Highlights
-
•
CCR5 regulates multiple cell types (e.g., T regulatory and Natural Killer cells) and immune responses.
-
•
The effects of CCR5, CCR5Δ32 (variant associated with reduced CCR5 expression) and CCR5 antagonists vary between infections.
-
•
CCR5 affects the pathogenesis of flaviviruses, especially in the brain.
-
•
The genetic variant CCR5Δ32 increases the risk of symptomatic West Nile virus infection.
-
•
The triad “CCR5, extracellular vesicles and infections” is an emerging topic.
Keywords: C-C chemokine receptor type 5, Chemokine, Host-pathogen interactions, Immunogenetics, Inflammation, Viral infection
Abstract
The interactions between chemokine receptors and their ligands may affect susceptibility to infectious diseases as well as their clinical manifestations. These interactions mediate both the traffic of inflammatory cells and virus-associated immune responses. In the context of viral infections, the human C-C chemokine receptor type 5 (CCR5) receives great attention from the scientific community due to its role as an HIV-1 co-receptor. The genetic variant CCR5Δ32 (32 base-pair deletion in CCR5 gene) impairs CCR5 expression on the cell surface and is associated with protection against HIV infection in homozygous individuals. Also, the genetic variant CCR5Δ32 modifies the CCR5-mediated inflammatory responses in various conditions, such as inflammatory and infectious diseases. CCR5 antagonists mimic, at least in part, the natural effects of the CCR5Δ32 in humans, which explains the growing interest in the potential benefits of using CCR5 modulators for the treatment of different diseases. Nevertheless, beyond HIV infection, understanding the effects of the CCR5Δ32 variant in multiple viral infections is essential to shed light on the potential effects of the CCR5 modulators from a broader perspective. In this context, this review discusses the involvement of CCR5 and the effects of the CCR5Δ32 in human infections caused by the following pathogens: West Nile virus, Influenza virus, Human papillomavirus, Hepatitis B virus, Hepatitis C virus, Poliovirus, Dengue virus, Human cytomegalovirus, Crimean-Congo hemorrhagic fever virus, Enterovirus, Japanese encephalitis virus, and Hantavirus. Subsequently, this review addresses the impacts of CCR5 gene editing and CCR5 modulation on health and viral diseases. Also, this article connects recent findings regarding extracellular vesicles (e.g., exosomes), viruses, and CCR5. Neglected and emerging topics in “CCR5 research” are briefly described, with focus on Rocio virus, Zika virus, Epstein-Barr virus, and Rhinovirus. Finally, the potential influence of CCR5 on the immune responses to coronaviruses is discussed.
1. Introduction
Inflammatory cells play a crucial role in protecting the host from viral infections. Leukocyte migration is a fundamental step of the inflammatory response to viruses, a process regulated by the interaction between chemokines and their receptors. Therefore, dysregulations in the chemokine-mediated inflammatory process may contribute to viral pathogenesis (Glass et al., 2003). The C-C chemokine receptor type 5 (CCR5) interacts primarily with the chemokines CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL5 (RANTES), which act as CCR5 agonists by stimulating cell migration and mediating inflammatory responses. On the other hand, the chemokine MCP-3/CCL7 is the main CCR5 antagonist ligand (Blanpain et al., 1999; Zlotnik and Yoshie, 2000; Glass et al., 2003; Alkhatib, 2009).
In addition to regulating the migration of non-specific leukocytes during inflammatory responses, CCR5 controls the action of specific cell types, including natural killer (NK) cells (Khan et al., 2006; Weiss et al., 2011) and regulatory T (Treg) cells (Wysocki et al., 2005; Tan et al., 2009; Dobaczewski et al., 2010). CCR5 is also expressed by tissue-resident memory T cells. These CCR5+ cells support barrier immunity (Davis et al., 2019).
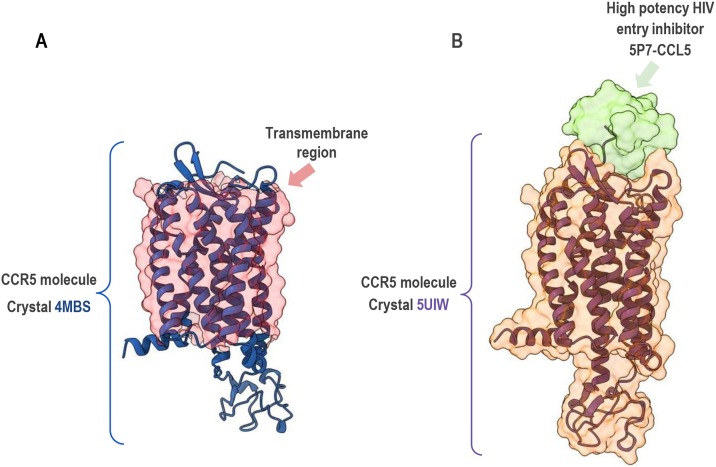
The CCR5 is a G-protein-coupled receptor (GPCR), containing seven transmembrane α-helices, three extracellular loops, and three intracellular loops (Tan et al., 2013). Fig. 1 shows the structure of CCR5, highlighting its transmembrane domains and extra and intracellular loops. The specificity of interaction between CCR5 and chemokines is mediated by the second extracellular loop (Samson et al., 1997). Helices 2 and 3 have a fundamental role in chemokine-induced CCR5 activation (Govaerts et al., 2003). The steps required from ligand binding culminating in cell migration encompass a series of intracellular interactions, including the G-protein heterotrimer and downstream effectors (Lacalle et al., 2017). After stimulation by chemokines or natural reactive antibodies and subsequent triggering of chemotaxis, CCR5 is phosphorylated and internalized in the cytoplasm (Signoret et al., 2000; Venuti et al., 2015, 2016; Lacalle et al., 2017; Venuti et al., 2017, 2018).
Fig. 1.
Crystal structure of CCR5. Crystal structure of CCR5 from RCSB PDB data bank (https://www.rcsb.org/) presented with ChimeraX molecular visualization program. A) 4MBS (CCR5, resolution 2.71 Angstroms) molecule, without contact with any ligand/inhibitor, display of the entire domain is shown in cartoon mode and colored in blue. The surface with transparency in red highlights only transmembrane regions of CCR5. B) 5UIW (CCR5 in complex with high potency HIV entry inhibitor 5P7−CCL5, resolution 2.20 Angstroms) molecule display of the entire domain is shown in cartoon mode and colored in purple. The surface with transparency (colored in orange) emphasizes subtle changes in the spatial conformation of the molecule when in contact with the inhibitor colored in green. Crystallography: Tan et al. (2013) for 4MBS and Zheng et al. (2017) for 5UIW.
The number of available receptors on the cell surface is related to the rate of internalization and recycling of CCR5, which affects the activation of CCR5 and consequent signaling of specific pathways that culminate in chemotaxis processes (Mueller et al., 2002). Of note, intracellular pools of CCR5 can be detected in the cells. These pools are probably formed by internalized or immature/precursor forms of CCR5 molecules (Mirzabekov et al., 1999; Kohlmeier et al., 2008; Achour et al., 2009; Guglielmi et al., 2011; Shirvani et al., 2011) that can be rapidly expressed on the cell surface in response to viral stimuli and inflammatory responses (Kohlmeier et al., 2008). In other words, CCR5 molecules are recycled by cells. Specifically, CCR5 recycling can be mediated by degradation followed by de novo synthesis (in response to stimulation by natural antibodies) or occur in the classic short-term system without de novo synthesis (in response to stimulation by CCL5, for example) (Venuti et al., 2015, 2016). The traffic of CCR5 between the plasma membrane and the intracellular medium is mediated by different molecules, including clathrins, β-arrestin 2, and extracellular signal-regulated kinase (ERK) 1 (Venuti et al., 2015, 2016; Venuti et al., 2018). Also, intracellular CD4 regulates the expression of CCR5 on the cell surface (Achour et al., 2009).
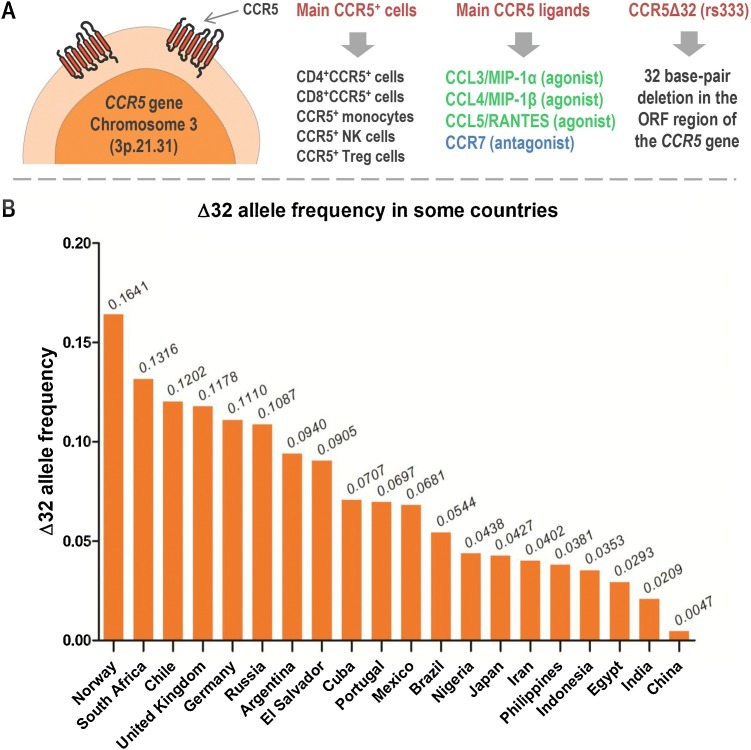
The human CCR5 protein (352 residues) is encoded by the CCR5 gene [Chromosome 3 (3p.21.31)], which is very polymorphic (Blanpain et al., 2000; Hoover, 2018). Among polymorphisms of the CCR5, the CCR5Δ32 (rs333) has been intensively studied in different human populations. The frequency of the CCR5Δ32 is quite variable. In general, the Δ32 allele frequency is high in European-derived populations (for example, 16 % in Norway and 11 % in Germany) and low or absent in African and Asian populations (Solloch et al., 2017). However, although the Δ32 allele is more frequent in European populations, there are exceptions due to migratory events. For example, the frequency of the Δ32 allele is high in South Africa (13 %) and Chile (12 %) (Solloch et al., 2017). Also, the frequency of the Δ32 allele can be quite variable within the same country. In Brazil, the frequency of the allele in the general population is around 4–5 % (Silva-Carvalho et al., 2016; Solloch et al., 2017). In the southern region of the country, the frequency can reach up to 9% due to the past migration of European populations to this region (Boldt et al., 2009; Pena et al., 2011; Schauren et al., 2013). Fig. 2 summarizes basic aspects of CCR5 and shows the frequency of the Δ32 allele in various countries.
Fig. 2.
