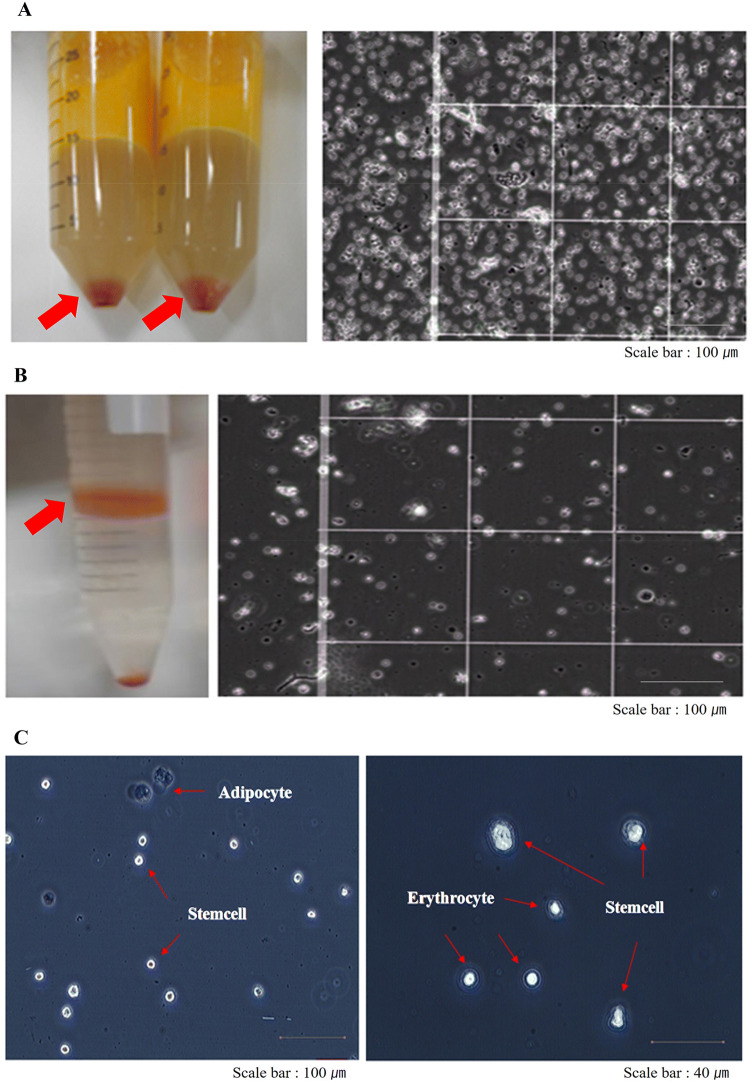
The average number of SVF cells isolated was 2.02 ± 0.95 × 105 cells (n = 10)/ml of lipoaspirated fat (Table 1). The tSVF exhibits a heterogeneous cell population by microscopic analysis (Fig. 1A), but the aSVF exhibits a relatively homogenous cell population (Fig. 1B) owing to the high quality of the purification method. The ASCs was identified 15–20 µm in size in SVF cells (Fig. 1C). The ASCs were easily expanded in vitro by culturing the SVF cells and exhibited a fibroblast-like morphology.
Table 1.
Isolated SVF details
| No. | Adipose tissue volume (ml) | Patient age (years) | SVF cell number | SVF cell viability (%) | ASC yield (%) |
|---|---|---|---|---|---|
| 1 | 4.5 | 20 | 1.5 × 105 | 80 | 36.8 |
| 2 | 103 | 39 | 2.7 × 107 | 72 | 30.2 |
| 3 | 35 | 49 | 1.32 × 107 | 82 | 41.6 |
| 4 | 56 | 49 | 8.63 × 106 | 73 | 25.3 |
| 5 | 44 | 18 | 7.6 × 106 | 72 | 33.5 |
| 6 | 40 | 20 | 6.0 × 106 | 80 | 29.4 |
| 7 | 60 | 58 | 1.68 × 107 | 90 | 28.5 |
| 8 | 45 | 57 | 9.0 × 106 | 90 | 32.5 |
| 9 | 42 | 27 | 6.0 × 106 | 85 | 30.2 |
| 10 | 40 | 32 | 1.0 × 107 | 82 | 33.9 |
Fig. 1.
