Abstract
Background:
Articular cartilage injury has a poor repair ability and limited regeneration capacity with therapy based on articular chondrocytes (ACs) implantation. Here, we validated the hypothesis that human nasal septum-derived chondrocytes (hNCs) are potent therapeutic agents for clinical use in cartilage tissue engineering using an injectable hydrogel, type I collagen (COL1).
Methods:
We manufactured hNCs incorporated in clinical-grade soluble COL1 and investigated their clinical potential as agents in an articular defect model.
Results:
The hNCs encapsulated in COL1 (hNC-collagen) were uniformly distributed throughout the collagen and showed much greater growth rate than hACs encapsulated in collagen for the 14 days of culture. Fluorescent staining of hNC-collagen showed high expression levels of chondrocyte-specific proteins under clinical conditions. Moreover, a negative mycoplasma screening result were obtained in culture of hNC-collagen. Notably, implantation of hNC-collagen increased the repair of osteochondral defects in rats compared with implantation of collagen only. Many human cells were detected within the cartilage defects.
Conclusion:
These results provide reliable evidences supporting for clinical applications of hNC-collagen in regenerative medicine for cartilage repair.
Electronic supplementary material
The online version of this article (10.1007/s13770-020-00261-9) contains supplementary material, which is available to authorized users.
Keywords: Cartilage regeneration, Chondrocytes, Human nasal septum, Soluble type I collagen, Tissue engineering
Introduction
The impairment of weight-bearing articular cartilage from trauma, degeneration or age-related disease can be encountered in the clinical field. In particular, the rising aging population seems to increase the articular cartilage defects or impairment that causes significant pain, decreased joint function and disability among affected patients [1]. These tissue defects require tissue regeneration, and the poor regenerative capacity of chondral defects has resulted in the use of autologous tissues in order to present an implant that can form the proper anatomical shape [2]. Several clinical trials have demonstrated the autologous chondrocyte transplantation (ACT) [3]. However, ACT is associated with a limited supply of chondrocytes and poor ability to restore cartilage structure and function. Therefore, it is crucial to identity an autologous cell source which shows a higher chondrogenic potential and lower donor-dependent cartilage restoration capacity has been crucial to enhancing the therapeutic potential of chondrocytes for the regeneration of cartilage defects.
Human chondrocytes derived from nasal septal cartilage (hNCs) represent a great potential as a clinically valuable source for regeneration of cartilage tissue. Compared with the acquisition of chondrocytes from articular cartilage, hNCs can be obtained relatively easier by minimally invasive collection procedures during nasal septum surgery for nasal obstruction with less morbidity [4]. Several studies have recently indicated that nasal chondrocytes also present a high potential for use in regenerative medicine [5–9]. Therefore, hNCs has been focused clinically to develop the cell therapy for treatment of musculoskeletal diseases, including cartilage defects and degenerative disorders [10].
The traditional ACT technique, which consists of injection of autologous cartilage cells into defect site of cartilage, has some disadvantages: the surgical exposure required for the watertight suture and incision for periosteum harvest. In addition, the injection of chondrocytes cannot cover the total condyle in an arthritic knee so that the implant is watertight, and there is also cell leakage and graft detachment after injection into the defect [11].
Recently, combination treatment of ACT with tissue engineering and bioactive, resorbable, and implantable biomaterials enhanced cartilage regeneration [12–14]. A collagen matrix is used for ACT as a useful membrane; chondrocytes are seeded on collagen matrix and then, the membrane seeded with cells, is implanted into the defect site. This technique shows some advantages to overcome the limitation of conventional ACT, but disadvantages still exist: cell loss from the membrane and detachment of the membrane [15]. The retention of implanted cells and their functional capacity around the implant site are very important for treating patients. The alternative ACT technique for clinical trials involves encapsulating chondrocytes in hydrogel for injection into the defect. Hydrogels such as alginate [16–18], collagen [19–21] and fibroin gel [22, 23] are widely used in field of tissue engineering due to their appropriate viscosity to allow these hydrogels can be injected easily and formed 3D structures quickly. Collagen gels are mainly used for injectable hydrogel systems [24]. Soluble type 1 collagen (COL1) is widely used substrate for cell adhesion in cultures and a bioengineered scaffold for regenerative medicine [25]. Collagen has been accepted to use in clinical trials for bone grafting, reconstructive surgery, cosmetic surgery, and dermal injections [26, 27].
This study investigated the behavior of hNCs in clinical-grade COLI for cartilage tissue engineering as clinically applicable agents in terms of cell viability, stability, phenotype, safety, and regeneration capacity in an articular defect model.
