Abstract
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), which presents an unprecedented challenge to medical providers worldwide. Although most SARS-CoV-2–infected individuals manifest with a self-limited mild disease that resolves with supportive care in the outpatient setting, patients with moderate to severe COVID-19 will require a multidisciplinary collaborative management approach for optimal care in the hospital setting. Laboratory and radiologic studies provide critical information on disease severity, management options, and overall prognosis. Medical management is mostly supportive with antipyretics, hydration, oxygen supplementation, and other measures as dictated by clinical need. Among its medical complications is a characteristic proinflammatory cytokine storm often associated with end-organ dysfunction, including respiratory failure, liver and renal insufficiency, cardiac injury, and coagulopathy. Specific recommendations for the management of these medical complications are discussed. Despite the issuance of emergency use authorization for remdesivir, there are still no proven effective antiviral and immunomodulatory therapies, and their use in COVID-19 management should be guided by clinical trial protocols or treatment registries. The medical care of patients with COVID-19 extends beyond their hospitalization. Postdischarge follow-up and monitoring should be performed, preferably using telemedicine, until the patients have fully recovered from their illness and are released from home quarantine protocols.
Abbreviations and Acronyms: AGP, aerosol-generating procedure; AKI, acute kidney injury; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; CBC, complete blood cell; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; ECG, electrocardiogram; ESR, erythrocyte sedimentation rate; FDA, Food and Drug Administration; GGO, ground-glass opacity; HRCT, high-resolution computed tomography; ICU, intensive care unit; IL, interleukin; LDH, lactate dehydrogenase; LFT, liver function test; PCR, polymerase chain reaction; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Article Highlights.
-
•
Supportive care is the standard approach to the management of coronavirus disease 2019 (COVID-19), with particular attention to respiratory support and early detection of potential complications, such as cytokine storm and organ dysfunction.
-
•
Therapeutic management of COVID-19 using antivirals and immunomodulators remains investigational and should be implemented using clinical trial protocols.
-
•
A multidisciplinary collaborative approach to the management of COVID-19 is an essential component of providing optimal care to patients.
Coronavirus disease 2019 (COVID-19) is a new acute respiratory illness caused by a novel zoonotic pathogen, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1, 2, 3, 4, 5 Since the first cases were reported in December 2019 from Wuhan, China,1 the infection has rapidly spread worldwide.6 Declared by the World Health Organization as a global pandemic on March 11, 2020, COVID-19 has altered societal norms and human behavior in very profound ways as communities attempt to “flatten the curve.”7 , 8 This public health strategy is an effort to minimize the effect on the health care system of a “surge” of patients who require hospitalization.7
Supportive care is the standard approach to the management of COVID-19. Because there is no proven effective antiviral treatment or vaccine, medical providers are scrambling to learn about this disease to provide the best management. Herein, a collaborative team of physicians review the rapidly evolving literature (published on PubMed, Medline, and various journal and society websites) on COVID-19 in their respective specialties. In the process, we provide a concise practical review that summarizes the clinical management of COVID-19 in the hospital setting, including the evaluation of patients, diagnostic testing, treatment strategies, and infection prevention measures.
SARS-CoV-2, the Virus
SARS-CoV-2 belongs to a family of large enveloped RNA viruses with a “crown” (corona)-like appearance.9 , 10 Until 2019 there were six coronaviruses that cause human disease. Four of these, human coronaviruses NL63, 229E, OC43, and HKU1, cause seasonal outbreaks of respiratory infections.11 The other two, SARS-CoV and Middle East respiratory virus syndrome coronavirus, caused pandemics in 2002 and 2012, respectively.12 The seventh member, SARS-CoV-2, was sequenced from a patient with unusual pneumonia in January 2020.1 , 13 Phylogenetic analysis shows 96% homology to a bat coronalike virus and 79% similarity to SARS-CoV.1 , 13 Its spike protein binds to the angiotensin-converting enzyme 2 receptor that is expressed on respiratory epithelium, type II pneumocytes, and epithelial cells of various organs, including the gastrointestinal tract and kidneys.14, 15, 16, 17
The reproduction number (or R0) of SARS-CoV-2 is 2 to 4, which means that every infected person can potentially transmit the virus to 2 to 4 susceptible individuals.18 , 19 Although initial cases were linked to a live animal and seafood market in Wuhan, person-to-person transmission in the community was quickly documented.20 The virus is transmitted between humans by respiratory droplets, aerosols, and fomites.21 , 22 The isolation of SARS-CoV-2 in stool suggests that it may be transmitted by fecal-oral route, although this has not yet been proven.23 , 24 Coughing, sneezing, singing, talking, and simply exhaling project infectious respiratory droplets into the environment,25 , 26 where the virus can survive for hours to days.21 , 27
COVID-19, the Disease
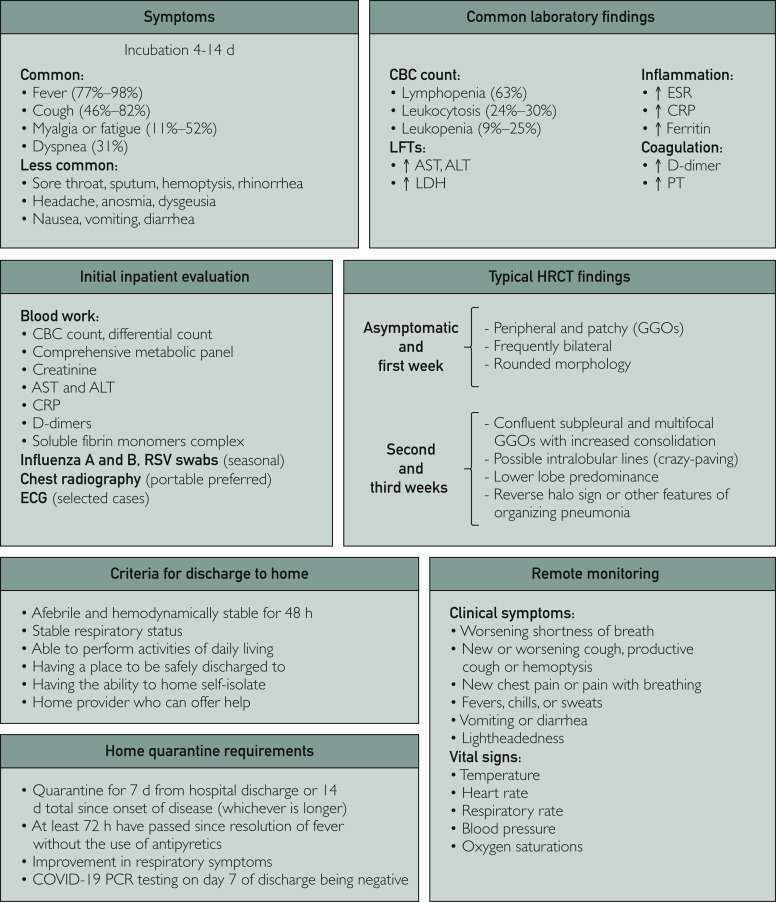
A large number of SARS-CoV-2–infected persons have no symptoms.28 Unaware of their infected status, asymptomatic persons can transmit the virus in the community, posing significant public health challenges.29, 30, 31, 32, 33 Among those who develop symptoms, the illness starts 4 to 14 days after exposure.5 Fever, dry cough, shortness of breath, and fatigue are common (Figure 1 ).2 , 3 , 5 Sore throat, rhinorrhea, nausea, vomiting, diarrhea,23 dysosmia, and dysgeusia have been reported.17 , 34 , 35
Figure 1.
