Abstract
BACKGROUND:
Human adipose tissue-derived stem cells (ADSCs) are attractive multipotent stem cell sources with therapeutic potential in various fields requiring repair and regeneration, such as acute and chronically damaged tissues. ADSC is suitable for cell-based therapy, but its use has been hampered due to poor survival after administration. Potential therapeutic use of ADSC requires mass production of cells through in vitro expansion. Many studies have consistently observed the tendency of senescence by mesenchymal stem cell (MSC) proliferation upon expansion. Hypoxia has been reported to improve stem cell proliferation and survival.
METHODS:
We investigated the effects of hypoxia pretreatment on ADCS proliferation, migration capacity, differentiation potential and cytokine production. We also analyzed the effects of vascular endothelial growth factor (VEGF) on osteogenic and chondrogenic differentiation of ADSCs by hypoxia pretreatment.
RESULTS:
Hypoxia pretreatment increased the proliferation of ADSCs by increasing VEGF levels. Interestingly, hypoxia pretreatment significantly increased chondrogenic differentiation but decreased osteogenic differentiation compared to normoxia. The osteogenic differentiation of ADSC was decreased by the addition of VEGF but increased by the depletion of VEGF. We have shown that hypoxia pretreatment increases the chondrogenic differentiation of ADSCs while reducing osteogenic differentiation in a VEGF-dependent manner.
CONCLUSION:
These results show that hypoxia pretreatment can provide useful information for studies that require selective inhibition of osteogenic differentiation, such as cartilage regeneration.
Electronic supplementary material
The online version of this article (10.1007/s13770-020-00265-5) contains supplementary material, which is available to authorized users.
Keywords: Adipose-derived stem cells (ADSCs), Hypoxia, VEGF, Differentiation, Cartilage regeneration
Introduction
Stem cells are distinguished from other cell types by two important characteristics: (1) they are unspecialized cells that are capable of self-renewal through proliferation (2) and can be induced to become tissue-specific cells under certain physiologic or experimental conditions [1]. Stem cells with these unique properties have great potential for tissue regenerative medicine [1].
Mesenchymal stem cells (MSCs) are pluripotent adult stem cells that can be isolated from various mesenchymal tissues in the adult body. MSC was first described by Friedenstein et al. as bone marrow-derived clonogenic: plastic-adherent cells capable of differentiating into osteoblasts, adipocytes and chondrocytes [2]. However, ideal stem cells for use in functional tissue engineering should be abundantly available, harvested with minimal morbidity, reliably differentiated in a variety of pathways, and safe and effective for transplantation. Recently, some reports have described the isolation of MSCs from human adipose tissue that appears to meet these criteria [3, 4]. These human adipose tissue-derived stem cells (ADSCs) are capable of forming mesodermal tissues such as bone, cartilage, tendons, skeletal muscle and fat when cultured under lineage-specific conditions, and can also form non-mesodermal tissues such as neurons and endocrine pancreatic cells [5, 6].
Recent studies revealed that low oxygen tension or hypoxia affects various types of stem cells, such as embryonic stem cells [7], induced pluripotent stem cells [8], and bone marrow-derived stem cells (BMSCs) [9–11]. A low oxygen environment is physiologically normal for most mammalian embryos as well as adult somatic stem cells [11]. In mammalian cells, the transcriptional response to oxygen deficiency is primarily mediated by hypoxia-inducible factor 1 (HIF-1), which gradually increases with decreasing oxygen concentration [12]. The expression of genes such as vascular endothelial growth factor (VEGF) and erythropoietin is induced to stimulate angiogenesis and hematopoiesis [12, 13]. In hypoxic conditions, ADSC proliferation is increased compared to normal oxygen conditions [10, 14]. Under hypoxic conditions, the secretion of VEGF and fibroblast growth factor (FGF)-2 proteins by ADSCs is increased [15].
Previous studies have shown that hypoxia promotes osteogenesis in human mesenchymal stromal cells [11, 16, 17]. However, another study reported that hypoxia inhibits bone formation in human mesenchymal stem cells [18]. Therefore, the effect of hypoxia on MSC differentiation remains ambiguous. We hypothesized that oxygen participates in the intricate balance between cellular proliferation and commitment toward differentiation. In this study, we investigated the effects of hypoxia pretreatment on ADCS proliferation, migration capacity, differentiation potential and cytokine production. We also analyzed the effects of VEGF on osteogenic differentiation of ADSC by hypoxia pretreatment and chondrogenic differentiation.
Materials and methods
Cell culture and in vitro differentiation
STEMPRO® human adipose-derived stem cells (hADSCs) purchased from Thermo Fisher Scientific (Carlsbad, CA, USA) were cultured in 75 cm2 flasks using a MesenPRO RS™ Medium Kit (Thermo Fisher Scientific) and l-glutamine (Thermo Fisher Scientific) according to the manufacturer’s instructions. Hypoxic culture experiments were performed in a multi-gas incubator (InvivO2; Baker Ruskinn, Sanford, ME, USA) at 37 °C in an atmosphere containing 5% CO2 balanced with nitrogen to reach an oxygen concentration of 1%. Normal oxygen culture experiments were performed in a standard incubator under conditions containing 5% CO2 and 20% O2. The ADSCs were differentiated by guidelines provided for inducing STEMPRO® ADSCs to differentiate towards osteoblasts, adipocytes, and chondrocytes using the StemPro Osteogenesis Differentiation Kit (Thermo Fisher Scientific), StemPro Adipogenesis Differentiation Kit (Thermo Fisher Scientific) and StemPro Chondrogenesis Differentiation Kit (Thermo Fisher Scientific), respectively, according to the manufacturer’s protocol. Alizarin Red (EMD Millipore, Billerica, MA, USA) staining was used to assess osteogenic differentiation, Oil Red O (Biovision, Mountain View, CA) staining to verify adipogenic differentiation, and Safranin-O (Sigma-Aldrich, St. Louis, MO, USA) staining to evaluate the ability of hADSCs to differentiate toward chondrocytes. To examine the direct effect of VEGF on osteogenic differentiation, we induced osteogenic differentiation by adding 50 ng/mL of VEGF (PeproTech, Rocky Hill, NJ, USA) and 50 µg/ml of anti-VEGF antibody (Avastin; Roche, Basel, Switzerland).
Proliferation assay
Cell proliferation under normoxic or hypoxic conditions was assessed using a Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Kumamoto, Japan). Briefly, ADSCs were seeded in 96-well plates at a density of 2.5 × 103 to 1 × 104 per well and incubated under normoxic (20% O2) or hypoxic (1% O2) conditions for 24 or 48 h. The cellular proliferation was measured by absorbance at 450 nm using VersaMax (Molecular Devices, San Jose, CA, USA) apparatus (n = 3) after adding 1/10 volume of CCK-8 reagent and incubating for 2 h.
Western blotting
Nuclear protein extracts were obtained using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific) for the detection of HIF-1α and histone H3. The protein concentrations were measured using a Bradford Protein Assay Kit (Bio-Rad Laboratories, Richmond, CA, USA). Ten micrograms of protein extracts were separated by SDS-PAGE using a NuPAGE electrophoresis system (Thermo Fisher Scientific). The proteins were transferred to polyvinylidene difluoride membranes using the iBlot Dry Blotting System (Thermo Fisher Scientific) in accordance with the manufacturer’s protocol. The membranes were blocked with 5% skim milk in Tris-buffered saline with 0.05% Tween 20 for 1 h and incubated with primary antibody overnight: anti-HIF-1α (Cell Signaling Technology, Beverly, MA, USA). Next, the membranes were incubated with the corresponding horseradish peroxidase-conjugated IgG secondary antibody (Cell Signaling Technology) for 1 h at room temperature. The proteins were detected using ECL reagent (GE Life Sciences, Issaquah, WA, USA) and the images were obtained using a ChemiDoc MP (Bio-Rad Laboratories) controlled by Image Lab software (Bio-Rad Laboratories).
