Abstract
Elevation of endothelial microparticles (EMPs) play an important role in the progression of inflammation-related vascular diseases such as cardiovascular diseases (CVDs). Thai perilla (Perilla frutescens) nutlets are rich in phenolic compounds and flavonoids that exert potent antioxidant and anti-inflammatory effects. We found that the ethyl acetate (EA) and ethanol (Eth) extracts of Thai perilla nutlets contain phenolic compounds such as luteolin, apigenin, chryseoriol and their glycosides, which exhibit antioxidant activity. The goal of the present study was to investigate the effects of the extracts on endothelial activation and EMPs generation in tumour necrosis factor-α (TNF-α)-induced EA.hy926 cells. We found that TNF-α (10 ng/ml) activated EA.hy926 cells and subsequently generated EMPs. Pre-treatment with the extracts significantly attenuated endothelial activation by decreasing the expression of the intracellular adhesion molecule-1 (ICAM-1) in a dose-dependent manner. Only the Eth extract showed protective effects against overproduction of interleukin-6 (IL-6) in the activated cells. Furthermore, the extracts significantly reduced TNF-α-enhanced EMPs generation in a dose-dependent manner. In conclusion, Thai perilla nutlet extracts, especially the Eth extract, may have potential to protect endothelium against vascular inflammation through the inhibition of endothelial activation and the generation of endothelial microparticles (EMPs).
Keywords: Perilla frutescens, endothelial cell, inflammation, microparticle, TNF-α, vessel
Introduction
Vascular inflammation plays an important role in the development of cardiovascular diseases (CVDs) such as atherosclerosis [1,2]. Accordingly, proinflammatory cytokines including tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) promote endothelial activation through the up-regulation of certain cell adhesion molecules (CAMs), such as the intracellular adhesion molecule-1 (ICAM-1), the vascular cell adhesion molecule-1 (VCAM-1) and E-selectin leading to the recruitment and adhesion of circulating leucocytes, mainly monocytes, to the site of inflammation [3]. Subsequently, the secreted cytokines and chemokines amplify the accumulation of the inflammatory cells and contribute to severe vascular inflammation [4,5]. Indeed, inhibition of endothelial activation is needed to prevent the development of inflammation-related diseases.
Moreover, activated endothelial cells release microparticles (MPs) in response to vascular inflammation. Many stimuli including TNF-α, cytokines, bacterial lipopolysaccharide (LPS) and reactive oxygen species (ROS) can stimulate the release of endothelial microparticles (EMPs) [6,7]. EMPs (<1 µm in diameter) are shed from the plasma membranes of endothelial cells, carrying proteins such as ICAM-1, and expose the anionic phosphatidylserine (PS) at their surface. Many studies have revealed that EMPs promote endothelial activation, coagulation and thrombosis through their PS membranes leading to the acceleration of the progression of vascular inflammation [8]. In particular, EMPs are considered a potential biomarker of endothelial dysfunction in CVDs [9]. TNF-α can induce oxidative stress and reduce nitric oxide (NO•) production resulting in an increase in the EMPs generation and the impaired function of endothelial (EA.hy926) cells [4].
Perilla frutescens (L.) Britton fruits have long been consumed medicinally and as a functional food in East Asian countries, including Thailand. Perilla nutlets are rich in α-linolenic acid (ALA) and contain phenolic compounds and flavonoids that exert antioxidant, anti-allergic, anti-cancer and anti-inflammatory effects [10,11]. Interestingly, the ethanol extract of perilla seeds was found to reduce levels of NO•, IL-6 and TNF-α in LPS-stimulated RAW264.7 macrophages [12]. Importantly, ethyl acetate and the ethanol extracts of Thai perilla nutlets containing the flavones apigenin, luteolin and chrysoeriol exhibit antioxidant and hepatic anti-lipid peroxidation activities [13]. However, the effects of Thai perilla fruits on cytokine-induced endothelial cell activation are less well known. The aim of the present study was to determine the effects of Thai perilla nutlet extracts on endothelial activation and EMPs production in TNF-α induced EA.hy926 cells.
Materials and methods
Chemicals and reagents
Calcein-acetomethoxy (Calcein-AM), CountBright™ absolute counting beads, 2′,7′–dichlorofluorescin diacetate (DCFH-DA), Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), human TNF-α, penicillin–streptomycin, RPMI 1640 and trypsin-EDTA were purchased from Thermo Fisher Scientific, Inc., Waltham, MA, U.S.A. Fluorescein isothiocyanate-Annexin V (FITC-Annexin V) and phycoerythrin (PE)-mouse anti-human CD54 were purchased from BD Biosciences, San Jose, CA, U.S.A. Additionally, 3-(4,5- Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and phosphate-buffered saline (PBS) at a pH of 7.0 were purchased from Sigma–Aldrich Inc., St. Louis, MO, U.S.A. An enzyme-linked immunosorbent assay (ELISA) kit for human IL-6 was purchased from Boster Biological Technology, Pleasanton, CA, U.S.A.
Preparation of perilla nutlet extracts
Thai perilla (P. frutescens) nutlets were harvested from Wienghang District, Chiang Mai Province, Thailand in 2014 and were botanically examined by Dr. C. Leon, Royal Botanic Gardens (RBG) Kew, Richmond, United Kingdom. These nutlets were compared botanically and chemically with authentic P. frutescens nutlets from the Royal Botanic Gardens Kew Economic Botany Collections, voucher reference: EBC 81840 TCMK 411. The nutlets were pressed to release oil and produce a residue, which sequential extracted according to our recently established method [13]. Briefly, the nutlet residue (500 g) was initially extracted twice with ethyl acetate (EA) at room temperature for 7 days. The EA extracts were combined and concentrated using a rotatory evaporator. The residue following the EA extraction was sequentially extracted with 80% ethanol (Eth) at room temperature for 7 days. Finally, the Eth extracts were combined and dried by rotary evaporation and lyophilisation. The EA extract (10% yield, w/w) containing apigenin, luteolin, chryseoriol and their glycosides, revealed total phenolic and flavonoid contents at 6.65 ± 0.05 mg gallic acid equivalent (GAE)/g and 4.52 ± 0.11 mg quercetin equivalent (QE)/g, respectively. In addition, the Eth extract (5% yield, w/w) containing rosmarinic acid, apigenin, luteolin, chryseoriol and their glycosides, revealed total phenolic and flavonoid contents at 70.77 ± 0.37 mg GAE/g and 19.97 ± 0.36 mg QE/g, respectively. The extracts were reconstituted in DMSO and diluted with culture medium at designated concentrations in the experiments.
