Abstract
Introduction and motivation
Since the end of 2019, the COVID-19 pandemic has affected millions of people worldwide. With the rapid spread of this virus, an immense burden has fallen upon both healthcare and economic systems. As a consequence, there is an unprecedented urgency for researchers and scientific committees from all over the world to find an effective treatment and vaccine.
Review Structure
Many potential therapies are currently under investigation, with some, like Hydroxychloroquine, being authorized for emergency use in some countries. The crucial issue is now clearly to find the suitable treatment strategy for patients given comorbidities and the timeline of the illness.
Vaccines are also under development and phase 1 clinical trials are rolling. Despite all efforts, no single drug or vaccine has yet been approved. In this review, we aim at presenting the proposed pathophysiological mechanisms of SARS-CoV-2 and to provide clinicians with a brief and solid overview of the current potential treatments classified according to their use at the three different currently proposed disease stages. In light of pathogenesis and proposed clinical classification, this review’s purpose is to summarize and simplify the most important updates on the management and the potential treatment of this emergent disease.
Keywords: COVID-19, Treatment, Pathophysiology
Introduction
Since the first identified case of Coronavirus Disease (COVID-19) in December 2019, the number of confirmed cases has dramatically increased all over the world, and as of April 21 st 2020, more than 2,397,216 cases worldwide have been confirmed, with, unfortunately, a rising death toll [1,2]. COVID-19 is caused by a novel coronavirus called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) with droplets and contact being the main route of transmission. Recently an airborne transmission route has been suggested [3,4]. Although 80% of infected people experience mild to moderate disease, the other 20% present severe cases leading to critically ill patients that represent a real concern due to the rapid spread of the virus and limited medical resources even in high income countries. The result has been an enormous challenge placed on the shoulders of healthcare systems [5,6]. With the higher mortality rate among severe and critically ill patients, and the relatively high rate of transmission, not to mention the economic burden and the absence of an effective vaccine, the need for an urgent and effective treatment proves urgently crucial. Several potential treatments have been proposed, and some have even been tested or still in ongoing trials [6,7].
Recently, one article has highlighted the importance of distinguishing between two different overlapping disease phases. The first phase is normally induced by the virus while the second is the result of host physiological response. In order to help with treatment decision, Siddiqi et al., proposed in their article three clinical stage classification for COVID-19 patients [8]. In light of pathogenesis and proposed clinical classification, this review’s purpose is to summarize and simplify the most important updates on the management and the potential treatment of this emergent disease.
Motivation and methodology
In this review, we aim to present the proposed pathophysiological mechanisms of SARS-CoV-2 and to provide clinicians with a brief and solid overview of current potential treatments classified according to their use at different disease stages. This manuscript may facilitate the process of knowledge acquisition for healthcare professionals while well-established strategies are still lacking.
As of the time of this manuscript writing, no clear consensus has been established about the use of these treatments; therefore, this cannot be considered as a set of formal recommendations. Rather, it is more a simplified guide to better understand the pathophysiological mechanisms of under-investigation treatments. To this end we searched major databases and research engines like PubMed and others for COVID-19 pathophysiology and for what may be considered as a “possible tracks for treatment development”.
Epidemiology
The first cases of COVID-19 were diagnosed in Wuhan, China. From there, the disease spread to all continents forming a pandemic with men being slightly more affected than women. Severe cases, which range between 20–30% depending on the population were reported especially among those who are older than 60 years old, those who are smokers or who have concomitant comorbidities such as hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), or those who are immunocompromised [1,9].
Overall mortality ratio was estimated to be 3–4% according to the World Health Organization (WHO) [10]. This rate gets significantly higher among patients with one or more of the aforementioned risk factors, and, according to some studies, it can be 10–27 % in patients older than 85 years old. On the other hand, younger and pediatric patients experience milder symptoms, and the mortality rate among patients under 19 years old is lower (<1 %) [11].
Diagnosis
Laboratory study
Complete blood count, coagulation profile, and serum biochemical tests are routinely performed for COVID-19 patients [12]. Lymphocytopenia is a common finding and the percentage of lymphocytes (LYM%) has been suggested as a predictive parameter during disease course. Patients with LYM% < 20 % on day 10–12 after symptom onset tend to have worse outcomes with higher mortality among those with LYM < 5 % [13]. A preliminary study proposed a neutrophil-to-lymphocyte ratio (NLR) of ≥ 3.13 as a predictor of severe disease specially in patients above 50 years old, and the authors recommended that these patients should be admitted early to intensive care units (ICU) [14].
Other common laboratory findings include elevated levels of the following: prothrombin time (PT), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH) and D-dimer. CRP levels seem to correlate directly with disease severity. In contrast, elevated procalcitonin levels are not a feature of COVID-19 and may suggest super infection [5].
Real-time reverse-transcription polymerase chain reaction (rRT-PCR) remains the gold standard for COVID-19 diagnosis. However, its sensitivity varies widely depending on the specimens’ type with the bronchoalveolar lavage fluid specimens demonstrating the highest sensitivity (93 %) while the reported sensitivity of the sputum (72 %), nasal (63 %) and pharyngeal (32 %) swabs are lower in sensitivity. Other concomitant samples (i.e. blood, feces, etc.) have been proposed as a way to improve sensitivity during COVID-19 diagnosis [15]. Some novel tests, like the rapid test using either by antigen or antibodies detection alongside enzyme linked immunosorbent assays (ELISA) testing for antibodies are available in some settings and seem to be effective and also have acceptable sensitivity and specificity [16].
Imaging studies
Chest computerized tomography (CT-scan) seems to have high sensitivity in all cases -including severe ones- in epidemic areas. The most common patterns on chest CT scans are ground-glass opacity, consolidation, bilateral patchy shadowing and peripheral and diffuse distribution. In one Chinese study, radiological changes were detected up to 5.1 ± 1.5 days before PCR turned positive in pharyngeal swabs, demonstrating a sensitivity of 97 % with low specificity of 25 %. The positive predictive value of CT scans is 65 %, and the negative predictive value is 83 % with the note that this study was conducted in Wuhan, China, the center of the epidemic, meaning these values might change in non-epidemic areas [17,18].
Contradictorily, other studies noticed that PCR positivity could precede radiological changes [19]. Therefore, making clinical decisions should be based on patient presentation and epidemiological criteria. Especially in cases of highly suspected patients, applying isolation or required infection-control procedures may be upheld upon the positivity of PCR and/or CT scan imaging studies [17,19].
Diagnostic criteria
Several guidelines have been proposed to define a “suspected case” [[20], [21], [22]]. Most of these rely on a combination of clinical and epidemiological criteria. For example, the WHO criteria recommend performing rRT-PCR for all those who fulfill “suspected case” criteria. But with the huge rising numbers, the lack of resources and the high cost of testing, many countries reserve PCR testing for severe and critically ill patients. This, according to some estimates, might mean that the actual numbers of infected individuals are way higher than those already diagnosed [21,23].
Pathophysiology and management
SARS-CoV-2 is an enveloped RNA virus that belongs to the beta-coronavirus family. It primarily infects the respiratory system causing acute respiratory distress syndrome (ARDS) and, in severe cases, mimicking previous SARS-CoV responsible for the Severe Acute Respiratory Syndrome (SARS) [5,24,25].
