Abstract
Background
Gastrectomy including D2 lymphadenectomy is regarded as the standard curative treatment for advanced gastric cancer in Asia. This procedure has also been adopted gradually in the West, despite lack of support from RCTs. This study sought to investigate any advantage for long‐term survival following D2 lymphadenectomy in routine gastric cancer surgery in a Western nationwide population‐based cohort.
Methods
All patients who had a gastrectomy for cancer in Sweden in 2006–2017 were included in the study. Prospectively determined data items were retrieved from the National Register of Oesophageal and Gastric Cancer. Extent of lymphadenectomy was categorized as D1+/D2 or the less extensive D0/D1 according to the Japanese Gastric Cancer Association classification. Overall survival was analysed and, in addition, a variety of possible confounders were introduced into the Cox proportional hazards regression model.
Results
A total of 1677 patients underwent gastrectomy, of whom 471 (28·1 per cent) were classified as having a D1+/D2 and 1206 (71·9 per cent) a D0/D1 procedure. D1+/D2 lymphadenectomy was not associated with higher 30‐ or 90‐day postoperative mortality. Median overall survival for D1+/D2 lymphadenectomy was 41·5 months with a 5‐year survival rate of 43·7 per cent, compared with 38·5 months and 38·5 per cent respectively for D0/D1 (P = 0·116). After adjustment for confounders, in multivariable analysis survival was significantly higher after D1+/D2 than following D0/D1 lymphadenectomy (hazard ratio 0·81, 95 per cent c.i. 0·68 to 0·95; P = 0·012).
Conclusion
This national registry study showed that long‐term survival after gastric cancer surgery was improved after gastrectomy involving D1+/D2 lymphadenectomy compared with D0/D1 dissection.
A nationwide prospective register‐based study examined the extent of lymphadenectomy in curative gastric cancer surgery and its effects on long‐term survival.
More evidence in favour of thorough lymphadenectomy
Antecedentes
En Asia, la gastrectomía con linfadenectomía D2 asociada se considera el tratamiento curativo estándar para el cáncer gástrico avanzado. Este procedimiento se ha adoptado gradualmente también en el mundo occidental a pesar de la falta de apoyo de los ensayos clínicos aleatorizados. En este estudio hemos tratado de investigar cualquier ventaja sobre la supervivencia a largo plazo tras la linfadenectomía D2 de rutina en una cohorte de base poblacional de cirugía del cáncer gástrico en un país occidental.
Métodos
Se incluyeron todos los pacientes que fueron sometidos a gastrectomía por cáncer en Suecia desde 2006‐2017. Se recuperaron datos registrados prospectivamente del Registro Nacional de Cáncer de Esófago y Estómago. La extensión de la linfadenectomía se categorizó en D1+/D2 o cuando fue menos amplia en D0/D1 de acuerdo con la clasificación de la Japanese Gastric Cancer Association. Se analizó la supervivencia global y, además, se introdujeron diversos factores de confusión en un modelo de regresión de riesgos proporcional de Cox.
Resultados
Un total de 1.677 pacientes fueron sometidos a gastrectomía, de los cuales 471 (28%) fueron clasificados como D1+/D2 y 1.206 (72%) como D0/D1. La linfadenectomía D1+/D2 no se asoció con una mayor mortalidad postoperatoria a los 30 y 90 días. La mediana de la supervivencia global para la linfadenectomía D1+/D2 fue de 41,5 meses con una tasa de supervivencia a los 5 años de 44% comparado con 38,5 meses y 39%, respectivamente, para D0/D1 (P = 0,116). Después de ajustar por los factores de confusión en el análisis multivariable, la supervivencia fue significativamente más alta en la linfadenectomía D1+/D2 comparada con D0/D1 (cociente de riesgos instantáneos, hazard ratio, HR 0,81 (i.c. del 95% 0,68‐0,95), P = 0,012)).
