Introduction
Granular cell tumors (GCTs) are rare, typically benign neoplasms considered to be derived from perineural Schwann cells.1 Classically, GCTs present as solitary, firm, flesh-colored nodules with a diameter less than 3 cm. They more commonly develop in the mucosa of the oral cavity, the tongue, and various anatomic locations on the body in the dermis and subcutis. GCTs have also been reported in the respiratory tract, gastrointestinal tract, and breast.2 They tend to develop in the fourth or fifth decade and have a slightly higher rate of incidence in women. The tumors are typically slow growing, but malignant GCTs can grow rapidly and have a small risk of metastasis (<2%). Malignant GCTs are usually larger and metastasis is more likely to occur when the tumor develops deep to the fascia.3 Histopathology demonstrates eosinophilic lysosome-containing granular cells that stain positively for S100 protein.
We report a case of an atypical, large, exophytic, verruciform, ulcerated plaque developing within a tattoo on the upper arm of a middle-aged man, confirmed by histopathology as a GCT.
Case report
A 43-year-old healthy white man presented with a 2-year history of a slow-growing mass on his right upper arm within a tattoo. The tattoo was completed more than 20 years before presentation. The patient endorsed mild pain and itching at the site. He also reported intermittent bleeding and purulent drainage. Before presentation, he was treating the site at home with 17% salicylic acid liquid wart treatment and 70% isopropyl alcohol. The patient denied any systemic symptoms, including fatigue, fever, chills, night sweats, or weight loss.
Physical examination showed a 2.6 by 2.5-cm exophytic, verruciform, ulcerated plaque on the right upper arm (Fig 1). The plaque had an erythematous rim with a small amount of yellow purulent fluid draining centrally. The deep aspect of the plaque was palpable beyond the base of the cutaneous portion and extended an additional 0.5 to 1 cm circumferentially in the subcutaneous tissue. Focused physical examination was negative for any clinically significant axillary lymphadenopathy or additional nodules. Because of the appearance of the mass, size, and depth of local invasion, our initial differential diagnosis included dimorphic fungal infections, atypical mycobacterial infections, squamous cell carcinoma, and sarcoidosis.
Fig 1.
Granular cell tumor presenting as a 2.6 × 2.5-cm exophytic, verruciform, ulcerated plaque on the right upper arm.
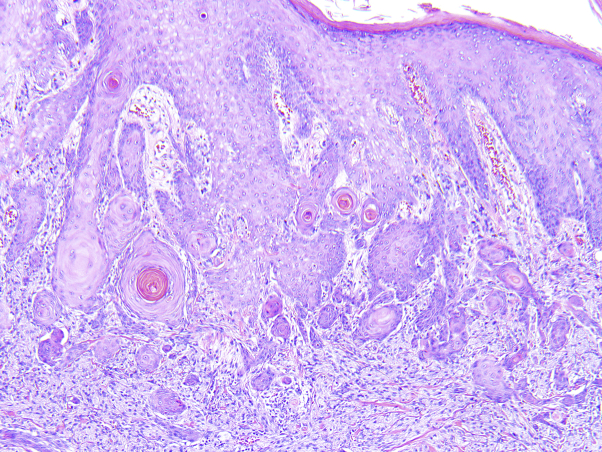
Histologic examination demonstrated large cells (histiocytes) with abundant slightly eosinophilic granular cytoplasm and uniform vesicular nuclei with a low mitotic rate (Fig 2). Immunohistochemistry was positive for S100 and CD68 and negative for cytokeratin 8/18 and Melan A (Figs 3 and 4). Based on immunohistochemistry and the histologic appearance, a diagnosis of GCT was made. Because of a lack of clinical features consistent with this diagnosis, a repeat biopsy was performed and resulted in the same histopathologic diagnosis. The tumor was fully excised and final pathology did not exhibit any malignant features. Tissue cultures of the mass were negative for deep fungal or atypical mycobacterial infections.
Fig 2.
Histopathology of granular cell tumor. Nests of cells with abundant eosinophilic granular cytoplasm separated by bands of collagenous stroma. Pseudoepitheliomatous hyperplasia can be observed in the overlying epidermis. (Hematoxylin-eosin stain; original magnification: ×40.)
Fig 3.
The tumor cells are diffusely positive for S100 protein by immunohistochemistry. (S100 immunohistochemistry stain; original magnification: ×200.)
Fig 4.
CD68 immunostain is diffusely positive and demonstrates the cytoplasmic granularity. (CD68 immunohistochemistry stain; original magnification ×200.)
Discussion
In contrast to the classic presentation of GCT as a small, flesh-colored, firm, subcutaneous nodule with overlying normal-appearing skin or mucosa (classically the tongue), our patient presented with a large ulcerated exophytic plaque with central purulent drainage on the upper arm in a previous site of trauma.