Based on experience with previous coronavirus infections, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV), several treatment options have been proposed to manage patients with COVID-19 [26]. Some of these options have already proved their efficacy in in-vitro studies, and even in several clinical trials. Until the time of writing this manuscript, no single drug has globally been approved as treatment for COVID-19. However, emergency authorization was given on a national level in some countries like the USA and Europe for the use of Chloroquine (CQ)/ Hydroxychloroquine (HCQ) in certain cases. Chinese guidelines in their 7th edition also authorized other therapies like Lopinavir/Ritonavir and Ribavirin+/- IFN(α-2 β) [7,22,27,28]. Depending on the three clinical stages of COVID-19, we present the potential therapeutic options for each stage, taking into consideration in the same time that these propositions may potentially take a cumulative form where some potential treatments of stage I, as an example, may be used also in more advanced stages, while the opposite scenario may be, in our opinion, considered as risky (Fig. 1 ).
Fig. 1.
Cumulative perspective of potential COVID-19 treatments based on three clinical stage classification. Symptomatic treatment is a basic step in all clinical stages of the disease, with oxygen therapy here reflecting all interventions that may be needed from nasal cannula to mechanical ventilation. While some argue that antiviral therapy (especially Remdesivir) could be considered for some patients in Stage I of the disease, other preserve it for the Stage II patients. Ribavirin and IFNα-2b in particular, in our opinion shouldn’t be considered in the management of Stage I. More severe cases (Stage III) need more extensive interventions by adding immunomodulatory treatments to the previous management steps in the attempt to contain the hyperinflammatory response.
*These treatments might also be investigated in both stage II and III.
**These treatments might also be investigated in stage III.
***These treatments are mainly investigated in stage III.
Abbreviations: LPV/RTV, Lopinavir/Ritonavir, LMWH, low molecular weight heparin, IVIG, Intravenous Immunoglobulin, CP Convalescent Plasma.
Stage 1 (mild COVID-19): viral invasion and replication
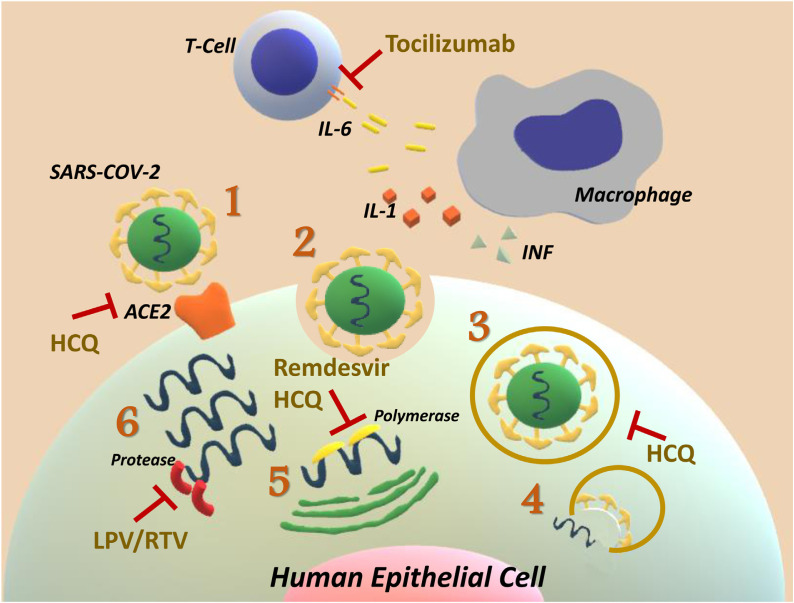
After an incubation period, SARS-CoV-2 invades the mucosal membranes, especially nasal and oral-pharyngeal membranes, causing upper respiratory infection with mild symptoms in the first phase. The virus binds to target cells through angiotensin-converting enzyme 2 (ACE2). This activates the serine protease TMPRSS2 for S protein priming (Fig. 2 ) [29]. ACE2 is expressed by epithelial cells of the intestine, lung, kidney, and blood vessels [30]. Treatment in this phase is based essentially on symptomatic relief, supportive and non-specific therapies, such as acetaminophen. The use of virus-targeting treatments may be beneficial in this period in order to stop viral replication [8].
Fig. 2.
Mechanism of action of drugs under investigation: Lopinavir/Ritonavir, Hydroxychloroquine, Remdisivir, and Tocilizumab. 1: SARS-CoV-2 attaches to the ACE2 receptor on human epithelial cells in order to gain entry to them. Once attached, it activates the TMPRSS2 which leads towards S protein activation by proteolytic cleavage. Camostat Mesylate is a therapeutic agent that inhibits TMPRSS2. 2 and 3: Endosomes, then, transfer the complex (virus-receptor) into the cell and 4: releases the virus genome (RNA) which undergoes 5 and 6 translation and transcription using the targeted cell transcription system. Remdisivir inhibits polymerase, while Lopinavir/Ritonavir (LPV/RTV) inhibits protease; the two fundamental enzymes during viral replication. Hydroxychloroquine (HCQ) targets several levels of viral infection. It changes the glycosylation of ACE2 to inhibit virus entry. Being a weak base, it also affects endosomal activity by increasing, the pH of acidic intracellular organelles. Finally, it interferes with RNA replication by targeting the polymerase. In severe and critical cases, macrophage activation leads to an uncontrolled and lethal inflammatory process known as “cytokine storm syndromes” driven by the release of multiple cytokines; IL-6 plays a key role in this process. Tocilizumab is a monoclonal antibody that inhibits the IL-6 Receptor which might mitigate the inflammatory response.
Although some argue that Hydroxychloroquin (HCQ) can be used early in Stage 1. This use may have negative effects regarding loss of immunization chances due not only to its reduction of viral load, but also to its mmuno-suppressive characteristics [31].
Standard of care management
Isolation remains the cornerstone intervention for containment of COVID-19 as no treatment or vaccination has yet been globally approved. Supplementary oxygen, acetaminophen, and antibiotics should be administered as required. Non-invasive ventilation, intubation and mechanical ventilation might be required for critically ill patients and in cases of respiratory failure in the advanced stages IIb and III. Close monitoring of organ function should be undertaken for early detection of organ failure or septic shock in predisposed patients [5,12].
Antiviral therapies
Remdesivir
Remdesivir is a phosphoramidate prodrug of an adenosine C–nucleoside that targets the viral RNA-dependent RNA polymerase (RdRp) proteins. Working as a nucleotide analogue, this drug has proved its broad-spectrum antiviral activity including the effect against several human and zoonotic coronaviruses including SARS-CoV-2. Since human safety data for Remdesivir are available from several clinical trials that tested Remdesivir’s efficacy against Ebola virus, several clinical trials are already being held in the United States and China to investigate its efficacy treating COVID-19 patients [7,26,32]. The dose under investigation for treatment of COVID-19 is 200 mg intravenously (IV) on day 1 followed by 100 mg IV daily for up to 10 days, infused over 30−60 min [7]. Several trials of Remdesivir treatment on few patients in the United States have shown early promising benefits in cases with severe pneumonia [33,34]. In a cohort study that included 61 severe COVID-19 patients from different countries, using Remdesivir (200 mg IV. on day 1, then 100 mg daily for 9 days) led to a clinical improvement in 63 % of patients with a median follow-up of 18 days. The total mortality rate in this study was 13 % [35].