Materials and methods
Cell isolation and expansion
hNCs were isolated as previously described [4]. This research was conducted in compliance with the Institutional Review Board of Catholic Medical Center Clinical Research Coordinating Center (IRB No. KC08TISS0341), Seoul St. Mary’s Hospital. Before surgery, the written informed consent from the donors was obtained to participate in this research. Nasal septal tissue was obtained during septoplasty from 10 donors (age range, 20–50 years). The obtained nasal septal tissue was washed with phosphate-buffered saline (PBS, Thermo Fisher Scientific, Waltham, MA, USA), cut into small pieces and digested with treatment of 0.2% (v/v) protease solution (Gibco-BRL, Grand Island, NY, USA) for 60 min. The tissue was then incubated in 0.3% (w/v) collagenase (Sigma-Aldrich Co., St. Louis, MO, USA) for 18 h at 37 °C. The cells isolated from nasal septal tissue were cultured in low-glucose Dulbecco’s Modified Eagle Medium (DMEM, Gibco-BRL) supplemented with 1% (v/v) penicillin/streptomycin (antibiotics, Thermo Fisher Scientific) and 10% (v/v) fetal bovine serum (FBS; Gibco-BRL) in a humidified atmosphere containing 5% (v/v) CO2 at 37 °C. Human articular chondrocytes (hACs) were obtained from Uijeongbu St. Mary’s Hospital. This research utilizing hACs was conducted in compliance with the Institutional Review Board of the Catholic Medical Center Clinical Research Coordinating Center, (UC14CNSI0150), Uijeongbu St. Mary’s Hospital. Before surgery, the written informed consent from the donors was obtained to participate in this research. The cartilage from a non-weight bearing area of the knee was collected and then digested with collagenase. The isolated cells were cultured in DMEM containing 1% (v/v) penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) and 10% (v/v) FBS (Thermo Fisher Scientific) in a humidified atmosphere containing 5% (v/v) CO2 at 37 °C.
hNCs encapsulated in COL1 (hNC-collagen)
A cell-laden collagen complex was formed using COL1 (Ubiosis, Seongnam, Korea, 30 mg/ml). hNCs prepared from the culture was mixed with the collagen solution to make a final concentration of 20 mg/ml (1 × 106 cells/ml). The mixture was then put in a culture plate and incubated for 30 min at room temperature to induce gelation of collagen [28]. The culture medium was added to culture plate and was incubated in a humidified atmosphere containing 5% (v/v) CO2 at 37 °C.
Immunofluorescent staining
For immunofluorescent staining, hNCs or hNC-collagen (1 × 105 cells/100μl) was fixed with 4% (w/v) PFA and then permeabilized by 0.03% (v/v) Triton X-100 solution. Then, hNCs or hNC-collagen complex was incubated with primary antibodies against type II collagen (COL2A, 1:500; Santa Cruz Biotechnology Inc., Dallas, Texas, USA), SRY-Box 9 (SOX9, 1:500; AbFrontier, Seoul, Korea), or Aggrecan (1:500; Santa Cruz Biotechnology Inc.), and then with secondary antibodies—Alexa Flour 546-conjugated goat anti-rabbit IgG or mouse IgG (1:1000; Molecular Probes, Eugene, OR, USA). 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich Co.) was used for counterstaining. Fluoresce was detected by a confocal microscope (LSM 510 Meta; Carl Zeiss Meditec AG, Jena, Germany).
Western blot
For western blotting, cultured hNCs were lysed in RIPA buffer (Thermo Fisher Scientific). The total lysates were centrifuged at 20,000 g for 15 min at 4 °C, and the supernatant was obtained. Protein samples were prepared with 5 × SDS-PAGE loading buffer (Dyne Bio, Seongnam, korea), separated on a Bio-Rad 4-20% (w/v) gel, and transferred to a PVDF membrane (Roche, Mannheim, Germany). The membranes were blocked and incubated with primary antibodies against COL2A (1:500; Santa Cruz Biotechnology Inc.), SOX9 (1:500; Abcam, Cambridge, UK), and GAPDH (1:1000; Santa Cruz Biotechnology Inc.) and the appropriate secondary antibodies. The membrane was then developed with ECL detection reagents (Thermo Fisher Scientific).
Imaging of cell movement in hNC-collagen
The cells moving in hNC-collagen complex were detected using a live cell imaging system (Lionheart FX; BioTek Instruments Inc., Winooski, VT, USA). Their movement was recorded at 20 min intervals for 72 h.
Cell viability and growth in hNC-collagen
The viability of cells in hNC-collagen complex (1 × 105 cells/100 μl) was analyzed using a live/dead viability kit (LIVE/DEAD® Viability/Cytotoxicity Kit; Thermo Fisher Scientific). The cultured hNC-collagen complex was treated with calcein AM and ethidium homodimer, followed by incubation for 30 min. The live cells were stained with calcein AM (green color) and the dead cells were stained with ethidium homodimer (red color). The fluorescence was detected by confocal laser scanning microscopy (LSM 510 Meta, Carl Zeiss). Cell proliferation in cultured hNCs and hNC-collagen was measured over 7 days and 14 days using an EZ-Cytox assay kit (Daeillab Co., Seoul, Korea). Absorbance was determined using a microplate reader (Molecular Devices Corporation, Sunnyvale, CA, USA). Proliferating cells in the cultures was observed by using a proliferation marker, 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich Co.). During the final 24 h of culture, 10 μM BrdU was treated into the culture plate. Cells were fixed, permeabilized, and then treated with fixative/denaturing solution at room temperature to denature DNA. Cells were incubated with a primary antibody against BrdU (1:200; AbD Serotec, Ltd., Oxford, UK) and then incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG secondary antibodies (1:1000; Molecular Probes). Nuclei were stained with DAPI (Sigma-Aldrich) and fluorescent was observed using a Zeiss LSM510 confocal microscope (Carl Zeiss).
Detection of mycoplasma contamination
Mycoplasma contamination of hNCs or hNC-collagen (1 × 105 cells/100 μl) in culture was evaluated by polymerase chain reaction (PCR) with a mycoplasma PCR detection kit (CS-D-50, CellSafe, Gyeonggi-do, Korea) according to the manufacturer’s instructions. Briefly, after 9 and 24 h of culturing, culture media was collected and centrifuged at 3000 rpm for 5 min. The supernatant was centrifuged again at 12,000 rpm for 10 min. Next, the mycoplasma pellet was collected and suspended in 100 μl of dH2O, and boiled at 98 °C for 10 min. The PCR reaction was conducted by mixing the supernatant with PCR template, primer mix, and 2X PCR premix. The PCR reactions were performed with following conditions: 1 cycle of predenaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s. The PCR products were loaded onto a 1.5% agarose gel.