Overview of the hospital management of patients with coronavirus disease 2019 (COVID-19). ALT = alanine aminotransferase; AST = aspartate aminotransferase; CBC = complete blood cell; CRP = C-reactive protein; ECG = electrocardiography; ESR = erythrocyte sedimentation rate; GGO = ground-glass opacity; HRCT = high-resolution computed tomography; LDH = lactate dehydrogenase; LFT = liver function test; PCR = polymerase chain reaction; RSV = respiratory syncytial virus.
Although respiratory symptoms are often the initial manifestations, gastrointestinal symptoms may predominate in up to 10% of patients.36 Eighty percent of symptomatic cases are mild and are managed with supportive care, without specific antiviral therapy or oxygen supplementation, in the outpatient setting.37
Approximately 20% of patients develop moderate to severe pneumonia that is best managed in the hospital.38 COVID-19 pneumonia is classified as moderate if a patient maintains oxygen saturation greater than 93% on room air and as severe if the respiratory rate is greater than 30 breaths/min, oxygen saturation is 93% or less on room air, Pao 2/Fio 2 is less than 300, or lung infiltrates are greater than 50%. The onset of respiratory failure may occur precipitously, often during the second week of illness. In 5% of patients, COVID-19 progresses to critical illness characterized by hyperinflammatory syndrome manifested as hypotension, shock, respiratory failure, and multiple organ failure.39 , 40 Risk factors for disease progression are older age, diabetes mellitus, chronic lung diseases, hypertension and cardiovascular diseases, and chronic liver and kidney diseases.22 , 41, 42, 43 An immunocompromised status and a high body mass index (>40 [calculated as weight in kilograms divided by height in meters squared]) have also been implicated.44, 45, 46
Initial Evaluation
COVID-19 should be suspected in any patient with fever, respiratory difficulties, or other symptoms (Figure 1). Most patients with suspected COVID-19 can be initially evaluated using phone or electronic visits to reduce the potential for viral transmission in the hospital or clinic (see the Telemedicine subsection).47 , 48 Once suspected, SARS-CoV-2 polymerase chain reaction (PCR) testing may be performed on nasopharyngeal swabs.49 , 50 Infection control measures, including handwashing, masking, and self-quarantining, should be implemented.51
Initial evaluation should define disease severity.36 , 52 Laboratory and radiographic evaluations are generally not required for patients with mild COVID-19.37 In patients who require hospitalization for moderate to severe and critical pneumonia,5 the initial laboratory evaluation includes a complete blood cell count with differential count, inflammatory markers (eg, C-reactive protein), and a metabolic profile that incudes assays of renal and liver status (Figure 1). D-dimer, fibrinogen, and soluble fibrin monomer complex levels and prothrombin time should be assessed to evaluate coagulopathy.52, 53, 54, 55 These initial tests are needed to stratify the risk of disease progression and guide supportive measures to prevent complications. Procalcitonin, interleukin (IL) 6, ferritin, B-type natriuretic peptide, troponin, and glucose-6-phosphase dehydrogenase levels are potentially helpful, when clinically indicated, to assess inflammatory status, consider potential complications, and guide treatment selection.53
The most common laboratory abnormalities are lymphopenia (63%-83%), leukopenia (9%-34%), leukocytosis (6%-30%), and thrombocytopenia (36%).2 , 3 , 5 , 53 A high neutrophil to lymphocyte ratio and eosinopenia are indicators of severe disease. Erythrocyte sedimentation rate, C-reactive protein, D-dimer, ferritin, and IL-6 levels are typically elevated,2 , 3 , 5 , 53 and the procalcitonin level is normal.56
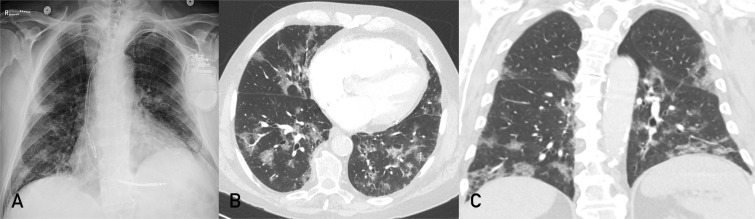
When respiratory co-infection is suspected, patients may be tested for other pathogens, particularly influenza.57 , 58 Portable chest radiography is suggested in patients with at least moderate respiratory symptoms to assess the severity of pneumonia and identify concomitant processes (Figure 2 ). Electrocardiography may also be considered, especially if using drug therapies that cause QTc prolongation or if there is concern for acute coronary syndrome or myocarditis.
Figure 2.
An elderly man with a history of congestive heart failure, atrial fibrillation, and hypertension presented to the emergency department for progressive dyspnea over 3 weeks. A, Portable chest radiography shows patchy peripheral opacities bilaterally. Chest computed tomographic angiogram was negative for pulmonary embolism or signs of heart failure but had typical findings commonly reported for coronavirus disease 2019. B and C, Axial and coronal computed tomographic images in lung windows show patchy bilateral ground-glass opacities with a peripheral and lower lung distribution.