Isolation of the stromal vascular fraction enhanced the yield of adipose-derived stem cells. A Heterogeneous cell population of SVF cell pellet (left arrow). We isolated a large cell population from adipose tissue via the traditional isolation method. The microscopic view of heterogeneous cells on the hemocytometer shows that most of the cells were erythrocytes (right). B SVF isolated by means of Ficoll gradient centrifugation show absence of most erythrocytes (left, arrow) and a high purity of the cell population on the hemocytometer (right). C High-purity SVF contained numerous ASCs (15–20 µm) with rod-shaped morphology. Erythrocytes seemed smaller in size than ASCs, and adipocytes seemed larger in size than ASCs
Yield of ASCs from SVF and characterization of ASCs
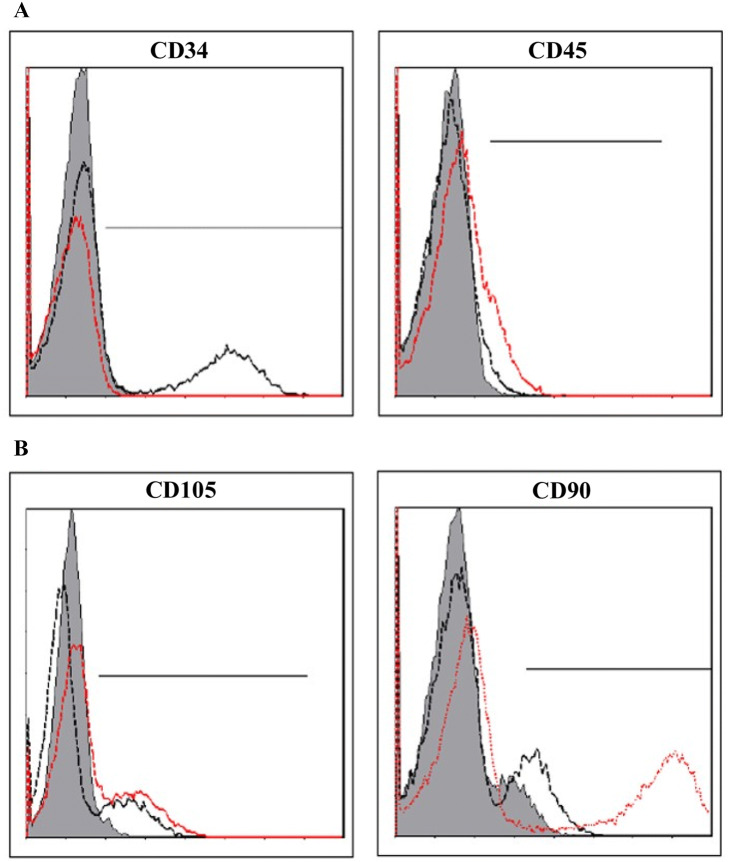
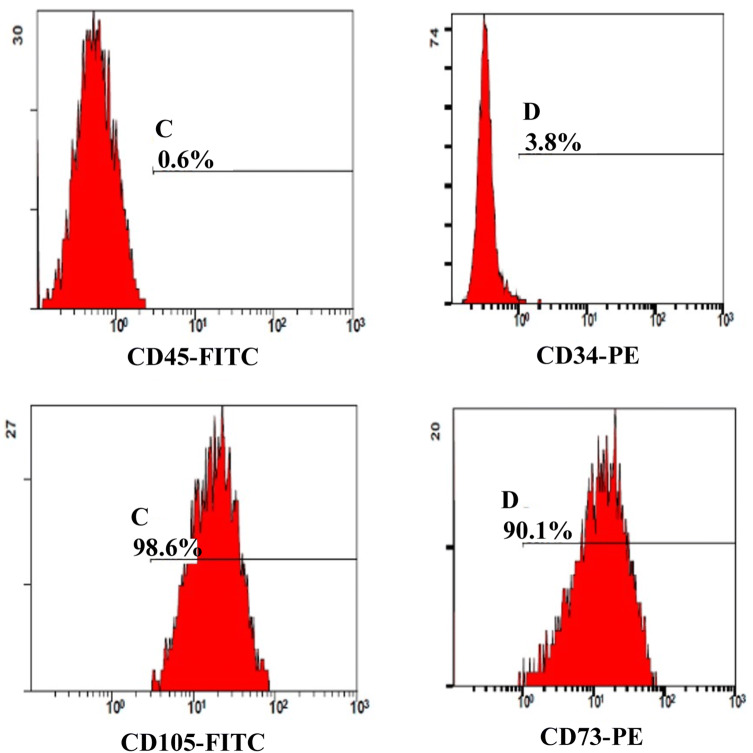
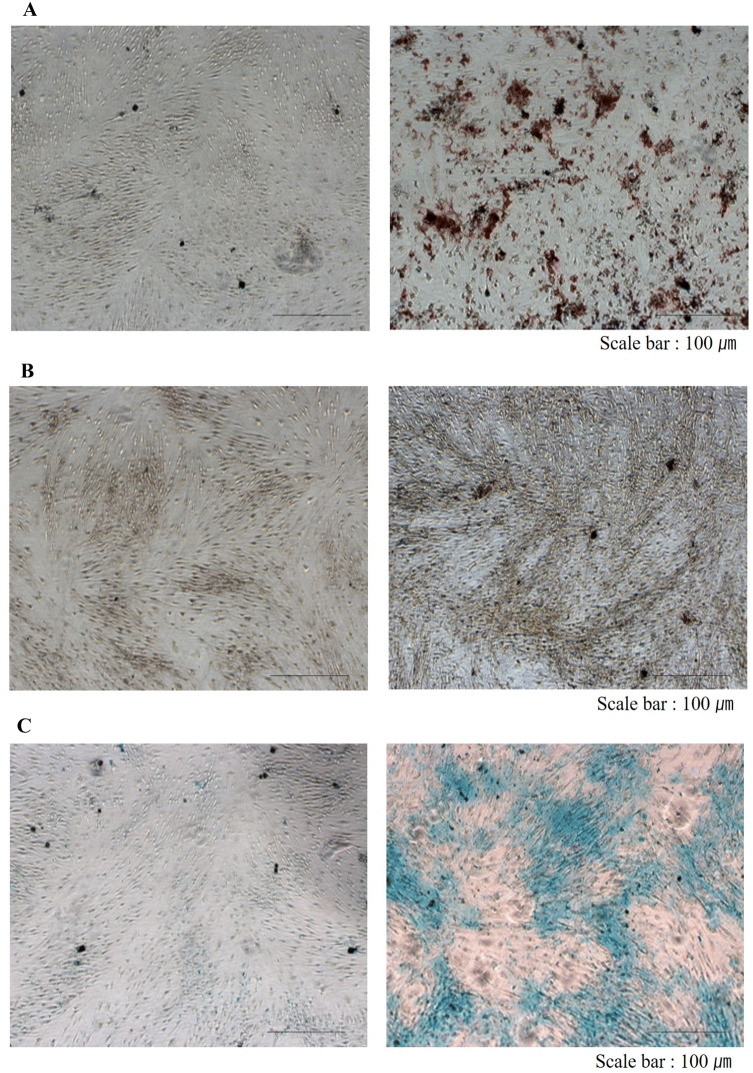
The stem cells isolated from the SVF using the improved technique was confirmed by two methods. The first method was confirmation of the markers of MSCs from isolated SVF using a flow cytometer. The expression ratios of CD34 and CD45, negative markers of ASCs, and CD90 and CD105, positive markers, were 7–14% and 21–24% in isolated SVF, respectively (Fig. 2). This result represented 10-fold increase in the amount of ASCs obtained compared with the previous method. The other stem cell confirmation method was counting of attached