Abstract
Authors have explored the photo-physical properties of Ho3+-Yb3+ doped BaTiO3 nanocrystals and proposed an intuitive method to probe temperature and crystal phase structure of the matrix. Structural phase change of doped crystals was analyzed in terms of their X-ray diffraction, and it was confirmed through second harmonic generation. We give insights on upconversion of energy of light-emission in Ho3+-Yb3+: BaTiO3 nanocrystals upon a 980 nm laser-light excitation and subsequently, the excited state dynamics were studied with the help of dependence of upconversion luminescence on excitation power and measuring-temperature. To understand the nature of occupancies of the Ho3+ ions at the Ti- and Ba-sites, we performed site-selective, time-resolved spectroscopic measurements at various crystal phases. Based on the lifetime analysis, it is inferred that the Ho3+ ions are present at two types of sites in barium titanate lattice. One of those is the 6-coordinated Ti-site of low symmetry, while the other one is the 12-coordinated Ba-site of higher symmetry. The upconversion emission of the nanocrystals are found to be temperature-sensitive (12 to 300 K), indicating possible use as a self-referenced temperature probe. An analysis of the temperature dependent emissions from 5F4 and 5S2 levels of Ho3+ ions, gives a maximum value of temperature sensitivity ~ 0.0095 K−1 at 12 K. Furthermore, we observe a sharp change in the luminescence intensity at ~180 K due to a ferroelectric phase change of the sample. The correlation of upconversion luminescence with the results of X-ray diffraction and second harmonic generation at different crystal phases implies that the frequency upconversion may be used as a probe of structural change of the lattice.
Subject terms: Optical physics, Photonic crystals, Chemical physics, Energy transfer, Optical spectroscopy, Nonlinear optics
Introduction
Ferroelectric titanates of a perovskite ABO3 structure (where A and B are usually divalent and tetravalent cations, respectively) are widely used in many applications due to their excellent dielectric, magnetic and electro-optic properties1–4. In general, a perfect perovskite has a cubic unit cell of a simple crystal structure of a CaTiO3- mineral perovskite. Here, the tolerance factor (Tf) governs the nature of distortions from its ideal shape, e.g., a rhombohedral or an orthorhombic GdFeO3-type crystal structure is observed on effectively small Tf ≤ 1, while polytypic structures turn up at Tf > 1. The wide range of physical properties of ABO3-type oxides are mainly dependent on the relative sizes of the ions, electronic configuration of the ions, dopants and substituents5,6. For example, Pandey et al. have investigated structure and electronic/magnetic properties of such oxides BiFeO3 − PbTiO3, BiFeO3-BaTiO3, etc. in this series7,8. Small ABO3 crystallites are particularly used in liquefied petroleum gas sensing and humidity sensing9–12.
Further, barium titanate (BaTiO3) is an important nonlinear optical material which has numerous applications in light modulation, optical memory storage, optical switching and electro-optic phase modulator13–16. Additionally, the high photorefractive sensitivity of barium titanate is beneficial for photonic applications15,16. Relation between phase transition and crystallite size of BaTiO3 is studied in many theoretical and experimental works17–20, which show that five phases, namely, rhombohedral, orthorhombic, tetragonal, cubic and hexagonal appear at different temperatures. A low temperature phase change is reported at ~183 K21. Considering radius of a rare-earth ion R3+ is within Ba2+(1.35 Å) and Ti4+(0.68 Å), the Ba2+ -site (A-site) is preferred to be occupied by a larger R3+ such as Nd3+ (1.08 Å) and La3+ (1.15 Å), while the intermediate ones such as Er3+ (0.89 Å) and Ho3+ (0.90 Å) may replace both the A- and B- sites22,23. BaTiO3 is a good optical host suitable for doping R3+ ions due to its low phonon cut-off frequency with excellent chemical and mechanical stability, which suits for upconversion luminescence. On the other hand, upconversion luminescence is an optical non-linear anti-Stokes process, which features conversion of low energy photons into high energy by utilizing the quantum mechanically forbidden 4f → 4f optical transitions of R3+ ions while doped in crystallite host materials24–27. Over the past decade, researchers have shown extensive range of advanced applications of upconversion nanoparticles, spanning from background noise-free biological imaging, theranostics, drug-delivery to photo-voltaic devices, and photochemical reactions28–33. To date, several excellent reviews on R3+ doped upconversion luminescence have been published to summarize the progress in this field along with demonstration of advanced applications34,35. The long-lived intermediate energy levels of the R3+ ions are favorable to achieve unique upconversion emission. The electronic transitions within a 4fn shell of R3+ ions greatly rely upon their site-symmetry in the crystal lattice of the host materials due to the difference in sensitivity to the crystal field. Therefore, the R3+ ions and their positions in different crystal structures may give distinct photoluminescence properties, and thus the knowledge of crystal structure of a given material may be realized by analyzing its luminescence behavior. Despite this interest, the explanation on the site symmetry of R3+ ions in BaTiO3 through luminescence measurements and the effect of phase transition on upconversion properties have not been studied in depth. Recently, the phase transition induced by high pressure in Eu3+: Bi2WO6 was investigated by Maczka et al. by utilizing the variation of emission intensity in downconversion luminescence measurements of Eu3+ ions36. Here, Bi2WO6 undergoes two phase transitions near 3.4 and 6.2 GPa pressure what is it clearly reflects in the Eu3+ light-emission. In a separate study, Yao et al.37 measured luminescence properties in the transition of phase structure of Er3+ doped Pb(Mg1/3Nb2/3O3)-PbTiO3 from a rhombohedral to a morphotropic phase boundary, consecutively to a tetragonal phase on increasing PbTiO3 content in this example. However, none of the earlier studies36–38 has taken into consideration studying such materials as a structural probe, and a major concern is that the basic luminescence properties in terms of excited state dynamics at steady-state or time-resolved mode with the crystal phase transition have not been addressed so far.
In this work, we report on the comprehensive upconversion properties of Ho3+-Yb3+: BaTiO3 nanocrystals. We have further characterized the structural phases by using not only the X-ray diffraction (XRD) patterns but also second harmonic generation (SHG). The temperature dependent population variation of 5F4 and 5S2 Ho3+-levels has been employed for phosphorescence intensity ratio based low-temperature thermometry. The variation of site-occupancy of Ho3+ ions in BaTiO3 of different phases is also investigated based on time-resolved spectroscopic analysis. This study suggests a general approach for sensibly probing crystal phase of this compound.