RNA isolation and quantitative polymerase chain reaction
Total RNA was isolated from the cells using an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The RNA concentrations were assessed using a Nanodrop spectrophotometer (ND-1000, Thermo Fisher Scientific). cDNA was synthesized from 1 μg of RNA using a Reverse Transcription Master Premix 1st strand cDNA synthesis kit (ELPIS, Daejeon, Korea). Quantitative polymerase chain reaction (qPCR) was performed using Power SYBR-Green Master Mix (Applied-Biosystems, Foster City, CA, USA) with triplicate reactions containing 1 × SYBR-Green Master Mix, 500 nM of each forward/reverse primer, and 15 ng of template DNA. The reactions were performed on an Applied Biosystems 7500 Fast Real-Time PCR System for 40 cycles. The fold-change in target gene expression was calculated using the 2−ΔΔCt method and normalized to the control housekeeping gene GAPDH. The primer sequences are shown in Table 1.
Table 1.
List of primers and sequences used for quantitative polymerase chain reactions
| Target | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Oct4 | ACATCAAAGCTCTGCAGAAAGAACT | CTGAATACCTTCCCAAATAGAACCC |
| Nanog | CCAACATCCTGAACCTCAGC | GCTATTCTTCGGCCAGTTG |
| Sox2 | TGCGAGCGCTGCACAT | GCAGCGTGTACTTATCCTTCTTCA |
| C-Myc | GGCCCCCAAGGTAGTTATCCTT | CGTTTCCGCAACAAGTCCTCT |
| P16INK4A | GAAGGTCCCTCAGACATCCCC | CCCTGTAGGACCTTCGGTGAC |
| Runx2 | TGAGAGCCGCTTCTCCAACC | GCGGAAGCATTCTGGAAGGA |
| ALP | ACCATTCCCACGTCTTCACA | CAGGCCCATTGCCATACA |
| Collagen1 | CACCAATCACCTGCGTACAG | GCAGTTCTTGGTCTCGTCAC |
| OPG | GGCAACACAGCTCACAAGAA | CTGGGTTTGCATGCCTTTAT |
| Osteocalcin | AGCAAAGGTGCAGCCTTTGT | CTGAAAGCCGATGTGGTCAG |
| Sialoprotein | ACCAGTACCAACAGCACAGA | TGGCTCCAGTGACACTTTCT |
Migration assay
ADSCs (5 × 105) were plated in 10 cm cell culture dishes (Corning, Corning NY, USA) and incubated for 48 h in 1% or 20% O2. Then, the ADSCs (1 × 103) were plated in 96-well plates and single cells were tracked using Operetta High‐Content imaging system (PerkinElmer, Waltham, MA, USA). We analyzed the movement of single cells over 12 h using Harmony software.
ELISA for secreted VEGF
ADSCs (4 × 105) were plated in 10 cm cell culture dishes and incubated for 48 h in 1% or 20% O2. The culture supernatants were collected and VEGF levels were analyzed using a Human VEGF Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The VEGF concentrations were quantified with a VersaMax apparatus at OD450 (n = 3).
Flow cytometry
Single-cell suspensions were obtained after pretreatment in hypoxic and normoxic conditions. The cells were washed twice with FACS buffer containing 1% fetal bovine serum in phosphate-buffered saline. The cells were counted and suspended at a concentration of 5 × 105 cells in 100 µl FACS buffer and incubated for 30 min at 4 °C in the dark with the following fluorescent antibodies: CD73-PE, CD90-FITC, CD105-APC, and CD45-PEcy7 (BD Biosciences, San Diego, CA, USA). The cells were washed twice with FACS buffer and then analyzed using flow cytometry (FACS CantoII; BD). The data were acquired, exported as FCS 3.0 files, and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Gene expression profiling
Genomic expression analysis was performed using the Human Mesenchymal Stem Cell RT2 Profiler PCR Array (PAHS-082Z, Qiagen). cDNA was synthesized from 800 ng of total RNA using the RT2 First Strand Kit (Qiagen). PCR arrays were performed in a CFX96 Touch™ Real-Time PCR Detection System (Qiagen) with RT2 SYBR® Green qPCR Master Mix (Qiagen). Each array consisted of a panel of 96 primer sets of 84 mesenchymal stem cell or cellular senescence genes, five housekeeping genes, and seven quality controls. The data were analyzed using web-based RT2 Profiler PCR Array Data Analysis v3.5 software (Qiagen). The fold-change in target gene expression was calculated using the 2−ΔΔCt method and normalized to the geometric mean of five housekeeping genes (ACTB, B2M, GAPDH, HPRT1, and RPLP0) according to manufacturer’s guide.
Cytokine profiling
Culture supernatants were obtained after the cells were incubated for 48 h in 1% or 20% O2. The cytokines secreted by ADSCs were measured using a Proteome Profiler Human XL Cytokine Array Kit (R&D systems) with 102 capture antibodies that were spotted in duplicate on a nitrocellulose membrane according to the manufacturer’s protocol. Then the immunospots were captured and processed using a ChemiDoc MP (Bio-Rad Laboratories) controlled by Image Lab software (Bio-Rad Laboratories). The intensities of the identified spots were quantified using NIH Image J software.
Transfection
VEGF siRNA (Santa Cruz) was transfected using HiperFect transfection reagent (Qiagen). Shortly before transfection, 2 × 105 cells were seeded per 6-well plate. siRNA (150 ng) was diluted in 100 μl of culture medium without serum. HiperFect Transfection Reagent (12 μl) was added to the diluted siRNA and mixed by vortexing. The samples were incubated for 5–10 min at room temperature to allow the formation of transfection complexes. The complexes were added dropwise to the cells and incubated for 24 h at normoxic conditions. Then, the medium was changed to osteogenic differentiation medium.
Statistical analysis
Student’s t test and one-way ANOVA were used for statistical analyses and significance was indicated by p < 0.05. All values are expressed as the means ± standard deviations. GraphPad Prism (GraphPad software) and Excel 2016 (Microsoft Corporation) were used for all the statistical analyses.
Results
Proliferation of ADSCs after hypoxia treatment
We first examined the effect of hypoxia on ADSC proliferation. As shown in Fig. 1A, the number of ADSCs at 1% O2 was higher than that at 20% O2 measured using the Cell Counting Kit-8. Compared to normoxia, the ADSCs cultured at 1% O2 showed significantly high proliferation at 24 and 48 h (Fig. 1A).
Fig. 1.
