Abstract
Background
Incisional hernia is a frequent complication after abdominal surgery. The aim of this study was to assess the efficacy of prophylactic mesh reinforcement (PMR) after midline laparotomy in reducing the incidence of incisional hernia.
Methods
A meta‐analysis was conducted following PRISMA guidelines. The primary outcome was the incidence of incisional hernia after follow‐up of at least 12 months. Secondary outcomes were postoperative complications. Only RCTs were included. A random‐effects model was used for the meta‐analysis, and trial sequential analysis was conducted.
Results
Twelve RCTs were included, comprising 1815 patients. The incidence of incisional hernia was significantly lower after PMR compared with sutured closure (risk ratio (RR) 0·35, 95 per cent c.i. 0·21 to 0·57; P < 0·001). Both onlay (RR 0·26, 0·11 to 0·67; P = 0·005) and retromuscular (RR 0·28, 0·10 to 0·82; P = 0·02) PMR led to a significant reduction in the rate of incisional hernia. The occurrence of seroma was higher in patients who had onlay PMR (RR 2·23, 1·10 to 4·52; P = 0·03). PMR did not result in an increased rate of surgical‐site infection.
Conclusion
PMR of a midline laparotomy using an onlay or retromuscular technique leads to a significant reduction in the rate of incisional hernia in high‐risk patients. Individual risk factors should be taken into account to select patients who will benefit most.
[Correction added on 19 February 2020, after first online publication: J. García Alamino has been amended to J. M. Garcia‐Alamino]
Prophylactic mesh reinforcement of a midline laparotomy leads to a significant reduction in the rate of incisional hernia in high‐risk patients, for both onlay and retromuscular mesh reinforcement.

Mesh high risk patients
Antecedentes
La eventración (hernia incisional) es una complicación frecuente de la cirugía abdominal. El objetivo es evaluar la eficacia de la inserción de una malla profiláctica de refuerzo (prophylactic mesh reinforcement, PMR) después de la laparotomía media para reducir la incidencia de eventración.
Métodos
Se realizó un metaanálisis siguiendo las recomendaciones PRISMA. La variable principal fue la incidencia de eventración después de un seguimiento mínimo de 12 meses. Las variables secundarias fueron las complicaciones postoperatorias. Solo se incluyeron ensayos controlados aleatorizados. Se utilizó un modelo de efectos aleatorios para el metaanálisis y se realizó un análisis secuencial de los ensayos.
Resultados
Se incluyeron 12 ensayos aleatorizados y controlados con 1.815 pacientes. La incidencia de eventración fue significativamente menor después de la PMR en comparación con el cierre simple (riesgo relativo, RR 0,35; i.c. del 95%: 0,21‐0,57, P < 0,0001). Hubo una reducción significativa de la tasa de eventración tanto si la PMR se colocó en posición supra‐aponeurótica (RR 0,26; i.c. del 95% 0,11‐0,67, P = 0,005) como retromuscular (RR 0,28; i.c. del 95% 0,0‐0,82, P = 0,02). La aparición de seromas fue mayor en los pacientes con RPM supra‐aponeurótica (RR 2,23; i.c. del 95% 1,10‐4,52, P = 0,03). La PMR no conllevó una mayor tasa de infecciones de la herida quirúrgica.
Conclusión
Una PMR en una laparotomía de la línea media, tanto en posición supra‐aponeurótica como retromuscular, reduce de forma significativa el desarrollo de eventraciones en pacientes de alto riesgo. Se deben considerar los factores de riesgo individuales para seleccionar a los pacientes que más puedan beneficiarse.
Introduction
Incisional hernia (IH) is a frequent complication after abdominal surgery, with an incidence ranging from 11 to 20 per cent in general surgical populations1, 2, 3, 4. The incidence of IH can increase up to 40 per cent in high‐risk groups, such as patients with an abdominal aortic aneurysm (AAA) or morbid obesity5, 6, 7, 8, 9, 10, 11, 12. IH may be asymptomatic, but can also lead to serious and potentially fatal complications, such as incarceration and strangulation of bowel. Furthermore, IH has a high impact on patients' quality of life and body image13, 14, and treatment of IH represents a financial burden on the healthcare system15.
Current treatment of IH is mesh repair, which has led to a lower recurrence rate compared with the primary suture technique16. However, the recurrence rate is still high, even when mesh is used. A Danish nationwide registry study17 reported a cumulative recurrence rate of 37 per cent at 3 years after IH repair. Currently, there is no definitive solution for the high recurrence rates and complications related to these recurrences. Prevention is therefore of paramount importance18. In the past few years, several studies on the prevention of IH with prophylactic mesh reinforcement (PMR) have been conducted. Most RCTs included a limited number of patients. Different surgical techniques of PMR, including mesh placement in onlay, retromuscular or intraperitoneal position, have been studied. The European Hernia Society (EHS) Guidelines Development Group19 on the closure of abdominal wall incisions made a recommendation in 2015: that PMR to reduce the incidence of IH after elective midline laparotomy in a high‐risk patient be suggested with a weak recommendation. The group also stated that larger trials were needed to make a strong recommendation.