SARS-CoV-2 Diagnostics
The diagnosis of COVID-19 requires laboratory confirmation of the presence of SARS-CoV-2 by PCR testing of clinical specimens.49 , 59 The Centers for Disease Control and Prevention (CDC) recommends that health care providers work with local health departments to refer patients for testing using an approved platform by the US Food and Drug Administration (FDA). Given the current limitations in testing resources and variable access, the CDC and the Infectious Diseases Society of America have prioritized patients who should be tested based on symptoms, risk factors, and demographic factors.60
A variety of commercial and laboratory-developed molecular assays (eg, PCR) have been developed to detect SARS-CoV-2, with variable performance characteristics.49 , 61 The sensitivity of the different SARS-CoV-2 reverse transcriptase PCR tests is variable.50 , 62 Although a positive SARS-CoV-2 PCR test result is diagnostic, a negative result does not exclude COVID-19. Factors that influence PCR sensitivity include specimen quality, viral load, and disease stage.49 , 62 Early during infection (eg, first 3-5 days), viral concentrations in the oro-nasopharynx are high.61 As lower respiratory tract disease develops later in the course, tracheal secretions, sputum, or bronchoalveolar lavage fluid samples have higher sensitivity.61 Caution should be applied while collecting samples by bronchoalveolar lavage or induced sputum because both are aerosolize-generating procedures (AGPs) that need to be performed using appropriate personal protective equipment.61 Serologic testing to detect antibodies against SARS-CoV-2 may be used to identify persons with recent or previous infection.59 , 63 , 64 However, serologic testing is not recommended to diagnose acute infection. Detection of SARS-CoV-2 immunoglobulin G may be used to determine community seroprevalence and for screening convalescent plasma donors. Whether antibody provides protective immunity against SARS-CoV-2 is unknown. Several point-of-care rapid serologic and molecular diagnostic tests are in development to facilitate for more time-expedient decision making regarding isolation precautions and management.59
Radiology
Chest radiography has poor sensitivity for detecting opacities early in COVID-19 and has limited utility as a screening test. Given the wide spectrum of presenting clinical symptoms and severity, including patients with nonpulmonary manifestations, radiography should not be ordered routinely in asymptomatic patients and those with mild disease. It is useful to guide management of moderately ill patients, as an additional indicator of disease severity, or for identification of alternate or additional conditions that require treatment, such as pneumothorax or congestive heart failure (Figure 2).
There is rapidly evolving literature on the role of chest computed tomography (CT).65 Early studies suggested CT as a standard for the diagnosis of COVID-19,66 but recent literature suggested that it should not be performed routinely. More than half of symptomatic patients have a negative chest CT within the first 2 days of infection.22 , 67, 68, 69 Even in a patient population with very high pretest probability (85% prevalence), chest CT has a positive predictive value of 92% but only a 42% negative predictive value.70 Thus, a negative chest CT cannot be used to exclude COVID-19, particularly within the first few days of infection.
The Radiological Society of North America published an expert consensus statement on reporting of CT findings of COVID-19.71 The most typical CT features are ground-glass opacities, with a peripheral, bilateral, and lower lung predominant pattern (Figure 2). Ground-glass opacities are commonly described to have rounded or nodular morphologic features. Progression of COVID-19 is characterized by increasing extent and density of opacities, consolidation and “crazy-paving” appearance, and organization with fibrosis.
Many CT findings are not commonly seen with COVID-19 and indicate alternate or additional disease processes, including upper lobe or perihilar distribution, “tree-in-bud” centrilobular nodularity, pleural effusions, cavitation, discrete solid nodules, and lymphadenopathy.
Critical Illness and Medical Complications
Hyperinflammatory Syndrome
In 5% to 10% of patients, COVID-19 progresses to a severe and critical hyperinflammatory stage that is associated with end-organ damage, most commonly with lung injury and acute respiratory distress syndrome (ARDS). Patients are usually admitted to the intensive care unit (ICU), where they receive respiratory and hemodynamic support.39 , 72 Severe lymphopenia is a common risk factor for severe COVID-19,39 with reduction in helper, suppressor, and regulatory T cells.53 , 73 , 74
Studies have reported elevated IL-6, C-reactive protein, and ferritin levels in patients with severe COVID-19, suggesting a proinflammatory process.28 , 52 , 53 Elevated levels of IL-2R, IL-7, IL-10, tumor necrosis factor α, granulocyte colony-stimulating factor, and macrophage inflammatory protein 1-α are also observed.75 It is hypothesized that this proinflammatory state triggers the critical illness in COVID-19, but whether these cytokines are the cause or the consequence of lung injury is not clear. Mortality in patients with severe and critical COVID-19 is 26%72 but is markedly higher in older individuals with significant comorbidity.39 Clinical trials of immunomodulatory drugs aimed at suppressing the cytokine release syndrome are underway (discussed later herein).
Respiratory Failure
Respiratory failure is the most common mechanism of death in COVID-19.2 , 3 , 5 , 28 Its onset is typically during the second week of illness, with a median time of ICU admission of 10 to 12 days.75 A subset of patients may progress rapidly to ARDS, with a median of 2.5 days from onset of dyspnea. Hypoxemic respiratory failure occurs in 10% to 20% of patients, and ARDS develops in 3% to 5%.2 , 3 , 5 , 28 Risk factors for ARDS are age 65 years or older, cardiovascular disease, diabetes mellitus, and hypertension.75 Patients with ARDS have a higher incidence of liver enzyme elevation, renal dysfunction, coagulopathy, and lymphopenia.75
Early identification of progressive respiratory failure is needed to escalate the level of care and apply important interventions such as high-flow oxygen, mechanical ventilation, prone position, and other supportive measurements. Classic ARDS is characterized by impaired gas exchange, decreased lung compliance, and increased pulmonary arterial pressure.76 In contrast, severe hypoxemia with well-preserved lung mechanics and high lung compliance have been described in COVID-19.77 As previously described in ARDS,78 microvascular thrombi in the lungs secondary to pulmonary microvascular coagulopathy is seen in COVID-19.79
Best practice for mechanical ventilation in ARDS recommends low tidal volumes (4-8 mL/kg predicted body weight), low end-inspiratory pressures (plateau pressure, <30 cmH2O), and low driving pressure (tidal volume over respiratory system compliance, <15 cmH2O) to prevent ventilator-induced lung injury in the setting of reduced lung compliance.80 , 81 Because some patients with COVID-19 hypoxemic respiratory failure have normal respiratory system compliance, higher tidal volumes may be acceptable in this subset of patients.41 For moderate or severe ARDS, neuromuscular blockade may be required to prevent ventilator asynchrony, decrease metabolic demand, and facilitate ventilation targets.82 Likewise, prone ventilation may improve ventilation-perfusion mismatch and oxygenation. Other aspects of classic ARDS management, such as fluid-restrictive strategies, need to be individualized because dehydration and intravascular depletion are frequent in COVID-19. It is important that we understand that the current ARDS guidelines are not tailored to all aspects of COVID-19–induced ARDS and they need to be individualized in the context of patient-specific presentation and other comorbidities.82 The use of noninvasive positive pressure ventilation or high-flow nasal cannula oxygen with self-proning in COVID-19 ARDS is controversial but may prevent intubation in some patients.83 The timing of intubation is debated because it must balance the risk of lung injury with the need for deep sedation, neuromuscular blockade, and prolonged mechanical ventilation. The optimal oxygenation goal should be targeted to oxygen saturation of 92% to 96% unless there is evidence of other chronic pulmonary conditions that may result in hypercapnic respiratory failure.82