Conclusión
Este estudio del registro nacional mostró que la supervivencia a largo plazo tras cirugía del cáncer gástrico mejoró después de una gastrectomía que incluya linfadenectomía D1+/D2 en comparación con la disección D0/D1.
Introduction
Radical resection for gastric cancer remains the mainstay for cure in patients with locally advanced disease. In the West it has been shown that long‐term survival can be improved further by adding perioperative chemotherapy1, 2, 3, whereas in studies from Japan, South Korea, China and Taiwan, adjuvant chemotherapy is usually recommended4, 5. Classification of the extent of lymphadenectomy has changed over time, and is now classified as D0, D1 and D2 according to the Japanese Gastric Cancer Association (JGCA)6. Three European RCTs7, 8, 9 have investigated D2 lymphadenectomy compared with a less extensive D1 lymphadenectomy. No study showed a survival advantage for D2 lymphadenectomy at 5 years, but the Dutch trial10 was able to show a benefit at 15 years of follow‐up after exclusion of postoperative deaths. Despite these results, D1+/D2 lymphadenectomy has become widely used in the West. The present nationwide cohort study was undertaken to see whether there was a long‐term survival benefit for patients with gastric cancer undergoing D1+/D2 lymphadenectomy.
Methods
This was a national quality register study including all patients in Sweden registered in the National Register of Oesophageal and Gastric cancer (NREV) from 2006 to 2017. The NREV, which has detailed clinical information on all patients with gastric cancer in Sweden, was cross‐matched with data from the Swedish National Patient Register, National Register of Education, Emigration Register and Death Register to obtain educational level, emigration status, time of death, and modified Charlson Co‐morbidity Index (CCI)11. The NREV has been validated previously and shown to be accurate for a variety of variables in more than 91 per cent of patients12. The Swedish National Patient Register has complete coverage of patient diagnostic codes in inpatient care from 1987, and for specialized outpatient care since 200113. The form and structure of the data collection have been described previously14.
The study was approved by the Regional Ethics Committee (EPN Stockholm Dnr: 2016/1486‐32 and 2013/596‐31/3).
Study population
All patients undergoing resection for adenocarcinoma of the stomach and gastro‐oesophageal junction cancer type III were included. Patients who had a previous gastric resection or who had undergone proximal gastrectomy or pylorus‐preserving gastrectomy were excluded, as were those who had a resection described as palliative. Patients were classified by the extent of lymphadenectomy as having a D0, D1, D1+ or D2 procedure according to the fourth English version of the JGCA treatment guidelines for distal and total gastrectomy6. An a priori decision grouped D1+ and D2 lymphadenectomies together as lymphadenectomy station criteria are similar and correspond to more radical resection, whereas D0 and D1 lymphadenectomy were grouped and analysed together as they represent limited lymphadenectomies.
Statistical analysis
Data are presented as mean(s.d.) values or as counts with percentages. Statistical analyses were performed with the χ2 test or Fisher's exact test for categorical variables, and Student's t test for continuous variables. Survival was calculated by use of the Kaplan–Meier method and analysed with the log rank test. Multivariable analysis of factors affecting survival was done by Cox proportional hazards modelling, and presented with hazard ratios (HRs) and 95 per cent confidence intervals. Variables in the model included: age (as a continuous variable), sex, CCI (categorized as 0–1, 2 and 3 or above), ASA grade (categorized as I, II, III, IV, V or missing), clinical tumour stage according to TNM8 (grouped as stage I, II, III, IV and missing), surgical procedure (distal or total gastrectomy), multivisceral resection (includes any additional organ resection in addition to gastrectomy, categorized as no or yes), preoperative chemotherapy (no, yes or missing), educational level (categorized as 9 years or less, 10–12 years, more than 12 years or missing), and calendar year of surgery (grouped as 2006–2009, 2010–2013 and 2014–2017). The variables were chosen based on clinical importance and statistical significance in the model. Educational level was included as it has been shown to be a possible confounder for survival15. The significance level was set at P = 0·100 or less for a variable to be included in the model.
