Abstract
Background
Stratification of the severity of infection is currently based on the Sequential Organ Failure Assessment (SOFA) score, which is difficult to calculate outside the ICU. Biomarkers could help to stratify the severity of infection in surgical patients.
Methods
Levels of ten biomarkers indicating endothelial dysfunction, 22 indicating emergency granulopoiesis, and six denoting neutrophil degranulation were compared in three groups of patients in the first 12 h after diagnosis at three Spanish hospitals.
Results
There were 100 patients with infection, 95 with sepsis and 57 with septic shock. Seven biomarkers indicating endothelial dysfunction (mid‐regional proadrenomedullin (MR‐ProADM), syndecan 1, thrombomodulin, angiopoietin 2, endothelial cell‐specific molecule 1, vascular cell adhesion molecule 1 and E‐selectin) had stronger associations with sepsis than infection alone. MR‐ProADM had the highest odds ratio (OR) in multivariable analysis (OR 11·53, 95 per cent c.i. 4·15 to 32·08; P = 0·006) and the best area under the curve (AUC) for detecting sepsis (0·86, 95 per cent c.i. 0·80 to 0·91; P < 0·001). In a comparison of sepsis with septic shock, two biomarkers of neutrophil degranulation, proteinase 3 (OR 8·09, 1·34 to 48·91; P = 0·028) and lipocalin 2 (OR 6·62, 2·47 to 17·77; P = 0·002), had the strongest association with septic shock, but lipocalin 2 exhibited the highest AUC (0·81, 0·73 to 0·90; P < 0·001).
Conclusion
MR‐ProADM and lipocalin 2 could be alternatives to the SOFA score in the detection of sepsis and septic shock respectively in surgical patients with infection.
In surgical patients, endothelial dysfunction is an early indicator differentiating sepsis from simple infection, whereas neutrophil degranulation is an early indicator differentiating septic shock from sepsis. Mid‐regional proadrenomedullin and lipocalin 2 are the most representative biomarkers of sepsis and septic shock respectively.

Useful biomarkers that merit validation elsewhere.
Antecedentes
La estratificación de la gravedad de una infección se basa actualmente en la puntuación SOFA (Sequential Organ Failure Assessment), que es difícil de calcular fuera de la unidad de cuidados intensivos. Los biomarcadores podrían ayudar a estratificar la gravedad de la infección en pacientes quirúrgicos.
Métodos
Se compararon las concentraciones de 10 biomarcadores que denotan disfunción endotelial, 22 que indican granulopoyesis de emergencia y 6 que expresan la degranulación de neutrófilos en tres grupos de pacientes de tres hospitales españoles (100 con infección, 95 con sepsis y 57 con shock séptico) en las primeras doce horas después del diagnóstico.
Resultados
Siete biomarcadores que expresan disfunción endotelial (proadrenomedulina, sindecan‐1, trombomodulina, angiopoyetina‐2, endocan‐1, molécula de adhesión endotelial 1 y E‐selectina) mostraron una fuerte asociación con la sepsis en comparación con la infección aislada. La proadrenomedulina presentó el valor más alto de la razón de oportunidades (odds ratio, OR) en el análisis multivariable (OR 11,53, i.c. del 95% 4,15‐32,08, P = 0,006) y la mejor área bajo la curva para detectar sepsis (AUC 0,86, i.c. del 95% 0,80‐0,91, P < 0,001). En la comparación entre sepsis y shock séptico, los biomarcadores que mostraron la asociación más estrecha con el shock séptico fueron dos biomarcadores de degranulación de neutrófilos (proteinasa‐3 y lipocalina‐2) (OR 8,09, i.c. del 9% 1,34‐48,91, P = 0,028; OR 6.62, i.c. del 95% 2,47‐17,77, P = 0,002), pero la lipocalina‐2 presentó la mejor AUC (0,81, i.c. del 95% 0,73‐0,90, P < 0,001).
Conclusión
la proadrenomedulina y la lipocalina‐2 podrían representar alternativas a la puntuación SOFA para detectar sepsis y shock séptico en pacientes quirúrgicos con infección.
Introduction
Sepsis and septic shock are major causes of morbidity and mortality in surgical patients1. In a patient with infection, prompt detection of sepsis is key to the initiation of early treatment with appropriate antimicrobials, elimination of the infectious source, administration of fluids and appropriate transfer to the ICU. In patients with sepsis, prompt detection of septic shock could imply a need to modify antibiotic treatment, seek alternative sources of
potentially infectious organisms not already identified, and adjust ICU support. Since publication of the Third International Consensus Definitions for Sepsis and Septic Shock (SEPSIS‐3) in 20162, severity stratification in patients with infection has been based on the Sequential Organ Failure Assessment (SOFA) score3. The problem with this score is that it is difficult to calculate in non‐ICU settings, such as surgical departments or the emergency room. The alternative proposed by the SEPSIS‐3 consensus for these settings, the quickSOFA (composed of three simple items: respiratory frequency, BP and the Glasgow Coma Scale score), is very specific but less useful for detecting sepsis4.
Biomarkers could contribute to stratification of the severity of infection. Sepsis is characterized by acute endothelial dysfunction, which increases vascular permeability, promotes activation of the coagulation cascade and tissue oedema, and compromises the perfusion of vital organs5. Biomarkers of endothelial responses can be used to categorize patients into homogeneous subgroups with different severity6. In turn, sepsis activates emergency granulopoiesis, inducing release of immature neutrophil precursor cells in the peripheral blood, an event related directly to severity7, 8, 9, 10. Emergency granulopoiesis can be detected by profiling the mRNA in blood of the genes that are expressed sequentially in the neutrophil precursors11, 12. Other molecules denoting severity during an infection are proteins released to the plasma during neutrophil degranulation13, 14. These include matrix metalloproteinase (MMP) 8, neutrophil gelatinase‐associated lipocalin and lactotransferrin, which have been shown to be closely related to the development of sepsis15, and levels of plasma MMPs 3, 7, 8 and 9 are increased in severe sepsis on admission to the ICU16.
In this study, 38 biomarkers of endothelial dysfunction, emergency granulopoiesis or neutrophil degranulation were evaluated to stratify severity in surgical patients with infection. The hypothesis was that these biomarkers might differentiate between three groups of patients: those with infection, those with sepsis, and those with septic shock.
