Abstract
Background
The brain's major inhibitory neurotransmitter gamma-aminobutyric acid (GABA) and the brain-derived neurotrophic factor (BDNF) play important roles in several stress-related disorders. Magnetic resonance spectroscopy (MRS) allows for non-invasive quantification of GABA concentration in the brain. We investigated the relationship between GABA concentration in the left dorsolateral prefrontal cortex (DLPFC) and BDNF concentration in the serum in a community-based sample of young subjects.
Methods
For the GABA measurement a single voxel MR spectrum was assessed in the prefrontal lobe (25 × 40 × 30 mm) using the MEGA-PRESS method in 276 subjects. BDNF serum concentrations were assessed with an ELISA kit. For 147 subjects we had both MRS and BDNF serum data, and for 79 subjects we had genotype data on the BDNF rs6265 polymorphism. Depressive psychopathology was assessed using Beck's Depression Inventory (BDI), Montgomery-Asberg Depression Rating Scale (MADRS) and Structured Clinical Interviews for Diagnostic and Statistical Manual of Mental Disorders (SCID) for DSM-IV.
Results
GABA concentration in the left DLPFC was negatively associated with BDNF serum concentration (r = -.264, p = .001). This correlation remained significant if corrected for sex (r = -.264, p = .001). BDNF serum concentration was also positively associated with volumes and surface areas of the left prefrontal cortex (p = .048, p = .005). There were no significant associations or interaction with depressive psychopathology (BDI, MADRS, SCID) or rs6265.
Conclusion
The results of this study suggest that GABA, BDNF and prefrontal brain volumes are interrelated, but do not show a strong association to depressive psychopathology, possibly due to the mild forms of psychiatric conditions present in our community-based sample.
Keywords: Neuroscience, Human genetics, Psychiatry, Depression, Psychopharmacology, Clinical psychology, BDNF, GABA, MRS, Neuroimaging, Stress
Neuroscience; Human Genetics; Psychiatry; Depression; Psychopharmacology; Clinical Psychology; BDNF; GABA; MRS; Neuroimaging; Stress
1. Introduction
There is growing evidence that the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) and the brain-derived neurotrophic factor (BDNF) play crucial roles in depression, anxiety, epilepsy, and in the treatment of these conditions (Butler et al., 2018; Gabbay et al., 2017; Hasler, van der Veen, Grillon, Drevets and Shen, 2010; Helms et al., 2006; Long et al., 2013; Moreira et al., 2019; Sanacora et al., 2012).
GABA is the primary inhibitory neurotransmitter in the adult brain and shapes the dynamic neural network via GABAergic interneurons (Fukuchi et al., 2014; Schur et al., 2016). Magnetic resonance spectroscopy (MRS) allows for a non-invasive measurement of the GABA concentration in the brain of living humans. Human and rat studies showed gender-related differences in GABA concentration, with men showing higher GABA concentrations compared to women (Al-Suwailem et al., 2018; Kirkovski et al., 2018; O'Gorman et al., 2011; Pandya et al., 2019).
MRS is an imaging technique to quantify metabolites such as GABA in the brain within one or several voxels of interest (Egerton et al., 2017; Lener et al., 2017; Long et al., 2013). With MRS, signals from total intra- and extracellular GABA as well as GABAergic interneurons across all tissue content are measured (Egerton et al., 2017; Schur et al., 2016). The MRS-visible GABA signal therefore reflects the dynamic balance between GABA synthesis, reuptake and degradation around the synapses (Hasler et al., 2010), and has been shown to be sensitive to medication-induced changes (e.g. from antiepileptic drugs like topiramate (Kuzniecky et al., 2002; Reis et al., 2002), which also induce changes in cortical excitability in humans (Dyke et al., 2017).
BDNF is a protein that belongs to the neurotrophin family of growth factors and it is involved in several aspects of neuronal functioning (Naegelin et al., 2018). BDNF can be found in the brain as well as in the blood, secreted by circulating platelets in humans, which release BDNF during blood coagulation. Its concentration can be measured non-invasively in serum (Lommatzsch et al., 2005; Yamamoto and Gurney, 1990). BDNF serum concentrations have been associated with the BDNF concentration within the brain (Karege et al., 2002; Klein et al., 2011). However, the mechanisms explaining this association remain to be fully elucidated (de Azevedo Cardoso et al., 2014; Karege et al., 2002; Naegelin et al., 2018; Vinberg et al., 2013).
The most plausible explanation for the presence of BDNF in the platelets are the platelet-generating megakaryocyte cells. These cells have been found to contain BDNF transcripts and may represent the link between brain and serum BDNF concentrations (Chacon-Fernandez et al., 2016; Naegelin et al., 2018). BDNF serum concentrations were shown to be decreased in untreated depressed participants compared to healthy subjects, and females tend to have lower BDNF serum concentrations compared to men (de Azevedo Cardoso et al., 2014; Elfving et al., 2012; Ellsworth et al., 2013; Kreinin et al., 2015; Lommatzsch et al., 2005; Molendijk et al., 2014; Ozan et al., 2010). Studies also found associations between BDNF and the volume of the prefrontal cortex (Forde et al., 2014; Frodl et al., 2008; Harrisberger et al., 2015; Matsuo et al., 2009; Pezawas et al., 2004), suggesting a link between prefrontal brain structure and function, and BDNF concentration in the serum.
Some rat and human studies have demonstrated interactions between GABA-ergic neurotransmission and BDNF release. On one hand, BDNF can suppress or presynaptically enhance GABA release through the activation of postsynaptic tropomyosin receptor kinase B (TrkB)-type receptors, which may result in an altered total GABA concentration (Bulleit and Hsieh, 2000; Colino-Oliveira et al., 2016; Kim et al., 2017; Pezet et al., 2002; Tanaka et al., 1997; Wardle and Poo, 2003). Conversely, changes in the balance between excitatory glutamatergic and inhibitory GABAergic neurotransmission has been shown to alter BDNF expression (Saffarpour et al., 2017). The activation of glutamate receptors enhances the synthesis of BDNF, whereas stimulation of the GABAergic system decreases BDNF synthesis and thus may alter BDNF concentration in the brain and in the blood (Fukuchi et al., 2014; Marmigere et al., 2003; Zafra et al., 1991).