Results and Discussion
Structural properties
The Ho3+-Yb3 doped BaTiO3 samples, prepared by a wet-chemical precipitation method (Section: Methods and analyses), were annealed at three different temperatures 873, 1173 and 1473 K for 6 h and were abbreviated as c-BT (cubic) q-BT (quasi-cubic) and t-BT (tetragonal), respectively, according to their XRD patterns. Figure 1(a) presents the XRD patterns of c-BT, q-BT and t-BT of Ho3+-Yb3+ doped BaTiO3. Following their positions and relative intensities, the individual XRD peaks can be indexed as in the standard cards (ICDD card no. 75-0461) for c- and t-BaTiO3 structures39–41. The c-BaTiO3 structure with the Pm3m space group gives lattice parameters a = 4.015 Å, while the t-BT sample with space group P4mm gives a = 3.994 Å and c = 4.022 Å. Tsur et al.22,23 have reported that Ho3+ can occupy both A-site or B-site (known as amphoteric behavior), whereas Yb3+ occupies B-site mainly because of its better stability in this site in BaTiO3. They have considered thermodynamics and Tf value to predict the R3+ occupancy in perovskites. An average crystallite size calculated by a well-known Scherrer formula42 was found to be 33, 39 and 54 nm (with an error bar ±3 nm) for the c-BT, q-BT and t-BT specimens, respectively. The patterns reveal that an increasing calcination temperature is promoting average crystallite size in a thermally promoted growth of small crystallites. The size of c-phase increased from 33 to 39 nm as the calcination temperature was raised from 873 K to 1173 K. The t-phase exhibits similar result with its crystallite size 54 nm (calcined at 1473 K). However, the crystallite size is unequally bigger in this phase, which is caused due to the fact that it involves a faster diffusion of atoms at so high temperature. That helps an increased formation of nuclei of the t-phase. The grain boundaries, which are among these nuclei, are separated by pores. At so high temperature, the crystallites grow rather rapidly at removal of grain boundaries43. A due c → t-BaTiO3 transition is demonstrated by splitting of (200) and (202) peaks at 2θ ≈ 45.71°. In the 1473 K sintered sample, two distinct peaks split (Fig. 1b) at 2θ ≈ 45.19° and 2θ ≈ 45.51° in (200) and (002) planes in place of a single (002) peak of c-BaTiO3. The specimen prepared at 1173 K owes the onset of t-phase formation. Looking closely to these two peaks, one can readily ascertain the existence of two phases in the q-BT. It is believed that as a result of cell elongation in the c-axis due to a ferroelectric transition at high temperature, the t-phase reforms over the c-phase. Co-existence of crystal phases in a similar perovskite material- barium titanate stannate was reported by Mueller et al.6 and the phenomenon was interpreted in terms of mechanically clamped c- and t-phases. A phase transition c → t-BaTiO3 in a similar range of calcining temperature was reported by Sengodan et al.41. Consistently, our findings are in this line as put in several reports of synthesis of this material21,39,40.
Figure 1.
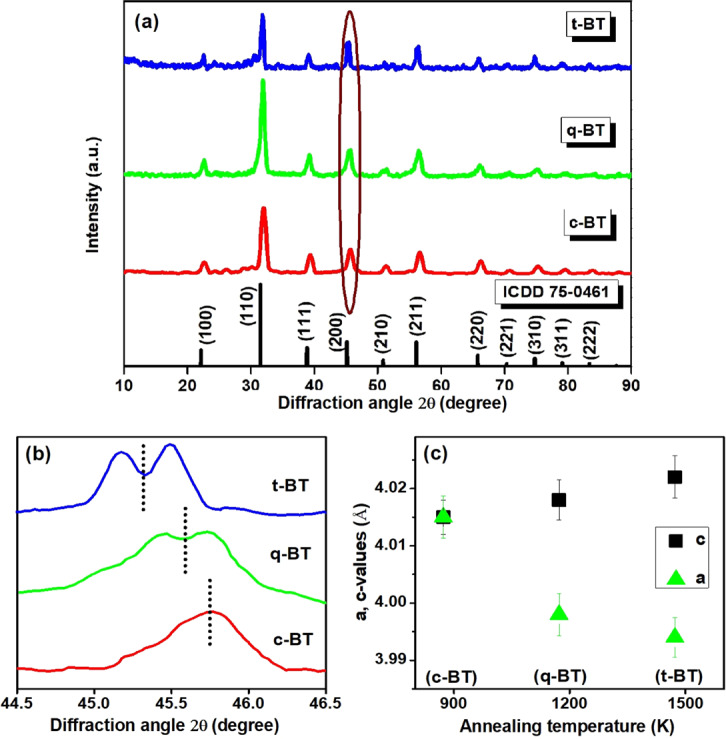
(a) XRD patterns of Ho3+-Yb3+:BaTiO3 synthesized at 873 K (c-BT), 1173 K (q-BT) and 1473 K (t-BT), (b) magnified XRD peaks around 2θ = 45.71°, and (c) variation of lattice parameters with increasing processing temperature, with a c → t-phase transition.
An analysis of XRD patterns on evolution of cubic, quasi-cubic and tetragonal lattice parameters with annealing temperature reveals due shifts in peak positions towards lower 2θ values. This results in a noticeble change of the unit cell volume as sintering temperature is increased from 873 K to 1473 K. What’s more, the effect of annealing temperature on the BaTiO3 lattice is reflected in the ratio of their lattice parameters (Fig. 1c). The tetragonality in terms of c/a ratio for the three samples- c-BT, q-BT and t-BT are calculated as 1, 1.005 and 1.007, unveiling intriguing correlation of increase in c/a ratio with lattice expansion44.
The evolution of crystallite size of Ho3+-Yb3+:BaTiO3 with annealing temperature is shown in Figure S1. It increases with increasing annealing temperature. Scanning electron microscopic (SEM) images (Figure S1) reveals that the small cystallites are joined one another in small assemblies. The results are similar to those of Er3+-Yb3+ doped BaTiO3 synthesized using the same procedure in our earlier study16. The energy-dispersive X-ray spectra for t-BT and c-BT are shown in Figure S2.
Optical properties
The optical absorption spectrum (Figure S3) of Ho3+-Yb3+: BaTiO3 powder in diffuse reflection mode shows a strong absorption in the Stark components of 2F7/2 level of Yb3+ ions at around 980 nm. The other R3+ absorption bands at 524, 655 and 800 nm are attributed to 5F4, 5S2 ← 5I8, 5F5 ← 5I8 and 5I5 ← 5I8 transitions of Ho3+ ions. Upon 980 nm laser-light excitation, the t-BT exhibits intense green (538 and 548 nm) and red (655 nm) frequency upconversions in the 5F4, 5S2 → 5I8 and 5F5 → 5I8 transitions, respectively, as shown in Fig. 2a and Figure S4. More details of mechanisms on the upconversion processes and photophysical pathways involved here are provided in the Supplementary Information.
Figure 2.
