Abstract
Background
The purpose of this study was to test use of the Dutch leakage score (DLS), serum C‐reactive protein (CRP) and serum procalcitonin (PCT) in the diagnosis of anastomotic leakage (AL) after elective colorectal resection in a prospective observational study.
Methods
Patients undergoing elective colorectal resection with anastomosis in 19 centres were enrolled over a 1‐year period from September 2017. The DLS and CRP and PCT levels were evaluated on postoperative day (POD) 2, POD3 and POD6. Statistical analysis, including determination of the area under the receiver operating characteristic (ROC) curve (AUC), was performed for the primary endpoint of AL; secondary endpoints were morbidity and mortality rates ( http://clinicaltrials.gov identifier: NCT03560180).
Results
Among 1546 patients enrolled, the AL rate was 4·9 per cent. Morbidity and mortality rates were 30·2 and 1·3 per cent respectively. With respect to AL, DLS performed better than CRP and PTC levels on POD2 and POD3 (AUC 0·75 and 0·84), whereas CRP levels were documented with better AUC values on POD6 (AUC 0·81). Morbidity was poorly predicted, whereas mortality was best predicted by PCT on POD2 (AUC 0·83) and by DLS on POD3 and POD6 (AUC 0·87 and 0·98 respectively). Overall, the combination of positive PCT, CRP and DLS values resulted in a probability of AL of 21·3 per cent on POD2, 33·4 per cent on POD3, and 47·1 per cent on POD6. However, the combination of their negative values excluded AL in 99·0 per cent of cases on POD2, 99·3 per cent on POD3, and 99·2 per cent on POD6.
Conclusion
DLS and CRP level are good positive and excellent negative predictors of AL; the addition of PCT improved the predictive value for diagnosis of AL.
Early diagnosis and treatment of anastomotic leakage (AL) is crucial to limit related mortality. In this prospective 1‐year multicentre study of 1546 patients who had an elective colorectal resection with anastomosis, the AL rate was 4·9 per cent. The Dutch leakage score was the best predictor of AL on days 2 and 3 after surgery.

Colorectal surgery and markers for leakage
Antecedentes
El objetivo de este estudio fue evaluar el sistema de puntuación de dehiscencia holandés (Dutch Leakage Score, DLS), la proteína C reactiva sérica (PCR) y la procalcitonina sérica (PCT) con el objetivo de diagnosticar la fuga anastomótica (anastomotic leakage, AL) después de una resección colorrectal electiva en un estudio prospectivo observacional.
Métodos
En este estudio multicéntrico, se incluyeron las resecciones colorrectales electivas con anastomosis efectuadas durante 1 año. El DLS, la PCR y la PCT se evaluaron en el postoperatorio al segundo día (post‐operative day, POD) 2, al tercer día POD3 y al sexto día POD6. El análisis estadístico, que incluye las características operativas del receptor (receiver‐operating characteristics, ROC) y el área bajo la curva (area under the curve, AUC) se realizó con la AL como criterio de valoración principal; los criterios de valoración secundarios fueron las tasas de morbilidad y mortalidad (http://ClinicalTrials.gov: NCT03560180).
Resultados
En los 1.546 pacientes incluidos en el estudio, la tasa de AL fue del 4,92%. Las tasas de morbilidad y mortalidad fueron del 30,20% y 1,29%, respectivamente. Con respecto a la AL, el DLS se comportó mejor que la PCR y la PCT en el POD2 y POD3 (AUC‐ROC: 0,74 y 0,83), mientras que los niveles de PCR se asociaron con mejores valores de AUC en el POD6 (AUC‐ROC: 0,81). La predicción de la morbilidad fue mala, mientras que la mortalidad se predijo mejor mediante la PCT en el POD2 (AUC‐ROC: 0,83) y el DLS en el POD3 y POD6 (AUC‐ROC: 0,87 y 0,98). En conjunto, la combinación de valores positivos de PCT, PCR y DLS resultó en una probabilidad de AL del 21,3% en POD2, 33,4% en POD3 y 47,1% en POD 6. Por otro lado, la combinación de sus valores negativos excluyó la AL en el 99,0% de los casos en el POD2, 99,3% en el POD3 y 99,2% en el POD6.
Conclusión
El DLS y la PCR son buenos predictores positivos y excelentes predictores negativos de AL. La adición de la PCT a los predictores anteriores mejora el valor predictivo para el diagnóstico de AL.
Introduction
Anastomotic leakage (AL) is a major complication after colorectal surgery1, with a reported incidence ranging from 2 to 7 per cent when surgery is performed by experienced surgeons2, 3, 4, 5, 6, 7. To limit the clinical consequences of this major complication (high reoperation rate, increased morbidity and mortality rates, and possibly worse long‐term outcome), AL should be detected and treated as soon as possible. Fever, pain, tachycardia, peritoneal purulent or faecal drain, and dynamic ileus have commonly been suggested as clinical signs of AL1, 2, 3, 4, 5, 6, 7. These signs and others were grouped into a clinical score (Dutch leakage score, DLS)8, useful for a specific diagnostic and therapeutic algorithm.
Moreover, several laboratory markers have been proposed, such as leucocyte count, serum procalcitonin (PCT) and C‐reactive protein (CRP) levels9, 10, 11. In a recent prospective multicentre investigation12, both CRP and PCT serum levels on postoperative day (POD) 3 and POD5 were strongly suggested to be included in decision‐making for patient discharge after colorectal surgery. It is still controversial whether adding PCT to CRP improves the overall accuracy of laboratory markers in the early diagnosis of AL13, 14; a recent meta‐analysis15 failed to confirm any added value of PCT, stressing the issue of increased costs as determination of serum PCT concentration is 4–20‐fold more expensive than estimation of CRP level.