Diminished GABA-ergic transmission in the prefrontal cortex has been related to circuit dysfunction in mood- and stress-related disorders including major depressive disorder (MDD) (Gerner et al., 1984; Ghosal et al., 2017; Hasler et al., 2010). Given the results from rat and human studies cited above, we hypothesized that a higher left dorsolateral prefrontal cortex (DLPFC) GABA concentration is associated with lower BDNF serum concentration, and that this association differs between genders. To elucidate this putatively inverse relationship between GABA and BDNF in healthy and depressed subjects, we investigated healthy subjects and current and remitted MDD patients in a community-based sample. In particular, we assessed left prefrontal GABA concentrations with single-voxel MRS and BDNF concentration in the serum. Clinical characterization of the research subjects included diagnostic interviews based on DSM-IV and standard rating instruments for depressive symptoms.
2. Methods and materials
2.1. Recruitment
Participants were recruited by advertisements in local newspapers or by blackboard webpages of the University of Zurich for this ongoing cohort study. This provided us with a sample of young adults from the general population. After a full explanation of the goals and risks of the study and after giving their consent, participants were admitted to the study. Inclusion criteria were age between 18 and 40 years and preferably being without any psychopharmacological medication for the last three months before testing to minimize the possible effects on the brain. Participants with current or remitted MDD were included into the study, but other severe psychiatric disorders, for example schizophrenia were excluded. The local ethics committee approved the study and the written consent (Kantonale Ethikkomission Zürich). The study described has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. In one of our previous studies (Hasler et al., 2019) we report data from a subset of the participants in the present study.
2.2. Procedure
After giving consent the participants were screened for magnetic resonance (MR) safety with a standard checklist and for psychological disorders with the screening part of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (SCID) for DSM-IV. Later the participants completed an extensive battery of psychological and physiological questionnaires. Validated German versions of all instruments were used. Finally, an approximately five hour long face-to-face session at the Children's Hospital in Zurich was conducted.
The face-to-face session, during which participants had to complete paper-pencil versions of psychological questionnaires, was supervised by a trained psychologist. Fifty ml blood samples were collected by trained staff from the hospital after the psychological assessments. Subjects underwent an MR session in a 3.0T GE Discovery MR750 scanner.
2.3. Sample
276 subjects were assessed with MRS, in 147 we had both MRS and BDNF serum data (67.6% females) and in 79 we also had genotype data on the polymorphism rs6265 of the BDNF gene.
Exclusion criteria were age outside the range of 18–40 years, preferably no use of psychopharmacological drugs in the past three months, severe psychiatric disorders such as schizophrenia, drug or alcohol abuse or and any illnesses or accidents affecting the brain or genetic variance, as for example brain injuries or diabetes.
Of the 276 subjects who underwent magnetic resonance imaging (MRI) scanning, two were excluded because they had mild abnormalities that could potentially influence the results (an arachnoid cyst in the right posterior lateral ventricle; a lipoma with dysplasia of the corpus callosum) leaving us with a sample of 274 subjects. Of these 274 subjects, 75 were diagnosed with MDD (16 currently depressed and 59 partially or fully remitted). Out of the reported 147 subjects with both GABA and BDNF serum data, 29 were diagnosed with MDD, including eight currently depressed and 21 partially or fully remitted (Table 1). Three of the MDD cases were on psychotropic medication (antidepressants or antipsychotics). Out of the 147 subjects, 99 were females and 54 of them were taking contraceptive medication.
Table 1.
Clinical characteristics of the original sample (N = 274) and the subsample (N = 147) with available data concerning GABA concentration and BDNF serum concentration used in this study.
| GABA sample | GABA-BDNF subsample | |
|---|---|---|
| N | 274 | 147 |
| Age Mean (SD) [years] | 24.68 (4.57) | 24.45 (4.35) |
| Females | 179 (65.3%) | 99 (67.3%) |
| MDD current or remitted (N) | 75 (27.4%) | 29 (19.7%) |
| Medicated Subjects | 3 (1.1%) | 2 (1.4%) |
| Mean BDNF serum concentration (pg/ml) | - | 11500 (3400) |
| BDNF rs6265 genotypes (subsample N = 109/79) | c/c 61 c/t 43 t/t 5 | c/c 43 c/t 33 t/t 3 |
| BDI Mean (SD) | 4.81 (6.87), 2 missing | 3.81 (6.53), 2 missing |
| MADRS Mean (SD) | 3.44 (5.56), 1 missing | 2.96 (5.90), 1 missing |
| Volume left prefrontal cortex (gyri and sulci, cm3) | 52.84 (6.10), 1 missing | 53.18 (6.17) |
| Surface left prefrontal cortex (gyri and sulci, cm2) | 165.16 (17.95), 1 missing | 165.71 (17.33) |
2.4. Blood sampling
Venous blood samples were taken on the day of MR scanning in the Children's Hospital (mean time 2:12 p.m., standard deviation 1.1 h). The blood samples were analysed by specialized laboratories in Basel (Switzerland) and New York (United States).
BDNF serum samples were stored at −80 °C before assaying BDNF content. An enzyme-linked immunosorbent assay (ELISA) kit (Biosensis® Mature BDNF RapidTM ELISA Kit: Human, Mouse, Rat; Thebarton, SA, Australia) was used to assess the serum BDNF concentrations. Serum samples were appropriately diluted (1:100) and detection of BDNF was carried out on a pre-coated mouse monoclonal anti-mature BDNF 96-well plate as described in the manufacturer's protocol. The absorbance was measured within 5 min after addition of the stop solution in a microplate reader set at 450nm and a correction wavelength set to 690nm, to determine BDNF concentrations according to the standard curve. All assays were carried out in duplicate and means were calculated.
