Abstract
The global pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), has been associated with worse outcomes in several patient populations, including the elderly and those with chronic comorbidities. Data from previous pandemics and seasonal influenza suggest that pregnant women may be at increased risk for infection-associated morbidity and mortality. Physiologic changes in normal pregnancy and metabolic and vascular changes in high-risk pregnancies may affect the pathogenesis or exacerbate the clinical presentation of COVID-19. Specifically, SARS-CoV-2 enters the cell via the angiotensin-converting enzyme 2 (ACE2) receptor, which is upregulated in normal pregnancy. Upregulation of ACE2 mediates conversion of angiotensin II (vasoconstrictor) to angiotensin-(1-7) (vasodilator) and contributes to relatively low blood pressures, despite upregulation of other components of the renin-angiotensin-aldosterone system. As a result of higher ACE2 expression, pregnant women may be at elevated risk for complications from SARS-CoV-2 infection. Upon binding to ACE2, SARS-CoV-2 causes its downregulation, thus lowering angiotensin-(1-7) levels, which can mimic/worsen the vasoconstriction, inflammation, and pro-coagulopathic effects that occur in preeclampsia. Indeed, early reports suggest that, among other adverse outcomes, preeclampsia may be more common in pregnant women with COVID-19. Medical therapy, during pregnancy and breastfeeding, relies on medications with proven safety, but safety data are often missing for medications in the early stages of clinical trials. We summarize guidelines for medical/obstetric care and outline future directions for optimization of treatment and preventive strategies for pregnant patients with COVID-19 with the understanding that relevant data are limited and rapidly changing.
Abbreviations and Acronyms: ACE2, angiotensin-converting enzyme 2; ACOG, American College of Obstetricians and Gynecologists; Ang, angiotensin; ARDS, acute respiratory distress syndrome; CD, cesarean delivery; CDC, Centers for Disease Control and Prevention; CL, cervical length; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; CVS, chorionic villus sampling; F2F, face to face; FDA, Food and Drug Administration; F/U, follow-up; GA, general anesthesia; GBS, group B streptococcus; HCQ, hydroxychloroquine; HCW, health care worker; HIV, human immunodeficiency virus; ICU, intensive care unit; IL, interleukin; IOL, induction of labor; ISUOG, International Society of Ultrasound in Obstetrics and Gynecology; NAFTNet, North American Fetal Therapy Network; NSAID, nonsteroidal anti-inflammatory drug; NST, nonstress test; PPE, personal protective equipment; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; RAAS, renin-angiotensin-aldosterone system; RCOG, Royal College of Obstetricians and Gynaecologists; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SMFM, Society for Maternal-Fetal Medicine; TMPRSS2, transmembrane serine protease 2; US, ultrasonography; VD, vaginal delivery; WHO, World Health Organization
Article Highlights.
-
•
Physiologic, metabolic, and vascular changes in normal and high-risk pregnancies may affect risks for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and modify/exacerbate the clinical presentation of coronavirus disease 2019 (COVID-19).
-
•
Pregnant women may be at greater risk for SARS-CoV-2 infection, with more severe COVID-19 symptoms and worse pregnancy outcomes.
-
•
Studies to date have reported higher risks of pregnancy complications, including preterm birth and preeclampsia, as well as higher rates of cesarean delivery.
-
•
Pharmacologic therapy is limited to medications with proven safety during pregnancy and lactation; safety data are often unavailable for medications in the early stages of clinical trials.
-
•
The current recommendations are based on a limited number of studies. Future large, likely multicenter, studies will be critical in improving our understanding of the pathophysiology and clinical characteristics of COVID-19 and pregnancy, which may optimize COVID-19 preventive and treatment strategies during normal and high-risk pregnancies.
Coronaviruses are a family of enveloped, single-stranded, positive-strand RNA viruses characterized by spherical morphologic features with surface spike projections. Human coronaviruses are divided into alphacoronaviruses and betacoronaviruses. The rapid emergence and human-to-human transmission of a virulent novel lineage B betacoronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in the global pandemic of coronavirus disease 2019 (COVID-19) associated with considerable morbidity and mortality.1 , 2 Worldwide population studies to date have identified several patient characteristics, including age and comorbid conditions, as risk factors for poor outcomes, but data on pregnant patients are limited. Based on data from previous pandemics, pregnant women3 are at higher risk for acquiring infection and dying compared with nonpregnant women. The current review provides a multidisciplinary summary of the course and management of COVID-19 during pregnancy using an evidence base that has been published since identification of the first patients in Wuhan, China, in December 2019.
Taxonomy and Phylogeny of Select Human Coronaviruses
Virion and Viral Life Cycle
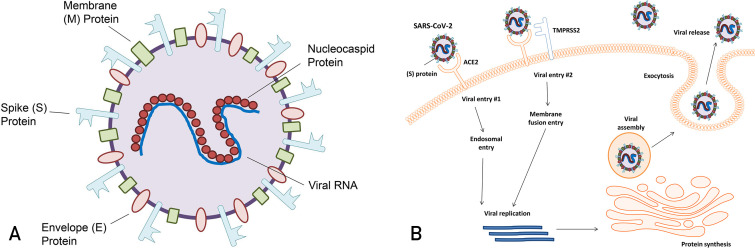
The capsid of SARS-CoV-2 contains an RNA genome complexed with a nucleocapsid protein. The membrane surrounding this nucleocapsid contains 3 proteins common to all coronaviruses: spike protein, membrane protein M, and small membrane protein E (Figure 1 A).4 Viral entry occurs via 2 routes. The first occurs when the spike protein attaches to the angiotensin-converting enzyme 2 (ACE2) receptor, releasing the viral genome and nucleocapsid protein into the host cell cytoplasm.5 The other pathway is the direct plasma membrane route via transmembrane serine protease 2 (TMPRSS2), which allows for proteolytic cleavage of the spike protein and mediation of fusion with the cell membrane.6 Intracellularly, the viral genome is translated into a replicase to produce more genome RNA, messenger RNA, and viral protein. Viral membrane proteins M, N, and E assemble on intracellular membranes. The nucleocapsid protein and viral RNA complex form a helical capsid structure, which buds between the endoplasmic reticulum and the Golgi apparatus. Mature viral particles are packaged in vesicles, transported to the cell membrane, and released from the cell (Figure 1B).5
Figure 1.
Features and life cycle of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A, Structure of the SARS-CoV-2 viron. B, Viral entry methods and replication of SARS-CoV-2.