All statistical analyses were performed with IBM SPSS® Statistics version 25 (IBM, Armonk, New York, USA).
Results
A total of 6761 patients were diagnosed with gastric and gastro‐oesophageal junction type III cancer. Among these, 1677 had non‐palliative distal or total gastrectomy for adenocarcinoma (Fig. 1). The extent of lymphadenectomy was D0/D1 in 1206 patients (71·9 per cent) and D1+/D2 in 471 (28·1 per cent). The complete distribution was 930 D0 (55·5 per cent), 276 D1 (16·5 per cent), 126 D1+ (7·5 per cent) and 345 D2 (20·6 per cent).
Figure 1.
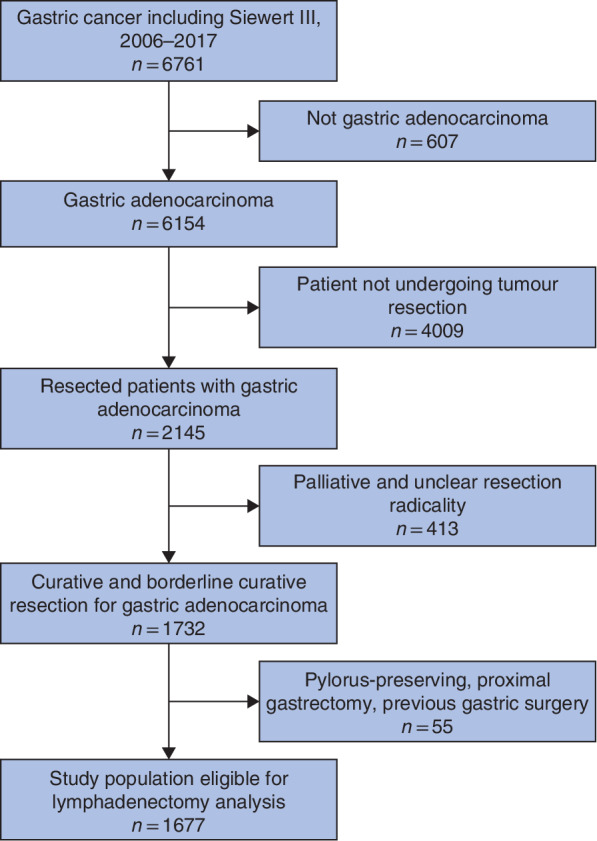
Flow chart of patient selection
Baseline characteristics of patients are presented in Table 1. In general, patients who had a D1+/D2 procedure were younger with a higher level of education, a slightly lower ASA grade and a more advanced tumour stage (stage III or above: 24·8 per cent compared with 14·6 per cent in the D0/D1 group). D1+/D2 lymphadenectomy became more popular during the latter part of the study period, comprising 20·8 per cent of resections in 2006–2009 versus 41·2 per cent in 2014–2017.
Table 1.