From a histopathologic perspective, the mass showed characteristic features observed with hematoxylin-eosin stain consistent with GCT. Although typically benign, malignant GCTs represent approximately 2% of reported cases, and histopathologic evaluation of undifferentiated growths is warranted to determine appropriate etiology and treatment.4,5 The histopathologic examination of the tissue did not reveal any malignant features such as necrosis, cellular atypia, high mitotic rate, or lymphovascular invasion, ruling out malignant GCT.3 The preferred treatment for GCTs is wide local excision with close follow-up to monitor for recurrence. The risk of local recurrence is estimated to be between 2% and 8% with negative margins and greater than 20% with positive ones.2
Our patient's GCT developed within a tattoo, raising the question of whether there is a role of trauma in the development of GCTs versus an incidental colocalization. There have been several other reports of GCTs developing in sites of previous localized trauma, including 2 cases of GCTs developing at vaccination sites and several cases of GCTs occurring at previous surgical sites.6, 7, 8, 9 In these cases, the temporal relationship between the traumatic insult and the development of the GCT varied between 2 weeks and greater than 8 years. Although none of the previous reported cases developed as far removed temporally from the initial dermal trauma as in our patient, it is possible that the chronic scar tissue or inflammation caused by the tattoo placement predisposed the patient to development of a GCT.10 Chasseuil et al9 hypothesized that the development of GCTs at previous sites of trauma could be due to a complication in neural cell regeneration and might explain why there is a higher propensity for the tongue mucosa because it is highly innervated tissue that undergoes repeated microtrauma secondary to mastication. Because tattoo placement requires hundreds to thousands of deep needle punctures, the role of dermal trauma secondary to tattoo placement in our patient should be considered a possible factor.10
Although GCTs are rare, the atypical clinical features in this case emphasize the importance of maintaining a broad differential diagnosis for growths of undetermined etiology. Because of the small risk of malignancy and high local recurrence rate without complete excision, timely and accurate diagnosis of GCTs is essential to appropriately treat these tumors. Additionally, further research into the possible role of dermal and mucosal trauma in the pathophysiology of GCT should be considered.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Rejas R.A., Campos M.S., Cortes A.R., Pinto D.D., De Sousa S.C. The neural histogenetic origin of the oral granular cell tumor: an immunohistochemical evidence. Med Oral Patol Oral Cir Bucal. 2011;16(1):e6–e10. doi: 10.4317/medoral.16.e6. [DOI] [PubMed] [Google Scholar]
- 2.Lack E.E., Worsham R.F., Callihan M.D. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13(4):301–316. doi: 10.1002/jso.2930130405. [DOI] [PubMed] [Google Scholar]
- 3.Fanburg-Smith J.C., Meis-Kindblom J.M., Fante R., Kindblom L.G. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998;22(7):779–794. doi: 10.1097/00000478-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Rose B., Tamvakopoulos G.S., Yeung E. Granular cell tumours: a rare entity in the musculoskeletal system. Sarcoma. 2009;2009:765927. doi: 10.1155/2009/765927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mobarki M., Dumollard J.M., Dal Col P., Camy F., Peoc'h M., Karpathiou G. Granular cell tumor: a study of 42 cases and systemic review of the literature. Pathol Res Pract. 2020;216(4):152865. doi: 10.1016/j.prp.2020.152865. [DOI] [PubMed] [Google Scholar]
- 6.Morvay O.M., Duek O.S., Bergman R. A granular cell tumor appearing at a vaccination site, a possible reaction to trauma? Am J Dermatopathol. 2019;41(10):780–781. doi: 10.1097/DAD.0000000000001291. [DOI] [PubMed] [Google Scholar]
- 7.Bandyopadhyay D., Sen S., Bandyopadhyay J.P. Granular cell tumour on vaccination scar in a young girl. Indian J Dermatol. 2006;51(3):196–197. [Google Scholar]
- 8.Murcia J.M., Idoate M., Laparte C., Baldonado C. Granular cell tumor of vulva on episiotomy scar. Gynecol Oncol. 1994;53(2):248–250. doi: 10.1006/gyno.1994.1125. [DOI] [PubMed] [Google Scholar]
- 9.Chasseuil H., Chasseuil E., Nadeau C., Frouin E., Hainaut E. Verrucous presentation of a granular cell tumour in scar tissue. Australas J Dermatol. 2020;61(1):e102–e104. doi: 10.1111/ajd.13103. [DOI] [PubMed] [Google Scholar]
- 10.Carlsen K.H., Sepehri M., Serup J. Tattooist-associated tattoo complications: “overworked tattoo,” “pigment overload” and infections producing early and late adverse events. Dermatology. 2019;9:1–8. doi: 10.1159/000501962. [DOI] [PubMed] [Google Scholar]