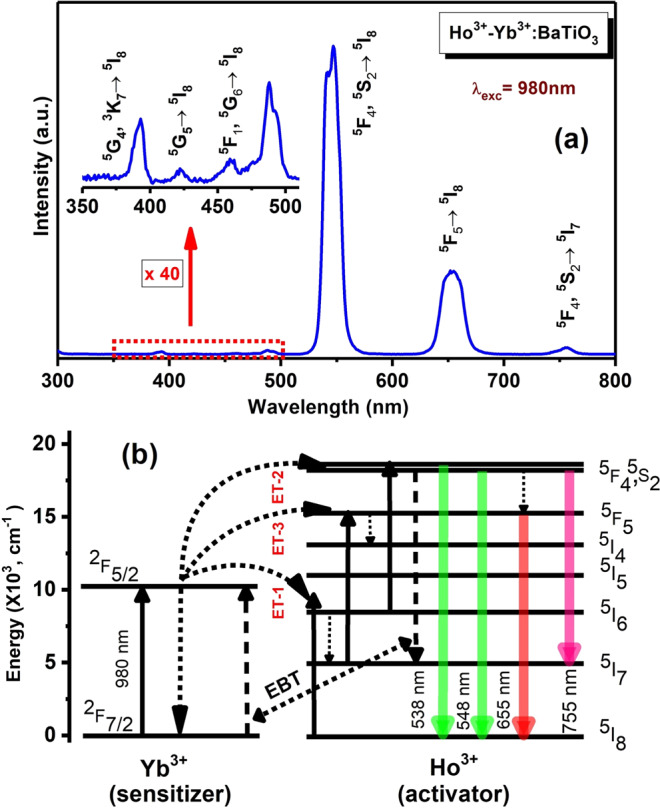
(a) Upconversion emission spectrum of Ho3+-Yb3+: t-BaTiO3 recorded at room temperature under 980 nm light excitation and (b) an energy-level diagram showing possible transition pathways.
Laser pulse energy dependent upconversion properties
In order to unravel the nature of upconversion processes, i.e., the number of photons involved in a particular transition in the upconversion emission, the pump power dependent upconversion (Figure S5a) was studied in a simplified model. As known from theoretical analysis, the slope of the logarithmic plot of pump power vs. intensity accounts the number of photons participated in a particular upconversion process45. In addition, to understand the mechanism and illustrate the experimental findings in Ho3+-Yb3+ doped BaTiO3, the population mechanism of 5F4, 5S2 and 5F5 levels have been inevitably established by solving the steady-state rate equations at an unsaturated stage of population of the ions. In the present case, the absorption cross-section of Yb3+ ions at 980 nm is much higher over the Ho3+ value28. Therefore, the steady-state rate equations of Ho3+-Yb3+ in BaTiO3, according to Fig. 2b can be set as follows:
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
where, NHo,i (i = 0, 1, 2, 3, 4, 5) are the density of population of the levels 5I8, 5I7, 5I6, 5I4, 5F5 and 5F4/5S2, respectively. The population densities of 2F7/2 (ground state) and 2F5/2 (excited state) of Yb3+ ions are expressed as NYb,i (i = 0,1). W1, W2, W4 and W5 are the non-radiative decay rates of 5I7, 5I6, 5F5 and 5F4/5S2 levels, while the radiative decay rates for the red, green and NIR emissions are expressed as Wʹ4, Wʹ5 and Wʹ51 from the levels 5F5 and 5F4/5S2. The decay rates of Yb3+ ions’ 2F5/2 → 2F7/2 is WYb, while the energy transfer (ET) rates to Ho3+ are K1, K2 and K3 corresponding to ET-1, ET-2, ET-3, respectively, as shown in Fig. 2b. The energy back transfer (EBT) from Ho3+ to Yb3+ is indicated by using Wʹ as an energy back transfer rate. The absorption cross-section of Ho3+ (between ‘i’ and ‘j’) and Yb3+ (between 2F5/2 and 2F7/2) are represented by σij and σYb, respectively, and the laser pump constant is ρP, which is dependent on incident pumping energy. The Eqs. (1) and (3) are associated with red upconversion emission, while the green upconversion emission is correlated with Eqs. (2) and (4). The frequency upconversion of Ho3+-Yb3+ is relatively cumbersome due to the involvement of the Ho3+ to Yb3+ back energy-transfer followed by emission from Yb3+. Therefore, the challenges in solving the rate equations are realized by neglecting the less impact terms in the equations. Since the Yb3+ ions have much higher absorption cross-section at 980 nm than the Ho3+ ions, the terms corresponding to ground state absorption (GSA) and excited state absoprtion (ESA) can be ruled out. In view of the fast multi-phonon relaxation between 5I4 and 5I5 levels, the 5I4 level can be considered as a short-lived level.
When the excitation pulse energy is low, the spontaneous decay of Ho3+ ions at the levels 5I6 and 5I7 are dominant through the energy-transfer processes ET-2 and ET-3. As a consequence, the relevant terms from Eqs. (2) and (1) can be omitted. Furthermore, the EBT is insignificant compared to the direct photon absorption of 980 nm wavelegth by the Yb3+ ions (2F7/2 to 2F5/2), allowing us to neglect the relevant term of EBT. From Eq. (5), we get,
| 6 |
From Eqs. (2) and (4), we consecutively get,
| 7 |
Using Eqs. (1) and (3), we obtain,
| 8 |
As the population density (NYb,1) of the level 2F5/2 of Ho3+ ion is proportional to the excitation power density, Eqs. (7) and (8) indicate green and red upconversion emission processes as quadratic power dependent.
On the other way, assuming the dominance of upconversion processes ET-2 and ET-3 over the linear decays at high excitation energy, the relevant terms- W1NHo,1 and W2NHo,2 can be neglected so to Eqs. (2) and (4) imply,
| 9 |
while Eqs. (1) and (3) consecutively imply,
| 10 |
Equations (9) and (10) indicate the linear nature of green and red upconversion processes at high pulse energy. However, our experimental observation (Figure S5b) shows the number of participated photons for 548, 655 and 755 nm light emissions as 1.54, 1.61 and 1.60, respectively, which are not directly in complete agreement with the theoretically predicted values. In the present case, a depletion of the 5I6 and 5I7 levels occurs due to the upconversion process and linear decay. The experimental observations show the value of involved photon numbers in the upconversion processes within the boundary values 1 and 2. Previous studies have reported that the 548, 655 and 755 nm emission bands are usually due to the absorption of two photons in YVO4, Y2O3, and other luminescent materials24,28,45,46.
Further observation on the excitation energy dependent upconversion emission of the bands located at 538 and 548 nm exhibits a systematic behavior of pump power with the ratio of these two bands (I538/I548) (Figure S5c). This type of variation is usually attributed to laser induced heating of the material, which led us to investigate the material extensively at various measuring-temperatures, as discussed as follows.
Effect of crystal phase transition on upconversion emission
The crystal structure of the doped BaTiO3 plays a crucial role in upconversion of energy of its light emission. Electronic transitions of the R3+ ions in the 4 f shells are strongly dependent on their site-symmetry in the crystal lattice and the position of the R3+ in the lattice is strikingly different in its different crystal structures. Consequently, the information on crystal structure may be derived by deciphering the photoluminescence properties of R3+-doped BaTiO3 of different structures. In order to realize the possibility of this material as a structural probe, we have thus further examined its 980 nm light excited frequency upconversion emission properties in its different crystal phases. The comparative upconversion spectra (Fig. 3) show largely enhanced upconversion emission intensity in the c → t-phase transition. Interestingly, the red emission is decreased in intensity over the green one (Figure S6a). In view of the overall emission, the maximum emission intensity is noted for the t-BT phase. Effects of grain-growth and elimination/creation of charge carriers in c-BT, q-BT and t-BT can be responsible for the tailored upconversion emission, wherein the XRD patterns and infrared bands did not indicate countable difference among the samples.