Cell culture
Human vascular endothelial (EA.hy926) cells were cultured in DMEM supplemented with 10% FBS and 100 IU/ml penicillin and streptomycin in a humidified 5% CO2 incubator at 37°C [4]. Human promyelocytic leukaemia (HL-60) cells were grown in RPMI 1640 medium that was supplemented with 10% FBS and 100 IU/ml penicillin and streptomycin in a humidified 5% CO2 incubator at 37°C [14].
Cell viability assay
Cell viability were enumerated based on the mitochondrial reducing power of living cells that could reduce the MTT substrate to a blue-coloured formazan product [14]. Briefly, EA and Eth extracts were solubilised with DMSO to achieve various concentrations. Consequently, EA.hy926 cells were treated with the extracts (50–400 µg/ml) at 37°C for 24 h. After incubation, the treated cells were incubated with MTT solution (5 mg/ml) for 4 h. The formazan crystals were then dissolved with DMSO and the optical density was measured at 570 nm using a 96-well microplate reader.
Determination of intracellular ROS production
Intracellular ROS production was measured by dichlorofluorescein (DCF) assay [15]. Briefly, EA.hy926 cells were incubated with DCFH-DA (10 μM) at 37 °C for 30 min. After incubation, the cells were washed with PBS and treated with ascorbic acid (200 µg/ml) or the extracts (50–200 µg/ml) for 1 h. The cells were then stimulated with TNF-α (10 ng/ml) for another 24 h. After stimulation, fluorescent intensity (FI) was measured using a fluorescence microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The results were expressed as percentage of fluorescence intensity relative to the control cells.
Detection of ICAM-1 expression
ICAM-1 plays an important role in mediating leucocyte tethering by rolling on the surface of the vascular endothelial cells. We used anti-CD54 antibody to assess ICAM-1 expression and flow cytometry to detect the red FI of PE [16]. Briefly, EA.hy926 cells were pre-treated with EA and Eth extracts (50–200 µg/ml) for 1 h and were stimulated with TNF-α (10 ng/ml) for 4 h. Cells were then collected and stained with PE–conjugated anti-CD54 antibody, which had previously been prepared in the complete medium for 30 min on ice. After incubation, the cells were washed twice with PBS and analysed (10000 cell events) using a flow cytometer (Beckman Coulter CyAn ADP, Brea, CA, U.S.A.) at 488 nm excitation and 570 nm emission wavelengths. Data were analysed using Kaluza 1.5a software. Fluorescent histogram was set to identify CD54 positive. ICAM-1 expression was expressed as mean FI.
Evaluation of HL-60 cell adhesion to EA.hy926 cells
The binding of human promyelocytic leukaemia cells (HL-60 cells) to EA.hy926 cells was determined using the previously described methods [3,17]. Briefly, EA.hy926 cells were pre-treated with EA and Eth extracts (50–200 µg/ml) at 37°C for 1 h, stimulated with TNF-α (10 ng/ml) for 4 h and washed with a serum-free medium. HL-60 cells labelled with calcein-AM (1 × 105 cells/well) were then co-cultured with the treated EA.hy926 cells for 30 min at 37°C. Non-adherent HL-60 cells were then removed by washing the cells three times with PBS. Mean FI of adherent HL-60 cells was measured with a 96-well plate spectrofluorometer (485 nm excitation wavelength/530 nm emission wavelength), and their fluorescent imaging was observed by employing the High-Content Analysis System.
Measurement of IL-6 levels using ELISA kit
IL-6 production was measured by commercial enzyme-linked immunosorbent assay (ELISA) kit. Briefly, EA.hy926 cells were pre-treated with EA and Eth extracts (50–200 µg/ml) for 1 h and then treated with TNF-α (10 ng/ml) for 24 h at 37°C. Afterwards, the culture medium was collected and centrifuged at 1500 rpm for 10 min at 4°C. Finally, the supernatant was quantified for IL-6 using an ELISA kit according to the manufacturer’s instructions.
Flow-cytometric analysis of EMPs
Amounts of EMPs were measured using the flow cytometry method [4,18]. In the assay, EA.hy926 cells were pre-treated with EA and Eth extracts (50–200 µg/ml) for 1 h and incubated with TNF-α (10 ng/ml) for 24 h. After incubation, the culture medium was collected and centrifuged at 5000×g for 15 min. The supernatant was collected and centrifuged at 20000×g for 40 min. The supernatant was discarded, and the EMPs pellet was reconstituted in Annexin V binding buffer and stained with FITC-Annexin V for 15 min in the dark at room temperature. Subsequently, CountBright™ counting beads were added in each sample as an internal control and 5000 counting bead events were analysed using FACSCalibur Flow Cytometer (BD) with BD CellQuest Pro Software (BD Biosciences, San Jose, CA, U.S.A.). For EMPs gating, calibrated latex beads (1.1 µm diameter) were used to set the population of particle size < 1 µm. The gated populations which stained with FITC-Annexin V were used to set the subpopulation of positive Annexin V EMPs. The numbers of positive Annexin V EMPs were normalised with the counting beads and were presented as absolute numbers of EMPs as well as the fold-change of EMPs relative to the control.
