Abstract
Objectives
To compare clinical, laboratory, and chest computed tomography (CT) findings in critically ill patients diagnosed with coronavirus disease 2019 (COVID-19) who survived and who died.
Methods
This retrospective study reviewed 60 critically ill patients (43 males and 17 females, mean age 64.4 ± 11.0 years) with COVID-19 pneumonia who were admitted to two different clinical centers. Their clinical and medical records were analyzed, and the chest CT images were assessed to determine the involvement of lobes and the distribution of lesions in the lungs between the patients who recovered from the illness and those who died.
Results
Compared with recovered patients (50/60, 83%), deceased patients (10/60, 17%) were older (mean age, 70.6 vs. 62.6 years, p = 0.044). C-reactive protein (CRP) (110.8 ± 26.3 mg/L vs 63.0 ± 50.4 mg/L, p < 0.001) and neutrophil-to-lymphocyte ratio (NLR) (18.7 ± 16.6 vs 8.4 ± 7.5, p = 0.030) were significantly elevated in the deceased as opposed to the recovered. Medial or parahilar area involvement was observed in all the deceased patients (10/10, 100%), when compared to only 54% (27/50) in the recovered. Ground-glass opacities (97%), crazy-paving pattern (92%), and air bronchogram (93%) were the most common radiological findings. There was significant difference in diabetes (p = 0.025) and emphysema (p = 0.013), and the odds ratio on a deceased patient having diabetes and emphysema was 6 times and 21 times the odds ratio on a recovered patient having diabetes and emphysema, respectively.
Conclusions
Older patients with comorbidities such as diabetes and emphysema, and higher CRP and NLRs with diffuse lung involvement were more likely to die of COVID-19.
Key Points
• Almost all patients critically ill with COVID-19 pneumonia had five lung lobes involved.
• Medial or parahilar area involvement and degree of lung involvement were more serious in the deceased patients when compared with those who recovered from treatment.
• Chronic lung disease, e.g., emphysema, diabetes, and higher serum CRP and NLR characterized patients who died of COVID-19.
Keywords: Coronavirus infections, Pneumonia, Comorbidity, Computed tomography, X-ray
Introduction
From early December 2019, coronavirus disease 2019 (COVID-19), which is caused by the novel coronavirus (2019-nCoV), has rapidly spread from Wuhan to other regions of China and countries around the world. According to the World Health Organization (WHO) report, by 4 April 2020, there were 1,000,000 confirmed cases globally, including 82,574 in China, and 56,985 deaths worldwide [1].
Management of critically ill patients is important to reduce the mortality of COVID-19. In China, the reported incidence of critical illness in COVID-19 patients was 17.7% in Wuhan, 10.4% in the Hubei Province, and 7.0% in areas outside the Hubei Province [2]. These figures necessitate attention since the incidence of critical illness among the Chinese medical staff afflicted with COVID-19 was 14.6% [2]. According to a recent study by Guan et al who reported the clinical characteristics of COVID-19 in China through an analysis of 1099 patients, 173 (15.7%) had severe disease with a mortality of 8.1%, which was significantly higher than that in the non-severe patients (0.1%) [3]. The mortality of critically ill COVID-19 patients in China was between 38.5 and 49.0% [2, 4, 5]. Thus, it is imperative to recognize both the clinical and imaging characteristics, thus achieving superior patient management.
Recently, several studies have reported chest computed tomography (CT) imaging features and changes during recovery in COVID-19 pneumonia patients without acute respiratory distress syndrome [6–13]. The common conclusion arising from these investigations is that CT is a useful imaging modality in the diagnostic evaluation of abnormal lung changes in the patients [11, 12, 14, 15]. However, CT manifestations in critically ill patients have not been described in the literature. Hence, this retrospective two-center case-control study investigated if clinical, laboratory, and chest CT findings in patients critically ill with COVID-19 differed between those who died and those who survived.
Materials and methods
Patients
We retrospectively reviewed the medical records of 60 critically ill COVID-19 pneumonia patients who were admitted to a hospital in Wuhan, Hubei Province, and another in Huaihua, Hunan Province, between 9 January 2020 and 19 February 2020. Non-contrast chest CT examinations were performed in all the patients. They were diagnosed by the local Centre for Disease Control and Prevention (CDC) (Hubei and Hunan Provinces) by using the real-time reverse transcription polymerase chain reaction (RT-PCR) assay with samples of bronchoalveolar lavage, endotracheal aspirate, nasopharyngeal swab, or oropharyngeal swab. According to the criteria for clinical severity of confirmed COVID-19 pneumonia (Table 1) [16], the patients of severe and critical type were defined as being critically ill. All CT scans were performed within 24 h for patients who met the clinical severity criteria. No exclusion criteria were applied since the selection criteria were strictly followed to include only the severely ill patients.
Table 1.
Criteria for determining clinical severity of COVID-19 pneumonia [16]
| Types | Findings |
|---|---|
| Mild | Mild clinical symptoms (fever < 38 °C (quelled without treatment), with or without cough, no dyspnea, no gasping, no chronic disease) |
| No imaging findings of pneumonia | |
| Moderate | Fever, respiratory symptoms, imaging findings of pneumonia |
| Severe | Meet any of the following: |
| a. Respiratory distress, RR ≥ 30 times/min | |
| b. SpO2 < 93% at rest | |
| c. PaO2/FiO2 ≤ 300 mmHg | |
| * Patients showing a rapid progression (> 50%) on CT imaging within 24–48 h should be managed as severe | |
| Critical | Meet any of the following: |
| a. Respiratory failure, need mechanical assistance | |
| b. Shock | |
| c. “Extra pulmonary” organ failure, intensive care unit is needed |
RR, respiratory rate; SpO2, oxygen saturation; PaO2, partial pressure of oxygen; FiO2, fraction of inspired oxygen
Patient characteristics including age, gender, clinical symptoms, time course of the symptoms before admission, medical history, laboratory results, and outcomes were recorded. Furthermore, neutrophil-to-lymphocyte ratio (NLR) was calculated. Owing to the retrospective and urgent nature of the data collection (both clinical and imaging data), ethical approval and informed consent were waived since this study did not involve any potential risk to the patients.