Cardiovascular Complications
The cardiovascular complications in COVID-19 can be due to cardiac injury secondary to SARS-CoV-2 infection and the increased risk in patients with cardiovascular diseases.84, 85, 86 Significant cardiovascular sequelae include myocardial injury (significant rise in serum troponin level) with acute cardiac injury and fulminant myocarditis, arrhythmias, and circulatory collapse,85 , 87, 88, 89 especially in patients with underlying cardiovascular diseases.85 , 86 , 90 Hypertension is the most common comorbidity.85 , 90, 91, 92 Less common cardiovascular comorbidities include coronary vascular and cerebrovascular disease and chronic heart failure.90 , 92
Clinical manifestations of myocardial injury in COVID-19 range from mild asymptomatic elevations in cardiac biomarkers to severe fulminant myocarditis and shock.28 , 91 , 93 In a study of 138 patients, acute cardiac injury (7.2%), shock (8.7%), and arrhythmia (16.7%) were frequent.91 In 113 patients who died of COVID-19, the incidence of acute cardiac injury, congestive heart failure, and shock were 77%, 49%, and 41%, respectively.94 New or worsening congestive heart failure occurred in 23% of 191 hospitalized patients.95 Acute cardiac injury may manifest as the phenomenon of ST-segment elevation without acute coronary artery occlusion, which may represent myocarditis.88 , 96
The mechanism of myocardial injury is likely multifactorial, including demand ischemia, toxic-metabolic effects, cytokine storm, direct viral cardiomyocyte injury, and inflammation triggering coronary plaque rupture.89 , 97 , 98 Cardiac pericytes have high expression of angiotensin-converting enzyme 22 receptors,99 but there is insufficient data to implicate this pathway. Although the pathogenesis of the cardiovascular sequelae is unclear, there is evidence that myocardial injury associated with acute respiratory illnesses is independently associated with mortality.100
It is important to differentiate acute coronary syndrome from other causes of troponin elevation in the setting of acute illness.87 Cardiology consultation is recommended when there are findings of myocardial ischemia (ie, angina, ST-segment changes on electrocardiogram, and/or regional wall motion abnormalities on echocardiogram) in the setting of myocardial injury, new or worsening heart failure, and arrhythmias. Treatment of the underlying disease and supportive care are recommended for myocardial injury in the absence of acute coronary syndrome.101 In patients with ST-segment elevation, a multidisciplinary evaluation should guide the decision for coronary angiography,102 taking into account the severity of illness and the potential benefit from coronary revascularization.103 No specific medical therapy is recommended for acute myocarditis, and routine endomyocardial biopsy is discouraged because it is unlikely to change management, and there are associated risks.104
Hepatic Dysfunction
Mild to moderate elevations in serum aminotransferase and bilirubin levels are common in hospitalized or critically ill patients with severe COVID-19.58 , 105 Although liver enzyme abnormalities are more common in severe disease, patients seldom develop acute liver failure or obvious intrahepatic cholestasis.52 , 106 Supportive care and avoidance of hepatotoxic drugs in patients with COVID-19 are recommended. Liver damage from virally induced cytotoxic T cells and dysregulation of the innate immune response has been proposed as the mechanism for liver enzyme elevation rather than viral hepatitis.107
Renal Dysfunction
Elevation of serum creatinine levels is frequent in patients with COVID-19 and is likely due to hypovolemia from poor oral intake, fever, and diarrhea.108 Elevated serum creatinine and blood urea nitrogen levels were reported in 15% of patients, and acute kidney injury (AKI) was reported in 1% to 15% of hospitalized patients and in 20% of critically ill patients.39 , 108 Proteinuria and hematuria were common.109 Acute kidney injury occurs 13 to 20 days after hospital admission and is associated with in-hospital mortality.55 , 109 , 110 Adequate assessment and monitoring of fluid status is, therefore, essential in all patients with COVID-19. Of those who develop AKI, approximately 1% to 5% may require renal replacement therapy.94 , 105 Autopsies of 6 patients with COVID-19 found severe acute tubular necrosis.111 Acute tubular necrosis is likely secondary to shock, hypotension, and/or hypovolemia, although direct tubular injury from the virus has been hypothesized.111 , 112 Angiotensin II pathway activation and coagulopathy leading to microvascular thrombosis are also likely contributors to AKI.113 , 114 Renal abnormalities often resolve within 3 weeks after onset of symptoms. Severity of pneumonia was correlated with lower odds of AKI recovery.110
Coagulopathy
COVID-19 has been associated with coagulopathy leading to microvascular thrombosis and consumption of coagulation factors often meeting the criteria for disseminated intravascular coagulation.54 , 115 , 116 Higher D-dimer levels have been observed in patients admitted to the ICU75 , 79 , 91 , 117 and in nonsurvivors.91 , 95 Additional features in some patients include prolonged prothrombin time and thrombocytopenia.118 COVID-19–associated coagulopathy has been associated with disease severity and mortality.93 , 119
The International Society of Thrombosis and Haemostasis recommends that all patients with COVID-19 undergo measurement of D-dimer level, prothrombin time, and platelet count for risk stratification and prognostic considerations.120 Fibrinogen and soluble fibrin monomer complex levels provide additional information on the potential for coagulopathy. Despite abnormal coagulation parameters, bleeding has not typically been described in COVID-19.54 If there is active bleeding, it is reasonable to consider transfusion of platelets (for counts <50,000×103/μL [to convert to ×109/L, multiply by 1]), fresh frozen plasma (if the international normalized ratio is >1.5-1.8), or cryoprecipitate (if the fibrinogen level is <51-68 mg/dL [to convert to g/L, multiply by 0.01]).
Thrombotic complications are common in patients with COVID-19 who are critically ill.121 Some data support the hypothesis that inhibiting thrombin generation with anticoagulation in patients with sepsis-associated coagulopathy may be associated with decreased mortality. One small study reported lower mortality rates among patients with COVID-19 and elevated D-dimer levels who received prophylactic heparin.79 Another study observed lower mortality rates in patients with high sepsis-induced coagulopathy scores or extremely elevated D-dimer levels who received prophylactic-dose low-molecular-weight heparin or unfractionated heparin.122 Prophylactic low-molecular-weight heparin should be considered in all patients requiring hospitalization for COVID-19 provided that platelet counts remain greater than 25,000/μL. However, despite prophylactic anticoagulation, thrombotic rates of up to 31% have been reported in critically ill patients in the ICU (27% venous thromboembolism, 3.7% arterial thrombosis).121 At this time, there is not enough evidence to recommend therapeutic-dose anticoagulation for all patients with severe COVID-19117; however, there are several ongoing studies exploring the role of intermediate-dose or full therapeutic-dose anticoagulation for critically ill patients even in the absence of thrombosis. The clinical benefit of extended-duration prophylaxis for patients who have recovered and are discharged from the hospital is also being debated.