Statistical analysis
Data are presented as mean ± SEM values. Statistical significance was analysed using one-way analysis of variance (ANOVA) with post hoc Tukey–Kramer’s test, for which P<0.05 was considered significant.
Results
Cytotoxic effect of perilla nutlet extracts
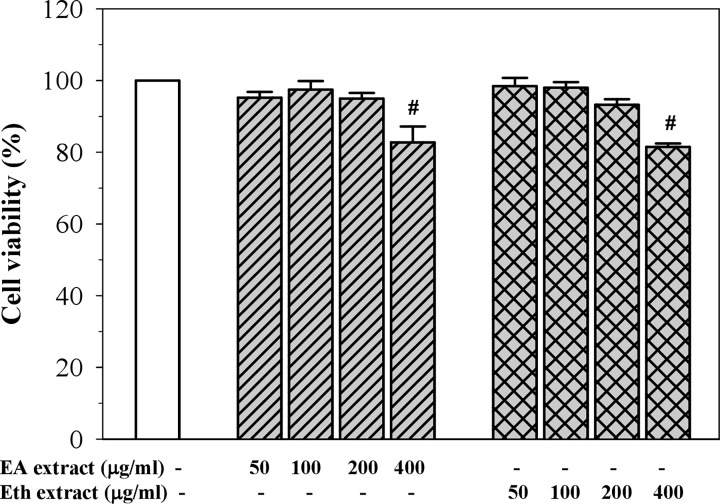
As shown in Figure 1, EA and Eth extracts at concentrations of 50–200 µg/ml were not found to be toxic to EA.hy926 cells (cell viability > 90%). As doses of the extracts were raised to 400 µg/ml, the number of viable cells were found to significantly decrease when compared with the control group (approximately 80% cell viability). Based on these results, further experiments were performed using the extracts at a concentration of 50–200 μg/ml.
Figure 1. Cell viability after treatment with EA and Eth extracts in EA.hy926 cells.
Data obtained from three independent experiments are expressed as mean ± SEM. #P<0.05 when compared with treatment without the extracts.
Inhibition of intracellular ROS production
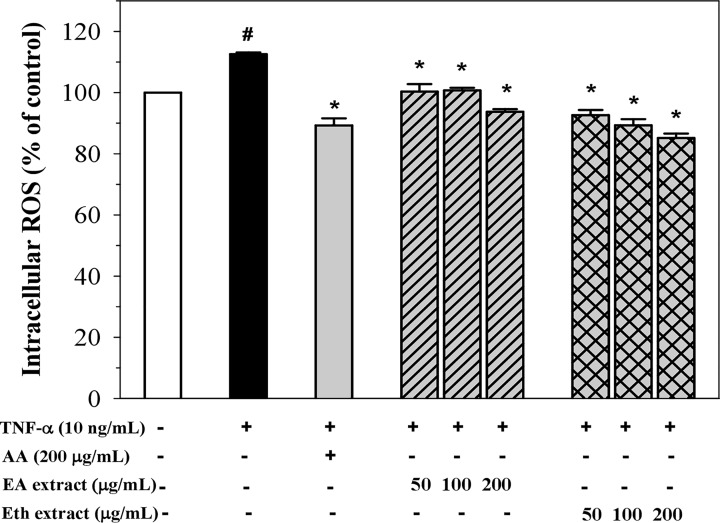
Stimulation of EA.hy926 cells with TNF-α (10 ng/ml) for 24 h significantly increased intracellular ROS production (Figure 2). Pre-treatment with ascorbic acid (200 µg/ml) significantly decreased the production of intracellular ROS. Indeed, both EA and Eth extracts significantly reduced the generation of intracellular ROS in a dose-dependent manner, where the Eth extracts were found to be more potent than those of the EA extract.
Figure 2. Levels of intracellular ROS production in TNF-α-induced EA.hy926 cells treated with ascorbic acid (AA), EA and Eth extracts.
Data obtained from three independent triplicate experiments are expressed as mean ± SEM. #P<0.05 when compared with nontreatment; *P<0.05 when compared with TNF-α-induced cells.
Inhibition of ICAM-1 expression
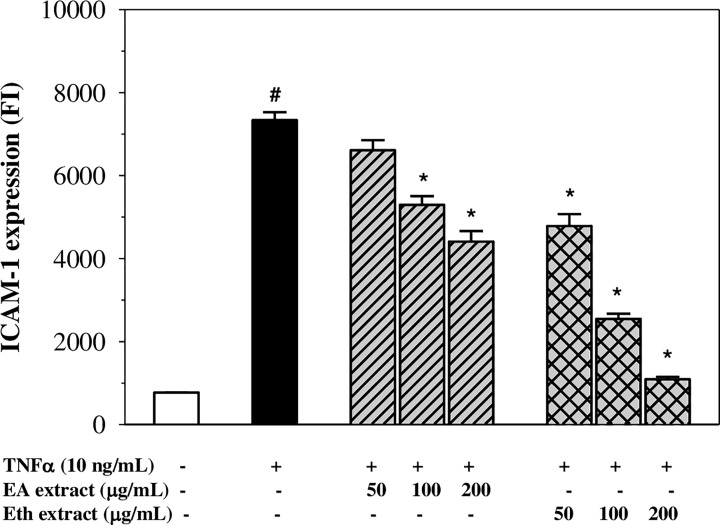
Exposure of EA.hy926 cells to TNF-α (10 ng/ml) for 24 h exhibited no cytotoxicity, whereas the exposure for 4 h was found to elevate the expression of ICAM-1 on the EA.hy926 cell membrane when compared with the control (P<0.05) (Figure 3). Pre-treatment of the cells with Eth extract (50–200 µg/ml) significantly reduced the expression of ICAM-1 in a concentration-dependent manner. Similarly, the EA extract decreased the expression of ICAM-1 in a dose-dependent manner with 100–200 µg/ml presenting significant inhibitory effects (decreasing expression to 30 and 15% of control, respectively). The results revealed that the EA and Eth extracts exhibited anti-inflammatory effects through the inhibition of adhesion molecule expression on TNF-α-stimulated endothelial cells.