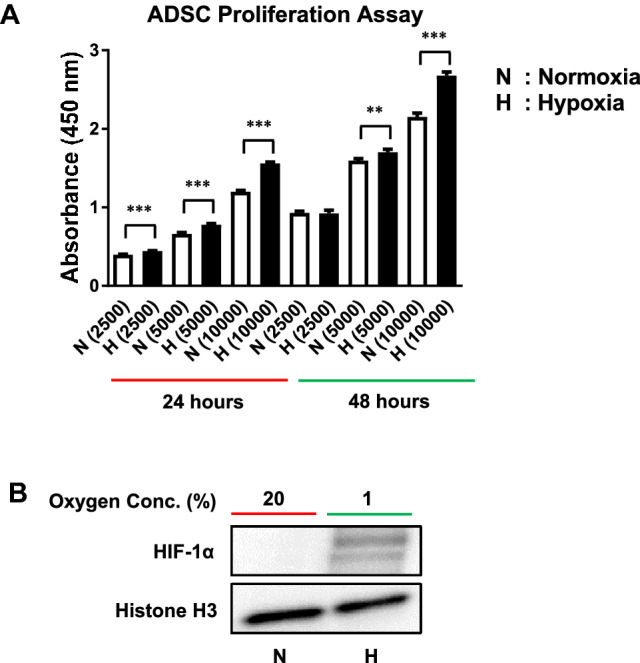
Effects of hypoxia pretreatment on the proliferation of ADSCs. A ADSC proliferation was significantly increased at 24 and 48 h by hypoxia compared to normoxia and measured using the CCK-8 kit. B Nuclear proteins were prepared from ADSCs exposed to hypoxia for 6 h and Western blotting was performed. The expression of HIF-1α was increased by hypoxia. The results are shown as the mean ± SD values. The statistical significance of hypoxia versus normoxia was determined by Student’s t-test (*p < 0.05 and **p < 0.01). N: normoxia (20% O2), H: hypoxia (1% O2)
HIF-1α expression in ADSCs after hypoxia treatment
We measured the amount of HIF-1α protein to demonstrate that the cells were actually exposed to hypoxia. HIF-1α protein was increased in cells incubated in hypoxic conditions for 6 h (Fig. 1B).
Expression of stem cell and senescence markers after hypoxia treatment
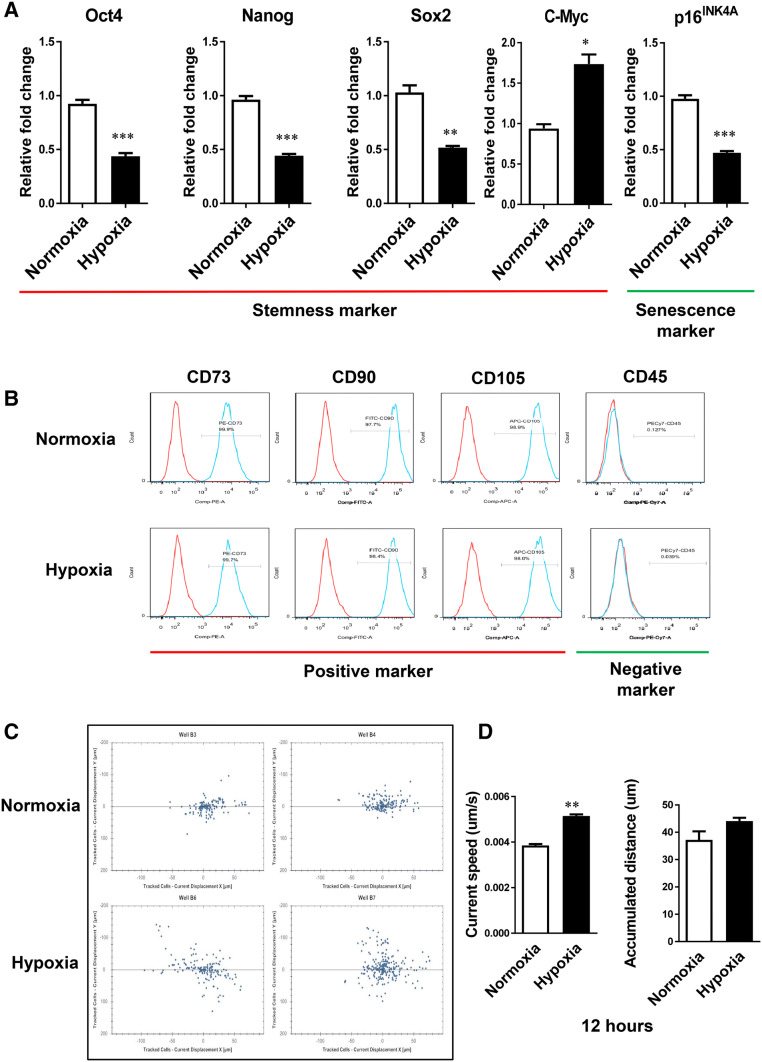
We analyzed the effects of hypoxia on the expression of stem cell markers such as Oct4, Nanog, Sox2 and c-Myc, and senescence marker p16INK4A in ADSCs. ADSCs have been reported to express several markers of pluripotent embryonic stem (ES) cells [19, 20]. We examined the expression of pluripotent marker Oct4, an important transcription factor in maintaining the undifferentiated status of ES cells. The expression of Oct4 was significantly decreased by hypoxia compared to normoxia. Similar patterns were observed in the expression of Nanog and Sox2 (Fig. 2A). In hypoxic conditions, the expression of c-Myc, one of stem cell markers, was significantly higher than in normoxic conditions (Fig. 2A). p16INK4A is known to contribute to cell and stem cell senescence and accumulates in many aged cells and tissues. Thus, p16INK4A is considered a biomarker of aging [21, 22]. The expression of senescence marker p16INK4a in hypoxia was significantly lower than in normoxia. The expression of mesenchymal surface markers in ADSCs under normoxic and hypoxic conditions was examined by FACS analysis (Fig. 2B). The expression of CD73, CD90, or CD105 in hypoxic conditions was similar to that in normoxic conditions (Fig. 2B).
Fig. 2.
Effect of hypoxia pretreatment on the properties of ADSCs. A The mRNA expression of the indicated gene was analyzed using real-time RT-PCR. The expression of stem cell markers Oct4, Nanog, and Sox2 in hypoxia was significantly lower than in normoxia. The expression of stem cell marker c-Myc in hypoxia was significantly higher than in normoxia. The expression of senescence marker p16INK4a in hypoxia was significantly lower than in normoxia. B In immunophenotype analysis, the expression of mesenchymal surface markers CD73, CD90, and CD105 was not significantly changed by hypoxia compared to normoxia. C The migration ability of ADSCs was increased in hypoxia compared to normoxia. D The current step and accumulated distance of ADSCs cultured in hypoxia were increased compared to normoxia at 12 h. The results are shown as the mean ± SD values. The statistical significance of hypoxia versus normoxia was determined by Student’s t-test (*p < 0.05 and **p < 0.01)
Increase in ADSC migration ability after hypoxia treatment
To evaluate the migration ability of ADSCs in hypoxic conditions, we incubated ADSC for 48 h in normal oxygen and hypoxic conditions and then plated single cells into 96-well plates to track the migration pathway for 12 h. The results showed that hypoxia increased the mobility of ADSCs by increasing the speed and cumulative distance (Fig. 2C, D).