Baseline characteristics of patients undergoing limited D0/D1 lymphadenectomy or more extensive D1+/D2 dissection
| D0/D1 (n = 1206) | D1+/D2 (n = 471) | Overall (n = 1677) | P † | |
|---|---|---|---|---|
| Age (years) * | 70(11) | 65(12) | 69(12) | < 0·001§ |
| Sex | 0·602 | |||
| M | 698 (57·9) | 266 (56·5) | 964 (57·5) | |
| F | 508 (42·1) | 205 (43·5) | 713 (42·5) | |
| BMI (kg/m2) * | 25·1(4·45) | 25·2(4·43) | 25·2(4·44) | 0·682§ |
| ASA grade | < 0·001‡ | |||
| I | 310 (25·7) | 168 (35·7) | 478 (28·5) | |
| II | 591 (49·0) | 223 (47·3) | 814 (48·5) | |
| III | 252 (20·9) | 66 (14·0) | 318 (19·0) | |
| IV | 24 (2·0) | 3 (0·6) | 27 (1·6) | |
| V | 0 (0) | 0 (0) | 0 (0) | |
| Missing | 29 (2·4) | 11 (2·3) | 40 (2·4) | |
| Clinical tumour stage | < 0·001 | |||
| I | 402 (33·3) | 120 (25·5) | 522 (31·1) | |
| II | 312 (25·9) | 167 (35·5) | 479 (28·6) | |
| III | 149 (12·4) | 100 (21·2) | 249 (14·8) | |
| IV | 27 (2·2) | 17 (3·6) | 44 (2·6) | |
| Missing | 316 (26·2) | 67 (14·2) | 383 (22·8) | |
| Charlson Co‐morbidity Index | 0·655 | |||
| 0–1 | 400 (33·2) | 147 (31·2) | 547 (32·6) | |
| 2 | 195 (16·2) | 83 (17·6) | 278 (16·6) | |
| ≥ 3 | 611 (50·7) | 241 (51·2) | 852 (50·8) | |
| Years of education | < 0·001 | |||
| ≤ 9 | 474 (39·3) | 149 (31·6) | 623 (37·1) | |
| 10–12 | 455 (37·7) | 193 (41·0) | 648 (38·6) | |
| > 12 | 186 (15·4) | 111 (23·6) | 297 (17·7) | |
| Missing | 91 (7·5) | 18 (3·8) | 109 (6·5) | |
| Tumour location | < 0·001 | |||
| GOJ Siewert III | 37 (3·1) | 52 (11·0) | 89 (5·3) | |
| Upper | 39 (3·2) | 34 (7·2) | 73 (4·4) | |
| Middle | 386 (32·0) | 205 (43·5) | 591 (35·2) | |
| Lower | 625 (51·8) | 123 (26·1) | 748 (44·6) | |
| Whole | 25 (2·1) | 23 (4·9) | 48 (2·9) | |
| Missing | 94 (7·8) | 34 (7·2) | 128 (7·6) | |
| Calendar year of surgery | < 0·001 | |||
| 2006–2009 | 468 (38·8) | 98 (20·8) | 566 (33·8) | |
| 2010–2013 | 469 (38·9) | 179 (38·0) | 648 (38·6) | |
| 2014–2017 | 269 (22·3) | 194 (41·2) | 463 (27·6) |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.). GOJ, gastro‐oesophageal junction.
χ2 test, except
Fisher's exact test and
Student's t test.
Surgical details are presented in Table 2. The distribution between total and distal gastrectomy was almost equal in the overall cohort. There was, however, a difference in the extent of lymphadenectomy related to the type of gastrectomy, with more D1+/D2 being performed in patients having a total gastrectomy (74·5 per cent) than in those having a distal gastrectomy (25·5 per cent). Preoperative chemotherapy was more commonly used in patients who subsequently underwent more extensive lymphadenectomy. Multivisceral resection included pancreatosplenectomy or splenectomy alone, as well as colectomy, adrenalectomy, cholecystectomy, and resection of the diaphragm, small bowel and liver. Multivisceral resection was associated more frequently with D1+/D2 lymphadenectomy. There was no significant difference regarding the use of laparoscopy or open surgery between the groups.
Table 2.