Figure 3. Levels of ICAM-1 expression in TNF-α-induced EA.hy926 cells treated with EA and Eth extracts.
Data obtained from three independent experiments are expressed as mean ± SEM. #P<0.05 when compared with nontreatment; *P<0.05 when compared with TNF-α-induced cells.
Effects on adhesion of HL-60 cells to TNF-α-activated EA.hy926 cells
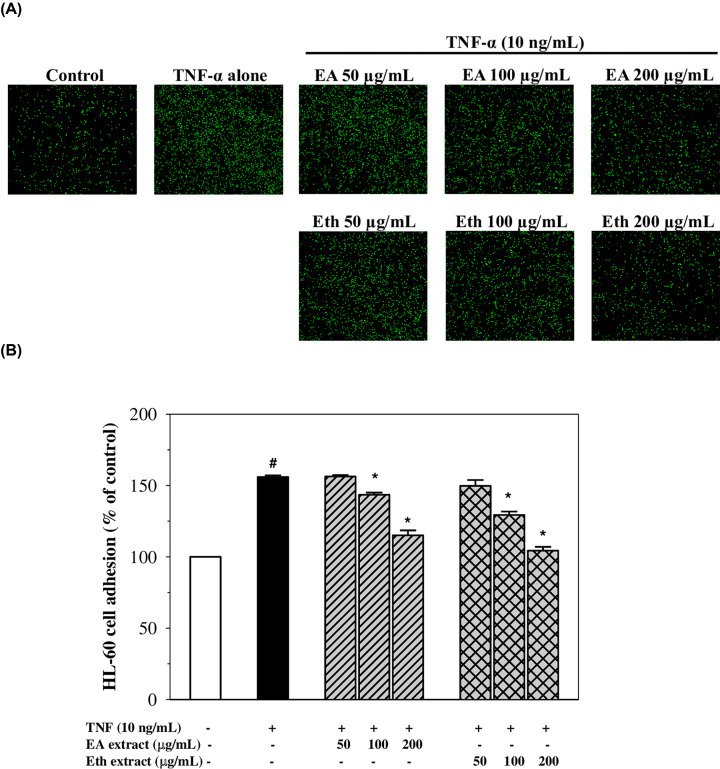
Clearly, TNF-α significantly induced adhesion of HL-60 cells to EA.hy926 cells by 1.56-fold when compared with the control (P<0.05). Indeed, the adhesion was decreased by pre-treatment with the EA and Eth extracts in a concentration-dependent manner with significant effects observed at concentrations of 100–200 µg/ml (decreasing adhesion to approximately 75 and 30% of TNF-α control for the EA extract, and 60 and 10% of TNF-α control for the Eth extract, respectively) (Figure 4). This broadly confirms the effects of the extracts on the ICAM-1 expression described above. The results suggest that the EA and Eth extracts could protect against the adhesion of leucocytes to vascular endothelial cells as a consequence of their anti-inflammatory effect.
Figure 4. Adhesion of HL-60 cells to TNF-α-activated EA.hy926 cells after treatment with EA and Eth extracts.
Fluorescent signal (A) and percentage (B) of HL-60 cells adhering to the TNF-α- activated EA.hy926 cells treated with EA and Eth extracts. Data obtained from three independent experiments are expressed as mean ± SEM. #P<0.05 when compared with nontreatment; *P<0.05 when compared with TNF-α-induced cells.
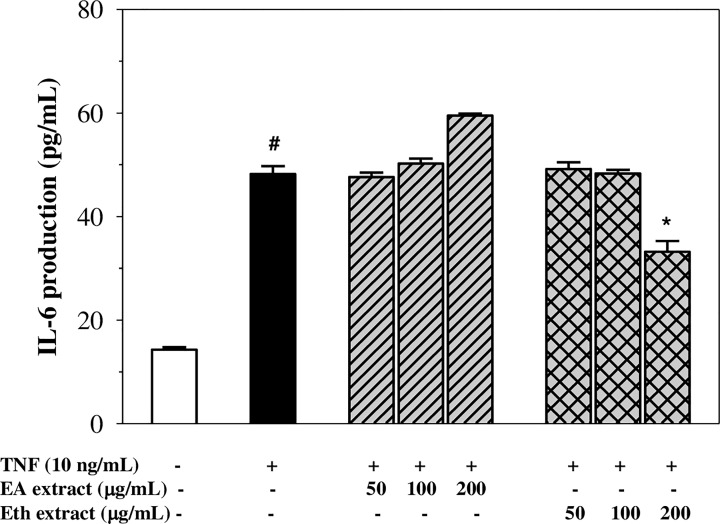
Inhibition of IL-6 secretion
As shown in Figure 5, exposure of EA.hy926 cells to TNF-α showed approximately a 3-fold increase in IL-6 secretion when compared with the control (P<0.05). Pre-treatment of the cells with the Eth extract suppressed IL-6 production; however, only a dose of 200 µg/ml presented a significant effect. In comparison, pre-treatment with the EA extract did not inhibit TNF-α-induced IL-6 secretion in the EA.hy926 cells. This result implies that the Eth extract, but not the EA extract, may exert the anti-inflammatory effect of reducing IL-6 production in TNF-α-activated EA.hy926 cells.
Figure 5. Levels of IL-6 production in TNF-α-activated EA.hy926 cells treated with EA and Eth extracts.
Data obtained from three independent experiments are expressed as mean ± SEM. #P<0.05 when compared with nontreatment; *P<0.05 when compared with TNF-α-induced cells.