Lopinavir/Ritonavir
Lopinavir (LPV) is a human immunodeficiency virus 1 (HIV-1) protease inhibitor which is administered in combination with the “booster” ritonavir (RTV), a potent CYP3A4 inhibitor that increases LPV half-life. Even though LPV/RTV was effective against SARS-CoV in tissue cultures, its efficacy against MERS-CoV was controversial. Results of a randomized, controlled, open-label trial that evaluated Lopinavir–Ritonavir vs. standard of care treatment in adults hospitalized adults with severe COVID-19 were recently published [36]. The two arms of this study included 199 confirmed COVID-19 patients and showed similar 28-day mortality rates and viral load at varying times since onset of symptoms. This study also showed withdrawal of medication in 13 patients in the LPV group due to adverse events (Table 1 ). However, the relatively long median time to randomization (13 days) after onset of symptoms is thought to partially explain lack of effectiveness in the COVID-19 arm [37].
Table 1.
Summary of clinical studies investigating proposed treatments of COVID-19.
| Investigation | Study | Country | Study type | Population (n patients) | Intervention group | Control group | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Anti-viral therapies | LPV/ RTV | Cao, B. et al. [37] | China | Open-label, randomized,controlled trial | >18 years, severe COVID-19 pneumonia cases. (n = 199) | LPV/ RTV (400 mg/100 mg) orally b.i.d. plus standard care for 14 days. | Standard care only for 14 days. | No benefit was observed with LPV/ RTV beyond standard care treatment. |
| Deng, L. et al. [40] | China | Retrospective cohort study | ≥18years, confirmed COVID-19 cases, without Invasive ventilation. (n = 33) | Umifenovir (Arbidol®) (200 mg/ 8 h) plus LPV/ RTV (400 mg/100 mg) for 5–21 days. | Oral LPV/ RTV only for 5–21 days. | Better clinical, radiological and laboratorial (RT-PCR negativity) response was observed in the (Umifenovir + lPV/ RTV) group. | ||
| Remdesivir | Grein, J. et al. [36] | Multi-national | Cohort study. | Hospitalized confirmed COVID-19 cases with O2 saturation ≤ 94% or receiving O2 support. (n = 61) | Remdesivir (200 mg IV. On D1 then 100 mg daily) total duration: 10 days. | NA | Clinical improvement was observed in 68 % of patients. Total mortality rate was 13 %. | |
| CQ/HCQ | Chen, J. et al. [59] | China | Randomized pilot study | Hospitalized confirmed COVID-19 cases. (n = 30) | HCQ (400 mg / day) for 5 days plus conventional treatments. | Conventional treatment only. | Duration from hospitalization to COVID-19 nucleic acid negativity, clinical improvement, and radiological changes were comparable with the control group. | |
| Chen, Z. et al.d [63] | China | Randomized clinical trial | ≥ 18 years, COVID-19 pneumonia cases. (n = 62) | HCQ (400 mg/day) orally for 5-days. | Standard treatment only. | HCQ significantly shortened TTCR and promote the absorption of pneumonia. | ||
| Gautret, P. et al. [64] | France | open-label Non-randomized clinical trial | >12 years, hospitalized confirmed COVID-19 cases. (n = 36) | HCQ sulfate orally (200 mg t.i.d./day) for ten days | Standard treatment only. | HCQ was significantly associated with viral load reduction/disappearance in COVID-19 patients and azithromycin reinforced its effect. | ||
| -Six patients received Azithromycin (500 mg) on D1 followed by (250 mg /day) the next four days. | ||||||||
| Gautret, P. et al.d [65] | France | Observational study | Hospitalized confirmed COVID-19 cases (n = 80) | HCQ sulfate orally (200 mg t.i.d./day) for ten days plus Azithromycin (500 mg on D1 followed by 250 mg/ day) for the next four days. | NA | Rapid fall of viral load (93 % negative PCR at D8). Virus cultures from respiratory samples were negative in 97.5 % patients at D5. | ||
| Anti-viral therapies | CQ/HCQ | Tang, W. et al.d [60] | China | Open-label, randomized, controlled trial. | ≥ 18 years, Hospitalized confirmed COVID-19 cases. (n = 150) | HCQ (1200 mg/day for 3 days, then 800 mg/day) total duration: 2−3 weeks for mild/moderate or severe patients, respectively. | Standard treatment only. | HCQ did not result in a higher negative conversion rate, but more clinical improvement than the control group was observed. |
| Adjunctive Therapy | Tocilizumab | Xu, X. et al. [82] | China | Observation study | Severe or critical COVID-19 patients | Tocilizumab IV. (400 mg once)a | NA | All patients had clinical, radiological and laboratory (LYM and CRP level) improvement. |
| (n = 21) | Three patients had another 400 mg within 12 h. | |||||||
| Siltuximab | Gritti, G. et al.d [91] | Italy | Retrospective study | Confirmed COVID-19 pneumonia cases with ARDS. (n = 21) | Siltuximab IV. (11 mg /kg /day) over 1 h plus standard treatment. | NA | Potential role in improving the clinical condition (33 % of patients improved, and 43 % stabilized, while 24 % had worsening in condition. | |
| convalescent plasma | Shen, C. et al. [81] | China | Case series | Confirmed critically ill COVID-19 cases on mechanical ventilation. with (n = 5) | Convalescent plasma (400 mL) with a SARS-CoV-2 specific antibody (IgG).b | NA | Clinical improvement was reported in all patients. | |
| Viral loads became negative within 12 days after transfusion. | ||||||||
| CS + IVIG | Zhou, Zhi-Guo. et al.d [78] | China | Retrospective study | Patients who failed to respond to low-dose CS (40−80 mg/day) and IVIG (10 g/day). (n = 3) | CS (160 mg/day) plus IVIG (20 g/d).c | NA | Moderate dose of CS + IVIG reversed the continued deterioration of COVID-19 patients who failed to respond to the low-dose therapy. | |
Abbreviations: n: number, LPV/ RTV: Lopinavir/Ritonavir, b.i.d.: Twice daily, h: Hour, O2: oxygen, CQ /HCQ: Hydroxychloroquine, TTCR: Time to clinical recovery, D: Day, PCR: Polymerase chain reaction, IV: Intravenous, LYM: Lymphocytes, CRP: C-reactive protein, CS: Corticosteroids, IVIG: Intravenous Immunoglobulins.
Patients also received standard care including Lopinavir, methylprednisolone, and oxygen therapy.
All patients received antiviral agents and methylprednisolone.
Patients also received, as required, oxygen therapy, antiviral treatment, antibiotic treatment, extracorporeal membrane oxygenation (ECMO), and continuous renal replacement therapy (CRRT).
Pre-print.