Surgical details of patients undergoing limited D0/D1 lymphadenectomy or more extensive D1+/D2 dissection
| D0/D1 (n = 1206) | D1+/D2 (n = 471) | Overall (n = 1677) | P * | |
|---|---|---|---|---|
| Surgical procedure | < 0·001 | |||
| Distal gastrectomy | 770 (63·8) | 120 (25·5) | 890 (53·1) | |
| Total gastrectomy | 436 (36·2) | 351 (74·5) | 787 (46·9) | |
| Laparoscopic surgery | 0·111 | |||
| No | 1171 (97·1) | 450 (95·5) | 1621 (96·7) | |
| Yes | 35 (2·9) | 21 (4·5) | 56 (3·3) | |
| Preoperative chemotherapy | < 0·001 | |||
| No | 870 (72·1) | 191 (40·6) | 1061 (63·3) | |
| Yes | 322 (26·7) | 280 (59·4) | 602 (35·9) | |
| Missing | 14 (1·2) | 0 (0) | 14 (0·8) | |
| Multivisceral resection | n = 1197 | n = 469 | n = 1666 | < 0·001 |
| No | 1004 (83·9) | 312 (66·5) | 1316 (79·0) | |
| Yes | 193 (16·1) | 157 (33·5) | 350 (21·0) | |
| Pancreatosplenectomy | n = 1197 | n = 469 | n = 1666 | < 0·001 |
| No | 1178 (98·4) | 446 (95·1) | 1624 (97·5) | |
| Yes | 19 (1·6) | 23 (4·9) | 42 (2·5) | |
| Splenectomy | n = 1197 | n = 469 | n = 1666 | < 0·001 |
| No | 1118 (93·4) | 369 (78·7) | 1487 (89·3) | |
| Yes | 79 (6·6) | 100 (21·3) | 179 (10·7) | |
| Emergency operation | n = 1198 | n = 1669 | 0·457 | |
| No | 1162 (97·0) | 460 (97·7) | 1622 (97·2) | |
| Yes | 36 (3·0) | 11 (2·3) | 47 (2·8) |
Values in parentheses are percentages.
χ2 test.
D1+/D2 lymphadenectomy resulted in a significantly greater lymph node yield and, although 30‐ and 90‐day postoperative mortality was similar, there was a slightly higher overall 30‐day complication rate in the D1+/D2 group (Table 3).
Table 3.
Lymph node yield and postoperative complications in patients undergoing limited D0/D1 lymphadenectomy or more extensive D1+/D2 dissection
| D0/D1 (n = 1206) | D1+/D2 (n = 471) | Overall (n = 1677) | P † | |
|---|---|---|---|---|
| No. of lymph nodes * | ||||
| Distal gastrectomy | 16(12) (n = 693) | 25(15) (n = 106) | 17(13) (n = 799) | < 0·001‡ |
| Total gastrectomy | 19(13) (n = 398) | 31(18) (n = 301) | 24(16) (n = 699) | < 0·001‡ |
| 30‐day postoperative mortality | ||||
| No | 1179 (97·8) | 460 (97·7) | 1639 (97·7) | 0·905 |
| Yes | 27 (2·2) | 11 (2·3) | 38 (2·3) | |
| 90‐day postoperative mortality | ||||
| No | 1143 (94·8) | 451 (95·8) | 1594 (95·1) | 0·407 |
| Yes | 63 (5·2) | 20 (4·2) | 83 (4·9) | |
| Overall complications | n = 1105 | n = 410 | n = 1515 | |
| No | 811 (73·4) | 264 (64·4) | 1075 (71·0) | 0·001 |
| Yes | 294 (26·6) | 146 (35·6) | 440 (29·0) |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d).
χ2 test, except
Student's t test.
Overall long‐term survival was slightly better after D1+/D2 lymphadenectomy, with a median overall survival of 41·5 months and 5‐year survival rate of 43·7 per cent compared with 38·5 months median overall survival and 5‐year survival rate of 38·5 per cent for D0/D1, although the difference was not statistically significant (P = 0·116) (Fig. 2). After adjustment for confounders, multivariable analysis indicated that survival was significantly longer after D1+/D2 lymphadenectomy compared with the D0/D1 procedure (HR 0·81, 95 per cent c.i. 0·68 to 0·95; P = 0·012) (Table 4).
Figure 2.