Reduction in osteogenic differentiation capability of ADSCs after hypoxia treatment
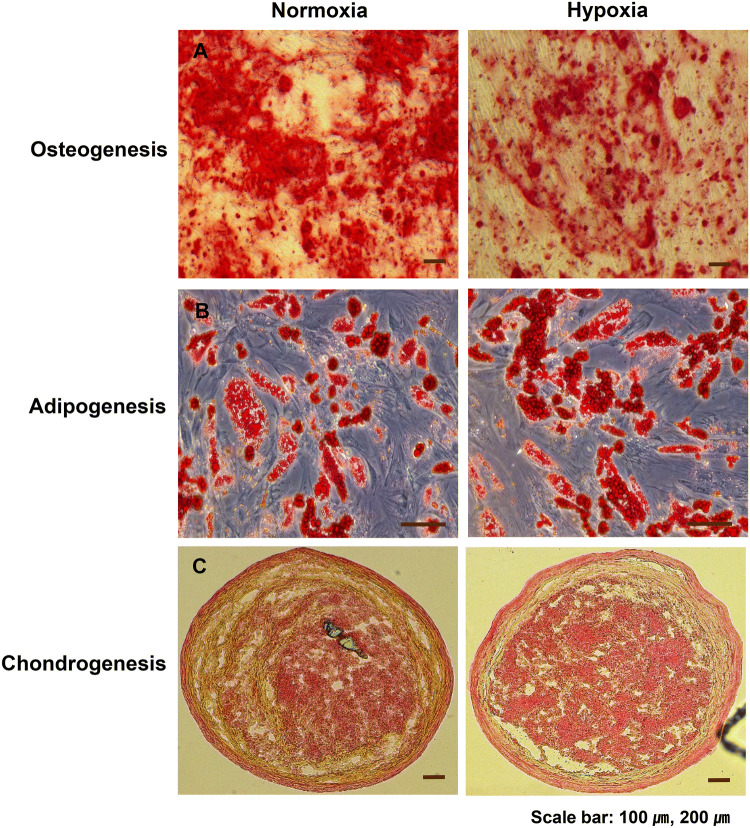
ADSCs are multipotent stem cells that can differentiate into mesenchymal and non-mesenchymal lineages [23]. To investigate the effect of hypoxic conditions on the differentiation of ADSCs, we evaluated the differentiation into adipocytes, osteoblasts and chondrocytes using ADSCs cultured under normal oxygen and hypoxic conditions. As determined by oil red O staining, the differentiation of ADSCs into adipocytes was not significantly different in hypoxia compared to normal oxygen conditions. As measured by Safranin O staining, the differentiation of ADSCs into chondrocytes was significantly increased in hypoxia compared to normoxia. As determined by Alizarin Red staining, the differentiation of ADSCs into osteoblasts was reduced in hypoxic conditions compared to normal oxygen conditions (Fig. 3).
Fig. 3.
Effects of hypoxia pretreatment on the differentiation potential of ADSCs. ADSCs were cultured at 4.5 × 105 cells/100 mm dish in hypoxia or normoxia for 48 h and induced to differentiate along adipogenic, osteogenic, and chondrogenic lines for 21 days. A Hypoxia inhibited the osteogenic differentiation efficiency compared to normoxia as shown by Alizarin Red staining. B No significant difference was observed in adipogenesis between normoxia and hypoxia by Oil Red O staining. C Hypoxia increased the chondrogenic differentiation efficiency compared to normoxia as shown by Safranin O staining
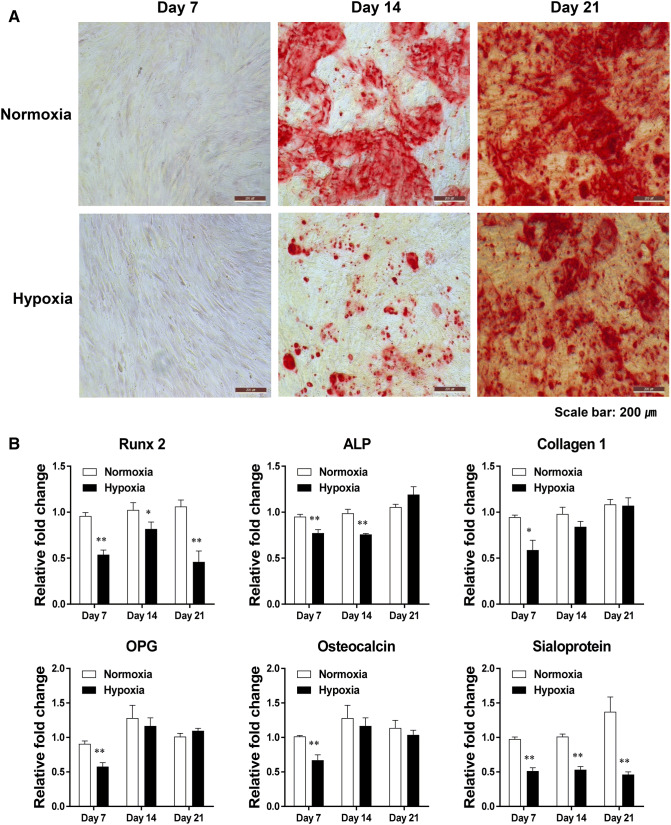
To determine the role of hypoxic culture conditions on the osteoblastic differentiation of ADSCs, Alizarin Red staining was performed at 7, 14, and 21 days after differentiation. We reconfirmed the reduction in the differentiation of ADSCs into osteoblasts after hypoxia treatment (Fig. 4A). The positively stained Alizarin Red area was decreased in hypoxia compared to cells cultured under normoxic conditions. In particular, the osteogenic differentiation capacity of ADSCs cultured in hypoxic conditions was observed to be significantly decreased on day 14 after differentiation (Fig. 4A).
Fig. 4.
Hypoxia reduced the mineralization capacity and the expression of osteogenesis-related genes in ADSCs. A Bone mineralization was reduced by hypoxia compared to normoxia as shown by Alizarin Red staining at 7, 14, and 21 days after the induction of osteogenesis. B The mRNA expression of the indicated genes was analyzed using real-time RT-PCR. The results are shown as the mean ± SD values. The statistical significance of hypoxia versus normoxia was determined by Student’s t-test (*p < 0.05 and **p < 0.01)
To investigate the changes in osteogenesis-related gene expression in osteoblast differentiation [24–29], we examined the expression of Runx2, ALP, collagen1, OPG, osteocalcin, and sialoprotein in osteoblasts differentiated from ADSCs cultured under normal oxygen and hypoxic conditions. The expression levels of Runx2 and sialoprotein were significantly decreased by hypoxia on days 7, 14, and 21 compared to normoxia (Fig. 4B). The expression of ALP was significantly decreased by hypoxia on days 7 and 14 compared to normoxia (Fig. 4B). The expression levels of collagen 1, OPG, and osteocalcin mRNA were decreased by hypoxia on day 7 compared to normoxia (Fig. 4B). These results indicated that hypoxic culture conditions could inhibit the osteogenic differentiation potential of ADSC.