Osteochondral defect model and hNC-collagen implantation
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Catholic University of Korea (2017-0040-04). Animals were maintained in air-conditioned rooms at controlled temperature (23 ± 3 °C) and humidity (50 ± 10%) with 12-h light/dark cycle. The animals were fed a standard rodent chow and purified water ad libitum. Fourteen-week-old (400–450 g) male Sprague–Dawley rats (Orient Bio Co., Seongnam, Korea) were used to evaluate the hNC-collagen complex in vivo. The animals were divided randomizely into four groups by different treatment: normal (n = 5), no treatment (n = 5), collagen only without cells (n = 5), or hNC-collagen (n = 5). The rats were anesthetized with a combination administration of ketamine (50 mg/kg; Zoletil, Virbac Laboratory, Carros, France) and xylazine (10 mg/kg; Rompun, Bayer, Mexico). After shaving around the knee joint, an articular cartilage defect was created by drilling 2 mm in depth and 2 mm in diameter using the Micromotor Hand Piece Control Box (Strong 207A, SAESHIN Precision Co., Ltd., Daegu, Korea). Osteochondral defects were created in the weight-bearing portion of the medial femoral condyle. After defect creation, collagen only or hNC-collagen (1 × 106 cells/20 μl) was implanted by injection to achieve full defect filling. The gel usually hardens within 5 min, and then hNC-collagen was fitted in the defect site. Once this was established, the knee joint was closed with sutures of absorbable 4-0 vicryl (Ethicon, Somerville, NJ, USA). The rats were allowed free movement in cages after recovering from anesthesia. At 8 weeks after implantation, the knee joint was surgically removed and the specimens fixed in 4% neutral buffered formalin were decalcified, and embedded in paraffin for histological examination.
Histological examination and immunohistofluorescent staining
5-mm paraffin sections were stained with hematoxylin and eosin (H&E) for general morphological evaluation. The GAG-rich ECM formation was detected with safranin O, and the proteoglycan with toluidine staining. For immunohistofluorescent staining, the sections were deparaffinized with xylene and rehydrated with 100% ethanol, 95% ethanol, and 80% ethanol, followed by antigen retrieval with proteinase K (Abcam) to expose the antigenic sites. The tissues were treated with 0.3% H2O2; incubated with primary antibodies against human nuclei (HuNu, 1:100, EMD Millipore, Billerica, MA, USA), COL2A (1:500; Santa Cruz Biotechnology Inc.), SOX9 (1:500; AbFrontier), or Aggrecan (1:500; Santa Cruz Biotechnology Inc.); and then incubated with secondary antibodies—Alexa Flour 546-conjugated goat anti-rabbit IgG or mouse IgG (1:1000; Molecular Probes) DAPI (Sigma-Aldrich) was used for counterstaining and fluorescent was observed by a Zeiss LSM510 confocal microscope (Carl Zeiss).
Quantification and statistical analysis
Data represent the mean ± SD or the mean ± SEM from three independent experiments. For multiple comparisons, ANOVA with Tukey’s post hoc test was used to determine for statistically significant differences among multiple groups. Differences between means of two samples were defined with Student’s t test. Probability values of < 0.05 were statistically significant. To determine the Aggrecan-, Sox9-, and Col2A-positive cells, the stained hNCs or hNC-collagen samples were analyzed under a confocal microscope, and the number of each type of marker-positive cell was calculated in four different confocal fields (Fig. 1).
Fig. 1.
Surgical procedure. A Images of the nasal septal tissue obtained from septoplasty. B Anatomical position of the nasal septum
Results
Proliferation in hNCs culture
The chondrocytes isolated from nasal cartilage were expanded from 2D monolayer culture. In this study, we investigated the proliferative ability of hNCs using BrdU, a proliferation marker. The cell proliferation of hNCs was compared with that of hACs, which are the most widely used cells for ACT in cartilage regeneration. Immunostaining for BrdU showed approximately 1.5–2.0-fold greater positive in hNCs obtained from three different donors compared with hACs from three different donors (Fig. 2A, B). Moreover, hNCs showed a faster cell growth compared with hACs during a culture period of 7 days (Fig. 2C).
Fig. 2.
Proliferation of hNCs in 2D monolayer culture. A Confocal microscopy images of hNCs and hACs in culture stained with BrdU (green), a proliferation marker, and DAPI (blue). Scale bars: 50 μm. B Positively stained cells were presented as percentage relative to total number of cells staining for DAPI. Te data are presented as the relative stained cells (± SEM). Statistical comparisons were made by t test. *p < 0.05. The results are representative of three independent experiments. C Growth ratios of hNCs and hACs during a culture period of 14 days after plating. The cell growth was examined using an EZ-Cytox assay kit. Each bar represents the relative cell growth (± SD). Two-way ANOVA followed by Bonferroni post hoc test was used for statistical analysis of cell growth rate comparing hNCs with hACs at all time points. *p < 0.05, **p < 0.01, ***p < 0.001. The results are representative of two independent experiments
Extracellular matrix (ECM) protein expression in hNCs culture
Immunostaining showed the expression of ECM components in both hNCs and hACs. Intracellular levels of COL2A and SOX9 were clearly detected by confocal microscopy in both hNCs and hACs (Fig. 3A). To further confirm the expression of ECM proteins in hNCs, hNCs samples derived from five different nasal septum donors were subjected to SDS-PAGE gels for western blotting analysis. COL2A and SOX9 were expressed in all five different donors, but their expression levels were different in all hNC samples after three passages of culture (Fig. 3B). These results indicate that hNCs exhibit a high proliferation capacity and low donor-dependent chondrogenic potency, suggesting that hNCs are a new alternative chondrocyte source for clinical application in cartilage regeneration.