Chest CT examinations
Of the 60 cases, 55 were obtained from WuGang General Hospital (Wuhan, Hubei Province), and the CT scans were performed on a 64-slice scanner (SOMATOM Definition AS, Siemens Healthineers) with data acquisition of 128 × 0.6 mm, pitch of 1.2. Volume data was reconstructed with a slice thickness of 1.5 mm for each scan. The remaining 5 cases were gathered from The First Affiliated Hospital of Hunan University of Medicine (Hunan Province) on a 16-slice CT scanner (LightSpeed, GE Medical Systems) with 1.3-mm-thick transverse slices. All the examinations were conducted with the patients in a supine position under single breath-hold at the end of inspiration. The tube voltage was 120 kVp, and automatic tube current modulation was used. All CT images were reconstructed with filtered back projection (FBP) algorithm with a matrix size of 512 × 512.
Chest CT evaluation
The CT images were independently reviewed by two cardiothoracic radiologists (having 20 years and 7 years of experience in the field), and the final decisions were reached by consensus. The following CT characteristics were recorded: (a) involvement of the pulmonary lobes and distribution of the lesion; (b) presence of lesion in terms of imaging appearances, such as ground-glass opacities (GGO), consolidation, and linear opacities; (c) accompanying signs such as crazy-paving pattern, air bronchogram, and margin of the lesion; (d) presence of underlying lung disease such as emphysema, fibrosis, and calcification; (e) presence of other abnormalities, including pleural effusion, pericardial effusion, and thoracic lymphadenopathy.
To further assess the abnormalities in CT images, the following parameters were analyzed: unilateral or bilateral lungs involved; number of lobes involved; number of peripheral or subpleural areas (peripheral 1/3), intermediate area (mid 1/3), and medial or parahilar region (medial 1/3) involved; degree of lung involvement; and consolidative degree of the lesions. Degree of involvement and consolidation were classified as none (0%), minimal (1–25%), mild (26–50%), moderate (51–75%), or severe (76–100%), which were recorded by assigning scores of 0, 1, 2, 3, and 4, respectively.
The terms of abnormal imaging appearances were defined according to the guidelines provided by the Fleischner Society [17]. GGO was defined as hazy increased lung density, with indistinct margins of bronchus and pulmonary vessels. Consolidation was defined as increased pulmonary parenchymal attenuation, with the margins of the bronchus and the pulmonary vessels being obscured. Crazy-paving pattern was defined as GGO combined with reticulation or/and interlobular septal thickening. An air bronchogram is an air-filled bronchus seen clearly with low density on a background of GGO or consolidation opacity. The margin of the lesion was defined as sharp or blurred. Thoracic lymphadenopathy was defined as the short-axis dimension of lymph node ≥ 10 mm.
Statistical analysis
The data were analyzed using SPSS 25.0 (SPSS, Inc.). Continuous variables were expressed as mean ± standard deviation (SD). The continuous variables were compared using the Mann-Whitney U test and categorical variables were compared using the odds ratio to determine their association with clinical outcomes in terms of deceased or recovered status. Findings should be interpreted as exploratory or descriptive. The differences were considered statistically significant at p ≤ 0.05.
Results
Patient characteristics
Of the 60 patients, 43 were males (71.7%), and their mean age was 64.4 ± 11.0 years. Patients who were deceased were older than those who recovered (mean age, 70.6 vs. 62.6 years, p = 0.044). The mean time between symptom onset to admission was 8.9 ± 5.0 days (Table 2).
Table 2.
Demographics and clinical symptoms of patients with critically ill COVID-19
| Basic characteristics | All patients | Recovery patients | Death patients | p | |
|---|---|---|---|---|---|
| n = 60 | n = 50 | n = 10 | |||
| Gender | Male | 43 (72%) | 36 (72%) | 7 (70%) | 1.000 |
| Female | 17 (28%) | 14 (28%) | 3 (30%) | 1.000 | |
| Age (years) | 64.4 ± 11.0 | 62.6 ± 11.0 | 70.6 ± 9.1 | 0.044 | |
| Time course of symptoms before hospital (days) | 8.9 ± 5.0 | 8.9 ± 4.9 | 8.9 ± 5.5 | 0.920 | |
| Highest temperature | 38.6 ± 0.7 | 38.6 ± 0.7 | 38.7 ± 0.7 | 0.723 | |
| Heart beat per minute (bpm) | 86.5 ± 14.3 | 87.2 ± 15.2 | 83.2 ± 9.3 | 0.471 | |
| Breath (bpm) | 20.3 ± 3.3 | 19.8 ± 2.7 | 22.2 ± 4.6 | 0.185 | |
| Systolic blood pressure (mmHg) | 114.4 ± 21.7 | 111.7 ± 22.7 | 126.6 ± 9.5 | 0.017 | |
| Diastolic blood pressure (mmHg) | 84.4 ± 25.8 | 87.1 ± 27.7 | 72.7 ± 7.6 | 0.279 | |
COPD, chronic obstructive pulmonary disease
Ten patients died in this study cohort during hospitalization for treatment, establishing the mortality of critically ill COVID-19 pneumonia patients to be 17%. The condition of the remaining 50 patients improved, and they were discharged from the hospitals. No significant difference was found in these basic clinical characteristics between the recovered and the deceased patients (Table 2).