Comparative gene expression profiles of ADSCs in normoxic and hypoxic conditions
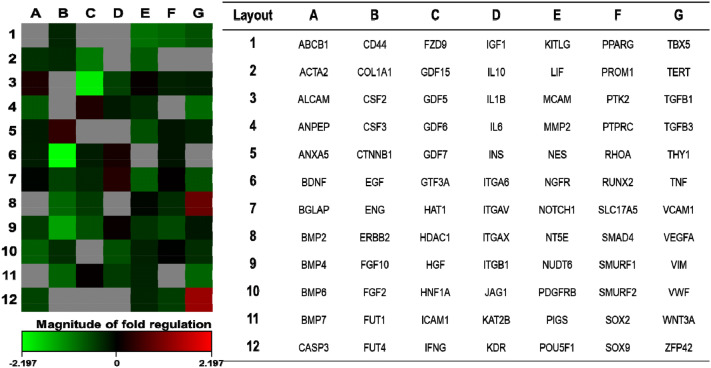
To understand the molecular basis of regulation of differentiation by hypoxia and to identify the stem cell-related genes that are selectively activated or repressed in response to hypoxia, ADSCs were analyzed using a Human Mesenchymal Stem Cell RT2 Profiler PCR Array. The expression profiles of 84 genes in ADSCs obtained after normoxia and hypoxia exposure are shown in Fig. 5. We identified 12 genes that were differentially expressed between normoxia and hypoxia with a minimal fold-change of 1.8 (Fig. 5, Table 2). Particularly, the expression of VEGFA gene was found to be upregulated in response to hypoxia. In contrast, EGF gene expression was mostly downregulated under hypoxic conditions (4.59-fold) (Fig. 5, Table 2).
Fig. 5.
Effect of hypoxia pretreatment on the differential expression of mesenchymal stem cell-related genes. The heat map shows the changes in mesenchymal stem cell-related genes in ADSCs cultured at 4.5 × 105 cells/100 mm dish for 48 h in hypoxia compared to normoxia. Gradient indicates the magnitude of the change in gene expression
Table 2.
Differential gene expression in ADSCs cultured in hypoxic conditions
| No. | Gene symbol | Fold change | Ref. seq. number |
|---|---|---|---|
| 1 | ZFP42 | 2.5 | NM_174900 |
| 2 | VEGFA | 1.87 | NM_003376 |
| 3 | EGF | − 4.59 | NM_001963 |
| 4 | GDF5 | − 4.1 | NM_000557 |
| 5 | FGF10 | − 2.63 | NM_004465 |
| 6 | GDF15 | − 2.08 | NM_004864 |
| 7 | KITLG | − 1.94 | NM_003994 |
| 8 | TGFB3 | − 1.88 | NM_003239 |
| 9 | ERBB2 | − 1.85 | NM_004448 |
| 10 | PPARG | − 1.84 | NM_015869 |
| 11 | WNT3A | − 1.82 | NM_033131 |
| 12 | FUT1 | − 1.8 | NM_000148 |
Profile of cytokines secreted by ADSCs cultured under normoxic and hypoxic conditions
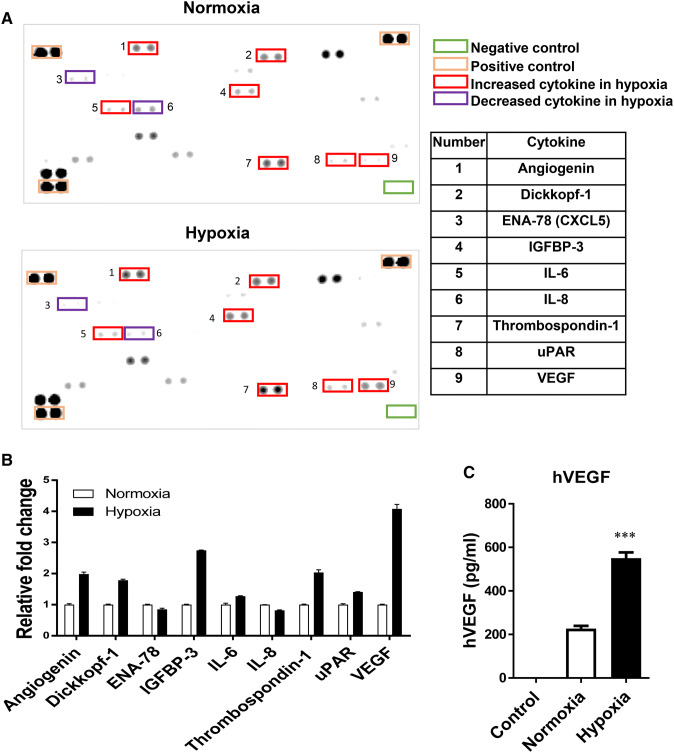
We further analyzed the cytokines secreted by ADSCs cultured under hypoxic culture conditions and compared them to those cultured under normoxic conditions using an antibody array. As shown in Fig. 6, the expression levels of several cytokines and growth factors were altered by hypoxia compared to normoxia (Fig. 6A, B). In particular, the production of VEGF and IGFBP-3 by ADSC was significantly higher in hypoxia compared to normal oxygen conditions (4.08- and 2.74-fold, respectively). Although not a significant increase, hypoxia increased the production of IL-6 by more than 1.2 fold. The amount of VEGF in conditioned medium was also measured by ELISA and the results were consistent with those of the cytokine array analysis (Fig. 6C). In all of the cytokine assays for VEGF, similar expression patterns were found at the mRNA and protein levels (Figs. 5, 6).
Fig. 6.
Effect of hypoxia on the secretion of cytokines and VEGF by ADSCs. ADSCs were cultured at 4.5 × 105 cells/100 mm dish for 48 h in hypoxia or normoxia. A Cytokines were measured in cell culture supernatants using the Human XL Cytokine Array Kit according to the manufacturer’s protocol. B The expression of angiogenin, Dickkopf-1, IGFBP-3, IL-6, thrombospondin-1, uPAR, and VEGF by ADSCs in hypoxia was higher than in normoxia. C The secretion of VEGF into the medium by ADSCs in hypoxia was significantly increased compared to normoxia. The results are shown as the mean ± SD values. The statistical significance of hypoxia versus normoxia was determined by Student’s t-test (*p < 0.05 and **p < 0.01)
Effects of VEGF on the osteoblastic differentiation of ADSCs
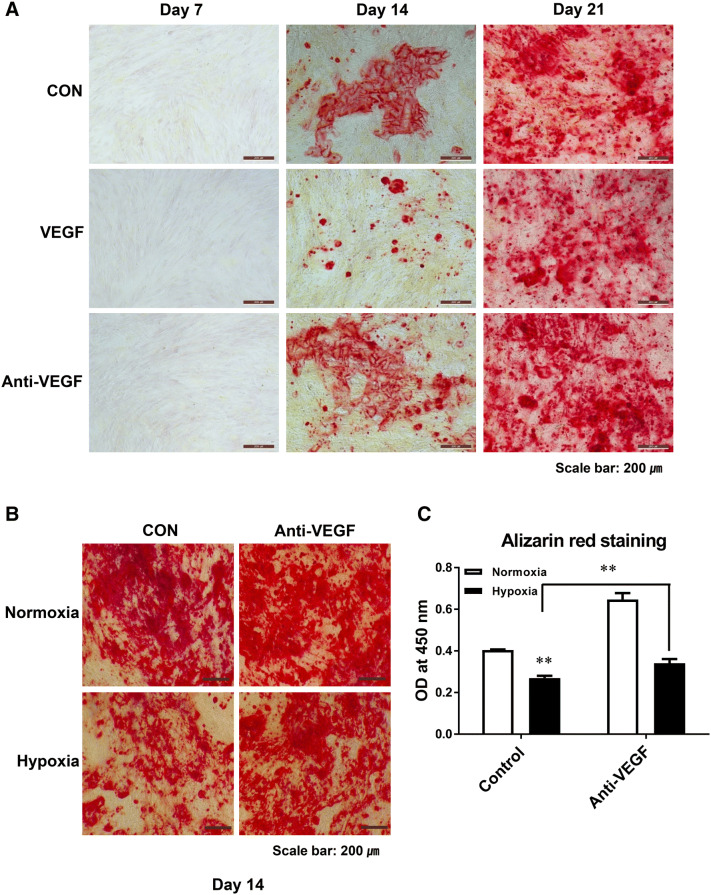
We examined whether increased VEGF inhibited the osteoblast differentiation by hypoxia. We assessed the direct effect of VEGF by adding VEGF to osteoblast differentiation medium of ADSC under normal oxygen conditions. The positively stained Alizarin Red area indicating osteogenic differentiation was smaller in the VEGF-treated group at days 14 and 21 compared to the control group (Fig. 7). In hypoxia, a decrease in the differentiation of ADSCs into osteoblasts was restored by Avastin (VEGF antibody) treatment (Fig. 7).