Fundamental aspects of CCR5 (panel A) and frequency of the Δ32 allele in selected countries (panel B). This figure was created using Servier Medical Art illustrations (available at https://smart.servier.com, under a Creative Commons Attribution 3.0 Unported License). The bar chart was plotted using GraphPad Prism 5.01 software (GraphPad Software, Inc., San Diego, CA, USA). The Δ32 allele frequencies were obtained from Solloch et al. (2017).
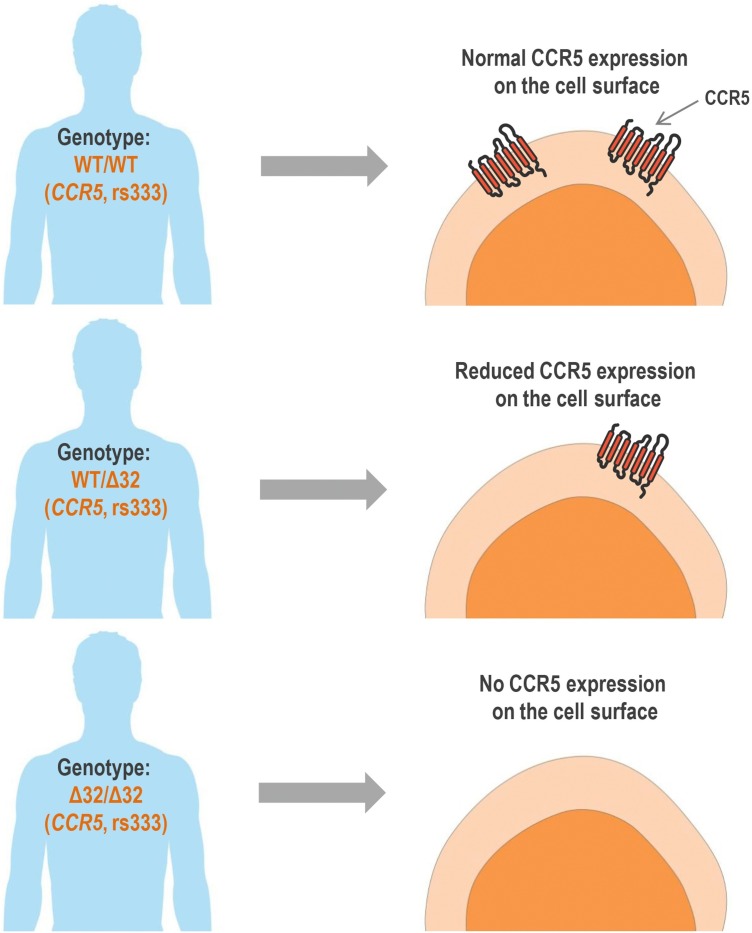
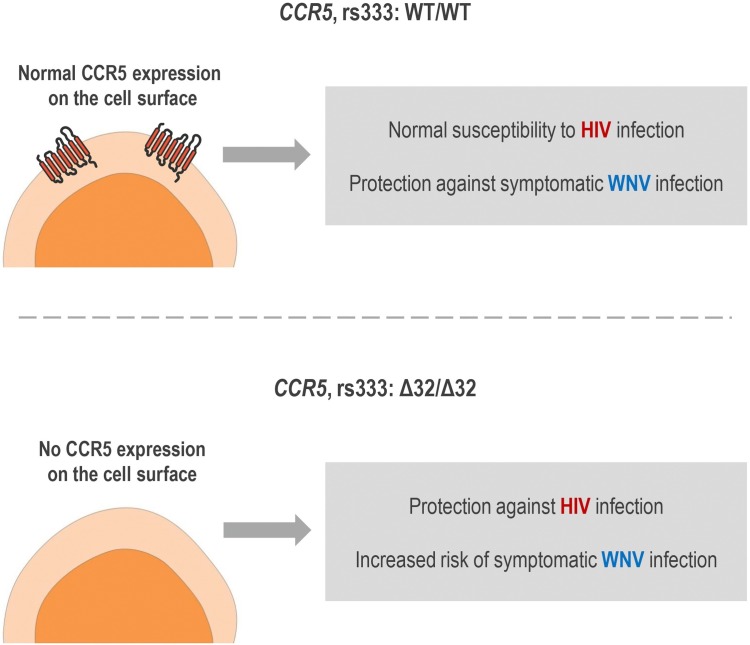
The CCR5Δ32 is the most studied genetic variant of the CCR5 gene because of its strong protective effect against HIV infection (considering susceptibility to CCR5-tropic strains). HIV entry into CD4+ T cells is mediated by the interaction of the virus with CD4 and with a co-receptor, usually CCR5. The CCR5Δ32 variant is a 32 base-pair deletion in the CCR5 coding region, which causes a frameshift, resulting in a truncated protein that is not directed to the cell surface. CCR5Δ32 in heterozygosis promotes a decrease in the expression of functional CCR5 on the cell surface compared to CCR5 wild-type cells. Therefore, individuals with heterozygous genotype for CCR5Δ32, if infected with HIV, have a small protection against disease progression due to the reduced expression of CCR5 on the surface of CD4+ T cells (reduced HIV−CCR5 interaction). In CCR5Δ32 homozygous cells, no CCR5 is expressed in the plasmatic membrane. Therefore, homozygous individuals for this polymorphism (Δ32/Δ32) show virtually total protection against HIV type 1 infection, since no CCR5 expression is verified on cell surface (no HIV−CCR5 interaction at cell surface is possible) (Deng et al., 1996; Dragic et al., 1996; Huang et al., 1996; Samson et al., 1996; Wu et al., 1997; Proudfoot, 2002; Venkatesan et al., 2002; Picton et al., 2012). Fig. 3 illustrates the phenotypic effects of CCR5Δ32 in human cells.
Fig. 3.
Phenotypic effects of the polymorphism CCR5Δ32 in human cells. WT/WT: wild-type homozygous genotype. WT/Δ32: heterozygous genotype. Δ32/Δ32: variant homozygous genotype. This figure was created using Servier Medical Art illustrations (available at https://smart.servier.com, under a Creative Commons Attribution 3.0 Unported License).
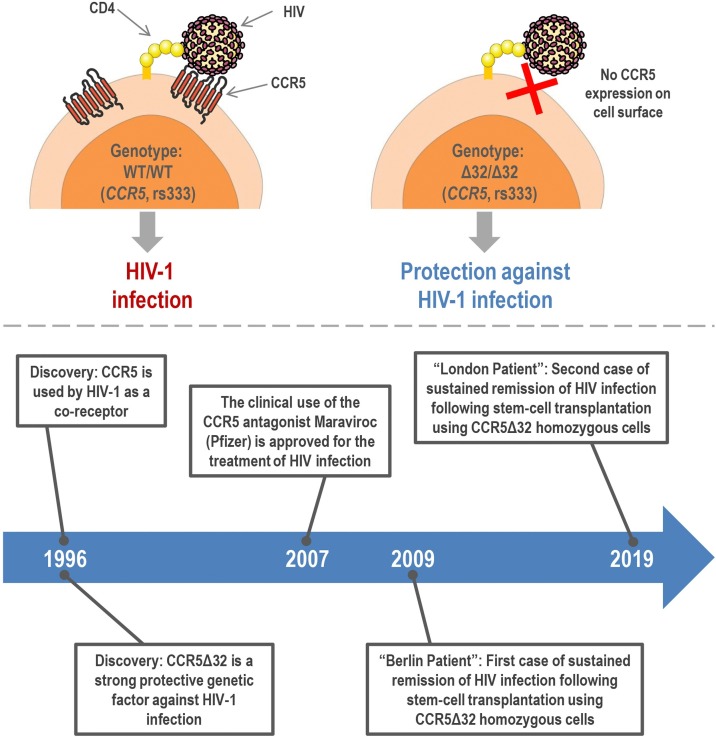
The main results involving the triad “CCR5, HIV, and CCR5Δ32″ were published in 1996 in Nature, Cell and Science papers by different groups (Parmentier, 2015). Since then, the research involving CCR5 has explored the role of the CCR5 protein and CCR5Δ32 polymorphism in different diseases, as well as the therapeutic potentials of CCR5 blockade. Currently, the physical interaction of CCR5 with HIV is known in detail (Shaik et al., 2019) and the research involving the impacts of CCR5Δ32 on HIV infection has led to the development of CCR5 antagonists: quite effective drugs used in the treatment of HIV-infected individuals, especially the licensed drug Maraviroc (Pfizer, Inc.), an allosteric modulator approved for clinical use in 2007 (Van Der Ryst, 2015; Latinovic et al., 2019). Also, a recent study has shown that molecules that inhibit CCR5 trafficking to the plasma membrane also have a therapeutic potential against HIV infection (Boncompain et al., 2019). Research involving CCR5 has also brought other important advances in combating HIV infection. Of note, there are already two cases of sustained remission of HIV infection following stem-cell transplantation using CCR5Δ32 homozygous donor, the “Berlin Patient” (Hütter et al., 2009) and the “London Patient” (Gupta et al., 2019, 2020). Fig. 4 summarizes the effects of CCR5Δ32 (homozygous genotype) on HIV infection and the main achievements of the research involving CCR5 and HIV infection.
Fig. 4.
Effects of the CCR5Δ32 (homozygous genotype) on HIV infection (upper panel) and the main achievements of the research involving CCR5 and HIV infection (bottom panel). This figure was created using Servier Medical Art illustrations (available at https://smart.servier.com, under a Creative Commons Attribution 3.0 Unported License).
Beyond the effects of CCR5/CCR5Δ32 on HIV infection, CCR5Δ32 impacts non-viral infectious diseases (Ungvári et al., 2007; Salnikova et al., 2013) and inflammation-related conditions (Chies and Nardi, 2001; Balistreri et al., 2009; Muntinghe et al., 2012; Kaminski et al., 2019a; Kulmann-Leal et al., 2020). Therefore, both the CCR5 molecule per se as well as the CCR5Δ32 variant, through the interference on the interaction of CCR5 with its ligands, may impact CCR5-mediated antiviral cellular activity and inflammatory reactions during host response against pathogens. Indeed, the CCL5−CCR5 axis influences virus-associated immune responses (Tyner et al., 2005; Grayson et al., 2007; Chen et al., 2017). Although the effects of CCR5Δ32 on CCR5 expression are clear, CCR5 and CCR5Δ32 have varied and even contrasting roles in different diseases. Due to interactions with other genetic variants as well as with environmental factors, the role of CCR5Δ32 may differ even within the same disease, depending on the ethnic group or population assessed (Rautenbach and Williams, 2020). Studying the involvement of CCR5 and the effects of CCR5Δ32 on infectious diseases may contribute to the understanding of the genetic factors involved in the susceptibility and progression of multiple viral diseases. Also, these studies can shed light on the use of CCR5 modulators/blockers in a broader perspective, beyond HIV infection. In this context, the aim of this review is to discuss the involvement of CCR5 and the effects of the CCR5Δ32 in human viral infections, covering West Nile virus, Influenza viruses, Human papillomavirus, Hepatitis B virus, Hepatitis C virus, Poliovirus, Dengue virus, Human cytomegalovirus, Crimean-Congo hemorrhagic fever virus, Enterovirus, Japanese encephalitis virus, and Hantavirus. This review also addresses the impacts of CCR5 gene editing and the CCR5 modulation on health and viral diseases. Recently, the role of extracellular vesicles has brought complexity to the fields of both immunology and virology. Therefore, this article connects recent findings regarding extracellular vesicles, viruses, and CCR5. In the last part of the review, neglected and emerging topics in “CCR5 research” are briefly discussed, with a focus on Rocio virus, Zika virus, Epstein-Barr virus, and Rhinovirus.