Other clinical trials including Lopinavir/Ritonavir either as monotherapy or mostly in combination with other drugs have been conducted or are still ongoing. Reported data from published investigations are difficult to interpret due to concomitant drug therapies, varying illness severity amongst patients and lack of comparison groups [7,38]. China, however, has included Lopinavir/Ritonavir in its guidelines at an oral dose of 400 mg/100 mg twice a day for no more than 10 days. But more data are needed from ongoing studies, and caution should be taken regarding adverse effects and the real advantage over standard of care management [7,22].
Umifenovir
Umifenovir is an antiviral agent that has been used for influenza treatment and has shown activity against SARS-CoV in vitro [7,39]. Its activity against SARS-CoV-2 was investigated recently at a dose of 200 mg every 8 h in combination with Lopinavir/Ritonavir in a retrospective cohort Chinese study [39]. Results showed better clinical response in the combination group in comparison with the Lopinavir/Ritonavir monotherapy group. Umifenovir is currently being tested in seven randomized trials for the treatment of COVID-19 [38].
Anti-endocytosis treatment
Baricitinib
Baricitinib is a selective Janus kinase 1 and Janus kinase 2 (JAK1 and JAK2) inhibitor. The mechanism of action is considered to be by modulation of viral endocytosis. But there are huge concerns that this blockage may potentiate SARS-Cov2 infection, and there are other safety issues related to this product’s administration [40].
Human recombinant soluble ACE2 (hrsACE2)
ACE2 was identified as a key receptor for SARS-CoV-2. Data regarding the interaction between COVID-19 severity and Angiotensin converting enzyme 2 (ACE2) receptor inhibition is conflict, and contrary theories have been proposed so far [41,42]. Initially, concerns were raised on the basis that ACEIs and ARBs led to an increase in the number of ACE2 receptors in the cardiopulmonary circulation in experimental animals, and that this might lead to more severe outcomes due to SARS-CoV-2 infection [43]. On the other hand, ACE2 has been found to protect the lungs from injury. And some data suggest that SARS-CoV-2 down-regulates ACE2 expression after initial engagement with the receptor. This down-regulation of ACE2 activity in the lungs might facilitate lung injury. In a recent in-vitro study hrsACE2 was shown to significantly block early stages of SARS-CoV-2 infections [41,42]. In a recent retrospective study that included 564 patients, hypertensive patients treated with ACEI/ARB were less likely to develop severe pneumonia, and the study concluded that these drugs might have a protective effect [44].
Until more data are available, there is no indication for patients treated with ACEIs or ARBS to withhold their treatment (Renin–Angiotensin–Aldosterone System Inhibitors in Patients with COVID-19 [45].
Camostat mesylate
Camostat Mesylate is a serine protease inhibitor, a drug approved in japan for use in pancreatic inflammation. It usually blocks TMPRSS2, a protease that was recently shown to be responsible for the coronavirus S protein priming, which is crucial for viral entry into target cells and for viral spread in the infected host as published by Hoffman et al., in Cell, from the German Primate center [29]. Several clinical trials are ongoing comparing Camostat Mesylate as a single agent vs. placebo or in combination with other drugs such as Hydroxychloroquin [46,47].
Stage IIa: moderate pulmonary involvement without hypoxia and stage IIb with hypoxia
In this stage, pulmonary involvement is well settled, while we note remarkable viral multiplication and pulmonary localized inflammation. Viral pneumonia, with cough, fever and hypoxia (stage IIb: hypoxia defined as a PaO2/FiO2 of <300 mmHg) identify this period [8]. Chest imaging reveals bilateral infiltrates or ground glass opacities [48]. Laboratory tests show increasing lymphopenia while inflammation markers may be normal or slightly elevated [5].
Normally most hospitalizations occur in this phase. With advanced disease, the virus infects the lower respiratory tract leading to pneumonia and worsens symptoms such as dyspnea and hypoxemia [4,5,49].
Treatment usually relies on supportive measures and the aforementioned anti-viral therapies. In early parts of this stage (IIa), the use of corticosteroids in patients with COVID-19 may be avoided. While in stage IIb, the use of anti-inflammatory treatment may be permitted, indeed, the presence of hypoxia may indicate the high probability of unfavorable systemic evolution [5,8].
Coagulopathy seems to be another issue that affects the prognosis in more severe cases. Importantly, elevated d-dimer levels seem to be a marker to predict severe illness and mortality. Other laboratory findings like hrombocytopenia and prolonged prothrombin time also indicate a hyper-coagulation state in COVID-19 patients. The use of anticoagulant therapy has improved mortality rates in hospitalized patients with markedly elevated D-dimer or those who have sepsis-induced coagulopathy (SIC) score ≥4. With all the emerging evidence, current guidelines recommend a prophylactic dose of low molecular weight heparin (LMWH) for all hospitalized patients in the absence of contraindications [[50], [51], [52]]
In order to eliminate the virus, a good immune status is essential in this phase [4,53]. Even in elderly and immunocompromised patients, these phases tend to be respected, albeit in presenting different severe clinical manifestations [8]. Treatments potentially investigable in this stage are the following:
Chloroquine and hydroxychloroquine
Chloroquine (CQ) is a 4-aminoquinoline antimalarial agent that has proven anti-inflammatory and immunomodulatory activities. CQ also has anti-viral properties due to the following mechanisms: blocking virus/cell fusion; interfering with the glycosylation of cellular receptors, lysosomes and autophagosomes impairment; inhibiting viral enzymes (i.e. viral DNA and RNA polymerase); increasing pH of intracellular vesicles which interferes with pH-dependent viral replication [[54], [55], [56]].
Hydroxychloroquine (HCQ) is a derivative of CQ with similar characteristics but with better safety profile [56,57]. Both drugs have narrow therapeutic range which makes drug-toxicity probable especially with uncontrolled usage. Cardiac toxicity (QT interval prolongation) is the major, and even lethal, concern about usage of these drugs [54,58].
In vitro studies have demonstrated CQ efficacy against SARS-CoV-2 [55]. With its known safety profile, clinical investigations have been held in different countries; and at least 18 trials evaluating HCQ or CQ are currently underway worldwide [38,58].
A pilot randomized Chinese study [59] investigated HCQ in 30 confirmed COVID-19 cases. Fifteen patients received 400 mg of HCQ per day for 5 days, while others received the conventional treatment. The primary endpoint was COVID-19 nucleic acid negativity in respiratory pharyngeal swab on day 7 after randomization. The results were comparable between the study and the control group as of day 7. Throat swabs were negative in 13 (86.7 %) cases in the HCQ group and in 14 (93.3 %) cases in the control group (P > 0.05). Furthermore, the median duration from hospitalization to virus nucleic acid negative conversion and the median time for body temperature normalization were also comparable between the two groups.
These results are comparable to another Chinese open-label, randomized, controlled trial that included 150 hospitalized COVID-19 patients. HCQ dosage in this study was 1, 200 mg daily for 3 days with a maintenance dose of 800 mg daily for 2 or 3 weeks depending on illness severity. No difference regarding negative viral conversion was observed between the HCQ and the standard of care group, but significant clinical improvement was noticed in the HCQ group [60].