cells, analysis of homology of stem cells via flow cytometry, and verification of differentiation potency of MSCs after incubation in non-serum media for 24 h. Consequently, the number of attached cells was 7.4 × 104 cells/g in average and 32.2% in aSVF. There was individual variations regarding ASCs contents in the aSVF ranging from 28.5 to 41.6% in aSVF. The attached cells exhibited homologous features like stem cells in flow cytometry (Fig. 3). Using flow cytometry, characteristic expression of stem cell-related surface markers were confirmed. ASCs expressed CD73 and CD105, and lacked CD34 and CD45 expression. The markers of SVF and ASCs are listed in Table 2. Also, attached cells differentiated into adipocytes, osteoblasts, and chondrocytes, and had features of stem cells. ASCs were treated with media known to induce adipogenic, osteogenic, or chondrogenic differentiation. Adipogenic differentiation was identified by Oil Red O staining of intracellular lipid droplets (Fig. 4A), osteogenic differentiation by Alizarin red S staining of mineralization of the matrix (Fig. 4B), and chondrogenic differentiation by Alcian blue staining of cartilage-specific proteoglycans (Fig. 4C) Therefore, the amount of ASCs in SVF isolated from adipose tissue increased over 10-fold in aSVF compared with tSVF, and the ASCs were confirmed by counting of attached cells, flow cytometry analysis, and differentiation potency.