Figure 3.

Comparative upconversion spectra upon 980 nm light excitation of Ho3+-Yb3+: BaTiO3 of different phases.
An electronic transition probability is not associated only with the electronic structure but also crystal structure in a solid material. The local R3+ crystal fields sensitively vary in its tailored crystal phases, which can promptly tune the electronic transition probability, finely tuning the upconversion emission properties. For example, electronic band-structure dependent frequency upconversion in monoclinic and t-LaVO4:Er3+ has been studied by Zhang et al.47, who observed nearly 10-times brighter green emission of t-LaVO4:Er3+ over its counterpart monoclinic phase, which is evidently due to a difference in the local structure. They have interpreted the origin of tailored light-emission in terms of a tailored local LaVO4:Er3+ structure. Not many reports are available on the upconversion emission in a phase transition suitable to compare these results more meticulously in terms of tailored charge carriers. Qualitatively, as the structure symmetry decreases in the t-BaTiO3, the Ho3+ doped sites acquire a larger perturbation field, which in turn arise in an enhanced electronic transition probability in the form of a duly enhanced light emission as observed in this example.
In accordance to the previous section- the solutions of the rate equations at low pump power (LP) and high pump power (HP), the ratios of Eqs. (7) to (8) and Eqs. (9) to (10) can be presented as
| 11 |
and
| 12 |
In this scenario, a considerable phenomenon is that the Ho3+ ions at the 5F4/5S2 levels depopulate radiatively at the 5I8 and 5I7 levels, producing the green and NIR emission bands and the branching ratio for these two emissions is a significant factor accounting in their intensities. In this context, we have calculated the ratio of green to NIR emission for the three specimens (Figure S6b), which reflects that the intensity increasing in both the emission bands almost linearly and, therefore, branching of Ho3+ ions’ populations at the 5F4/5S2 level is not responsible for a change in a ratio of green to red emission band (IG/IR). Thus, based on the results illustrated in the rate equation model, an increased IG/IR ratio could be due to several combined effects (i) a decrease of non-radiative decay from 5F4/5S2 to 5F5, (ii) an increase of non-radiative decay from 5F5 to 5I4 in a c → t-BaTiO3 phase transition, and (iii) an induced crystal field on the Ho3+ doped sites.
Effect of temperature on upconversion emission and temperature sensing
In order to understand the Ho3+-Yb3+:BaTiO3 properties at temperatures below room temperature, we have further investigated the upconversion emission as a function of temperature over 12 to 300 K (Fig. 4a). The emission intensity is increased by a large amount at low temperatures due to a deactivation of non-radiative channels. A closer observation of intensities of green and red emissions at selective temperatures reveals that at ~ 180 K, there is a sharp jump in their values, as shown in Figure S7. This abnormal intensity change in these upconversion emission bands at this specific temperature could be associated to a structural change in BaTiO3 with its ferroelectric phase transition and Ho3+crystal field symmetry is altered in the transition. Recently, Zuo and coworkers48 have reported a similar investigation of Er3+-emission intensity change at Curie transition temperature (395 K) of the BaTiO3 matrix. Figure S7 also illustrates that the upconversion emission from 5F4 (538 nm) level is notably suppressed, while it is increased remarkably from the 5S2 (548 nm) level. The 5F4 state is quenched thermally to 5S2 what is it contributes an enhanced 5S2 → 5I8 emission on cooling the sample. Alternatively, as the temperature increases, the intensities of 538 nm and 548 nm bands tend to be equal at ~ 260 K (Figure S7) and it anticipates that at higher temperature (≥ 300 K), the 538 nm band will be starting raising its intensity on hot phonons, which help to populate Ho3+ ions in the 5F4 level from the 5S2 level. However, the ratio 538 nm/548 nm (Fig. 4b) is not affected due to the phase transition, which infers that the influence of thermal quenching is more effective than that of the phase transition for the 5F4/5S2 levels. Additionally, the temperature dependent emissions from 5F4 and 5S2 levels reflect that this material could be utilized for non-contact thermometry. Therefore, to investigate its temperature sensing ability, its temperature dependent upconversion emission spectra were inspected over 12 K to 300 K. The 5F4 and 5S2 levels of Ho3+ are closely located so as to thermally exchange couple each other. Thus, the variations of relative intensities of these bands were studied at different temperatures. It is observed that the overall intensity increases as the sample cools down with no any shift in the two bands. The ratio of intensities of 538 nm and 548 nm emission bands is seen to be increasing in the sample warming from 12 K to 300 K. The variation of relative intensities of these two emission bands at different temperatures was calculated and the graph is shown in Fig. 4b.
Figure 4.
(a) Temperature dependent upconversion spectra of t-BT, (b) plot of ln(FIR) against temperature, and (c) variation of absolute sensitivity with temperature.
The fluorescence intensity ratio (FIR) of the two bands can be written as49,50,
| 13 |
where, I538 and I548 are the integrated intensities corresponding to the 5F4 → 5I8 and 5S2 → 5I8 transitions, respectively; B is dependent on radiative probabilities, degeneracies and emitted photon energies of the associated levels; ΔE is the energy gap between the 5F4 and 5S2 levels; k is the Boltzmann constant and T is the absolute temperature. Figure 4b shows the variation of FIR with temperature. The fitting of experimental data according to Eq. (13), gives B = 0.75 ± 0.01 and ΔE = 120 ± 10 cm−1.
Being an important parameter of sensor materials, the sensitivity (absolute) has been calculated as the rate of change of FIR with temperature and can be described as51,52
| 14 |
where all the terms have their usual meanings as defined in Eq. (13). The values of FIR and ΔE as obtained above were used to calculate the S-value. The sensor sensitivity, as presented in Fig. 4c as a function of temperature, confers that the sensitivity of the material is reasonably good and it decreases rapidly as temperature rises towards room temperature. A maximum value of S ~ 0.0095 K−1 is thus found at 12 K, which is decreased to 0.0002 K−1 at 300 K. Eventually, the material is very well sensitive to temperature especially at low temperatures. A similar trend of thermal S variation was observed for YVO4:Ho3+/Yb3+ as on excited by an ultraviolet light of 266 nm wavelength in our recent studies28.