2.5. MRI data acquisition and analyses
MRI data were obtained with a 3.0 GE Discovery MR750 scanner equipped with an eight-channel receive-only head coil. The measurements included a localizer and a 3D T1-weighted spoiled gradient recalled (SPGR) sequence for the assessment of global and local brain volumes (162 slices of 256 x 256 voxels, 1 × 1 × 1 mm resolution; TR = 11 ms, TE = 5 ms, TI = 600 ms, flip angle 8°), which was used for the localization of the MR spectra.
T1-weighted images were segmented and warped into MNI space using “new segment” procedure in SPM12 for Matlab. For volumetric measurements, freesurfer (version 5.3.0) was run over the 3-dimensional T1-weighted images (Dale et al., 1999; Fischl et al., 1999). Volumes of subcortical structures, whole brain volumes and areas and thicknesses of neocortical areas can be analysed by this software.
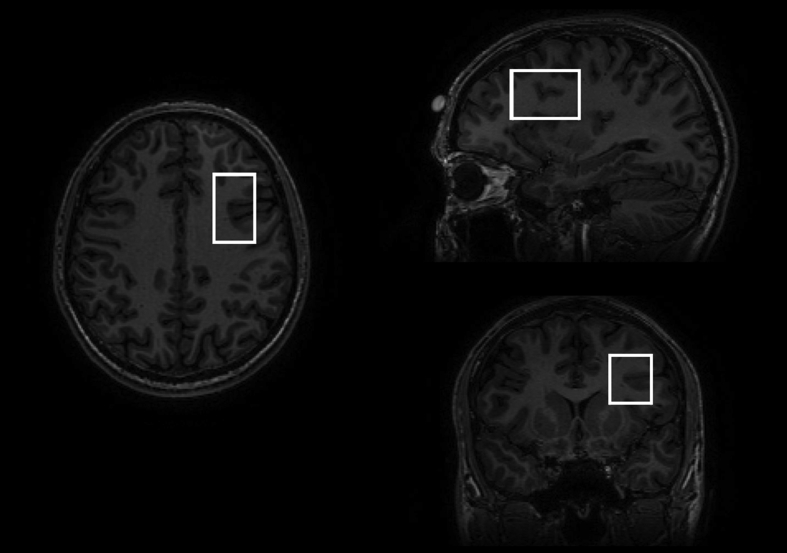
MR spectra were acquired from a 25 × 40 × 30 mm voxel in the left frontal lobe using the MEGA-PRESS method (TE = 69 ms, TR = 2000 ms, 320 averages and eight-step phase cycle; Figure 1). We chose the left DLPFC (and not the right one) because most MRS studies examined the left side (Moriguchi et al., 2018), and because of the default direction of the chemical shift effect on the scanner, which by results in fat being shifted to the left (so for a voxel on the left side of the brain, scalp fat is shifted away from the voxel), and water being shifted to the right (so for a voxel on the left side of the brain, ventricular water is also shifted away from the voxel). Due to the major overlapping signals for GABA with those from N-acetyl aspartate (NAA) and Creatine, the MEGA-PRESS technique was used to edit the GABA signal (Schur et al., 2016). Due to the overlap of the edited GABA peak with co-edited macromolecules, findings represent GABA+ rather than pure GABA values.
Figure 1.
MR images showing the position of the measured voxel in the left DLPFC.
MR spectra were preprocessed with a vendor-provided pipeline, which included steps for coil combination, frequency alignment, phasing, residual water removal, and averaging, and both the edited data and water reference data were saved as.RAW files for processing with LCModel. Spectral fitting for the edited data was performed with LCModel version 6.3 using a simulated basis set, including basis spectra for GABA, Glutamate, Glutamine, Glutathione, N-acetyl-aspartate (NAA), and N-acetyl-aspartly-glutamate (NAAG). The control parameter sptype was set to ‘mega-press-2’ to control the baseline fitting, and spectra were visually inspected for the quality of the GABA fit.
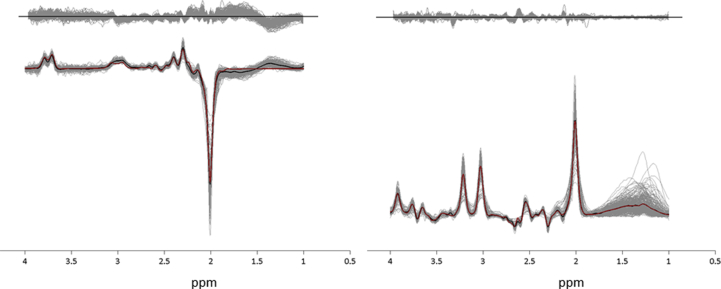
Metabolite levels with a Cramer-Rao lower bound (CRLB) of more than 20% were excluded from the analysis. Water-scaled GABA+ concentrations were calculated in institutional units after correcting the GABA+ and estimated water concentrations for cerebrospinal fluid contamination (atrophy) within the voxel (Chowdhury et al., 2015). Figure 2 shows the edited and unedited spectra for all participants, together with the group mean spectra and the mean LCModel fit across the group.
Figure 2.
Edited and unedited spectra for all participants. The individual spectra for all participants are shown in grey, with the group-mean spectra overlaid in black and the mean LCModel fit overlaid in red. The residuals from each participant are also plotted in grey above the spectra.
2.6. Psychological measurements
Subjects filled out the Becks Depression Inventory (BDI) (Beck et al., 1961) and the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) shortly before MR scanning at the Children's Hospital in Zurich, using the validated German versions. Psychiatric diagnoses were made based on the SCID for DSM-IV (First et al., 2001).
2.7. Statistics
In explorative analyses, we tested associations between GABA+ concentration, biological measures (BDNF serum concentrations and left DLPFC volumes) and depressive psychopathology (BDI, MADRS, MDD DSM-IV diagnosis) using Pearson's bivariate correlation analyses in SPSS (IBM SPSS Statistics, Version 24).