Since the publication of these EHS guidelines, three meta‐analyses20, 21, 22 have been published, together with the long‐term data from the largest multicentre RCT on IH prevention after midline laparotomy, comparing PMR with primary suture (the PRIMA trial)23. However, in one of these meta‐analyses20, RCTs and observational studies were mixed. In the meta‐analysis of Wang and colleagues21, some studies on non‐midline incisions were included and none of the meta‐analyses included long‐term data from the PRIMA trial.
The aim of this meta‐analysis was to assess the safety and efficacy of PMR, both onlay and retromuscular, in reducing the IH incidence after elective midline laparotomy.
Methods
A meta‐analysis was conducted and reported following the PRISMA guidelines24. This meta‐analysis was registered prospectively at the Prospero database on 5 November 2015 (CRD42015027079) with the acronym MARIA review. The meta‐analysis was finalized after publication of the final results of the PRIMA trial on 19 June 2017.
Information sources and search terms
A systematic computerized literature search was performed until 1 January 2017, using 12 databases: Embase, MEDLINE, Web of Science, SCOPUS, Cochrane Library, CINAHL, PubMed Publisher, LILACS (Latin American and Caribbean Literature on Health Sciences), SciELO (Scientific Electronic Library Online), ScienceDirect, ProQuest and Google Scholar. The Biomedical Information Specialist of the Medical Library (Erasmus University Medical Centre, Rotterdam, the Netherlands) prepared the search strategy. The syntax with search terms is shown in Appendix S1 (supporting information).
Study selection, data extraction and quality assessment
Three reviewers independently screened all records by title and abstract for eligibility. After this first screening, the full text of records was assessed. Only eligible RCTs were included. The methodological quality of RCTs was assessed using SIGN (Scottish Intercollegiate Guidelines Network) checklists. Risk‐of‐bias assessment was done with the Cochrane Collaboration tool25, in which the following aspects are assessed: random sequence generation, allocation concealment, blinding of patients, personnel or outcome assessors, incomplete outcome data and selective reporting. Assessment of both methodological quality and risk of bias was performed by three independent reviewers. Studies were assessed as having either a low or high risk of bias.
RCTs were included if they met the following inclusion criteria: patients aged at least 18 years, undergoing midline laparotomy, for all types of indication, with all types of mesh and all types of mesh position, and follow‐up of at least 12 months. The primary outcome was the incidence of IH. Secondary outcomes were postoperative complications: seroma, surgical‐site infection (SSI), haematoma and burst abdomen. No language restrictions were used.
All required data were extracted and collected in a standard manner by at least two authors independently. Any disagreements during the data extraction phase were resolved through discussion and by consulting a third investigator. A summary‐of‐findings table was created, in which the following information was collected: study characteristics (title, year of publication, study design, number of included patients), indication for midline laparotomy, description of intervention and description of the compared intervention (‘control group’), type of mesh used, mesh placement, length of follow‐up and outcome measurements. When a paper included data for different mesh positions, the data for these were described separately per group in the summary‐of‐findings table. For duplicate data reported by the same author(s), the article with the longest follow‐up period was selected.
Statistical analysis
A meta‐analysis, pooling the results of the retrieved studies, was performed. A sensitivity analysis was conducted to reduce the risk of possible bias for primary and secondary outcomes. Meta‐analyses that combined other subgroups (mesh position) were also performed. A random‐effects model was used and presented as risk ratios (RRs) with 95 per cent confidence intervals. Effects were considered statistically significant if the 95 per cent c.i. of the overall effect estimate did not overlap. The I 2 statistic was used to assess heterogeneity. Groups with zero events were adjusted with a constant continuity adjustment of 0·5 in each arm (as per the default adjustment in the software used). Publication bias was assessed by a funnel plot. Analyses were performed using Review Manager software (RevMan version 5.3; The Nordic Cochrane Centre, Copenhagen, Denmark). Two‐sided P < 0·050 was considered statistically significant.
Conducting a meta‐analysis can lead to type I errors (false‐positives) or overestimation of treatment effects due to systematic errors (bias) and random errors (play of change). To avoid this, trial sequential analysis (TSA) was conducted. TSA can provide a required information size (RIS). The RIS is the required number of patients that needs to be included in the meta‐analysis to provide firm evidence26, 27. Control event rate (CER) and relative risk reduction (RRR) values were calculated. CER is the proportion of participants in the control group who have the outcome. RRR can be interpreted as the reduction in the relative risk of the specified outcome in the treatment group, compared with the control group. TSA was planned for all retrieved studies, and for the group of studies with low risk of bias. TSA was performed using the TSA software v0.9 (http://www.ctu.dk/tsa/downloads.aspx).