Site-selective and phase dependent time-resolved luminescence
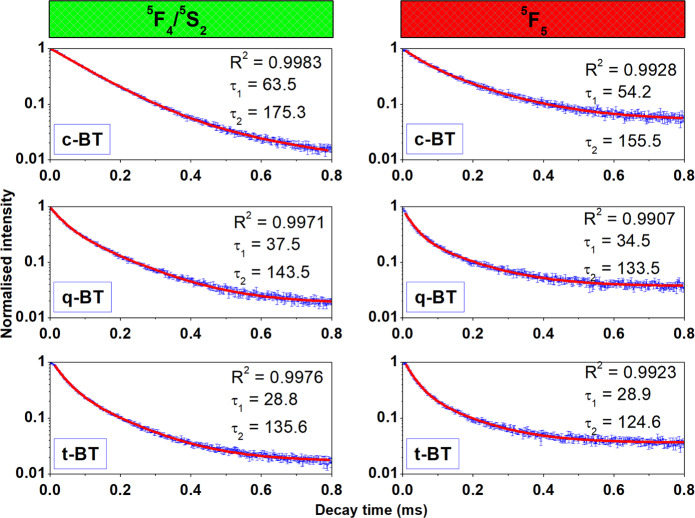
In 1965, a preliminary report53 on emission spectra of Sm3+ in BaTiO3 had described two separate series of spectral bands in the Sm3+ present in two nonequivalent sites in the lattice of Ba2+ and Ti4+ sites. Therefore, in order to examine the variation of lifetime of the green upconversion (5F4/5S2) and red upconversion (5F5) emitting levels and to light on the site selective occupancy of Ho3+ ions in a BaTiO3 lattice, the time-resolved spectroscopic measurements were conducted by exciting the selective specimens with a 980 nm laser-light. The streak camera images (survey spectra) related to the 5F4/5S2 → 5I8 and 5F5 → 5I8 transitions of Ho3+ ions are portrayed in Fig. 5. The decay profiles of 5F4/5S2 and 5F5 levels (Fig. 6) can be described in terms of an empirical relation as follows54,
| 15 |
where I(t) and I0 are the upconversion intensities at time ‘t’ and ‘0’ (zero), respectively, with A1 and A2 as the fitting parameters at fast τ1 and slow τ2 components of the luminescence lifetimes. The values of decay times so calculated are summarized in Table 1 for c-BT, q-BT and t-BT samples.
Figure 5.
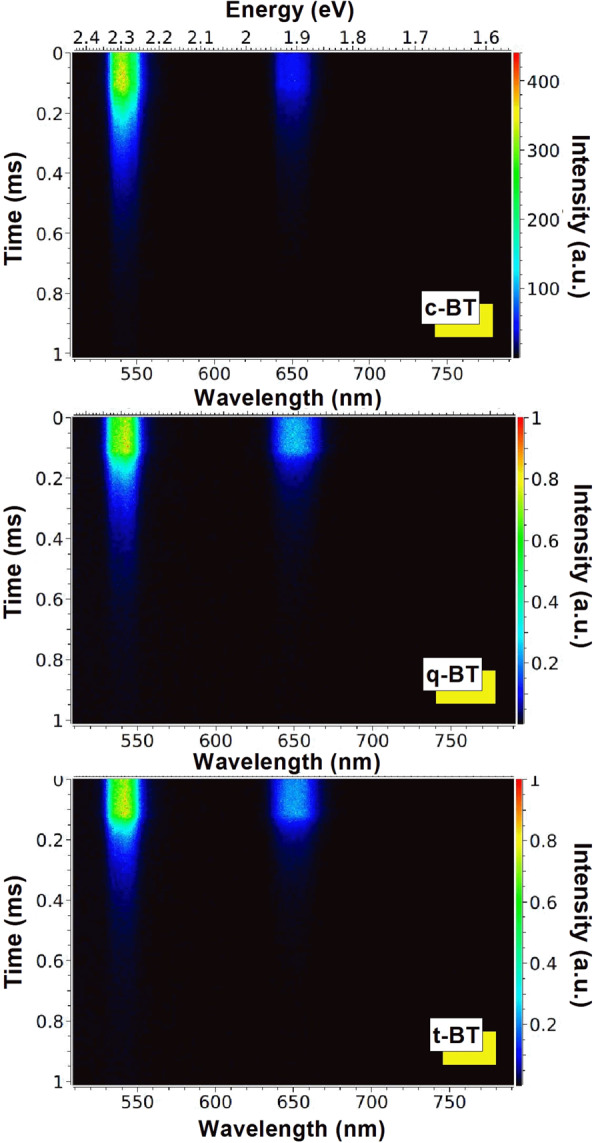
Streak camera evolved images of Ho3+-Yb3+: BaTiO3 in c-BT, q-BT and t-BT phases.
Figure 6.
Time-resolved luminescence decay transients of 5F4/5S2 and 5F5 levels of Ho3+ in Ho3+-Yb3+: BaTiO3 upon a 980 nm laser-light excitation at room temperature. τ1,2 values in the insets are in μs.
Table 1.
Short and long components of lifetime of 5F4/5S2 and 5F5 levels of Ho3+ ions co-doped in BaTiO3 of different phases.
| Energy state | Lifetime components | Ho3+-Yb3+:BaTiO3 | ||
|---|---|---|---|---|
| c-BT (μs) | q-BT (μs) | t-BT (μs) | ||
| 5F4/5S2 | τ1 | 63.5 | 37.5 | 28.8 |
| τ2 | 175.3 | 143.5 | 135.6 | |
| 5F5 | τ1 | 54.2 | 34.5 | 28.9 |
| τ2 | 155.5 | 133.5 | 124.6 | |
Reported values have an error bar ≤ 2%.
The fraction (ϕ) of site-occupancy of Ho3+ ions of a characteristic lifetime value can be determined in a formula16,55,
| 16 |
The short-lived and long-lived components of lifetime can be interpreted by considering the existence of two types of emitting sites in a Ho3+-Yb3+: BaTiO3 sample. The Ho3+ (ionic size: 0.901 Å) can occupy the Ba2+ and Ti4+ sites with two unequal co-ordinations at the two sites; which in turn adapt different modulated lifetimes. As we know, the co-ordination numbers of Ba2+ and Ti4+ ions thereby are 12 and 6, respectively (Figure S8), which render a large difference in ionic size of Ho3+ and 12-coordinated Ba2+ sites, so as Ho3+ occupied Ba2+ sites form largely distorted octahedrons.
Conversely, if a produced defect in a charge difference is large, the local site is expected to lose its inversion symmetry. Nonetheless, while a Ho3+ ion occupies a 6-coordinated Ti4+ site, the ionic size difference is smaller over the former one and results in a lesser lattice distortion. In general, a comparatively short component of lifetime is associated with a low symmetric position of largely relaxed electronic transition rules, while a long component of lifetime is usually attributed to a relatively high symmetric position owing to the forbidden 4f-4f transitions55. Therefore, in this purview, it is proposed that the short component of lifetime arises in Ho3+ occupied Ti4+ sites (6-coordinated), while the long component of lifetime arises in Ho3+ occupied Ba2+ sites (12-coordinated). The fraction of Ho3+ ions at Ba2+ sites is calculated as 73% leaving behind a residual 27% value that in the Ti4+ sites in a c-BT sample. Further, as the Ho3+ site-occupancy reorders in a phase transition, it becomes 64% and 36% in the respective sites in a t-BT sample. A due change in the coordination numbers with a crystal phase is responsible for switching the site-occupancy, which eventually tunes local crystal fields around the R3+ carriers. It is also possible that part of Ho3+ ions occupies Ti4+ interstitial sites and some others occupy the surfaces in the small crystallites. The site occupancy of Sm3+ ions is reported in a similar SrZrO3 host55 in which Sr2+ sites serve as a minor and Zr4+ sites as a major host of Sm3+ dopants. In another example, Eu3+ ions doped in α-Zn2P2O756 yield short and long lifetimes as they order at two differently coordinated Zn-sites.