Methods
Surgical patients with infection, sepsis or septic shock were recruited prospectively from the surgery departments and surgical ICUs of the three participating hospitals (Hospital Clínico Universitario de Valladolid, Hospital Universitario Río Hortega de Valladolid and Hospital Clínico Universitario de Salamanca), between January 2017 and January 2019. Infection was defined according to the US Centers for Disease Control and Prevention National Surveillance Definitions for Specific Types of Infections17. Sepsis and septic shock were defined using the SEPSIS‐3 consensus definitions2, 18. A specific standard survey was employed in the three participating hospitals to collect clinical data along with results of haematological, biochemical, radiological and microbiological investigations. Healthy controls with similar age and sex characteristics to the patients were recruited from the Centro de Hemoterapia y Hemodonación de Castilla y León (CHEMCYL, Valladolid, Spain).
Ethical approval
The study was approved by the respective Committees for Ethics in Clinical Research of the three participating hospitals. Methods were carried out in accordance with current Spanish law for Biomedical Research, fulfilling the standards indicated by the Declaration of Helsinki. Written informed consent was obtained from patients' relatives or their legal representative before enrolment.
Microbiology
Standard cultures in biological samples, guided by the presumptive source of the infection, were performed to assess the presence of the causal pathogen. Potentially contaminant microorganisms were not considered.
Biomarker profiling
Thirty‐eight biomarkers (10 denoting endothelial dysfunction, 22 indicating emergency granulopoiesis and 6 denoting neutrophil degranulation) were profiled in the three patient groups (infection, sepsis or septic shock) in the first 12 h after diagnosis (Tables S1 and S2 , supporting information). The methods used to profile these biomarkers are detailed in Appendix S1 (supporting information). Blood from healthy individuals was collected as part of their blood donation.
Statistical analysis
Statistical analysis was performed with IBM SPSS® version 20 (IBM, Armonk, New York, USA). Box plots were represented using Minitab® 19.2 (Minitab, Coventry, UK). For demographic and clinical characteristics of the patients, differences between groups were assessed using the χ2 test for categorical variables. Differences between groups for continuous variables were assessed with the Kruskal–Wallis test, with post hoc tests adjusting for multiple comparisons. In the comparison of infection and sepsis, multivariable logistic regression analysis was employed to evaluate the association between biomarker levels and the presence of sepsis. In the comparison of sepsis and septic shock, the same type of analysis was employed to evaluate the association between biomarker levels and the presence of septic shock. Only biomarkers yielding P ≤ 0·050 in univariable analysis were tested in multivariable analyses. Potential confounding clinical factors that yielded P ≤ 0·100 in univariable analysis were introduced as adjusting variables in multivariable analyses, followed by multiple testing correction by the false discovery rate using the Benjamini–Hochberg procedure. The optimal operating point in the area under the curve (AUC) analysis was identified as described previously19.
Results
There was a total of 100 patients with infection, 95 with sepsis and 57 with septic shock. Patients with infection were significantly younger than those in the other groups (Table 1), and the healthy controls. Proportions of men to women were similar in all patient groups and control subjects. Patients with sepsis and septic shock had more antecedent cardiovascular, respiratory or renal disease. The proportion of patients needing urgent surgery was similar in the three groups. Abdominal surgery was the most frequent type, and the abdomen was the predominant source of infection in all three patient groups.
Table 1.
Clinical characteristics of the patients
| Infection (n = 100) | Sepsis (n = 95) | Septic shock (n = 57) | P † (infection versus sepsis) | P † (infection versus septic shock) | P † (sepsis versus septic shock) | |
|---|---|---|---|---|---|---|
| Age (years) * | 57·0 (39·25–70·50) | 73·0 (59–80) | 74·0 (68–78·5) | < 0·001‡ | < 0·001‡ | 1·000 |
| Male sex | 60 (60·0) | 62 (65) | 31 (54) | 0·448 | 0·493 | 0·183 |
| Co‐morbidity | ||||||
| Chronic cardiovascular disease | 13 (13·0) | 28 (29) | 21 (37) | 0·005 | < 0·001 | 0·347 |
| Chronic respiratory disease | 2 (2) | 13 (14) | 6 (11) | 0·003 | 0·022 | 0·569 |
| High BP | 30 (30·0) | 47 (49) | 34 (60) | 0·009 | < 0·001 | 0·223 |
| Chronic renal failure | 2 (2·0) | 9 (9) | 7 (12) | 0·024 | 0·004 | 0·388 |
| Chronic hepatic failure | 4 (4·0) | 2 (2) | 1 (2) | 0·422 | 0·423 | 0·880 |
| Diabetes mellitus | 14 (14·0) | 20 (21) | 17 (30) | 0·194 | 0·017 | 0·222 |
| Cancer | 13 (13·0) | 18 (19) | 13 (23) | 0·256 | 0·112 | 0·568 |
| Immunosuppression | 5 (5·0) | 13 (14) | 6 (11) | 0·037 | 0·205 | 0·553 |
| Surgery type | ||||||