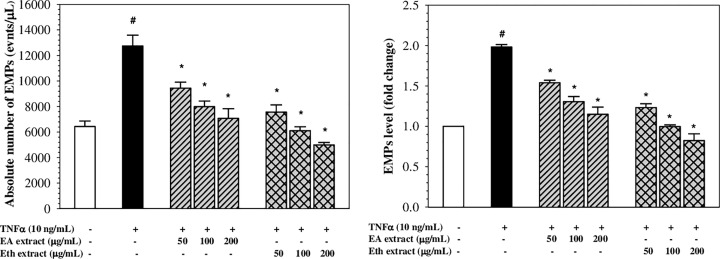
Inhibition of EMPs generation
Absolute numbers and fold changes of Annexin V+ EMPs are shown in Figure 6. Interestingly, TNF-α (10 ng/ml) was found to increase levels of EMPs generation by 1.98 ± 0.05-fold in EA.hy926 cells when compared with the control (P<0.05). Pre-treatment with EA and Eth extracts (50–200 µg/ml) significantly decreased the levels of EMPs generation in a concentration-dependent manner in TNF-α-induced EA.hy926 cells. The Eth extract was found to be more potent than the EA extract, in which the Eth extract at 100-200 µg/ml completely abrogated TNF-α-induced EMPs generation. The results imply that EA and Eth extracts may protect endothelial cells from TNF-α-induced EMPs generation.
Figure 6. Generation of EMPs in TNF-α-induced EA.hy926 cells treated with EA and Eth extracts.
Data obtained from three independent experiments are expressed in absolute numbers and fold change as mean ± SEM. #P<0.05 when compared with nontreatment; *P<0.05 when compared with TNF-α-induced cells.
Discussion
Previous studies have demonstrated that perilla fruits are rich in phenolic compounds including flavonoids that exhibit antioxidant and anti-inflammatory activities [10–12]. Recently, we have reported that the flavones such as apigenin, luteolin and chrysoeriol, and their glycosides were detected in the EA and Eth extracts of Thai perilla nutlets [13]. However, rosmarinic acid and its glycoside were found only in the Eth extract [13]. Moreover, the Eth extract revealed higher phenolic and flavonoid contents, higher compound diversity and greater antioxidant activity than the EA extract [13]. In the present study, the EA and Eth extracts in doses up to 200 μg/ml were found to be nontoxic (cell viability > 90%) to human endothelial (EA.hy926) cells but were significantly toxic at 400 μg/ml. Consistently, we have shown in our previous study that both extracts at doses up to 200 μg/ml were nontoxic to human hepatocellular carcinoma (Huh7) cells and expressed cytotoxicity at higher doses. According to the findings of our previous study, both extracts inhibited intracellular ROS in FeCl3-induced HuH7 cells. In this study, we also evaluated the antioxidant activity of the extracts by assessing their ability to inhibit intracellular ROS production in TNF-α-induced EA.hy926 cells. TNF-α is one of the stimulators of ROS generation that can cause cellular damage as well as inflammation. Our results revealed that the EA and Eth extracts significantly inhibited the generation of ROS in TNF-α-induced EA.hy926 cells in a concentration-dependent manner. However, only the Eth extracts at a dose of 200 μg/mL displayed more potency in terms of antioxidant activity than those of ascorbic acid, which is known as a standard antioxidant. The Eth extract also had more potency than those of the EA extract, which are in accordance with those of our previous study [13]. It is possible that the anti-oxidant activity of the perilla extracts depended upon the detected compounds and contents of phenolics and flavonoids. These findings suggest that antioxidants contained in both extracts could prevent oxidative stress in human endothelial cells.
In the present study, the EA and Eth extracts exhibited anti-inflammatory activity through the inhibition of endothelial activation in TNF-α-induced EA.hy926 cells. Notably, the Eth extract was found to be more potent than the EA extract. It is possible that the anti-inflammatory effect may occur in accordance with their phenolic and flavonoid contents and their respective free-radical scavenging activity. Phenolic compounds have been reported to display anti-inflammatory activity in both in vitro and in vivo experiments [19–21]. During the inflammation response, ROS are generated from activated inflammatory cells, resulting in oxidative stress that can amplify the damage to the tissues. Thus, phenolic compounds including flavonoids are desirable in inhibiting the progression of inflammation due to their antioxidant activity and anti-inflammatory activity. In particular, rosmarinic acid, mostly existing in rosemary and ethanolic extracts obtained from plants, was found to exert anti-inflammatory effects on cells, possibly through inactivation of enzymes such as 5-lipoxygenase, inducible nitric oxide synthase and cyclooxygenase-2 (COX2), and signal proteins such as TNF-α, mitogen-activated protein kinases (MAPKs), nuclear factor kapper B (NF-kB), E-cadherin, vimentin, p38, c-Jun NH2-terminal kinase 1/2 (JNK1/2) and Janus kinase 2 (JAK2), as well as signal transducers and activators of transcription 1/3 (STAT1/3) [22–24]. Interestingly, previous studies found that perilla extract or rosmarinic acid obviously decreased the degree of gene expression of ICAM-1 and COX-2 in 12-tetradecanoylphorbol 13-acetate treated animals, suggesting anti-inflammatory and ROS scavenging effects [25,26].