Results
A total of 1498 records were identified after removal of duplicates. After screening of title and abstract, 49 articles were found relevant for full‐text assessment. After full‐text assessment, 29 articles were excluded, leaving 13 RCTs and seven non‐randomized trials for the qualitative synthesis (Fig. 1 ). In total, 13 RCTs5, 6, 7, 8, 9, 10, 11, 23, 28, 29, 30, 31, 32 fulfilled the inclusion criteria, but the study of Timmermans and colleagues32 was excluded because only the short‐term results (postoperative complications in the first month) were discussed; thus 12 RCTs were analysed. Six studies7, 8, 11, 23, 29, 31 were considered to have a low risk of bias, and six5, 6, 9, 10, 28, 30 to have a high risk of bias (Table 1 ).
Figure 1.

PRISMA diagram for the review
Table 1.
Risk‐of‐bias assessment for prevention of incisional hernia with prophylactic mesh reinforcement in midline laparotomy
| Reference | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) |
|---|---|---|---|---|---|---|
| Abo‐Ryia et al.9 | − | − | − | − | + | |
| Bali et al.10 | − | − | − | − | + | + |
| Bevis et al.8 | + | + | + | + | + | + |
| Caro‐Tarrago et al.29 | + | + | + | + | ||
| El‐Khadrawy et al.28 | − | − | − | − | + | |
| García‐Ureña et al.31 | + | + | + | + | + | |
| Gutiérrez de la Peña et al.6 | − | − | − | − | + | |
| Jairam et al.23 | + | + | + | + | + | |
| Muysoms et al.11 | + | + | + | + | + | |
| Pans et al.5 | − | − | − | − | + | |
| Sarr et al.30 | + | − | − | − | − | + |
| Strzelczyk et al.7 | + | + | + | + |
+, Low risk of bias; −, high risk of bias.
The 12 RCTs comprised a total of 1815 patients. Study and patient characteristics are shown in Table 2 . Inclusion criteria for PMR of the midline laparotomy in the individual RCTs were either the presence of an AAA8, 10, 11, 23, morbid obesity5, 7, 9, 30, colorectal cancer surgery31 or a mixture of operative indications6, 28, 29. Most studies placed a polypropylene mesh in an onlay6, 10, 23, 29, 31 or retromuscular7, 8, 11, 23 position. Two studies10, 30 used biological meshes, and one5 used a rapidly absorbable intraperitoneal mesh.
Table 2.
Summary of findings for included studies on the prevention of incisional hernia with prophylactic mesh reinforcement in midline laparotomies
| Reference | n | Indication for midline laparotomy | Type of mesh | Mesh position | Follow‐up (months) | Outcome measurements | Diagnosis of IH (clinical/radiological) |
|---|---|---|---|---|---|---|---|
| Pans et al.5 | 288 | Morbid obesity | Polyglactin | Intraperitoneal | 29·8 | IH, postoperative morbidity | Not reported |
| Gutiérrez de la Peña et al.6 | 88 | Colorectal cancer, gastric cancer, cholelithiasis, diverticulosis, Crohn's disease, pancreatic cystadenoma, gastric ulcer, cancer of small intestine | Polypropylene | Onlay | 36 | IH, haematoma, seroma, infection, pain | Clinical; if not conclusive, CT |
| Strzelczyk et al.7 | 74 | Gastric bypass surgery | Polypropylene | Retromuscular | 28 | IH, wound leak, bleeding, other surgical complication | Clinical plus ultrasound imaging |
| El‐Khadrawy et al.28 | 40 | High risk | Polypropylene | Preperitoneal | 36·7 | IH, seroma | Clinical |
| SSI, wound disruption, chronic wound pain, cardiac, pulmonary problems, DVT, ascites | |||||||
| Bevis et al.8 | 80 | AAA | Polypropylene | Retromuscular | Mesh 30·2 | IH, wound infection, hernia operation | Clinical; if doubt, ultrasound imaging |
| No mesh 19·6 | |||||||
| Abo‐Ryia et al.9 | 64 | Open bariatric surgery | Polypropylene | Preperitoneal | Mesh 48 | Safety and efficacy of preperitoneal prosthetic enforcement, seroma, infection, partial dehiscence | Clinical; ultrasound imaging in suspected cases |
| No mesh 49 | |||||||
| Caro‐Tarrago et al.29 | 160 | Colorectal and general surgery | Polypropylene | Onlay | Mesh 14·8 | IH, all adverse events, postoperative complications | Clinical and CT |
| No mesh 12·5 | |||||||
| Sarr et al.30 | 280 | Open RYGB | Biological | Intraperitoneal | 24 | IH, wound infection, wound dehiscence, wound sinus tract, wound erythema, seroma | Clinical, phone call, primary care physician |
| Bali et al.10 | 40 | Open AAA | Biological | Onlay | 36 | IH, duration of surgery, postoperative complications, reoperation rate | Clinical and CT |
| García‐Ureña et al.31 | 107 | Colorectal surgery | Polypropylene | Onlay | 24 | IH, incidence of local complications: SSI, seroma, evisceration, mesh rejection | Clinical and CT |
| Systemic complications | |||||||
| Muysoms et al.11 | 114 | AAA and ASA grade < IV | Partially absorbable polypropylene | Retromuscular | 24 | IH | Clinical and, if available, ultrasound imaging or CT |
| Postoperative complications | |||||||
| SSI | |||||||
| Duration of surgery | |||||||
| Jairam et al.23 | 480 | Open AAA surgery or midline laparotomy in patients with BMI > 27 kg/m2 | Polypropylene | Onlay (188 patients) | 24 | IH, postoperative complications | Clinical and ultrasound imaging or CT |
| Quality of life | |||||||
| Cost‐effectiveness | |||||||
| Retromuscular (185 patients) |
IH, incisional hernia; SSI, surgical‐site infection; DVT, deep vein thrombosis; AAA, abdominal aortic aneurysm; RYGB, Roux‐en‐Y gastric banding.