| Urgent | 70 (70·0) | 74 (78) | 41 (72) | 0·210 | 0·798 | 0·407 |
| Abdominal | 65 (65·0) | 54 (57) | 24 (42) | 0·243 | 0·005 | 0·078 |
| Cardiothoracic | 0 (0) | 14 (15) | 14 (25) | < 0·001 | < 0·001 | 0·130 |
| Vascular | 1 (1·0) | 3 (3) | 1 (2) | 0·288 | 0·685 | 0·601 |
| Urological/renal | 0 (0) | 0 (0) | 1 (2) | § | 0·184 | 0·195 |
| Other | 13 (13·0) | 4 (4) | 4 (7) | 0·030 | 0·246 | 0·453 |
| Time course and outcome | ||||||
| Length of hospital stay (days)* | 5 (2–12) | 15 (8–29·5) | 31 (18·50–48·75) | < 0·001‡ | < 0·001‡ | < 0·001‡ |
| Length of ICU stay (days)* | 0·5 (0–2) | 3 (1–6·75) | 7 (3–13) | < 0·001‡ | < 0·001‡ | 0·001‡ |
| Hospital mortality | 0 (0) | 7 (7) | 22 (39) | 0·006 | < 0·001 | < 0·001 |
| Source of infection | ||||||
| Respiratory tract | 4 (4·0) | 15 (16) | 14 (25) | 0·006 | < 0·001 | 0·183 |
| Abdomen | 67 (67·0) | 48 (51) | 19 (33) | 0·019 | < 0·001 | 0·039 |
| Urinary tract | 0 (0) | 4 (4) | 6 (11) | 0·038 | 0·001 | 0·128 |
| Surgical site | 12 (12·0) | 16 (17) | 13 (23) | 0·335 | 0·075 | 0·365 |
| Bacteraemia | 0 (0) | 6·30 (6) | 12 (21) | 0·011 | < 0·001 | 0·006 |
| Other | 13 (13·0) | 16 (17) | 4 (7) | 0·451 | 0·246 | 0·083 |
| Microbiology | ||||||
| Positive culture | 29 (29·0) | 54 (57) | 43 (75) | < 0·001 | < 0·001 | 0·021 |
| Gram‐positive | 13 (13·0) | 29 (31) | 21 (37) | 0·003 | < 0·001 | 0·422 |
| Gram‐negative | 23 (23·0) | 31 (33) | 34 (60) | 0·133 | < 0·001 | 0·001 |
| Fungal | 4 (4·0) | 8 (8) | 8 (14) | 0·199 | 0·023 | 0·275 |
| Measurements at diagnosis * | ||||||
| SOFA score | 0 (0–1) | 6 (3–8) | 9 (7–11) | < 0·001‡ | < 0·001‡ | < 0·001‡ |
| Total bilirubin (mg/dl) | 0·70 (0·4–1·03) | 0·70 (0·43–1·78) | 1·00 (0·56–1·89) | 1·000‡ | 0·013‡ | 0·072‡ |
| Glucose level (mg/dl) | 118 (105–140) | 158 (117·5–184) | 163 (128–232·50) | < 0·001‡ | < 0·001‡ | 1·000‡ |
| Sodium level (mmol/l) | 139·00 (136–141·25) | 138·00 (135–141·25) | 129·00 (134–142) | 0·008‡ | < 0·001‡ | 0·001‡ |
| Potassium level (mmol/l) | 3·90 (3–4·10) | 4·00 (3·50–4·20) | 3·85 (3–4·12) | § | § | § |
| Platelet count (cells/mm3) | 221 500 (186 250–299 250) | 184 000 (105 250–276 000) | 123 000 (88 500–258 000) | 0·023‡ | 0·001‡ | 0·794‡ |
| INR | 1·15 (1·03–1·26) | 1·27 (1·16–1·35) | 1·44 (1·27–1·81) | 0·002‡ | < 0·001‡ | < 0·001‡ |
| Albumin (mg/dl) | 3480 (2737·5–4135) | 2445 (2132·50–3130) | 2340 (1837·5–2752·5) | < 0·001‡ | < 0·001‡ | 1·000‡ |
| Lactate (mmol/l) | 1·50 (1·06–1·85) | 1·50 (1·23–2) | 3·55 (2·46–5·19) | 1·000‡ | < 0·001‡ | < 0·001‡ |
| White blood cell count (cells/mm3) | 13 070 (9187·5–16 347·5) | 14 050 (9410–18 290) | 14 550 (7525–19 880) | § | § | § |
| Lymphocytes (cells/mm3) | 1383·50 (911·50–1806·36) | 940 (600·10–1453·86) | 592 (410·08–1103·39) | 0·021‡ | < 0·001‡ | 0·004‡ |
| Monocytes (cells/mm3) | 795·52 (466·07–1099) | 632 (345–962) | 411·25 (245–791·56) | 0·049‡ | 0·002‡ | 0·196‡ |
| Neutrophils (cells/mm3) | 10 144 (6327·70–13 864·48) | 12 100 (7857–15 195) | 12 240 (5647·50–18 177·50) | 0·091‡ | 0·199‡ | 1·000‡ |
| Eosinophils (cells/mm3) | 43 (12·50–117·41) | 19 (0–57·52) | 10·92 (0–47·53) | § | § | § |
| Basophils (cells/mm3) | 33·60 (18–59·73) | 26·90 (11·76–54·80) | 20 (10·54–49·92) | 0·827‡ | 0·042‡ | 0·196‡ |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r.). SOFA, Sequential Organ Failure Score; INR, international normalized ratio.
χ2 test, except
Kruskal–Wallis test;
absence of P value for χ2 or Kruskal–Wallis test, as appropriate.
Respiratory infection was more common in patients with sepsis or septic shock than in patients with infection alone. The prevalence of bacteraemia was highest in patients with septic shock, where Gram‐negative bacteria dominated (Table 1).
Patients with septic shock showed the highest degree of organ failure as assessed by the SOFA score. Duration of hospital stay was directly associated with severity. No patient in the infection group died in hospital, compared with seven of 95 (7 per cent) patients with sepsis and 22 of 57 (39 per cent) with septic shock (Table 1).
Coagulopathy (as assessed by the international normalized ratio) and decreased lymphocyte and monocyte counts were related to increasing severity. Biomarker levels showed a generalized trend to increase with disease severity (Figs 1 and 2; Table S3 , supporting information).
Figure 1.
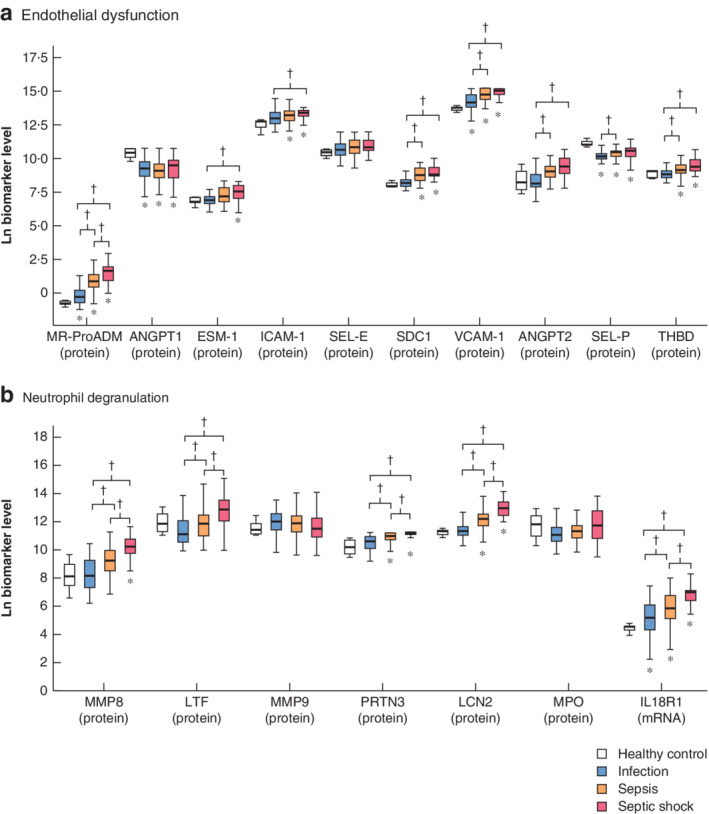
Levels of endothelial dysfunction and neutrophil degranulation biomarkers in healthy control, infection, sepsis and septic shock groups Biomarkers of a endothelial dysfunction and b neutrophil degranulation. Levels of mid‐regional proadrenomedullin (MR‐ProADM) are in nmol/l, those of interleukin‐18 receptor type 1 (IL18R1) are copies of cDNA per ng mRNA, and those of the remaining biomarkers are in pg/ml. ANGPT, angiopoietin; ESM, endothelial cell‐specific molecule; ICAM, intercellular adhesion molecule; SEL‐E, E‐selectin; SDC, syndecan; VCAM, vascular cell adhesion molecule; SEL‐P, P‐selectin; THBD, thrombomodulin; MMP, matrix metalloproteinase; LTF, lactoferrin; PRTN, proteinase; LCN, lipocalin; MPO, myeloperoxidase. *P ≤ 0·050 versus healthy control; †P ≤ 0·050 (Kruskal–Wallis test).