Studies involving HIV will not be included in this article because different aspects of the relationship between HIV and CCR5/CCR5Δ32 are well established and have already been intensely explored in different reviews (Allen et al., 2018; Brelot and Chakrabarti, 2018; Ndung’u et al., 2019). Also, the impacts of CCR5 and CCR5Δ32 on tick-borne encephalitis virus (TBEV) infection will not be addressed in this article because our group has recently reviewed these topics in a recent publication (Ellwanger and Chies, 2019a).
2. Methodological notes
This article is characterized as a narrative review. The selection of the articles was carried out from an initial search on PubMed (https://pubmed.ncbi.nlm.nih.gov/), using the terms “CCR5”, “CCR5Δ32”, “virus”, “viral infection”, “exosomes”, and “extracellular vesicles” (separately and in association). After extensive analysis of article abstracts, it was established that the review would address the following viruses: West Nile virus, Influenza viruses, Human papillomavirus, Hepatitis B virus, Hepatitis C virus, Poliovirus, Dengue virus, Human cytomegalovirus, Crimean-Congo hemorrhagic fever virus, Enterovirus, Japanese encephalitis virus, Hantavirus, Rocio virus, Zika virus, Epstein-Barr virus, and Rhinovirus. All original articles that evaluated the involvement of CCR5 or CCR5Δ32 in the infections caused by the mentioned viruses were analyzed. Subsequently, the Google Scholar (https://scholar.google.com.br/) was consulted to detect relevant papers that were not indexed in PubMed, using the terms “CCR5” in association with the name of each of the viruses covered in the review. The authors of this review tried to include the largest number of original articles that addressed the topic covered in this work, intending to write a broad and complete article. However, some papers were not included because it was not possible to obtain clear conclusions from the studies. The reference lists of the consulted articles were also used to complement the selection of articles for this review.
As previously mentioned in the introduction, a discussion addressing the involvement of CCR5 in TBEV infection was not included in this work. Articles addressing the involvement of CCR5 and CCR5Δ32 in HIV infection were included only to present to the reader some basic and historical aspects related to such topics, cited mainly in the introduction section and figures, but not included as a major section of the article. Considering the importance of the coronavirus disease 19 (COVID-19) pandemic, a section addressing the potential influence of CCR5 on the immune responses to coronaviruses was included in this article. Review articles were also selected in PubMed and Google Scholar for writing the sections and paragraphs that address the basic aspects of viruses, exosomes, and diseases (e.g., epidemiological, molecular, clinical aspects). Exceptionally, review articles with outstanding discussions regarding the role of CCR5 in viral infections were also cited in this work. Regarding tables, it is important to note that the data available in the “Population” columns are limited and often represent only general characteristics of the evaluated population. In many studies, a population can be composed of individuals from various ethnic groups. This limitation must be taken into account when evaluating the results of the studies cited in the tables. Finally, also in “Population” columns, “information not available” was used when this information was not clearly described in the cited article.
3. CCR5Δ32 and viral infections
3.1. West Nile virus
West Nile virus (WNV) is a neurotropic positive-sense single-stranded RNA flavivirus endemic in various parts of the world. WNV transmission to humans occurs through the bite of infected mosquitoes, especially species of the Culex genus (Suthar et al., 2013). Although different animals can participate in the WNV transmission cycle, birds are the classic amplifier hosts. Humans, horses and other mammals are dead-end hosts (Kramer et al., 2008; CDC, 2018). Among humans, blood transfusion, organ transplantation, and breast milk can also transmit the virus. Vertical transmission may also occur. However, compared to transmission by mosquito bites, these routes of transmission are rare (Kramer et al., 2008).
About 25 % of WNV-infected individuals develop West Nile fever, a clinical condition with variable symptoms and severity. In less than 1% of the infected individuals, WNV invades the central nervous system (CNS), causing neurological manifestations (neuroinvasive disease), including meningitis, encephalitis, and acute flaccid paralysis. Of note, West Nile neuroinvasive disease shows a 10%–20% fatality rate. Severe illness is associated with older age and other factors, including genetic traces (Petersen et al., 2013; Sejvar, 2016). WNV infection is considered the leading cause of arboviral encephalitis in the world (Ciota, 2017). The treatment of WNV infection is supportive (Petersen et al., 2013).
It is known that the CCR5 protein interferes in the clinical course of WNV infection (Glass et al., 2005; Diamond, 2009; Michlmayr and Lim, 2014), but the effects of CCR5Δ32 on the susceptibility to this infection and disease progression are different. According to Lim et al. (2010) and Danial-Farran et al. (2015), CCR5Δ32 has no important effect on susceptibility to WNV infection. In accordance, Loeb et al. (2011) found no association between CCR5Δ32 and WNV infection. Conversely, there is robust population-based data showing a strong association between the CCR5Δ32 homozygous genotype and increased risk of developing symptomatic WNV infection (Glass et al., 2006; Lim et al., 2008, 2010). Also, Bigham et al. (2011) showed that the CCR5Δ32 variant was associated with symptomatic WNV infection when the dominant model of inheritance was considered in the analysis. A recent meta-analysis confirmed the role of the CCR5 gene in WNV infection, specifically the association between the CCR5Δ32 with severe disease (Cahill et al., 2018). Table 1 summarizes the results of the studies that evaluated the CCR5Δ32 genetic variant in the context of human WNV infection.
Table 1.
Impacts of CCR5Δ32 on West Nile virus (WNV) infection.
| Population | Sample | Main findings | References |
|---|---|---|---|
| American | 395 symptomatic WNV + individuals; 145 symptomatic but non-infected individuals; 1318 controls (blood donors) | The CCR5Δ32 homozygous genotype was strongly associated with increased risk of symptomatic WNV infection | Glass et al. (2006) |
| American | 224 symptomatic WNV + individuals; 1318 controls (blood donors) | Corroborating data from Glass et al. (2006), the CCR5Δ32 homozygous genotype was strongly associated with increased risk of symptomatic WNV infection | Lim et al. (2008) |
| American | 634 WNV + individuals; 422 non-infected individuals | CCR5Δ32 homozygous genotype was associated with clinical symptoms of WNV infection; No association between CCR5Δ32 and susceptibility to WNV infection | Lim et al. (2010) |
| American, Canadian | 385 symptomatic WNV + individuals; 328 asymptomatic WNV + individuals; 1318 controls [blood donors from Glass et al. (2006)] | No association of CCR5Δ32 and WNV infection considering symptomatic and asymptomatic WNV + individuals; Considering a dominant model of inheritance of CCR5Δ32 and using controls from Glass et al. (2006), the CCR5Δ32 was associated with symptomatic WNV infection and infection risk | Bigham et al. (2011) |
| American, Canadian | 821 WNV + individuals with severe infection; 1233 individuals with non-severe infection | No association between CCR5Δ32 and WNV infection severity | Loeb et al. (2011) |
| Israeli (Ashkenazi Jews) | 39 symptomatic WNV + individuals; 61 non-infected individuals | No association between CCR5Δ32 and WNV infection | Danial-Farran et al. (2015) |
In agreement with studies showing that CCR5Δ32 homozygous genotype is a risk factor for symptomatic WNV infection in humans, Ccr5-/- WNV-infected mice showed a reduced capacity of viral control, increased disease severity, impaired leukocyte trafficking towards the brain, and high mortality rates than Ccr5 wild-type mice. These findings reinforce that Ccr5 is a key molecule in the immune response against WNV (Glass et al., 2005; Durrant et al., 2015). Taking together, these pieces of evidence robustly support the role of CCR5 as a protective molecule on WNV pathogenesis. Specifically, CCR5+ leukocytes play a fundamental role in combating WNV in the brain (Glass et al., 2005; Lim et al., 2006; Michlmayr and Lim, 2014; Durrant et al., 2015). In this sense, CCR5 plays a specific and non-redundant role in controlling WNV infection (Lim and Murphy, 2011; Durrant et al., 2015; Ellwanger et al., 2020a).
Based on the data mentioned above, the lack of CCR5 expression linked to CCR5Δ32 homozygosis is an important risk factor for increased severity of WNV-associated disease. The opposite effects of the CCR5Δ32 genetic variant on both HIV and WNV infections are summarized in Fig. 5 . Hereupon, individuals homozygous for CCR5Δ32 and living in endemic areas of the WNV should take additional care to prevent WNV infection (e.g., use of repellents, mosquito nets). Also, the use of CCR5 blockers to treat HIV infection may have a negative impact on populations living in WNV-endemic areas. To avoid this negative impact, HIV-infected individuals who live in such areas and who use CCR5 blockers must apply robust measures against mosquito bites (Glass et al., 2006; Lim et al., 2006; Lim and Murphy, 2011).
Fig. 5.
Effects of the polymorphism CCR5Δ32 on HIV and WNV infections. Upper panel: effects observed in individuals with the wild-type homozygous genotype (WT/WT). Bottom panel: effects observed in individuals with the homozygous genotype variant (Δ32/Δ32). This figure was created using Servier Medical Art illustrations (available at https://smart.servier.com, under a Creative Commons Attribution 3.0 Unported License).
3.2. Influenza virus
Influenza infection affects humans seasonally, causing recurrent epidemics and even pandemics in some years. In humans, the infection is caused basically by influenza A and influenza B, both enveloped negative-sense single-stranded RNA viruses belonging to Orthomyxoviridae family (Krammer et al., 2018; Petrova and Russell, 2018). Influenza A is a zoonotic disease, and influenza B circulates primarily in humans. Influenza infection affects mainly the respiratory tract, which can cause mild to severe disease depending on viral and host characteristics. Secondary bacterial infection may also occur (Krammer et al., 2018). Human co-infection with multiple influenza types is an important neglected problem (Gregianini et al., 2019). Influenza is a prevalent infection worldwide, and new vaccines are produced annually, based on strains circulating each year in the Northern and Southern hemispheres (Krammer et al., 2018; Petrova and Russell, 2018). Antivirals can be used in the treatment of influenza infection (Krammer et al., 2018). Investments in new vaccines, antiviral drugs, and surveillance systems are needed to reduce the global burden associated with influenza infection (Petrova and Russell, 2018).