Table 3 is a summary statistics of demographics and clinical signs and symptoms. The only significant difference was found in diabetes (p = 0.025), and the odds ratio on a deceased patient having diabetes was 6 times the odds ratio on a recovered patient having diabetes.
Table 3.
Qualitative features of demographics and clinical signs and symptoms—summary statistics
| Clinical demographics | Features | % of (n = 10) deceased patients with feature | % of (n = 50) recovered patients with feature | Odds ratio | Lower 95% confidence limit | Upper 95% confidence limit | Wald statistic | p value |
|---|---|---|---|---|---|---|---|---|
| Comorbidity | Hypertension | 40 | 36 | 1.185 | 0.295 | 4.762 | 0.239 | 0.811 |
| CHD | 30 | 22 | 1.519 | 0.336 | 6.871 | 0.543 | 0.587 | |
| COPD | 20 | 8 | 2.875 | 0.449 | 18.395 | 1.115 | 0.264 | |
| Peptic ulcer | 10 | 16 | 0.583 | 0.065 | 5.265 | − 0.480 | 0.631 | |
| Stroke | 20 | 8 | 2.875 | 0.449 | 18.395 | 1.115 | 0.265 | |
| Diabetes | 40 | 10 | 6.000 | 1.253 | 28.742 | 2.242 | 0.025 | |
| Signs and symptoms | Fever | 90 | 82 | 1.976 | 0.221 | 17.623 | 0.610 | 0.542 |
| Cough | 80 | 76 | 1.263 | 0.235 | 6.777 | 0.273 | 0.785 | |
| Phlegm | 50 | 56 | 0.786 | 0.202 | 3.060 | − 0.348 | 0.728 | |
| Shortness of breath | 40 | 46 | 0.783 | 0.196 | 3.117 | − 0.348 | 0.728 | |
| Myalgia | 0 | 12 | - | - | - | - | - | |
| Fatigue | 40 | 58 | 0.483 | 0.121 | 1.927 | − 1.031 | 0.302 | |
| Headache and dizziness | 0 | 6 | - | - | - | - | - | |
| Sore throat | 0 | 2 | - | - | - | - | - | |
| Loss of appetite | 40 | 48 | 0.722 | 0.181 | 2.875 | − 0.462 | 0.644 | |
| Nausea and vomit | 10 | 8 | 1.278 | 0.127 | 12.806 | 0.208 | 0.835 | |
| Diarrhea | 10 | 14 | 0.683 | 0.074 | 6.253 | − 0.338 | 0.735 |
Statistics are not computed where there are cells containing 0% (or 100%)
CHD, congenital heart disease; COPD, chronic obstructive pulmonary disease
Laboratory results
As shown in Table 4, C-reactive protein (CRP) (67.9 ± 50.5 mg/L) was elevated in all the patients, but it was significantly greater in the deceased than in the recovered (p < 0.001). Similarly, the NLR and white blood cell counts were significantly higher in the deceased when compared with the recovered (p = 0.03).
Table 4.
Laboratory results of patients with critically ill COVID-19
| Characteristics | All patients | Recovered patients | Deceased patients | p |
|---|---|---|---|---|
| n = 60 | n = 50 | n = 10 | ||
| CRP (mg/L) | 67.9 ± 50.5 | 63.0 ± 50.4 | 110.8 ± 26.3 | < .001 |
| WBC count (109/L) | 6.7 ± 3.6 | 6.3 ± 3.3 | 9.6 ± 5.3 | 0.030 |
| LYM count (109/L) | 0.81 ± 0.52 | 0.83 ± 0.53 | 0.61 ± 0.43 | 0.108 |
| NEUT count (109/L) | 5.6 ± 3.7 | 5.2 ± 3.4 | 8.6 ± 5.1 | 0.056 |
| PaO2 (mmHg) | 78.5 ± 28.9 | 80.3 ± 28.2 | 62.5 ± 34.6 | 0.160 |
| SpO2 (%) | 92.7 ± 14.2 | 93.4 ± 14.2 | 84.0 ± 15.6 | 0.006 |
| NLR | 9.7 ± 9.5 | 8.4 ± 7.5 | 18.7 ± 16.6 | 0.030 |
CRP, C-reactive protein; WBC, white blood cell; LYM, lymphocyte; NEUT, neutrophils; NLR, neutrophil-to-lymphocyte ratio
CT findings
Bilateral lungs were involved in all the patients. Almost all the 60 patients (98%) exhibited involvement of all 5 lobes, while only 4 lobes were involved in 1 patient. In the deceased patients, the lesions included the peripheral, intermediate, and medial areas for all the patients. Medial or parahilar area involvement was highly prevalent in the deceased patients (Fig. 1). The degree of lung involvement was more severe in the deceased than in recovered (mean score, 3.3 ± 0.5 vs. 2.0 ± 0.7, p < 0.001), with no significant difference in the consolidative degree of the lesions (1.5 ± 1.4 vs. 1.6 ± 1.1, p = 0.662). GGO appearances (Fig. 2), crazy-paving pattern, and air bronchogram were discernable in almost all the patients (Figs. 3 and 4). Consolidation and linear opacities were also found in some patients with no significant difference between the deceased and the recovered patients (Fig. 5). The margin of the lesions was blurred in 98% of the patients and clear in only 20% (Fig. 6). Table 5 is a summary statistics of CT findings and underlying diseases. The only significant difference was found in emphysema (p = 0.013), and the odds ratio on a deceased patient having emphysema was 21 times the odds ratio on a recovered patient having emphysema.