A preliminary Brazilian randomized, double-blinded, clinical trial has investigated two different CQ dosages (600 mg twice daily for 10 days vs. 450 mg for 5 days, twice daily only on the first day) in 81 patients with severe COVID-19. All patients received ceftriaxone and azithromycin as well. The high dosage CQ arm had higher QTc prolongation and mortality rates, with no apparent benefit of CQ regarding viral clearance or mortality in either study arms [61]. Of note, only 62 out of the enrolled 81 patients had been confirmed by RT-PCR.
On the other hand, data emerging from other ongoing Chinese trials have demonstrated that CQ phosphate is superior to a control treatment in the following areas: pneumonia exacerbation inhibition, imaging findings improvement, virus negative conversion promoting, and disease course shortening [62]. Additionally, a randomized Chinese cohort of 62 in-hospital patients with COVID-19 showed that HCQ may help shorten the time to clinical recovery [63]. Gautret, P. et al., reported promising results in two studies in France [64,65]. In these studies, researchers investigated the efficacy of HCQ in combination with azithromycin for the treatment of confirmed COVID-19 patients. In the first study, a significant reduction in viral carriage on day 6 post inclusion compared to control group was noticed. Meanwhile, the second study reports a rapid fall in nasopharyngeal viral load on day 7 (83 %) and day 8 (93 %) which was confirmed by PCR. In addition, virus cultures were negative in 97.5 % patients on day 5. This allowed rapid discharge with a mean length of stay of five days. HCQ dosage used in these studies was 600 mg per day for 10 days.
Combining all in-vitro and in-vivo data, some countries have authorized treating hospitalized patients, with some conditions, with CQ and HCQ [22,27,28]. However, there are serious concerns raised regarding its usage. For example, clinical data from reliable randomized controlled studies are still missing, and data published to date lacks homogeneity in terms of recommended dose concentration, treatment duration, and severity of patient illness [58].
Recently, the American Thoracic Society has released interim guidance on treating COVID-19. For hospitalized patients with pneumonia, HCQ and CQ may be used on a case-by-case basis, but clinicians must consider the potential benefit/risk ratio and the patient's condition must be severe. For outpatients with COVID-19 or hospitalized patients without pneumonia there are no specific recommendations [66]. In contrast, the Surviving Sepsis Campaign announced that there’s insufficient evidence to declare a recommendation on the use of CQ and HCQ in critically ill adults with COVID-19 [67]. The Infectious Diseases Society of America (IDSA), on the other hand, in its recently published guidelines [68] has recommended HCQ/CQ (+/-azithromycin) in the context of clinical trials only.
Ribavirin and IFNα-2b
Ribavirin is an antiviral compound. It works as a guanosine analogue and usually in combination with IFN (α, β). Preclinical studies with Ribavirin have shown promise, but clinical data in the treatment of MERS-CoV epidemic was conflicting. Interferons (α, β), however, demonstrated positive action against MERS-CoV [7,26]. Clinical data regarding this combination in the treatment of COVID-19 patients is still lacking, but several clinical trials are currently being conducted [38,69].
The WHO has launched a multinational trial called “solidarity trial”. This trial will test the four most promising coronavirus treatments remdesivir, lopinavir/ritonavir, lopinavir/ritonavir plus interferon b, Hydroxychloroquin with the aim to end the pandemic [70].
Ivermectin
Ivermectin is a broad spectrum anti-parasitic drug, but it has also shown anti-viral activity in vitro with its confirmed ability to inhibit integrase protein (IN) nuclear import and HIV-1 replication. It has also shown its ability to inhibit nuclear import of host and viral proteins [71,72]. A recent in vitro study tested the antiviral activity of ivermectin against SARS-CoV-2 [72]. Ivermectin treatment resulted in 99.8 % reduction in all viral material within 48 h compared to control samples. These results made the authors propose Ivermectin as a possible treatment for COVID-19 since it’s an FDA approved drug with a known safety profile.
Nitazoxanide
Nitazoxanide has shown in vitro activity against SARS CoV-2 with known broad antiviral activity against other viruses like influenza and rotavirus. The mechanism of action is believed to be due to interference with viral replication by targeting host regulated pathways rather than virus-specific pathways. In addition to its antiviral activity, it inhibits the production of pro-inflammatory cytokines TNF-, IL-2, IL-4, I-5, IL-6, IL-8 and IL10. No clinical information is yet available on the efficacy of Nitazoxanide in the treatment of COVID-19 [7,73].
Stage III: hyperinflammation
This is the most severe stage in this disease stage classification and luckily it concerns a fewer number of patients with ARDS and cytokine storm syndrome (CSS) being the hallmark of the pathogenesis [8,74]. In these severe cases, virus replication and tissue damage continue, especially in the lungs and other ACE2 expressing organs, which leads to an even higher increase in pro-inflammatory cytokines released by macrophages and granulocytes [49,53]. High levels of pro-inflammatory cytokines such as Interleukin 1b (IL-1b), IFN-c, IP-10, MCP-1, granulocyte macrophage colony stimulating factor (GM-CSF), platelet derived growth factor (PDGF), IL-4, and IL-10 are reported in patients infected with COVID-19. In addition, in severe cases of COVID-19 admitted to the ICU the following are present: increased IL-2, IL-6, IL-7, IL-8, IL-9, and IL-10; fibroblast growth factor (FGF); granulocyte colony stimulating factor (G-CSF); IP-10; macrophage inflammatory protein 1 alpha (MIP1A); tumor necrosis factor (TNFα). The cytokine profile in these severe cases indicates cytokine-release syndrome (CRS) which is a life-threatening condition that leads to ARDS the main cause of death in critically ill COVID-19 patients [25,53,[74], [75], [76]].
Among these cytokines, several have been suggested as a potential therapeutic target. IL-1, for example, is activated after the binding between COVID-19 and toll-like receptors (TLR). Released IL-1 mediates lung inflammation, fever and fibrosis. IL-1 is also known for its important role in the progression of pulmonary fibrosis [49]. IL-6 is also an important cytokine during respiratory viral infections, and it has been reported that, during this COVID-19 pandemic, it has been correlated with pulmonary infection severity in ICU patients [49,75]. Both IL-1 and IL-6 have been presented as potential therapeutic targets [74,75].
In addition to potential shock, stage III is also marked by extra-pulmonary involvement, such as vasoplegia, myocarditis, and organ failure. Treatment is essentially based on the use of immunomodulatory therapies in order to improve systemic inflammation and to block consequent organ failure. The use of corticosteroids may be helpful in this phase, generally in tandem with the use of cytokine inhibitors. Intravenous immune globulin (IVIG) may also be considered as an immune system modulator. Rapid application of such a treatment plan can potentially enhance patient prognosis, which is basically poor in this stage [8,77,78].
Corticosteroids
Using corticosteroids to treat severe pneumonia due to COVID-19 is still controversial. The WHO recommends against the routine use of corticosteroids in these patients [12]. Data from clinical trials are widely variable in terms of participants included and results reported, that’s why clinical judgment should be based on a case-by-case approach. Delayed viral clearance and infection susceptibility are two major concerns that come with corticosteroid usage in COVID-19 patients [7,45,69]. In a recent release, the Surviving Sepsis Campaign has suggested systemic corticosteroid administration for ARDS adult patients with COVID-19 who are mechanically ventilated. On the other hand, recommends against corticosteroid use in adults without ARDS [67]. Five randomized controlled trials investigating methylprednisolone in COVID-19 patients are also currently registered [38].