The severity of influenza infection is related to the intensity of proinflammatory responses and the predominant profile of cytokine production by the host (Liu et al., 2016). A body of evidence has shown that both CCL5 and CCR5 participate in the modulation of the immune response to influenza virus infection (Matsukura et al., 1998; Tyner et al., 2005; Sládková and Kostolanský, 2006; Kohlmeier et al., 2008; Oslund and Baumgarth, 2011). Of note, CCR5 mediates the recruitment of NK cells to the lungs in influenza A infection (Carlin et al., 2018) and participates in neutrophil action in the lungs during influenza pneumonia (Rudd et al., 2019).
Although flow cytometry data did not indicate significant changes regarding CCR5 expression on the surface of human monocytes after experimental influenza A infection (Salentin et al., 2003), various studies have shown that, in mice, the lack of CCR5 expression is associated with a higher risk of death by influenza infection (Dawson et al., 2000; Tyner et al., 2005; Fadel et al., 2008; Tavares et al., 2020). Based on these findings, it was postulated that pharmacological CCR5 blockade may have some undesirable effect on the immune response against the influenza virus in humans (Fadel et al., 2008). Importantly, more research on this aspect is needed since this data suggests that, in humans, the CCR5 absence due to the CCR5Δ32 polymorphism could affect pathogenesis and the lethality rate of influenza infection.
Falcon et al. (2015) evaluated the frequency of the CCR5Δ32 in pandemic H1N1-infected Spanish individuals and revealed an association between the polymorphism with fatal outcome. The CCR5Δ32 was also associated with increased disease severity in other studies (Keynan et al., 2010; Rodriguez et al., 2013). However, these results should be interpreted with caution since both studies were based on very small sample sizes (Keynan et al., 2010; Rodriguez et al., 2013). Importantly, other studies addressing humans reported no association between CCR5Δ32 and severity of influenza infection (Sironi et al., 2014; Maestri et al., 2015; Matos et al., 2019). Taking together, the body of evidence suggests that CCR5Δ32 has little influence on severity of influenza infection (Table 2 ). Results described by Falcon et al. (2015) seem to be specific to that studied population, composed of individuals from 13 regions of Spain.
Table 2.
Impacts of CCR5Δ32 on Influenza virus infection.
| Population | Sample | Main findings | References |
|---|---|---|---|
| Canadian | 20 individuals infected with 2009 pandemic H1N1 | The CCR5Δ32 allele was considered a risk factor for severe infection among Caucasian individuals | Keynan et al. (2010) |
| Spanish | 1 fatal case and 1 mild disease case (2009 pandemic H1N1) | The patient who died from the infection had CCR5Δ32 homozygous genotype | Rodriguez et al. (2013) |
| Mostly European | 29 individuals (27 Italian; 1 Spanish; 1 Chinese) infected with 2009 pandemic H1N1 | No association between the CCR5Δ32 and H1N1 infection | Sironi et al. (2014) |
| Spanish | 171 individuals infected with 2009 pandemic H1N1 | The CCR5Δ32 was associated with increased risk for fatal outcome | Falcon et al. (2015) |
| Brazilian | 156 infected/hospitalized individuals; 174 infected (mild symptoms) but non-hospitalized individuals (2009 pandemic Influenza A H1N1) | No association between the CCR5Δ32 and H1N1 infection severity | Maestri et al. (2015) |
| Brazilian | 153 influenza-like illness cases; 173 severe acute respiratory infection cases; 106 fatal cases (2009 pandemic H1N1) | No association between the CCR5Δ32 and H1N1 infection severity or mortality | Matos et al. (2019) |
3.3. Human papillomavirus
Human papillomavirus (HPV) is a double-stranded DNA virus belonging to the papillomavirus family. HPV is transmitted by direct contact (for example, through sexual intercourse). The HPV infection is quite common worldwide, and is usually controlled by the immune system. Viral clearance occurs in most cases within 1–2 years after infection. However, if the infection is not controlled, HPV can cause non-cancerous mucosal lesions or different types of malignant lesions such as anal cancer, penile cancer, vulvar cancer, head cancer, neck cancer, and especially cervical cancer. HPV is an oncogenic pathogen because it inhibits the activity of p53 and Prb (retinoblastoma protein) tumor suppressor molecules through the action of E6 and E7 viral proteins, respectively. These proteins also exhibit other oncogenic mechanisms. Worldwide, between 5–10 % of all cancers in women are due to HPV infection (Schiffman et al., 2016; Sanjosé et al., 2018). There are several HPV genotypes (>200), and those most associated with cancer are: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and probably 68 (Schiffman et al., 2016). Of note, the genotype 16 (HPV16) has a prominent role in cancer development. Vaccination is one of the most effective ways to prevent HPV infection, having important positive impacts on multiple aspects of population health. HPV vaccine protects the vaccinated individual from infection per se and from HPV-related cancer (Schiffman et al., 2016; Sanjosé et al., 2018).
The expression of CCR5 is increased in cervical cancer tissues (Sales et al., 2014; Che et al., 2016). Also, in vitro growth, proliferation, and invasive capacity of cervical cancer cells can be inhibited through CCR5 downregulation (Che et al., 2016). These findings suggest the involvement of CCR5 in the development of cervical lesions. In a study performed with Indian individuals (Singh et al., 2008), the genotype and allele frequencies of CCR5Δ32 were not different between cervical cancer patients and controls. However, when the patients were stratified by cancer stage (stages 1B to 4), the CCR5 heterozygous genotype was associated with stage 1B of cervical cancer. The HPV positivity rate among the evaluated patients was not described (Singh et al., 2008).
The relationship between CCR5Δ32, HPV infection, and cervical lesions is addressed in other studies (Table 3 ). CCR5Δ32 homozygous genotype was associated with increased susceptibility to HPV infection in a study performed with Swedish individuals (Zheng et al., 2006). Mangieri et al. (2019) investigated the potential influence of CCR5Δ32 on susceptibility to HPV infection and cervical lesions in Brazilian women. However, the genetic variant was not significantly associated with susceptibility to HPV infection (considering allele frequency, codominant model and dominant model) or HPV-associated lesions (Mangieri et al., 2019). In accordance, previous studies performed with Brazilian women did not find an association between CCR5Δ32 and susceptibility to HPV infection (Suzuki et al., 2008) or between the polymorphism and HPV-related cervical lesions (Suzuki et al., 2008; Santos et al., 2016). Lastly, CCR5Δ32 did not affect susceptibility to HPV infection in Lithuanian individuals with laryngeal cancer (Stumbrytė-Kaminskienė et al., 2020). In conclusion, although tissue analysis and evidence obtained in vitro suggest that the CCR5 is potentially involved in the pathogenesis of HPV, most studies point to a lack of involvement of CCR5Δ32 in susceptibility to HPV infection or HPV-associated diseases.
Table 3.
Impacts of CCR5Δ32 on Human papillomavirus (HPV) infection.
| Population | Sample | Main findings | References |
|---|---|---|---|
| Swedish | 126 HPV-infected individuals; 173 non-infected individuals | CCR5Δ32 homozygous genotype was associated with increased risk of HPV infection | Zheng et al. (2006) |
| Brazilian | 74 HPV-infected individuals; 43 non-infected individuals | No association between CCR5Δ32 and HPV infection; No association between CCR5Δ32 and HPV-related cancer | Suzuki et al. (2008) |
| Brazilian | 139 HPV-infected individuals with cervical intraepithelial neoplasia or cervical cancer; 151 HPV-infected individuals without cervical lesions | No association between CCR5Δ32 and HPV-related lesions; No association between CCR5Δ32 and HPV genotypes | Santos et al. (2016) |
| Brazilian | 164 HPV-infected individuals; 185 non-infected individuals | No statistically significant effect of CCR5Δ32 on susceptibility to HPV infection or HPV-related cervical lesions (low‐grade squamous intraepithelial lesion or high‐grade squamous intraepithelial lesion) | Mangieri et al. (2019) |
| Lithuanian | 49 laryngeal squamous cell carcinoma patients (21 HPV-infected patients and 28 non-infected patients) | No statistically significant effect of CCR5Δ32 on susceptibility to HPV infection, clinical-pathological characteristics or surviving rates | Stumbrytė-Kaminskienė et al. (2020) |
3.4. Hepatitis B virus
The Hepatitis B virus (HBV) is a hepatotropic DNA virus member of the Hepadnaviridae family. In adults, approximately 95 % of HBV infections are resolved naturally (viral clearance). Individuals who have not cleared the virus after acute infection become chronic HBV carriers. Of note, chronic HBV infection affects 3.5 % of the world population. A portion of individuals who develop chronic infection have a variety of liver diseases: different levels of inflammation and fibrosis, cirrhosis, and cancer. The outcome of the infection (acute or chronic) as well as the course of liver diseases, depends on the interaction between host and viral factors. The pharmacological therapies available to treat HBV are not satisfactory, since frequently the infection is only controlled, and cure is rarely achieved. There is, therefore, a need to develop new anti-HBV therapies. Currently, HBV infection can be prevented through vaccination (Yuen et al., 2018; Lang et al., 2019; Lee and Banini, 2019).
The CCR5 and its ligands regulate the action of T cells and other leukocytes in the liver. Thus, CCR5 regulates liver inflammation and participates in the local immune response against viruses (Ajuebor et al., 2006; Sanchooli et al., 2014). In mice models of hepatitis, CCR5 deficiency was associated with increased liver inflammation, tissue injury, and liver failure (Ajuebor et al., 2005; Moreno et al., 2005; Stevens et al., 2019). Deficiency of CCR5 expression is generally associated with reduced migration of inflammatory cells, which would translate into less inflammation. This reasoning is correct and applies to different situations and tissues (Braunersreuther et al., 2007; Muntinghe et al., 2009; Kaminski et al., 2019a). However, CCR5 is an immunoregulatory molecule (Doodes et al., 2009; Dobaczewski et al., 2010; Christmann et al., 2011; Hütter et al., 2011) and therefore its deficiency can also cause deregulation in the action of various immune cell types (e.g., NK and Treg cells), increasing the inflammatory status in some tissues. In humans, multiple evidence has shown the involvement of CCR5 (protein and gene) in distinct aspects of HBV infection (Ahn et al., 2006; TrehanPati et al., 2009; Ahmadabadi et al., 2013; Yang et al., 2018). Interestingly, CCR5Δ32 and other host genetic factors can affect the immunogenicity of the HBV vaccine (Ganczak et al., 2017; Ellwanger and Chies, 2019b). Considering these aspects, the influence of the CCR5Δ32 on susceptibility/resistance to HBV and disease severity is quite plausible.