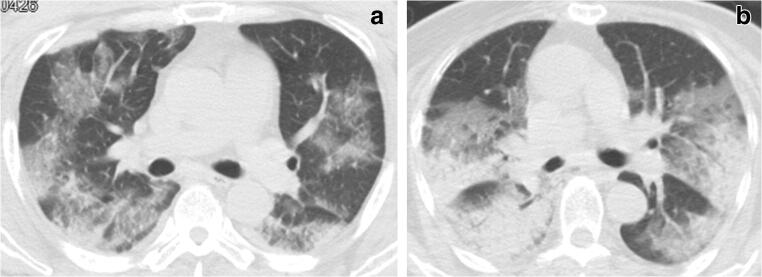
Fig. 1.
Lesion distribution in the lung fields of critically ill patients with COVID-19. a A 53-year-old male critically ill COVID-19 patient whose symptoms improved and was discharged from the hospital after treatment. CT shows the lesions infiltrating the peripheral and intermediate lung fields, with a lung involvement degree of 26–50% and lesion consolidation degree of 1–25%. b A 77-year-old female critically ill patient with COVID-19 who eventually died. Peripheral, intermediate, and medial areas were seen to be simultaneously involved on CT, with lung involvement and lesion consolidation degrees of 51–75%
Fig. 2.
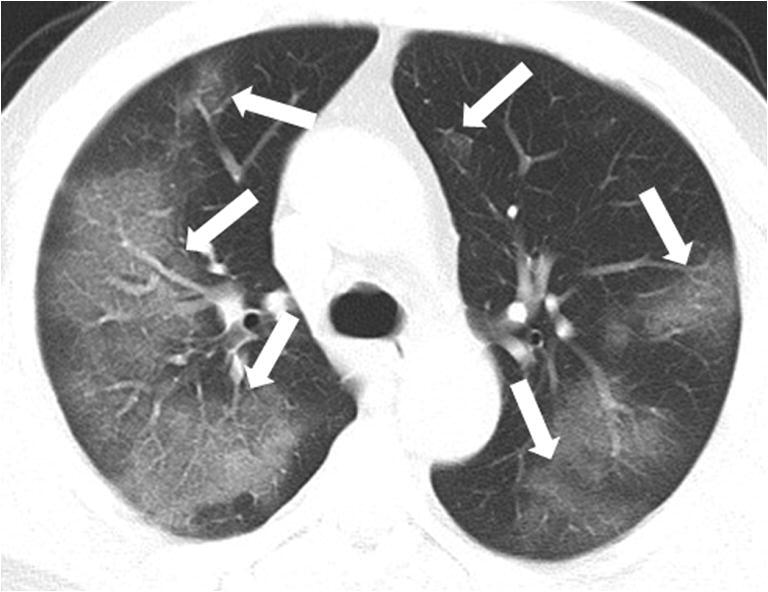
A 52-year-old male critically ill patient with COVID-19 who recovered after treatment. Multiple pure GGO lesions were seen in the peripheral and intermediate lung fields (arrows) on the CT image. GGO, ground-glass opacities
Fig. 3.
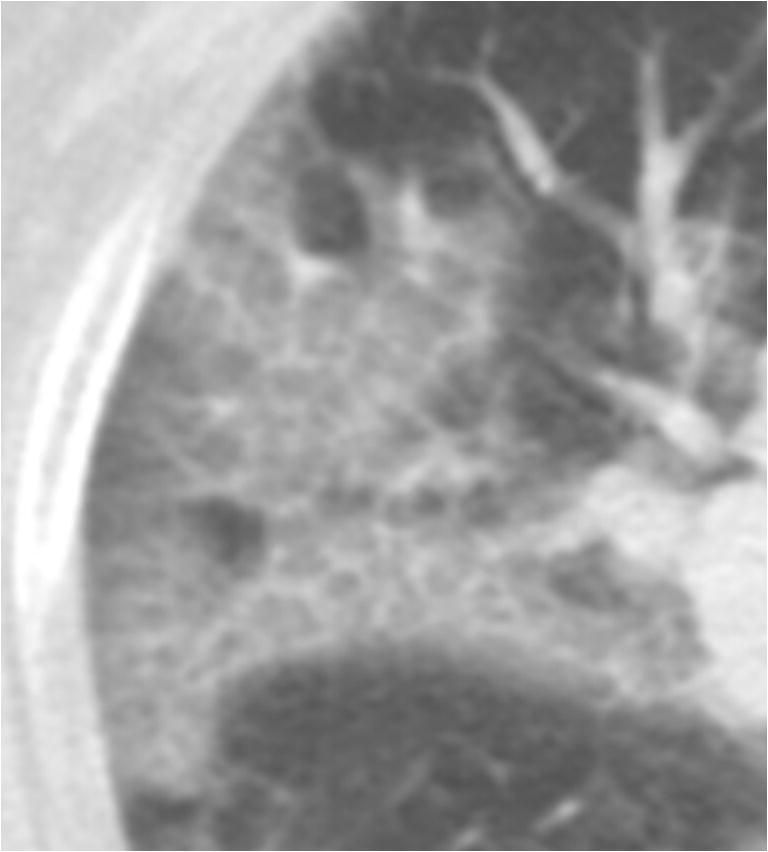
Crazy-paving pattern in a 65-year-old female critically ill COVID-19 patient in the recovery group. A typical crazy-paving pattern is visualized on the CT image
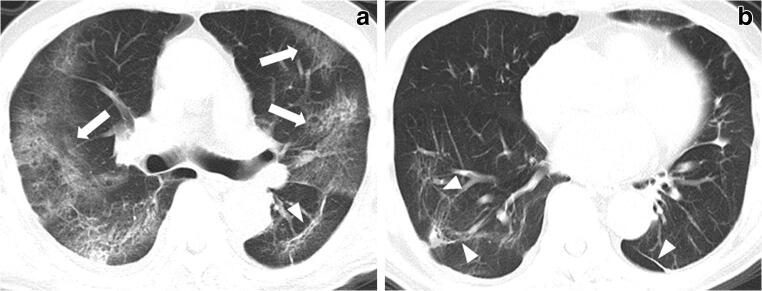
Fig. 4.