Figure 2.
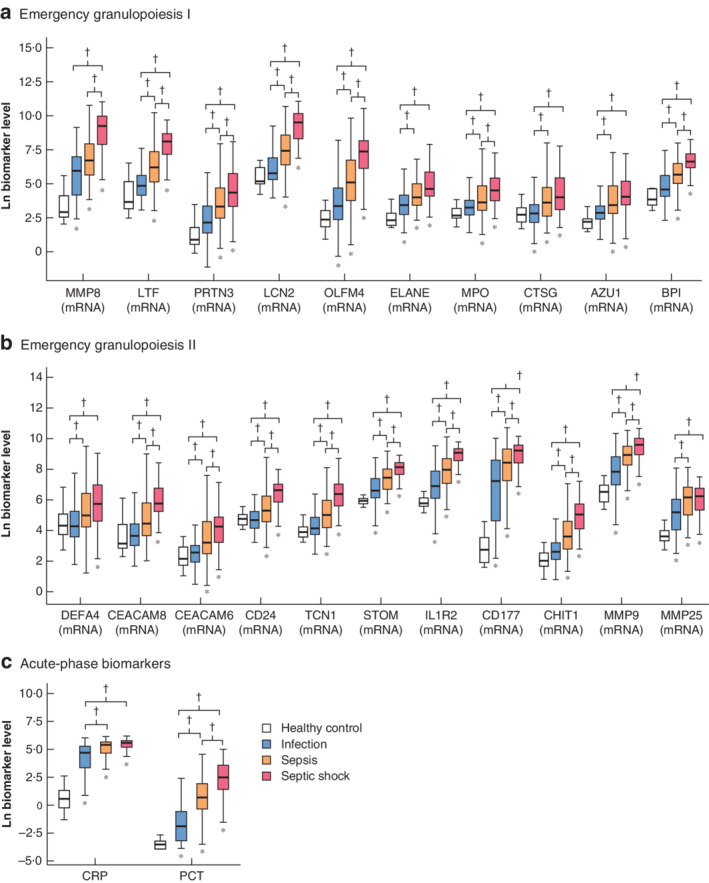
Levels of emergency granulopoiesis and acute‐phase response biomarkers in healthy control, infection, sepsis and septic shock groups Biomarkers of a,b emergency granulopoiesis and c acute‐phase response. Levels of C‐reactive protein (CRP) are in mg/l, those of procalcitonin (PCT) are in ng/ml, and those of the remaining biomarkers are copies of cDNA per ng mRNA. MMP, matrix metalloproteinase; LTF, lactoferrin; PRTN, proteinase; LCN, lipocalin, OLFM, olfactomedin; ELANE, elastase, neutrophil expressed; MPO, myeloperoxidase; CTSG, cathepsin G; AZU, azurocidin; BPI, bactericidal/permeability‐increasing protein; DEFA, defensin α; CEACAM, carcinoembryonic antigen‐related cell adhesion molecule; CD, cluster of differentiation; TCN, transcobalamin; STOM, stomatin; IL1R2, interleukin‐1 receptor type 2; CHIT, chitinase. *P ≤ 0·050 versus healthy control; †P ≤ 0·050 (Kruskal–Wallis test).
Confounding factors from Table 1 that yielded P ≤ 0·100 in univariable analysis, to be introduced as adjusting variables in multivariable analyses, are shown in Table S4 (supporting information).
Multivariable analysis of biomarker levels to evaluate the risk of sepsis versus infection identified seven biomarkers of endothelial dysfunction, two of neutrophil degranulation and 13 of emergency granulopoiesis as independent risk factors for sepsis (Table 2).
Table 2.