During inflammation, vascular endothelial cells become activated by TNF-α that is secreted from immune cells (e.g. macrophages, monocytes, mast cells, T cells and natural killer cells) and ultimately respond to express ICAM-1 molecules. Accordingly, ICAM-1 will be expressed on the surface of the endothelial cells and function to mediate an interaction between the activated endothelial cells and leucocytes, leading to infiltration of the immune cells [1,27]. Moreover, the aberrant expression of ICAM-1 is associated with other inflammatory diseases such as atherosclerosis, arthritis and cancer [28,29]. In the present study, we have found that the Eth extract containing rosmarinic acid, apigenin and luteolin revealed more potent anti-inflammatory effects than the EA extract containing only apigenin and luteolin. These findings are also in accordance with the suppression of ICAM-1 expression by the EA and Eth extracts, in which rosmarinic acid in the Eth extract may cooperate with apigenin and luteolin in the inhibition of ICAM-1 expression and the amelioration of TNF-α-induced inflammation. Evidently, rosmarinic acid of Perilla frutescent leaves displayed anti-inflammatory effects via the potent inhibition of TNF-α-induced endothelial protein C receptor shedding by suppression of the TNF-α-converting enzyme [30]. Previous study found that polyphenols, including rosmarinic acid, caffeic acid, ferulic acid and quercetin, were present in the butanol extract of mullein (Verbascum phlomoides L.) flowers and were found to exert anti-inflammatory action by inhibiting ICAM-1 expression on TNF-α-induced human umbilical vein endothelial cells (HUVECs) [26]. In addition, apigenin was found to be an anti-allergic inflammatory agent by decreasing the degree of expression of ICAM-1 and IL-6 within the mRNA and protein levels in di-(2-ethylhexyl) phthalate (DEHP)-stimulated HUVECs [31]. Notably, luteolin inhibited ICAM-1 expression in TNF-α-induced cells and mice [3,32,33]. Moreover, rosmarinic acid suppressed ICAM-1 expression in TNF-α-stimulated human fibroblast cells [34,35].
Here, we have documented that EA and Eth extracts were able to potently block the adhesion of HL-60 cells to TNF-α-stimulated EA.hy926 cells in a concentration-dependent manner with even higher inhibitory effects being observed in the Eth extract. In previous studies, apigenin was found to reduce the binding of monocytes to DEHP-induced HUVECs [31] and luteolin suppressed adhesion of monocytes to TNF-α-induced EA.hy926 cells and HUVECs [3]. In these findings, the bioactive compounds present in the EA and Eth extracts may exert an anti-inflammatory effect by inhibiting the interaction between endothelial cells and leucocytes, consequently resulting in a decrease in CAM expression on the endothelial cell membrane.
Pro-inflammatory cytokines such as IL-6 are key mediators involved in enhancing vascular inflammation [36]. The release of IL-6 from activated endothelial cells results in the activation of the immune system through the recruitment of leucocytes and by promoting neutrophil apoptosis [37,38]. Our results have shown that the Eth extract decreased the production of IL-6 in TNF-α-induced EA.hy926 cells with a significant effect occurring only at a dose of 200 µg/ml. However, previous studies have revealed that apigenin inhibited the secretion of IL-6 in THP-1-derived macrophages and compound 48/80-induced mice [31,39], whereas luteolin reduced the release of IL-6 and TNF-α in LPS-stimulated RAW264.7 cells [40]. Indeed, rosmarinic acid reduced the production of IL-6 in LPS-induced acute lung injuries and dextran sulphate-induced colitis in mice [41,42]. Our observations have identified the anti-inflammatory effects of the polyphenolics that are present in Eth extract in the inhibition of IL-6 production.
It has been widely documented that EMPs are involved in the regulation of inflammation, vascular injury and thrombosis [6,9]. Similar findings have demonstrated that EMPs exerted pro-coagulant activity through their PS-rich outer membrane leaflet, which was bound to coagulation factors II, Va and Xa, and subsequently initiated thrombosis [7]. EMPs can promote vascular inflammation through their endothelial adhesion molecules leading to the recruitment of inflammatory cells [2,6]. In our present study, TNF-α (10 ng/ml) significantly induced the release of EMPs in EA.hy926 cells; however, pre-treatment of the cells with EA and Eth extracts potently inhibited the TNF-α-induced generation of EMPs in a dose-dependent manner. These results confirm the anti-inflammatory effects of the EA and Eth extracts of perilla nutlets on TNF-α-induced endothelial cell activation, the inhibitory effects on EMPs generation and attenuation in the severity of endothelial activation and vascular inflammation-related diseases.
Conclusions
Thai perilla nutlet extracts, especially the Eth extract containing rosmarinic acid, apigenin and luteolin, potentially protect endothelial cell activation via inhibition of ICAM-1 expression and IL-6 production, adherence of leucocytes to endothelial cells, and EMPs generation. Conclusively, these events indicate the relevant inhibitory effects of Thai perilla nutlet extracts on vascular inflammation and CVDs.
Abbreviations
- calcein-AM
calcein-acetomethoxy
- CAM
cell adhesion molecule
- CVD
cardiovascular disease
- DEHP
di-(2-ethylhexyl) phthalate
- DMEM
Dulbecco’s modified Eagle’s medium
- EA
ethyl acetate
- ELISA
enzyme-linked immunosorbent assay
- EMP
endothelial microparticle
- Eth
ethanol
- FBS
fetal bovine serum
- FI
fluorescent intensity
- FITC
fluorescein isothiocyanate
- GAE
gallic acid equivalent
- HL-60 cell
human promyelocytic leukaemia cell
- HUVEC
human umbilical vein endothelial cell
- IC50
inhibitory concentration at 50%
- ICAM-1
intracellular adhesion molecule-1
- IL
interleukin
- LPS
lipopolysaccharide
- MP
microparticle
- MTT
3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NO•
nitric oxide
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- PS
phosphatidylserine
- QE
quercetin equivalent
- ROS
reactive oxygen species
- TNF-α
tumour necrosis factor-α
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by Research and Researchers for Industries (RRI), the Thailand Research Fund (TRF) [grant number PHD56I0016 (to Narisara Paradee)]; the Newton Fund Ph.D. Placement 2016, British Council Thailand (to Narisara Paradee)]; the Medical Faculty Endowment Fund, Chiang Mai University [Research ID: 2558 03504/Study Code: BIO 2558 03504 (to Somdet Srichairatanakool)]; and the Tipco BioTech Company, Prachuapkhirikhan, Thailand.
Author Contribution
Narisara Paradee designed and conducted experiments, analysed data, and wrote the draft manuscript. Niramon Utama-ang designed and advised on experiments involving food and natural products. Chairat Uthaipibull designed experiments and shared in the discussion. John B. Porter and Maciej W. Garbowski oversaw the assay of MPs and contributed to the discussion. Somdet Srichairatanakool conceived and designed the study and experiments and contributed to the discussion. All authors have read and approved the manuscript.