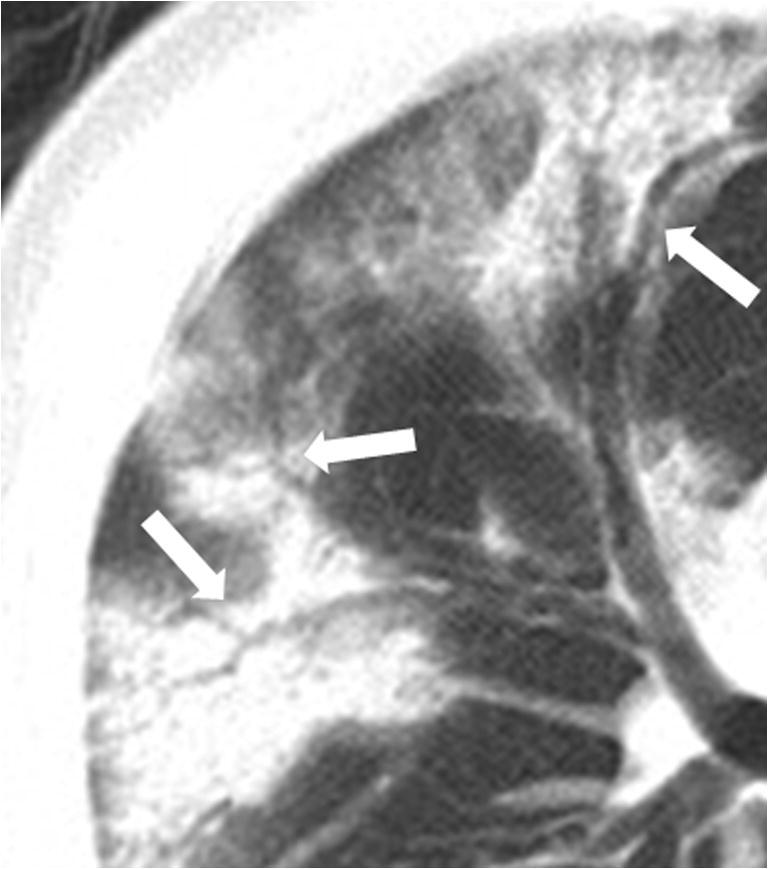
Air bronchogram appearance in a 73-year-old male critically ill COVID-19 patient in the recovery group. Multiple bronchogram signs are seen in the GGO and consolidation lesions (arrows). GGO, ground-glass opacities
Fig. 5.
A 77-year-old male critically ill COVID-19 patient who recovered after treatment. GGO (arrows in a) and linear opacities (arrowheads in b) are presented in the same patient on the CT images. GGO, ground-glass opacities
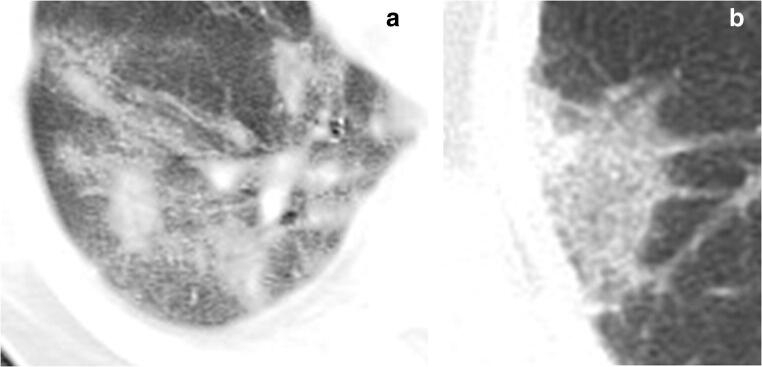
Fig. 6.
GGO with different margins in a 78-year-old male critically ill COVID-19 patient in the recovery group. a GGO with blurred margin. b GGO with clear margin. GGO, ground-glass opacities
Table 5.
