Patients with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) pneumonia with SARS-CoV-2 present with coagulation disorders and marked susceptibility to thrombosis (1,2). However, the exact prevalence of deep vein thrombosis (DVT) has been poorly investigated, although the risk seems increased in intensive care unit (ICU) patients (3). Therefore, we decided to perform routine duplex ultrasound examination of the lower limb veins systematically in order to administer appropriate anticoagulation in all intubated and mechanically ventilated patients with SARS-CoV-2 pneumonia.
We conducted a prospective observational study in the medical and surgical critical care departments of Lariboisière University Hospital, Paris, France. Consecutive adults receiving invasive mechanical ventilation for SARS-CoV-2 pneumonia were included. Patients with previously diagnosed DVT or pulmonary embolism were excluded. During the hospital stay, prophylactic anticoagulation was administered as daily subcutaneous 4,000 IU enoxaparin and, if the glomerular filtration rate was <15 ml/min, as continuous intravenous infusion of daily 15,000 IU unfractionated heparin. Duplex ultrasonography and plasma D-dimer assessment (STA-Liatest-DDI-Plus, Stago, Asnières sur Seine, France) were performed in all patients during the first week of ICU admission. In patients without DVT on the initial ultrasound, a second ultrasound examination was performed ∼7 days later. Quantitative variables are expressed as median (interquartile range) and categoric variables as percentages. The study was part of the French coronavirus disease 2019 cohort registry and was approved by our institutional ethics committee (IDRCB, 2020-A00256-33; CPP, 11-20 20.02.04.68737). When possible, signed informed consent was obtained from the patients or the next of kin.
From March 13 to April 3, 2020, 56 patients with SARS-CoV-2 pneumonia were included. Most of the patients were male (75%) with hypertension (46%), diabetes (45%), obesity (30%), and ischemic heart disease (20%). They required vasopressors in 32% of the cases. Prophylactic anticoagulation using enoxaparin or unfractionated heparin was administered in 41 patients (73%) and 8 patients (14%), respectively. Therapeutic anticoagulation was used in 7 patients (13%) to treat atrial fibrillation (n = 2) and manage extracorporeal membrane oxygenation (n = 5).
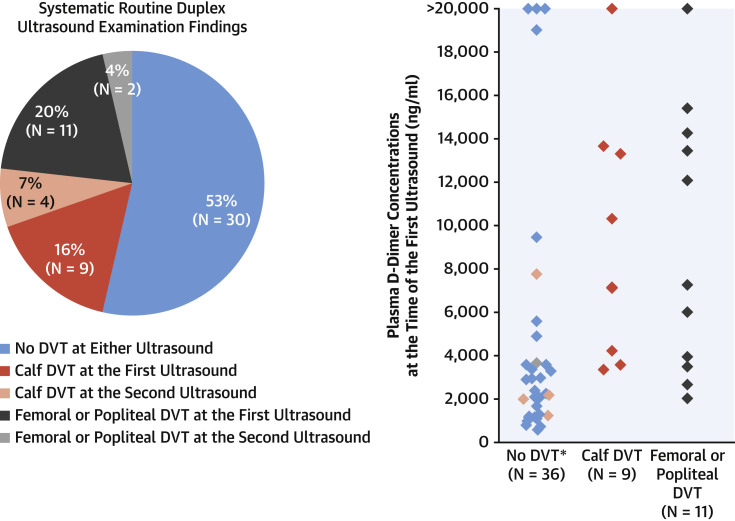
The initial ultrasound was performed 3 days (interquartile range: 2 to 4 days) post-intubation, corresponding to 10 days (interquartile range: 8 to 13 days) after the onset of the first symptoms. Twenty (36%) patients had DVT, among which 11 (20%) cases were proximal (popliteal or femoral) DVT. A second ultrasound examination was performed in 17 patients 8 days (interquartile range: 5 to 9 days) post-intubation, corresponding to 14 days (interquartile range: 11 to 15 days) after the first symptoms. Six patients (35%) acquired DVT; 2 (12%) cases were proximal despite prophylactic anticoagulation in 3 patients and therapeutic anticoagulation in the other 3. Overall, 26 of 56 patients (46%) were diagnosed with DVT, either proximal (n = 13, 23%) or calf (n = 13, 23%) (Figure 1 ). DVT patients had significantly higher plasma D-dimer compared with non-DVT patients (7,210 ng/ml [interquartile range: 3,770 to 13,550 ng/ml] vs. 2,225 ng/ml [interquartile range: 1,195 to 3,630 ng/ml], p = 0.0002) with no significant difference in plasma fibrinogen (7.4 g/l [interquartile range: 5.8 to 8.9 g/l] vs. 7.6 g/l [interquartile range: 5.5 to 8.5 g/l], p = 0.7).
Figure 1.
DVT and Plasma D-dimer in 56 Mechanically Ventilated SARS-CoV-2 Patients
(Left) Study groups as percentages of the total (numbers) according to the deep vein thrombosis (DVT) presence and site at the initial and second ultrasound. Color significance is detailed in the figure. (Right)Diamonds represent individual concentrations of D-dimer at the initial ultrasound according to the DVT presence and site, with the same color significance as in the left panel. In the “No DVT” group, the salmondiamonds and the light bluediamonds represent D-dimer in patients who developed femoral and calf DVT, respectively, at the second ultrasound. ∗Missing D-dimer in 1 patient with popliteal DVT at the second ultrasound. SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2.
Studies have reported a highly variable prevalence of DVT (between 2.0% [2] and 14.8% [3]) in ICU patients, most likely because of the absence of consistent screening. To the best of our knowledge, this is the first study performing systematic ultrasound examination for DVT diagnosis, thus providing data free of selection biases. Our data showed a remarkably high DVT prevalence (46%) and revealed the rapid time course of thrombus formation despite prophylactic anticoagulation. Importantly, 50% of the DVTs were popliteal or femoral, which are most often associated with thromboembolic events, consistent with the unexpectedly high number of pulmonary embolisms (21%) reported in SARS-CoV-2 pneumonia patients admitted to the ICU and occurring within a median time from ICU admission of 6 days (interquartile range: 1 to 18 days) (4).
Our data suggest that close monitoring of DVT occurrence is necessary in mechanically ventilated SARS-CoV-2 patients, and because ultrasound may not always be available, especially in epidemic settings, larger studies may investigate the diagnostic performance of D-dimers for DVT diagnosis in these patients. Moreover, the intensity of anticoagulation may need to be reconsidered based on future investigations to ensure more effective prevention (1).
In conclusion, we demonstrated a very high DVT prevalence including a high proportion of potentially life-threatening proximal DVT in mechanically ventilated SARS-CoV-2 patients despite standard prophylactic anticoagulant treatment, suggesting the need for close DVT monitoring and assessment of the risks/benefits of more intense anticoagulation regimens in this population.
Footnotes
Please note: The authors have reported that they have no relationships relevant to the contents of this paper to disclose. The authors thank Marie Neuwirth, Maxime Delrue, Caroline Grant, and Edwige Matera for helping with data gathering and Siemens Healthineers France for kindly lending Lariboisière hospital ultrasound machines for the duration of the pandemic. The authors would also like to thank Mrs. Alison Good (Scotland, UK) for her helpful review of the manuscript.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
References
- 1.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tavazzi G., Civardi L., Caneva L., Mongodi S., Mojoli F. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020;46:1121–1123. doi: 10.1007/s00134-020-06040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poissy J., Goutay J., Caplan M. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020 Apr 24 doi: 10.1161/CIRCULATIONAHA.120.047430. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]