Multivariable analysis for risk of sepsis versus infection
| Biomarker* | Indicator for | Odds ratio | P | Benjamini–Hochberg P |
|---|---|---|---|---|
| Mid‐regional proadrenomedullin (nmol/l) | ED | 11·53 (4·15, 32·08) | < 0·001 | 0·006 |
| Syndecan 1 (pg/ml) | ED | 9·48 (2·86, 31·38) | < 0·001 | 0·006 |
| Thrombomodulin (pg/ml) | ED | 4·14 (1·28, 13·39) | 0·018 | 0·040 |
| Angiopoietin 2 (pg/ml) | ED | 3·70 (1·80, 7·59) | < 0·001 | 0·006 |
| Endothelial cell‐specific molecule 1 (pg/ml) | ED | 3·58 (1·45, 8·83) | 0·006 | 0·022 |
| Vascular cell adhesion molecule 1 (pg/ml) | ED | 2·72 (1·10, 6·76) | 0·031 | 0·047 |
| E‐selectin (pg/ml) | ED | 2·32 (1·12, 4·81) | 0·023 | 0·041 |
| Lipocalin 2 (pg/ml) | ND | 2·27 (1·16, 4·44) | 0·016 | 0·040 |
| MMP8 (pg/ml) | ND | 1·90 (1·23, 2·96) | 0·004 | 0·016 |
| Procalcitonin (ng/ml) | AR | 1·83 (1·41, 2·37) | < 0·001 | 0·006 |
| Chitinase 1 (CHIT1) (copies/ng) | EG | 1·81 (1·24, 2·64) | 0·002 | 0·009 |
| Stomatin (STOM) (copies/ng) | EG | 1·68 (1·09, 2·60) | 0·020 | 0·040 |
| MMP9 (MMP9) (copies/ng) | EG | 1·67 (1·14, 2·44) | 0·008 | 0·026 |
| Interleukin‐1 receptor type 2 (IL1R2) (copies/ng) | EG | 1·64 (1·12, 2·42) | 0·011 | 0·006 |
| MMP8 (MMP8) (copies/ng) | EG | 1·64 (1·23, 2·19) | 0·001 | 0·033 |
| Lipocalin 2 (LCN2) (copies/ng) | EG | 1·62 (1·23, 2·15) | 0·001 | 0·006 |
| Transcobalamin 1 (TCN1) (copies/ng) | EG | 1·56 (1·07, 2·27) | 0·021 | 0·040 |
| Lactoferrin (LTF) (copies/ng) | EG | 1·55 (1·16, 2·06) | 0·002 | 0·009 |
| Bactericidal/permeability‐increasing protein (BPI) (copies/ng) | EG | 1·52 (1·07, 2·17) | 0·020 | 0·040 |
| CD24 (CD24) (copies/ng) | EG | 1·51 (1·05, 2·17) | 0·026 | 0·043 |
| C‐reactive protein (mg/l) | AR | 1·51 (1·05, 2·18) | 0·028 | 0·044 |
| MMP25 (MMP25) (copies/ng) | EG | 1·46 (1·05, 2·05) | 0·026 | 0·043 |
| CD177 (CD177) (copies/ng) | EG | 1·31 (1·05, 1·65) | 0·020 | 0·040 |
| Olfactomedin 4 (OLFM4) (copies/ng) | EG | 1·28 (1·05, 1·55) | 0·012 | 0·033 |
Values in parentheses are 95 per cent confidence intervals.
Biomarker values correspond to napierian logarithms. Variables adjusted for gene expression biomarkers were age, cardiovascular disease, immunosuppression, high BP, chronic respiratory disease, chronic renal disease, abdominal surgery, other surgery, respiratory source of infection and abdominal source of infection; variables adjusted for protein biomarkers were age, immunosuppression, high BP, chronic respiratory disease, chronic renal disease, urgent surgery, abdominal surgery, other surgery, respiratory source of infection and abdominal source of infection (Table S4 , supporting information). ED, endothelial dysfunction; ND, neutrophil degranulation; MMP, matrix metalloproteinase; AR, acute‐phase response; EG, emergency granulopoiesis; CD, cluster of differentiation.
Multivariable analysis to evaluate the risk of septic shock versus sepsis revealed four biomarkers of endothelial dysfunction, six of neutrophil degranulation and 14 of emergency granulopoiesis as independent risk factors for septic shock (Table 3).
Table 3.
Multivariable analysis for risk of septic shock versus sepsis
| Biomarker* | Indicator for | Odds ratio | P | Benjamini–Hochberg P |
|---|---|---|---|---|
| Proteinase 3 (pg/ml) | ND | 8·09 (1·34, 48·91) | 0·023 | 0·028 |
| Lipocalin 2 (pg/ml) | ND | 6·62 (2·47, 17·77) | < 0·001 | 0·002 |
| Syndecan 1 (pg/ml) | ED | 6·10 (1·77, 21·06) | 0·004 | 0·006 |
| Mid‐regional proadrenomedullin (nmol/l) | ED | 4·58 (1·99, 10·58) | < 0·001 | 0·002 |
| Thrombomodulin (pg/ml) | ED | 4·52 (1·42, 14·34) | 0·011 | 0·014 |
| Interleukin‐18 receptor type 1 (IL18R1) (copies/ng) | ND | 4·22 (2·26, 7·85) | < 0·001 | 0·002 |
| Stomatin (STOM) (copies/ng) | EG | 3·74 (1·87, 7·45) | < 0·001 | 0·002 |
| Interleukin‐1 receptor type 2 (IL1R2) (copies/ng) | EG | 3·72 (2·10, 6·58) | < 0·001 | 0·002 |
| Angiopoietin 2 (pg/ml) | ED | 3·02 (1·29, 7·10) | 0·011 | 0·014 |
| MMP8 (pg/ml) | ND | 2·97 (1·55, 5·67) | 0·001 | 0·002 |
| MMP9 (MMP9) (copies/ng) | EG | 2·67 (1·55, 4·59) | < 0·001 | 0·002 |
| Lipocalin 2 (LCN2) (copies/ng) | EG | 2·45 (1·71, 3·50) | < 0·001 | 0·002 |
| MMP8 (MMP8) (copies/ng) | EG | 2·43 (1·74, 3·38) | < 0·001 | 0·002 |
| Transcobalamin 1 (TCN1) (copies/ng) | EG | 2·36 (1·62, 3·44) | < 0·001 | 0·002 |
| Lactoferrin (pg/ml) | ND | 2·30 (1·38, 3·84) | 0·001 | 0·002 |
| Myeloperoxidase (pg/ml) | ND | 2·26 (1·20, 4·25) | 0·011 | 0·014 |
| Lactoferrin (LTF) (copies/ng) | EG | 2·24 (1·59, 3·15) | < 0·001 | 0·002 |
| Bactericidal/permeability‐increasing protein (BPI) (copies/ng) | EG | 2·23 (1·49, 3·36) | < 0·001 | 0·002 |
| CD24 (CD24) (copies/ng) | EG | 2·15 (1·47, 3·16) | < 0·001 | 0·002 |
| Chitinase 1 (CHIT1) (copies/ng) | EG | 2·01 (1·42, 2·83) | < 0·001 | 0·002 |
| CD177 (CD177) (copies/ng) | EG | 1·97 (1·30, 2·99) | 0·001 | 0·002 |
| Olfactomedin 4 (OLFM4) (copies/ng) | EG | 1·85 (1·42, 2·40) | < 0·001 | 0·002 |
| Carcinoembryonic antigen‐related cell adhesion molecule 8 (CEACAM8) (copies/ng) | EG | 1·78 (1·31, 2·41) | < 0·001 | 0·002 |
| Procalcitonin (ng/ml) | AR | 1·73 (1·31, 2·29) | < 0·001 | 0·002 |
| Myeloperoxidase (MPO) (copies/ng) | EG | 1·36 (1·02, 1·81) | 0·038 | 0·044 |
Values in parentheses are 95 per cent confidence intervals.