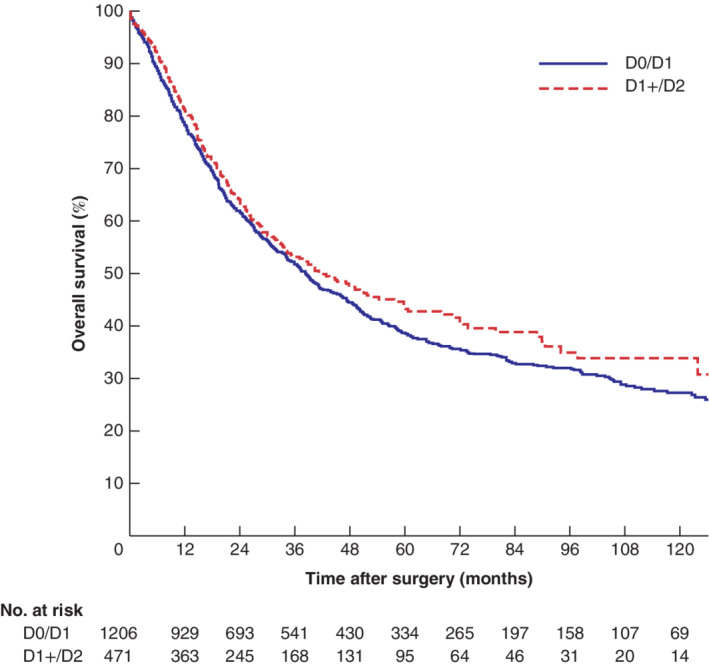
Kaplan–Meier analysis of overall survival in patients undergoing limited or more extensive lymphadenectomy D0/D1, limited lymphadenectomy; D1+/D2, more extensive dissection. P = 0·116 (log rank test).
Table 4.
Cox proportional hazards analysis of the impact of lymphadenectomy on survival
| D1+/D2 versus D0/D1 | ||||
|---|---|---|---|---|
| Crude HR | P | Adjusted HR | P | |
| All patients | 0·89 (0·77, 1·03) | 0·107 | 0·81 (0·68, 0·95) | 0·012 |
| Distal gastrectomy | 0·61 (0·44, 0·84) | 0·003 | 0·75 (0·54, 1·06) | 0·100 |
| Total gastrectomy | 0·86 (0·72, 1·03) | 0·108 | 0·85 (0·70, 1·04) | 0·111 |
Values in parentheses are 95 per cent confidence intervals. Adjusted for age, sex, Charlson Co‐morbidity Index, ASA grade, clinical tumour stage, surgical procedure (in analysis of all cases), multivisceral resection, preoperative chemotherapy, educational level, and calendar year of surgery. HR, hazard ratio.
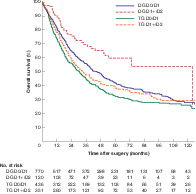
When stratified by surgical procedure, significantly better survival was found in patients having a distal gastrectomy and D1+/D2 lymphadenectomy compared with D0/D1 (5‐year survival rate of 59·6 versus 41·8 per cent respectively; P = 0·002). After total gastrectomy median overall survival was 33·6 months with a 5‐year survival rate of 38·7 per cent in the D1+/D2 group, compared with 30·8 months and 32·6 per cent respectively after D0/D1 lymphadenectomy (P = 0·125) (Fig. 3).
Figure 3.