References
- 1.Liao J.K. (2013) Linking endothelial dysfunction with endothelial cell activation. J. Clin. Invest. 123, 540–541 10.1172/JCI66843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rautou P.E., Leroyer A.S., Ramkhelawon B., Devue C., Duflaut D., Vion A.C. et al. (2011) Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ. Res. 108, 335–343 10.1161/CIRCRESAHA.110.237420 [DOI] [PubMed] [Google Scholar]
- 3.Jia Z., Nallasamy P., Liu D., Shah H., Li J.Z., Chitrakar R. et al. (2015) Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IKappaBalpha/NF-kappaB signaling pathway. J. Nutr. Biochem. 26, 293–302 10.1016/j.jnutbio.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kam A., Li K.M., Razmovski-Naumovski V., Nammi S., Chan K., Grau G.E. et al. (2015) Curcumin reduces tumour necrosis factor-enhanced annexin V-positive microparticle release in human vascular endothelial cells. J. Pharm. Pharm. Sci. 18, 424–433 10.18433/J3ZC8G [DOI] [PubMed] [Google Scholar]
- 5.Liu S., Dong Y., Wang T., Zhao S., Yang K., Chen X. et al. (2014) Vaspin inhibited proinflammatory cytokine induced activation of nuclear factor-kappa B and its downstream molecules in human endothelial EA.hy926 cells. Diabetes Res. Clin. Pract. 103, 482–488 10.1016/j.diabres.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 6.Chironi G.N., Boulanger C.M., Simon A., Dignat-George F., Freyssinet J.M. and Tedgui A. (2009) Endothelial microparticles in diseases. Cell Tissue Res. 335, 143–151 10.1007/s00441-008-0710-9 [DOI] [PubMed] [Google Scholar]
- 7.Dignat-George F. and Boulanger C.M. (2010) The many faces of endothelial microparticles. Arterioscler. Thromb. Vasc. Biol. 31, 27–33 10.1161/ATVBAHA.110.218123 [DOI] [PubMed] [Google Scholar]
- 8.Curtis A.M., Wilkinson P.F., Gui M., Gales T.L., Hu E. and Edelberg J.M. (2009) p38 mitogen-activated protein kinase targets the production of proinflammatory endothelial microparticles. J. Thromb. Haemost. 7, 701–709 10.1111/j.1538-7836.2009.03304.x [DOI] [PubMed] [Google Scholar]
- 9.Boulanger C.M., Amabile N. and Tedgui A. (2006) Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension 48, 180–186 10.1161/01.HYP.0000231507.00962.b5 [DOI] [PubMed] [Google Scholar]
- 10.Asif M. (2011) Health effects of omega-3,6,9 fatty acids: Perilla frutescens is a good example of plant oils. Orient Pharm. Exp. Med. 11, 51–59 10.1007/s13596-011-0002-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.H., Park K.H., Lee M.H., Kim H.T., Seo W.D., Kim J.Y. et al. (2013) Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity- containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 136, 843–852 10.1016/j.foodchem.2012.08.057 [DOI] [PubMed] [Google Scholar]
- 12.Lee H.A. and Han J.S. (2012) Anti-inflammatory effect of Perilla frutescens (L.) Britton var. frutescens extract in LPS-stimulated RAW 264.7 macrophages. Prev. Nutr. Food Sci. 17, 109–115 10.3746/pnf.2012.17.2.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paradee N., Howes M.R., Utama-Ang N., Chaikitwattna A., Hider R.C. and Srichairatanakool S. (2019) A chemically characterized ethanolic extract of Thai Perilla frutescens (L.) Britton fruits (nutlets) reduces oxidative stress and lipid peroxidation in human hepatoma (HuH7) cells. Phytother. Res. 33, 2064–2074 10.1002/ptr.6396 [DOI] [PubMed] [Google Scholar]
- 14.Chao C.Y., Lii C.K., Tsai I.T., Li C.C., Liu K.L., Tsai C.W. et al. (2011) Andrographolide inhibits ICAM- 1 expression and NF-kappaB activation in TNF-alpha-treated EA.hy926 cells. J. Agric. Food Chem. 59, 5263–5271 10.1021/jf104003y [DOI] [PubMed] [Google Scholar]
- 15.Wang S., Sarria B., Mateos R., Goya L. and Bravo-Clemente L. (2019) TNF-alpha-induced oxidative stress and endothelial dysfunction in EA.hy926 cells is prevented by mate and green coffee extracts, 5-caffeoylquinic acid and its microbial metabolite, dihydrocaffeic acid. Int. J. Food Sci. Nutr. 70, 267–284 10.1080/09637486.2018.1505834 [DOI] [PubMed] [Google Scholar]
- 16.Amrani Y., Lazaar A.L. and Panettieri R.A. Jr (1999) Up-regulation of ICAM-1 by cytokines in human tracheal smooth muscle cells involves an NF-kappa B-dependent signaling pathway that is only partially sensitive to dexamethasone. J. Immunol. 163, 2128–21034 [PubMed] [Google Scholar]
- 17.Li S., Xu J., Yao W., Li H., Liu Q., Xiao F. et al. (2015) Sevoflurane pretreatment attenuates TNF- alpha-induced human endothelial cell dysfunction through activating eNOS/NO pathway. Biochem. Biophys. Res. Commun. 460, 879–886 10.1016/j.bbrc.2015.03.126 [DOI] [PubMed] [Google Scholar]
- 18.Dey-Hazra E., Hertel B., Kirsch T., Woywodt A., Lovric S., Haller H. et al. (2010) Detection of circulating microparticles by flow cytometry: influence of centrifugation, filtration of buffer, and freezing. Vasc. Health Risk Manag. 6, 1125–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serafini M., Peluso I. and Raguzzini A. (2010) Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 69, 273–278 10.1017/S002966511000162X [DOI] [PubMed] [Google Scholar]