Viral Tropism and Normal and High-Risk Pregnancies
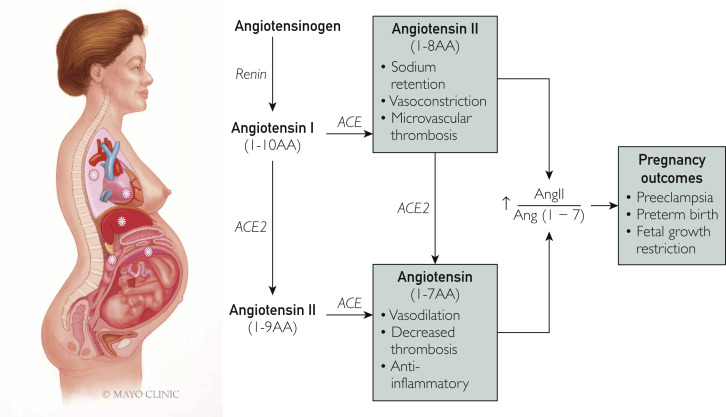
The ACE2 enzyme plays a key role in the conversion of angiotensin Ang I to Ang-(1-9) and Ang II to Ang-(1-7) (vasodilatory, antithrombotic, and anti-inflammatory activities) (Figure 2 ). The hormonal profile of normal gestation is characterized by an early increase of all the components of the renin-angiotensin-aldosterone system (RAAS), including ACE2.7 This raises the possibility that pregnant women may be at a greater risk for SARS-CoV-2 infection. In addition, low blood pressure in pregnant women is maintained through a balance between being refractory to the pressor effects of Ang II and increased levels of Ang-(1-7), which exhibit systemic vasodilatory responses.8 , 9 In preeclampsia, a pregnancy-specific hypertensive disorder that affects 3.5% of all pregnancies10 clinically is characterized by multisystem involvement and, commonly, proteinuria; this balance is lost, with an overexaggerated Ang II blood pressure response.11 Preeclampsia has also been associated with decreased maternal plasma Ang-(1-7) levels.9 Because SARS-CoV-2 not only binds to ACE2 but also causes its downregulation,12 infections during pregnancy may potentiate the RAAS abnormalities, ie, increased Ang II relative to decreased Ang-(1-7), that are present in preeclampsia. COVID-19 and preeclampsia share additional common mechanisms, including endothelial cell dysfunction and coagulation abnormalities. Notably, ACE2 receptors are also expressed by endothelial cells,13 and endothelial cell infection and immune cell–mediated endothelial injury have been recently described in COVID-19.14 Because the hallmark of preeclampsia is endothelial dysfunction,15 infection with SARS-CoV-2 during pregnancy could mimic and/or initiate microvascular dysfunction by causing endotheliitis. Systemic inflammation and microcirculatory dysfunction, characterized by vasoconstriction and resultant ischemia, ensue. This can further contribute to a pro-coagulopathic state, as demonstrated by high rates of deep vein thrombosis, stroke, and pulmonary embolism, which are increasingly reported in patients with COVID-19.16, 17, 18 Infection with SARS-CoV-2 during pregnancy can be particularly prothrombotic because coagulation abnormalities may potentiate a hypercoagulable state, which is already present in uncomplicated pregnancy and exacerbated by preeclampsia.15 Similarly, complement activation, which is present in both preeclampsia19 and COVID-19,20 may result in particularly severe thrombotic vascular injury when these disease states are present concurrently. In summary, RAAS abnormalities, endothelial dysfunction, complement activation, and the pro-coagulopathic effects of COVID-19 are similar to those occurring in preeclamptic pregnancies, potentially resulting in progressive vascular damage. Therefore, pregnancy and its complications represent a vulnerable state for invasive infection with SARS-CoV-2, reflecting several overlapping cellular mechanisms.
Figure 2.
Pregnancy, coronavirus disease 2019 (COVID-19), and mechanisms of vascular damage. Upregulation of angiotensin-converting enzyme 2 (ACE2) receptor in pregnancy may increase the risk of severe acute respiratory syndrome coronavirus 2 infection. Binding of virus to ACE2 causes its downregulation and may increase angiotensin (Ang) II relative to Ang-(1-7), thus favoring vasoconstriction, which can mimic/worsen vascular dysfunction in preeclampsia.
In addition to the direct cytotoxic effect of the virus, tissue injury in COVID-19 is mediated through an excessive inflammatory response, commonly referred to as cytokine storm. Cytokine storm is mediated via immune responses, which are significantly modified in pregnancy, and may contribute to COVID-19 laboratory and clinical characteristics during pregnancy.
Immune Responses to COVID-19
During pregnancy, the maternal immune system must adjust to tolerate the semiallogeneic fetus while maintaining its ability to respond to pathogenic insult.21 , 22 This is also known as T helper 2 polarization. However, near the end of pregnancy a switch to T helper 1 immunity occurs and the maternal immune system becomes proinflammatory, leading to the sequence of events that occur before parturition (ie, cervical dilation, contractions). Data on immune responses to SARS-CoV-2 in pregnant women are lacking at this time, and data from previous pandemics, suggest that pregnancy may increase the risk of acquiring infection and dying compared with nonpregnant women.3 The timing of infection during gestation may induce differences in maternal immune responses, viral clearance, and, ultimately, perinatal outcomes. Because the first and third trimesters are proinflammatory to promote implantation and labor,23 pregnant women infected with SARS-CoV-2 during these trimesters may be at higher risk for exaggerated responses to virus (cytokine storm). Furthermore, high levels of stress and inflammation occur during labor, and the physiologic changes that occur in a mother’s body after the baby is born could lead to poor maternal COVID-19 outcomes postpartum. This has been observed clinically, where pregnant women with mild symptoms on admission to the hospital for delivery required postpartum hospital admission for respiratory symptoms.24 , 25
Conflicting data exist regarding vertical transmission of the virus; however, research on other coronavirus infections during pregnancy suggests that in utero transmission does not occur. Mouse models and epidemiologic data have shown that inflammatory immune responses generated by viral infection during pregnancy can result in negative effects on fetal brain development.26, 27, 28 During the H1N1 pandemic, infected women had higher rates of preterm birth.29 Therefore, although placental transmission of the virus may not occur with SARS-CoV-2 infection, other short- and long-term effects from inflammation may adversely affect the developing fetus. These require further characterization. Maternal immunity may be passed on to protect the fetus, conferring passive immunity. Immunoglobulin G specific to the 2003 SARS-CoV outbreak strain was found not only in maternal blood, but also in amniotic fluid and cord blood.30 Another possible source of antibodies could be breast milk, but this has yet to be determined.
Maternal Physiology and Clinical Characteristics of COVID-19 During Pregnancy
Significant physiologic changes to respiration occur during pregnancy,31 including increased secretions and congestion in the upper airways, increased chest wall circumference, and upward displacement of the diaphragm. These changes result in decreased residual volume and increased tidal volume and air trapping, slightly decreased airway resistance, stable diffusion capacity, increased minute ventilation, and increased chemosensitivity to carbon dioxide. Hemodynamic changes include increased plasma volume of 20% to 50%, increased cardiac output, and decreased vascular resistance.31 These changes result in a state of physiologic dyspnea and respiratory alkalosis as well as an increased susceptibility to respiratory pathogens. As has been seen with other viral respiratory infections, the early symptoms of SARS-CoV-2 infection may mimic physiologic dyspnea in pregnancy, which could result in delayed diagnosis and more severe disease.32
Pregnant women with SARS-CoV-2 infection may experience more severe symptoms compared with nonpregnant women. Existing limited data have reported on rapid deterioration in women who had no symptoms on arrival and were subsequently diagnosed as having severe COVID-19.24 In some, but not all, patients, maternal comorbidities were present (hypertension, diabetes, cholestasis of pregnancy).24 , 33 Case reports have also described cases of quickly worsening maternal status with the ultimate diagnosis of cardiomyopathy.34 Unfortunately, these rapidly progressive maternal complications have led to a high rate of cesarean deliveries (CDs) for either worsening maternal status or nonreassuring fetal status secondary to the worsening maternal clinical state.
Preeclampsia is an example of a common pregnancy-related complication that may be exacerbated by, or may exacerbate, COVID-19, as previously discussed. The picture becomes further complicated because the two processes share common laboratory abnormalities. Thus, it may be difficult to discern whether certain abnormal laboratory findings are due to SARS-CoV-2 infection or preeclampsia, and this interplay may have treatment implications. For example, thrombocytopenia35 and liver function abnormalities,36 both of which are diagnostic criteria for preeclampsia with severe features, are also associated with worsening COVID-19.
Maternal Disease and Outcomes
Physiologic changes in normal pregnancy and metabolic and vascular changes in high-risk pregnancies may affect the pathogenesis or exacerbate the clinical presentation of COVID-19 during pregnancy. A systematic review by Di Mascio et al37 evaluating and comparing obstetric outcomes in combined coronavirus infections (SARS, Middle East respiratory syndrome, and SARS-CoV-2) found that SARS-CoV-2 alone resulted in higher rates of preterm birth (24.3% [95% CI, 12.5% to 38.6%] for <37 weeks’ gestation and 21.8% [95% CI, 12.5% to 32.9%] for <34 weeks’ gestation), preeclampsia (16.2% [95% CI, 4.2% to 34.1%]), and CD (83.9% [95% CI, 73.8% to 91.9%]).