Fig. 7.
Effect of VEGF and anti-VEGF antibody on the differentiation of ADSCs into osteoblasts. A The differentiation of ADSCs into osteoblasts was induced by the addition of VEGF to the medium. The positively stained Alizarin Red area was smaller in cells treated with VEGF compared to the control at 14 and 21 days. B, C The differentiation of ADSCs into osteoblasts in hypoxia was restored by VEGF antibody. The results are shown as the mean ± SD values. The statistical significance of hypoxia versus normoxia was determined by Student’s t-test (*p < 0.05 and **p < 0.01)
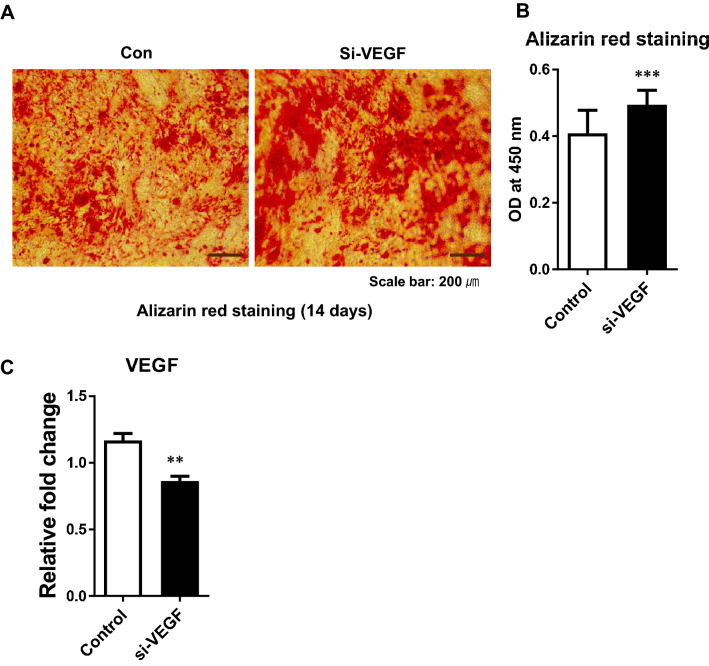
We examined whether the inhibition of VEGF expression by transfected VEGF siRNA increases osteoblast differentiation. The differentiation of ADSC into osteoblasts was induced after transfection with VEGF siRNA. The positively stained Alizarin Red area was increased after transfection with VEGF siRNA at day 14 compared to the control (Fig. 8).
Fig. 8.
Effect of VEGF siRNA on the differentiation of ADSCs into osteoblasts. The differentiation of ADSCs to osteoblasts was induced after transfection with VEGF siRNA. A, B The positively stained Alizarin Red area was increased in cells transfected with VEGF siRNA (si-VEGF) compared to control at 14 days. C The mRNA expression of VEGF gene was analyzed using real-time RT-PCR after transfection with VEGF siRNA. The results are shown as the mean ± SD values. The statistical significance of hypoxia versus normoxia was determined by Student’s t-test (*p < 0.05 and **p < 0.01)
Increase in chondrogenic differentiation potential of ADSCs by hypoxia pretreatment
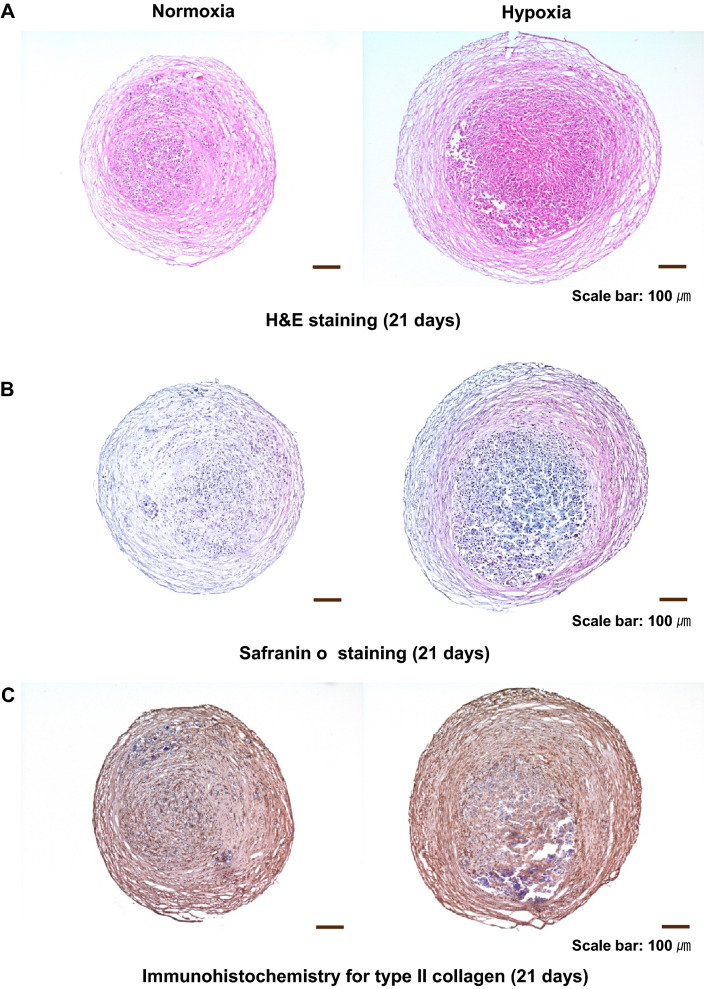
We investigated whether the differentiation of ADSC to chondrocytes was increased by hypoxia pretreatment. In H&E staining, the mass of chondrocytes differentiated from ADSC was increased by hypoxia compared to normoxia (Fig. 9A). In Safranin O staining (Fig. 9B) and immunohistochemical analysis for type II collagen (Fig. 9C), hypoxia pretreatment increased the efficiency of ADSC differentiation into chondrocytes compared to normoxia.
Fig. 9.