Fig. 2.
Flow cytometry histograms of human SVF. Red and black colored lines represent the specific antibodies indicated. Gray color indicates an isotype of each specific antibody. A Both CD34 and CD45 markers are highly expressed in the hematopoietic cell population. CD34 and CD45 cells have been described as representing about 14% and 7% of the SVF, respectively. B Both CD105 and CD90 markers are highly expressed on ASCs. CD105 and CD90 cells have been described as representing about 24% and 21% of the SVF, respectively
Fig. 3.
Flow cytometry histograms of human ASCs. ASCs were selected by adherent capacity on a flask. Attached cells were harvested by trypsinization, and the expression levels were analyzed by MSC positive markers (CD73, CD105) and negative markers (CD34, CD45), respectively
Table 2.
Surface marker expression differences between SVF cells and ASCs
| Marker | SVF | ASCs |
|---|---|---|
| CD 34 | ± | – |
| CD 45 | ± | – |
| CD 73 | + | ++ |
| CD 90 | + | ++ |
| CD 105 | + | ++ |
++, > 70%; +, 20–30%; ±, 5–20%; –, < 5%
Fig. 4.
In vitro multi-lineage differentiation potential of ASCs was confirmed. A Adipogenesis was demonstrated by Oil Red O staining, B osteogenesis by Alizarin red S staining, and C chondrogenesis by Alcian blue staining. Cells were treated with either control (left panels) or differentiation media (right panels)
Evaluation of co-culture conditions
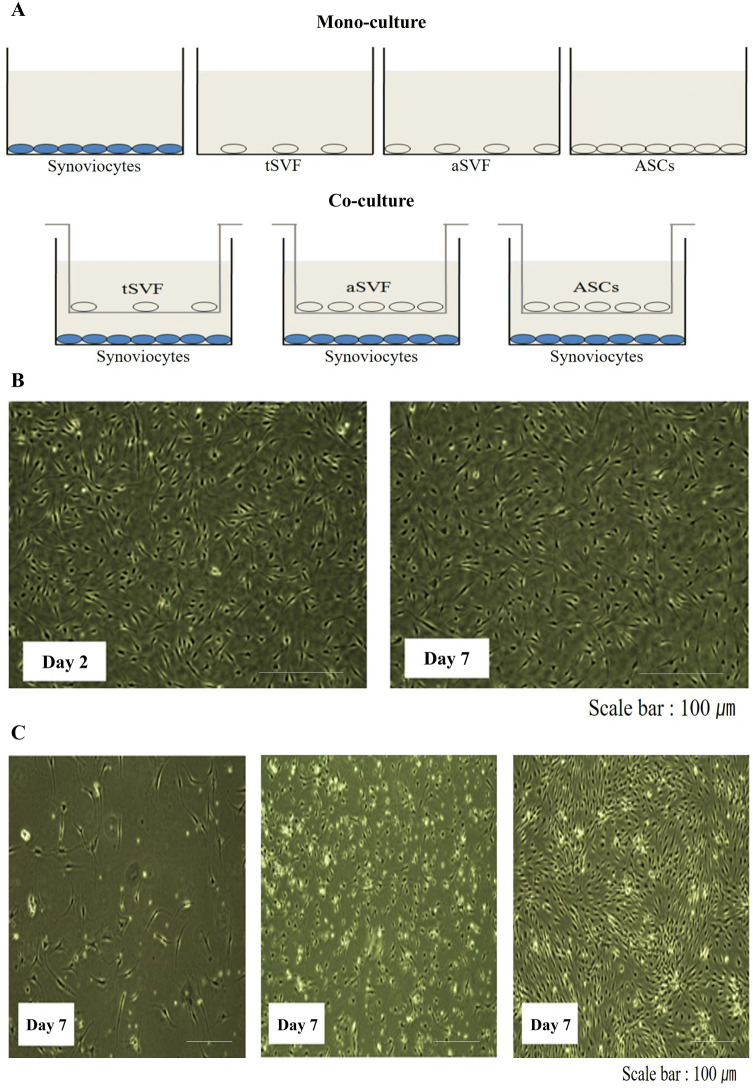
To evaluate co-culture conditions, we performed preliminary experiments to select a culture medium that did not induce proliferation of synoviocytes, tSVF cells, aSVF cells, or ASCs until day 7. F12/DMEM with ascorbic acid, proline, and sodium pyruvate was used since, as shown in Fig. 5, it did not induce cell proliferation until day 7. Moreover, the cells were all viable until day 7 and did not show any morphologic changes (Fig. 5B). We used a Transwell system to avoid cell-to-cell contact (Fig. 5A). On the basis of these results, the subsequent experiments were performed on day 7.