Due to previously reported gender differences concerning both GABA and BDNF serum concentrations we repeated the analyses in the female and male subsample. MDD was added to the analyses as confounding parameter in a partial correlation. Post-hoc analyses were also conducted to examine the link between BDNF and other neuro-metabolites present in the edit OFF spectra, namely, NAA, Creatine, Choline, and myo-inositol.
3. Results
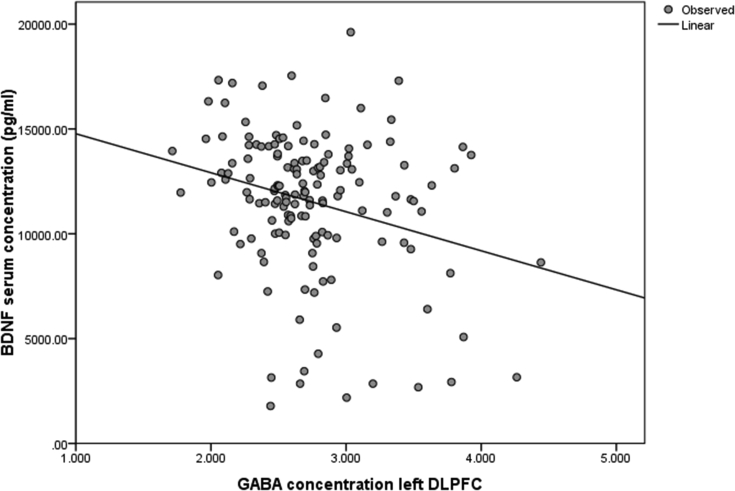
Figure 3 shows that GABA+ concentration in the DLPFC was significantly negatively associated with the BDNF serum concentration (r = -.264, p = .001, N = 147; Figure 3). This negative association was significant in the female subsample (r = -.306, p = .002, N = 99) but not in the male subsample (r = -.189, p = .197, N = 48).
Figure 3.
Bivariate correlation between GABA concentration in the left prefrontal cortex, corrected for atrophy and water scaling, and BDNF serum concentration, normalized and averaged (r = -.264, p = .001, N = 147).
BDNF serum concentrations were positively associated with the volumes of the left prefrontal cortex (gyri and sulci; r = .164, p = .048, N = 147), and the surface area of the left prefrontal cortex (gyri and sulci; r = .231, p = .005, N = 147). GABA+ concentration showed no significant association with the volume or the surface of the left prefrontal cortex (volume: r = -.128, p = .122; surface: r = -.089, p = .282; N = 147).
GABA+ concentration in the left DLPFC was not significantly associated with the diagnosis of MDD (r = -.092, p = .268, N = 147), BDI sum score (r = -.043, p = .604, N = 145) and MADRS total score (r = -.081, p = .333, N = 146, 1 missing). There was no significant association between BDNF concentration in the serum and MDD diagnosis (r = .105, p = .203, N = 147), BDI sum score (r = -.031, p = 709, N = 145) and MADRS total score (r = .099, p = .236, N = 146). BDNF concentration in the serum was not significantly associated with hippocampal volumes (left hippocampus volume: r = -.058, p = .488; right hippocampus volume: r = .041, p = .618; N = 147). In N = 79 subjects we had genotype data on the BDNF polymorphism rs6265. We did not find a significant association between this polymorphism and bilateral hippocampal brain volumes (left hippocampus volume: r = .159, p = .161; right hippocampus volume: r = .170, p = .135; N = 79), BDNF serum concentration (r = .059, p = .608, N = 79) or prefrontal GABA+ concentration (r = -.038, p = .742, N = 79). This result remained unchanged after excluding the three medicated subjects (data not shown). Using age, BMI or sports activity as a control variable did not change the results either.
The bivariate correlations were also calculated for genders separately, due to reported sex-differences in GABA and BDNF systems. There was a trend for an association between left DLPFC GABA+ concentration and MADRS total score in male subjects (r = -.267, p = .067, N = 48) but not in female subjects (r = -.010, p = .922, N = 98). The partial correlation showed a significant association between prefrontal GABA+ and BDNF serum concentration if corrected for sex (r = -.264, p = .001).
The partial correlation revealed that MDD did not change the significant results between GABA+ and BDNF serum concentration (r = -.257, p = .002, N = 144), between BDNF concentration in the serum and left prefrontal volume and surface-area (volume: r = .197, p = .017; surface-area: r = .266, p = .001; N = 144). The use of contraceptives was not associated with the BDNF polymorphism (r = .032, p = .780, N = 79), BDNF serum concentration (r = .097, p = .241, N = 147) and prefrontal GABA+ concentration (r = -.053, p = .525, N = 147). The post-hoc tests for a link between BDNF and other neuro-metabolites present in the edit OFF spectra did not reveal any significant associations (all p > 0.12). Using Creatinine instead of H2O as reference signal did not change the main findings of this study.
4. Discussion
In a cohort of young adults from the general population, we investigated the link between GABA+ levels in the left DLPFC, prefrontal brain volumes and surface-areas, serum BDNF concentrations, and depressive psychopathology.
The main finding of the present study is that higher GABA+ concentrations in the left DLPFC were associated with lower BDNF serum concentrations in young adults. No association was found between the BDNF polymorphism and GABA+ concentration. In the gender-specific analyses, the significant association between GABA+ and BDNF serum concentration held true in the female but not in the male subsample. BDNF serum concentration was associated with increased brain volumes and surface-areas in the left prefrontal cortex. No associations were found between GABA+ concentration, BDNF polymorphism and left prefrontal volumes and surface-areas. Depressive symptoms as measured by BDI and MADRS and MDD diagnosis were not associated with GABA+, BDNF polymorphism or BDNF serum concentrations, but we found a negative trend towards a correlation between GABA+ concentration and MADRS total score in males but not in females. Depression did not affect the association between GABA and BDNF.