Outcome measurements
The primary outcome was the incidence of IH after follow‐up of at least 12 months. Twelve RCTs were included in the overall quantitative analysis for the primary outcome and publication bias33 was evaluated. The meta‐analysis showed a significant reduction of IH in patients with PMR compared with that in patients who had a primary suture (RR 0·35; P < 0·001) (Fig. 2 a). The funnel plot was slightly asymmetrical, indicating a possible publication bias for studies favouring mesh prophylaxis (Fig. 3 ). Analysis of the primary outcome for studies with a low risk of bias (6 RCTs) showed that the occurrence of IH was significantly lower (RR 0·23; P < 0·001) in the PMR group than in the primary suture group (Fig. 2 b). Both onlay and retromuscular PMR led to a significant reduction of the IH incidence compared with primary suture, with RRs of 0·26 (P = 0·005) and 0·28 (P = 0·02) respectively (Fig. 4 ).
Figure 2.
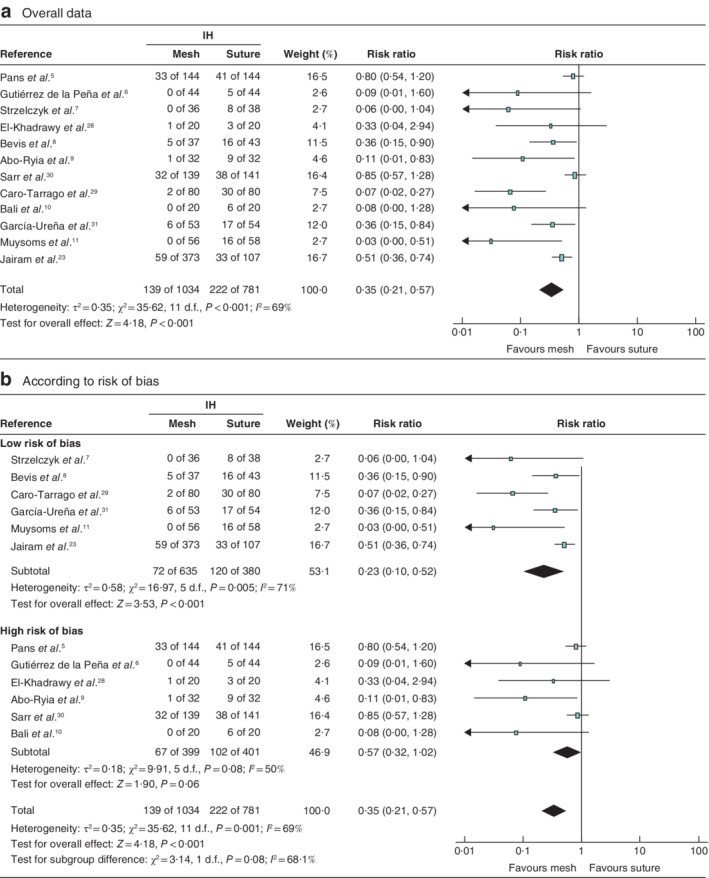
Forest plots of incisional hernia after prophylactic mesh reinforcement of a midline laparotomy versus primary suture a Overall data from all 12 included studies; b data for studies with a low or high risk of bias. A Mantel–Haenszel random‐effects model was used for meta‐analysis. Risk ratios are shown with 95 per cent confidence intervals. IH, incisional hernia.
Figure 3.

Funnel plot for low and high risk of bias studies RR, risk ratio.
Figure 4.