Effect of hypoxia pretreatment on the chondrogenic differentiation potential of ADSC. A The mass of cells differentiated into chondrocytes was increased by hypoxia compared to normoxia. B, C Hypoxia increased the chondrogenic differentiation efficiency compared to normoxia as shown by Safranin O staining and immunohistochemical analysis for type II collagen
Discussion
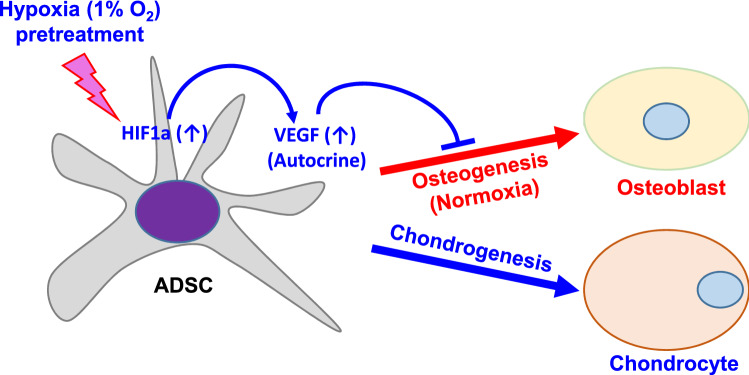
MSCs are of interest for therapeutic purposes for tissue repair, but an expansion of cells through in vitro culture is required for use in therapy. In vitro culture conditions, however, change at both cellular and molecular levels, reducing the proliferation and differentiation potential of MSCs [30, 31]. ADSC has recently been isolated from human subcutaneous adipose tissue [5, 32, 33] and is considered an alternative to BMSC in regenerative medicine in that cells are readily available (Fig. 10).
Fig. 10.
Schematic diagram of regulation by hypoxia pretreatment in the differentiation of ADSC into osteoblasts and chondrocytes. Hypoxia (1% O2) pretreatment increases VEGF through increased expression of HIF1a, and increased VEGF inhibits osteogenic differentiation but promotes chondrogenic differentiation
Changes in oxygen concentration in culture conditions affect stem cell proliferation and differentiation. The proliferation of human ADSCs has been reported to be increased by hypoxia (2% O2) [11, 15]. Rasmussen et al. reported that 5% of oxygen culture conditions increased the proliferation of ADSC [34]. In this study, it was confirmed that hypoxia (1% O2) increased the proliferation of ADSC compared to normoxia. From these results, it was confirmed that hypoxic culture conditions could be usefully used for regenerative medicine which requires the production of a large number of ADSCs from a small number of donor cells.
HIF-1α improves the oxygen environment by promoting angiogenesis through induction of expression of VEGF, platelet-derived growth factor and basic fibroblast growth factor [35]. Hypoxic conditions have been reported to increase the expression of HIF-1α in ADSC by increasing the expression of VEGF [11, 36]. In this study, we also confirmed that hypoxic conditions increased the expression of HIF-1α and VEGF in ADSC.
In this study, changes in stem cell characteristics of ADSC caused by hypoxia were investigated by expression of common phenotypic markers (CD73, CD90 and CD105) of MSC. Hypoxic conditions did not affect the expression level of stem cell common phenotypic markers. Next, we investigated whether hypoxia can affect the expression of stem cell-related genes in ADSC. Indeed, several previous reports have described that hypoxia (1–2% O2) increases the expression of stem cell-related genes including Oct4, Nanog and Klf4 in various types of stem cells [10, 11]. In this study, as in previous studies by Kwon SY et al. [16], the expression of Nanog, Sox2, and Oct4 in ADSC was significantly decreased in hypoxia compared to normoxia, while c-Myc expression was increased. The reason why the expression pattern of MSC stem cell markers in our results differs from the previous study results is presumed to be due to the difference between the stem cell origin used in the experiment, the concentration of oxygen in culture, and the timing and duration of hypoxia exposure. The difference between this study and the previous studies is thought to be that hypoxia was used only as a pretreatment condition in ADSC culture. Furthermore, it is assumed that the increased expression of c-Myc, a key regulator of cell survival, is associated with an increase in ADSC proliferation in hypoxia [37, 38].
In contrast, the expression of the senescence marker, p16INK4a, was significantly reduced. Therefore, hypoxic conditions could improve the viability of transplanted cells when ADSC is used for clinical treatment.
Hypoxia has been reported to significantly increase the migration of ADSC [10, 39]. In this study, we also found that hypoxia increased the migration of ADSC. Based on previous studies and our findings, it was confirmed that hypoxia influences the movement of ADSC as well as the secretion of various growth factors and cytokines.
Hypoxia is known to affect the expression of genes and proteins in cells. As previously reported [40–42], we found that the expression of VEGF by ADSC in hypoxia was increased at both gene (mRNA) and protein levels. Since VEGF is known to play a pivotal role in angiogenesis and cardiac repair, increased VEGF expression in hypoxia can be clinically important [43, 44]. We also confirmed that hypoxic culture conditions induced down-regulation of the EGF gene and up-regulation of IGFBP-3 protein in ADSC through PCR and cytokine array experiments, respectively. EGF has been reported to significantly increase BMP9-induced osteogenic differentiation of MSCs [45] and IGFBP-3 has been reported to reduce osteogenic differentiation [46]. In this study, we confirmed that hypoxia can inhibit the differentiation of ADSC into osteoblasts by regulating the expression of VEGF, EGF and IGFBP-3.
Hypoxia has been reported to inhibit [18, 47] or promote [10, 16, 17] osteogenesis in mesenchymal stem cells. In this study, we found that hypoxia pretreatment inhibited osteogenic differentiation of ADSC but increased chondrogenic differentiation (Fig. 10). We confirmed for the first time that VEGF increased by hypoxia pretreatment inhibited osteogenic differentiation and that decreased osteoblast differentiation was increased by treatment with Avastin, a VEGF inhibitor. In addition, we confirmed that the differentiation of ADSC into osteoblast was increased by inhibition of VEGF expression using VEGF siRNA. These results indicate that VEGF is a key regulator of osteoblast differentiation of ADSC in hypoxia pretreatment. Malladi et al. reported that severe hypoxic conditions strongly inhibited the osteogenesis and chondrogenesis of ADSC [48], but hypoxia pretreatment increased the chondrogenic differentiation of ADSC. In addition, the use of VEGF has been reported to directly improve the differentiation efficiency from adipose-derived stem cells to chondrocytes [49]. These results showed that the effects of hypoxia on MSC may vary depending on the origin of the cells and the culture conditions, and it is necessary to confirm the characteristics of the cells by the culture conditions in order to use stem cells for therapeutic purposes.
ADSC can be considered as a promising candidate for various therapeutic purposes, such as tissue regeneration because it is relatively easy to acquire and isolate and manipulate in vitro compared to other stem cells. A better understanding of the biological properties and differentiation potential of ADSC will make it possible to culture them in a form suitable for transplantation. In this study, we showed that hypoxia pretreatment of ADSC can promote chondrogenic differentiation while inhibiting osteogenic differentiation, which can be useful for cartilage regeneration (Fig. 10). In order to use ADSC for therapeutic purposes, further studies are needed to establish the culture strategies that allow for the expansion of ADSC while retaining their differentiation potential.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean government (Grant Numbers 2016R1A6A3A04011000 and 2015M3A9C7030190). This research was supported by Chungbuk National University (2018).
Compliance with ethical standards
Conflict of interest
The authors have no conflicts to disclose.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ok Kyung Hwang and Young Woock Noh have contributed equally to this work.
Contributor Information
Jin Tae Hong, Email: jinthong@chungbuk.ac.kr.
Je-Wook Lee, Email: jwl@kbiohealth.kr.