Co-infections
Patients with COVID-19 may develop co-infection with other respiratory pathogens. The rate of co-infection ranges from 4% to 35% of patients depending on the series and the season.58 , 123 In 1 study, 24 of 116 specimens (21%) had SARS-CoV-2 co-infection with rhinovirus, enterovirus, respiratory syncytial virus, and other coronaviruses. In another study, the most commonly reported co-infection was influenza.58 Early detection of coexisting influenza infection is critical for the prompt initiation of appropriate influenza-specific therapy. Currently there are limited data on bacterial or fungal co-infection, but suspicion for this possibility must remain high, especially in patients with COVID-19 on extended mechanical ventilation. Once bacterial superinfection is suspected, empirical antibiotics may be initiated while microbiologic evaluation is undertaken. Suspicion for superimposed co-infection may be suggested by “indeterminate” or “atypical” radiographic features.
Isolation Precautions
Person-to-person spread of SARS-CoV-2 occurs mainly by respiratory droplets27 and direct contact with infected surfaces.27 Patients who are suspected or confirmed of having COVID-19 should, therefore, be managed under appropriate infection prevention and control practices, specifically droplet and contact precautions, as outlined by the CDC (https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html).27 Respiratory droplets typically do not linger in the air and, thus, airborne precautions are generally not needed. However, airborne precautions are necessary when AGPs are performed, including sputum induction, endotracheal intubation and extubation, bronchoscopy, open tracheal suctioning, esophageal procedures, and cardiopulmonary resuscitation. During the performance of AGPs, N95 plus eyewear protection or powered air-purifying respirator and contact precautions are required.
Patients with SARS-CoV-2 infection should remain on droplet and contact precautions for the duration of hospitalization. Discontinuation of isolation precautions requires clinical symptom resolution and demonstration of 2 consecutive negative SARS-CoV-2 PCR test results.
Treatment Considerations
The standard of care for COVID-19 is supportive management, including analgesics, fluids, electrolytes, and oxygen supplementation.124 There is no solid evidence to suggest that nonsteroidal anti-inflammatory drugs worsen COVID-19 symptoms.124 , 125
Antiviral Therapeutics
Pharmaceutical management of COVID-19 takes into account the stage of illness. During the early stage (eg, during the first week), when the pathology is driven mainly by viral replication, an effective antiviral therapy is desired. The goal of management is to suppress viral replication to prevent systemic or end-organ disease. However, there is no drug proven to be clinically effective.124 Thus, participation in controlled clinical trials of experimental and repurposed drugs is recommended.124, 125, 126 Remdesivir, an RNA-dependent RNA polymerase inhibitor, was found to have modest efficacy in a cohort of 53 patients who participated in a compassionate-use program.127 A placebo-controlled trial (which was prematurely stopped because of poor enrollment because the pandemic was slowing down in China) found that remdesevir was not associated with significant clinical improvement.128 However, the (unpublished) clinical trial sponsored by the National Institutes of Health found that remdesivir was significantly associated with faster time to clinical improvement.129 The US FDA has granted emergency use authorization for remdesivir for the treatment of COVID-19.130
Favipiravir is another viral RNA polymerase inhibitor undergoing controlled clinical trials.124 The anti–human immunodeficiency virus protease inhibitor lopinavir-ritonavir, the antimalarial chloroquine, and anti-inflammatory hydroxychloroquine are being repurposed and evaluated for their efficacy in COVID-19.126 , 131 , 132 The randomized controlled trial that evaluated lopinavir-ritonavir in patients with severe COVID-19 did not find significant clinical benefit compared with supportive care.126
Convalescent plasma has been suggested for the treatment of COVID-19. It is hypothesized that antibody to SARS-CoV-2 in the plasma of persons who have recovered from the illness may be efficacious in controlling viral infection. The FDA has authorized an expanded access program on the use of convalescent plasma for the treatment of COVID-19. In their initial experience, 5 patients with COVID-19 pneumonia reported fever resolution, improvement in sequential organ failure assessment scores, decline in viral load, and resolution of ARDS.133 The outcome of the ongoing registry of thousands of patients who received convalescent plasma using the expanded access program in the United States is eagerly awaited.
Immunomodulators
For the 5% of patients who progress to develop severe systemic hyperinflammatory syndrome, the goal of treatment is to control the proinflammatory state. In this regard, immunomodulators such as IL-6 inhibitors (sarilumab, tocilizumab, and others), granulocyte-macrophage colony-stimulating factor inhibitors (lenzilumab), IL-1 receptor antagonist (anakinra), and others are proposed.124 Several clinical trials are underway to assess the safety and efficacy of these immunomodulators for the treatment of severe, critical, and life-threatening COVID-19.125 Current evidence suggests against routine corticosteroid therapy in COVID-19 unless it is used for other clinical indications or under a clinical trial protocol.134
Hospital Discharge and Follow-up
Early hospital discharge may result in repeated admissions when COVID-19 worsens during the second week of illness. In addition to its progression to lower respiratory tract infection, COVID-19 may result in cardiac injury, AKI, electrolyte disturbances, and coagulation disorders,92 which have postdischarge implications. Other factors that may impair health recovery are the limited physical therapy that these patients receive during hospitalization due to their respiratory compromise, the need for being in isolation precautions, and a decrease in health care staff availability. All of these factors contribute to the longer hospital stay, risk of postdischarge complications, and increased risk of repeated admissions.
Safe criteria for hospital discharge include having been afebrile and stable hemodynamically for at least 48 hours, a stable and safe respiratory status whether or not requiring oxygen, able to conduct activities of daily living, alert cognition, having a place to be safely discharged to, the ability to self-isolate at home to protect other family members, and the presence of a home provider who can continue the care for these patients (Figure 1). Once discharged from the hospital, patients should have quick access to health care in case they develop clinical deterioration.