Biomarker values correspond to napierian logarithms. Variables adjusted for gene expression biomarkers were age, abdominal surgery, abdominal source of infection, bacteraemia, other sources of infection, presence of Gram‐negative organisms and presence of polymicrobial infection; variables adjusted for protein biomarkers were surgical‐site source of infection, bacteraemia, presence of Gram‐negative organisms, and presence of polymicrobial infection (Table S4 , supporting information). ND, neutrophil degranulation; ED, endothelial dysfunction; EG, emergency granulopoiesis; MMP, matrix metalloproteinase; CD, cluster of differentiation; AR, acute‐phase response.
The AUC analysis to assess biomarker sensitivity and specificity indicated that mid‐regional proadrenomedullin (MR‐ProADM) was the best biomarker for differentiating sepsis from infection, whereas lipocalin 2 in plasma was the best biomarker for distinguishing septic shock from sepsis (Fig. 3).
Figure 3.
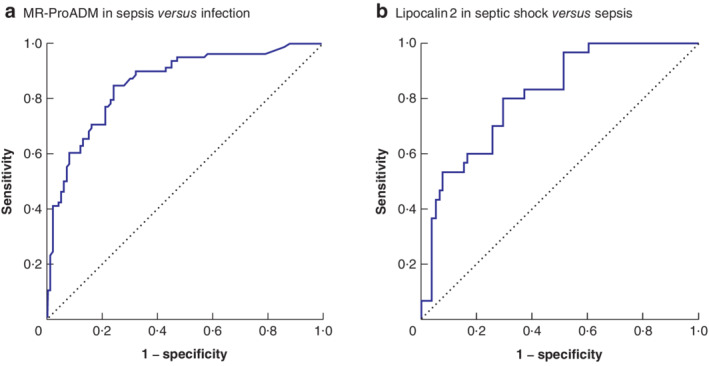
Area‐under‐the‐curve analysis evaluating the accuracy of two biomarkers in differentiating sepsis from infection or from septic shock a Accuracy of mid‐regional proadrenomedullin (MR‐ProADM) in differentiating sepsis from infection. Area under the curve (AUC): 0·86, 95 per cent c.i. 0·80 to 0·91 (P < 0·001); optimal operating point (OOP): 1·165 nmol/l; sensitivity: 84·6 per cent; specificity: 76·0 per cent. b Accuracy of lipocalin 2 in differentiating septic shock from sepsis. AUC: 0·81, 95 per cent c.i. 0·73 to 0·90 (P < 0·001); OOP: 246 346 pg/ml; sensitivity: 80·0 per cent; specificity: 70·5 per cent.
Discussion
This study found that a panel of seven biomarkers related to endothelial dysfunction (MR‐ProADM, syndecan (SDC) 1, thrombomodulin (THBD), angiopoietin (ANGPT) 2, endothelial cell‐specific molecule 1, vascular cell adhesion molecule 1 and E‐selectin) were associated with the presence of sepsis in patients with infection. This suggests that induction of endothelial injury is an early event as organ dysfunction develops.
SDC1 and MR‐proADM were the biomarkers showing the highest odds ratios for sepsis. SDC1 is a glycosaminoglycan shed from the endothelial glycocalyx during sepsis, and levels in plasma correlate with the SOFA score20, 21. In the present study, MR‐proADM was the biomarker of endothelial dysfunction showing not only the strongest association but also the best balance between sensitivity and specificity for sepsis, with an AUC of 0·86. Adrenomedullin is secreted from various organs and tissues, including vascular endothelial cells. It regulates vascular tone and endothelial permeability22. MR‐proADM, the mid‐regional fragment of proadrenomedullin, is more stable and directly reflects levels of the rapidly degraded active adrenomedullin peptide23. There is growing evidence of the value of MR‐ProADM as an early marker of severity in patients with infection24 and as a predictor of organ failure in patients with community‐acquired pneumonia25.
In the comparison of sepsis and septic shock, the number of biomarkers of endothelial dysfunction independently associated with septic shock dropped to four (SDC1, MR‐ProADM, THBD and ANGPT2). In contrast, six biomarkers denoting neutrophil degranulation were associated with septic shock: proteinase 3 (a serine protease), lipocalin 2 (a neutrophil gelatinase‐associated protein), interleukin‐18 receptor type 1 (an inductor of neutrophil degranulation)26, matrix metalloproteinase (MMP) 8 (a neutrophil collagenase), lactoferrin (a major iron‐binding protein) and myeloperoxidase (a heme protein). Only two of these biomarkers seemed relevant to differentiate sepsis from plain infection (lipocalin 2 and MMP8), suggesting that neutrophil degranulation may be important in the pathogenesis of septic shock. Proteins released from neutrophil granules could be mediating antibacterial effects8, 27, 28, 29, 30, and may participate in tissue remodelling31, attenuation of inflammation32 and preventing the deleterious effects of neutrophil extracellular traps33. However, increased intravascular levels of degranulated proteins could induce enhanced proteolysis34, endothelial injury and organ failure35, 36, 37, 38, 39.
Proteinase 3 and lipocalin 2 had strongest associations with the presence of septic shock. Neutrophil degranulation can lead to increased endothelial permeability via a mechanism that, in part, involves the actions of proteinase 340, and a multimarker model containing proteinase 3 was able to predict the risk of septic acute kidney injury in patients with septic shock41. In the present study, lipocalin 2 was the marker showing the best balance between sensitivity and specificity in detecting septic shock. Lipocalin 2 has been used for risk stratification, early diagnosis and prognostication of sepsis in the emergency department42, 43. This protein is associated with mortality and multiple organ dysfunction syndrome in severe sepsis and septic shock44. Lipocalin 2 has been promoted as a relatively robust predictor of 28‐day mortality in severe sepsis45.
The present study has shown that emergency granulopoiesis is a preserved signature of both sepsis and septic shock, although to a greater degree in septic shock. The observed parallel between emergency granulopoiesis signatures and severity is in agreement with a previous study9 demonstrating that, in sepsis, the increased presence of circulating immature granulocytes is linked to clinical deterioration.
Regarding acute‐phase biomarkers, procalcitonin showed modest associations with the risk of sepsis and septic shock, while C‐reactive protein showed a mild association, exclusively with the risk of sepsis. These results indicate that neither procalcitonin nor C‐reactive protein is suitable for severity stratification in patients with infection.
Profiling protein levels in plasma of MR‐ProADM and lipocalin 2 could contribute to stratification of the severity of infection, particularly in settings where calculation of the SOFA score is not feasible. Evaluation of protein biomarkers is technically easier than evaluating those of transcriptomic nature. Emerging point‐of‐care devices could result in evaluation of these biomarkers in clinical practice as results can be obtained in less than 1 h46.