References
- 1.Dao LT, Park EY, Hwang OK, Cha JY, Jun HS. Differentiation potential and profile of nuclear receptor expression during expanded culture of human adipose tissue-derived stem cells reveals PPARgamma as an important regulator of Oct4 expression. Stem Cells Dev. 2014;23:24–33. doi: 10.1089/scd.2013.0137. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 3.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–675. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- 5.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, et al. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341:1135–1140. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 7.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 10.Hung SP, Ho JH, Shih YR, Lo T, Lee OK. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J Orthop Res. 2012;30:260–266. doi: 10.1002/jor.21517. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto Y, Fujita M, Tanaka Y, Kojima I, Kanatani Y, Ishihara M, et al. Low oxygen tension enhances proliferation and maintains stemness of adipose tissue-derived stromal cells. Biores Open Access. 2013;2:199–205. doi: 10.1089/biores.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakudo N, Morimoto N, Ogawa T, Taketani S, Kusumoto K. Hypoxia enhances proliferation of human adipose-derived stem cells via HIF-1a activation. PLoS One. 2015;10:e0139890. doi: 10.1371/journal.pone.0139890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irigoyen M, García-Ruiz JC, Berra E. The hypoxia signalling pathway in haematological malignancies. Oncotarget. 2017;8:36832–36844. doi: 10.18632/oncotarget.15981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dos Santos F, Andrade PZ, Boura JS, Abecasis MM, da Silva CL, Cabral JM. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010;223:27–35. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]
- 15.Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 16.Kwon SY, Chun SY, Ha YS, Kim DH, Kim J, Song PH, et al. Hypoxia enhances cell properties of human mesenchymal stem cells. Tissue Eng Regen Med. 2017;14:595–604. doi: 10.1007/s13770-017-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagegg M, Gaber T, Lohanatha FL, Hahne M, Strehl C, Fangradt M, et al. Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner. PLoS One. 2012;7:e46483. doi: 10.1371/journal.pone.0046483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang DC, Yang MH, Tsai CC, Huang TF, Chen YH, Hung SC. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS One. 2011;6:e23965. doi: 10.1371/journal.pone.0023965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang HM, Kim J, Park S, Kim J, Kim H, Kim KS, et al. Insulin-secreting cells from human eyelid-derived stem cells alleviate type I diabetes in immunocompetent mice. Stem Cells. 2009;27:1999–2008. doi: 10.1002/stem.127. [DOI] [PubMed] [Google Scholar]
- 20.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata KR, Aoyama T, Shima Y, Fukiage K, Otsuka S, Furu M, et al. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells. 2007;25:2371–2382. doi: 10.1634/stemcells.2007-0225. [DOI] [PubMed] [Google Scholar]
- 23.Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 24.Hunter GK, Goldberg HA. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A. 1993;90:8562–8565. doi: 10.1073/pnas.90.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lian JB, Stein GS. Runx2/Cbfa1: a multifunctional regulator of bone formation. Curr Pharm Des. 2003;9:2677–2685. doi: 10.2174/1381612033453659. [DOI] [PubMed] [Google Scholar]
- 26.Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S, et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89:773–779. doi: 10.1016/s0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- 27.Pavlin D, Gluhak-Heinrich J. Effect of mechanical loading on periodontal cells. Crit Rev Oral Biol Med. 2001;12:414–424. doi: 10.1177/10454411010120050401. [DOI] [PubMed] [Google Scholar]
- 28.Ryoo HM, Hoffmann HM, Beumer T, Frenkel B, Towler DA, Stein GS, et al. Stage-specific expression of Dlx-5 during osteoblast differentiation: involvement in regulation of osteocalcin gene expression. Mol Endocrinol. 1997;11:1681–1694. doi: 10.1210/mend.11.11.0011. [DOI] [PubMed] [Google Scholar]
- 29.Shui C, Spelsberg TC, Riggs BL, Khosla S. Changes in Runx2/Cbfa1 expression and activity during osteoblastic differentiation of human bone marrow stromal cells. J Bone Miner Res. 2003;18:213–221. doi: 10.1359/jbmr.2003.18.2.213. [DOI] [PubMed] [Google Scholar]
- 30.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1:19. doi: 10.1186/scrt19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kakudo N, Shimotsuma A, Kusumoto K. Fibroblast growth factor-2 stimulates adipogenic differentiation of human adipose-derived stem cells. Biochem Biophys Res Commun. 2007;359:239–244. doi: 10.1016/j.bbrc.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 33.Kakudo N, Shimotsuma A, Miyake S, Kushida S, Kusumoto K. Bone tissue engineering using human adipose-derived stem cells and honeycomb collagen scaffold. J Biomed Mater Res A. 2008;84:191–197. doi: 10.1002/jbm.a.31311. [DOI] [PubMed] [Google Scholar]
- 34.Rasmussen JG, Frøbert O, Pilgaard L, Kastrup J, Simonsen U, Zachar V, et al. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy. 2011;13:318–328. doi: 10.3109/14653249.2010.506505. [DOI] [PubMed] [Google Scholar]
- 35.Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86:236–242. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stubbs SL, Hsiao ST, Peshavariya HM, Lim SY, Dusting GJ, Dilley RJ. Hypoxic preconditioning enhances survival of human adipose-derived stem cells and conditions endothelial cells in vitro. Stem Cells Dev. 2012;21:1887–1896. doi: 10.1089/scd.2011.0289. [DOI] [PubMed] [Google Scholar]
- 37.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang LE. Carrot and stick: HIF-alpha engages c-Myc in hypoxic adaptation. Cell Death Differ. 2008;15:672–677. doi: 10.1038/sj.cdd.4402302. [DOI] [PubMed] [Google Scholar]
- 39.Kim JH, Park SH, Park SG, Choi JS, Xia Y, Sung JH. The pivotal role of reactive oxygen species generation in the hypoxia-induced stimulation of adipose-derived stem cells. Stem Cells Dev. 2011;20:1753–1761. doi: 10.1089/scd.2010.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antebi B, Rodriguez LA, 2nd, Walker KP, 3rd, Asher AM, Kamucheka RM, Alvarado L, et al. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res Ther. 2018;9:265. doi: 10.1186/s13287-018-1007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Xu Y, Zhao J, Zhang Z, Yang R, Xie J, et al. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014;9:e96161. doi: 10.1371/journal.pone.0096161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 43.Song YH, Gehmert S, Sadat S, Pinkernell K, Bai X, Matthias N, et al. VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2007;354:999–1003. doi: 10.1016/j.bbrc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 44.Zisa D, Shabbir A, Suzuki G, Lee T. Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochem Biophys Res Commun. 2009;390:834–838. doi: 10.1016/j.bbrc.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Qin J, Luo Q, Bi Y, Zhu G, Jiang W, et al. Cross-talk between EGF and BMP9 signalling pathways regulates the osteogenic differentiation of mesenchymal stem cells. J Cell Mol Med. 2013;17:1160–1172. doi: 10.1111/jcmm.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JH, Yoon SM, Song SU, Park SG, Kim WS, Park IG, et al. Hypoxia suppresses spontaneous mineralization and osteogenic differentiation of mesenchymal stem cells via IGFBP3 up-regulation. Int J Mol Sci. 2016;17:E1389. doi: 10.3390/ijms17091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang YC, Zhu HM, Cai JQ, Huang YZ, Xu J, Zhou Y, et al. Hypoxia inhibits the spontaneous calcification of bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2012;113:1407–1415. doi: 10.1002/jcb.24014. [DOI] [PubMed] [Google Scholar]
- 48.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:C1139–C1146. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- 49.Pawitan JA, Suryani D, Lilianty J, Purwoko RY, Liem IK. The use of VEGF supplemented media for chondrogenic differentiation of adipose derived mesenchymal stem cells. Biotechnol (Rajkot) 2013;7:169–173. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.