Patients who were residents of congregate settings, such as skilled nursing facilities, before hospitalization pose additional discharge challenges. These patients can return to their place of residence if the facility has the ability to isolate and care for the patient. If that is not feasible, many of these patients may not be discharged from the hospital until they have recovered from the illness and tested negative for the SAR-CoV-2 by nasopharyngeal PCR.135 , 136
Telemedicine
During this pandemic of a highly infectious virus, the need to limit patients’ movements and to manage them remotely is desired. Telemedicine is essential to monitor the patient’s clinical status remotely to identify early clinical warning signs and to decrease potential exposure to others, including health care workers. Telemedicine in this context not only includes phone calls and video visits but also remote monitoring of symptoms and vital signs such as temperature, heart and respiratory rates, blood pressure, and oxygen saturations from home via devices or smartphone apps.47 , 48 In March 2020, the FDA allowed manufacturers of certain noninvasive, vital sign measuring devices to expand their use so that health care providers can use them to monitor patients remotely. In addition, during this pandemic, privacy and data protection regulations for video and remote communications with patients have been relaxed to allow quicker and easier access of patients to their health care team. These in-home monitoring devices can collect data intermittently or continuously and can be programed to gather more or less intensive data, depending on the patient’s risk category. Some of these patients may require remote cardiac rhythm monitoring, such as LifeVest (ZOLL Medical Corp) or BodyGuardian (Preventice Solutions), especially if they developed new arrhythmia or new heart failure with low ejection fraction (<35%) during hospitalization. These remote monitoring tools allow the clinical team to identify abnormal biometrics early to rapidly triage and escalate care with either in-home management or transfer to an emergency department or an infusion center for management. Remote monitoring devices and clinical teams also serve to reinforce to the patient to remain in home isolation and, at the end of their illness, assist in releasing the patient from isolation precautions.135 , 136
Conclusion
COVID-19 presents a significant challenge to medical providers worldwide. Management of the disease is mostly supportive care with antipyretics, hydration, and oxygen supplementation, as dictated by clinical need. For patients with moderate to severe COVID-19 that requires hospitalization, medical complications affecting various organ systems are not uncommon and may lead to critical illness and multiple organ failure. Hence, the medical care of patients with COVID-19 is best optimized by the collaboration among various health care providers from different specialties. As illustrated in this management review, clinical expertise in hospital medicine, infectious diseases, clinical microbiology, radiology, pulmonary and critical care medicine, cardiology, hematology, and primary care are essential in ensuring that medical complications are prevented or treated early and aggressively. Finally, when patients are medically ready for hospital discharge, telemedicine will provide proper follow-up and monitoring until the patients have medically recovered from their illness and are ready to be released from home quarantine protocols.
Footnotes
Potential Competing Interests: Dr Razonable has received grant support from Roche Regeneron, Aptima. Dr Carmona has received payment for manuscript presentation from Elsevier, payment for development of educational presentations from CHEST and SEPAR (sponsored by Boehringer Ingelheim), and travel/accommodations/meeting expenses from the American Thoracic Society. The other authors report no competing interests.
Supplemental Online Material
References
- 1.Wu F., Zhao S., Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen S.J., Chen M.Y., Liu Y.L. Early introduction of severe acute respiratory syndrome coronavirus 2 into Europe [published online March 20, 2020] https://doi.org/10.3201/eid2607.200359 Emerg Infect Dis. [DOI] [PMC free article] [PubMed]
- 7.Pan A., Liu L., Wang C. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323(19):1–9. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson L.M., Simard J.F., Oluyomi A. US public concerns about the COVID-19 pandemic from results of a survey given via social media [published online March 7, 2020] https://doi.org/10.1001/jamainternmed.2020.1369 JAMA Intern Med. [DOI] [PMC free article] [PubMed]
- 9.Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E. A novel coronavirus emerging in China: key questions for impact assessment. N Engl J Med. 2020;382(8):692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 10.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaunt E.R., Hardie A., Claas E.C., Simmonds P., Templeton K.E. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol. 2010;48(8):2940–2947. doi: 10.1128/JCM.00636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;525(1):135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu H., Zhong L., Deng J. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai A., Bergna A., Acciarri C., Galli M., Zehender G. Early phylogenetic estimate of the effective reproduction number of SARS-CoV-2 [published online February 25, 2020] https://doi.org/10.1002/jmv.25723 J Med Virol. [DOI] [PMC free article] [PubMed]
- 20.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernheim A., Mei X., Huang M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3):200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin L., Jiang X., Zhang Z. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 24.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung N.H.L., Chu D.K.W., Shiu E.Y.C. Respiratory virus shedding in enhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asadi S., Wexler A.S., Cappa C.D., Barreda S., Bouvier N.M., Ristenpart W.D. Aerosol emission and superemission during human speech increase with voice loudness. Sci Rep. 2019;9(1):2348. doi: 10.1038/s41598-019-38808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [published online February 24, 2020] https://doi.org/10.1001/jama.2020.2648 JAMA. [DOI] [PubMed]
- 29.Lai C.C., Liu Y.H., Wang C.Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths [published online March 4, 2020] https://doi.org/10.1016/j.jmii.2020.02.012 J Microbiol Immunol Infect. [DOI] [PMC free article] [PubMed]
- 30.Li G., Li W., He X., Cao Y. Asymptomatic and presymptomatic infectors: hidden sources of COVID-19 disease [published online April 9, 2020] https://doi.org/10.1093/cid/ciaa418 Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 31.Wang Y., Liu Y., Liu L., Wang X., Luo N., Ling L. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who where asymptomatic at hospital admission in Shenzhen, China. J Infect Dis. 2020;221(11):1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C., Ji F., Wang L. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, Xuzhou, China [published online March 31, 2020] https://doi.org/10.3201/eid2607.200718 Emerg Infect Dis. [DOI] [PMC free article] [PubMed]
- 33.Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and ageusia: common findings in COVID-19 patients [published online April 1, 2020] https://doi.org/10.1002/lary.28692 Laryngoscope. [DOI] [PMC free article] [PubMed]
- 35.Gane S.B., Kelly C., Hopkins C. Isolated sudden onset anosmia in COVID-19 infection: a novel syndrome [published online April 2, 2020]? https://doi.org/10.4193/Rhin20.114 Rhinology. [DOI] [PubMed]
- 36.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis [published online March 30, 2020] https://doi.org/10.1016/S1473-3099(20)30243-7 Lancet Infect Dis. [DOI] [PMC free article] [PubMed]