As of April 22, 2020, a total of 23 studies25 , 34 , 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 (excluding overlapping of case reports) addressing obstetrical and neonatal outcomes of SARS-CoV-2 infection in pregnancy have been published in English. These studies span January 1, 2020, to April 22, 2020, and include 185 patients. The abstracted information is presented in Table 1 , which summarizes maternal and neonatal outcomes. Briefly, most of the diagnoses occurred in the third trimester. Fever was the most common presenting symptom, followed by cough, dyspnea, and gastrointestinal alterations. Slightly more than 25% of patients were asymptomatic at diagnosis. The most common laboratory findings were lymphopenia and neutrophilia. Pneumonia was a common diagnosis (40%), and a small percentage (3.24%) required intensive care unit admission.
Table 1.
| Characteristic |
Value (N=185) |
|---|---|
| Maternal data | |
| Age (y), mean (range) | 29.6 (20-41) |
| Trimester (No./total No. [%]) | |
| First | 3/185 (1.62) |
| Second | 5/185 (2.70) |
| Third | 177/185 (95.68) |
| Signs and symptoms (No./total No. [%]) | |
| Fever | 90/169 (53.25) |
| Pneumonia | 75/184 (40.76) |
| Cough | 56/169 (33.13) |
| Asymptomatic | 44/169 (26.03) |
| Dyspnea/shortness of breath | 22/169 (13.01) |
| Gastrointestinal alterations | 9/169 (5.32) |
| ICU admission | 6/185 (3.24) |
| Diagnostic method (No./total No. [%]) | |
| qRT-PCR SARS-CoV-2 only | 179/185 (96.75) |
| CT changes only | 6/185 (3.24) |
| qRT-PCR SARS-CoV-2 and CT changes | 100/185 (54.05) |
| Laboratory alterations | |
| Lymphopenia | 32/93 (34.40) |
| Neutrophilia | 8/93 (8.60) |
| Interventions (No./total No. [%]) | |
| Antibiotics | 64/145 (44.13) |
| Supportive measures | 41/145 (28.27) |
| Antiviral therapy | 39/145 (26.90) |
| Corticosteroids | 12/145 (8.28) |
| Obstetric comorbidities (No./total No. [%])c | |
| Gestational hypertension | 6/182 (3.29) |
| Preeclampsia | 4/182 (2.20) |
| Gestational diabetes | 11/182 (6.04) |
| Prelabor rupture of membranes | 13/184 (7.07) |
| Fetal distress | 23/184 (12.50) |
| Patient status (No./total No. [%]) | |
| Delivered | 152/185 (82.16) |
| Still pregnant | 33/185 (17.83) |
| Mode of delivery (No./total No. [%]) | |
| Cesarean delivery | 129/152 (84.86) |
| Vaginal delivery | 19/152 (12.50) |
| Pregnancy termination | 4/152 (2.63) |
| Gestational age at delivery of viable pregnancies (No./total No. [%]) | |
| <28 wk | 0/148 (0.00) |
| 28-31 6/7 wk | 2/148 (1.35) |
| 32-35 6/7 wk | 26/148 (17.56) |
| ≥36 wk | 96/148 (64.86) |
| Missing data | 24/148 (16.21) |
| Neonatal data | |
|---|---|
| Neonates reported (No./total No. [%]) | |
| Total | 146 (100) |
| Live births | 145/146 (99.31) |
| Stillbirths | 1/146 (0.68) |
| Comorbidities after live birth (No./total No. [%]) | |
| Neonatal ICU admission | 27/145 (18.62) |
| Low birth weight | 15/145 (10.34) |
| Pneumonia | 9/145 (6.20) |
| qRT-PCR SARS-CoV-2 positive | 2/145 (1.37) |
| Neonatal death | 1/145 (0.69) |
COVID-19 = coronavirus disease 2019; CT = computed tomography; ICU = intensive care unit; qRT-PCR = quantitative reverse transcriptase polymerase chain reaction; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Note that certain parameters were not evaluated or reported in all patients, so the denominators used for calculations represent only the numbers for which data are available.
Thirty-three of 185 patients (17.8%) were still pregnant at the end of this study; therefore, rates of complications occurring in late pregnancy or close to delivery, such as preeclampsia, might have been underestimated.
Management of patients varied according to institution. Most were treated with medications that are considered to be relatively safe during pregnancy: antibiotics (cefoperazone, sulbactam, ceftriaxone, cefazolin, and azithromycin), antiviral therapy (lopinavir, ritonavir, oseltamivir, and ganciclovir), and a few were treated with corticosteroids (dexamethasone, methylprednisolone).
Due to the high false-negative rates of the nasopharyngeal swab for the quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) for the SARS-CoV-2 test,59 computed tomography may be required to confirm the diagnosis in cases of high suspicion, as seen in 4 cases reported by Wu et al.53 There were no patients who delivered before 28 weeks’ gestation, and most patients delivered at 36 0/7 weeks or later. The impact of infection on timing of delivery is still unclear. Liu et al41 reported a 46% preterm labor rate between 32 and 36 weeks of gestation in 10 patients admitted with positive SARS-CoV-2 infection, and Zhang et al44 reported no difference in mean ± SD gestational age at delivery for 16 women with SARS-CoV-2 infection (38.7±1.4 weeks) and 45 women without SARS-CoV-2 infection (37.9±1.6 weeks).
A systematic review by Zaigham and Andersson60 including 108 pregnant women reported that CD was the most common mode of delivery, with a rate of 92%. It can be speculated that SARS-CoV-2 infections are more likely to result in maternal hypoxia or increased oxygen requirements, resulting in a nonreassuring fetal heart tracing, warranting expedited delivery. There may also be lack of SARS-CoV-2 screening in some health care settings, resulting in selection bias for CD in severe cases. The indication for CD needs to be further evaluated because current guidelines indicate that SARS-CoV-2 infection alone is not an indication for CD.61 , 62
A recent multicenter cohort study of severe COVID-19 in pregnant patients from 12 US institutions reported that patients were usually admitted to the hospital with severe disease 7 days after the onset of symptoms and typically were intubated 2 days after admission.63 Fifty percent of women required delivery, resulting in a high rate of preterm birth.
Neonatal Outcomes
Neonatal outcomes are shown in Table 1. There was 1 reported stillbirth41 (<1%) due to severe maternal disease with multiple organ failure and 1 neonatal death38 (<1%) due to refractory shock with multiple organ failure after delivery at 34 5/7 weeks’ gestation. Among the 145 live births, 2 neonates tested positive for SARS-CoV-2 infection. Both did well with supportive therapy and observation and were discharged from the hospital in stable condition.48 , 58
Di Mascio et al37 reported increased perinatal mortality and higher rates of neonatal intensive care unit admissions, but all neonates tested negative for SARS-CoV-2 infection. Chen et al43 confirmed no morphologic changes related to infection in 3 placentas of COVID-19–positive mothers. All 3 neonates also tested negative for SARS-CoV-2. Although these findings are consistent with reports suggesting minimal to no risk of vertical transmission,42 , 43 , 64 Penfield et al65 reported positive SARS-CoV-2 results in 3 of 11 placental swabs from COVID-19–positive mothers. All 3 of the neonates also tested negative. Whether vertical transmission truly occurred, or whether neonates were swabbed too early (during the incubation period) is unclear.
Shah et al66 published a well-structured classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates that gives the opportunity to consider the risk of maternal to fetal or neonatal transmission beyond just vertical transmission. The classification includes congenital infection from intrauterine death/stillbirth, congenital infection in live-born infants, neonatal infection acquired intrapartum, or neonatal infection acquired postnatally. In addition, several professional societies have provided guidelines for the management of COVID-19 during pregnancy. The overall summaries from these professional bodies are consistent, with some variation in the strength of recommendations.119
Current Guidelines for COVID-19 Management in Pregnancy
Professional perinatal societies, including the Society for Maternal-Fetal Medicine (SMFM)62 , 67 and the American College of Obstetricians and Gynecologists (ACOG)68 , 69 from the United States, the Royal College of Obstetricians and Gynaecologists (RCOG)61 from the United Kingdom, the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG),70 the Centers for Disease Control and Prevention (CDC),71 , 72 and the World Health Organization (WHO),73 have developed guidelines for the care of pregnant patients. Herein we summarize the most current guidelines, updated as of April 22, 2020. A total of 9 papers were identified from 6 societies: SMFM, ACOG, RCOG, ISUOG, CDC, and WHO.