Previous studies investigating the relationship between GABA and BDNF have suggested a complex and not yet fully understood interplay between the two factors, as discussed in the introduction (Bulleit and Hsieh, 2000; Colino-Oliveira et al., 2016; Marmigere et al., 2003; Pezet et al., 2002; Saffarpour et al., 2017; Zafra et al., 1991). The connection between GABA and BDNF may depend on pre- and postsynaptic effects of the two factors and may differ considerably between brain regions (Marmigere et al., 2003; Saffarpour et al., 2017; Tanaka et al., 1997; Zafra et al., 1991). Both GABA and BDNF serum concentrations have been shown to be reduced in subjects with MDD and subjects after acute and chronic stress (Fogaca and Duman, 2019; Hasler et al., 2010; Kishi et al., 2017). However, in remitted subjects with MDD, prefrontal GABA concentration was found to be normal (Hasler et al., 2005; Schur et al., 2016). Our findings of normal BDNF and GABA concentrations in MDD may therefore arise as a result of our community-based sampling method, leading to the inclusion of subjects with rather mild, partly remitted and unmedicated MDD.
Previous studies on serum BDNF concentrations have found gender differences, with men showing higher BDNF serum concentrations compared to women (de Azevedo Cardoso et al., 2014; Ozan et al., 2010). Our study suggests that gender differences in BDNF concentrations are unrelated to depression-related disorders, and could possibly be related to the effects of sex hormones (Elfving et al., 2012).
To date, our knowledge about sex-related differences concerning the GABAergic system is limited. Some studies have shown higher GABA concentrations in men compared to women (O'Gorman et al., 2011; Pandya et al., 2019). Our significant correlation between GABA+ and BDNF serum concentration in the female but not in the male subsample may reflect both GABA and BDNF specific gender differences. The negative trend in our results between GABA+ concentrations and MADRS scores in the male but not in the female subsample may also reflect the gender differences concerning GABA but should be interpreted with caution. Although many previous studies investigated GABA and BDNF serum concentrations in subjects with psychiatric disorders, the apparent gender differences in both GABA+ and BDNF seen in our study of young adults from the general population indicates that gender should also be considered in group comparisons of healthy samples with psychiatric groups (Molendijk et al., 2014). In addition, since BDNF concentrations can be normalized by antidepressant treatment (Kreinin et al., 2015), medication status should be taken into account to avoid potential confounds from medication effects.
BDNF is strongly expressed in the limbic brain regions, hence many studies have tested for associations between hippocampal volumes and BDNF serum concentrations, yielding inconsistent results (Frodl et al., 2014; Harrisberger et al., 2015). In our study, we did not find a significant correlation between BDNF concentration in the serum or the BDNF polymorphism rs6265 and hippocampal brain volumes.
With regard to the BDNF polymorphism, we did not find a significant association with BDNF concentration in the serum. The BDNF gene is located on chromosome 11p13 and encodes proBDNF, which generates mature BDNF, which can be measured in plasma and serum (Elfving et al., 2012). Most studies have researched the single nucleotide polymorphism (SNP) rs6265 (Val66Met) of the BDNF gene, but the association between this polymorphism and BDNF serum concentrations show divergent results and the mechanism has yet to be demonstrated in detail (Karege et al., 2002; Kishi et al., 2017; Naegelin et al., 2018; Ozan et al., 2010). The Met allele of the rs6265 polymorphism is thought to impair the intracellular trafficking of proBDNF into the dendrites and vesicles, thus this SNP seems to play an important role in the regulation of extracellular BDNF concentrations (Kishi et al., 2017). A series of studies have examined the SNP rs6265 regarding the risk of depression and hippocampal volumes (Elfving et al., 2012). In healthy controls, homozygous Val/Val carriers of the rs6265 polymorphism showed larger hippocampal volumes compared to Met carriers (Hajek et al., 2012; Kishi et al., 2017). However, Harrisberger et al. found no such association between the rs6265 polymorphism and hippocampal volume in patients with MDD (Harrisberger et al., 2015). Forde et al. showed that the BDNF Val66Met polymorphism may not be as specific as previously supposed, but may have a global effect on the cortical morphology in humans (Forde et al., 2014). Matsuo et al. presented smaller DLPFC volumes in Val/Met carriers as compared to the Val/Val subjects (Matsuo et al., 2009). Our results of increased BDNF serum concentration being associated with larger volumes and surface-areas in the prefrontal cortex in young adults may reflect the association between BDNF polymorphism and prefrontal brain volume, but still it remains unclear whether and how BDNF serum concentration is linked to BDNF in the brain (Naegelin et al., 2018).
Recently it has been suggested that, aside from platelet-generating megakaryocytes, epigenetics may play an important role in the association between BDNF serum and brain BDNF concentrations (Chacon-Fernandez et al., 2016; Chen and Chen, 2017; Mallei et al., 2015; Naegelin et al., 2018). Mallei et al. showed that homozygous mice Met carriers displayed repressive neuronal activation of BDNF and repressive epigenetic changes in certain BDNF promoters, which is accompanied by reduced transcription of BDNF promoters (Mallei et al., 2015).
Our non-significant results regarding serum BDNF concentration and depression-related symptoms may indicate that the downregulation of BDNF serum concentration may not be associated with healthy or mostly remitted MDD participants, but may be specific to severe MDD and reflect the complex molecular pathologies of psychiatric disorders (Guilloux et al., 2012).
The present study provides insight into the association of prefrontal GABA concentration, BDNF serum concentration, prefrontal brain volumes, and depressive symptoms in a cohort of young adults from the general population. The approach of a community-based sample of young subjects should be considered a strength of the present study, as the comparability to the general population is higher than in studies that included severely depressed participants under psychopharmacological drugs. To our knowledge, the association between prefrontal GABA and BDNF serum concentration has not been examined in in humans, although animal studies have suggested an important connection between central GABA concentration and BDNF. Furthermore, our study was large enough to study the sexes separately. We sought to keep the time of the blood and MRS measurements constant, because BDNF shows a strong circadian variation (Begliuomini et al., 2008).