Fig. 5.
Evaluation of co-culture conditions. A Schematic of the co-culture system is shown. B Morphology of synoviocytes. There was no difference in cell proliferation on day 2 and 7, and C tSVF(left), aSVF(middle), and ASCs (right) were evaluated on day 7. Original magnification: 40×
SVF exerts anti-inflammatory effects on synoviocytes
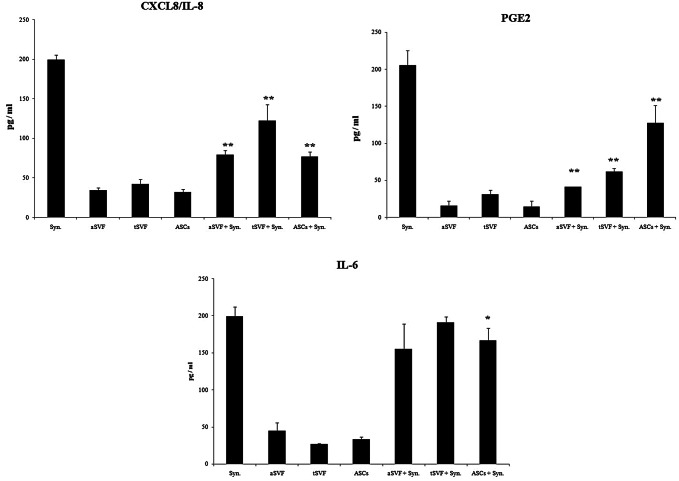
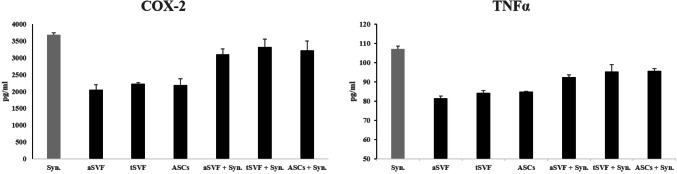
First, the release of the major inflammatory factors prostaglandin E2, IL-6, and CXCL8/IL-8 was analyzed on day 7. aSVF in co-culture with synoviocytes showed a significantly decreased release of prostaglandin E2, IL-6, and CXCL8/IL-8 from synoviocytes (Fig. 6). As shown in Fig. 6, the expression of PGE2 was significantly down-regulated in all experimental groups; the inhibition of IL-6 and CXCL8/IL-8 production was also confirmed as shown Fig. 6. In addition, COX2 and TNFα showed seemingly down-regulated but there was no statistical difference under these conditions (Fig. 7). The analysis of other inflammatory chemokines (CCL2/MCP-1 and CCL5/RANTES) revealed the same trend (data not shown) under all culture conditions tested but also there was no statistical significance (p = 0.36).
Fig. 6.
Anti-inflammatory effects of SVF on synoviocytes secreted PGE2, CXCL8/IL-8 and IL-6. aSVF significantly down-regulates the expression of the main osteoarthritis inflammatory cytokines and chemokines. PGE2, CXCL8/IL-8 and IL-6 release is decreased by tSVF, aSVF, and ASCs co-culture. *p < 0.05, **p < 0.01 compared with each monoculture of synoviocytes
Fig. 7.