Little is known about the diagnostic accuracy of the combination of clinical and laboratory markers for the diagnosis of AL, or whether the addition of the DLS to CRP and PCT may improve early AL diagnosis. A previous small (100 patients) single‐centre series16 identified cumulative (POD1–4) area under the receiver operating characteristic curve (AUC) values at 0·8357 for DLS, 0·8035 for CRP and 0·7188 for PCT. This study was conducted to evaluate AL prospectively after colorectal resection, aiming to determine the specificity and sensitivity of DLS, CRP and PCT.
Methods
A 1‐year prospective observational study was conducted from September 2017 in 19 Italian centres. The study protocol (http://clinicaltrials.gov NCT03560180) was registered and published beforehand17, 18. Briefly, all consecutive patients undergoing elective colorectal surgery with anastomosis were enrolled following written informed consent. Patients were included if undergoing elective minimally invasive (laparoscopic or robotic) or open/converted ileocolorectal resection with intracorporeal or extracorporeal anastomosis, including Hartmann reversal. Exclusion criteria were: ASA grade IV–V; patients with a stoma before or at operation; stoma reversal without resection; transanal resection; pregnancy; ongoing infection before surgery; and hyperthermic intraperitoneal chemotherapy (HIPEC) for carcinomatosis.
CRP, PCT and DLS8 were measured before surgery and on POD2, POD3 and (optional) POD6.
Postoperative clinical examination was conducted daily by the surgical team for the following signs: fever (above 38°C), pulse rate, abdominal pain, bowel movements, volume and aspect of drainage (if present). Any complementary investigation was decided according to the attending surgeon's own criteria, and imaging was not performed routinely to detect leakage.
All complications were registered according to the Clavien–Dindo classification19, 20, including leak, wound infection21, pneumonia, central line and urinary tract infection. Follow‐up was conducted in the outpatient clinic for up to 6 weeks after discharge.
The primary endpoint was AL, defined as any deviation from the planned postoperative course related to the anastomosis, presence of pus or enteric fluid in drains or an abdominal/pelvic collection in the area of the anastomosis on CT, contrast leakage through the anastomosis during administration of an enema, or anastomotic dehiscence at reoperation for postoperative peritonitis.
Secondary endpoints were morbidity and mortality rates. Readmissions and reoperations were also recorded, as was postoperative length of hospital stay. All data were recorded prospectively, transmitted to the coordinating centre monthly, and incorporated into a spreadsheet. Any discrepancy was checked and addressed.
The ethics committee of the Comitato Etico Regionale delle Marche reviewed and approved the study protocol on 7 September 2017 (protocol number 2017‐0244‐AS). All participating centres submitted the protocol and obtained authorization from the local institutional review board.
Statistical analysis
As the presence of comorbidities is one of the most significant risk factors for AL3, 5, 22, 23, the estimated sample size was calculated on the basis of an estimated odds ratio of 5·622 for ASA grade I–II versus grade III; assuming a 95 per cent c.i. for the estimation and a maximum error of 0·04, the required sample size was 1062. Quantitative values are expressed as mean(s.d.) or median values, with ranges and 95 per cent c.i.. Categorical data are shown with percentage frequencies. Differences in continuous variables were analysed with the Mann–Whitney U test.
AUC values were calculated for DLS, CRP and PCT on POD2, POD3 and POD6 for all endpoints; values of 0·7–0·8 were considered acceptable, 0·8–0·9 as excellent, and those above 0·9 as outstanding24. Differences in AUC values were analysed with the χ2 test. With respect to the primary endpoint of AL, optimal cut‐off points for DLS, CRP and PCT were obtained by applying Youden's index (sensitivity + specificity − 1), choosing AUC values where the index was maximal.
Negative (NPVs) and positive (PPVs) predictive values were also calculated. All patients with AL as defined above and with values of the three potential predictors (DLS, CRP and PCT) above the cut‐off point were considered to be true positives, and all patients without AL and with potential predictor values below the cut‐off point were considered true negatives. A logistic regression model was built using the presence or absence of AL as the dependent variable, and CRP, PCT and DLS values equal to or less than, or greater than their respective cut‐offs as explanatory factors. Using logistic transformation of the linear predictors, the probability of AL related to different combinations of predictor values was determined.
For all statistical tests the significance level was fixed at P < 0·050. Statistical analyses were carried out using STATA® software (StataCorp, College Station, Texas, USA).
Results
From September 2017 to September 2018, 1546 patients were enrolled in the study from a total of 2717 resections performed. Reasons for exclusion of 1171 patients were: stoma before or at operation, 53·9 per cent; refusal of consent, 14·7 per cent; urgent or emergency procedure, 8·0 per cent; stoma closure without resection, 5·6 per cent; transanal procedure, 3·0 per cent; ongoing infection, 1·8 per cent; ASA grade IV–V, 1·6 per cent; HIPEC, 1·0 per cent; and other reason (pregnancy, inability to complete all data requested in the case report form), 10·3 per cent. Preliminary epidemiological data have been published in a previous report18. Overall, neoplastic disease accounted for 68·8 per cent of the resections, and minimally invasive surgery was employed in 83·5 per cent of procedures.
