Abstract
Coronavirus disease outbreak caused a severe public health burden all over the world. Salinomycin (SAL) is a broad-spectrum antibiotic that had drawn attention in selective targeting of cancer and viral infections. Recent drug screen identified SAL as a potent antiviral agent against SARS-CoV-2. In this hypothesis, we discuss the potential of pulmonary delivery of SAL using nanostructured lipid carriers (NLCs) against SARS-CoV-2.
Keywords: COVID-19, SARS-CoV-2, Salinomycin, ACE-2, Endocytosis
Introduction
Coronaviruses (CoVs) are enveloped, single-stranded RNA viruses belonging to the family Coronaviridae within the order Nidovirales. Coronaviruses (CoV) are of four different genera, α, β, γ, and δ CoVs. Among these β CoVs like SARS-CoV, Middle East respiratory syndrome (MERS-CoV), and novel coronavirus (SARS-CoV-2) is responsible for severe and potentially fatal respiratory infections [1], [2], [3] Table 1 ..
Table 1.
FDA approved drugs used for treating SARS-CoV-2 infection.
| Drug | Clinical use | Possible mechanism against SARS-CoV-2 | Ref. |
|---|---|---|---|
| Arbidol | Influenza | Disruption of binding of viral envelope protein to host cells | [10] |
| baricitinib | Rheumatoid arthritis | JAK inhibition, anti-inflammatory effects | [11] |
| chloroquine | malaria | Alteration of endosomal pH | [12] |
| favipiravir | HIV | Inhibition of viral RNA synthesis | [13] |
| galidesivir | hepatitis C, Ebola and Marburg virus | Inhibition of viral nucleotide synthesis | [14] |
| lopinavir | HIV | Inhibition of viral protease | [15] |
| remdesivir | Ebola | Inhibition of viral nucleotide synthesis | [16] |
| ribavirin | RSV infection, hepatitis C, hemorrhagic fever | Inhibition of viral nucleotide synthesis | [14], [17] |
It was reported that CoVs enter host cells Angiotensin-Converting Enzyme-2 (ACE-2) receptor-mediated endocytosis, which is a pH-dependent process. In this process, Spike (S) protein plays a major role in receptor binding and membrane fusion of SARS-CoV-2 for entry into host cells. It contains a large ectodomain, transmembrane anchor, and a short intracellular tail. The ectodomain of SARS-CoV-2 consists of two subunits. S1 subunit of SARS-CoV-2 is responsible for binding with the ACE-2 receptor for viral entry to host cells. Following receptor binding, the SARS-CoV-2 enter the cytosol of the host cell, by pH-dependent proteolytic cleavage of spike protein by TMPRSS2. Then the fusion of viral and host cell membranes occurs in acidified endosomes, allowing viral genomes to affect host cells with the aid of the S2 subunit [1], [4], [5].
Coronavirus disease 19 (COVID-19) caused by SARS-CoV-2 was the pandemic, affected nearly 1,400,000 people worldwide as on 7 April 2020, according to WHO. However, rapid spread, potential mortality, and lack of clinically approved drugs and vaccines against COVID-19 are the major challenges. There is a need, therefore, for quick discovery of drugs against this emerging infectious disease. However, the slow phase of discovery and associated costs are the major challenges in discovering drugs against SARS-CoV-2.
Drug repurposing using existing drugs is an attractive strategy to accelerate drug discovery against COVID-19. Recently researchers focused on screening of FDA approved drugs against SARS-CoV-2 [6], [7], [8]. Salinomycin (SAL) is a carboxylic polyether ionophore isolated from Streptomyces albus. Ionophores show a broad spectrum of bioactivity, like antibacterial, antifungal, antiparasitic, antiviral, and recently, they are also used as anti-tumor agents [9]. However, poor absorption, low bioavailability, and off-target effects are the potential limitations for effective repurposing of SAL as an antiviral agent against SARS-CoV-2. In the present study, we, therefore, propose the pulmonary delivery of SAL using nanostructured lipid carriers (NLCs).
Hypothesis
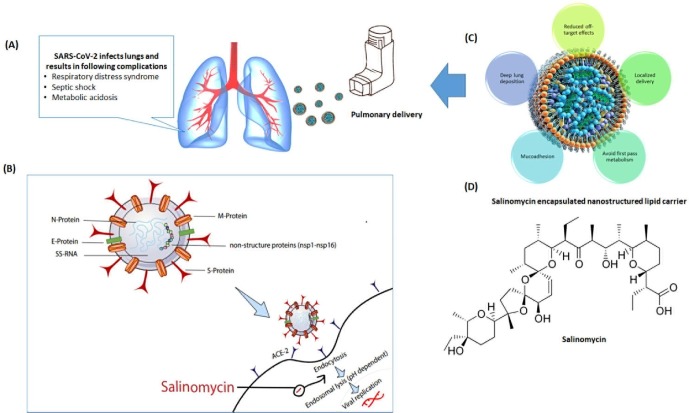
In the present study, we proposed to prepare NLCs for intra-pulmonary delivery of SAL to prevent SARS-CoV-2 infection (Fig. 1 ). The proposed drug delivery system with the following advantages:
-
•
Noninvasive means of administration
-
•
Localized delivery to lung epithelium directly
-
•
Avoid first-pass metabolism
-
•
Reduced off-target effects
-
•
Rapid and effective drug absorption
Fig. 1.
A. Respiratory complications due to SARS-CoV-2; B. Mechanism of action SAL as an antiviral agent against SARS-CoV-2; C. Advantages of inhaled NLCs, D. Structure of SAL.