Fig. 3.
Chondrocyte-specific protein expression of hNCs in 2D monolayer culture. A Confocal images of cultured hNCs and hACs double stained with the chondrocyte-specific markers Sox9 (green), type II collagen (red), and DAPI (blue). Scale bars: 50 μm. B Western blots of SDS-PAGE gels of hNCs extracts obtained from five different donors and immunodetection using antibodies against Sox9 and type II collagen. β-actin was used as a loading control
Cell movement, viability, and growth of hNC-collagen in culture
After encapsulating hNCs in collagen, cell movement was detected by microscopy, suggesting that collagen does not affect cell migration (Fig. 4A and Supplemental Videos 1–2). The viability of hNC-collagen and the cell growth in in vitro culture were analyzed using a live/dead viability and an EZ-Cytox assay during a culture period of 14 days in vitro. Figure 4B and C show the viability staining of hNC-collagen, with green indicating the cytoplasm of living cells and red indicating the nuclei of dead cells. Cell viability of hNC-collagen was great and hNC-collagen exhibited a faster growth rate than that of hACs encapsulated in COL1 (hAC-collagen) during the 14 days of culture (Fig. 4D). These data have impact on the therapeutic use of hNC-collagen in clinically cartilage regenerative medicine.
Fig. 4.
Survival and growth of hNC-collagen after collagen encapsulation in vitro. A Image of cell movement in hNCs after collagen encapsulation (supplemental video 1–2). B Growth ratios of hNC-collagen and hAC-collagen on culture plates 14 days after collagen encapsulation. The cell growth in collagen was examined using an EZ-Cytox assay kit. The graph represents the relative cell growth (± SD). Two-way ANOVA followed by Bonferroni post hoc test was used for statistical analysis of cell growth rate comparing hNC-collagen with hAC-collagen at all time points. *p < 0.05, **p < 0.01, ***p < 0.001. The results are representative of two independent experiments. C Image of cultured hNC-collagen and hAC-collagen stained with calcein AM (live cells as green fluorescence) and ethidium homodimer (dead cells as red fluorescence) in an in vitro live/dead cell assay carried out 14 days after collagen encapsulation, as observed by confocal microscopy. Scale bars: 50 μm. All images and results are representative of two or three independent experiments
ECM protein expression in hNC-collagen in the absence of growth factor
To evaluate the clinical applicability of hNC-collagen, the expression of ECM proteins in hNC-collagen was demonstrated by immunostaining with chondrocyte-specific antibodies in the absence of growth factor, FBS. Immunofluorescent staining for COL1A, SOX9 and Aggrecan showed that approximately 90–95% of hNCs expressed these proteins immediately after encapsulation (1 h), and the expression level of ECM proteins was maintained from 1 to 24 h after collagen encapsulation (Fig. 5).
Fig. 5.
Chondrocyte-specific protein expression of hNC-collagen after collagen encapsulation. A, B Confocal microscopy images of hNC-collagen in PBS in the absence of growth medium and stained with the chondrocyte-specific markers COL2A (red), SOX9 (red), Aggrecan (red). Nuclei were counterstained with DAPI (blue). Scale bars: A 50 μm; B 20 μm. All images are representative of three independent experiments. C–E The number of SOX9-, Aggrecan-, COL2A-positive cells were counted at 1 h, 9 h, and 24 h after collagen encapsulation. The data are presented as the percentage of Sox9-, aggrecan-, type II collagen-positive cells to DAPI (± SEM). Statistical comparisons were made by one-way ANOVA. There was no consistent change in the number of positive cells. The results are representative of two independent experiments. NC; negative control, PC; positive control, Col; collagen only, Mix; hNC-collagen mixture, Cell; cell only
Mycoplasma detection in hNC-collagen in culture
Mycoplasma testing is required for the therapeutic use of hNC-collagen. To evaluate mycoplasma contamination in hNC-collagen, mycoplasma detection is conducted using PCR detection kit with specific primers designed to detect highly conserved coding region in the mycoplasma genome. The supernatant obtained from the cultured hNCs or hNC-collagen mixture was tested for mycoplasma contamination, and all tested samples showed one PCR product band with an approximate size of 700 bp, which is an internal DNA standard used to confirm the successful PCR (Fig. 6); therefore, collagen did not cause mycoplasma contamination of hNCs.
Fig. 6.