A summary of these guidelines is outlined in Table 2 , divided into three sections: antepartum, intrapartum, and postpartum care. The guidelines provide practical management recommendations that institutions can adapt to their infrastructures and resource availability. The recommendations from the SMFM focus on high-risk pregnancies, and those from the ACOG and the RCOG focus on all pregnancies. The WHO and the CDC focus on recommendations that can be generalized across all patient populations, and ISUOG focuses on sonography and care of ultrasound equipment.
Table 2.
Consensus on Recommendations Classified by Phase of Care of Pregnancy
| Antepartum care | |||||||
|---|---|---|---|---|---|---|---|
| Title | Prenatal infection screening | Prenatal appointment | US frequency | US equipment/patient rooms | Antenatal surveillance | Antenatal corticosteroids | GBS screening |
| Consensus on recommendations | Triage symptomatic patients via telehealth Test anyone with new flulike symptoms Prioritize high-risk patients: older, immunocompromised, advanced HIV, homeless, hemodialysis Use drive-through or standalone testing area All suspected cases should be screened using qRT-PCR Symptomatic patients should be treated as positive until results are back Repeat testing in 24 h if negative but still high suspicion |
Elective and nonurgent appointments should be postponed or completed by telehealth Encourage use of telehealth for all visits HCW meetings should be conducted via virtual/audio platform, if feasible Reserve F2F visits for 11-13, 20, 28, 36 wk and weekly after 37 wk Complete laboratory tests and US on same visit day Limit support person at outpatient F2F visits |
Consensus: Continue US as medically indicated when possible SMFM suggestions: Combine dating and nuchal translucency US in first trimester Anatomy scan at 20-22 wk Consider stopping serial CL after anatomy US if transvaginal US CL ≥35 mm, previous preterm birth at >34 wk Body mass index >40: schedule at 22 wk to reduce risk of suboptimal views/need for follow-up Single growth F/U at 32 wk Low-lying placenta F/U 34-36 wk |
Must be cleaned with disinfectant per manufacturer guidelines after EVERY use Deep clean all instruments and room in the case of a positive patient |
Reserve for medically indicated screening Limit NST <32 wk Twice weekly NST only for fetal growth restriction with abnormal umbilical arterial Doppler studies, complicated monochorionic twins, or Kell-sensitized patients with significant titers If patient needs US, perform biophysical profile instead of NST Kick counts instead of NST for low-risk patients Daily NST if patient hospitalized |
Should continue if <34 wk, even if tested positive for COVID-19 Balance risks and benefits for 34 0/7 to 36 6/7 wk Other modifications should be individualized |
As indicated between 36 0/7 and 37 6/7 wk gestation Consider grouping with other visits in the same time frame Patients can self-collect with proper instructions if the resources and infrastructure allow |
| Intrapartum care | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Predelivery preparation/screening | Delivery location | Delivery time | Mode of delivery | Support person | Obstetric analgesia and anesthesia | Oxygen use | Second stage of labor | Third stage of labor | Umbilical cord clamping | PPE |
| Consensus on recommendations | Social distancing and off work for 2 wk before anticipated delivery (start at ∼37 wk) Screen patient and partner on phone day before admission Limit HCW staffing to only essential staff |
Designated isolation room, for suspected or confirmed cases of COVID-19 | Based on routine obstetric indications Early delivery should be considered for critically ill patients No contraindications to IOL unless there are limited beds |
Based on routine obstetric indications COVID-19 infection is not an indication for CD Expedite delivery by CD in the setting of fetal distress or maternal deterioration Water births should be avoided |
Allowed 1 consistent asymptomatic support person | No evidence against regional or general anesthesia Epidural analgesia is recommended to women with suspected or confirmed COVID-19 to minimize the need for GA if urgent delivery is needed Avoid use of nitrous oxide |
Do not use oxygen for intrauterine resuscitation Consider aerosolizing HCWs must wear appropriate PPE (N95) |
Do not delay pushing Consider shortening with operative delivery to minimize aerosolization and maternal respiratory effort |
Consider active management to reduce blood loss (national blood shortage) | Delayed cord clamping is still recommended in the absence of contraindications Avoid delayed cord clamping in confirmed and suspected cases |
-Asymptomatic or negative patients: Patient and provider wear surgical mask Aerosolizing procedures: N95 for patient and N95, gown, gloves, face shield for provider |
| Postpartum care | ||||||
|---|---|---|---|---|---|---|
| Title | Placental and fetal tissue | Length of stay | Breastfeeding | Skin to skin | Postpartum pain control | Postpartum visit |
| Consensus on recommendations | ISUOG recommendations: Should be handled as infectious tissue in positive patients Consider qRT-PCR on placenta |
Expedited discharge should be considered if stable. VD → 1 d CD → 2 d |
Limited evidence to advise against breastfeeding Advise patients to (1) practice respiratory hygiene during feeding, (2) wear a mask, (3) wash hands before and after touching the baby, (4) routinely clean and disinfect surfaces they have touched During separation, encourage dedicated breast pumping |
Routine precautionary separation of a healthy baby and mother is not advised Encourage good hygiene and appropriate PPE for COVID-19–positive patients |
No contraindication to NSAID use | Encourage telehealth for postpartum visit Limit F2F visits only for medically necessary concerns |
CD = cesarean delivery; CL = cervical length; COVID-19 = coronavirus disease 2019; F2F = face to face; F/U = follow-up; GA = general anesthesia; GBS = group B streptococcus; HCW = health care worker; HIV = human immunodeficiency virus; IOL = induction of labor; ISUOG = International Society of Ultrasound in Obstetrics and Gynecology; NSAID = nonsteroidal anti-inflammatory drug; NST = nonstress test; PPE = personal protective equipment; qRT-PCR = quantitative reverse transcriptase polymerase chain reaction; SMFM = Society for Maternal-Fetal Medicine; US = ultrasonography; VD = vaginal delivery.
Prenatal/Antepartum Care
The consensus among all societies recommends the use of telehealth for prenatal visits. Ultrasound and antenatal surveillance should be combined with visits for laboratory tests or prenatal care. Patients should be screened for symptoms, travel history, and contact history before any face-to-face visits; those who are symptomatic or meet the criteria should undergo testing for SARS-CoV-2 using qRT-PCR. Appropriate personal protective equipment (PPE) should be worn by patients and health care workers. Administration of antenatal corticosteroids for fetal lung maturation should still be considered if a pregnancy is between 24 0/7 and 33 6/7 weeks’ gestation, but the risk/benefit balance needs to be discussed by the multidisciplinary team. Data on the use of corticosteroids during late preterm (34 0/7 to 36 6/7 weeks) are still controversial, but routine administration is not advised.67
Intrapartum Care
Institutions should have a designated area for triaging, screening, and admitting SARS-CoV-2–positive patients. The mode and timing of delivery should follow routine obstetric indications, keeping in mind that COVID-19 alone is not an indication for CD, unless there is fetal distress or deteriorating maternal clinical status. Societies recommend that only 1 consistent healthy asymptomatic individual providing support should be present during labor and delivery. Aerosol-generating procedures, including forceful pushing during the second stage of labor and oxygen supplementation for intrauterine resuscitation, should be limited and appropriate PPE (N95) worn. Water births are contraindicated due to the limited ability to monitor mother and baby, and the risk of fecal transmission.