Seventy‐six patients (4·9 per cent) reported AL, with a mean(s.d.) time to diagnosis of 5·94(4·97) (range 1–31) days. Diagnosis was reached by CT with water‐soluble contrast enema (30 patients, 39 per cent) or intravenous contrast (22, 29 per cent), clinical evaluation (23, 30 per cent) and endoscopy (1 patient, 1 per cent). The degree of severity of AL was Clavien–Dindo grade IIIa in one patient (1 per cent), grade IIIb in 69 patients (91 per cent), and grade IVb in six (8 per cent).
The overall morbidity rate was 30·2 per cent, and included 447 grade I–II and 185 grade III–IVb complications18. The operative mortality rate was 1·3 per cent. Fourteen patients were readmitted to hospital, and 107 (6·9 per cent) required reoperation. The mean(s.d.) duration of postoperative hospital stay was 7·89(5·97) (median 6, 95 per cent c.i. 7·59 to 8·19, range 1–120) days.
Basal and postoperative values of CRP, PCT and DLS according to the endpoints are shown in Table 1 and Fig. 1.
Table 1.
Basal and postoperative values of C‐reactive protein, procalcitonin and Dutch leakage score according to the endpoints
| Anastomotic leakage | Morbidity | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P † | Yes | No | P † | Yes | No | P † | |
| CRP | |||||||||
| Basal | 10·32(28·43) | 10·76(22·42) | 0·544 | 14·35(28·79) | 9·16(19·67) | 0·003 | 25·35(40·35) | 10·54(22·57) | 0·009 |
| POD2 | 176·51(126·69) | 109·01(68·01) | < 0·001 | 143·57(89·08) | 96·49(61·79) | < 0·001 | 201·22(83·91) | 111·27(72·61) | < 0·001 |
| POD3 | 212·29(111·94) | 98·61(71·51) | < 0·001 | 143·83(94·95) | 86·45(61·12) | < 0·001 | 209·33(109·22) | 102·80(76·51) | < 0·001 |
| POD6* | 156·06(89·11) | 61·36(57·81) | < 0·001 | 97·79(78·71) | 45·67(41·17) | < 0·001 | 192·66(74·43) | 67·94(65·05) | 0·001 |
| PCT | |||||||||
| Basal | 0·11(0·21) | 0·13(0·40) | 0·648 | 0·14(0·35) | 0·12(0·41) | 0·089 | 0·31(1·00) | 0·13(0·38) | 0·265 |
| POD2 | 5·65(12·04) | 1·36(3·26) | < 0·001 | 2·81(6·59) | 1·03(2·37) | < 0·001 | 12·49(19·06) | 1·45(3·58) | < 0·001 |
| POD3 | 5·56(11·05) | 0·94(2·54) | < 0·001 | 2·24(5·86) | 0·67(1·56) | < 0·001 | 11·39(19·56) | 1·03(2·76) | < 0·001 |
| POD6* | 4·12(6·19) | 0·86(5·89) | 0·002 | 1·87(8·15) | 0·52(3·31) | < 0·001 | 12·70(12·93) | 1·02(5·83) | 0·002 |
| DLS | |||||||||
| Basal | 0·39(0·89) | 0·25(0·72) | 0·123 | 0·36(0·89) | 0·21(0·65) | 0·001 | 1·20(1·91) | 0·24(0·69) | < 0·001 |
| POD2 | 3·48(2·79) | 1·53(1·39) | < 0·001 | 2·35(2·06) | 1·31(1·12) | < 0·001 | 4·55(3·48) | 1·59(1·48) | < 0·001 |
| POD3 | 4·07(3·08) | 1·15(1·31) | < 0·001 | 2·25(2·22) | 0·87(0·90) | < 0·001 | 5·62(3·93) | 1·24(1·46) | < 0·001 |
| POD6* | 3·40(3·51) | 0·87(1·22) | < 0·001 | 1·79(2·22) | 0·50(0·61) | < 0·001 | 7·64(4·40) | 1·01(1·47) | < 0·001 |
Values are mean(s.d.).
Data available for 566 patients. CRP, C‐reactive protein; PCT, procalcitonin; DLS, Dutch leakage score.
Mann–Whitney U test.
Figure 1.
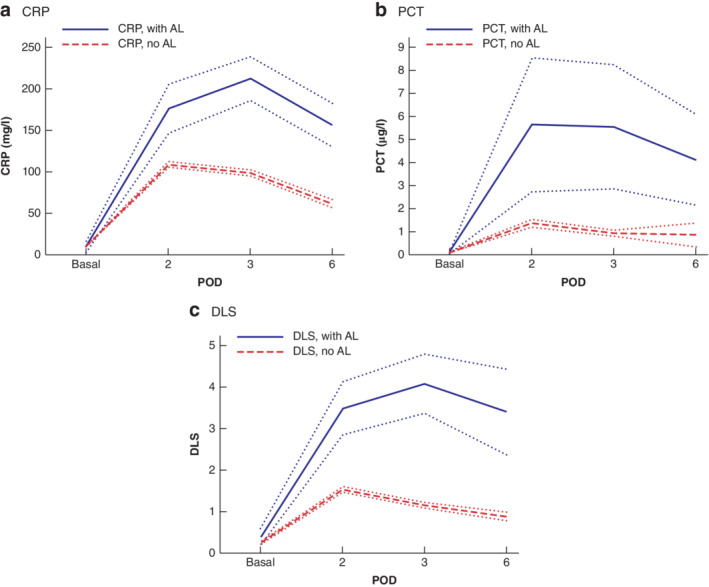
Basal and postoperative C‐reactive protein, procalcitonin and Dutch leakage score values according to the presence or absence of anastomotic leakage a C‐reactive protein (CRP), b procalcitonin (PCT) and c Dutch leakage score (DLS). Mean values are shown with 95 per cent confidence intervals. AL, anastomotic leakage; POD, postoperative day.