Qualitative features of CT findings—summary statistics
| Abnormal lung changes | Features | % of (n = 10) deceased patients with feature | % of (n = 50) recovered patients with feature | Odds ratio | Lower 95% confidence limit | Upper 95% confidence limit | Wald statistic | p value |
|---|---|---|---|---|---|---|---|---|
| Involvement and distribution | Bilateral lungs | 100 | 100 | - | - | - | - | - |
| 4 lobes | 0 | 2 | - | - | - | - | - | |
| 5 lobes | 100 | 98 | - | - | - | - | - | |
| Peripheral area | 100 | 100 | - | - | - | - | - | |
| Intermediate area | 100 | 84 | - | - | - | - | - | |
| Medial area | 100 | 54 | - | - | - | - | - | |
| Presence of lesion types | GGO | 100 | 96 | - | - | - | - | - |
| Consolidation | 60 | 70 | 0.643 | 0.158 | 2.613 | − 0.618 | 0.643 | |
| Linear opacities | 0 | 26 | - | - | - | - | - | |
| Accompanied signs | Crazy-paving pattern | 100 | 90 | - | - | - | - | - |
| Air bronchogram | 100 | 92 | - | - | - | - | - | |
| Clear margin | 20 | 20 | 1.000 | 0.183 | 5.460 | 0.000 | 1.000 | |
| Blur margin | 100 | 98 | - | - | - | - | - | |
| Underlying lung diseases | Emphysema | 30 | 2 | 21.000 | 1.909 | 230.962 | 2.489 | 0.013 |
| Fibrosis | 0 | 2 | - | - | - | - | - | |
| Calcification | 30 | 20 | 1.714 | 0.375 | 7.836 | 0.695 | 0.487 | |
| Other abnormalities | Pleural effusion | 30 | 24 | 1.357 | 0.303 | 6.083 | 0.399 | 0.690 |
| Pericardial effusion | 0 | 4 | - | - | - | - | - |
Statistics are not computed where there are cells containing 0% (or 100%)
GGO, ground-glass opacities
Discussion
In this study, we focused on the analysis of basic clinical information, comorbidity, symptoms, laboratory results, clinical outcomes, and CT findings in 60 critically ill COVID-19 pneumonia patients. In CT images, multilobular infiltrates were noticed in all the critically ill patients with COVID-19 pneumonia. Almost all of the patients (98%, 59/60) manifested 5 lobes involvement, with 4 lobes involvement observed in only 1 patient. Medial or parahilar area involvement and degree of lung involvement were more serious in the deceased patients when compared with those who recovered from treatment. Underlying lung disease, such as emphysema, may significantly affect the outcome of critically ill patients. In addition to these CT features, patient’s age, diabetes, CRP, and NLR were higher in the deceased of critically ill patients with COVID-19 pneumonia.
This investigation is different from the previous ones which had included different categories of COVID-19 patients and compared mild with moderate or severe, severe with non-severe, and intensive care unit (ICU) with non-ICU patients [2, 18–21]. Of the 60 patients in our study, 10 (17%) patients died; thus, the mortality rate is similar to the 15% that was reported by Huang et al [4] and Cao et al [22], but much higher than the 1.4% for all the patients and 8.1% for the severe patients as documented by Guan et al [3]. However, most of the patients (93.6%) remained in the hospital in the study by Guan et al; therefore, the clinical outcomes were unknown at the time of publication despite the analysis of a large sample size. The mortality rate in our cohort is lower than the 38.5% and 49% that was reported in China [2, 4, 5, 23]. The low mortality rate recorded in these investigations could have been due to enrolment with the exclusion of critically ill COVID-19 patients. It could also have been due to the fact that not all the patients received CT scans within 24 h, considering the emergency of the situation.
Since the outbreak of COVID-19, several studies are available in the literature and some of them have focused on the description of chest imaging features [6–15, 18–21, 24]. Typical chest CT findings include GGO and consolidation, which are seen in most of the patients, while other findings include crazy-paving pattern, interlobular thickening, and linear opacities. These reported findings are based on patients with mild and moderate disease. However, there is a lack of information about analyzing the imaging features in severely ill patients. In such a context, our study has uniquely presented the imaging findings regarding the involvement and distribution patterns in critically ill or severe COVID-19 pneumonia patients. In the mild and moderate types of patients, only few pulmonary lobes were involved according to the previous studies (1–3 lobes involvement, 29–44.4%; 4 lobes involvement, 11.1–19%; 5 lobes involvement, 38–44.4%) [6, 7]. However, in this study of the critically ill patients, involvement of all 5 lobes was observed in 98% of the patients. A larger proportion of intermediate (87%) and medial (62%) areas was involved in our recruited critically ill patients, which was in contrast with the predominant peripheral involvement in the mild and moderate patients [7, 11, 13, 25]. Among the critically ill patients in this study, the mean degree of lung involvement was 2.2 ± 0.9 with a range of 0–4, which represents nearly 50% of the lung field being involved. In the deceased group, the mean score was 3.3 ± 0.5, indicating a higher degree of lung involvement. In the research by Chung et al [7], a total of the lung severity scores of mild and moderate patients were calculated and a summation of each lobe score (with similar 0–4 scales) was performed to determine the degree of involvement of the lung field. Their results showed that the mean total lung severity score was 9.9 in a range of 0–20. Our results aid in predicting the extent of disease in these critically ill patients by analyzing the degree of lung involvement based on chest CT images.
According to the MuLBSTA scoring system [26], multilobular infiltrates, lymphocyte ≤ 0.8 × 109/L, bacterial coinfection, acute-smoker, quit-smoker, hypertension, and age ≥ 60 years are the mortality risks for viral pneumonia. In this work, although 5 lobes were infiltrated in most patients, medial or parahilar area involvement and the degree of lung involvement were higher in the deceased patients. Involvement range and degree might be the potential risk predictors on CT images in the COVID-19 pneumonia patients. Our patient sample is different from those in other studies as we evaluated the clinical and imaging features in the critically ill patients with pneumonia. Since emphysema was more common in the deceased patients, it could be hypothesized that an underlying lung disease may also affect the clinical outcome. Furthermore, patients with low lymphocyte count, hypertension, and old age appeared more frequently in the deceased.