Postpartum Care
Breastfeeding should not be discouraged, and mother and baby separation is not advised, unless the mother is acutely ill. Mothers are advised to follow appropriate respiratory hygiene by wearing masks during skin-to-skin contact and breastfeeding. Mothers should wash hands before handling their babies or touching pumps or bottles and should avoid coughing while their babies are feeding. All surfaces and breast pumps should be sanitized after each use. In an effort to limit infection exposure, hospital length of stay should be decreased to 1 day for vaginal deliveries and 2 days for CDs. Postpartum visits should be performed through telehealth and patients advised to continue compliance with social distancing after discharge. The method of telehealth should be individualized based on institution resources and availability.
Implications of COVID-19 in Special Pregnant Patient Populations
Evidence on the potential outcomes of SARS-CoV-2 infection in pregnancies already complicated by congenital anomalies is lacking. Given the severity of some potentially life-threatening congenital conditions as well as the disease-altering effects of fetal interventions, these procedures are considered urgent essential medical services. Therefore, necessary adjustments to the prenatal evaluation and selection of fetal intervention candidates have been proposed to better adapt this essential service to the ongoing pandemic. Perhaps the most important factor to consider is the potential risk of vertical transmission induced by the invasive nature of these procedures.
There is no definitive evidence of in utero transmission from SARS-CoV-2 to date. Some case reports48 , 51 , 58 have reported possible vertical transmission due to positive amniotic fluid SARS-CoV-2 PCR test results, but most of the limited patient series reported in the literature indicate a low to negligible risk.74 , 75 Evidence is rapidly accumulating, and this consensus may change as more patients with COVID-19 in pregnancy are reported.
Prenatal Diagnosis
In the event of a suspected or confirmed fetal anomaly, additional evaluation (fetal echocardiography, amniocentesis, chorionic villus sampling [CVS], or cordocentesis) may be indicated to identify patients who could benefit from fetal interventions.
Prenatal diagnostic evaluations may be classified as invasive or noninvasive depending on the risk of vertical transmission and exposure of patients and health care workers to SARS-CoV-2. Imaging studies, including ultrasonography and fetal echocardiography, are considered noninvasive (with no risk of vertical transmission), but specific precautions, including hygiene and use of appropriate PPE, should be applied to the patient and examiner, as well as proper care of the sonogram and ultrasound suite.76 For patients with suspected or confirmed SARS-CoV-2 infection, consideration should be given to postponing prenatal imaging until asymptomatic, if safely feasible.
Invasive diagnostic tests (CVS, amniocentesis, and cordocentesis) are associated with a theoretical risk of vertical transmission because these procedures may directly correlate with the risk of fetomaternal hemorrhage.74 Chorionic villus sampling, which is usually performed between 10 0/7 and 13 6/7 weeks’ gestation, may be offered to patients with a low risk of SARS-CoV-2 infection (asymptomatic or negative screening result). For symptomatic patients with suspected or confirmed SARS-CoV-2, invasive diagnostic tests can be delayed if safely feasible. If genetic testing cannot be delayed, amniocentesis (usually performed after 14 0/7 weeks’ gestation) should be performed instead of CVS owing to the theoretical lower risk of vertical transmission if transplacental access is avoided. Amniocentesis can also be offered to all asymptomatic or confirmed SARS-CoV-2–negative patients.74 Fetal blood sampling/transfusion is another invasive procedure with a theoretical risk of vertical transmission. This intervention may be offered to patients with confirmed negative SARS-CoV-2 PCR but should be delayed (if feasible and safe) in those who are symptomatic or positive for SARS-CoV-2.74
Fetal Therapy
The Mayo Clinic Fetal Center follows the recommendations of the North American Fetal Therapy Network (NAFTNet), which currently recommends that fetal interventions be provided as much as resources allow due to the time-sensitive nature of conditions amenable to fetal therapy.77 Specific institutional policies may vary, but, in general, all fetal interventions that have been established as the standard of care (for select patients) should continue to be provided, taking the necessary perioperative precautions. Conversely, innovative or experimental procedures that are yet to show proven benefit should be individualized. In general, for patients with asymptomatic SARS-CoV-2 infection, fetal intervention can be offered. For symptomatic patients, it is recommended that fetal therapy be postponed until maternal conditions stabilize and patients have recovered from the disease. Some examples of fetal surgeries that are still currently offered at Mayo Clinic include fetoscopic laser ablation of placental anastomoses for twin-to-twin transfusion syndrome,78 in utero repair of spina bifida,79 intrauterine fetal blood transfusion,80 in utero intervention for lower urinary tract obstruction,81 fetal endoscopic tracheal occlusion for congenital diaphragmatic hernia,82 in utero procedure for fetal tumors associated with hydrops,83 and in utero intervention for severe congenital heart defects.84
Treatment of COVID-19 in Pregnant Patients
No drugs have been proved to be effective and safe to use for the treatment of COVID-19 to date. Table 3 outlines the medications or therapies used in various research protocols under investigation, as well as their safety for use in pregnancy. In addition, because the pro-coagulatory state of pregnancy may contribute to thrombotic risks associated with COVID-19, thromboprophylaxis, which is currently advised for patients with COVID-19,113 should be considered for pregnant patients as well.
Table 3.
Treatment Options for COVID-19
| Treatment strategy | Mechanism of action | Effectiveness | Safety in pregnancy |
|---|---|---|---|
| HCQ/chloroquine85 | Reduces inflammatory cytokines86; interferes with ACE2 receptor synthesis.87,88 | Reduction of body temperature recovery time and cough remission, pneumonia recovery, improved CT findings, nasopharyngeal viral clearance.89, 90, 91 | Generally considered safe in pregnancy and frequently used for patients with autoimmune disease.92 Efficacy unproven. Concern for prolonged QTc. |
| HCQ and azithromycin | Reduction of viral replication and IL-6 and IL-8 production.87,93 | Improved nasopharyngeal viral clearance.90 | HCQ: as above. Azithromycin: considered safe.94 |
| Lopinavir/rotinavir | Inhibition of 3-chymotrypsin-like protease.86,95,96 | Reduced mortality.97 | Good safety profile in pregnant patients with HIV.98 |
| Remdesivir | Inhibition of viral RNA-dependent RNA polymerase.99 | Clinical trial still underway. Reduction in duration of hospital stay and mortality.100 | Not yet FDA approved. |
| Anakinra | IL-1 inhibitor. | Clinical trial still underway. | Insufficient data to determine risk in pregnancy.101 |
| Siltuximab | Human-mouse chimeric monoclonal antibody against IL-6. | Improvement in clinical condition in one-third of patients.102 | Insufficient data to determine risk in pregnancy.103 |
| Sarilumab | Recombinant IL-6 receptor monoclonal antibody. | No data yet from randomized clinical trials or observational studies.85 | Insufficient data to determine risk in pregnancy.85 |
| Tocilizumab | Recombinant IL-6 receptor monoclonal antibody. | No data yet from randomized clinical trials or observational studies.85 | Insufficient data to determine risk in pregnancy.85 |
| Interferon | Antiviral cytokines. | No data yet from randomized clinical trials or observational studies.85 | Varying adverse effect profiles in various preparations. |
| Corticosteroid | Anti-inflammatory actions.104 | Reduced mortality in patients with ARDS.105 Faster improvement in patients with severe COVID pneumonia.106 | Considered safe, approved for lung maturation in preterm birth.107 |
| ACE inhibitors or angiotensin receptor blockers | ACE2 receptor is the cell receptor for viral entry for COVID-19 virus.108,109 | No data yet from randomized clinical trials or observational studies85 | Contraindicated in pregnancy.110,111 |
| Convalescent plasma | Convalescent plasma from recently recovered donors targeting COVID-19 virus. | 10 Patients with clinically severe COVID-19 were given 200 mL of convalescent plasma. Increase in oxyhemoglobin saturation by day 3, and improved lymphocyte count as well as CRP levels were noted.Several studies are currently underway.112 | No data on safety in pregnancy. However, specific immunoglobulins as for varicella are used in pregnancy.103 |
ACE = angiotensin-converting enzyme; ARDS, acute respiratory distress syndrome; COVID-19 = coronavirus disease 2019; CRP = C-reactive protein; CT = computed tomography; FDA = Food and Drug Administration; HCQ = hydroxychloroquine; HIV = human immunodeficiency virus; IL = interleukin.