This study has an important limitation in that biomarkers were compared only at diagnosis of infection, sepsis or septic shock. Further prospective follow‐up studies with serial sampling should validate the potential role of MR‐ProADM and lipocalin 2 in predicting clinical worsening of patients with infection or sepsis.
Supporting information
Table S1. Description of endothelial dysfunction biomarkers
Table S2. Description of emergency granulopoiesis biomarkers
Appendix S1. Methods for biomarker profiling
Table S3. Biomarker levels across groups
Table S4. Univariable analysis selecting confounding variables to be entered into multivariable analyses
Acknowledgements
M.M.‐F, L.M.V.‐R. and R.A. are joint first authors of this article, and J.F.B.‐M., C.A. and M.H.‐R. contributed equally.
The authors thank the nursing teams of the participating clinical services for their continuous support to the research programme. They also thank M. J. Garcia Salgado, from the Biobanco Hospital Clínico Universitario de Salamanca, for assistance with sample storing, and CHEMCYL (Valladolid, Spain) for providing the samples from healthy controls.
The authors thank the Instituto de Salud Carlos III for financial support (grant numbers PI15/01959, PI15/01451 and PI16/01156) and Consejería de Educación de Castilla y Leon/Fondo social Europeo for supporting M.M.‐F. This research was also supported by funds from the European Union (Fondo Europeo de Desarrollo Regional, Una manera de hacer Europa).
J.F.B.‐M. is the inventor of a patent with Thermo‐Fisher on MR‐proADM for prognosis prediction in sepsis. J.F.B.‐M. and R.A. are inventors of a patent with Thermo‐Fisher on MMP8 for diagnosis and severity assessment of sepsis.
Disclosure: The authors declare no other conflict of interest.
Funding information
Instituto de Salud Carlos III, PI15/01959, PI15/01451, PI16/01156
Consejería de Educación de Castilla y León/Fondo social Europeo
European Union (Fondo Europeo de Desarrollo Regional, Una manera de hacer Europa)
References
- 1. Angus DC, Linde‐Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 2. Singer M, Deutschman CS, Seymour CW, Shankar‐Hari M, Annane D, Bauer M et al The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H et al The SOFA (Sepsis‐related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis‐Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 4. Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the quick‐SOFA and systemic inflammatory response syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta‐analysis. Chest 2018; 153: 646–655. [DOI] [PubMed] [Google Scholar]
- 5. Bermejo‐Martin JF, Martín‐Fernandez M, López‐Mestanza C, Duque P, Almansa R. Shared features of endothelial dysfunction between sepsis and its preceding risk factors (aging and chronic disease). J Clin Med 2018; 7: E400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clark DV, Banura P, Bandeen‐Roche K, Liles WC, Kain KC, Scheld WM et al Biomarkers of endothelial activation/dysfunction distinguish sub‐groups of Ugandan patients with sepsis and differing mortality risks. JCI Insight 2019; 23: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol Rev 2016; 273: 11–28. [DOI] [PubMed] [Google Scholar]
- 8. Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol 2014; 14: 302–314. [DOI] [PubMed] [Google Scholar]
- 9. Daix T, Guerin E, Tavernier E, Mercier E, Gissot V, Hérault O et al; Septiflux Trial Group . Multicentric standardized flow cytometry routine assessment of patients with sepsis to predict clinical worsening. Chest 2018; 154: 617–627. [DOI] [PubMed] [Google Scholar]
- 10. Mare TA, Treacher DF, Shankar‐Hari M, Beale R, Lewis SM, Chambers DJ et al The diagnostic and prognostic significance of monitoring blood levels of immature neutrophils in patients with systemic inflammation. Crit Care 2015; 19: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cowland JB, Borregaard N. The individual regulation of granule protein mRNA levels during neutrophil maturation explains the heterogeneity of neutrophil granules. J Leukoc Biol 1999; 66: 989–995. [DOI] [PubMed] [Google Scholar]
- 12. Almansa R, Heredia‐Rodríguez M, Gomez‐Sanchez E, Andaluz‐Ojeda D, Iglesias V, Rico L et al Transcriptomic correlates of organ failure extent in sepsis. J Infect 2015; 70: 445–456. [DOI] [PubMed] [Google Scholar]
- 13. Sônego F, Castanheira FV, Ferreira RG, Kanashiro A, Leite CA, Nascimento DC et al Paradoxical roles of the neutrophil in sepsis: protective and deleterious. Front Immunol 2016; 7: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost 2018; 16: 231–241. [DOI] [PubMed] [Google Scholar]
- 15. Tong Y, Ku X, Wu C, Liu J, Yang C, Tang W et al Data‐independent acquisition‐based quantitative proteomic analysis reveals differences in host immune response of peripheral blood mononuclear cells to sepsis. Scand J Immunol 2019; 89: e12748. [DOI] [PubMed] [Google Scholar]
- 16. Yazdan‐Ashoori P, Liaw P, Toltl L, Webb B, Kilmer G, Carter DE et al Elevated plasma matrix metalloproteinases and their tissue inhibitors in patients with severe sepsis. J Crit Care 2011; 26: 556–565. [DOI] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention . CDC/NHSN Surveillance Definitions for Specific Types of Infections; updated 2014. https://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf [accessed 10 January 2017].