Second harmonic generation
In SHG, two identical photons effectively combine giving rise to a single photon of twice the energy of the initial value. Nanocrystals exhibiting SHG are nowadays emerging in a versatile optical probe57. These nanocrystals are comprised of non-centrosymmetric crystallites and their overall contribution from the asymmetric unit cells give the SHG. Using SHG, BT nanocrystals have recently been demonstrated in stem cell labelling with high contrast images58. Thus, a phase transformation in Ho3+-Yb3+: BaTiO3 is not probed only in terms of its XRD but also its second harmonic signal. The magnitude of eccentricity of distortion in the crystal system is correlated with the intensity of the SHG signal. The SHG under a 1064 nm laser-light excitation for three BT specimens, which were annealed at 873 K (c-BT), 1173 K (q-BT) and 1473 K (t-BT), are shown in Fig. 7.
Figure 7.
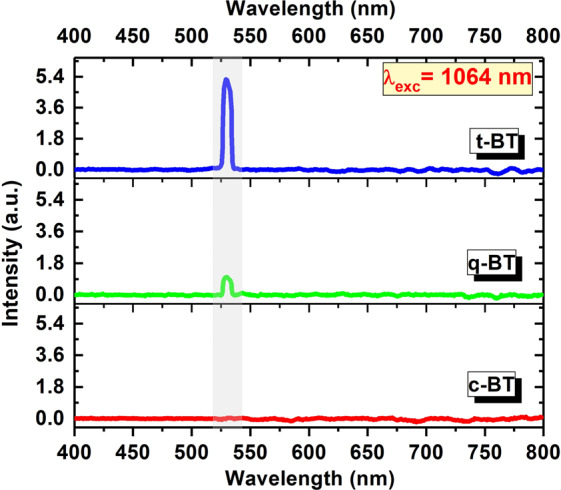
Second harmonic emission spectra of c-BT, q-BT and t-BT upon exciting with a 1064 nm pulsed laser (7 ns pulse width).
The presence of a green light at 532 nm in a half of the IR excitation wavelength supports the exploration of a non-linear effect in the BT samples. A markedly enhanced SHG signal intensity is exhibited for t-BT specimen. No such signal is visible in c-BT in view of its center of symmetry. As an eccentric distortion rises-up, the SHG turns-up in a t-BT phase. Our results of SHG, energy-upconversion of luminescence, and XRD patterns are consistent one another, and thus infer that our approach would lend itself for the use of a ‘structural probe’ employing the upconversion luminescence for identifying a crystal phase of the Ho3+-Yb3+ doped BTs.
Conclusions
In conclusion, upconverted light emission from Ho3+-Yb3+: BaTiO3 is studied with the steady-state and time-resolved photoluminescence properties. The green to red emission intensity ratio varies across a phase transition of the crystallites on a due change in Ho3+ positions in the final lattice, with modified population of the Ho3+ energy levels. The experimental results on the number of photons involved in the upconversion processes were described in support with the theoretical approach of the rate equations. Effect of thermal annealing of the samples has been illustrated based on the Ho3+ emission spectra and the rate equations. It is found that the SHG signal intensity is a measure of eccentricity present in the non-centro-symmetric BaTiO3 crystals. Further, an analysis of decay time of 5F4/5S2 and 5F5 levels exhibits double decay characteristic of these levels. The Ho3+ occupancies of 73% and 27% found in the Ba2+ and Ti4+ sites in a cubic phase get modified to 64% and 36%, respectively, as it transforms to a tetragonal phase. The temperature dependent luminescence of this material can be used as a low-temperature probe, with a sensitivity as much as 0.0095 K−1 found at 12 K. Furthermore, consistent with XRD and SHG results, this work suggests a general approach to use upconversion luminescence of these samples in identifying their crystal structure and phase transitions.
Methods and analyses
Synthesis of Ho3+-Yb3+: BaTiO3 small crystallites
A doped BaTiO3 with Ho3+-Yb3+ ions, was prepared via a wet-chemical co-precipitation method of an optimized composition, 96.8 mol% BaTiO3 + 0.2 mol% Ho2O3 + 3.0 mol% Yb2O3, suitable for the upconversion emission28,46.
In a typical reaction batch16,59, BaCO3, acetic acid (CH3COOH), titanium tetra-isopropoxide C12H28O4Ti, ytterbium acetate Yb(CH3COO)3 and holmium acetate Ho(CH3COO)3 of purity better than 99% were used as raw materials. A 1.5 g of barium carbonate was dissolved in 15 mℓ of acetic acid and warmed on a hot-plate of a magnetic stirrer at 80 °C, and then, cooled down to room temperature. Required amounts of 0.2 mol% of holmium acetate and 3.0 mol% of ytterbium acetate were added to this precursor in a homogeneous solution. As a source of Ti4+ ions, 2.0 mℓ titanium tetra-isopropoxide was mixed in the above solution and stirred on a magnetic stirrer for 1 h. At the final stage, 10 mℓ of distilled water was added to this solution for precipitating small slurries capping the cations of hydroxides, which were recovered and finally washed repeatedly in ethanol. So obtained sample was dried in ambient atmosphere over days and then annealed at 873, 1173 and 1473 K for 6 h, here after called as c-BT, q-BT and t-BT, respectively.
Light-emission and other measurements
The XRD patterns were measured on a Bruker D8 Advance X-ray diffractometer using Cu-Kα (1.5405 Å) radiation. The absorption spectrum was taken in a diffuse reflectance mode using a Lambda 950, UV-Vis-NIR spectrophotometer (Perkin Elmer). The upconversion emission spectra at different pulse energies were recorded using a 980 nm diode laser on SP2300 grating spectrometer (Princeton Instruments, USA)51. The measurements of temperature-dependent upconversion spectra from 12 K to 300 K were performed on a SPEX 1000 M spectrometer28. A He-closed-cycle refrigerator at 10−7 mbar pressure was used to cool down the sample in a chamber. The time-resolved spectroscopic measurements were carried out under excitation at a 980 nm laser-light by a Ti-sapphire laser, Mira 900-F (Coherent) pumped by Verdi 10–532 nm laser with an experimental set up consisting of a streak camera (Hamamatsu C10910), a synchronous delay generator (Hamamatsu C10647-01), water-cooled CCD (Hamamatsu Orca R2), and a delay unit (Hamamatsu C1097-05), as used in our earlier studies16,28. Furthermore, a chopper wheel at 200 Hz frequency was employed for generating laser ‘pulses’. The SHG measurements were performed using a 1064 nm Nd-YAG laser of a pulse width of 7 ns (laser model: SplitLight 600, InnoLas Laser GmbH, Germany). A suitable filter was used to block 1064 nm radiation to pass at the CCD detector.
Supplementary information
Acknowledgements
This project has been funded with support from the European Commission. K. Kumar acknowledges Department of Science and Technology (DST), New Delhi for financial support for project nos. EMR/000228/2017 and SR/FST/PSI-184/2013.