- 20.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C. and Rahu N. (2016) Oxidative stress and inflammation: what polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 7432797 10.1155/2016/7432797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azab A., Nassar A. and Azab A.N. (2016) Anti-inflammatory activity of natural products. Molecules 21, 1321 10.3390/molecules21101321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geller F., Schmidt C., Gottert M., Fronza M., Schattel V., Heinzmann B. et al. (2010) Identification of rosmarinic acid as the major active constituent in Cordia americana. J. Ethnopharmacol. 128, 561–566 10.1016/j.jep.2010.01.062 [DOI] [PubMed] [Google Scholar]
- 23.Luo J., Zhang L., Zhang X., Long Y., Zou F., Yan C. et al. (2019) Protective effects and active ingredients of Salvia miltiorrhiza Bunge extracts on airway responsiveness, inflammation and remodeling in mice with ovalbumin-induced allergic asthma. Phytomedicine 52, 168–177 10.1016/j.phymed.2018.09.170 [DOI] [PubMed] [Google Scholar]
- 24.Lin J.T., Chang Y.Y., Chen Y.C., Shen B.Y. and Yang D.J. (2017) Molecular mechanisms of the effects of the ethanolic extract of Muntingia calabura Linn. fruit on lipopolysaccharide-induced pro- inflammatory mediators in macrophages. Food Funct 8, 1245–1253 10.1039/C6FO01735E [DOI] [PubMed] [Google Scholar]
- 25.Osakabe N., Takano H., Sanbongi C., Yasuda A., Yanagisawa R., Inoue K. et al. (2004) Anti- inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. Biofactors 21, 127–131 10.1002/biof.552210125 [DOI] [PubMed] [Google Scholar]
- 26.Grigore A., Colceru-Mihul S., Litescu S., Panteli M. and Rasit I. (2013) Correlation between polyphenol content and anti-inflammatory activity of Verbascum phlomoides (mullein). Pharm. Biol. 51, 925–929 10.3109/13880209.2013.767361 [DOI] [PubMed] [Google Scholar]
- 27.Turner M.D., Nedjai B., Hurst T. and Pennington D.J. (2014) Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 1843, 2563–2582 10.1016/j.bbamcr.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 28.Lee S.Y., Zaske A.M., Novellino T., Danila D., Ferrari M., Conyers J. et al. (2011) Probing the mechanical properties of TNF-alpha stimulated endothelial cell with atomic force microscopy. Int. J. Nanomedicine 6, 179–195 10.2147/IJN.S12760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shishodia S. (2012) Molecular mechanisms of curcumin action: gene expression. Biofactors 39, 37–55 10.1002/biof.1041 [DOI] [PubMed] [Google Scholar]
- 30.Ku S.K., Yang E.J., Song K.S. and Bae J.S. (2013) Rosmarinic acid down-regulates endothelial protein C receptor shedding in vitro and in vivo. Food Chem. Toxicol. 59, 311–315 10.1016/j.fct.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 31.Wang J., Liao Y., Fan J., Ye T., Sun X. and Dong S. (2012) Apigenin inhibits the expression of IL-6, IL-8, and ICAM-1 in DEHP-stimulated human umbilical vein endothelial cells and in vivo. Inflammation 35, 1466–1476 10.1007/s10753-012-9460-7 [DOI] [PubMed] [Google Scholar]
- 32.Kotanidou A., Xagorari A., Bagli E., Kitsanta P., Fotsis T., Papapetropoulos A. et al. (2002) Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression of proinflammatory molecules in mice. Am. J. Respir. Crit. Care Med. 165, 818–823 10.1164/ajrccm.165.6.2101049 [DOI] [PubMed] [Google Scholar]
- 33.Shimoi K., Saka N., Kaji K., Nozawa R. and Kinae N. (2000) Metabolic fate of luteolin and its functional activity at focal site. Biofactors 12, 181–186 10.1002/biof.5520120129 [DOI] [PubMed] [Google Scholar]
- 34.Lee J., Jung E., Kim Y., Park J., Hong S., Hyun C.G. et al. (2006) Rosmarinic acid as a downstream inhibitor of IKK-beta in TNF-alpha-induced upregulation of CCL11 and CCR3. Br. J. Pharmacol. 148, 366–375 10.1038/sj.bjp.0706728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swamy M.K., Sinniah U.R. and Ghasemzadeh A. (2018) Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl. Microbiol. Biotechnol. 102, 7775–7793 10.1007/s00253-018-9223-y [DOI] [PubMed] [Google Scholar]
- 36.Tanaka T., Narazaki M. and Kishimoto T. (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6, a016295 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheller J., Chalaris A., Schmidt-Arras D. and Rose-John S. (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813, 878–888 10.1016/j.bbamcr.2011.01.034 [DOI] [PubMed] [Google Scholar]
- 38.Barnes T.C., Anderson M.E. and Moots R.J. (2011) The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int. J. Rheumatol. 2011, 721608 10.1155/2011/721608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X., Wang G., Gurley E.C. and Zhou H. (2014) Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 9, e107072 10.1371/journal.pone.0107072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang B.C., Li Z., Xu W., Xiang C.H. and Ma Y.F. (2018) Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Am. J. Transl. Res. 10, 265–273 [PMC free article] [PubMed] [Google Scholar]
- 41.Chu X., Ci X., He J., Jiang L., Wei M., Cao Q. et al. (2012) Effects of a natural prolyl oligopeptidase inhibitor, rosmarinic acid, on lipopolysaccharide-induced acute lung injury in mice. Molecules 17, 3586–3598 10.3390/molecules17033586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin B.R., Chung K.S., Cheon S.Y., Lee M., Hwang S., Noh Hwang S. et al. (2017) Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-kappaB and STAT3 activation. Sci. Rep. 7, 46252 10.1038/srep46252 [DOI] [PMC free article] [PubMed] [Google Scholar]