The NLR was identified as the independent risk factor for predicting critical illness in the COVID-19 pneumonia patients, with 3.13 serving as a good cut-off value [27]. In this research, the average NLR of the 60 patients was 9.7 ± 9.5, which is significantly higher than the recommended value of 3.13. Moreover, the NLR was significantly higher in the deceased patients (nearly double the value of the recovered patients), indicating its potential to predict not only critical illness but also death in the severely ill patients.
Despite numerous reports on COVID-19 being available in the literature, to our knowledge, only the clinical factors or imaging features associated with disease severity have been documented [28, 29]. For instance, Liu et al analyzed 78 COVID-19 pneumonia patients, focusing only on the clinical factors with regard to their effects on disease progression [28]. In their group, 11 patients demonstrated deterioration of the situation, while 67 exhibited improvement or stabilization. Akin to our findings, patient age, CRP, pre-existing conditions such as smoking history and respiratory failure, albumin, and maximum body temperature on admission were identified as the factors responsible for COVID-19 progression. Li et al compared 25 critically ill COVID-19 pneumonia patients with 55 ordinary patients in terms of their clinical and CT imaging features [29]. In addition to the common CT imaging findings associated with COVID-19 patients, they used the CT scores to determine the diagnostic value of this technique in differentiating these two situations. Older age, comorbidities, increased CRP and neutrophil ratio, decreased lymphocytes, and higher CT scores were directly related to severe or critical illness. These findings, along with ours, further confirm the value of these combined factors in assisting clinical decision-making while evaluating the disease severity for prediction of outcomes.
Regarding some therapeutic measures to potentially prevent critically ill patients with COVID-19 from succumbing to the disease, according to our experience and others, a combination of ribavirin, lopinavir/ritonavir, and interferon is the most common and effective treatment for COVID-19 patients to fight against coronavirus. Cardiac suppressive effects should be paid attention to, because of use of lopinavir/ritonavir [30–32].
In the center of Huaihua, Hunan Province, for severe and critically ill patients, glucocorticoids are used in combination to deal with uncontrollable high fever and inflammatory factor storms. During the ICU period, the body temperature of critically ill COVID-19 patients could reach normal level within the next 24 h after glucocorticoid treatment is received. The inflammatory index, including CRP, lymphocyte count, and oxygenation index, is significantly improved within 5 days after glucocorticoid combined treatment. Hyperglycemia is the most common complication of glucocorticoid therapy. Almost all the critically ill COVID-19 patients have elevated blood glucose in varying degrees, regardless of having diabetes or not, during glucocorticoid therapy. The elevated blood glucose lacks sensitivity to insulin treatment in this scenario. Thus, we recommend low dose (40 mg/12 h) glucocorticoid therapy with short term (5 days) for critically ill COVID-19 patients to control uncontrollable high fever and inflammatory factor storms avoiding severe adverse reaction. Closely frequent blood glucose monitoring should be performed for hospitalized patients with severe disease [31]. It is important that the long-term use of corticosteroids should be avoided because of delay in virus clearance due to immunosuppression.
Lymphocytopenia is considered an important indicator of poor prognosis in patients with severe or critically ill status. This is due to the fact that SARS-CoV-2 may act mainly on T lymphocytes, resulting in T lymphocyte damage. Patients with critical illness may develop bacterial infection due to low immune function. Therefore, antibiotics can be used as appropriate to treat and prevent the occurrence of mixed infection.
Some limitations exist in this study. First, this was a preliminary description of the CT findings in critically ill COVID-19 pneumonia patients. The sample size was relatively small owing to the strict selection criteria applied to these patients. Thus, further studies with the inclusion of more cases should be conducted so that robust conclusions could be drawn. Second, as the CT follow-up scans were done at different clinical sites, only the first scan taken within 24 h of critical illness onset was described in this work. Change of chest CT findings in critically ill patients, including the recovery and death process, can provide additional information for clinical outcome prediction and management assessment. Besides, we did not follow-up on the recovered patients. A recent research reported that some recovered patients may still be carriers of the virus [33]. Therefore, long-term follow-up of these patients is necessary, which should be the focus of future research. Lastly, no autopsy was performed in the deceased patient group. Upon comparison with the pathological results, additional and a more precise interpretation of the CT image signs will be available in the future work.
In conclusion, this study involving critically ill COVID-19 patients has revealed that ground-glass opacities, crazy-paving pattern, and air bronchogram represent the most common findings, with more of the pulmonary lobes involved in the patients. Medial and intermediate area involvement in the lungs was more often seen in the deceased than in the recovered patients. Chest CT plays an important role in assessing the severity of COVID-19. Additionally, some clinical and laboratory factors such as patient age, co-existing conditions including diabetes and emphysema, CRP, and NLR could be combined to predict the disease outcomes as they were significantly higher in the patients who succumbed to the disease than in those who recovered from it. While clinical trials are underway to investigate the new antiviral drugs, currently no vaccine or specific effective antiviral therapies are available for COVID-19. Medical interventions in dealing with patients with COVID-19 include general treatments comprising nutritional interventions and immune enhancers; antiviral treatments as discussed above with use of different antiviral drugs; vaccine and therapies for coronavirus, which are being developed; and other supportive treatments for elderly and vulnerable patients with comorbidities to reduce the risk of complications or mortality associated with these pre-existing respiratory or cardiovascular diseases.
Acknowledgments
We would like to thank Mr. Gil Stevenson for assistance in the statistical analysis.