Anti-inflammatory effects of SVF on synoviocytes secreted COX-2 and TNFα. aSVF, tSVF and ASCs does not significantly down-regulate the expression of COX-2 and TNFα
Discussion
OA is the most common joint disease, but there is only symptomatic treatment to manage the disease condition. Inflammation has been proved as a key factor in the progression of OA, suggesting that targeting inflammation in OA could be a promising therapeutic strategy [17, 18]. MSCs have been identified and isolated from different tissues (bone marrow, adipose tissue, umbilical cord vein) [11, 19, 20] and have been shown to exhibit important immunomodulatory properties in vitro [21] as well as in vivo in various clinical trials [22, 23]. With this in mind, researchers have paid considerable attention to several inflammatory cytokines, including interleukin (IL)-12, IL-6, IL-1β, and tumor necrosis factor (TNF)-α, increase in synovial fluid in patients with TMJ OA (25). In addition, the levels of monocyte chemoattractant protein (MCP)-1 in patients with TMJ OA is also elevated in inflammatory synovial tissue, which is highly upregulated in IL-1β-stimulated synovial cells [24, 25]. A recent report suggested that ASCs could have promising therapeutic effects in OA patients by reducing inflammation and promoting regeneration of the cartilage [8, 26]. However, the clinical application of ACSs entails a costly and long-term legal licensing process. From a doctor’s and patient’s point of view, it is important to find an inexpensive and available treatment option. Therefore, the use of SVF with high stem cell content without above drawbacks of ASCs will be helpful for many patients suffering from TMJ-OA. In this study, by applying the improved SVF separation technology that increased the adipose derived stem cell content, the stem cell content was increased by 28.5–41.6% in SVF. This result might be an important progress in the popular use of uncultured SVF for the treatment of TMJ-OA because the amount of ASCs in the SVF might be a critical factor affecting its therapeutic effect on a disease condition.
In this study, as an initial step for further clinical trials using SVF for confirming TMJ-OA treatment, we analyzed the in vitro effects of SVF containing a significant amount of ASCs on synoviocytes obtained from TMJ-OA patients. The synoviocytes isolated from the joint tissues of TMJ OA patients expressed major inflammatory cytokines such as PGE2, IL-6, and CXCL8/IL-8 and the SVF inhibited the release of these inflammatory factors, in vitro. Interestingly, the decrease in the expression of PGE2 was more prevalent in aSVF than in ASC in this study. It is difficult to figure out which property of the SVF resulted in this most down-regulation effect but it might be due to a certain heterogenic component of the SVF. Regarding this issue, further study is necessary. As in an acute asthma lung model [27], we also found that the anti-inflammatory effects of SVF seemed to depend on the degree of inflammation of the TMJ-OA tissue although the number of patients in this study was not enough to make a statistics(data not shown). It has been reported that human MSCs require activation in vitro, and activating stimuli appear to include pro-inflammatory cytokines (IL-1, IFN, TNF) or interaction with monocytes [28]. Under the present culture conditions, SVF and synoviocytes either in monoculture or co-culture did not produce IL-1, TNF, or IFN(data not shown). Therefore, the SVF in this study might be activated by other factors specifically produced by inflamed synoviocytes and a different pathway may play a role in the anti-inflammatory effect in this study [29–32]. Generally, the inflammatory cascade is driven by a variety of enzymes, among which COX is prominent and catalyzes the formation of prostaglandins and thromboxanes from arachidonic acid. COX has at least two isoforms, a constitutive form (COX-1) and an inflammation-induced form (COX-2) [33, 34]. In this study, COX-2 expression by synovial cells in coculture was inhibited by SVF, suggesting an independent involvement of this pathway in the anti-inflammatory effects exerted by these cells.
However, it has also been shown that human OA cartilage explants express COX-2 and spontaneously produce PEG2 [35] and ASCs also produce PGE2 [36]. In this study, the increase in PGE2 in the synoviocytes measured in a single culture was basic release from these cells, and thus the decrease in PGE2 in mixed cultures with the SVF is significantly related to the SVF, which is important for immunosuppressive properties through PGE2 [37].
Interestigly, our data showed that inhibition of PGE2 was specifically observed separately from COX-2 when co-cultured with SVF and inflammatory synovial cells from TMJ-OA patients. These results seem paradoxical because it has been known that the inhibition of COX-2 in the inflammatory response depends on the inhibition of PGE2. This phenomenon may be a technical problem of the experiment. Further research on this will be needed in the future.