Immunomodulatory therapies
A- Bio- immune-modulatory treatments
Intravenous immunoglobulin IVIG
Immunoglobulins have been widely used in medical practice, and their use has shown clinical benefits in previous studies of SARS and MERS. IVIG is currently under investigation for treatment of COVD-19 [38,69,77]. A case series of 3 severe COVID-19 patients who received high-dose IVIG (0.3–0.5 g/kg/day) for five days was reported [77]. All patients experienced clinical improvement shortly after administration. However, other therapeutic agents were administered for these patients including antivirals and a short course of steroids.
In a pre-print retrospective study [78], authors reported the data of 10 severe COVID-19 patients who didn’t respond to a combination of low-dose corticosteroid (40−80 mg/d) and immunoglobulin (10 g/d) but have significantly improved after receiving a short-term moderated dose of corticosteroid (160 mg/d) plus immunoglobulin (20 g/d). Again, all patients received concomitant therapies including oxygen therapy, antiviral treatment, and antibiotic treatment.
Pentaglobin®
Considering the genetic relation between SARS-CoV-2 and SARS-CoV-1 some argue that it may be considered for further verification as a potential therapeutic, as 10 of 12 SARS patients were reported, in a 2004 publication, to have an uneventful recovery after treatment with this product [4,79].
Convalescent plasma
The FDA has recently approved convalescent plasma for serious or immediately life-threatening COVID-19 infections under emergency Investigational New Drug Application (eINDs) [80]. Convalescent plasma has been previously studied during other epidemics including H1N1 influenza virus pandemic, SARS-CoV-1 epidemic, and the MERS-CoV epidemic. Recently, a preliminary case series of five intubated COVID-19 patients with ARDS showed promising results. These patients received 400 ml of convalescent plasma containing neutralizing SARS-CoV-2–specific antibody (IgG) from recovered COVID-19 donors. All patients had gradual clinical and radiological improvement within 3 days and four patients no longer required respiratory support by day 9, viral loads also became negative within 12 days after transfusion. Seven clinical trials are currently registered [7,38,81].
B- Monoclonal antibodies
Tocilizumab
Tocilizumab is a monoclonal antibody (mAb) that inhibits the IL-6 Receptor. As mentioned earlier, IL-6 plays a key role in respiratory viral infections and in CRS which is linked to poorer prognosis and higher mortality rate in COVID-19 patients. Tocilizumab is approved for the treatment of rheumatoid arthritis and its safety and efficacy profile has previously been well studied [7,74].
Preliminary data [82] included cases of 21 severe and critical pneumonia in COVID-19 confirmed patients who showed clinical and radiological improvement in 75.0 % (severe pneumonia) and 90.5 % (critical pneumonia) of participants. Lymphocyte count also returned to normal in 52.6 % of cases on day 5 of drug administration. All patients received 400 mg of intravenous Tocilizumab once, and three patients, due to persistent fever, received another 400 mg dose after 12 h.
Despite promising primary data, when it comes to studies regarding optimal timing and IL-6 threshold of Tocilizumab administration during the course of COVID-19, information is still lacking from larger, controlled, long-term studies. Moreover, IL-6 monitoring might be an obstacle in some institutions, and its adverse effects bacterial infection susceptibility, neutropenia and thrombocytopenia should be considered when a clinical decision is to be made. Two clinical trials are ongoing right now which might provide further needed information [7,38].
Of note Surviving Sepsis Campaign has provided no recommendation regarding Tocilizumab use [67].
Leronlimab
Leronlimab is a humanized IgG4 monoclonal antibodies that blocks CC chemokine cellular receptor 5 (CCR5) and plays a key role in several immunological processes. Leronlimab is being evaluated for HIV and breast cancer treatment and it is believed to have an antiviral activity while mitigating the cytokine storm. Some claim that Leronlimab has achieved preliminary results in a few severe cases of COVID-19 [83,84].
Bevacizumab
Bevacizumab is an approved treatment for multiple cancers. It is a humanized monoclonal antibody that prevents the association between vascular endothelial growth factor (VEGF) and endothelial receptors Flt-1 and KDR, and it may reduce the levels of VEGF caused by hypoxia and severe inflammation. Eventually, it might suppress the edema in patients with COVID-19. Bevacizumab is currently being tested in a Chinese randomized controlled trial [7,69].
C-anti-interleukin 1
Anakinra®
Anakinra® is an IL-1 receptor antagonist. As mentioned earlier, IL-6 seems to have a key role in the respiratory viral infections and in progression of pulmonary fibrosis. So Anakinra® has been suggested as possible adjunctive treatment. But, to the best of our knowledge, no in-vitro or clinical data are available [7,49]
D-cell-based therapy
Expanded umbilical cord mesenchymal stem cells (US-MSC)
The Italian College of Anesthesia, Analgesia Resuscitation and Intensive Care has reported recent guidelines to treat coronavirus patients. It includes a statement that stem cells could have a serious potential to treat COVID-19 by decreasing the number of patients admitted to the ICU and discharging them quickly [85].
MSC can theoretically inhibit immune system overreaction and improve the microenvironment that promotes endogenous repair which could potentially protect alveolar epithelial cells. This would, in theory, prevent pulmonary fibrosis and also improve lung function [86,87].
The published studies are from China, including a small cohort 7 in the treatment group and 3 in the control group [86] and a case report [88]. Some expect that clinical trials may be, nowadays or very soon, recruiting. The available data shows some promise in managing COVID-19 illness, especially in critically ill patients that could be treated under a compassionate-use protocol [89].
CYNK-001
CYNK-001 (intravenous infusion of natural killer (NK) cells) is the name of yet another trial that has enrolled COVID-19 patients. It aims to boost immune systems of patients at risk of more severe disease and those starting to show symptoms by applying CYNK-001. The mechanism has been slowing virus replication [90].
Multiple other treatments are currently under investigation. The following are a few examples: Darunavir/cobicistat (HIV-1 protease inhibitor): Eculizumab (humanized monoclonal IgG antibody against complement protein C5); Favipiravir (RNA polymerase inhibitor); Nelfinavir (HIV-1 protease inhibitor); Sarilumab (IL-6 receptor antagonist); Sofosbuvir (Antiviral used to treat hepatitis C); TZLS-501 (anti-IL6R); Vitamin C; Siltuximab (Sylvant®; a chimeric mAb that targets IL-6); Xuebijing (XBJ), ribonucleoside analog β-D-N4-hydroxycytidine (NHC, EIDD-1931); zinc sulfate [7,69,[91], [92], [93]].
Prevention
Vaccines
An urgent response to the brisk expansion of COVID-19 has been evoked throughout scientific communities in order to develop an effective vaccine. Rapid genome sequencing and potential molecular targets have been identified, and more than 20 vaccines are currently under investigation. Some of these vaccines have shown effectiveness in preclinical studies, while still others are already in phase I clinical trials [[94], [95], [96]].