- 18. Shankar‐Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS et al; Sepsis Definitions Task Force . Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA 2016; 315: 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Almansa R, Ortega A, Ávila‐Alonso A, Heredia‐Rodríguez M, Martín S, Benavides D et al Quantification of immune dysregulation by next‐generation polymerase chain reaction to improve sepsis diagnosis in surgical patients. Ann Surg 2019; 269: 545–553. [DOI] [PubMed] [Google Scholar]
- 20. Nelson A, Berkestedt I, Schmidtchen A, Ljunggren L, Bodelsson M. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock 2008; 30: 623–627. [DOI] [PubMed] [Google Scholar]
- 21. Sallisalmi M, Tenhunen J, Yang R, Oksala N, Pettilä V. Vascular adhesion protein‐1 and syndecan‐1 in septic shock. Acta Anaesthesiol Scand 2012; 56: 316–322. [DOI] [PubMed] [Google Scholar]
- 22. Mebazaa A, Geven C, Hollinger A, Wittebole X, Chousterman BG, Blet A et al; AdrenOSS‐1 study investigators . Circulating adrenomedullin estimates survival and reversibility of organ failure in sepsis: the prospective observational multinational Adrenomedullin and Outcome in Sepsis and Septic Shock‐1 (AdrenOSS‐1) study. Crit Care 2018; 22: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Struck J, Tao C, Morgenthaler NG, Bergmann A. Identification of an adrenomedullin precursor fragment in plasma of sepsis patients. Peptides 2004; 25: 1369–1372. [DOI] [PubMed] [Google Scholar]
- 24. Saeed K, Wilson DC, Bloos F, Schuetz P, van der Does Y, Melander O et al The early identification of disease progression in patients with suspected infection presenting to the emergency department: a multi‐centre derivation and validation study. Crit Care 2019; 23: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menéndez R, Méndez R, Almansa R, Ortega A, Alonso R, Suescun M et al Simultaneous depression of immunological synapse and endothelial injury is associated with organ dysfunction in community‐acquired pneumonia. J Clin Med 2019; 8: E1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leung BP, Culshaw S, Gracie JA, Hunter D, Canetti CA, Campbell C et al A role for IL‐18 in neutrophil activation. J Immunol 2001; 167: 2879–2886. [DOI] [PubMed] [Google Scholar]
- 27. Standish AJ, Weiser JN. Human neutrophils kill Streptococcus pneumoniae via serine proteases. J Immunol 2009; 183: 2602–2609. [DOI] [PubMed] [Google Scholar]
- 28. Cramer EP, Dahl SL, Rozell B, Knudsen KJ, Thomsen K, Moser C et al Lipocalin‐2 from both myeloid cells and the epithelium combats Klebsiella pneumoniae lung infection in mice. Blood 2017; 129: 2813–2817. [DOI] [PubMed] [Google Scholar]
- 29. Atkinson SJ, Varisco BM, Sandquist M, Daly MN, Klingbeil L, Kuethe JW et al Matrix metalloproteinase‐8 augments bacterial clearance in a juvenile sepsis model. Mol Med 2016; 22: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miethke M, Skerra A. Neutrophil gelatinase‐associated lipocalin expresses antimicrobial activity by interfering with l‐norepinephrine‐mediated bacterial iron acquisition. Antimicrob Agents Chemother 2010; 54: 1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blázquez‐Prieto J, López‐Alonso I, Huidobro C, Albaiceta GM. The emerging role of neutrophils in repair after acute lung injury. Am J Respir Cell Mol Biol 2018; 59: 289–294. [DOI] [PubMed] [Google Scholar]
- 32. González‐López A, Aguirre A, López‐Alonso I, Amado L, Astudillo A, Fernández‐García MS et al MMP‐8 deficiency increases TLR/RAGE ligands S100A8 and S100A9 and exacerbates lung inflammation during endotoxemia. PLoS One 2012; 7: e39940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okubo K, Kamiya M, Urano Y, Nishi H, Herter JM, Mayadas T et al Lactoferrin suppresses neutrophil extracellular traps release in inflammation. EBioMedicine 2016; 10: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bauzá‐Martinez J, Aletti F, Pinto BB, Ribas V, Odena MA, Díaz R et al Proteolysis in septic shock patients: plasma peptidomic patterns are associated with mortality. Br J Anaesth 2018; 121: 1065–1074. [DOI] [PubMed] [Google Scholar]
- 35. Becker BF, Jacob M, Leipert S, Salmon AHJ, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol 2015; 80: 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuravi SJ, Bevins A, Satchell SC, Harper L, Williams JM, Rainger GE et al Neutrophil serine proteases mediate inflammatory cell recruitment by glomerular endothelium and progression towards dysfunction. Nephrol Dial Transplant 2012; 27: 4331–4338. [DOI] [PubMed] [Google Scholar]
- 37. Schubert‐Unkmeir A, Konrad C, Slanina H, Czapek F, Hebling S, Frosch M. Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: a role for MMP‐8. PLoS Pathog 2010; 6: e1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Macdonald SPJ, Bosio E, Neil C, Arendts G, Burrows S, Smart L et al Resistin and NGAL are associated with inflammatory response, endothelial activation and clinical outcomes in sepsis. Inflamm Res 2017; 66: 611–619. [DOI] [PubMed] [Google Scholar]
- 39. Solan PD, Dunsmore KE, Denenberg AG, Odoms K, Zingarelli B, Wong HR. A novel role for matrix metalloproteinase‐8 in sepsis. Crit Care Med 2012; 40: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patterson EK, Cepinskas GK, Inoue K, Fraser DD. Sepsis‐associated elastase and proteinase 3 induce endothelial permeability. FASEB J 2017; 31: 978.11–978.11. [Google Scholar]
- 41. Wong HR, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT et al A multibiomarker‐based model for estimating the risk of septic acute kidney injury. Crit Care Med 2015; 43: 1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang M, Zhang Q, Zhao X, Dong G, Li C. Diagnostic and prognostic value of neutrophil gelatinase‐associated lipocalin, matrix metalloproteinase‐9, and tissue inhibitor of matrix metalloproteinases‐1 for sepsis in the emergency department: an observational study. Crit Care 2014; 18: 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Macdonald SPJ, Stone SF, Neil CL, van Eeden PE, Fatovich DM, Arendts G et al Sustained elevation of resistin, NGAL and IL‐8 are associated with severe sepsis/septic shock in the emergency department. PLoS One 2014; 9: e110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang B, Chen G, Zhang J, Xue J, Cao Y, Wu Y. Increased neutrophil gelatinase‐associated lipocalin is associated with mortality and multiple organ dysfunction syndrome in severe sepsis and septic shock. Shock 2015; 44: 234–238. [DOI] [PubMed] [Google Scholar]
- 45. Chang W, Zhu S, Pan C, Xie J‐F, Liu S‐Q, Qiu H‐B et al Predictive utilities of neutrophil gelatinase‐associated lipocalin (NGAL) in severe sepsis. Clin Chim Acta 2018; 481: 200–206. [DOI] [PubMed] [Google Scholar]
- 46. Reddy B, Hassan U, Seymour C, Angus DC, Isbell TS, White K et al Point‐of‐care sensors for the management of sepsis. Nat Biomed Eng 2018; 2: 640–648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Description of endothelial dysfunction biomarkers
Table S2. Description of emergency granulopoiesis biomarkers
Appendix S1. Methods for biomarker profiling
Table S3. Biomarker levels across groups
Table S4. Univariable analysis selecting confounding variables to be entered into multivariable analyses