The following limitation of our study merits comment. MRS total GABA signal was measured within a relatively large voxel. Our method does not allow for discrimination between GABA concentrations of different cell types. The spectral overlap between GABA and other metabolites remain a challenging issue with MRS. We used the MEGA-PRESS technique to address this limitation, but it is important to note that some macromolecular contribution is likely to be present in the GABA signal detected by MEGA-PRESS, and this signal should therefore be regarded as GABA+ rather than as pure GABA.
In the present study, two specific aspects of BDNF, namely its concentration in the serum and the rs6265 polymorphisms were investigated. The relationship between brain and serum BDNF has still to be elucidated and thus the results should be considered with caution. Although contraceptive medication does not seem to impact our results, we cannot rule out the possibility that differences between females and males are moderated by sex-hormones. As our sample consisted of young subjects from the general population with low depression scores, our results may differ from samples with current MDD subjects.
In conclusion, our data suggest a complex but important association between GABA+ concentration and BDNF serum concentration, prefrontal brain volumes and possible gender related differences in young adults. To understand the mechanisms concerning the interaction between BDNF and GABA concentration better, it would be interesting to combine the factors investigated in this study with stress-related epigenetics in a bigger sample. We are planning to conduct follow-up assessments of our cohort to evaluate the predictive value of our measures. For the gender differences concerning GABA and BDNF concentration future studies should focus on gender-matching between groups and should consider the menstrual cycle, contraceptive use and sex-hormones of female participants. In addition, in future studies it would be interesting to examine the effects of neurotrophic factors and epigenetic markers and their influence on depression and stress-related disorders.
Declarations
Author contribution statement
Sabrina Theresia Müller, Christopher Ritter: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Andreas Buchmann, Carmen Ghisleni: Performed the experiments; Wrote the paper.
Melanie Haynes, Gregor Hasler: Conceived and designed the experiments; Wrote the paper.
Ruth Tuura: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the University of Bern, Switzerland, and the University Children’s Hospital Zurich, Switzerland.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to all the participants who took part in this study. We thank Fabia Colombo, Isabelle Fischer and Sarela Brechbühl for data acquisition and data entry. We would like to express our gratitude to Steffen Bollmann and Yosuke Morishima for their highly valued support in the technical setup of the study.
References
- Al-Suwailem E., Abdi S., El-Ansary A. Sex differences in the glutamate signaling pathway in juvenile rats. J. Neurosci. Res. 2018;96(3):459–466. doi: 10.1002/jnr.24144. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatr. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Begliuomini S., Lenzi E., Ninni F., Casarosa E., Merlini S., Pluchino N.…Genazzani A.R. Plasma brain-derived neurotrophic factor daily variations in men: correlation with cortisol circadian rhythm. J. Endocrinol. 2008;197(2):429–435. doi: 10.1677/JOE-07-0376. [DOI] [PubMed] [Google Scholar]
- Bulleit R.F., Hsieh T. MEK inhibitors block BDNF-dependent and -independent expression of GABA(A) receptor subunit mRNAs in cultured mouse cerebellar granule neurons. Brain Res. Dev. Brain Res. 2000;119(1):1–10. doi: 10.1016/s0165-3806(99)00119-4. [DOI] [PubMed] [Google Scholar]
- Butler K.M., Moody O.A., Schuler E., Coryell J., Alexander J.J., Jenkins A., Escayg A. De novo variants in GABRA2 and GABRA5 alter receptor function and contribute to early-onset epilepsy. Brain. 2018;141(8):2392–2405. doi: 10.1093/brain/awy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon-Fernandez P., Sauberli K., Colzani M., Moreau T., Ghevaert C., Barde Y.A. Brain-derived neurotrophic factor in megakaryocytes. J. Biol. Chem. 2016;291(19):9872–9881. doi: 10.1074/jbc.M116.720029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.W., Chen L. Epigenetic regulation of BDNF gene during development and diseases. Int. J. Mol. Sci. 2017;18(3) doi: 10.3390/ijms18030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury F.A., O'Gorman R.L., Nashef L., Elwes R.D., Edden R.A., Murdoch J.B.…Richardson M.P. Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. J. Magn. Reson. Imag. 2015;41(3):694–699. doi: 10.1002/jmri.24611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colino-Oliveira M., Rombo D.M., Dias R.B., Ribeiro J.A., Sebastiao A.M. BDNF-induced presynaptic facilitation of GABAergic transmission in the hippocampus of young adults is dependent of TrkB and adenosine A2A receptors. Purinergic Signal. 2016;12(2):283–294. doi: 10.1007/s11302-016-9502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- de Azevedo Cardoso T., Mondin T.C., Wiener C.D., Marques M.B., Fucolo Bde A., Pinheiro R.T., Oses J.P. Neurotrophic factors, clinical features and gender differences in depression. Neurochem. Res. 2014;39(8):1571–1578. doi: 10.1007/s11064-014-1349-4. [DOI] [PubMed] [Google Scholar]