Until the final approval of a given vaccine and its effective distribution, an approach based on early case detection and isolating, laboratory testing, contact tracing and quarantining (as recommended by the WHO) seem the most effective way to prevent further spread of SARS-CoV-2 [6,97].
BCG vaccination policy and COVID-19
Growing evidence suggests a link between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19. Comparing Italy to Japan, for example, the first case of COVID-19 appeared in Japan earlier than in Italy, but Japan has maintained a low mortality rate despite not implementing restrictive social isolation measures [98]. Another study found that the mortality rate has been 4.28/million in countries with a BCG program compared to 40/million in countries without such a program [99].
To date, the efficacy of BCG vaccine on preventing COVID-19 is controversial due to many limitations, one being the reality of comparing countries in different phases of the pandemic. This association might be clearer with upcoming data.
Tuberculosis vaccine (VPM1002), on the other hand, is a new vaccine based on the old BCG vaccine. The idea is that in many studies conducted on mice, the vaccinated mice had lower influenza, lower serum viral load, and less lung damage. It is claimed that the BCG vaccine may activate the immune system against viruses and possibly decrease COVID-19 mortality rates [100].
Conclusion
While the complete pathophysiology of COVID-19 needs to be better understood, urgent research for rapid solutions based on already established knowledge is still ongoing.
For many physicians, these new potential strategies may be seductive. Although the disastrous situation currently faced by many countries may explain the attractiveness of such treatments, the urgent need for a cure does not justify any use which is unauthorized by national health regulatory authorities.
Meanwhile, as the world waits for a widely approved treatment, preventive interventions coupled with clear local and international management guidelines must be always respected in order to lessen the damage and permit more exhaustive and conclusive research to be conducted.
Declaration of Competing Interest
TA has received honorarium from Biotest France SAS. Biotest AG commercializes Pentaglobin®.
LA, BA, MS, AA declare no conflict of interest.
Acknowledgment
The authors thank Rachel Tipton, Hasan Iessa, PhD., and Danah El-Refaie for the proof reading of this manuscript and Layth Sliman for the technical support.
Contributor Information
Tamim Alsuliman, Email: dr.tameem.soliman@gmail.com.
Lugien Alasadi, Email: lugienalasadi@gmail.com.
Banan Alkharat, Email: Banankharat88@hotmail.com.
Micha Srour, Email: micha.srour@chru-lille.fr.
Ali Alrstom, Email: Ali.alrstom@spu.edu.sy.
References
- 1.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–Infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . 2020. Coronavirus disease 2019 (COVID-19) Situation Report – 92.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200421-sitrep-92-covid-19.pdf?sfvrsn=38e6b06d_4 (Accessed April 21, 2020) [Google Scholar]
- 3.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection—a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020:1–14. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan S.A., Sheikh F.N., Jamal S., Ezeh J.K., Akhtar A. Coronavirus (COVID-19): a review of clinical features, diagnosis, and treatment. Cureus. 2020 doi: 10.7759/cureus.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395:931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCreary E.K., Pogue J.M. COVID-19 treatment: a review of early and emerging options. Open Forum Infect Dis. 2020 doi: 10.1093/ofid/ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . 2020. Coronavirus disease 2019 (COVID-19) Situation Report – 46.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200306-sitrep-46-covid-19.pdf?sfvrsn=96b04adf_4 [Google Scholar]
- 11.2020. CDC COVID-19 response team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — united States, February 12–march 16; p. 2020. https://www.whitehouse.gov/wp-content/uploads/2020/03/03.16.20_ coronavirus-guidance_8.5x11_315PM.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO . 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected Interim guidance.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Google Scholar]
- 13.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.-Q.-Q. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5 doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C. medRxiv; 2020. Neutrophil-to-Lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. 2020.02.10.20021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020 doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO . 2020. Global surveillance for COVID-19 caused by human infection with COVID-19 virus Interim guidance.https://www.who.int/publications-detail/global-surveillance-for-human-infection-with-novel-coronavirus-(2019-ncov) [Google Scholar]
- 21.CDC . 2020. Updated guidance on evaluating and testing persons for coronavirus disease 2019 (COVID-19) 2020.https://emergency.cdc.gov/han/2020/HAN00429.asp?fbclid=IwAR1nH3P3F6mAkhF-cdBnJsZvYcqJIib7A54n6Ahfm8PcalwiWThe1KhSYiE (Accessed April 8, 2020) [Google Scholar]
- 22.China National Health Commission . 7th edition. 2020. Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment.http://kjfy.meetingchina.org/msite/news/show/cn/3337.html?fbclid=IwAR0dVMG0GjMgNP0J4iL4v4_2GuuviMQBMR0uP-A8eZiYIsSlvICAg3a9Owo 抗击新冠肺炎 (Accessed April 8, 2020) [Google Scholar]
- 23.Vincent J.-L.-L., Taccone F.S. Understanding pathways to death in patients with COVID-19. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30165-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020 doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020 doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FDA . 2020. Fact sheet for health care providers emergency use authorization (EUA) of hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of COVID-19 in certain hospitalized patients.https://www.fda.gov/media/136537/download [Google Scholar]
- 28.EMA . 2020. COVID-19: chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programmes.https://www.ema.europa.eu/en/documents/press-release/covid-19-chloroquine-hydroxychloroquine-only-be-used-clinical-trials-emergency-use-programmes_en.pdf [Google Scholar]
- 29.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yakoub-Agha I. Hydroxychloroquine in Covid-19: Does the end justify the means? Curr Res Transl Med. 2020 doi: 10.1016/j.retram.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko W.-C.-C., Rolain J.-M.-M., Lee N.-Y.-Y., Chen P.-L.-L., Huang C.-T.-T., Lee N.-Y.-I. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kujawski S.A., Wong K.K., Collins J.P., Epstein L., Killerby M.E., Midgley C.M. medRxiv; 2020. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. 2020.03.09.20032896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 38.Belhadi D., Peiffer-Smadja N., Lescure F.-X.-X., Yazdanpanah Y., Mentré F., Laouénan C. medRxiv; 2020. A brief review of antiviral drugs evaluated in registered clinical trials for COVID-19. 2020.03.18.20038190. [DOI] [Google Scholar]
- 39.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Favalli E.G., Biggioggero M., Maioli G., Caporali R. Baricitinib for COVID-19: a suitable treatment? Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–Angiotensin–Aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diaz J.H. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med. 2020 doi: 10.1093/jtm/taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng Z., Li J., Yao S., Yu Q., Zhou W., Mao X. medRxiv; 2020. The use of adjuvant therapy in preventing progression to severe pneumonia in patients with coronavirus disease 2019: a multicenter data analysis. 2020.04.08.20057539. [DOI] [Google Scholar]
- 45.2020. BC centre for disease control. Unproven therapies for COVID-19.http://www.bccdc.ca/Health-Professionals-Site/Documents/Guidelines_Unproven_Therapies_COVID-19.pdf [Google Scholar]