In this study, PGE2 expression was more inhibited in the SVF co-culture group than in the co-culture group with ASCs. In general, it is known that ASC in SVF has a pharmacological effect, and this phenomenon is very unusual. This is probably due to the positive interaction of ASCs and other cells contained in SVF. In fact, it has shown that when cells are transplanted with niche in the vicinity of cells, cell survival and therapeutic effects are improved [38].
Although limited experimental results, our study showed that aSVF showed a better immunosuppressive effect than that of tSVF, which would be more useful as a new treatment option of TMJ-OA in the future.
In conclusion, in this study, we showed that our modified method could produce SVF from the patient’s abdominal adipose with more content of ASCs. And the SVF significantly downregulated the expression of main OA inflammatory cytokines such as PGE2 and IL-8 in synoviocytes from TMJ-OA patients. Our preliminary data suggest that the COX-2/PGE2 pathway might be one of the key modulators that plays a role in this anti-inflammatory mechanism of action of SVF in TMJ-OA patients. With this in mind, the SVF might be clinically feasible and a potential therapeutic option for TMJ-OA. However, further study is needed to confirm more detailed mechanism of the therapeutic effect of the SVF for treating TMJ-OA. And further clinical trials are necessary.
Acknowledgements
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10V-0090-010014). This study was also supported by the Korean Incorporated Association of Temporomandibular Joint.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical statements
This study was approved by the institutional review board (IRB) of Asan Medical Center (S2013-0409-0001). Informed written consent was provided by each patient involved in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hyungki Kim, Email: lines77@daum.net.
Bu-Kyu Lee, Email: bukyu.lee@gmail.com.
References
- 1.Stohler CS. Craniofacial pain and motor function: pathogenesis, clinical correlates, and implications. Crit Rev Oral Biol Med. 1999;10:504–518. doi: 10.1177/10454411990100040601. [DOI] [PubMed] [Google Scholar]
- 2.Dimitroulis G. The role of surgery in the management of disorders of the temporomandibular joint: a critical review of the literature: part 2. Int J Oral Maxillofac Surg. 2005;34:231–237. doi: 10.1016/j.ijom.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 5.Goldring M, Otero M, Tsuchimochi K, Ijiri K, Li Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis. 2008;67:iii75–iii82. doi: 10.1136/ard.2008.098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter DJ. Pharmacologic therapy for osteoarthritis—the era of disease modification. Nat Rev Rheumatol. 2011;7:13–22. doi: 10.1038/nrrheum.2010.178. [DOI] [PubMed] [Google Scholar]
- 8.Jorgensen C, Noël D. Mesenchymal stem cells in osteoarticular diseases. Regen Med. 2011;6:44–51. doi: 10.2217/rme.11.80. [DOI] [PubMed] [Google Scholar]
- 9.ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64:3604–3613. doi: 10.1002/art.34626. [DOI] [PubMed] [Google Scholar]
- 10.Desando G, Cavallo C, Sartoni F, Martini L, Parrilli A, Veronesi F, et al. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res Ther. 2013;15:R22. doi: 10.1186/ar4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez AM, Elabd C, Amri EZ, Ailhaud G, Dani C, et al. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:125–128. doi: 10.1016/j.biochi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak FJB. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 14.Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 15.Grigolo B, Roseti L, Neri S, Gobbi P, Jensen P, Major EO, et al. Human articular chondrocytes immortalized by HPV-16 E6 and E7 genes: maintenance of differentiated phenotype under defined culture conditions. Osteoarthritis Cartilage. 2002;10:879–889. doi: 10.1053/joca.2002.0836. [DOI] [PubMed] [Google Scholar]
- 16.Lisignoli G, Grassi F, Piacentini A, Cocchini B, Remiddi G, Bevilacqua C, et al. Hyaluronan does not affect cytokine and chemokine expression in osteoarthritic chondrocytes and synoviocytes. Osteoarthritis Cartilage. 2001;9:161–168. doi: 10.1053/joca.2000.0372. [DOI] [PubMed] [Google Scholar]