With respect to AL, DLS performed better than CRP and PTC (highest AUC value) on POD2 and POD3, whereas CRP level had better sensitivity and specificity curves on POD6 (Fig. 2). Morbidity was predicted poorly by all laboratory values and clinical score, whereas mortality was predicted best by PCT on POD2 and by DLS on POD3 and POD6. No differences between AUCs were statistically significant (Table 2).
Figure 2.
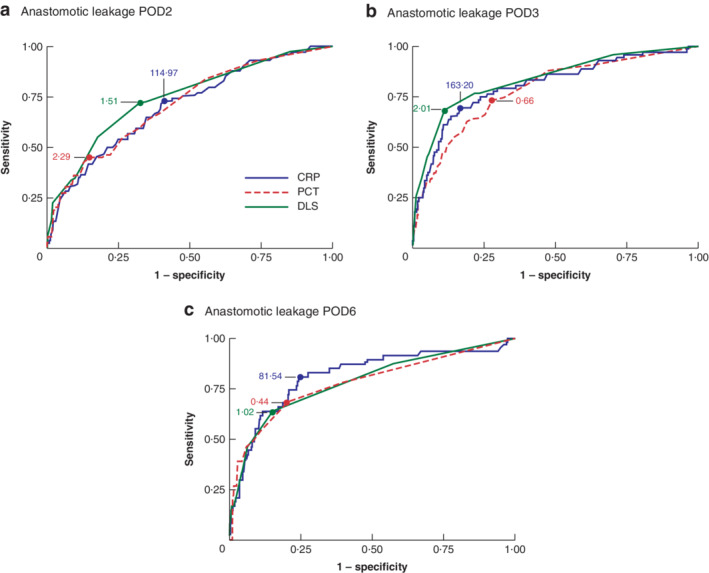
Areas under the receiver operating characteristic (ROC) curve for anastomotic leakage for C‐reactive protein, procalcitonin and the Dutch leakage score on postoperative days 2, 3 and 6 a Postoperative day (POD) 2, b POD3 and c POD6. Optimal cut‐off values for C‐reactive protein (CRP; mg/l), procalcitonin (PCT; μg/l) and the Dutch leakage score (DLS) are indicated.
Table 2.
Receiver operating characteristic (ROC) curve analysis
| AUC | Optimal cut‐off value* | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | P ‡ | |
|---|---|---|---|---|---|---|---|
| Anastomotic leakage | |||||||
| POD2 | 0·451 | ||||||
| CRP | 0·706 (0·637, 0·773) | 114·97 | 73·0 | 59·0 | 08·3 | 97·7 | |
| PCT | 0·712 (0·642, 0·782) | 2·29 | 45·0 | 85·3 | 13·1 | 96·9 | |
| DLS | 0·747 (0·682, 0·812) | 1·51 | 72·4 | 67·3 | 10·3 | 97·9 | |
| POD3 | 0·481 | ||||||
| CRP | 0·807 (0·744, 0·868) | 163·20 | 69·4 | 83·4 | 17·5 | 98·2 | |
| PCT | 0·784 (0·723, 0·854) | 0·66 | 73·1 | 72·2 | 9·2 | 98·2 | |
| DLS | 0·836 (0·777, 0·893) | 2·01 | 68·5 | 88·8 | 23·8 | 98·2 | |
| POD6† | 0·894 | ||||||
| CRP | 0·815 (0·738, 0·891) | 81·54 | 80·9 | 75·4 | 23·0 | 97·7 | |
| PCT | 0·780 (0·704, 0·874) | 0·44 | 68·3 | 80·0 | 22·0 | 96·8 | |
| DLS | 0·786 (0·705, 0·865) | 1·02 | 63·8 | 85·0 | 27·8 | 96·3 | |
| Morbidity | |||||||
| POD2 | 0·915 | ||||||
| CRP | 0·661 (0·630, 0·692) | 114·97 | 61·0 | 65·4 | 43·1 | 79·6 | |
| PCT | 0·666 (0·636, 0·699) | 0·29 | 74·3 | 50·5 | 39·5 | 81·8 | |
| DLS | 0·667 (0·636, 0·697) | 1·51 | 56·2 | 74·7 | 49·1 | 79·8 | |
| POD3 | 0·764 | ||||||
| CRP | 0·688 (0·657, 0·718) | 104·23 | 61·1 | 68·9 | 46·6 | 80·0 | |
| PCT | 0·684 (0·654, 0·716) | 0·26 | 69·1 | 59·3 | 42·7 | 81·4 | |
| DLS | 0·721 (0·691, 0·750) | 1·51 | 50·2 | 87·3 | 63·7 | 79·8 | |
| POD6† | 0·462 | ||||||
| CRP | 0·567 (0·517, 0·615) | 57·34 | 70·9 | 42·3 | 28·4 | 81·8 | |
| PCT | 0·518 (0·461, 0·567) | 1·34 | 94·7 | 10·7 | 33·0 | 81·3 | |
| DLS | 0·532 (0·480, 0·582) | 0·03 | 63·1 | 42·7 | 35·2 | 70·1 | |
| Mortality | |||||||
| POD2 | 0·340 | ||||||
| CRP | 0·807 (0·680, 0·932) | 126·93 | 88·6 | 64·2 | 2·3 | 99·8 | |
| PCT | 0·829 (0·703, 0·954) | 1·01 | 81·3 | 70·5 | 3·0 | 99·7 | |
| DLS | 0·823 (0·704, 0·942) | 2·52 | 72·2 | 81·0 | 4·3 | 99·6 | |
| POD3 | 0·251 | ||||||
| CRP | 0·793 (0·667, 0·918) | 128·22 | 83·3 | 70·4 | 3·3 | 99·7 | |
| PCT | 0·843 (0·719, 0·963) | 1·06 | 75·0 | 77·7 | 3·6 | 99·6 | |
| DLS | 0·869 (0·759, 0·979) | 2·02 | 82·4 | 86·3 | 6·7 | 99·8 | |
| POD6† | 0·072 | ||||||
| CRP | 0·913 (0·754, 1·000) | 107·09 | 100 | 80·6 | 5·3 | 100 | |
| PCT | 0·954 (0·801, 1·000) | 0·897 | 100 | 86·8 | 5·4 | 100 | |
| DLS | 0·978 (0·894, 1·000) | 3·51 | 100 | 94·5 | 18·4 | 100 |
Values in parentheses are 95 per cent confidence intervals.