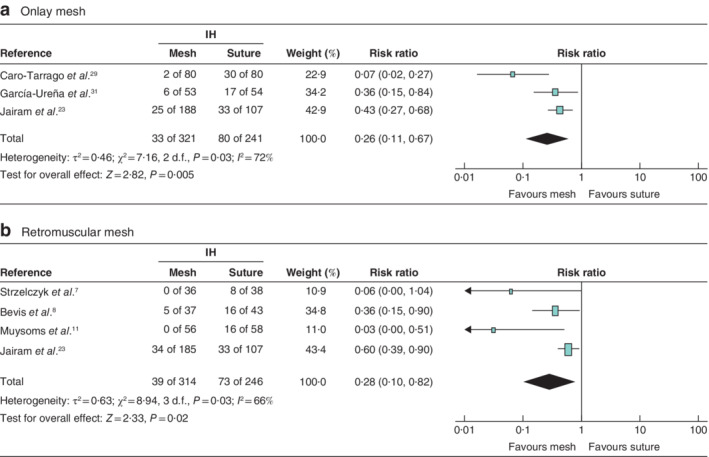
Forest plots of incisional hernia after prophylactic mesh reinforcement of a midline laparotomy versus primary suture a Data from studies using onlay mesh reinforcement; b data from studies using retromuscular mesh reinforcement. A Mantel–Haenszel random‐effects model was used for meta‐analysis. Risk ratios are shown with 95 per cent confidence intervals. IH, incisional hernia.
TSA (for the primary outcome) was done for all 12 included studies. The CER proportion was 28 per cent, the RRR was 65 per cent, and a constant continuity adjustment was set at 0·5 events per group. The accrued information size (n = 1815) was 273·3 per cent of the estimated RIS (n = 664). This means that firm evidence was available. For low‐risk‐of‐bias studies (6 RCTs), the CER proportion was 31 per cent and RRR 77 per cent. The accrued information size (n = 1015) was 283·5 per cent of the estimated RIS (n = 358) (Fig. 5 ).
Figure 5.

Trial sequential analysis curves of the incidence of incisional hernia after prophylactic mesh reinforcement of a midline laparotomy versus primary suture
a Overall data from all 12 included studies; b data from studies with a low risk of bias. a,b Both graphs are two‐sided. RIS, required information size.
Secondary outcomes were analysed for the low‐risk‐of‐bias studies (6 RCTs). Patients with an onlay PMR had a higher risk of developing seroma (RR 2·23; P = 0·030) compared with patients who had a primary suture. Patients who had a retromuscular PMR had no greater chance of developing seroma than those with a primary suture (RR 1·67; P = 0·17) (Fig. 6 ). The occurrence of SSI was not significantly higher for onlay PMR (RR 0·82; P = 0·33) or retromuscular PMR (RR 0·85; P = 0·55) than for primary suture (Fig. 7 ). There were insufficient data to analyse the incidence of haematoma and burst abdomen.
Figure 6.
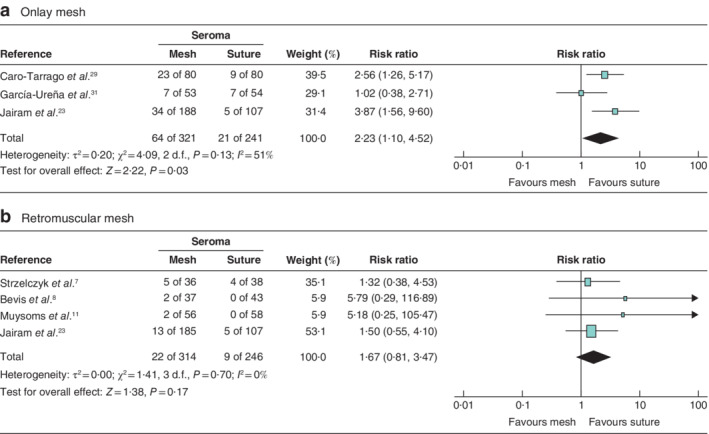
Forest plots of postoperative seroma after prophylactic mesh reinforcement of a midline laparotomy versus primary suture a Data from studies using onlay mesh reinforcement; b data from studies using retromuscular mesh reinforcement. A Mantel–Haenszel random‐effects model was used for meta‐analysis. Risk ratios are shown with 95 per cent confidence intervals.
Figure 7.
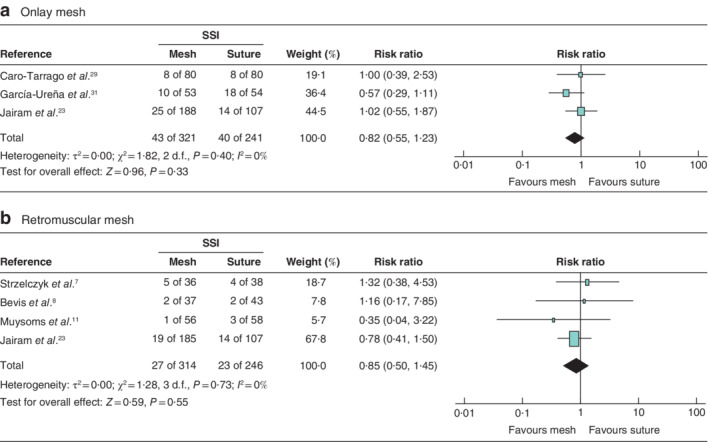
Forest plots of surgical‐site infection after prophylactic mesh reinforcement of a midline laparotomy versus primary suture a Data from studies using onlay mesh reinforcement; b data from studies using retromuscular mesh reinforcement. A Mantel–Haenszel random‐effects model was used for meta‐analysis. Risk ratios are shown with 95 per cent confidence intervals. SSI, surgical‐site infection.