There are 6 candidate vaccines under phase 1 or 2 clinical trials and 77 more candidate vaccines in preclinical evaluation as of April 23, 2020.114 Many vaccines use the spike protein (S protein) as their platform and present as forms of recombinant protein-based vaccines, live attenuated vaccines, inactive viral vaccines, and viral-vector–based vaccines.115 Live attenuated vaccines are generally contraindicated in pregnancy, but exceptions may be made during pandemic situations (exception for smallpox vaccine). As with any drug under development, assessment for safety in pregnancy is conducted after initial safety data become available from clinical studies.116 Although it is essential to guarantee safety, an unfortunate impact of delaying research in pregnancy is that vaccinations for pregnant women may also be delayed. This is especially problematic during a pandemic or epidemic, as evident from lessons learned from the Ebola outbreak.117
Future Perspectives
The presented data are preliminary, collected over 4 months and likely to change once large data sets become available. However, the projected course of COVID-19 on the morbidity and mortality of pregnant patients during these challenging times is unprecedented. Racial disparities are known to exist in the obstetric literature.118 Global health crises subject racial and ethnic minorities, as well as patients with immunocompromised comorbidities, to poorer outcomes. We envision that national and international perinatal societies will focus on the unique challenges faced by vulnerable patient populations that are burdened with physical, emotional, and social crises, with a focus on improving outcomes for all pregnant patients.
Conclusion
Given differing physiology during gestation, pregnancy represents a vulnerable state that may be associated with a greater risk of SARS-CoV-2 infection and subsequent worse COVID-19 outcomes. Global efforts to fast track publication of data on COVID-19 in pregnancy, albeit limited, have allowed us to form a framework to care for these patients. Early reports suggest higher rates of preeclampsia and other pregnancy-related complications with SARS-CoV-2 infection during pregnancy, thus adding urgency to the pursuit of research into optimal COVID-19 treatment and preventive strategies during pregnancy.
Acknowledgments
We thank Margaret A. McKinney, MS, for assistance with media and illustration support.
Footnotes
Grant Support: This work was supported by grant R01-HL136348 from the National Institutes of Health.
Potential Competing Interests: Dr Chakraborty has received grants from the National Institutes of Health and research support from Gilead. The other authors report no competing interests.
Supplemental Online Material
References
- 1.Loeffelholz M.J., Tang Y.-W. Laboratory diagnosis of emerging human coronavirus infections: the state of the art. Emerg Microbes Infect. 2020;9(1):747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya A.E., Baker S.C., Baric R.S. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen S.A., Jamieson D.J., Macfarlane K., Cragan J.D., Williams J., Henderson Z. Pandemic Influenza and Pregnancy Working Group. Pandemic influenza and pregnant women: summary of a meeting of experts. Am J Public Health. 2009;99(suppl 2):S248–S254. doi: 10.2105/AJPH.2008.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prajapat M., Sarma P., Shekhar N. Drug targets for corona virus: a systematic review. Indian J Pharmacol. 2020;52(1):56–65. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Molec Biol Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brosnihan K.B., Neves L.A., Anton L., Joyner J., Valdes G., Merrill D.C. Enhanced expression of Ang-(1-7) during pregnancy. Braz J Med Biol Res. 2004;37(8):1255–1262. doi: 10.1590/s0100-879x2004000800017. [DOI] [PubMed] [Google Scholar]
- 8.West C.A., Sasser J.M., Baylis C. The enigma of continual plasma volume expansion in pregnancy: critical role of the renin-angiotensin-aldosterone system. Am J Physiol Renal Physiol. 2016;311(6):F1125–F1134. doi: 10.1152/ajprenal.00129.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merrill D.C., Karoly M., Chen K., Ferrario C.M., Brosnihan K.B. Angiotensin-(1-7) in normal and preeclamptic pregnancy. Endocrine. 2002;18(3):239–245. doi: 10.1385/ENDO:18:3:239. [DOI] [PubMed] [Google Scholar]
- 10.Garovic V.D., White W.M., Vaughan L. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020;75(18):2323–2334. doi: 10.1016/j.jacc.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lumbers E.R., Delforce S.J., Arthurs A.L., Pringle K.G. Causes and consequences of the dysregulated maternal renin-angiotensin system in preeclampsia. Front Endocrinol (Lausanne) 2019;10:563. doi: 10.3389/fendo.2019.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glowacka I., Bertram S., Herzog P. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84(2):1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrario C.M., Trask A.J., Jessup J.A. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289(6):H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garovic V.D., Hayman S.R. Hypertension in pregnancy: an emerging risk factor for cardiovascular disease. Nat Clin Pract Nephrol. 2007;3(11):613–622. doi: 10.1038/ncpneph0623. [DOI] [PubMed] [Google Scholar]
- 16.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19 [published online April 10, 2020] https://doi.org/10.1016/j.thromres.2020.04.013 Thromb Res. [DOI] [PMC free article] [PubMed]
- 17.Xie Y., Wang X., Yang P., Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiol Cardiothorac Imag. 2020;2(2):e200067. doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China [published online April 10, 2020] https://doi.org/10.1001/jamaneurol.2020.1127 JAMA Neurol. [DOI] [PMC free article] [PubMed]
- 19.Alrahmani L., Willrich M.A.V. The complement alternative pathway and preeclampsia. Curr Hypertens Rep. 2018;20(5):40. doi: 10.1007/s11906-018-0836-4. [DOI] [PubMed] [Google Scholar]
- 20.Risitano A.M., Mastellos D.C., Huber-Lang M. Complement as a target in COVID-19 [published online April 23, 2020]? https://doi.org/10.1038/s41577-020-0320-7 Nat Rev Immunol. [DOI] [PMC free article] [PubMed]
- 21.Aghaeepour N., Ganio E.A., McIlwain D. An immune clock of human pregnancy [published online April 10, 2020] Sci Immunol. 2017;2(15):eaan2946. doi: 10.1126/sciimmunol.aan2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enninga E.A., Nevala W.K., Creedon D.J., Markovic S.N., Holtan S.G. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol. 2015;73(3):251–262. doi: 10.1111/aji.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mor G., Aldo P., Alvero A.B. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17(8):469–482. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 24.Breslin N., Baptiste C., Miller R. COVID-19 in pregnancy: early lessons. Am J Obstet Gynecol MFM. 2020;2(2):100111. doi: 10.1016/j.ajogmf.2020.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslin N., Baptiste C., Gyamfi-Bannerman C. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals [published online April 9, 2020] https://doi.org/10.1016/j.ajogmf.2020.100118 Am J Obstet Gynecol MFM. [DOI] [PMC free article] [PubMed]
- 26.Choi G.B., Yim Y.S., Wong H. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351(6276):933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei J., Vermillion M.S., Jia B. IL-1 receptor antagonist therapy mitigates placental dysfunction and perinatal injury following Zika virus infection. JCI Insight. 2019;4(7):e122678. doi: 10.1172/jci.insight.122678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mednick S.A., Machon R.A., Huttunen M.O., Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45(2):189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 29.Siston A.M., Rasmussen S.A., Honein M.A. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303(15):1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang X., Gao X., Zheng H. Specific immunoglobulin G antibody detected in umbilical blood and amniotic fluid from a pregnant woman infected by the coronavirus associated with severe acute respiratory syndrome. Clin Diagn Lab Immunol. 2004;11(6):1182–1184. doi: 10.1128/CDLI.11.6.1182-1184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegewald M.J., Crapo R.O. Respiratory physiology in pregnancy. Clin Chest Med. 2011;32(1):1–13. doi: 10.1016/j.ccm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Society for Maternal-Fetal Medicine Management considerations for pregnant patients with COVID-19 developed with guidance from Torre Halscott, MD, MS and Jason Vaught, MD. https://s3.amazonaws.com/cdn.smfm.org/media/2334/SMFM_COVID_Management_of_COVID_pos_preg_patients_4-29-20_final.pdf