- 17.Lee YH, Park HK, Auh QS, Nah H, Lee JS, Moon HJ, et al. Emerging potential of exosomes in regenerative medicine for temporomandibular joint osteoarthritis. Int J Mol Sci. 2020;24:E1541. doi: 10.3390/ijms21041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63:391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 20.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 21.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 22.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 23.Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 24.Ogura N, Tobe M, Sakamaki H, Nagura H, Abiko Y, Kondoh T. Tumor necrosis factor-alpha increases chemokine gene expression and production in synovial fibroblasts from human temporomandibular joint. J Oral Pathol Med. 2005;34:357–363. doi: 10.1111/j.1600-0714.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 25.Ogura N, Satoh K, Akutsu M, Tobe M, Kuyama K, Kuboyama N, et al. MCP-1 production in temporomandibular joint inflammation. J Dent Res. 2010;89:1117–1122. doi: 10.1177/0022034510376041. [DOI] [PubMed] [Google Scholar]
- 26.Garza JR, Campbell RE, Tjoumakaris FP, Freedman KB, Miller LS, Santa Maria D, et al. Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: a double-blinded prospective randomized controlled clinical trial. Am J Sports Med. 2020;48:588–598. doi: 10.1177/0363546519899923. [DOI] [PubMed] [Google Scholar]
- 27.Bonfield TL, Nolan Koloze MT, Lennon DP, Caplan AI. Defining human mesenchymal stem cell efficacy in vivo. J Inflamm (Lond) 2010;7:51. doi: 10.1186/1476-9255-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui SJ, Zhang T, Fu Y, Liu Y, Gan YH, Zhou YH, et al. DPSCs attenuate experimental progressive TMJ arthritis by inhibiting the STAT1 pathway. J Dent Res. 2020;99:446–455. doi: 10.1177/0022034520901710. [DOI] [PubMed] [Google Scholar]
- 30.Jiang L, Xu K, Li J, Zhou X, Xu L, Wu Z, et al. Nesfatin-1 suppresses interleukin-1β-induced inflammation, apoptosis, and cartilage matrix destruction in chondrocytes and ameliorates osteoarthritis in rats. Aging (Albany NY) 2020;12:1760–1777. doi: 10.18632/aging.102711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia L, Zhang HX, Xing ML, Xu YB, Li P, Huang LK, et al. Knockdown of PRMT1 suppresses IL-1β-induced cartilage degradation and inflammatory responses in human chondrocytes through Gli1-mediated Hedgehog signaling pathway. Mol Cell Biochem. 2018;438:17–24. doi: 10.1007/s11010-017-3109-7. [DOI] [PubMed] [Google Scholar]
- 32.Xue XT, Kou XX, Li CS, Bi RY, Meng Z, Wang XD, et al. Progesterone attenuates temporomandibular joint inflammation through inhibition of NF-κB pathway in ovariectomized rats. Sci Rep. 2017;7:15334. doi: 10.1038/s41598-017-15285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appleton I, Tomlinson A, Willoughby DA. Induction of cyclo-oxygenase and nitric oxide synthase in inflammation. Adv Pharmacol. 1996;35:27–78. doi: 10.1016/S1054-3589(08)60274-4. [DOI] [PubMed] [Google Scholar]
- 34.Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, El Hachem K, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–20. doi: 10.22203/eCM.v021a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caron MM, Emans PJ, Sanen K, Surtel DA, Cremers A, Ophelders D, et al. The role of prostaglandins and COX-enzymes in chondrogenic differentiation of ATDC5 progenitor cells. PLoS One. 2016;11:e0153162. doi: 10.1371/journal.pone.0153162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrio L, Cuevas VD, Menta R, Mancheño-Corvo P, delaRosa O, Dalemans W, et al. Human adipose tissue-derived mesenchymal stromal cells promote B-cell motility and chemoattraction. Cytotherapy. 2014;16:1692–1699. doi: 10.1016/j.jcyt.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Yañez R, Oviedo A, Aldea M, Bueren JA, Lamana ML. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010;316:3109–3123. doi: 10.1016/j.yexcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Lee CC, Hirasawa N, Garcia KG, Ramanathan D, Kim KD. Stem and progenitor cell microenvironment for bone regeneration and repair. Regen Med. 2019;14:693–702. doi: 10.2217/rme-2018-0044. [DOI] [PubMed] [Google Scholar]