Values are mg/l for C‐reactive protein (CRP) and μg/l for procalcitonin (PCT).
Data available for 566 patients. AUC, area under the receiver operating characteristic curve; PPV, positive predictive value; NPV, negative predictive value; POD, postoperative day; DLS, Dutch leakage score.
χ2 test (comparison of AUC values).
Logistic regression confirmed the statistical significance of all the three predictors with regard to AL (Table 3). The probability of AL according to CRP, PCT and DLS values below or above their cut‐off points are shown in Table 4. The maximal PPVs (all predictor values above the cut‐off points) were 21·3 per cent on POD2, 33·4 per cent on POD3, and 47·1 per cent on POD6. The maximal NPVs (all predictor values equal to or less than cut‐off points, last row) were 99·0, 99·3 and 99·2 per cent on POD2, POD3 and POD6 respectively.
Table 3.
Logistic regression analysis for the presence of anastomotic leakage according to the optimal cut‐off point for the three predictors
| Coefficient | s.e. | Z | P | |
|---|---|---|---|---|
| POD2 | ||||
| CRP | 0·920 | 0·297 | 3·09 | 0·002 |
| PTC | 0·895 | 0·271 | 3·30 | 0·001 |
| DLS | 1·520 | 0·292 | 5·20 | < 0·001 |
| Constant | −4·640 | 0·307 | −15·11 | < 0·001 |
| POD3 | ||||
| CRP | 1·553 | 0·322 | 4·83 | 0·001 |
| PTC | 1·046 | 0·329 | 3·18 | 0·001 |
| DLS | 2·214 | 0·297 | 7·46 | < 0·001 |
| Constant | −5·005 | 0·309 | −16·20 | < 0·001 |
| POD6 | ||||
| CRP | 1·482 | 0·465 | 3·19 | 0·001 |
| PTC | 1·474 | 0·415 | 3·55 | < 0·001 |
| DLS | 1·705 | 0·401 | 4·25 | < 0·001 |
| Constant | −4·775 | 0·413 | −10·98 | < 0·001 |
POD, postoperative day; CRP, C‐reactive protein; PTC, procalcitonin; DLS, Dutch leakage score.
Table 4.
Probability of the presence or absence of anastomotic leakage according to combinations of predictor values in relation to their optimal cut‐off points
| Probability of AL (%) | ||||||
|---|---|---|---|---|---|---|
| POD2 | POD3 | POD6 | ||||
| Combination* | PPV | NPV | PPV | NPV | PPV | NPV |
| CRP>, PCT>, DLS> | 21·3 | 78·7 | 33·4 | 66·6 | 47·1 | 52·9 |
| CRP>, PCT≤, DLS> | 10·0 | 90·0 | 15·0 | 85·0 | 17·0 | 83·0 |
| CRP≤, PCT>, DLS> | 9·8 | 90·2 | 14·8 | 85·2 | 16·9 | 83·1 |
| CRP>, PCT>, DLS≤ | 5·6 | 94·4 | 5·8 | 94·2 | 14·0 | 86·0 |
| CRP≤, PCT≤, DLS> | 4·2 | 95·8 | 5·2 | 94·8 | 4·4 | 95·6 |
| CRP>, PCT≤, DLS≤ | 2·4 | 97·6 | 1·9 | 98·1 | 3·6 | 96·4 |
| CRP≤, PCT>, DLS≤ | 2·3 | 97·7 | 1·9 | 98·1 | 3·6 | 96·4 |
| CRP≤, PCT≤, DLS≤ | 1·0 | 99·0 | 0·7 | 99·3 | 0·8 | 99·2 |
>, Indicates value above optimal cut‐off point; ≤, indicates value equal to or less than optimal cut‐off point. AL, anastomotic leakage; POD, postoperative day; PPV, positive predictive value; NPV, negative predictive value; CRP, C‐reactive protein; PCT, procalcitonin; DLS, Dutch leakage score.
Discussion
This multicentre prospective study has demonstrated that the combination of laboratory markers and clinical scoring system allows the exclusion of AL in the early postoperative period (NPV 99·0 per cent on POD2).