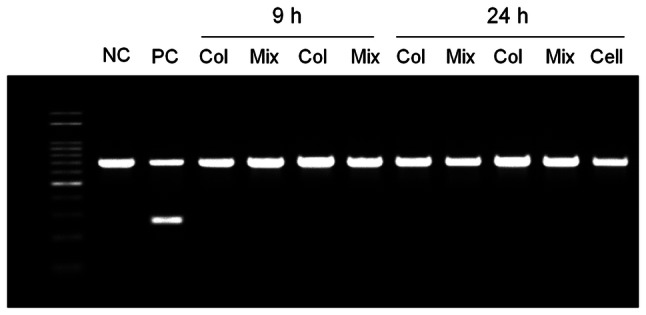
Mycoplasma contamination analysis of hNCs and hNC-collagen in culture. PCR analysis of samples of collagen, 2D cultured hNCs, and hNC-collagen cultured under growth conditions for 24 h. The results are representative of three independent experiments
Expression of TIMP-2 in hNC-collagen culture medium
Secretion of tissue inhibitor of metalloproteinase (TIMP)-2 from culture medium of hNC-collagen was assayed by ELISA. TIMPs show a direct inhibitory effect on matrix metalloproteinase (MMP) activity [29, 30]. MMPs derived from chondrocytes, synovium, and polymorphonuclear leukocytes play a major role in cartilage degradation of osteoarthritis (OA) [31, 32]. After 4 days of culture, the culture medium from hNCs or hNC-collagen were obtained and analyzed for TIMP-2 expression. The results showed comparable levels of TIMP2 secretion both in hNCs and hNC-collagen culture medium (Fig. 7). The concentration of TIMP-2 in hNCs and hNC-collagen culture medium was range of 1.2–1.4 ng/ml and 1.2–1.6 ng/ml, respectively.
Fig. 7.

TIMP-2 expression of hNCs and hNC-collagen. Comparison of the TIMP-2 levels in the culture medium of hNCs and hNC-collagen. The TIMP-2 concentration was detected by ELISA in the supernatants of hNCs or hNC-collagen cultures over 4 days. Each bar represents TIMP2 expression levels (± SEM). Statistical comparisons were made by paired t-tests. There was no consistent change in the expression levels of TIMP-2. The results are representative of three independent experiments
Implantation of hNC-collagen in an osteochondral defect model
To evaluate the therapeutic effect of hNC-collagen for cartilage regeneration in an osteochondral defect model, collagen only or hNC-collagen was implanted in the osteochondral defect of a rat (Fig. 8A). At 8 weeks after implantation, H&E staining showed that the osteochondral defect treated with only collagen induced little tissue infiltration into the defect area (Fig. 8B, c). In contrast, hNC-collagen treatment caused greatly enhanced tissue infiltration into the defect area and resulted in the appearance of an articular-like surface (Fig. 8B, d), which is similar to the normal appearance (Fig. 8B, a). Higher magnification revealed a smooth surface without an obvious border with the surrounding normal articular cartilage. The hNC-collagen-implanted tissue sections demonstrated safranin O staining which is relatively heavier (Fig. 8C, d) compared with the collagen-implanted defect (Fig. 8C, c), indicating a higher concentration of proteoglycans, one of the essential components for normal function of cartilage tissue. Further analysis was performed using toluidine blue to visualize the GAG content. The osteochondral defect treated with only collagen showed very little staining (Fig. 8D, c). However, the defect filled with hNC-collagen showed higher GAG accumulation around the periphery and within the defect area (Fig. 8D, d). Moreover, at 8 weeks post implantation, several implanted human cells were still observed within the defect areas in the animal models implanted with hNC-collagen (Fig. 8E, b and F, b).
Fig. 8.
Evaluation of osteochondral defects of a rat knee after hNC-collagen implantation. A Implantation of hNC-collagen into rat osteochondral defects. B H&E staining, C Safranin O staining, and D Toluidine blue staining of normal cartilage, an osteochondral defect, an osteochondral defect filled with collagen, and an osteochondral defect filled with hNC-collagen at 8 weeks postimplantation. The defect is marked with * and defect filled with collagen or hNC-collagen is marked with **. Scale bars: (a–d) 100 μm; (a’–d’) 50 μm. E Immunohistochemical staining of an osteochondral defect filled with collagen (a) and hNC-collagen (b) with antibody against HuNu at 8 weeks after implantation. HuNu-positive cells were observed for hNC-collagen. Scale bars: 50 μm. F Confocal microscopy images of defective sections stained with antibodies against HuNu. Nuclei were counterstained with DAPI (blue). Scale bars: 20 μm
Discussion
Articular cartilage is aneural and avascular environment nourished by synovial fluid alone, suggesting minimal endogenous ability for repair of articular surface defects [33, 34]. Brittberg et al. demonstrated the treatment of deep cartilage defects with ACT, via a method first published in 1994 [35]. However, this method has limitations, such as cell leakage from the implantation site and uneven cell distribution [36]. Thus, matrix-assisted autologous chondrocyte transplantation (MACT) procedures have been developed to address most of these problems. The scaffold serves as a biodegradable 3D structure to support in vitro cell growth of living cells and their subsequent transplantation [3]. Because liquid or gel scaffolds can be injected into the site of cartilage injury to avoid invasive clinical procedure, hydrogels recently considered as attractive scaffold materials in tissue engineering of cartilage [3]. Following this trend, MACT utilizing a collagen gel scaffold has been found to exhibit good therapeutic potential for the implantation of engineered tissue [36].
Septum surgery is one of the most common procedures in otorhinolaryngology performed worldwide to improve symptoms of nasal obstruction [37]. The septal cartilage is discarded as surgical waste during the surgical procedure (Fig. 1). Recently, the promising potential of hNCs was recognized [38]. The hNCs are easily accessible via minimally invasive surgery of the nasal septum as well as from the discarded tissue after surgery, with minimal donor site morbidity [39]. Moreover, hNCs show a significantly high chondrogenic capacity in in vitro constructs [40] and consistent behavior in cartilage engineering regardless of the age of the donor [41]. Based on these favorable findings, hNCs represents a valuable cellular source in engineering of autologous cartilage grafts [6, 40, 41]. Here, we aimed to test the role of hNCs and collagen gel scaffolds as effective therapeutic agents for treatment of articular surface defects in cartilage tissue engineering for clinical applications. Collagen is the main protein of connective tissue that supports tissue morphology, and widely used for bone grafting, reconstructive surgery, cosmetic surgery, and dermal injections [26, 27]. Atelocollagen is a highly purified type I collagen extracted from the skin dermis by treatment of pepsin and removal of telopeptide which is the immunologically active component. This makes it non-immunogenic and an ideal scaffold for tissue regeneration [28].