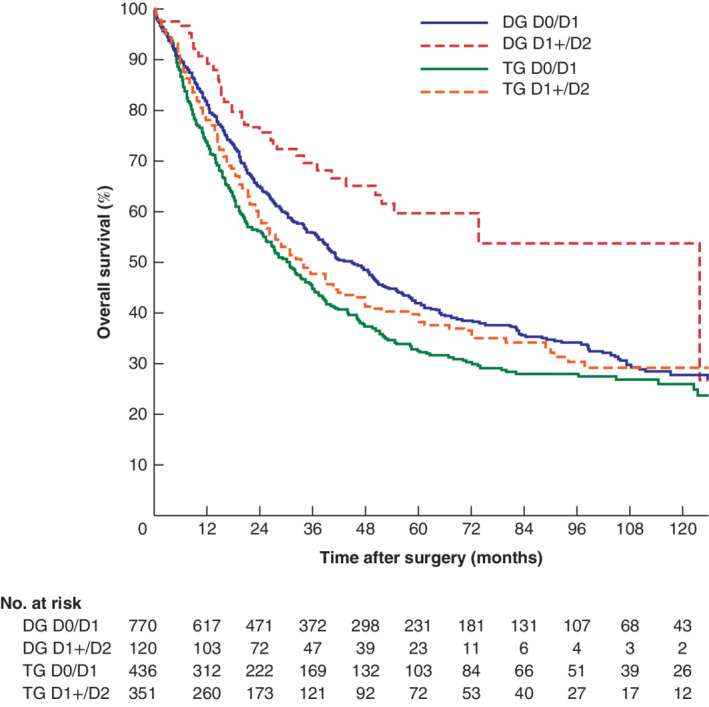
Kaplan–Meier analysis of overall survival after lymphadenectomy, stratified for surgical procedure D0/D1, limited lymphadenectomy; D1+/D2, more extensive dissection. Distal gastrectomy (DG): P = 0·002; total gastrectomy (TG): P = 0·125 (log rank test).
Discussion
This study, based on a prospectively developed database from routine gastric cancer surgery in a Western population, has shown that extended lymphadenectomy (D1+/D2) performed during curatively intended gastrectomy offered better long‐term survival than limited lymphadenectomy (D0/D1). Although none of the three European randomized trials7, 8, 9 detected a long‐term survival advantage following gastrectomy and D2 lymphadenectomy, compared with D1 lymphadenectomy, in two early trials16, 17 D2 lymphadenectomy was associated with high postoperative morbidity and mortality, mainly due to the routine inclusion of splenectomy and pancreatic tail resection. In the latest randomized trial conducted in Italy18, where splenectomy and pancreatic tail resection were not standard in D2 lymphadenectomy, postoperative morbidity and mortality were substantially lower. In the Dutch trial10, after 15 years of follow‐up and with exclusion of postoperative deaths, a survival benefit was detected. The notion that D2 dissection can nowadays be safely carried out in Western centres was supported by a previous national registry study14 showing acceptable complication rates.
The traditional view is that the highest level of evidence is obtained from well designed and adequately powered RCTs. Such studies are not, however, always generalizable owing to specific patient entry criteria. On the other hand, well defined population‐based studies using data retrieved from well validated national registers, with minimal selection bias and accurate follow‐up information, reflect standard practice. The national NREV database includes detailed surgical data regarding the type of resection, including dissected lymph node stations. Most importantly, the validity and quality of the data in the NREV register have been recognized as high12. Some potential weaknesses must, however, be acknowledged. Detailed information on non‐surgical oncological treatments has been included only recently in the database, and was not available at the time of the present study. Although a significantly greater proportion of patients having the D1+/D2 procedure received chemotherapy, survival benefit estimates among this group remained after adjustment in the multivariable model. This suggests that chemotherapy might best be considered an adjunct to adequate lymphadenectomy as a means of improving survival after gastric cancer surgery.
Another potential problem is the risk of misclassification of the lymphadenectomy groups. There is a possibility of yielding no lymph nodes from a specific station, and of removal of nodes outside the registered extent of the resection. Information on specific lymphadenectomy stations was entered into the registry by the operating surgeon, but there has been no external validation of this. The study period covered modifications to the lymphadenectomy classifications from the second to the fourth English editions of the JGCA guidelines, with substantial change between the second and third editions6, 19, 20. Despite these changes, it was notable that a significantly higher lymph node harvest was found in the D1+/D2 group in the present study than in the D0/D1 group. It seems unlikely that misclassification of lymphadenectomy would have a large impact on the results of this study, as such a misclassification would most likely occur randomly, affecting each study group equally.