The main potential source of bias in this prospective study was the exclusion of 1171 patients (43·1 per cent), mainly due to the presence of a diverting stoma (53·9 per cent of exclusions), in the evaluation of the diagnostic yield of clinical and serum biomarkers for AL25. Another possible confounder was the recorded proportion of minimally invasive resections (83·5 per cent), which was higher than that reported (40–46·7 per cent) in the same period in a nationwide database26. This finding probably reflects the exclusion of urgent resections and the particular interest in colorectal resection and minimally invasive surgery in participating centres.
AL was detected in 76 patients (4·9 per cent), well within the range (2·7–10 per cent) reported previously in large cohort studies22, 27, 28, 29, 30. The mean time to diagnosis of AL was 5·94 days (median POD5), compared with 12·7 days (median POD8) in open colorectal resections4. It remains difficult to establish whether this was actually earlier diagnosis, as the use of both clinical and serological markers for AL diagnosis in the present study may have focused special attention on this diagnosis, or whether this was simply an earlier appearance of AL due to the high rate of minimally invasive resections (earlier return of bowel function compared with open surgery). In any case, this finding deserves particular attention when considering the increasing use of enhanced recovery programmes in colorectal surgery31, 32.
With regard to laboratory markers, significant differences in postoperative values were observed in patients with AL and those without. Both CRP and PCT levels showed their typical course as acute‐phase proteins induced by the systemic inflammation response, peaking at POD2 (PCT) and at POD3 (CRP), as reported previously10, 11, 12, 13, 14, 33.
Different clinical scoring systems for early diagnosis of AL have been suggested8, 34, externally validated16 and further modified35. Although not statistically significant as a predictor of AL, the clinical score (DLS) appeared to be the best predictor of AL on POD2 and POD3. This score was devised to reduce the delay in the diagnosis of AL and related mortality; it was originally based8 on 11 easily accessible and recordable clinical parameters and on two laboratory tests (a 5 per cent increase in leucocytes or CRP, and a 5 per cent increase in blood urea or creatinine), with scores ranging from 0 to 20 (cut‐off 4), and then simplified and revalidated using three clinical parameters and one laboratory test (CRP levels above 250 mg/l), with scores ranging from 0 to 4 (cut‐off 1), maintaining the same diagnostic accuracy35. In the present study protocol17, the original DLS was applied.
The addition of PCT to DLS and CRP improved both the cumulative PPV and NPV of tests for the presence or absence of AL. However, PCT was the best predictor of mortality on POD2 (AUC 0·829; cut‐off 1·01 μg/l; NPV 0·997), confirming its role in patients with ongoing sepsis36. Although previous studies14, 15 suggested that PCT should not be used routinely as a biochemical marker for early diagnosis of AL after elective colorectal surgery, owing to its poor cost‐effectiveness, the combined use of DLS, CRP and PCT on POD2 and POD3 may be useful to predict or exclude patients with clinically severe AL and potentially uncontrolled sepsis and death.
Collaborators
iCral Study Group members: M. Catarci (study coordinator; General Surgery Unit, C. e G. Mazzoni Hospital, Ascoli Piceno); G. Ruffo (General Surgery Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Sacro Cuore Don Calabria Hospital, Negrar di Valpolicella); F. Borghi (Department of Surgery, Santa Croce e Carle Hospital, Cuneo); A. Patriti (Department of Surgery, Marche Nord Hospital, Pesaro e Fano); P. Delrio (Colorectal Surgical Oncology, IRCCS G. Pascale Foundation, Naples); M. Scatizzi (General and Oncological Surgery Unit, Santo Stefano Hospital, Prato); S. Mancini (General and Oncological Surgery Unit, San Filippo Neri Hospital, Rome); G. Garulli (General Surgery Unit, Ceccarini Hospital, Riccione); A. Carrara (Department of Surgery, Santa Chiara Hospital, Trento); F. Pirozzi (Abdominal Surgery Unit, IRCCS Casa Sollievo della Sofferenza Foundation, San Giovanni Rotondo); S. Scabini (General and Oncological Surgery Unit, National Cancer Centre San Martino, Genoa); A. Liverani (General Surgery Unit, Regina Apostolorum Hospital, Albano Laziale); G. Baiocchi (General Surgery Unit 3, University and Spedali Civili of Brescia, Brescia); R. Campagnacci (Department of Surgery, C. Urbani Hospital, Jesi); A. Muratore (General Surgery Unit, E. Agnelli Hospital, Pinerolo); G. Longo (General Surgery Unit, Policlinico Casilino, Rome); M. Caricato (Geriatric Surgery Unit, Policlinico Campus BioMedico, Rome); R. Macarone Palmieri (General and Oncological Surgery Unit, Belcolle Hospital, Viterbo); N. Vettoretto (General Surgery Unit, Spedali Civili of Brescia, Montichiari); P. Ciano (General Surgery Unit, C. e G. Mazzoni Hospital, Ascoli Piceno); E. Bertocchi (General Surgery Unit, IRCCS Sacro Cuore Don Calabria Hospital, Negrar di Valpolicella); D. Cianflocca (Department of Surgery, Santa Croce e Carle Hospital, Cuneo); M. Lambertini (Department of Surgery, Marche Nord Hospital, Pesaro e Fano); U. Pace (Colorectal Surgical Oncology, IRCCS G. Pascale Foundation, Naples); M. Baraghini (General and Oncological Surgery Unit, Santo Stefano Hospital, Prato); R. Angeloni (General and Oncological Surgery Unit, San Filippo Neri Hospital, Rome); A. Lucchi (General Surgery Unit, Ceccarini Hospital, Riccione); G. Tirone (Department of Surgery, Santa Chiara Hospital, Trento); A. Sciuto (Abdominal Surgery Unit, IRCCS Casa Sollievo della Sofferenza Foundation, San Giovanni Rotondo); A. Martino (General and Oncological Surgery Unit, National Cancer Centre San Martino, Genoa); T. di Cesare (General Surgery Unit, Regina Apostolorum Hospital, Albano Laziale); S. Molfino (General Surgery Unit 3, University and Spedali Civili of Brescia, Brescia); A. Maurizi (Department of Surgery, C. Urbani Hospital, Jesi); P. Marsanic (General Surgery Unit, E. Agnelli Hospital, Pinerolo); F. Tomassini (General Surgery Unit, Policlinico Casilino, Rome); G. T. Capolupo (Geriatric Surgery Unit, Policlinico Campus BioMedico, Rome); P. Amodio (General and Oncological Surgery Unit, Belcolle Hospital, Viterbo); E. Arici (General Surgery Unit, Spedali Civili of Brescia, Montichiari); B. Ruggeri (Clinical Governance Unit, C. e G. Mazzoni Hospital, Ascoli Piceno); and G. Guercioni (General Surgery Unit, C. e G. Mazzoni Hospital, Ascoli Piceno).