Several studies investigated the role of the CCR5Δ32 polymorphism in HBV infection. Some of them found no association between those variables (Arababadi et al., 2010; Khorramdelazad et al., 2013; Safari et al., 2017; Zhang et al., 2018; Moudi et al., 2019). In other studies, the absence of CCR5Δ32 allele in the sample, due to ethnic features of the evaluated population, precluded the analysis concerning the potential impact of this genetic variant on HBV infection (Ahn et al., 2006; Li et al., 2011).
Some authors have observed significant influences of CCR5Δ32 on HBV infection. Suneetha et al. (2006) reported an association between the CCR5Δ32 heterozygous genotype and chronic HBV infection. In contrast, Thio et al. (2007) found an association between the CCR5Δ32 allele and infection recovery in a study that analyzed individuals with persistent HBV infection and individuals who recovered from the infection. Subsequently, Thio et al. (2008) associated the HBV infection recovery with an epistatic effect between the CCR5Δ32 and the RANTES-403A promoter polymorphisms. Finally, Abdolmohammadi et al. (2016) found a protective effect of the CCR5Δ32 genetic variant against HBV infection, once the Δ32 allele was more frequent in controls compared to HBV-infected individuals.
Recently, we investigated the frequency of CCR5Δ32 in HBV mono-infected and HBV/HIV co-infected Brazilian individuals (Ellwanger et al., 2020b). A control group and HIV mono-infected individuals were also evaluated in our study. A total of 1113 individuals were studied, which represents the largest study involving CCR5Δ32 and HBV infection to date (see Table 4 for comparisons with other studies). We found a significant protective effect of CCR5Δ32 on HBV/HIV co-infection, a result probably due to the partial protective effect of CCR5Δ32 against HIV infection since no important impact of CCR5Δ32 on susceptibility to HBV mono-infection was observed (Ellwanger et al., 2020b).
Table 4.
Impacts of CCR5Δ32 on HBV infection.
| Population | Sample | Main findings | References |
|---|---|---|---|
| Korean | 349 chronic HBV-infected individuals; 243 individuals with spontaneous recover; 106 non-infected individuals | The CCR5Δ32 allele was not found in any studied individual | Ahn et al. (2006) |
| Information not available | 214 chronic HBV-infected individuals; 408 non-infected individuals | The CCR5Δ32 heterozygous genotype was associated with chronic HBV infection; The CCR5Δ32 wild-type allele was associated with severe liver disease when analyzed in combination with other genetic variants (a/a-T/T-Wt/Wt model from VDR-Apa1/Taq1/CCR5Δ32 polymorphisms) | Suneetha et al. (2006) |
| American | 190 individuals with persistent HBV infection; 336 individuals with infection recover | The CCR5Δ32 allele was associated with recovery from the infection | Thio et al. (2007) |
| American | 181 individuals with persistent HBV infection; 316 individuals with infection recover | Infection recovery was associated with an epistatic interaction between the CCR5Δ32 and the RANTES-403A polymorphism | Thio et al. (2008) |
| Iranian | 57 individuals with occult HBV infection; 100 non-infected individuals | The CCR5Δ32 heterozygous genotype was found in 3 controls and no individuals with occult HBV infection carried the allele (small sample size study); No association between the CCR5Δ32 and occult HBV infection | Arababadi et al. (2010) |
| Chinese | 185 individuals who underwent liver transplantation due to HBV-related disease | The CCR5Δ32 allele was not found in any studied individual | Li et al. (2011) |
| Iranian | 60 chronic HBV-infected individuals; 60 non-infected individuals | The CCR5Δ32 heterozygous genotype was found in 3 controls and no infected individual carried the allele (small sample size study); No association between the CCR5Δ32 and HBV infection | Khorramdelazad et al. (2013) |
| Iranian | 357 HBV-infected individuals; 455 non-infected individuals | The CCR5Δ32 was associated with protection against HBV infection | Abdolmohammadi et al. (2016) |
| Iranian | 60 chronic HBV-infected individuals; 120 individuals with infection recover | The CCR5Δ32 heterozygous genotype was found in 1 individual with infection recover and no HBV-infected individual carried the allele (small sample size study); No association between the CCR5Δ32 and HBV infection | Safari et al. (2017) |
| Chinese | 263 HBV-infected individuals; 141 non-infected individuals | No association between the CCR5Δ32 and HBV infection | Zhang et al. (2018) |
| Iranian | 100 chronic HBV-infected individuals; 40 individuals with infection recover; 100 non-infected individuals | No association between the CCR5Δ32 (as an individual factor) and HBV infection; The WtAGCC haplotype (from CCR5Δ32, CCR5−2459A/G, MCP-1−2518A/G, VDR-APa1A/C and VDR-Taq1T/C polymorphisms, respectively) was associated with HBV infection | Moudi et al. (2019) |
| Brazilian | 335 HBV mono-infected individuals; 144 HBV/HIV co-infected individuals; 300 HIV mono-infected individuals; 334 non-infected individuals | The CCR5Δ32 was a protective factor against HBV/HIV co-infection, but the CCR5Δ32 did not affect susceptibility or resistance to HBV mono-infection | Ellwanger et al. (2020b) |
3.5. Hepatitis C virus
The Hepatitis C virus (HCV) was formally described in 1989 (Choo et al., 1989) and, currently, HCV infection is one of the most important infectious diseases in terms of global public health burden. HCV is an enveloped single-stranded positive-sense RNA virus, has seven genotypes, and belongs to the Flaviviridae family, Hepacivirus genus (Pietschmann and Brown, 2019). It is estimated that 71 million individuals are chronically infected by HCV worldwide (Viganò et al., 2019).
Similar to the HBV infection, HCV-infected individuals can eliminate the virus naturally or develop chronic infection, which occurs in 55–85 % of the cases. Chronic infection can cause liver inflammation, cirrhosis, and hepatocarcinoma (Lingala and Ghany, 2015). In addition to liver damage, HCV causes a series of immune-mediated extrahepatic manifestations, including rheumatologic, dermatologic, ophthalmologic, renal, pulmonary, neuropsychiatric, cardiovascular, and hematologic manifestations, especially mixed cryoglobulinemia (Romano et al., 2018). HCV therapy using direct-acting antivirals (DAAs) shows cure rates over 95 % (Pietschmann and Brown, 2019). Early treatment of infected patients decreases death rates from HCV-associated liver disease, reduces disease transmission, and alleviates extrahepatic health problems. Focusing the efforts on HCV treatment is extremely important because there is no effective HCV vaccine (Viganò et al., 2019).
HCV seropositivity is an important risk factor for HIV infection (Zwolińska et al., 2013). Like HIV, HCV is primarily transmitted through blood transfusion and sexual intercourse, and HCV/HIV co-infection is a major problem worldwide. Depression of the immune system due to uncontrolled HIV infection may contribute to HCV progression (Schlabe and Rockstroh, 2018). Both susceptibility to HCV infection and disease progression are affected by viral and environmental factors and physio-metabolic, immune, and genetic components of the host (Ellwanger et al., 2018a).
Infiltration of inflammatory cells in the hepatic tissue is a classic observation in HCV infection. Chemokines and chemokine receptors participate in the recruitment and activity of inflammatory cells in the liver, acting on anti−HCV immune responses and ultimately modifying the rate of inflammation and other histological manifestations observed during infection. Based on this rationale, the CCR5 molecule was postulated as having an impact on HCV-induced liver injury, susceptibility to HCV infection, and modulation of the possibilities of viral clearance. The downregulation of CCR5 due to CCR5Δ32 may interfere in these processes (Ahlenstiel et al., 2004; Coenen and Nattermann, 2010). In agreement, several polymorphisms in other immune system genes [especially human leukocyte antigen (HLA), mannose-binding lectin (MBL), toll-like receptor (TLR), interleukins (IL), and interferon (IFN) gene families] indeed modify both susceptibility to HCV infection and disease progression (Ellwanger et al., 2018a). Especially the focus of this review, clinical response to HCV therapy is influenced by CCR5 gene polymorphisms (Konishi et al., 2004; Omran et al., 2013). Also, there is evidence showing that CCR5 haplotypes can affect susceptibility to HCV infection (Huik et al., 2013).
A recent in vitro study suggested that CCR5 blockage could have a beneficial effect on the treatment of HCV infection since CCR5 antagonists (maraviroc and cenicriviroc) inhibit HCV replication (Blackard et al., 2019). The use of CCR5 antagonists in humans is safe (Fätkenheuer et al., 2010; Gulick et al., 2014; Giaquinto et al., 2018) and these drugs have the potential to treat a number of diseases in which CCR5 is involved, including HCV-associated liver disease. Although the use of CCR5 antagonists on HCV mono-infection is not yet approved, it can be useful specifically for co-infected HIV/HCV patients, where CCR5 blocking (maraviroc) is already recommended (Haïm-Boukobza et al., 2013; Blackard et al., 2019). However, the detailed patterns of CCR5 expression in different tissues and at various points in the clinical course of HCV infection are still poorly understood. According to a recent study, the expression of CCR5 in CD8 + T cells is increased in the liver of chronic HCV-infected patients (Pirozyan et al., 2019), but other studies have found mixed results regarding CCR5 expression on T cells in the context of HCV infection (Lichterfeld et al., 2002; Vincent et al., 2005; Larrubia et al., 2007; Zahran et al., 2020). Therefore, considering that the role of CCR5 in HCV infection is still uncertain, the potential use of CCR5 blockers to treat HCV mono-infection should be cautious.
The influence of the CCR5Δ32 variant on HCV infection susceptibility was investigated by several studies. Woitas et al. (2002) found a significantly higher frequency of the CCR5Δ32 homozygous genotype in HCV-infected individuals compared to controls, HIV-infected and HCV/HIV co-infected individuals, suggesting the CCR5Δ32 as a risk factor for HCV infection. In agreement, the Δ32 allele was a significant risk factor for infection when the authors compared the HCV-infected group to both controls and HIV-infected individuals. Moreover, the CCR5Δ32 homozygous genotype was associated with increased HCV loads. In their study, it was observed an important deviation from the Hardy-Weinberg equilibrium in data from HCV-infected individuals; and a high portion of the individuals included in the study was hemophiliac (Woitas et al., 2002). Hemophiliac individuals were at high risk of exposure to HCV and HIV until the mid-1980s (Promrat et al., 2003; Zhang et al., 2003). Considering that the CCR5Δ32 homozygous genotype provides protection against HIV infection, a high frequency of this genotype in an HCV-infected group may be due to HIV resistance, but not to HCV, among individuals highly exposed to both viruses. Due to those and other reasons, the results of Woitas et al. (2002) were criticized by different authors (Klein, 2003; Mangia et al., 2003; Poljak et al., 2003; Promrat et al., 2003; Zhang et al., 2003). In this sense, no influence of CCR5Δ32 on susceptibility to HCV infection were reported in studies performed with various populations (Glas et al., 2003; Mangia et al., 2003; Poljak et al., 2003; Promrat et al., 2003; Zhang et al., 2003; Ruiz-Ferrer et al., 2004; Wald et al., 2004; Wasmuth et al., 2004; Thoelen et al., 2005; Goyal et al., 2006). Reinforcing the observations of those different studies, our group found no association between the CCR5Δ32 and susceptibility to HCV infection or HCV/HIV co-infection in a study that evaluated a large number of Brazilian individuals (Ellwanger et al., 2018b). Bineshian et al. (2018) did not detect the CCR5Δ32 allele in any Iranian HCV-infected individual and controls included in their study, preventing any conclusion in terms of susceptibility in that population. Finally, it is important to mention that in addition to the study by Woitas et al. (2002), a study performed in 2013 also found an association between the CCR5Δ32 homozygous genotype with chronic HCV infection in Europeans, but the authors of the study mentioned that specific factors regarding selection bias (e.g., co-exposure to HIV) may have influenced their results (Suppiah et al., 2013). Taken together, the above-mentioned results called attention for the importance of performing genetic variant studies in different populations, exposed to different social and environmental factors and presenting distinct ethnic backgrounds.