Highly proliferative capacity is needed to obtain enough number of cells in tissue engineering [42]. In this study, hNCs showed greater and faster cell growth compared with hACs at fourth passage of culture (Fig. 2). Notably, hNC-collagen 3D culture showed great proliferation compared with the hAC-collagen when monitored over a culture period of 14 days (Fig. 4B). This difference could be explained by the rapid proliferation of human adult nasal chondrocytes compared with hACs in culture. Additionally, over 14 days of viability monitoring, hNCs in the collagen complex showed greater survival than hACs during cultivation (Fig. 4C, D). Considering that it is important to obtain sufficient number of chondrocytes for clinical use, hNCs exhibit great clinical potential in tissue engineering of cartilage defects.
In this study, two kinds of xenogeneic materials such as injectable porcine collagen and FBS were used for cell culture and preparation of the cell-collagen complex. Injectable porcine collagen has been used for various cosmetic and reconstructions [36] and several clinical trials have shown the use of FBS without any adverse side effects [43]. However, it is suggested to wash the cells enough to decrease the residual xenogenic protein levels for acceptable levels [44]. Based on the above considerations, the chondrogenic property of hNCs in the complex was evaluated in the absence of FBS. At each time point, the expression of the chondrocyte-specific markers Aggrecan, COL2A, and SOX9 were detected in hNC-collagen by immunofluorescent staining. Approximately 90% of hNCs expressed the chondrogenic markers, and this tendency persisted from 1 to 24 h after collagen encapsulation (Fig. 5). Furthermore, the cultured hNCs and hNC-collagen complex were examined for mycoplasma contamination at the gene level for therapeutic cartilage regeneration. A negative mycoplasma screening result was obtained in all specimens, suggesting that the cultured chondrocytes and the manufacture of hNC-collagen would be safe for clinical use (Fig. 6).
Chondrocytes maintain the homeostasis of ECM by regulating the levels of matrix proteinase and protease inhibitors to maintain the composition and structural integrity of the ECM in articular cartilage. TIMPs inhibit the catabolic activity of MMP, which plays an important role in the pathogenesis of OA [29–32]. In hNC-collagen complexes, the expression levels of TIMPs resembled those of chondrocytes alone, suggesting that the cultured chondrocytes in the complex exhibit similar metabolic activity, with potential application in the regeneration of osteochondral defects (Fig. 7).
Notably, in the rodent model of osteochondral cartilage defect, histological analysis clearly demonstrated articular-like surface production that resembled articular cartilage and a higher concentration of proteoglycans in the osteochondral defects after implantation of hNC-collagen complex compared with the collagen (Fig. 8B, C). Moreover, hNC-collagen implantation resulted in substantial GAG-rich ECM synthesis compared with the collagen implantation (Fig. 8D). Histological staining revealed the implanted hNCs in the collagen complex, as cells distributed relatively homogeneously, with several vital cells maintained at the site of implantation at 8 weeks (Fig. 8E).
In this study, hNCs proliferated rapidly in the collagen gel scaffold while maintaining great chondrogenic potency and showed excellent properties for integration into host tissue and high ECM production. Based on our results, we conclude that the production of hNCs and manufacturing of hNC-collagen complex is a safe and valuable treatment for cartilage engineering. In the future, the banking of hNCs obtained from discarded tissue during surgery may be utilized for application of cell therapy.
In conclusion, this study described the potential of hNCs and hNC-collagen as effective therapeutic agents for treatment of articular surface defects in cartilage tissue engineering for clinical applications. The hNCs are easily accessible via minimally invasive surgery of the nasal septum as well as normally discarded tissue from surgery, with minimal donor site morbidity. Here, hNCs proliferated rapidly in the collagen gel scaffold while maintaining great chondrogenic potency in vitro. Notably, implantation of hNC-collagen led to great chondrogenic repair of osteochondral defects in rats, which could be explained by excellent properties of hNC-collagen for integration into host tissue and high ECM production. Considering that it is important to secure a sufficient amount of chondrocytes for clinical application, hNCs exhibit great potential for therapeutic use in regenerative medicine of cartilage defects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B07045421), the Bio & Medical Technology Development Program of the NRF funded by the Ministry of Science & ICT(2018M3A9E8020856, 2019M3A9H2032424, 2019M3E5D5064110), and the Institute of Clinical Medicine Research of Bucheon St. Mary’s Hospital, Research Fund (2017, 2018). This research was also supported by a grant from the E.N.T. Fund of the Catholic University of Korea (program years 2017–2018). The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dr Jung-Min Yon and Mrs Hyun A Bae (The Catholic University of Korea) for helpful discussion.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This research was conducted in compliance with the Institutional Review Board of Catholic Medical Center Clinical Research Coordinating Center (IRB No. KC08TISS0341), Seoul St. Mary’s Hospital. Before surgery, the written informed consent from the donors was obtained to participate in this research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Se Hwan Hwang, Jung Yeon Lim, and Sung Won Kim have contributed equally to this work.
Contributor Information
Se Hwan Hwang, Email: yellobird@catholic.ac.kr.
Jung Yeon Lim, Email: jylim8921@gmail.com.