- 37.Xiao Y., Tan C., Duan J., Wu A., Li C. An effective model for the outpatient management of COVID-19 [published online March 26, 2020] https://doi.org/10.1017/ice.2020.92 Infect Control Hosp Epidemiol. [DOI] [PMC free article] [PubMed]
- 38.Liang W.H., Guan W.J., Li C.C. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicenter) and outside Hubei (non-epicenter): a nationwide analysis of China [published online April 8, 2020] https://doi.org/10.1183/13993003.00562-2020 Eur Respir J. [DOI] [PMC free article] [PubMed]
- 39.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region: case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region. Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo W., Li M., Dong Y. Diabetes is a risk factor for the progression and prognosis of COVID-19 [published online March 31, 2020] https://doi.org/10.1002/dmrr.3319 Diabetes Metab Res Rev. [DOI] [PMC free article] [PubMed]
- 43.Lippi G., Wong J., Henry B.M. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130(4):304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 44.Lighter J., Phillips M., Hochman S. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission [published online April 9, 2020] https://doi.org/10.1093/cid/ciaa415 Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 45.Pereira M.R., Mohan S., Cohen D.J. COVID-19 in solid organ transplant recipients: initial report from the US epicenter [published online April 24, 2020] https://doi.org/10.1111/ajt.15941 Am J Transplant. [DOI] [PMC free article] [PubMed]
- 46.Akalin E., Azzi Y., Bartash R. COVID-19 and kidney transplantation [published online April 24, 2020] https://doi.org/10.1056/NEJMc2011117 N Engl J Med. [DOI] [PMC free article] [PubMed]
- 47.Greenhalgh T., Koh G.C.H., Car J. COVID-19: a remote assessment in primary care [published online March 25, 2020] https://doi.org/10.1136/bmj.m1182 BMJ. [DOI] [PubMed]
- 48.Greenhalgh T., Wherton J., Shaw S., Morrison C. Video consultations for covid-19 [published online March 12, 2020] https://doi.org/10.1136/bmj.m998 BMJ. [DOI] [PubMed]
- 49.Binnicker M.J. Emergence of a novel coronavirus disease (COVID-19) and the importance of diagnostic testing: why partnership between clinical laboratories, public health agencies, and industry is essential to control the outbreak. Clin Chem. 2020;66(5):664–666. doi: 10.1093/clinchem/hvaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases [published online February 26, 2020] https://doi.org/10.1148/radiol.2020200642 Radiology. [DOI] [PMC free article] [PubMed]
- 51.Shah A., Kashyap R., Tosh P., Sampathkumar P., O'Horo J.C. Guide to understanding the 2019 novel coronavirus. Mayo Clin Proc. 2020;95(4):646–652. doi: 10.1016/j.mayocp.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao Y., Li T., Han M. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19 [published online March 17, 2020] https://doi.org/10.1002/jmv.25770 J Med Virol. [DOI] [PMC free article] [PubMed]
- 53.Chen G., Wu D., Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terpos E., Ntanasis-Stathopoulos I., Elalamy I. Hematological findings and complications of COVID-19 [published online April 13, 2020] https://doi.org/10.1002/ajh.25829 Am J Hematol. [DOI] [PMC free article] [PubMed]
- 55.Deng Y., Liu W., Liu K. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study [published online March 20, 2020] https://doi.org/10.1097/CM9.0000000000000824 Chin Med J (Engl) [DOI] [PMC free article] [PubMed]
- 56.Lippi G., Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bajema K.L., Oster A.M., McGovern O.L. Persons evaluated for 2019 novel coronavirus - United States, January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(6):166–170. doi: 10.15585/mmwr.mm6906e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding Q., Lu P., Fan Y., Xia Y., Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China [published online March 20, 2020] https://doi.org/10.1002/jmv.25781 J Med Virol. [DOI] [PMC free article] [PubMed]
- 59.Patel R., Babady E., Theel E.S. Report from the American Society for Microbiology COVID-19 International Summit, 23 March 2020: value of diagnostic testing for SARS-CoV-2/COVID-19 [published online March 26, 2020] https://doi.org/10.1128/mBio.00722-20 mBio. [DOI] [PMC free article] [PubMed]
- 60.Hanson K.E., Caliendo A.M., Arias C.A. 2020. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management. Accessed June 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu F., Yan L., Wang N. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients [published online March 28, 2020] https://doi.org/10.1093/cid/ciaa345 Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 62.Xie C., Jiang L., Huang G. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int J Infect Dis. 2020;93:264–267. doi: 10.1016/j.ijid.2020.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan Y., Li X., Yang G. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients [published online April 10, 2020] https://doi.org/10.1016/j.jinf.2020.03.051 J Infect. [DOI] [PMC free article] [PubMed]
- 64.Xu Y., Xiao M., Liu X. Significance of serology testing to assist timely diagnosis of SARS-CoV-2 infections: implication from a family cluster. Emerg Microbes Infect. 2020;9(1):924–927. doi: 10.1080/22221751.2020.1752610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fang Y., Zhang H., Xie J. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020] https://doi.org/10.1148/radiol.2020200432 Radiology. [DOI] [PMC free article] [PubMed]
- 66.Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of Chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 67.Pan F., Ye T., Sun P. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y., Dong C., Hu Y. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study [published online March 19, 2020] https://doi.org/10.1148/radiol.2020200843 Radiology. [DOI] [PMC free article] [PubMed]
- 69.Song F., Shi N., Shan F. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen Z., Chi Y., Zhang L. Coronavirus disease 2019: initial detection on chest CT in a retrospective multicenter study of 103 Chinese subjects [published online April 6, 2020] https://doi.org/10.1148/ryct.2020200092 Radiol Cardiothorac Imaging. [DOI] [PMC free article] [PubMed]
- 71.Simpson S., Kay F.U., Abbara S. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19: endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA [published online April 28, 2020] https://doi.org/10.1097/RTI.0000000000000524 J Thorac Imaging. [DOI] [PMC free article] [PubMed]
- 72.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response [published online March 13, 2020]. JAMA. doi:10.1001/jama.2020.4031. [DOI] [PubMed]
- 73.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China [published online March 12, 2020] https://doi.org/10.1093/cid/ciaa248 Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 74.Wang F., Nie J., Wang H. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [published online March 13, 2020] https://doi.org/10.1001/jamainternmed.2020.0994 JAMA Intern Med. [DOI] [PMC free article] [PubMed]
- 76.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 77.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 does not lead to a "typical" acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herold S., Gabrielli N.M., Vadasz I. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2013;305(10):L665–L681. doi: 10.1152/ajplung.00232.2013. [DOI] [PubMed] [Google Scholar]
- 79.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2 [published online April 3, 2020] https://doi.org/10.1007/s11239-020-02105-8 J Thromb Thrombolysis. [DOI] [PMC free article] [PubMed]