Considering multiple clinical and histological parameters, two main different results were obtained when the CCR5Δ32 genetic variant was evaluated in the context of HCV-related diseases: reduced liver inflammation in Δ32 allele carriers (Hellier et al., 2003; Wald et al., 2004; Goulding et al., 2005); and no association between the CCR5Δ32 and clinical variables (Glas et al., 2003; Mangia et al., 2003; Promrat et al., 2003; Goyal et al., 2006; Mascheretti et al., 2004; Ruiz-Ferrer et al., 2004; Morard et al., 2014; Ellwanger et al., 2018b).
In the context of persistence/resolution of HCV infection and viral control, in the one hand, studies described association of the CCR5Δ32 allele with reduced rates of spontaneous viral clearance (Nattermann et al., 2011; Morard et al., 2014), higher viral load (Yilmaz et al., 2014), and reduced anti−HCV immune response (Ahlenstiel et al., 2009). On the other hand, studies have reported association between the CCR5Δ32 variant and increased rates of spontaneous viral clearance (Goulding et al., 2005; El-Moamly et al., 2013). No significant effect of the CCR5Δ32 on viral clearance was reported by other authors (Mascheretti et al., 2004).
The potential impact of the CCR5Δ32 polymorphism on response to HCV therapy was also evaluated by some authors. Ahlenstiel et al. (2003) found an association between the CCR5Δ32 allele and reduced response rates to interferon-α monotherapy, but the polymorphism did not affect the response to the combined interferon/ribavirin therapy. This finding shows that the use of more robust therapeutic regimens compensates the undesirable effects of CCR5Δ32 on HCV therapy with interferon-α monotherapy. Of note, the effect of CCR5Δ32 may be negligible in the context of modern HCV therapies (Ahlenstiel et al., 2003). Other studies did not report significant effects of the polymorphism on response to HCV therapy (Glas et al., 2003; Promrat et al., 2003; Goyal et al., 2006; Mascheretti et al., 2004; Suppiah et al., 2013; Morard et al., 2014). Again, it is important to mention that the ethnic distribution of the CCR5Δ32 allele could interfere with study results. Konishi et al. (2004), for example, did not detect the CCR5Δ32 allele in any Japanese individual included in their study focused on host genetic factors involved in the response to interferon therapy. Lastly, the polymorphism also does not appear to be associated with any specific HCV genotype (Glas et al., 2003; Wasmuth et al., 2004; Goyal et al., 2006).
Already in 2004, Ahlenstiel et al. (2004) highlighted that the impact of CCR5 on HCV infection was controversial. In 2020, many controversies remain, although some points are better defined. Table 5 summarizes the main findings of the studies that evaluated CCR5Δ32 on HCV infection. Although some studies indicate an influence of the polymorphism on susceptibility to infection, most studies indicate that the Δ32 allele has little (or no) influence on HCV susceptibility. The impact of the CCR5Δ32 on HCV-related liver disease is quite variable and context-dependent. Finally, available data suggest some benefit of CCR5 antagonists for the treatment of HCV mono-infection. However, these data are still limited and further studies evaluating this topic are needed.
Table 5.
Impacts of CCR5Δ32 on HCV infection.
| Population | Sample | Main findings | References |
|---|---|---|---|
| German | 153 HCV-infected individuals; 102 HIV-infected individuals; 130 HCV/HIV co-infected individuals; 102 non-infected individuals | The CCR5Δ32 polymorphism (Δ32 allele and homozygous genotype) was associated with increased susceptibility to HCV infection; The CCR5Δ32 homozygous genotype was associated with increased HCV loads | Woitas et al. (2002) |
| German | 156 chronic HCV-infected individuals | The CCR5Δ32 allele was associated with reduced response rates to interferon-α monotherapy | Ahlenstiel et al. (2003) |
| Caucasian | 62 chronic HCV-infected individuals; 119 non-infected individuals | No association between the CCR5Δ32 and susceptibility to HCV infection, HCV-related liver disease or therapy response | Glas et al. (2003) |
| European | 544 HCV-infected individuals with persistent infection; 128 individuals who cleared the virus (547 individuals were genotyped for CCR5Δ32) | The CCR5Δ32 polymorphism was associated with reduced portal inflammation and increased liver fibrosis | Hellier et al. (2003) |
| Information not available | 235 chronic HCV-infected individuals; 96 non-infected individuals | No association between the CCR5Δ32 and susceptibility to HCV infection or HCV-related disease progression | Mangia et al. (2003) |
| Slovenian | 150 HCV-infected individuals; 101 HIV-infected individuals; 385 non-infected individuals | No association between the CCR5Δ32 and susceptibility to HCV infection | Poljak et al. (2003) |
| American | 417 individuals with liver disease (including 339 HCV-infected individuals); 2380 non-infected individuals | No association between the CCR5Δ32 and susceptibility to HCV infection, HCV-related liver disease or therapy response | Promrat et al. (2003) |
| Caucasian (American; European) | 1419 hemophiliacs (stratified in the analyzes according to serological status for HCV and HIV) | No association between the CCR5Δ32 and susceptibility to HCV infection; This study indicated that the results of Woitas et al. (2002) was affected by the inclusion of hemophiliacs in the HCV-infected group | Zhang et al. (2003) |
| Japanese | 105 chronic HCV-infected individuals; 53 non-infected individuals | The CCR5Δ32 allele was not detected in any individuals included in the study | Konishi et al. (2004) |
| German | 257 chronic HCV-infected individuals; 250 non-infected individuals | No association between the CCR5Δ32 and susceptibility to HCV infection, HCV-related liver disease or therapy response | Mascheretti et al. (2004) |
| Spanish | 139 HCV-infected individuals; 100 non-infected individuals | No association between the CCR5Δ32 and susceptibility to HCV infection or liver injury | Ruiz-Ferrer et al. (2004) |
| Israeli | 127 chronic HCV-infected individuals; 48 HCV-infected individual who had undergone liver transplantation due to liver cirrhosis; 75 non-infected individuals | No association between the CCR5Δ32 and susceptibility to HCV infection; The CCR5Δ32 allele was associated with reduced liver inflammation | Wald et al. (2004) |
| German | 333 HCV-infected individuals; 125 non-infected individuals | No association between the CCR5Δ32 and susceptibility to HCV infection; No major association between the CCR5Δ32 and HCV-related liver disease or therapy response | Wasmuth et al. (2004) |
| Irish | 196 chronic HCV-infected individuals; 88 individuals who cleared the virus; 120 non-infected individuals | The CCR5Δ32 polymorphism was associated with HCV clearance and less severe hepatic inflammatory scores | Goulding et al. (2005) |
| Belgian | 163 HCV-infected individuals; 310 non-infected individuals | No association between the CCR5Δ32 and susceptibility to HCV infection | Thoelen et al. (2005) |
| Information not available | 252 chronic HCV-infected individuals; 408 non-infected individuals | No association between the CCR5Δ32 and susceptibility to HCV infection, HCV-related liver disease or therapy response | Goyal et al. (2006) |
| Information not available | 21 HCV-infected hemophiliacs | The CCR5Δ32 allele was associated with reduced anti-viral (mediated by interferon-γ) response | Ahlenstiel et al. (2009) |
| German | 277 chronic HCV-infected individuals; 119 individuals who cleared the virus; 105 non-infected individuals | The CCR5Δ32 wild-type genotype was associated with spontaneous viral clearance. Therefore, CCR5Δ32 allele can be considered a risk factor for persistent infection | Nattermann et al. (2011) |
| Egyptian | 190 Schistosoma mansoni/HCV co-infected individuals; 220 S. mansoni-infected individuals | The CCR5Δ32 allele was associated with spontaneous viral clearance in co-infected individuals | El-Moamly et al. (2013) |
| Australian; European | 813 chronic HCV-infected individuals; 836 non-infected individuals | The CCR5Δ32 homozygous genotype was associated with chronic HCV infection (especially in Europeans); No association between the CCR5Δ32 and therapy response | Suppiah et al. (2013) |
| Swiss; Italian | 1290 chronic HCV-infected individuals; 160 individuals who cleared the virus | The CCR5Δ32 allele was associated with decreased rates of spontaneous viral clearance; No association between the CCR5Δ32 and HCV-related liver disease or therapy response | Morard et al. (2014) |
| Turkish | 58 chronic HCV-infected individuals; 58 non-infected individuals | The CCR5Δ32 allele was associated with higher HCV load and reduced histology activity index in liver samples | Yilmaz et al. (2014) |
| Iranian | 100 HCV-infected individuals; 100 non-infected individuals | The CCR5Δ32 was not detected in any individuals included in the study | Bineshian et al. (2018) |
| Brazilian | 674 HCV-infected individuals; 104 HCV/HIV co-infected individuals; 300 HIV-infected individuals; 274 non-infected individuals | No association between the CCR5Δ32 and susceptibility to HCV infection or HCV/HIV co-infection; No association between the CCR5Δ32 and HCV-related fibrosis, cirrhosis or hepatocarcinoma | Ellwanger et al. (2018b) |
3.6. Poliovirus
Poliovirus (PV) is a single-stranded RNA enterovirus of the family Picornaviridae. There are three types of PV: wild PV type 1 (WPV1), type 2 (WPV2), and type 3 (WPV3). The PV replicates in the tonsils and intestinal tract. In few infection cases (∼1%), the virus invades the CNS and can cause poliomyelitis resulting in paralysis. Poliomyelitis is a condition characterized by inflammation of the gray matter of the spinal cord and muscle paralysis unleashed by PV replication in motor neurons (Racaniello, 2006; Kew and Pallansch, 2018; Keohane et al., 2020). Polioencephalitis can also occur and is characterized by the inflammation of the gray matter of the brain (Keohane et al., 2020). PV infection is preventable through vaccination. The global PV vaccination program (Global Polio Eradication Initiative) has virtually eliminated cases of PV-derived paralysis, which currently occur to a limited extent in some countries, such as Pakistan and Afghanistan, and are caused by WPV1, while WPV2 and WPV3 have not circulated in human populations since 1999 and 2012, respectively (Kew and Pallansch, 2018).