Discussion
This meta‐analysis has shown that the use of PMR in patients undergoing midline laparotomy leads to a significantly lower occurrence of IH compared with a primary suture closure, with TSA indicating that the evidence was firm. This significant effect was shown for both onlay and retromuscular PMR. PMR was found to be safe, with no increase in SSI, but an increased risk of seroma formation for onlay PMR only.
This meta‐analysis has a few limitations. Substantial statistical heterogeneity was seen in the studies regarding the primary outcome (I 2 = 71 per cent). This probably reflects the variability of surgical technique and methodological approach across studies.
Although the technique of PMR in the treatment arm of the RCTs is often well described, there is less information on the control group undergoing primary suture. Protocols often describe the use of a suture : wound length ratio (SL : WL) greater than 4 : 1, but data for the SL : WL ratio were recorded and reported in only a few studies11, so that adherence to the optimal primary suture technique was unclear in most studies. Moreover, the short‐stitch technique, which is currently considered the best evidenced technique with the lowest incidence of IH3, was not used in any of the RCTs in this meta‐analysis. Therefore, it could be argued that the treatment effect of PMR is increased because of a suboptimal suturing technique in the control groups.
Of the 12 RCTs included in this meta‐analysis, half were considered to have a high risk of bias. Owing to the use of sensitivity analysis, however, the treatment effect was maintained, and was even greater when only studies with a low risk of bias were analysed.
Only RCTs with a minimum follow‐up of 12 months were included in the meta‐analysis, with all but one29 having follow‐up of at least 24 months. This is still too short, however, to evaluate the long‐term efficacy or potential late adverse effects of PMR.
In most studies, physical examination was used to detect IH during follow‐up. Some added selective or systematic medical imaging for the evaluation of IH, using either ultrasound imaging or CT. Imaging will increase the number of patients diagnosed with IH by detection of subclinical IHs, and this might overestimate the importance of PMR in providing clinical benefit.
Most of the 12 included studies used a polypropylene mesh in either an onlay or a retromuscular position; two10, 30 used a biological mesh and one5 used an absorbable synthetic mesh. Two of these studies did not show PMR to be effective, and all three were considered to have a high risk of bias. All studies with a low risk of bias used either an onlay or a retromuscular mesh position, and all included trials were performed in an elective surgery setting. The results of this meta‐analysis on PMR can therefore be considered valid only for synthetic, non‐absorbable mesh in either an onlay or a retromuscular position in an elective setting.
The slightly asymmetrical funnel plot for IH indicates possible publication bias towards studies that favour PMR, resulting in an overestimation of the underlying beneficial effect of the intervention.
Most studies included only patients considered at high risk for developing an IH. It is difficult to identify the individual risk factors whereby patients would benefit from PMR. The guidelines on the closure of abdominal wall incisions from the EHS19 state that the evidence is weak for the use of PMR in patients at high risk of IH development. Such a guideline could be implemented only if the EHS Guidelines Development Group also described the exact criteria for considering patients to be at high risk. From this perspective, the study performed by Fischer et al.18 is interesting, as these authors stratified patients into four IH risk groups (low, moderate, high, extreme), based on characteristics of the patient and the surgical procedure. From this meta‐analysis, it seems clear that patients undergoing AAA repair and those having bariatric surgery through a midline incision will benefit from PMR, although these are now less common as many patients with an AAA are treated with endovascular procedures, and bariatric surgery is often performed by a laparoscopic approach.
Selection based on patient characteristics needs cut‐off values. The appropriate cut‐off value for BMI is unclear. The PRIMA trial23 used a BMI of at least 27 kg/m2 as an inclusion criterion for PMR, but further research is needed to identify an appropriate BMI cut‐off point at which the risk of developing IH increases. Data from large clinically oriented prospective registries34 might be helpful to explore which factors might lead to an increased risk of IH.
PMR can be considered safe for elective laparotomy. The only adverse event detected in this meta‐analysis was an increased rate of seroma formation after onlay PMR, related to the subcutaneous dissection required. However, even though the occurrence of seroma was higher in the onlay PMR group, there was no increase in SSI. There were insufficient data to analyse the number of other adverse events, such as haematoma or burst abdomen.
One of the main strengths of this meta‐analysis is the fact that RCTs with a low risk of bias were analysed separately35. Further, the most up‐to‐date RCTs were included23. TSA showed firm evidence in favour of PMR for midline laparotomy in high‐risk patients, suggesting that no further trials are required to address the effects of PMR in this population. RCTs in other patient populations with differing levels of risk would, however, be helpful to evaluate the effectiveness of PMR.