Acknowledgements
Medtronic SI® Italy provided liberal and unconditional economic support for the organization of three investigator meetings, held in Milan, Italy, in September 2017 and June 2018, and in Rome, Italy, in October 2018.
Disclosure: The authors declare no conflict of interest.
Funding information
No funding
Contributor Information
The Italian ColoRectal Anastomotic Leakage (iCral) Study Group, Email: marco.catarci@sanita.marche.it.
The Italian ColoRectal Anastomotic Leakage (iCral) Study Group:
M. Catarci, G. Ruffo, F. Borghi, A. Patriti, P. Delrio, M. Scatizzi, S. Mancini, G. Garulli, A. Carrara, F. Pirozzi, S. Scabini, A. Liverani, G. Baiocchi, R. Campagnacci, A. Muratore, G. Longo, M. Caricato, R. Macarone Palmieri, N. Vettoretto, P. Ciano, E. Bertocchi, D. Cianflocca, M. Lambertini, U. Pace, M. Baraghini, R. Angeloni, A. Lucchi, G. Tirone, A. Sciuto, A. Martino, T. di Cesare, S. Molfino, A. Maurizi, F. Tomassini, G. T. Capolupo, P. Amodio, E. Arici, B. Ruggeri, and G. Guercioni
References
- 1. Boushey R, Williams LJ. Management of Anastomotic Complications of Colorectal Surgery; 2018. https://www.uptodate.com/contents/management-of-anastomotic-complications-of-colorectal-surgery [accessed 11 September 2018]. [Google Scholar]
- 2. Slieker JC, Komen N, Mannaerts GH, Karsten TM, Willemsen P, Murawska M et al Long‐term and perioperative corticosteroids in anastomotic leakage: a prospective study of 259 left‐sided colorectal anastomoses. Arch Surg 2012; 147: 447–452. [DOI] [PubMed] [Google Scholar]
- 3. Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 2009; 208: 269–278. [DOI] [PubMed] [Google Scholar]
- 4. Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it's later than you think. Ann Surg 2007; 245: 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruce J, Krukowski ZH, Al‐Khairy G, Russell EM, Park KG. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001; 88: 1157–1168. [DOI] [PubMed] [Google Scholar]
- 6. Russ A, Kennedy GD. Postoperative complications In ASCRS Textbook of Colon and Rectal Surgery (3rd edn), Steele SR, Hull TL, Read TE, Saclarides TJ, Senagore AJ, Whitlow CB. (eds). Springer: New York, 2016; 121–140. [Google Scholar]
- 7. Platell C, Barwood N, Dorfmann G, Makin G. The incidence of anastomotic leaks in patients undergoing colorectal surgery. Colorectal Dis 2007; 9: 71–79. [DOI] [PubMed] [Google Scholar]
- 8. den Dulk M, Noter SL, Hendriks ER, Brouwers MA, van der Vlies CH, Oostenbroek RJ et al Improved diagnosis and treatment of anastomotic leakage after colorectal surgery. Eur J Surg Oncol 2009; 35: 420–426. [DOI] [PubMed] [Google Scholar]
- 9. Giaccaglia V, Salvi PF, Cunsolo GV, Sparagna A, Antonelli MS, Nigri G et al Procalcitonin, as an early biomarker of colorectal anastomotic leak, facilitates enhanced recovery after surgery. J Crit Care 2014; 29: 528–532. [DOI] [PubMed] [Google Scholar]
- 10. Ortega‐Deballon P, Radais F, Facy O, d'Athis P, Masson D, Charles PE et al C‐reactive protein is an early predictor of septic complications after elective colorectal surgery. World J Surg 2010; 34: 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oberhofer D, Juras J, Pavicić AM, Rancić Zurić I, Rumenjak V. Comparison of C‐reactive protein and procalcitonin as predictors of postoperative infectious complications after elective colorectal surgery. Croat Med J 2012; 53: 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giaccaglia V, Salvi PF, Antonelli MS, Nigri G, Pirozzi F, Casagranda B et al Procalcitonin reveals early dehiscence in colorectal surgery: the PREDICS study. Ann Surg 2016; 263: 967–972. [DOI] [PubMed] [Google Scholar]
- 13. Garcia‐Granero A, Frasson M, Flor‐Lorente B, Blanco F, Puga R, Carratalá A et al Procalcitonin and C‐reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis Colon Rectum 2013; 56: 475–483. [DOI] [PubMed] [Google Scholar]
- 14. Facy O, Paquette B, Orry D, Binquet C, Masson D, Bouvier A et al; IMACORS Study. Diagnostic accuracy of inflammatory markers as early predictors of infection after elective colorectal surgery: results from the IMACORS study. Ann Surg 2016; 263: 961–966. [DOI] [PubMed] [Google Scholar]
- 15. Cousin F, Ortega‐Deballon P, Bourredjem A, Doussot A, Giaccaglia V, Fournel I. Diagnostic accuracy of procalcitonin and C‐reactive protein for the early diagnosis of intra‐abdominal infection after elective colorectal surgery: a meta‐analysis. Ann Surg 2016; 264: 252–256. [DOI] [PubMed] [Google Scholar]