Host genetics and profile of immune responses affect the course of PV infection, determining if a particular individual will have neurological impairment (Andersen et al., 2019). Considering that CCR5 affects the course of other neuroinvasive viral infections such as TBEV and WNV, it was hypothesized that CCR5Δ32 could influence the development of neurological impairment associated with PV infection, as this only occurs in a portion of infected individuals (Rosenberg et al., 2013). In this context, Rosenberg et al. (2013) evaluated the effect of the CCR5Δ32 on severe cases of PV infection through a retrospective study performed in Finland, including rare samples of individuals with neurological symptoms from the 1984–1985 PV outbreak occurred in that country (Table 6 ). The authors found no statistically significant effect of the CCR5Δ32 on PV infection; only a trend of association between the Δ32 allele and increased risk of PV infection was observed (Rosenberg et al., 2013). However, this study had a very small sample size (only seven cases of severe PV infection were evaluated) and therefore the results were not conclusive. In addition, due to the declining number of PV infection cases in the world, the effect of CCR5Δ32 will be increasingly difficult to be assessed in population-based studies.
Table 6.
Impacts of CCR5Δ32 on various viral infections.
| Pathogen/Disease | Population | Sample | Main findings | References |
|---|---|---|---|---|
| Poliovirus (PV) infection | Finnish | 7 PV-infected individuals with neurological symptoms;79 non-infected individuals or asymptomatic infected individuals | No association between CCR5Δ32 and severe PV-associated neurologic disease (small sample size study) | Rosenberg et al. (2013) |
| Dengue virus (DENV) infection | Brazilian | 87 DENV-infected children (severe cases); 326 controls | No statistical association between CCR5Δ32 and DENV infection | Xavier-Carvalho et al. (2013) |
| Australian | 56 DENV-infected individuals; 91 non-infected individuals | No statistical association between CCR5Δ32 and DENV infection | Brestovac et al. (2014) | |
| Indian | 42 DENV-infected individuals (33 mild cases and 9 severe cases); 90 non-infected individuals | The CCR5Δ32 allele was not detected in any individuals included in the study | Islam et al. (2019) | |
| Human cytomegalovirus (CMV) infection | European-American; African-American | 203 European-American and 117 African-American individuals with AIDS and CMV retinitis | No statistical association between CCR5Δ32 (as an individual factor) and mortality, retinitis progression or retinal detachment | Sezgin et al. (2011) |
| Polish | 72 children with intrauterine CMV infection; 398 non-infected children | No statistical association between CCR5Δ32 and CMV infection | Kasztelewicz et al. (2017) | |
| Crimean-Congo hemorrhagic fever virus (CCHFV) infection | Turkish | 3 CCHFV fatal cases; 12 CCHFV non-fatal cases | All individuals had the wild-type genotype | Engin et al. (2009) |
| Turkish | 133 CCHFV-infected individuals; 97 non-infected individuals | The CCR5Δ32 heterozygous genotype and the Δ32 allele were associated with protection against CCHFV infection (both factors found in increased frequency in control group) | Rustemoglu et al. (2017) | |
| Enterovirus (EV) infection | German | 97 individuals with enteroviral cardiomyopathy (23 individuals with persistent EV infection; 42 individuals who spontaneously cleared the virus; 32 individuals with persistent EV infection who received interferon-β therapy to clear the virus) | CCR5Δ32 was associated with spontaneous viral clearance and better clinical outcome (all Δ32 allele carriers showed viral clearance and none of them died during the study period) | Lassner et al. (2018) |
| Japanese encephalitis virus (JEV) infection | Indian | 183 JEV-infected individuals; 361 non-infected individuals | No statistical association between the CCR5Δ32 and Japanese encephalitis considering the CCR5Δ32 as an individual factor | Deval et al. (2019) |
| Nephropathia epidemica (linked to hantavirus infection) | Russian (Republic of Tatarstan) | 98 nephropathia epidemica cases; 592 controls | CCR5Δ32 did not affect susceptibility to hantavirus infection. However, the wild-type homozygous genotype was associated with more severe disease | Kletenkov et al. (2019) |
3.7. Dengue virus
Dengue virus (DENV) is a single-stranded RNA virus that belongs to the Flaviviridae family, Flavivirus genus. There are four DENV serotypes, being all transmitted to humans by Aedes mosquitoes (Aedes aegypti and Aedes albopictus) (Guzman et al., 2016). DENV infection is a global health problem with huge impacts on public health systems, especially in tropical countries (Bhatt et al., 2013), being considered the most common arbovirosis in the world (Stanaway et al., 2016). Globally, the incidence of symptomatic DENV infection is within the range of 50–100 million cases per year, resulting in ∼10,000 deaths each year (Stanaway et al., 2016).
Clinically, dengue illness is divided into three basic phases: acute febrile phase, critical phase, and recovery (convalescent) phase. Dengue disease occurs with/without warning signs or severe dengue. Warning signs (suggestive signs or symptoms of important fluid loss, capillary leakage, and shock, such as severe abdominal pain and mucosal bleeding; observed at the end of the febrile phase) allow the rapid identification of patients who need more clinical attention and supportive therapy, in an attempt to avoid severe dengue. When severe disease occurs, this condition can lead to serious organ involvement, shock, and hemorrhage, among other signals and symptoms (Guzman et al., 2016). Infection with a DENV serotype triggers long-term immunity to that specific serotype (homotypic DENV). Immunity to heterotypic DENV also occurs, but it is transitory. Therefore, an individual can have dengue disease more than once. Severe dengue occurs more frequently in recurrent infection with a different viral serotype (Murphy and Whitehead, 2011; St John and Rathore, 2019).
The immune response mediates DENV clearance and the resolution of dengue diseases, but it is also involved in the disease pathogenesis (Murphy and Whitehead, 2011). Some evidence suggests the participation of CCL5/CCR5 axis in the protection against DENV (Sierra et al., 2014), as well as in the pathogenesis of Dengue disease (Islam et al., 2019). Indeed, DENV infection is associated with increased frequency of human CCR5 + T cells (de-Oliveira-Pinto et al., 2012; Badolato-Corrêa et al., 2018). In a study performed by Marques et al. (2015), lower viral replication was found in macrophages treated with CCR5 blockers. In the same study, CCR5-/- mice were protected from DENV infection. These findings suggest that CCR5Δ32 could be a protective factor against DENV infection. However, Xavier-Carvalho et al. (2013) found no statistical difference in the frequency of CCR5Δ32 polymorphism between Brazilian children with severe DENV infection and healthy controls. In the same direction, no effect of CCR5Δ32 on susceptibility to DENV infection was found in a small sample-size study performed with individuals from Western Australia (Brestovac et al., 2014) and recent data suggested no important involvement of CCR5 gene or CCR5 polymorphisms in DENV infection (Cahill et al., 2018; Ornelas et al., 2019). Finally, the CCR5Δ32 allele was not identified in a small group of Indian DENV-infected individuals (Islam et al., 2019). Table 6 shows some details of the studies involving CCR5Δ32 and DENV infection.
Taking together, the data mentioned above indicate that the effects of CCR5 on DENV infection are very different between humans and rodents. However, it should be noted that the approach of each mentioned study is quite particular, and we cannot exclude some potential effects of CCR5 and CCR5Δ32 on DENV infection in humans.
3.8. Human cytomegalovirus
Human cytomegalovirus (CMV) is an enveloped double-stranded DNA virus belonging to the Herpesviridae family (Crough and Khanna, 2009; Herbein, 2018). The infection in immunocompetent adults is usually asymptomatic. However, in some cases, it can cause mononucleosis syndrome or other rare complications, including meningitis and myocarditis. CMV is an opportunistic pathogen and, therefore, symptomatic infection occurs more frequently in immunocompromised individuals (HIV infected or transplanted patients on immunosuppressive drugs). Congenital CMV infection is associated with morbidity and mortality in newborns, being associated with neurodevelopmental problems (Crough and Khanna, 2009). CMV is also linked to cancer development, once several CMV proteins (e.g., pUL122, pUL123, pUS28, pUL83, pUL111A) activate pro-oncogenic pathways, including angiogenesis, escape of immune control and tumor suppressors, tumoral inflammation, invasion and metastasis, genome instability, and increased cell survival and proliferation (Herbein, 2018). Poor socioeconomic condition favors CMV infection. Antibodies indicating past CMV infection are found in ∼60 % of adults from high-income countries. In low-income countries, the rate of past infections can reach 100 % (Griffiths et al., 2015).
CMV can manipulate the immune system producing virokines (virus-encoded cytokine/chemokine homologs) and viroceptors (virus-encoded cytokine/chemokine receptor homologs), molecules that enable the virus to evade host immune defenses. Such molecules can also facilitate viral replication (Lucas et al., 2001; Froberg, 2004; Vomaske et al., 2012). Importantly, CMV-encoded proteins can interact with CCR5, modulating its function and cell migration (Tadagaki et al., 2012; Vomaske et al., 2012), potentially affecting the pathogenesis of diseases in which CCR5 have an involvement, especially HIV infection. CMV infection in immunocompromised HIV/AIDS patients can cause different problems, including retinitis, esophagitis, gastritis, pneumonitis, and hepatitis (Crough and Khanna, 2009). CMV proteins can have an inhibitory effect on HIV−CCR5 interaction, hampering cell infection by HIV (Tadagaki et al., 2012). In the opposite direction, Johnson et al. (2018) have shown that CMV upregulates CCR5 expression in vitro, suggesting a potential contribution to HIV susceptibility in vivo. In accordance, a previous study has indicated that CMV infection upregulates CCR5 expression (Johnson et al., 2015). However, mixed results were reported regarding the effect of CMV on CCR5 expression since there is evidence indicating that CMV infection may reduce CCR5 expression in various cell types (Lecointe et al., 2002; Varani et al., 2005). Interestingly, these mixed results may not be contradictory. CMV-infected cells may indeed exhibit decreased CCR5 expression, limiting HIV infection in these cells. However, CMV-infected cells release CMV-associated soluble factors that increase CCR5 expression in non-infected bystander cells, then facilitating HIV replication in such cells and, consequently, contributing to HIV pathogenesis (King et al., 2006).
There is evidence showing that variants in the CCR5 gene can influence multiple aspects of CMV infection (Loeffler et al., 2006; Sezgin et al., 2011). For example, the CCR5 promoter polymorphism rs1800023 affects CMV replication (Bravo et al., 2014; Corrales et al., 2015). In a study evaluating children, Kasztelewicz et al. (2017) found no influence of CCR5Δ32 on susceptibility to congenital CMV infection, severity of congenital CMV disease, or CMV-related sensorineural hearing loss at birth. As an individual genetic factor, CCR5Δ32 was not statistically associated with the progression of CMV retinitis, a condition that CMV can cause in immunocompromised individuals (Sezgin et al., 2011) (Table 6).