- 33.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes [published online March 17, 2020] https://doi.org/10.5858/arpa.2020-0901-SA Arch Pathol Lab Med. [DOI] [PubMed]
- 34.Juusela A., Nazir M., Gimovsky M. Two cases of coronavirus 2019–related cardiomyopathy in pregnancy [published online April 3, 2020] https://doi.org/10.1016/j.ajogmf.2020.100113 Am J Obstet Gynecol MFM. [DOI] [PMC free article] [PubMed]
- 35.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Mascio D., Khalil A., Saccone G. Outcome of coronavirus spectrum infections (SARS, MERS, COVID 1 -19) during pregnancy: a systematic review and meta-analysis [published online March 25, 2020] https://doi.org/10.1016/j.ajogmf.2020.100107 Am J Obstet Gynecol MFM. [DOI] [PMC free article] [PubMed]
- 38.Zhu H., Wang L., Fang C. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery [published online March 25, 2020] https://doi.org/10.1093/cid/ciaa200 Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 40.Chen S., Huang B., Luo D.J. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases [in Chinese] Zhonghua Bing Li Xue Za Zhi. 2020;49(5):418–423. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020] https://doi.org/10.1016/j.jinf.2020.02.028 J Infect. [DOI] [PMC free article] [PubMed]
- 42.Li Y., Zhao R., Zheng S. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020;26(6):1335–1336. doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L., Jiang Y., Wei M. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese] Zhonghua Fu Chan Ke Za Zhi. 2020;55(3):166–171. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 45.Liu D., Li L., Wu X. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis [published online March 18, 2020] https://doi.org/10.2214/AJR.20.23072 AJR Am J Roentgenol. [DOI] [PubMed]
- 46.Wen R., Sun Y., Xing Q.-S. A patient with SARS-CoV-2 infection during pregnancy in Qingdao, China [published online March 10, 2020] https://doi.org/10.1016/j.jmii.2020.03.004 J Microbiol Immunol Infect. [DOI] [PMC free article] [PubMed]
- 47.Li N., Han L., Peng M. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study [published online March 30, 2020] https://doi.org/10.1093/cid/ciaa352 Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 48.Yu N., Li W., Kang Q. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20(5):559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia H., Zhao S., Wu Z., Luo H., Zhou C., Chen X. Emergency Caesarean delivery in a patient with confirmed COVID-19 under spinal anaesthesia. Br J Anaesth. 2020;124(5):e216–e218. doi: 10.1016/j.bja.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen R., Zhang Y., Huang L., Cheng B-h, Xia Z-y, Meng Q-t. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patients. Can J Anesth. 2020;67(6):655–663. doi: 10.1007/s12630-020-01630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong L., Tian J., He S. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zambrano L.I., Fuentes-Barahona I.C., Bejarano-Torres D.A. A pregnant woman with COVID-19 in Central America [published online March 25, 2020] https://doi.org/10.1016/j.tmaid.2020.101639 Travel Med Infect Dis. [DOI] [PMC free article] [PubMed]
- 53.Wu X., Sun R., Chen J., Xie Y., Zhang S., Wang X. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia [published April 8, 2020] https://doi.org/10.1002/ijgo.13165 Int J Gynecol Obst. [DOI] [PMC free article] [PubMed]
- 54.Xiong X., Wei H., Zhang Z. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID-19 [published online April 10, 2020] https://doi.org/10.1002/jmv.25857 J Med Virol. [DOI] [PMC free article] [PubMed]
- 55.Peng Z., Wang J., Mo Y. Unlikely SARS-CoV-2 vertical transmission from mother to child: a case report. J Infect Public Health. 2020;13(5):818–820. doi: 10.1016/j.jiph.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lowe B., Bopp B. COVID-19 vaginal delivery: a case report [published online April 15, 2020] https://doi.org/10.1111/ajo.13173 Aust N Z J Obstet Gynaecol. [DOI] [PMC free article] [PubMed]
- 57.Vlachodimitropoulou Koumoutsea E., Vivanti A.J., Shehata N. COVID19 and acute coagulopathy in pregnancy [published online April 17, 2020] https://doi.org/10.1111/jth.14856 J Thromb Haemost. [DOI] [PMC free article] [PubMed]
- 58.Zamaniyan M., Ebadi A., Aghajanpoor Mir S., Rahmani Z., Haghshenas M., Azizi S. Preterm delivery in pregnant woman with critical COVID-19 pneumonia and vertical transmission [published online April 17, 2020] https://doi.org/10.1002/pd.5713 Prenat Diagn. [DOI] [PMC free article] [PubMed]
- 59.Kelly J.C., Dombrowksi M., O’Neil-Callahan M., Kernberg A.S., Frolova A.I., Stout M.J. false-negative COVID-19 testing: considerations in obstetrical care [published online April 28, 2020] https://doi.org/10.1016/j.ajogmf.2020.100130 Am J Obstet Gynecol MFM. [DOI] [PMC free article] [PubMed]
- 60.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies [published online April 7, 2020] https://doi.org/10.1111/aogs.13867 Acta Obstet Gynecol Scand. [DOI] [PMC free article] [PubMed]
- 61.Royal College of Obstetricians and Gynaecologists Coronavirus (COVID-19) infection in pregnancy: information for healthcare professionals. https://www.rcog.org.uk/coronavirus-pregnancy
- 62.Boelig R.C., Saccone G., Bellussi F., Berghella V. MFM Guidance for COVID-19 [published online March 19, 2020] https://doi.org/10.1016/j.ajogmf.2020.100106 Am J Obstet Gynecol MFM. [DOI] [PMC free article] [PubMed]
- 63.Pierce-Williams R.A.M., Burd J., Felder L. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study [published online May 8, 2020] https://doi.org/10.1016/j.ajogmf.2020.100134 Am J Obstet Gynecol MFM. [DOI] [PMC free article] [PubMed]
- 64.Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections [published online April 7, 2020] Viruses. 2020;12(2):194. doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Penfield C.A., Brubaker S.G., Limaye M.A. Detection of SARS-COV-2 in placental and fetal membrane samples [published online May 8, 2020] https://doi.org/10.1016/j.ajogmf.2020.100133 Am J Obstet Gynecol MFM. [DOI] [PMC free article] [PubMed]
- 66.Shah P.S., Diambomba Y., Acharya G., Morris S.K., Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99(5):565–568. doi: 10.1111/aogs.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boelig R.C., Manuck T., Oliver E.A. Labor and delivery guidance for COVID-19. Am J Obstet Gynecol MFM. 2020:100110. doi: 10.1016/j.ajogmf.2020.100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.American College of Obstetricians and Gynecologists . 2020. Novel coronavirus COVID-19, a practice advisory. [Google Scholar]
- 69.American College of Obstetricians and Gynecologists COVID-19 FAQs for obstetrician-gynecologists, obstetrics. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics
- 70.Poon L.C., Yang H., Lee J.C.S. ISUOG interim guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet Gynecol. 2020;55(5):700–708. doi: 10.1002/uog.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Centers for Disease Control and Prevention . 2020. Information for healthcare providers: COVID-19 and pregnant women. [Google Scholar]
- 72.Centers for Disease Control and Prevention . 2019. Pregnancy & breastfeeding: information about coronavirus disease; p. 2020. [Google Scholar]
- 73.World Health Organization Q&A on COVID-19, pregnancy, childbirth and breastfeeding. https://preventionconversation.org/2020/03/21/world-health-organization-qa-on-covid-19-pregnancy-childbirth-and-breastfeeding Published March 21, 2020. Dupe 49.