Sung Won Kim, Email: kswent@catholic.ac.kr.
References
- 1.Mobasheri A, Csaki C, Clutterbuck A, Rahmanzadeh M, Shakibaei M. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: applications in cartilage repair and osteoarthritis therapy. Histol Histopathol. 2009;24:347–366. doi: 10.14670/HH-24.347. [DOI] [PubMed] [Google Scholar]
- 2.Nam BM, Kim BY, Jo YH, Lee S, Nemeno JG, Yang W, et al. Effect of cryopreservation and cell passage number on cell preparations destined for autologous chondrocyte transplantation. Transplant Proc. 2014;46:1145–1149. doi: 10.1016/j.transproceed.2013.11.117. [DOI] [PubMed] [Google Scholar]
- 3.Kon E, Filardo G, Di Matteo B, Perdisa F, Marcacci M. Matrix assisted autologous chondrocyte transplantation for cartilage treatment: a systematic review. Bone Joint Res. 2013;2:18–25. doi: 10.1302/2046-3758.22.2000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DH, Lim JY, Kim SW, Lee W, Park SH, Kwon MY, et al. Characteristics of nasal septal cartilage-derived progenitor cells during prolonged cultivation. Otolaryngol Head Neck Surg. 2018;159:774–782. doi: 10.1177/0194599818777195. [DOI] [PubMed] [Google Scholar]
- 5.Pelttari K, Mumme M, Barbero A, Martin I. Nasal chondrocytes as a neural crest-derived cell source for regenerative medicine. Curr Opin Biotechnol. 2017;47:1–6. doi: 10.1016/j.copbio.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Candrian C, Vonwil D, Barbero A, Bonacina E, Miot S, Farhadi J, et al. Engineered cartilage generated by nasal chondrocytes is responsive to physical forces resembling joint loading. Arthritis Rheum. 2008;58:197–208. doi: 10.1002/art.23155. [DOI] [PubMed] [Google Scholar]
- 7.Rotter N, Bonassar LJ, Tobias G, Lebl M, Roy AK, Vacanti CA. Age dependence of cellular properties of human septal cartilage: implications for tissue engineering. Arch Otolaryngol Head Neck Surg. 2001;127:1248–1252. doi: 10.1001/archotol.127.10.1248. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Lim MH, Jeun JH, Park SH, Lee W, Park SH, et al. Evaluation of polycaprolactone-associated human nasal chondrocytes as a therapeutic agent for cartilage repair. Tissue Eng Regen Med. 2019;16:605–614. doi: 10.1007/s13770-019-00210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon JH, Yun BG, Lim MJ, Kim SJ, Lim MH, Lim JY, et al. Rapid cartilage regeneration of spheroids compose of human nasal septum-derived chondrocytes in rat osteochondral defect model. Tissue Eng Regen Med. 2020;17:81–90. doi: 10.1007/s13770-019-00231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idrusa RBH, Huia CK, Ibrahimb FW, Husseinc FN, Saimd AB. The expansion potential of human nasal septum chondrocytes for the formation of engineered cartilage. Sci Asia. 2007;33:145–152. [Google Scholar]
- 11.Steinwachs M. New technique for cell-seeded collagen matrix-supported autologous chondrocyte transplantation. Arthroscopy. 2009;25:208–211. doi: 10.1016/j.arthro.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18:519–527. doi: 10.1007/s00167-009-1028-1. [DOI] [PubMed] [Google Scholar]
- 13.Erggelet C, Sittinger M, Lahm A. The arthroscopic implantation of autologous chondrocytes for the treatment of full-thickness cartilage defects of the knee joint. Arthroscopy. 2003;19:108–110. doi: 10.1053/jars.2003.50025. [DOI] [PubMed] [Google Scholar]
- 14.Della Villa S, Kon E, Filardo G, Ricci M, Vincentelli F, Delcogliano M, et al. Does intensive rehabilitation permit early return to sport without compromising the clinical outcome after arthroscopic autologous chondrocyte implantation in highly competitive athletes? Am J Sports Med. 2010;38:68–77. doi: 10.1177/0363546509348490. [DOI] [PubMed] [Google Scholar]
- 15.Choi NY, Kim BW, Yeo WJ, Kim HB, Suh DS, Kim JS, et al. Gel-type autologous chondrocyte (Chondron™) implantation for treatment of articular cartilage defects of the knee. BMC Musculoskelet Disord. 2010;11:103. doi: 10.1186/1471-2474-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bichara DA, Zhao X, Hwang NS, Bodugoz-Senturk H, Yaremchuk MJ, Randolph MA, et al. Porous poly (vinyl alcohol)-alginate gel hybrid construct for neocartilage formation using human nasoseptal cells. J Surg Res. 2010;163:331–336. doi: 10.1016/j.jss.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 17.Markstedt K, Mantas A, Tournier I, Martínez Ávila H, Hägg D, Gatenholm P. 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16:1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 18.Mannoor MS, Jiang Z, James T, Kong YL, Malatesta KA, Soboyejo WO, et al. 3D printed bionic ears. Nano Lett. 2013;13:2634–2639. doi: 10.1021/nl4007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiffel AJ, Kafka C, Hernandez KA, Popa S, Perez JL, Zhou S, et al. High-fidelity tissue engineering of patient-specific auricles for reconstruction of pediatric microtia and other auricular deformities. PLoS One. 2013;8:e56506. doi: 10.1371/journal.pone.0056506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajan N, Habermehl J, Coté MF, Doillon CJ, Mantovani D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat Protoc. 2006;1:2753–2758. doi: 10.1038/nprot.2006.430. [DOI] [PubMed] [Google Scholar]