Author contributions
M.K.M. prepared the Ho3+-Yb3+ doped BaTiO3 samples. M.K.M. and K.K. performed the upconversion measurements. M.K.M., T.K. and U.V. carried out time-resolved spectroscopic measurements and M.K.M. performed the analysis. U.V. and H.H. supervised the work.
Data availability
The experimental data is available upon request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-65149-z.
References
- 1.Ohtomo A, Muller DA, Grazul JL, Hwang HY. Artificial charge-modulationin atomic-scale perovskite titanate superlattices. Nature. 2002;419:378. doi: 10.1038/nature00977. [DOI] [PubMed] [Google Scholar]
- 2.Tang P, Towner DJ, Meier AL, Wessels BW. Low-voltage, polarization-insensitive, electro-optic modulator based on a polydomain barium titanate thin film. Appl. Phys. Lett. 2004;85:4615. [Google Scholar]
- 3.Scott JF. Applications of modern ferroelectrics. Science. 2007;315:954. doi: 10.1126/science.1129564. [DOI] [PubMed] [Google Scholar]
- 4.Xu, Y. Ferroelectric materials and their applications. Elsevier (2013).
- 5.Ramadass N. ABO3-type oxides—Their structure and properties—A bird’s eye view. Mater. Sci. Eng. . 1978;36(2):231. [Google Scholar]
- 6.Mueller V, Beige H, Abicht HP, Eisenschmidt C. X-ray diffraction study revealing phase coexistence in barium titanate stannate. J. Mater. Res. 2004;19(10):2834. [Google Scholar]
- 7.Singh A, Patel JP, Pandey D. High temperature ferroic phase transitions and evidence of paraelectric cubic phase in the multiferroic 0.8 BiFeO3–0.2 BaTiO3. Appl. Phys. Lett. 2009;95(14):142909. [Google Scholar]
- 8.Bhattacharjee S, Senyshyn A, Krishna PSR, Fuess H, Pandey D. Simultaneous changes of nuclear and magnetic structures across the morphotropic phase boundary in (1−x)BiFeO3− xPbTiO3. Appl. Phys. Lett. 2010;97(26):262506. [Google Scholar]
- 9.Verma N, Singh S, Yadav BC. Experimental investigations on barium titanate nanocomposite thin films as an opto-electronic humidity sensor. J. Exp. Nanosci. 2014;9(7):669. [Google Scholar]
- 10.Sikarwar S, Yadav BC. Opto-electronic humidity sensor: A review. Sens. Actuator A-Phys. 2015;233:54. [Google Scholar]
- 11.Singh M, Yadav BC, Ranjan A, Sonker RK, Kaur M. Detection of liquefied petroleum gas below lowest explosion limit (LEL) using nanostructured hexagonal strontium ferrite thin film. Sens. Actuator B-Chem. 2017;249:96. [Google Scholar]
- 12.Manikandan V, Singh M, Yadav BC, Vigneselvan S. Room-temperature gas sensing properties of nanocrystalline-structured indium-substituted copper ferrite thin film. J. Electron. Mater. 2018;47(11):6366. [Google Scholar]
- 13.Ye, Z. G. (Ed.). Handbook of advanced dielectric, piezoelectric and ferroelectric materials: Synthesis, properties and applications. Elsevier (2008).
- 14.Yu K, Wang H, Zhou Y, Bai Y, Niu Y. Enhanced dielectric properties of BaTiO3/poly (vinylidene fluoride) nanocomposites for energy storage applications. J. Appl. Phys. 2013;113:034105. [Google Scholar]
- 15.Xie L, Huang X, Huang Y, Yang K, Jiang P. Core@ double-shell structured BaTiO3–polymer nanocomposites with high dielectric constant and low dielectric loss for energy storage application. J. Phys. Chem. C. 2013;117:22525. [Google Scholar]
- 16.Mahata MK, et al. Incorporation of Zn2+ ions into BaTiO3: Er3+/Yb3+ nanophosphor: an effective way to enhance upconversion, defect luminescence and temperature sensing. Phys. Chem. Chem. Phys. 2015;17:20741. doi: 10.1039/c5cp01874a. [DOI] [PubMed] [Google Scholar]
- 17.Mitoseriu L, et al. Grain size dependence of switching properties of ferroelectric BaTiO3 ceramics. Jpn. J. Appl. Phys. 1996;35:5210. [Google Scholar]
- 18.Ram S, Jana A, Kundu TK. Ferroelectric BaTiO3 phase of orthorhombic crystal structure contained in nanoparticles. J. Appl. Phys. 2007;102(5):054107. [Google Scholar]
- 19.Jana A, Ram S, Kundu TK. BaTiO3 nanoparticles of orthorhombic structure following a polymer precursor. Part I. X-ray diffraction and electron paramagnetic resonance. Philos. Mag. 2007;87(35):5485. [Google Scholar]
- 20.Tan Y, et al. Unfolding grain size effects in barium titanate ferroelectric ceramics. Sci. Rep. 2015;5:9953. doi: 10.1038/srep09953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoneda Y, Sakaue K, Terauchi H. Phase transition of BaTiO3 thin films. J. Phys. Condens. Mat. 2001;13:9575. [Google Scholar]
- 22.Tsur Y, Dunbar TD, Randall CA. Crystal and defect chemistry of rare earth cations in BaTiO3. J. Electroceram. 2001;7:25. [Google Scholar]
- 23.Tsur Y, Hitomi A, Scrymgeour I, Randall CA. Site occupancy of rare-earth cations in BaTiO3. Jpn. J. Appl. Phys. 2001;40:255. [Google Scholar]
- 24.Auzel F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 2004;104:139. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]
- 25.Ram, S., Mishra, A., & Fecht, H. J. Radiative emissions in rare-earth ions in Al2O3 and nanocomposites. In Encyclopedia of Nanoscience and Nanotechnology (Vol. 22, No. 288, pp. 179-288). American Scientific Publishers (2011).
- 26.Mahata, M. K., Hofsäss, H. C. & Vetter, U. Photon-upconverting materials: advances and prospects for various emerging applications (pp. 109–131). Luminescence: An Outlook on the Phenomena and their Applications (ed. J. Thirumalai). InTech (2016).