- Dyke K., Pepes S.E., Chen C., Kim S., Sigurdsson H.P., Draper A.…Jackson S.R. Comparing GABA-dependent physiological measures of inhibition with proton magnetic resonance spectroscopy measurement of GABA using ultra-high-field MRI. Neuroimage. 2017;152:360–370. doi: 10.1016/j.neuroimage.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A., Modinos G., Ferrera D., McGuire P. Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Transl. Psychiatry. 2017;7(6):e1147. doi: 10.1038/tp.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfving B., Buttenschon H.N., Foldager L., Poulsen P.H., Andersen J.H., Grynderup M.B.…Mors O. Depression, the Val66Met polymorphism, age, and gender influence the serum BDNF level. J. Psychiatr. Res. 2012;46(9):1118–1125. doi: 10.1016/j.jpsychires.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Ellsworth K.A., Moon I., Eckloff B.W., Fridley B.L., Jenkins G.D., Batzler A.…Wang L. FKBP5 genetic variation: association with selective serotonin reuptake inhibitor treatment outcomes in major depressive disorder. Pharmacogenetics Genom. 2013;23(3):156–166. doi: 10.1097/FPC.0b013e32835dc133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research, New York State Psychiatric Institute; New York: 2001. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fogaca M.V., Duman R.S. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front. Cell. Neurosci. 2019;13:87. doi: 10.3389/fncel.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde N.J., Ronan L., Suckling J., Scanlon C., Neary S., Holleran L.…Cannon D.M. Structural neuroimaging correlates of allelic variation of the BDNF val66met polymorphism. Neuroimage. 2014;90:280–289. doi: 10.1016/j.neuroimage.2013.12.050. [DOI] [PubMed] [Google Scholar]
- Frodl T., Carballedo A., Frey E.M., O'Keane V., Skokauskas N., Morris D.…Connor T. Expression of glucocorticoid inducible genes is associated with reductions in cornu ammonis and dentate gyrus volumes in patients with major depressive disorder. Dev. Psychopathol. 2014;26(4 Pt 2):1209–1217. doi: 10.1017/S0954579414000972. [DOI] [PubMed] [Google Scholar]
- Frodl T., Moller H.J., Meisenzahl E. Neuroimaging genetics: new perspectives in research on major depression? Acta Psychiatr. Scand. 2008;118(5):363–372. doi: 10.1111/j.1600-0447.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- Fukuchi M., Kirikoshi Y., Mori A., Eda R., Ihara D., Takasaki I.…Tsuda M. Excitatory GABA induces BDNF transcription via CRTC1 and phosphorylated CREB-related pathways in immature cortical cells. J. Neurochem. 2014;131(2):134–146. doi: 10.1111/jnc.12801. [DOI] [PubMed] [Google Scholar]
- Gabbay V., Bradley K.A., Mao X., Ostrover R., Kang G., Shungu D.C. Anterior cingulate cortex gamma-aminobutyric acid deficits in youth with depression. Transl. Psychiatry. 2017;7(8):e1216. doi: 10.1038/tp.2017.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner R.H., Fairbanks L., Anderson G.M., Young J.G., Scheinin M., Linnoila M.…Cohen D.J. CSF neurochemistry in depressed, manic, and schizophrenic patients compared with that of normal controls. Am. J. Psychiatr. 1984;141(12):1533–1540. doi: 10.1176/ajp.141.12.1533. [DOI] [PubMed] [Google Scholar]
- Ghosal S., Hare B., Duman R.S. Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Curr. Opin. Behav. Sci. 2017;14:1–8. doi: 10.1016/j.cobeha.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux J.P., Douillard-Guilloux G., Kota R., Wang X., Gardier A.M., Martinowich K.…Sibille E. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol. Psychiatr. 2012;17(11):1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek T., Kopecek M., Hoschl C. Reduced hippocampal volumes in healthy carriers of brain-derived neurotrophic factor Val66Met polymorphism: meta-analysis. World J. Biol. Psychiatr. 2012;13(3):178–187. doi: 10.3109/15622975.2011.580005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrisberger F., Smieskova R., Schmidt A., Lenz C., Walter A., Wittfeld K.…Borgwardt S. BDNF Val66Met polymorphism and hippocampal volume in neuropsychiatric disorders: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2015;55:107–118. doi: 10.1016/j.neubiorev.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Hasler G., Buchmann A., Haynes M., Müller S., Ghisleni C., Brechbühl S., Tuura R. Association between prefrontal glutamine levels and neuroticism determined using proton magnetic resonance spectroscopy. Transl. Psychiatry. 2019 doi: 10.1038/s41398-019-0500-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G., Neumeister A., van der Veen J.W., Tumonis T., Bain E.E., Shen J.…Charney D.S. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol. Psychiatr. 2005;58(12):969–973. doi: 10.1016/j.biopsych.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Hasler G., van der Veen J.W., Grillon C., Drevets W.C., Shen J. Effect of acute psychological stress on prefrontal GABA concentration determined by proton magnetic resonance spectroscopy. Am. J. Psychiatr. 2010;167(10):1226–1231. doi: 10.1176/appi.ajp.2010.09070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms G., Ciumas C., Kyaga S., Savic I. Increased thalamus levels of glutamate and glutamine (Glx) in patients with idiopathic generalised epilepsy. J. Neurol. Neurosurg. Psychiatry. 2006;77(4):489–494. doi: 10.1136/jnnp.2005.074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F., Perret G., Bondolfi G., Schwald M., Bertschy G., Aubry J.M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatr. Res. 2002;109(2):143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Kim J., Lee S., Kang S., Kim S.H., Kim J.C., Yang M., Moon C. Brain-derived neurotropic factor and GABAergic transmission in neurodegeneration and neuroregeneration. Neural Regen. Res. 2017;12(10):1733–1741. doi: 10.4103/1673-5374.217353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkovski M., Suo C., Enticott P.G., Yucel M., Fitzgerald P.B. Short communication: sex-linked differences in gamma-aminobutyric acid (GABA) are related to social functioning in autism spectrum disorder. Psychiatry Res. Neuroimaging. 2018;274:19–22. doi: 10.1016/j.pscychresns.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Kishi T., Yoshimura R., Ikuta T., Iwata N. Brain-derived neurotrophic factor and major depressive disorder: evidence from meta-analyses. Front. Psychiatr. 2017;8:308. doi: 10.3389/fpsyt.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A.B., Williamson R., Santini M.A., Clemmensen C., Ettrup A., Rios M.…Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int. J. Neuropsychopharmacol. 2011;14(3):347–353. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- Kreinin A., Lisson S., Nesher E., Schneider J., Bergman J., Farhat K.…Pinhasov A. Blood BDNF level is gender specific in severe depression. PLoS One. 2015;10(5):e0127643. doi: 10.1371/journal.pone.0127643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzniecky R., Ho S., Pan J., Martin R., Gilliam F., Faught E., Hetherington H. Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology. 2002;58(3):368–372. doi: 10.1212/wnl.58.3.368. [DOI] [PubMed] [Google Scholar]