Laparotomies are performed by a variety of surgical specialties, such as vascular surgeons, colorectal surgeons, gynaecologists and urologists. Many of those surgeons have little experience in treating abdominal wall hernias with mesh, particularly for retromuscular mesh placement. This meta‐analysis has shown that onlay PMR, which is easier to perform, is also effective, and likely to be more acceptable to these surgeons.
This meta‐analysis has provided evidence in favour of closure of midline laparotomies with PMR in high‐risk patients. Individual risk factors should be taken into account to select patients who will benefit (most) from PMR, which should become standard treatment for high‐risk groups.
Supporting information
Appendix S1. Supporting Information
Acknowledgements
A.P.J. and M.L.‐C. contributed equally to this study.
Disclosure: The authors declare no conflict of interest.
Funding information
No funding
References
- 1. Höer J, Lawong G, Klinge U, Schumpelick V. Factors influencing the development of incisional hernia. A retrospective study of 2983 laparotomy patients over a period of 10 years. Chirurg 2002; 73: 474–480. [DOI] [PubMed] [Google Scholar]
- 2. Mudge M, Hughes LE. Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg 1985; 72: 70–71. [DOI] [PubMed] [Google Scholar]
- 3. Millbourn D, Cengiz Y, Israelsson LA. Effect of stitch length on wound complications after closure of midline incisions: a randomized controlled trial. Arch Surg 2009; 144: 1056–1059. [DOI] [PubMed] [Google Scholar]
- 4. Bosanquet DC, Ansell J, Abdelrahman T, Cornish J, Harries R, Stimpson A et al Systematic review and meta‐regression of factors affecting midline incisional hernia rates: analysis of 14 618 patients. PLoS One 2015; 10: e0138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pans A, Elen P, Dewé W, Desaive C. Long‐term results of polyglactin mesh for the prevention of incisional hernias in obese patients. World J Surg 1998; 22: 479–482. [DOI] [PubMed] [Google Scholar]
- 6. Gutiérrez de la Peña C, Medina Achirica C, Domínguez‐Adame E, Medina Díez J. Primary closure of laparotomies with high risk of incisional hernia using prosthetic material: analysis of usefulness. Hernia 2003; 7: 134–136. [DOI] [PubMed] [Google Scholar]
- 7. Strzelczyk JM, Szymański D, Nowicki ME, Wilczyński W, Gaszynski T, Czupryniak L. Randomized clinical trial of postoperative hernia prophylaxis in open bariatric surgery. Br J Surg 2006; 93: 1347–1350. [DOI] [PubMed] [Google Scholar]
- 8. Bevis PM, Windhaber RA, Lear PA, Poskitt KR, Earnshaw JJ, Mitchell DC. Randomized clinical trial of mesh versus sutured wound closure after open abdominal aortic aneurysm surgery. Br J Surg 2010; 97: 1497–1502. [DOI] [PubMed] [Google Scholar]
- 9. Abo‐Ryia MH, El‐Khadrawy OH, Abd‐Allah HS. Prophylactic preperitoneal mesh placement in open bariatric surgery: a guard against incisional hernia development. Obes Surg 2013; 23: 1571–1574. [DOI] [PubMed] [Google Scholar]
- 10. Bali C, Papakostas J, Georgiou G, Kouvelos G, Avgos S, Arnaoutoglou E et al A comparative study of sutured versus bovine pericardium mesh abdominal closure after open abdominal aortic aneurysm repair. Hernia 2015; 19: 267–271. [DOI] [PubMed] [Google Scholar]
- 11. Muysoms FE, Detry O, Vierendeels T, Huyghe M, Miserez M, Ruppert M et al Prevention of incisional hernias by prophylactic mesh‐augmented reinforcement of midline laparotomies for abdominal aortic aneurysm treatment: a randomized controlled trial. Ann Surg 2016; 263: 638–645. [DOI] [PubMed] [Google Scholar]
- 12. Deerenberg EB, Harlaar JJ, Steyerberg EW, Lont HE, van Doorn HC, Heisterkamp J et al Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double‐blind, multicentre, randomised controlled trial. Lancet 2015; 386: 1254–1260. [DOI] [PubMed] [Google Scholar]
- 13. van Ramshorst GH, Eker HH, Hop WC, Jeekel J, Lange JF. Impact of incisional hernia on health‐related quality of life and body image: a prospective cohort study. Am J Surg 2012; 204: 144–150. [DOI] [PubMed] [Google Scholar]
- 14. van Ramshorst GH, Eker HH, van der Voet JA, Jeekel J, Lange JF. Long‐term outcome study in patients with abdominal wound dehiscence: a comparative study on quality of life, body image, and incisional hernia. J Gastrointest Surg 2013; 17: 1477–1484. [DOI] [PubMed] [Google Scholar]
- 15. Gillion JF, Sanders D, Miserez M, Muysoms F. The economic burden of incisional ventral hernia repair: a multicentric cost analysis. Hernia 2016; 20: 819–830. [DOI] [PubMed] [Google Scholar]