- 74.Deprest J., Van Ranst M. , Lannoo L, et al. SARS-CoV2 (COVID-19) infection: is fetal surgery in times of national disasters reasonable [published online April 11, 2020]? https://doi.org/10.1002/pd.5702 Prenat Diagn. [DOI] [PMC free article] [PubMed]
- 75.Karimi-Zarchi M., Neamatzadeh H., Dastgheib S.A. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review [published online April 2, 2020] https://doi.org/10.1080/15513815.2020.1747120 Fetal Pediatr Pathol. [DOI] [PMC free article] [PubMed]
- 76.Abu-Rustum R.S., Akolekar R., Sotiriadis A. ISUOG consensus statement on organization of routine and specialist obstetric ultrasound services in the context of COVID-19 [published online March 31, 2020] https://doi.org/10.1002/uog.22029 Ultrasound Obstet Gynecol. [DOI] [PubMed]
- 77.Bahtiyar M.O., Baschat A., Deprest J. Fetal interventions in the setting of COVID-19 pandemic: statement from the North American Fetal Therapy Network (NAFTNet) https://doi.org/10.1016/j.ajog.2020.04.025 Am J Obstet Gynecol. [DOI] [PMC free article] [PubMed]
- 78.Ruano R., Rodo C., Peiro J.L. Fetoscopic laser ablation of placental anastomoses in twin-twin transfusion syndrome using 'Solomon technique. Ultrasound Obstet Gynecol. 2013;42(4):434–439. doi: 10.1002/uog.12492. [DOI] [PubMed] [Google Scholar]
- 79.Ruano R., Daniels D.J., Ahn E.S. In utero restoration of hindbrain herniation in fetal myelomeningocele as part of prenatal regenerative therapy program at Mayo Clinic. Mayo Clinic Proc. 2020;95(4):738–746. doi: 10.1016/j.mayocp.2019.10.039. [DOI] [PubMed] [Google Scholar]
- 80.Lindenburg I.T., van Kamp I.L., Oepkes D. Intrauterine blood transfusion: current indications and associated risks. Fetal Diagn Ther. 2014;36(4):263–271. doi: 10.1159/000362812. [DOI] [PubMed] [Google Scholar]
- 81.Ruano R., Sananes N., Wilson C. Fetal lower urinary tract obstruction: proposal for standardized multidisciplinary prenatal management based on disease severity. Ultrasound Obstet Gynecol. 2016;48(4):476–482. doi: 10.1002/uog.15844. [DOI] [PubMed] [Google Scholar]
- 82.Ruano R., Yoshisaki C.T., da Silva M.M. A randomized controlled trial of fetal endoscopic tracheal occlusion versus postnatal management of severe isolated congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2012;39(1):20–27. doi: 10.1002/uog.10142. [DOI] [PubMed] [Google Scholar]
- 83.Nassr A.A., Ness A., Hosseinzadeh P. Outcome and treatment of antenatally diagnosed nonimmune hydrops fetalis. Fetal Diagn Ther. 2018;43:123–128. doi: 10.1159/000475990. [DOI] [PubMed] [Google Scholar]
- 84.Said S.M., Qureshi M.Y., Taggart N.W. Innovative 2-step management strategy utilizing EXIT procedure for a fetus with hypoplastic left heart syndrome and intact atrial septum. Mayo Clinic Proc. 2019;94(2):356–361. doi: 10.1016/j.mayocp.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 85.NIH COVID-19 treatment guidelines. 2020. https://www.covid19treatmentguidelines.nih.gov
- 86.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vincent M.J., Bergeron E., Benjannet S. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen J., Liu D., Liu Let al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ (Med Sci) 2020;49(2):215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gautret P., Lagier J.-C., Parola P. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study [published online April 11, 2020] https://doi.org/10.1016/j.tmaid.2020.101663 Travel Med Infect Dis. [DOI] [PMC free article] [PubMed]
- 91.Li Y., Xie Z., Lin W. An exploratory randomized controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI) [published online April 15, 2020] https://doi.org/10.1101/2020.03.19.20038984 medRxiv.
- 92.Food and Drug Administration Plaquenil® hydroxychloroquine sulfate, USP. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/009768s041lbl.pdf
- 93.Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. 2010;36(3):646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 94.Food and Drug Administration Zithromax® (azithromycin tablets) and (azithromycin for oral suspension) https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf
- 95.Ul Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.-L. Structural basis of SARS-CoV-2 3CL(pro) and anti-COVID-19 drug discovery from medicinal plants [published online March 26, 2020] https://doi.org/10.1016/j.jpha.2020.03.009 J Pharm Anal. [DOI] [PMC free article] [PubMed]
- 96.Liu X., Wang X.-J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J Genet Genom. 2020;47(2):119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.NIH. Recommendations for the use of antiretroviral drugs in pregnant women with HIV infection and interventions to reduce perinatal HIV transmission in the United States. https://aidsinfo.nih.gov/guidelines/html/3/perinatal/449
- 99.Warren T.K., Jordan R., Lo M.K. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marchione M. A 1st: US study finds Gilead drug works against coronavirus. https://abcnews.go.com/Health/wireStory/company-drug-proved-effective-virus-us-study-70405458
- 101.Anakinra. Drugs.com website, https://www.drugs.com/ppa/anakinra.html#moreResources. Accessed July 8, 2020.
- 102.Gritti G., Raimondi F., Ripamonti D. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support [published online May 22, 2020] https://doi.org/10.1101/2020.04.01.20048561 medRxiv.
- 103.National Institutes of Health. Table 3b. Host modifiers and immune-based therapy under evaluation for treatment of COVID-19. https://covid19treatmentguidelines.nih.gov/immune-based-therapy/table-3b-characteristics-of-immune-based-therapy
- 104.Barnes P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol. 2006;148(3):245–254. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [published online March 13, 2020] https://doi.org/10.1001/jamainternmed.2020.0994 JAMA Intern Med. [DOI] [PMC free article] [PubMed]
- 106.Wang Y., Jiang W., He Q. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China [published online March 12, 2020] medRxiv. [DOI]
- 107.American College of Obstetricians and Gynecologistsy Practice advisory: novel coronavirus 2019 (COVID-19) https://www.acog.org/en/Clinical/Clinical%20Guidance/Practice%20Advisory/Articles/2020/03/Novel%20Coronavirus%202019
- 108.Chan J.F., Kok K.H., Zhu Z. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gralinski L.E., Menachery V.D. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12(2):135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Food and Drug Aministration Angiotensin-converting enzyme inhibitors and pregnancy: FDA public health advisory. http://www.ncbop.org/PDF/FDAAdvisoryACEIuseDuringPregnancyJune2006.pdf
- 111.Barreras A., Gurk-Turner C. Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent) 2003;16(1):123–126. doi: 10.1080/08998280.2003.11927893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duan K., Liu B., Li C. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thachil J., Tang N., Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.World Health Organization Draft landscape of COVID-19 candidate vaccines. https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines
- 115.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52(4):583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Food and Drug Administration Postapproval pregnancy safety studies: guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/postapproval-pregnancy-safety-studies-guidance-industry Published May 2019.
- 117.Gomes M.F., de la Fuente-Núñez V., Saxena A., Kuesel A.C. Protected to death: systematic exclusion of pregnant women from Ebola virus disease trials. Reprod Health. 2017;14(suppl 3):172. doi: 10.1186/s12978-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.ACOG Committee Opinion No 649: racial and ethnic disparities in obstetrics and gynecology. Obstet Gynecol. 2015;126(6):e130–e134. doi: 10.1097/AOG.0000000000001213. [DOI] [PubMed] [Google Scholar]
- 119.Narang K., Ibirogba E.R., Elrefaei A., Trad A.T.A., Theiler R., Nomura R. SARS-CoV-2 in pregnancy: a comprehensive summary of current guidelines. J Clin Med. 2020;9(5):1521. doi: 10.3390/jcm9051521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.