- 46.2020. The impact of camostat mesilate on COVID-19 infection - full text view - ClinicalTrials.gOv.https://clinicaltrials.gov/ct2/show/NCT04321096 (Accessed May 3, 2020) [Google Scholar]
- 47.2020. Combination therapy with camostat mesilate + hydroxychloroquine for COVID-19 - full text view - ClinicalTrials.gOv.https://clinicaltrials.gov/ct2/show/NCT04338906 (Accessed May 3, 2020) [Google Scholar]
- 48.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N. Chest CT findings in coronavirus Disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell B., Moss C., George G., Santaolalla A., Cope A., Papa S. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kearney J. 2020. Chloroquine as a potential treatment and prevention measure for the 2019 novel coronavirus: a review. [DOI] [Google Scholar]
- 55.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6 doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu T.Y., Frieman M., Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat Nanotechnol. 2020 doi: 10.1038/s41565-020-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Touret F., De Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020;177 doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.CHEN J., LIU D., LIU L., LIU P., XU Q., XIA L. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ. 2020;49 doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. medRxiv; 2020. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial. 2020.04.10.20060558. [DOI] [Google Scholar]
- 61.Borba M.G.S., Val F de A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M. medRxiv; 2020. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study) 2020.04.07.20056424. [DOI] [Google Scholar]
- 62.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 63.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D. medRxiv; 2020. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. 2020.03.22.20040758. [DOI] [Google Scholar]
- 64.Gautret P., Lagier J.-C.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Gautret P., Lagier J.-C.-C., Parola P., Hoang V.T., Meddeb L., Sevestre J. 2020. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: an observational study. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson K.C., Chotirmall S.H., Bai C., Rello J. COVID‐19: Interim Guidance on Management Pending Empirical Evidence. From an American Thoracic Society‐led International Task Force. Am Thorac Soc. 2020 [Google Scholar]
- 67.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020 doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C.-C. Infectious Diseases Society of America; 2020. Infectious diseases society of america guidelines on the treatment and management of patients with COVID-19. Version 1.0.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Revista Panamericana De Salud Pública. 2020;44:1. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.WHO . 2020. Solidarity” clinical trial for COVID-19 treatments.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments (Accessed April 7, 2020) [Google Scholar]
- 71.Wagstaff K.M., Sivakumaran H., Heaton S.M., Harrich D., Jans D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443:851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rossignol J.-F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health. 2016;9:227–230. doi: 10.1016/j.jiph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., qi Y. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020 doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao W., Liu X., Bai T., Fan H., Hong K., Song H. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou Z.-G.-G., Xie S.-M.-M., Zhang J., Zheng F., Jiang D.-X.-X., Li K.-Y.-Y. 2020. Short-term moderate-dose corticosteroid plus ImmunoglobuliK.-Y. Effectively reverses COVID-19 patients who have failed low-dose therapy. [DOI] [Google Scholar]
- 79.Ho J.C., Wu A.Y., Lam B., Ooi G.C., Khong P.L., Ho P.L. Pentaglobin in steroid-resistant severe acute respiratory syndrome. Int J Tuberc Lung Dis. 2004;8:1173–1179. [PubMed] [Google Scholar]
- 80.FDA; 2020. Research C for BE and. Revised information for investigational COVID-19 convalescent plasma.https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/revised-information-investigational-covid-19-convalescent-plasma (Accessed April 7, 2020) [Google Scholar]
- 81.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu X., Han M., Li T., Sun W., Wang D., Fu B. Chinaxiv; 2020. Effective treatment of severe COVID-19 patients with tocilizumab. https://doi.org/ChinaXiv:20200300026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaplon H., Muralidharan M., Schneider Z., Reichert J.M. Antibodies to watch in 2020. mAbs. 2020;12 doi: 10.1080/19420862.2019.1703531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.CytoDyn Inc . 2020. Leronlimab used in seven patients with severe COVID-19 demonstrated promise with two intubated patients in ICU, removed from ICU and extubated with reduced pulmonary inflammation.https://content.equisolve.net/_1c2b15dbeefa8a3170ccfdf955e184f3/cytodyn/news/2020-03-27_Leronlimab_Used_in_Seven_Patients_with_Severe_399.pdf [Google Scholar]
- 85.di Anestesia SocietàItaliana, Analgesia Rianimazionee Terapia Intensiva. 2020. RACCOMANDAZIONI DI ETICA CLINICA PER L’AMMISSIONE A TRATTAMENTI INTENSIVI E PER LA LORO SOSPENSIONE, IN CONDIZIONI ECCEZIONALI DI SQUILIBRIO TRA NECESSITÀ E RISORSE DISPONIBILI.https://www.siaarti.it/SiteAssets/News/COVID19%20-%20documenti%20SIAARTI/SIAARTI%20-%20Covid19%20-%20Raccomandazioni%20di%20etica%20clinica.pdf (Accessed April 9, 2020) [DOI] [PubMed] [Google Scholar]
- 86.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Behnke J., Kremer S., Shahzad T., Chao C.-M.-M., Böttcher-Friebertshäuser E., Morty R.E. MSC based therapies—new perspectives for the injured lung. J Clin Med. 2020;9:682. doi: 10.3390/jcm9030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang B., Chen J., Li T., Wu H., Yang W., Li Y. Chinaxiv; 2020. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. https://doi.org/chinaXiv:202002.00084v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Atluri S., Manchikanti L., Hirsch J.A. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Phys. 2020;23:E71–E83. [PubMed] [Google Scholar]
- 90.Center for Drug Evaluation and Research . 2020. Investigational new drug (IND) application. FDA.https://www.fda.gov/drugs/types-applications/investigational-new-drug-ind-application (Accessed April 7, 2020) [Google Scholar]
- 91.Gritti G., Raimondi F., Ripamonti D., Riva I., Landi F., Alborghetti L. medRxiv; 2020. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. 2020.04.01.20048561. [DOI] [Google Scholar]
- 92.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020:eabb5883. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.NIH . National Institutes of Health (NIH); 2020. NIH clinical trial of investigational vaccine for COVID-19 begins.https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins (accessed April 7, 2020) [Google Scholar]
- 95.Kim E., Erdos G., Huang S., Kenniston T.W., Balmert S.C., Carey C.D. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.WHO . 2020. DRAFT landscape of COVID-19 candidate vaccines.https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf?ua=1 (Accessed April 9, 2020) [Google Scholar]
- 97.WHO . 2020. Critical preparedness, readiness and response actions for COVID-19 Interim guidance.https://www.who.int/publications-detail/critical-preparedness-readiness-and-response-actions-for-covid-19 [Google Scholar]
- 98.Miller A., Reandelar M.J., Fasciglione K., Roumenova V., Li Y., Otazu G.H. medRxiv; 2020. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. 2020.03.24.20042937. [DOI] [Google Scholar]
- 99.Hegarty P., Kamat A., Zafirakis H., Dinardo A. 2020. BCG vaccination may be protective against Covid-19. [Google Scholar]
- 100.Angelidou A., Diray-Arce J., Conti M.G., Smolen K.K., Van Haren S.D., Dowling D.J. BCG as a case study for precision vaccine development: lessons from vaccine heterogeneity, trained immunity, and immune ontogeny. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]