- 16. Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long‐term follow‐up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg 2004; 240: 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Helgstrand F, Rosenberg J, Kehlet H, Strandfelt P, Bisgaard T. Reoperation versus clinical recurrence rate after ventral hernia repair. Ann Surg 2012; 256: 955–958. [DOI] [PubMed] [Google Scholar]
- 18. Fischer JP, Basta MN, Mirzabeigi MN, Bauder AR, Fox JP, Drebin JA et al A risk model and cost analysis of incisional hernia after elective, abdominal surgery based upon 12 373 cases: the case for targeted prophylactic intervention. Ann Surg 2016; 263: 1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muysoms FE, Antoniou SA, Bury K, Campanelli G, Conze J, Cuccurullo D et al; European Hernia Society. European Hernia Society guidelines on the closure of abdominal wall incisions. Hernia 2015; 19: 1–24. [DOI] [PubMed] [Google Scholar]
- 20. Borab ZM, Shakir S, Lanni MA, Tecce MG, MacDonald J, Hope WW et al Does prophylactic mesh placement in elective, midline laparotomy reduce the incidence of incisional hernia? A systematic review and meta‐analysis. Surgery 2017; 161: 1149–1163. [DOI] [PubMed] [Google Scholar]
- 21. Wang XC, Zhang D, Yang ZX, Gan JX, Yin LN. Mesh reinforcement for the prevention of incisional hernia formation: a systematic review and meta‐analysis of randomized controlled trials. J Surg Res 2017; 209: 17–29. [DOI] [PubMed] [Google Scholar]
- 22. Payne R, Aldwinckle J, Ward S. Meta‐analysis of randomised trials comparing the use of prophylactic mesh to standard midline closure in the reduction of incisional herniae. Hernia 2017; 21: 843–853. [DOI] [PubMed] [Google Scholar]
- 23. Jairam AP, Timmermans L, Eker HH, Pierik REGJM, van Klaveren D, Steyerberg EW et al; PRIMA Trialist Group. Prevention of incisional hernia with prophylactic onlay and sublay mesh reinforcement versus primary suture only in midline laparotomies (PRIMA): 2‐year follow‐up of a multicentre, double‐blind, randomised controlled trial. Lancet 2017; 390: 567–576. [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J et al The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis – a simulation study. PLoS One 2011; 6: e2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA) Copenhagen Trial Unit, Centre for Clinical Intervention Research: Copenhagen, 2011.
- 28. El‐Khadrawy OH, Moussa G, Mansour O, Hashish MS. Prophylactic prosthetic reinforcement of midline abdominal incisions in high‐risk patients. Hernia 2009; 13: 267–274. [DOI] [PubMed] [Google Scholar]
- 29. Caro‐Tarrago A, Olona Casas C, Jimenez Salido A, Duque Guilera E, Moreno Fernandez F, Vicente Guillen V. Prevention of incisional hernia in midline laparotomy with an onlay mesh: a randomized clinical trial. World J Surg 2014; 38: 2223–2230. [DOI] [PubMed] [Google Scholar]
- 30. Sarr MG, Hutcher NE, Snyder S, Hodde J, Carmody B. A prospective, randomized, multicenter trial of Surgisis Gold, a biologic prosthetic, as a sublay reinforcement of the fascial closure after open bariatric surgery. Surgery 2014; 156: 902–908. [DOI] [PubMed] [Google Scholar]
- 31. García‐Ureña MÁ, López‐Monclús J, Hernando LA, Montes DM, Valle de Lersundi AR, Pavón CC et al Randomized controlled trial of the use of a large‐pore polypropylene mesh to prevent incisional hernia in colorectal surgery. Ann Surg 2015; 261: 876–881. [DOI] [PubMed] [Google Scholar]
- 32. Timmermans L, Eker HH, Steyerberg EW, Jairam A, de Jong D, Pierik EG et al Short‐term results of a randomized controlled trial comparing primary suture with primary glued mesh augmentation to prevent incisional hernia. Ann Surg 2015; 261: 276–281. [DOI] [PubMed] [Google Scholar]
- 33. Schünemann H, Brozek J, Guyatt G, Oxman A (eds). GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; 2013. http://gdt.guidelinedevelopment.org/app/handbook/handbook.html [accessed 1 July 2017].
- 34. Sedrakyan A, Campbell B, Graves S, Cronenwett JL. Surgical registries for advancing quality and device surveillance. Lancet 2016; 388: 1358–1360. [DOI] [PubMed] [Google Scholar]
- 35. Roberts I, Ker K, Edwards P, Beecher D, Manno D, Sydenham E. The knowledge system underpinning healthcare is not fit for purpose and must change. BMJ 2015; 350: h2463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information