- 16. Martin G, Dupré A, Mulliez A, Prunel F, Slim K, Pezet D. Validation of a score for the early diagnosis of anastomotic leakage following elective colorectal surgery. J Visc Surg 2015; 152: 5–10. [DOI] [PubMed] [Google Scholar]
- 17. Benedetti M, Pergolini I, Ciano P, Ciotti S, Guercioni G, Ruffo G et al Early diagnosis of anastomotic leakage after colorectal surgery by the Dutch leakage score, serum procalcitonin and serum C‐reactive protein: study protocol of a prospective multicentre observational study by the Italian ColoRectal Anastomotic Leakage (iCral) study group. G Chir 2019; 40: 20–25. [PubMed] [Google Scholar]
- 18. Italian ColoRectal Anastomotic Leakage (iCral) study group . Colorectal surgery in Italy: a snapshot from the iCral study group. Updates Surg 2019; 71: 339–347. [DOI] [PubMed] [Google Scholar]
- 19. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al The Clavien–Dindo classification of surgical complications: five‐year experience. Ann Surg 2009; 250: 187–196. [DOI] [PubMed] [Google Scholar]
- 21. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992; 13: 606–608. [PubMed] [Google Scholar]
- 22. Bakker IS, Grossmann I, Henneman D, Havenga K, Wiggers T. Risk factors for anastomotic leakage and leak‐related mortality after colonic cancer surgery in a nationwide audit. Br J Surg 2014; 101: 424–432. [DOI] [PubMed] [Google Scholar]
- 23. Choi HK, Law WL, Ho JW. Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: analysis of risk factors. Dis Colon Rectum 2006; 49: 1719–1725. [DOI] [PubMed] [Google Scholar]
- 24. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 2010; 5: 1315–1316. [DOI] [PubMed] [Google Scholar]
- 25. Sciuto A, Merola G, De Palma GD, Sodo M, Pirozzi F, Bracale UM et al Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol 2018; 24: 2247–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agenzia Nazionale per i Servizi Sanitari Regionali Piano Nazionale Esiti Ed; 2017. http://pne2017.agenas.it/ [accessed 7 August 2019].
- 27. Gessler B, Eriksson O, Angenete E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int J Colorectal Dis 2017; 32: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY et al Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg 2013; 257: 665–671. [DOI] [PubMed] [Google Scholar]
- 29. Frasson M, Flor‐Lorente B, Rodríguez JL, Granero‐Castro P, Hervás D, Alvarez Rico MA et al; ANACO Study Group. Risk factors for anastomotic leak after colon resection for cancer: multivariate analysis and nomogram from a multicentric, prospective, national study with 3193 patients. Ann Surg 2015; 262: 321–330. [DOI] [PubMed] [Google Scholar]
- 30. Midura EF, Hanseman D, Davis BR, Atkinson SJ, Abbott DE, Shah SA et al Risk factors and consequences of anastomotic leak after colectomy: a national analysis. Dis Colon Rectum 2015; 58: 333–338. [DOI] [PubMed] [Google Scholar]
- 31. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg 2017; 152: 292–298. [DOI] [PubMed] [Google Scholar]
- 32. Braga M, Beretta L, Pecorelli N, Maspero M, Casiraghi U, Borghi F et al; PeriOperative Italian Society Group. Enhanced recovery pathway in elderly patients undergoing colorectal surgery: is there an effect of increasing ages? Results from the perioperative Italian Society Registry. Updates Surg 2018; 70: 7–13. [DOI] [PubMed] [Google Scholar]
- 33. Barbić J, Ivić D, Alkhamis T, Drenjancević D, Ivić J, Harsanji‐Drenjancević I et al Kinetics of changes in serum concentrations of procalcitonin, interleukin‐6, and C‐ reactive protein after elective abdominal surgery. Can it be used to detect postoperative complications? Coll Antropol 2013; 37: 195–201. [PubMed] [Google Scholar]
- 34. Er S, Özden S, Koca F, Yıldız BD, Yüksel BC, Tez M. External validation of anastomotic leakage risk analysis system in patients who underwent colorectal resection. Turk J Med Sci 2019; 49: 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. den Dulk M, Witvliet MJ, Kortram K, Neijenhuis PA, de Hingh IH, Engel AF et al The DULK (Dutch leakage) and modified DULK score compared: actively seek the leak. Colorectal Dis 2013; 15: e528–e533. [DOI] [PubMed] [Google Scholar]
- 36. Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta‐analysis. PLoS One 2015; 10: e0129450. [DOI] [PMC free article] [PubMed] [Google Scholar]