- Lener M.S., Niciu M.J., Ballard E.D., Park M., Park L.T., Nugent A.C., Zarate C.A., Jr. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol. Psychiatr. 2017;81(10):886–897. doi: 10.1016/j.biopsych.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M., Zingler D., Schuhbaeck K., Schloetcke K., Zingler C., Schuff-Werner P., Virchow J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging. 2005;26(1):115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Long Z., Medlock C., Dzemidzic M., Shin Y.W., Goddard A.W., Dydak U. Decreased GABA levels in anterior cingulate cortex/medial prefrontal cortex in panic disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;44:131–135. doi: 10.1016/j.pnpbp.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallei A., Baj G., Ieraci A., Corna S., Musazzi L., Lee F.S.…Popoli M. Expression and dendritic trafficking of BDNF-6 splice variant are impaired in knock-in mice carrying human BDNF Val66Met polymorphism. Int. J. Neuropsychopharmacol. 2015;18(12) doi: 10.1093/ijnp/pyv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmigere F., Rage F., Tapia-Arancibia L. GABA-glutamate interaction in the control of BDNF expression in hypothalamic neurons. Neurochem. Int. 2003;42(4):353–358. doi: 10.1016/s0197-0186(02)00100-6. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Walss-Bass C., Nery F.G., Nicoletti M.A., Hatch J.P., Frey B.N.…Soares J.C. Neuronal correlates of brain-derived neurotrophic factor Val66Met polymorphism and morphometric abnormalities in bipolar disorder. Neuropsychopharmacology. 2009;34(8):1904–1913. doi: 10.1038/npp.2009.23. [DOI] [PubMed] [Google Scholar]
- Molendijk M.L., Spinhoven P., Polak M., Bus B.A., Penninx B.W., Elzinga B.M. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484) Mol. Psychiatr. 2014;19(7):791–800. doi: 10.1038/mp.2013.105. [DOI] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Moreira F.P., de Azevedo Cardoso T., Mondin T.C., Wiener C.D., de Mattos Souza L.D., Oses J.P.…da Silva R.A. Serum level of nerve growth factor is a potential biomarker of conversion to bipolar disorder in women with major depressive disorder. Psychiatr. Clin. Neurosci. 2019 doi: 10.1111/pcn.12896. [DOI] [PubMed] [Google Scholar]
- Moriguchi S., Takamiya A., Noda Y., Horita N., Wada M., Tsugawa S.…Nakajima S. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol. Psychiatr. 2018 doi: 10.1038/s41380-018-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naegelin Y., Dingsdale H., Sauberli K., Schadelin S., Kappos L., Barde Y.A. Measuring and validating the levels of brain-derived neurotrophic factor in human serum. eNeuro. 2018;5(2) doi: 10.1523/ENEURO.0419-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gorman R.L., Michels L., Edden R.A., Murdoch J.B., Martin E. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J. Magn. Reson. Imag. 2011;33(5):1262–1267. doi: 10.1002/jmri.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozan E., Okur H., Eker C., Eker O.D., Gonul A.S., Akarsu N. The effect of depression, BDNF gene val66met polymorphism and gender on serum BDNF levels. Brain Res. Bull. 2010;81(1):61–65. doi: 10.1016/j.brainresbull.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Pandya M., Palpagama T.H., Turner C., Waldvogel H.J., Faull R.L., Kwakowsky A. Sex- and age-related changes in GABA signaling components in the human cortex. Biol. Sex Differ. 2019;10(1):5. doi: 10.1186/s13293-018-0214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L., Verchinski B.A., Mattay V.S., Callicott J.H., Kolachana B.S., Straub R.E., Weinberger D.R. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J. Neurosci. 2004;24(45):10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S., Cunningham J., Patel J., Grist J., Gavazzi I., Lever I.J., Malcangio M. BDNF modulates sensory neuron synaptic activity by a facilitation of GABA transmission in the dorsal horn. Mol. Cell. Neurosci. 2002;21(1):51–62. doi: 10.1006/mcne.2002.1166. [DOI] [PubMed] [Google Scholar]
- Reis J., Tergau F., Hamer H.M., Muller H.H., Knake S., Fritsch B.…Rosenow F. Topiramate selectively decreases intracortical excitability in human motor cortex. Epilepsia. 2002;43(10):1149–1156. doi: 10.1046/j.1528-1157.2002.09902.x. [DOI] [PubMed] [Google Scholar]
- Saffarpour S., Shaabani M., Naghdi N., Farahmandfar M., Janzadeh A., Nasirinezhad F. In vivo evaluation of the hippocampal glutamate, GABA and the BDNF levels associated with spatial memory performance in a rodent model of neuropathic pain. Physiol. Behav. 2017;175:97–103. doi: 10.1016/j.physbeh.2017.03.025. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Treccani G., Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62(1):63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur R.R., Draisma L.W., Wijnen J.P., Boks M.P., Koevoets M.G., Joels M.…Vinkers C.H. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum. Brain Mapp. 2016;37(9):3337–3352. doi: 10.1002/hbm.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Saito H., Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J. Neurosci. 1997;17(9):2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinberg M., Bukh J.D., Bennike B., Kessing L.V. Are variations in whole blood BDNF level associated with the BDNF Val66Met polymorphism in patients with first episode depression? Psychiatr. Res. 2013;210(1):102–108. doi: 10.1016/j.psychres.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Wardle R.A., Poo M.M. Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J. Neurosci. 2003;23(25):8722–8732. doi: 10.1523/JNEUROSCI.23-25-08722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Gurney M.E. Human platelets contain brain-derived neurotrophic factor. J. Neurosci. 1990;10(11):3469–3478. doi: 10.1523/JNEUROSCI.10-11-03469.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F., Castren E., Thoenen H., Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc. Natl. Acad. Sci. U. S. A. 1991;88(22):10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]