- 80.Amato M.B., Meade M.O., Slutsky A.S. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 81.Fan E., Del Sorbo L., Goligher E.C. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 82.Alhazzani W., Moller M.H., Arabi Y.M. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caputo N.D., Strayer R.J., Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED's experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375–378. doi: 10.1111/acem.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zeng J.H., Liu Y.X., Yuan J. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights [published online April 10, 2020] https://doi.org/10.1007/s15010-020-01424-5 Infection. [DOI] [PMC free article] [PubMed]
- 85.Du Y., Tu L., Zhu P. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study [published online April 3, 2020] https://doi.org/10.1164/rccm.202003-0543OC Am J Respir Crit Care Med. [DOI] [PMC free article] [PubMed]
- 86.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 88.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) [published online March 27, 2020] https://doi.org/10.1001/jamacardio.2020.1096 JAMA Cardiol. [DOI] [PMC free article] [PubMed]
- 89.Dong N., Cai J., Zhou Y., Liu J., Li F. End-stage heart failure with COVID-19: strong evidence of myocardial injury by 2019-nCoV [published online April 7, 2020] https://doi.org/10.1016/j.jchf.2020.04.001 JACC Heart Fail. [DOI] [PMC free article] [PubMed]
- 90.Li X., Wang L., Yan S. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China [published online March 25, 2020] https://doi.org/10.1001/jamacardio.2020.0950 JAMA Cardiol. [DOI] [PMC free article] [PubMed]
- 93.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis [published online March 10, 2020] https://doi.org/10.1016/j.pcad.2020.03.001 Prog Cardiovasc Dis. [DOI] [PMC free article] [PubMed]
- 94.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study [published online March 26, 2020] https://doi.org/10.1136/bmj.m1091 BMJ. [DOI] [PMC free article] [PubMed]
- 95.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin [published online March 16, 2020] https://doi.org/10.1093/eurheartj/ehaa190 Eur Heart J. [DOI] [PMC free article] [PubMed]
- 97.Corrales-Medina V.F., Madjid M., Musher D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10(2):83–92. doi: 10.1016/S1473-3099(09)70331-7. [DOI] [PubMed] [Google Scholar]
- 98.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review [published online March 27, 2020] https://doi.org/10.1001/jamacardio.2020.1286 JAMA Cardiol. [DOI] [PubMed]
- 99.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Warren-Gash C., Smeeth L., Hayward A.C. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9(10):601–610. doi: 10.1016/S1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 101.Sandoval Y., Jaffe A.S. Type 2 myocardial infarction: JACC review topic of the week. J Am Coll Cardiol. 2019;73(14):1846–1860. doi: 10.1016/j.jacc.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 102.Szerlip M., Anwaruddin S., Aronow H.D. Considerations for cardiac catheterization laboratory procedures during the COVID-19 pandemic perspectives from the Society for Cardiovascular Angiography and Interventions Emerging Leader Mentorship (SCAI ELM) Members and Graduates [published online March 25, 2020] https://doi.org/10.1002/ccd.28887 Catheter Cardiovasc Interv. [DOI] [PubMed]
- 103.Bennett C.M., Anavekar N.S., Gulati R. ST-segment elevation, myocardial injury, and suspected or confirmed COVID-19 patients: diagnostic and treatment uncertainties [published online April 11, 2020] https://doi.org/10.1016/j.mayocp.2020.04.005 Mayo Clin Proc. [DOI] [PMC free article] [PubMed]
- 104.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T., Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome [published online April 16, 2020] https://doi.org/10.1161/CIRCULATIONAHA.120.047349 Circulation. [DOI] [PMC free article] [PubMed]
- 105.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li J., Fan J.G. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol. 2020;8(1):13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang L., Li X., Chen H. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pei G., Zhang Z., Peng J. Renal involvement and early prognosis in patients with COVID-19 pneumonia [published online April 28, 2020] https://doi.org/10.1681/ASN.2020030276 J Am Soc Nephrol. [DOI] [PMC free article] [PubMed]
- 111.Diao M., Zhang S., Chen D., Hu W. The novel coronavirus (COVID-19) infection in Hangzhou: an experience to share [published online March 5, 2020] https://doi.org/10.1017/ice.2020.62 Infect Control Hosp Epidemiol. [DOI] [PMC free article] [PubMed]
- 112.Pan X.W., Xu D., Zhang H., Zhou W., Wang L.H., Cui X.G. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis [published online March 31, 2020] https://doi.org/10.1007/s00134-020-06026-1 Intensive Care Med. [DOI] [PMC free article] [PubMed]
- 113.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Batlle D., Soler M.J., Sparks M.A. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology [published online May 4, 2020] https://doi.org/10.1681/ASN.2020040419 J Am Soc Nephrol. [DOI] [PMC free article] [PubMed]
- 115.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia [published online April 9, 2020] https://doi.org/10.1111/jth.14830 J Thromb Haemost. [DOI] [PMC free article] [PubMed]
- 116.Han H., Yang L., Liu R. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection [published online March 16, 2020] https://doi.org/10.1515/cclm-2020-0188 Clin Chem Lab Med. [DOI] [PubMed]
- 117.Wang J., Hajizadeh N., Moore E.E. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series [published online April 8, 2020] https://doi.org/10.1111/jth.14828 J Thromb Haemost. [DOI] [PMC free article] [PubMed]
- 118.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thachil J., Tang N., Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19 [published online April 10, 2020] https://doi.org/10.1016/j.thromres.2020.04.013 Thromb Res. [DOI] [PMC free article] [PubMed]
- 122.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens [published online April 15, 2020] https://doi.org/10.1001/jama.2020.6266 JAMA. [DOI] [PMC free article] [PubMed]
- 124.Vijayvargiya P., Esquer-Garrigos Z., Castillo N., Gurram P., Stevens R., Razonable R.R. Treatment considerations for COVID-19: a critical review of the evidence (or lack thereof) [published online April 30, 2020] https://doi.org/10.1016/j.mayocp.2020.04.027 Mayo Clin Proc. [DOI] [PMC free article] [PubMed]
- 125.Bhimraj A., Morgan R.L., Shumaker A.H. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19 [published online April 17, 2020] https://doi.org/10.1093/cid/ciaa478 Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 126.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe Covid-19 [published online April 10, 2020] https://doi.org/10.1056/NEJMoa2007016 N Engl J Med. [DOI] [PMC free article] [PubMed]
- 128.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. NIH website. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19
- 130.Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment. FDA website. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment
- 131.Perinel S., Launay M., Botelho-Nevers E. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients [published online April 7, 2020] https://doi.org/10.1093/cid/ciaa394 Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 132.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yuan J., Kou S., Liang Y., Zeng J., Pan Y., Liu L. PCR assays turned positive in 25 discharged COVID-19 patients [published online April 8, 2020] https://doi.org/10.1093/cid/ciaa398 Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 136.Xu K., Chen Y., Yuan J. Factors associated with prolonged viral RNA shedding in patients with COVID-19 [published online April 9, 2020] https://doi.org/10.1093/cid/ciaa351 Clin Infect Dis. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.