- 27.Wen S, et al. Advances in highly doped upconversion nanoparticles. Nat. Commun. 2018;9:2415. doi: 10.1038/s41467-018-04813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahata MK, Koppe T, Kumar K, Hofsäss H, Vetter U. Demonstration of temperature dependent energy migration in dual-mode YVO4: Ho3+/Yb3+ nanocrystals for low temperature thermometry. Sci. Rep. 2016;6:36342. doi: 10.1038/srep36342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorris HH, Resch-Genger U. Perspectives and challenges of photon-upconversion nanoparticles-Part II: bioanalytical applications. Anal. Bioanal. Chem. 2017;409:5875. doi: 10.1007/s00216-017-0482-8. [DOI] [PubMed] [Google Scholar]
- 30.Soni AK, Mahata MK. Photoluminescence and cathodoluminescence studies of Er3+-activated strontium molybdate for solid-state lighting and display applications. Mater. Res. Express. 2017;4(12):126201. [Google Scholar]
- 31.Tiwari SP, et al. Future prospects of fluoride based upconversion nanoparticles for emerging applications in biomedical and energy harvesting. J. Vac. Sci. Technol. B. 2018;36(6):060801. [Google Scholar]
- 32.Kumar V, Pandey A, Ntwaeaborwa OM, Swart HC. Energy transfer upconversion in Er3+-Tm3+ codoped sodium silicate glass. Physica B. 2018;535:330. [Google Scholar]
- 33.Balakrishna A, et al. Synthesis, structure and optical studies of ZnO: Eu3+, Er3+, Yb3+ thin films: Enhanced up-conversion emission. Colloid. Surf. A. 2018;540:123. [Google Scholar]
- 34.Chen G, Qiu H, Prasad PN, Chen X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem. Rev. . 2014;114:5161. doi: 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou B, Shi B, Jin D, Liu X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 2015;10:924. doi: 10.1038/nnano.2015.251. [DOI] [PubMed] [Google Scholar]
- 36.Maczka M, et al. Lattice dynamics and pressure-induced phase transitions in Bi2W2O9: High-pressure Raman study. Phys. Rev. B. 2010;81:104301. [Google Scholar]
- 37.Yao Y, Luo L, Li W, Zhou J, Wang F. An intuitive method to probe phase structure by upconversion photoluminescence of Er3+ doped in ferroelectric Pb (Mg1/3Nb2/3)O3-PbTiO3. Appl. Phys. Lett. 2015;106:082906. [Google Scholar]
- 38.Sukul PP, Mahata MK, Ghorai UK, Kumar K. Crystal phase induced upconversion enhancement in Er3+/Yb3+ doped SrTiO3 ceramic and its temperature sensing studies. Spectrochim. Acta A. 2019;212:78. doi: 10.1016/j.saa.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 39.Ohara Y, Koumoto K, Yanagida H. Barium titanate ceramics with high piezoelectricity fabricated from fibrous particles. J. Am. Ceram. Soc. 1985;68:108. [Google Scholar]
- 40.Li B, Zhang S, Zhou X, Chen Z, Wang S. Microstructure and dielectric properties of Y/Zn codoped BaTiO3 ceramics. J. Mater. Sci. 2007;42:5223. [Google Scholar]
- 41.Sengodan R, Shekar B, Chandar Sathish S. Structure, surface morphology and optical properties of BaTiO3 powders prepared by wet chemical method. Indian J. Pure Appl. Phys. 2014;52(12):839–845. [Google Scholar]
- 42.Scherrer P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen, Mathematisch-Physikalische Klasse. 1918;1918:98. [Google Scholar]
- 43.Chen YF, Lee CY, Yeng MY, Chiu HT. The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth. 2003;247(3-4):363–370. [Google Scholar]
- 44.Rao F, Kim M, Freeman AJ, Tang S, Anthony M. Structural and electronic properties of transition-metal/BaTiO3(001) interfaces. Phys. Rev. B. 1997;55(20):13953. [Google Scholar]
- 45.Pollnau M, Gamelin DR, Luethi SR, Guedel HU. Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys. Rev. B. 2000;61:3337. [Google Scholar]
- 46.Mahata MK, Koppe T, Hofsäss H, Kumar K, Vetter U. Host sensitized luminescence and time-resolved spectroscopy of YVO4: Ho3+ nanocrystals. Phys. Procedia. 2015;76:125. [Google Scholar]
- 47.Zhang F, Li G, Zhang W, Yan YL. Phase-Dependent Enhancement of the Green-Emitting Upconversion Fluorescence in LaVO4: Yb3+, Er3+ Inorg. Chem. 2015;54:7325. doi: 10.1021/acs.inorgchem.5b00851. [DOI] [PubMed] [Google Scholar]
- 48.Zuo Q, Luo L, Li W, Wang F. An effective method to detect the Curie transition of Er3+/Yb3+ co-doped BaTiO3 ceramics by up-conversion photoluminescence intensity ratio. J. . 2016;49:265303. [Google Scholar]
- 49.Mahata MK, Kumari A, Rai VK, Kumar K. Er3+, Yb3+ doped yttrium oxide phosphor as a temperature sensor. AIP Conf. Proc. 2013;1536:1270. [Google Scholar]
- 50.Sinha S, Mahata MK, Kumar K. Up/down-converted green luminescence of Er3+–Yb3+ doped paramagnetic gadolinium molybdate: a highly sensitive thermographic phosphor for multifunctional applications. RSC Adv. 2016;6:89642. [Google Scholar]
- 51.Mahata MK, Kumar K, Rai VK. Er3+-Yb3+ doped vanadate nanocrystals: a highly sensitive thermographic phosphor and its optical nanoheater behavior. Sensor. Actuat. B-Chem. 2015;209:775. [Google Scholar]
- 52.Sinha S, Mahata MK, Kumar K. Comparative thermometric properties of bi-functional Er3+–Yb3+ doped rare earth (RE = Y, Gd and La) molybdates. Mater. Res. Express. 2018;5(2):026201. [Google Scholar]
- 53.Makishim S, Yamamoto H, Tomotsu T, Shionoya S. Luminescence spectra of Sm3+ in BaTiO3 host lattice. J. Phys. Soc. Jpn. 1965;20:2147. [Google Scholar]
- 54.Sinha S, Mahata MK, Kumar K. Enhancing the upconversion luminescence properties of Er3+–Yb3+ doped yttrium molybdate through Mg2+ incorporation: effect of laser excitation power on temperature sensing and heat generation. New J. Chem. 2019;43(15):5960–5971. [Google Scholar]
- 55.Gupta SK, Ghosh PS, Pathak N, Aryab A, Natarajana V. Understanding the local environment of Sm3+ in doped SrZrO3 and energy transfer mechanism using time-resolved luminescence: a combined theoretical and experimental approach. RSC Adv. 2014;4:29202. [Google Scholar]
- 56.Gupta SK, Mohapatra M, Godbole SV, Natarajan V. On the unusual photoluminescence of Eu3+ in α-Zn2P2O7: a time resolved emission spectrometric and Judd-Ofelt study. RSC Adv. 2013;3:20046. [Google Scholar]
- 57.Kim E, et al. Second-harmonic generation of single BaTiO3 nanoparticles down to 22 nm diameter. ACS Nano. 2013;7:5343. doi: 10.1021/nn401198g. [DOI] [PubMed] [Google Scholar]
- 58.Sugiyama N, Sonay AY, Tussiwand R, Cohen BE, Pantazis P. Stem Cell Imaging: Effective Labeling of Primary Somatic Stem Cells with BaTiO3 Nanocrystals for Second Harmonic Generation Imaging. Small. 2018;14:1870036. doi: 10.1002/smll.201703386. [DOI] [PubMed] [Google Scholar]
- 59.Mahata MK, Kumar K, Rai VK. Structural and optical properties of Er3+/Yb3+ doped barium titanate phosphor prepared by co-precipitation method. Spectrochim. Acta A. 2014;124:285. doi: 10.1016/j.saa.2014.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The experimental data is available upon request from the corresponding author.