In Sweden, D1+/D2 lymphadenectomy was performed more commonly during total rather than distal gastrectomy, yet the greatest impact of the extent of lymphadenectomy on survival was seen in patients who had a distal gastrectomy. This difference accounts for a major part of the survival advantage in the adjusted Cox proportional hazards modelling, although it should be noted that lack of awareness of the latest JGCA guidelines on adequate lymphadenectomy during distal gastric cancer surgery may be important. Despite these limitations, this population‐based registry study of a nation's routine practice shows that long‐term survival after gastric cancer surgery is improved following gastrectomy with D1+/D2 compared with less extensive lymphadenectomy.
Acknowledgements
This study was funded by a research grant from the Swedish Cancer Society.
J.A.T. is currently employed by Sanofi Genzyme.
Disclosure: The authors declare no other conflict of interest.
Funding information
Swedish Cancer Society
References
- 1. Al‐Batran SE, Homann N, Pauligk C, Goetze TO , Meiler J, Kasper S et al; FLOT4‐AIO Investigators . Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro‐oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019; 393: 1948–1957. [DOI] [PubMed] [Google Scholar]
- 2. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M et al; MAGIC Trial Participants . Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20. [DOI] [PubMed] [Google Scholar]
- 3. Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G et al Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011; 29: 1715–1721. [DOI] [PubMed] [Google Scholar]
- 4. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW et al; CLASSIC trial investigators . Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5‐year follow‐up of an open‐label, randomised phase 3 trial. Lancet Oncol 2014; 15: 1389–1396. [DOI] [PubMed] [Google Scholar]
- 5. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A et al; ACTS‐GC Group . Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. N Engl J Med 2007; 357: 1810–1820. [DOI] [PubMed] [Google Scholar]
- 6. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017; 20: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I et al; Dutch Gastric Cancer Group . Extended lymph‐node dissection for gastric cancer. N Engl J Med 1999; 340: 908–914. [DOI] [PubMed] [Google Scholar]
- 8. Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V et al Patient survival after D1 and D2 resections for gastric cancer: long‐term results of the MRC randomized surgical trial. Surgical Co‐operative Group. Br J Cancer 1999; 79: 1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Degiuli M, Sasako M, Ponti A, Vendrame A, Tomatis M, Mazza C et al; Italian Gastric Cancer Study Group . Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014; 101: 23–31. [DOI] [PubMed] [Google Scholar]
- 10. Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15‐year follow‐up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010; 11: 439–449. [DOI] [PubMed] [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 12. Linder G, Lindblad M, Djerf P, Elbe P, Johansson J, Lundell L et al Validation of data quality in the Swedish National Register for Oesophageal and Gastric Cancer. Br J Surg 2016; 103: 1326–1335. [DOI] [PubMed] [Google Scholar]
- 13. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C et al External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kung CH, Song H, Ye W, Nilsson M, Johansson J, Rouvelas I et al Extent of lymphadenectomy has no impact on postoperative complications after gastric cancer surgery in Sweden. Chin J Cancer Res 2017; 29: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Linder G, Sandin F, Johansson J, Lindblad M, Lundell L, Hedberg J. Patient education‐level affects treatment allocation and prognosis in esophageal‐ and gastroesophageal junctional cancer in Sweden. Cancer Epidemiol 2018; 52: 91–98. [DOI] [PubMed] [Google Scholar]
- 16. Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT et al Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995; 345: 745–748. [DOI] [PubMed] [Google Scholar]
- 17. Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V et al Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996; 347: 995–999. [DOI] [PubMed] [Google Scholar]
- 18. Degiuli M, Sasako M, Ponti A. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg 2010; 97: 643–649. [DOI] [PubMed] [Google Scholar]
- 19. Japanese Gastric Cancer Association . Japanese Classification of Gastric Carcinoma – 2nd English Edition. Gastric Cancer 1998; 1: 10–24. [DOI] [PubMed] [Google Scholar]
- 20. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14: 113–123. [DOI] [PubMed] [Google Scholar]