Abbreviations
- CDC
Centre for Disease Control and Prevention
- COPD
Chronic obstructive pulmonary disease
- COVID-19
Coronavirus disease 2019
- CRP
C-reactive protein
- CT
Computed tomography
- FiO2
Fraction of inspired oxygen
- GGO
Ground-glass opacities
- ICU
Intensive care unit
- LYM
Lymphocyte
- NEUT
Neutrophils
- NLR
Neutrophil-to-lymphocyte ratio
- PaO2
Partial pressure of oxygen
- RR
Respiratory rate
- RT-PCR
Reverse transcription polymerase chain reaction
- SD
Standard deviation
- SpO2
Oxygen saturation
- WBC
White blood cell
- WHO
World Health Organization
Funding information
The authors declare that they have not received any funding related to this work.
Compliance with ethical standards
Guarantor
The scientific guarantor of this study is Zhonghua Sun.
Conflict of interest
The authors declare no conflict of interest in this work.
Ethical approval
Owing to the retrospective and urgent nature of the data collection (both clinical and imaging data), ethical approval and informed consent were waived since this study did not involve any potential risk to the patients.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dr Nan Zhang and Dr Xunhua Xu contributed equally as co-first authors to this work.
Contributor Information
Yu Li, Email: 1523115105@qq.com.
Huiming Yin, Email: 1976841746@qq.com.
Zhonghua Sun, Email: z.sun@curtin.edu.au.
References
- 1.World Health Organization (2020) Novel coronavirus (2019-nCoV) situation report-75. https://www.who.int/docs/default-source/coronaviruse/20200302-sitrep-75-covid-19.pdf?sfvrsn=99251b2b_2
- 2.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention (2020) The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 41(2):145–151 [DOI] [PubMed]
- 3.Guan W-j, Ni Z-Y, Hu Y et al (2020) Clinical characteristics of coronavirus disease in China. N Engl J Med. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Sun W, Li J, et al (2020) Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. medRxiv. 02.17.20024166
- 6.Pan Y, Guan H, Zhou S et al (2020) Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 10.1007/s00330-020-06731-x [DOI] [PMC free article] [PubMed]
- 7.Chung M, Bernheim A, Mei X et al (2020) CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology:200230. 10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed]
- 8.Fang Y, Zhang H, Xu Y, Xie J, Pang P, Ji W (2020) CT manifestations of two cases of 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology:200280. 10.1148/radiol.2020200280 [DOI] [PMC free article] [PubMed]
- 9.Lei J, Li J, Li X, Qi X (2020) CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology:200236. 10.1148/radiol.2020200236 [DOI] [PMC free article] [PubMed]
- 10.Liu P, Tan XZ (2020) 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology:200257. 10.1148/radiol.2020200257
- 11.Pan F, Ye T, Sun P et al (2020) Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology:200370. 10.1148/radiol.2020200370
- 12.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J (2020) Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology:200343. 10.1148/radiol.2020200343 [DOI] [PMC free article] [PubMed]
- 13.Ai T, Yang Z, Hou H et al (2020) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology:200642. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed]
- 14.Fang Y, Zhang H, Xie J et al (2020) Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology:200432. 10.1148/radiol.2020200432 [DOI] [PMC free article] [PubMed]
- 15.Huang P, Liu T, Huang L, et al. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology. 2020;12:200330. doi: 10.1148/radiol.2020200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.General Office of National Health Committee, Office of State Administration of Traditional Chinese Medicine. Notice on the issuance of a programme for the diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (trial sixth edition). http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2shtml. Accessed 19 Feb 2020
- 17.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Yu C, Zhang L et al (2020) Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 10.1007/s00259-020-04735-9 [DOI] [PMC free article] [PubMed]
- 19.Wu J, Liu J, Zhao X et al (2020) Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 10.1093/cid/ciaa199 [DOI] [PMC free article] [PubMed]
- 20.Xu YH, Dong JH, An WM et al (2020) Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. 10.1016/j.jinf.2020.02.017 [DOI] [PMC free article] [PubMed]
- 21.Zhang JJ, Dong X, Cao YY et al (2020) Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy 10.1111/all.14238 [DOI] [PubMed]
- 22.Cao J, Tu WJ, Cheng W et al (2020) Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 10.1093/cid/ciaa243 [DOI] [PMC free article] [PubMed]
- 23.Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortality due to COVID-19 based on analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed]
- 24.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song F, Shi N, Shan F, et al. Emerging coronavirus 2019-nCoV pneumonia. Radiology. 2020;6:200274. doi: 10.1148/radiol.2020200274. [DOI] [Google Scholar]
- 26.Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA Score. Front Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Liu Y, Xiang P, et al (2020) Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv. 02.10.20021584
- 28.Liu W, Tao Z, Wang L et al (2020) Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 10.1097/CM9.0000000000000775 [DOI] [PMC free article] [PubMed]
- 29.Li K, Wu J, Wu F et al (2020) The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 10.1097/RLI.0000000000000672 [DOI] [PMC free article] [PubMed]
- 30.Gupta R, Misra A. Contentious issues and evolving concepts in the clinical presentation and management of patients with COVID-19 infection with reference to use of therapeutic and other drugs used in co-morbid diseases (hypertension, diabetes etc) Diabetes Metab Syndr. 2020;14(3):251–254. doi: 10.1016/j.dsx.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta R, Ghosh A, Singh AK, Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr. 2020;14(3):211–212. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;28:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan L, Xu D, Ye G et al (2020) Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 10.1001/jama.2020.2783 [DOI] [PMC free article] [PubMed]