- 21.Gallop PM, Seifter S. [93] Preparation and properties of soluble collagens. Methods Enzymol. 1963;6:635–641. [Google Scholar]
- 22.Lee SJ, Broda C, Atala A, Yoo JJ. Engineered cartilage covered ear implants for auricular cartilage reconstruction. Biomacromol. 2011;12:306–313. doi: 10.1021/bm100856g. [DOI] [PubMed] [Google Scholar]
- 23.Neumeister MW, Wu T, Chambers C. Vascularized tissue-engineered ears. Plast Reconstr Surg. 2006;117:116–122. doi: 10.1097/01.prs.0000195071.01699.ce. [DOI] [PubMed] [Google Scholar]
- 24.Rao KP. Recent developments of collagen-based materials for medical applications and drug delivery systems. J Biomater Sci Polym Ed. 1996;7:623–645. doi: 10.1163/156856295x00526. [DOI] [PubMed] [Google Scholar]
- 25.Pacak CA, MacKay AA, Cowan DB. An improved method for the preparation of type I collagen from skin. J Vis Exp. 2014;83:e51011. doi: 10.3791/51011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patino MG, Neiders ME, Andreana S, Noble B, Cohen RE. Collagen as an implantable material in medicine and dentistry. J Oral Implantol. 2002;28:220–225. doi: 10.1563/1548-1336(2002)028<0220:CAAIMI>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Nair R, Sevukarajan M, Mohammed T, Badivaddin C, Kumar A. Collagen based drug delivery systems: a review. J Innov Trends Pharm Sci. 2010;1:288–304. [Google Scholar]
- 28.Shetty AA, Kim SJ, Shetty V, Jang JD, Huh SW, Lee DH. Autologous collagen induced chondrogenesis (ACIC: Shetty-Kim technique)–A matrix based acellular single stage arthroscopic cartilage repair technique. J Clin Orthop Trauma. 2016;7:164–169. doi: 10.1016/j.jcot.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cawston TE. Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther. 1996;70:163–182. doi: 10.1016/0163-7258(96)00015-0. [DOI] [PubMed] [Google Scholar]
- 30.Knäuper V, López-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 31.Murphy G, Hembry RM, Hughes CE, Fosang AJ, Hardingham TE. Role and regulation of metalloproteinases in connective tissue turnover. Biochem Soc Trans. 1990;18:812–815. doi: 10.1042/bst0180812. [DOI] [PubMed] [Google Scholar]
- 32.Woessner JF, Jr, Gunja-Smith Z. Role of metalloproteinases in human osteoarthritis. J Rheumatol Suppl. 1991;27:99–101. [PubMed] [Google Scholar]
- 33.Wylie JD, Hartley MK, Kapron AL, Aoki SK, Maak TG. What is the effect of matrices on cartilage repair? A systematic review. Clin Orthop Relat Res. 2015;473:1673–1682. doi: 10.1007/s11999-015-4141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costa E, González-García C, Gómez Ribelles JL, Salmerón-Sánchez M. Maintenance of chondrocyte phenotype during expansion on PLLA microtopographies. J Tissue Eng. 2018;9:2041731418789829. doi: 10.1177/2041731418789829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 36.Tohyama H, Yasuda K, Minami A, Majima T, Iwasaki N, Muneta T, et al. Atelocollagen-associated autologous chondrocyte implantation for the repair of chondral defects of the knee: a prospective multicenter clinical trial in Japan. J Orthop Sci. 2009;14:579–588. doi: 10.1007/s00776-009-1384-1. [DOI] [PubMed] [Google Scholar]
- 37.Ketcham AS, Han JK. Complications and management of septoplasty. Otolaryngol Clin North Am. 2010;43:897–904. doi: 10.1016/j.otc.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Shafiee A, Seyedjafari E, Sadat Taherzadeh E, Dinarvand P, Soleimani M, Ai J. Enhanced chondrogenesis of human nasal septum derived progenitors on nanofibrous scaffolds. Mater Sci Eng C. 2014;40:445–454. doi: 10.1016/j.msec.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 39.Pelttari K, Pippenger B, Mumme M, Feliciano S, Scotti C, Mainil-Varlet P, et al. Adult human neural crest–derived cells for articular cartilage repair. Sci Transl Med. 2014;6:251ra119. doi: 10.1126/scitranslmed.3009688. [DOI] [PubMed] [Google Scholar]
- 40.Kafienah W, Jakob M, Démarteau O, Frazer A, Barker MD, Martin I, et al. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002;8:817–826. doi: 10.1089/10763270260424178. [DOI] [PubMed] [Google Scholar]
- 41.Rotter N, Bonassar LJ, Tobias G, Lebl M, Roy AK, Vacanti CA. Age dependence of biochemical and biomechanical properties of tissue-engineered human septal cartilage. Biomaterials. 2002;23:3087–3094. doi: 10.1016/s0142-9612(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 42.Shafiee A, Kabiri M, Ahmadbeigi N, Yazdani SO, Mojtahed M, Amanpour S, et al. Nasal septum-derived multipotent progenitors: a potent source for stem cell-based regenerative medicine. Stem Cells Dev. 2011;20:2077–2091. doi: 10.1089/scd.2010.0420. [DOI] [PubMed] [Google Scholar]
- 43.de Lima M, McMannis J, Gee A, Komanduri K, Couriel D, Andersson BS, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transpl. 2008;41:771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