Justification of the proposed hypothesis
For all enveloped viruses, the significant step of entry into host cell is fusion. SARS-CoV-2 fusion occurs in low pH with a half-maximal rate of fusion at pH 5.5. A compelling body of evidence suggests, SAL inhibits replication of viral RNA in the cytoplasm by altering the pH. SAL, therefore, has the potential to prevent the entry of SARS-CoV-2 into the cytosol and prevent membrane fusion (a pH-dependent process) [18]. It was reported that SAL has antiviral propensity by preventing the migration of nuclear protein (NP) to form a viral ribonuclear complex (VNP). This fails to acidify the endosomal-lysosomal compartments due to cytoplasmic accumulation of NP in the host cells [18], [19]. It was also reported that SAL could interact with S- protein, and influence ACE2 binding and prevent the release of viral RNA into the cytoplasm [20]. Besides, a recent drug screen identified SAL as a potential antiviral agent against SARS-CoV-2 (IC50 = 0.24 µM) [19]. However, the clinical efficacy of SAL against SARS-CoV-2 needs to be evaluated.
Pulmonary delivery is an attractive strategy for localized delivery of therapeutics to infection sites. Besides, inhaled nanoparticles overcomes the limitations of poor bioavailability and drug absorption. NLCs are biocompatible nanocarriers with good tolerability for pulmonary delivery. Due to their size in nanometers, NLCs can be easily aerosolized into droplets with suitable aero-dynamical properties. This enables deep lung deposition of an active compound. Furthermore, NLCs adhere to the mucosal surface of the lung for a more extended period compared to larger particles due to the small size. Particle adhesion, accumulation, and retention in the lung as well as prolonged-release due to NLCs results in enhanced and sustained therapeutic effects. NLCs have advantage of better patient compliance [21], [22], [23], [24], [25]. All these advantages of inhaled NLCs play an essential role in the treatment of respiratory infections like COVID-19.
Conclusion
There are several investigational drugs and drugs under preclinical trials for prevention/curative purposes of COVID-19, but still, there is no current approved drug. SAL has the ability to disruption concentration across the cell membrane and prevents virus entry into the host cells by selective targeting of SAL. Encapsulation of SAL in NLC for pulmonary delivery is an attractive approach for effective repurposing of SAL as an antiviral agent by improving its absorption at the infection site.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.109858.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res. 2020;7(1):1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIntosh K. Springer; 1974. Coronaviruses: a comparative review, Current Topics in Microbiology and Immunology/Ergebnisse der Mikrobiologie und Immunitätsforschung; pp. 85–129. [Google Scholar]
- 3.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson G. COVID-19 social distancing, ACE 2 receptors, protease inhibitors and beyond? Int J Clin Pract. 2020 doi: 10.1111/ijcp.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greener M. Old drug, new tricks: realising the potential of repurposing. Prescriber. 2017;28(10):34–38. [Google Scholar]
- 7.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 8.Pindiprolu S.K.S., Pindiprolu S.H. Plausible mechanisms of Niclosamide as an antiviral agent against COVID-19. Med Hypotheses. 2020;109765 doi: 10.1016/j.mehy.2020.109765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Zhang H., Wang X., Wang J., Zhang X., Zhang Q. The eradication of breast cancer and cancer stem cells using octreotide modified paclitaxel active targeting micelles and salinomycin passive targeting micelles. Biomaterials. 2012;33(2):679–691. doi: 10.1016/j.biomaterials.2011.09.072. [DOI] [PubMed] [Google Scholar]
- 10.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favalli E.G., Biggioggero M., Maioli G., Caporali R. Baricitinib for COVID-19: a suitable treatment? Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;105932(10.1016) doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elfiky A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020;117592 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko W.-C., Rolain J.-M., Lee N.-Y., Chen P.-L., Huang C.-T., Lee P.-I. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;117477 doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang Y., Shin J.S., Yoon Y.-S., Go Y.Y., Lee H.W., Kwon O.S. Salinomycin inhibits influenza virus infection by disrupting endosomal acidification and viral matrix protein 2 function. J Virol. 2018;92(24):e01441–18. doi: 10.1128/JVI.01441-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko M., Chang S.Y., Byun S.Y., Choi I., d'Alexandry A.L.P.H., Shum D., Min J.-Y., Windisch M.P. Screening of FDA-approved drugs using a MERS-CoV clinical isolate from South Korea identifies potential therapeutic options for COVID-19. bioRxiv. 2020 doi: 10.3390/v13040651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Clercq E. Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Rev Anti-infective Therapy. 2006;4(2):291–302. doi: 10.1586/14787210.4.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain K., Sood S., Gowthamarajan K. Optimization of artemether-loaded NLC for intranasal delivery using central composite design. Drug Deliv. 2015;22(7):940–954. doi: 10.3109/10717544.2014.885999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber S., Zimmer A., Pardeike J. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 2014;86(1):7–22. doi: 10.1016/j.ejpb.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Beloqui A., Solinís M.Á., Rodríguez-Gascón A., Almeida A.J., Préat V. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomed Nanotechnol Biol Med. 2016;12(1):143–161. doi: 10.1016/j.nano.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Patlolla R.R., Chougule M., Patel A.R., Jackson T., Tata P.N., Singh M. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J Control Release. 2010;144(2):233–241. doi: 10.1016/j.jconrel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao Z., Shao J., Tan B., Guan S., Liu Z., Zhao Z. Targeted lung cancer therapy: preparation and optimization of transferrin-decorated nanostructured lipid carriers as novel nanomedicine for co-delivery of anticancer drugs and DNA. Int J Nanomed. 2015;10:1223. doi: 10.2147/IJN.S77837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.