3.9. Crimean-Congo hemorrhagic fever virus
Crimean-Congo hemorrhagic fever virus (CCHFV) belongs to the genus Nairovirus, family Bunyaviridae. CCHFV circulates in Africa, Europe, Middle East, and Asia countries, and can infect a variety of domestic animals and wild species, but without causing symptomatic illness. Humans are accidental hosts of CCHFV, for which the virus is transmitted mainly by tick-bites (especially ticks of the genus Hyalomma), although other routes of transmission also exist, such as exposure to blood of infected animals. Most CCHFV-infected individuals have no symptoms or have mild nonspecific febrile syndrome. However, in some individuals, the infection can cause the Crimean-Congo hemorrhagic fever, a severe disease characterized by fever, myalgia, hemorrhage, among other manifestations. Neurological complications can also occur, being the spectrum and intensity of the disease quite variable (Ergönül, 2006; Bente et al., 2013; Garrison et al., 2019).
Deregulation of several cytokines and chemokines is involved in the pathogenesis of Crimean-Congo hemorrhagic fever (Ergönül, 2006; Saksida et al., 2010; Bente et al., 2013; Garrison et al., 2019). In a study performed by Arasli et al. (2015), expression of the CCR5 ligands CCL2, CCL3, and CCL4 was increased in CCHFV-infected adults compared to controls. Considering these same chemokines in CCHFV-infected children, only CCL4 was significantly increased compared to pediatric controls (Arasli et al., 2015).
Engin et al. (2009) evaluated the CCR5Δ32 in 15 Turkish CCHFV-infected individuals and observed the wild-type homozygous genotype in all cases. In a subsequent study evaluating the Turkish population, Rustemoglu et al. (2017) found a protective effect of CCR5Δ32 heterozygous genotype and Δ32 allele on CCHFV infection, since the genotype and allele frequencies were higher in controls than in CCHFV-infected individuals. Conversely, the wild-type genotype (normal CCR5 expression) was prevalent among infected individuals. These findings suggest that CCR5 contributes to susceptibility to CCHFV infection and that CCR5 down-regulation due to CCR5Δ32 results in some protection against the infection. However, further studies are needed to explain the mechanisms by which CCR5 participates in CCHFV infection. In the same study, the CCR5Δ32 was not significantly associated with disease severity, clinical parameters, or mortality rate (Rustemoglu et al., 2017). Together, these findings (Table 6) indicate that the effect of CCR5Δ32 is given specifically on resistance against CCHFV infection, without affecting the pathogenesis/outcome of Crimean-Congo hemorrhagic fever.
3.10. Enterovirus
The genus Enterovirus is composed of non-enveloped, positive-stranded RNA viruses, belonging to the Picornaviridae family. Enteroviruses (EV) can infect the gastrointestinal tract, CNS, and other organs, including heart (Tapparel et al., 2013). Cardiomyopathy is a common consequence of EV infection in the heart. EV infection is associated with myocardial inflammation (myocarditis) and other damages to heart tissues (Badorff et al., 2000; Cooper, 2009; Sagar et al., 2012; Tapparel et al., 2013; Weintraub et al., 2017). A portion of EV-infected individuals clears the virus, while others develop persistent infection that can damage heart tissues. Viral persistence in individuals with enteroviral cardiomyopathy is associated with an increased mortality rates (Kühl et al., 2005; Lassner et al., 2018).
Some studies suggest that CCR5 influences different aspects of the pathogenesis of viruses belonging to the Picornaviridae family, including Encephalomyocarditis virus (Christmann et al., 2011; Shaheen et al., 2015), Coxsackievirus B3 (Valaperti et al., 2013), and Rhinovirus (Muehling et al., 2018), once CCR5 participates in the regulation of the host immune response during infection by these viruses. Considering the effects of the genetic variant CCR5Δ32 on CCR5 expression and CCR5-related immune responses, it is possible that CCR5Δ32 also shows some impact on EV-related diseases.
In a German study that evaluated patients with enteroviral (chronic/inflammatory) cardiomyopathy (Lassner et al., 2018), the CCR5Δ32 was strongly associated with spontaneous viral clearance and better clinical outcome (reduced mortality rate) (Table 6). These findings indicate a critical involvement of the CCR5 molecule in the pathogenesis of EV cardiomyopathy. It was suggested that the CCR5Δ32 genotyping could be used to assist in the prediction of the clinical progression of enteroviral cardiomyopathy: the Δ32 allele as a predictor of a better prognosis, without the need of antiviral interferon-β (IFN-β) therapy; and the wild-type genotype as a predictor of a worse prognosis and immediate need of antiviral IFN-β therapy (Lassner et al., 2018). The clinical use of IFN-β is effective to eliminate the virus, avoid irreversible cardiac injury, and reduce mortality rates, but it is also associated to numerous adverse effects (Kühl et al., 2012; Lassner et al., 2018). Considering the prognostic value of the CCR5Δ32 on the clinical course of enteroviral cardiomyopathy, it is necessary to evaluate the relationship between the CCR5Δ32 and the disease in different human populations, mainly through genetic association studies. If this association is confirmed in other populations, the CCR5Δ32 genotyping will be a broad-spectrum clinical tool, enhancing and driving the treatment of enteroviral cardiomyopathy.
3.11. Japanese encephalitis virus
Japanese encephalitis virus (JEV) contains a single-stranded positive-sense RNA genome and belongs to the family Flaviviridae, genus Flavivirus. Birds and pigs are the JEV amplifying hosts. JEV is transmitted by Culex mosquitoes and occur especially in Australia and Asian countries. Humans and horses are dead-end JEV hosts. The human infection causes a broad range of clinical manifestations, varying from asymptomatic infection (most cases) to mild febrile syndrome and even lethal meningomyeloencephalitis. JEV is the etiological agent of most cases of viral encephalitis in many countries, reaching ∼30 % mortality rate (van den Hurk et al., 2009; Tiwari et al., 2012; Le Flohic et al., 2013).
Some evidence obtained in laboratory conditions (in vitro analysis and murine models) showed that JEV infection induces the up-regulation of CCR5 gene (Gupta and Rao, 2011; Pareek et al., 2014; Zhang et al., 2019). Also, increased infiltration of CCR5+ CD8+ T cells was observed in the brains of JEV-infected mice (Zhang et al., 2019). In this context, using a mouse model of Japanese encephalitis, Larena et al. (2012) showed that CCR5 protects the host against JEV infection in the CNS, being essential for disease recovery. CCR5-deficient mice showed higher viral loads in the brain and spinal cord as well as increased mortality rate, as compared to control mice (Larena et al., 2012). Also using a mouse model of Japanese encephalitis, Kim et al. (2016) demonstrated that CCR5 controls the infiltration of CD4+Foxp3+ T regulatory (Treg) cells in the CNS, contributing to protection against Japanese encephalitis. The infiltration and action of inflammatory cells in the brain are important to limit neuroinvasive infections, processes that are regulated by multiple factors, especially cytokines, chemokines and their receptors, including CCR5. However, the uncontrolled action of inflammatory cells can cause damage to the CNS. Of note, CD4+Foxp3+ Treg cells regulate the immune responses, avoiding undesirable or excessive action of inflammatory cells (Bardina and Lim, 2012; Veiga-Parga et al., 2013; Simonetta and Bourgeois, 2013; Campbell, 2015; Kim et al., 2016). According to these pieces of evidence, CCR5-mediated action of Treg cells is critical for the control of Japanese encephalitis. However, the role of CCR5 in JEV infection may be more complex than it appears at first. Liu et al. (2018) demonstrated that the use of the CCR5 antagonist maraviroc reduces the JEV-induced inflammation in mice brain, increasing survival rate. This result suggests that potential deleterious effects of CCR5-mediated inflammation in the brain may override the effects of CCR5-mediated action of Treg cells and, therefore, the use of CCR5 antagonists to treat Japanese encephalitis may be beneficial. Based on these complex and contradictory results, it can be concluded that CCR5 indeed has an important influence on Japanese encephalitis, but it is not yet possible to state its specific roles, once they are varied and appear to be context-dependent. Also, the results obtained in animal models may not fully represent the disease course in humans.
Indeed, some evidences suggest the involvement of CCR5 in the pathogenesis of Japanese encephalitis in humans. In Indian individuals with mild cases of Japanese encephalitis, a significant dowregulation of the CCR5 gene was observed as compared to healthy controls (Chowdhury and Khan, 2019). However, the CCR5Δ32 does not have a relevant influence on the disease. Deval et al. (2019) investigated the effect of various genetic variants, including CCR5Δ32, on the development of Japanese encephalitis in individuals from North India (Table 6). No statistically significant difference was found when the CCR5Δ32 (as an individual factor) was compared between cases and controls (Deval et al., 2019). Considering that both studies mentioned above were performed in the Indian population, studies evaluating the potential effects of CCR5 and CCR5Δ32 on Japanese encephalitis in other populations are necessary.
3.12. Hantavirus infection
Puumala virus (PUV) infection is a zoonosis endemic in Europe. PUV is an enveloped single-stranded RNA virus, belonging to the Bunyaviridae family, genus Hantaviridae. Most PUV infections are mild or subclinical. In some infected individuals, PUV is responsible for the development of nephropathia epidemica, a milder form of hemorrhagic fever with renal syndrome, a condition typically caused by other Hantaviruses (Settergren, 2000; Mustonen et al., 2013; Lebecque and Dupont, 2019). An in vitro study found increased CCR5 gene expression in primary monocytes infected by hantaviruses (Hantaan virus, Sin Nombre virus and Andes virus) (Markotić et al., 2007). In this sense, host genetic factors and the immune responses affect different aspects of PUV infection and progression of nephropathia epidemica (Mustonen et al., 2013), although the role of CCR5 in this disease is little known.
Kletenkov et al. (2019) evaluated the potential influence of the CCR5Δ32 on nephropathia epidemica in individuals from Republic of Tatarstan, Russia (Table 6). Of note, in their study, the authors stated hantavirus infection as the cause of Nephropathia epidemica, not specifying the PUV detection. No statistical difference was observed in CCR5Δ32 genotype frequencies between cases and controls, indicating that CCR5Δ32 does not influenced the susceptibility to hantavirus infection in the studied population. Considering only nephropathia epidemica cases, the study revealed an association between the wild-type homozygous genotype (functional CCR5) and increased severity of the disease. Conversely, the heterozygous genotype was considered a protective factor against increased disease severity (Kletenkov et al., 2019). To the best of our knowledge, there are no other studies that have evaluated CCR5Δ32 in nephropathia epidemica. However, the results presented by Kletenkov et al. (2019) suggest that further studies evaluating the roles of CCR5 and CCR5Δ32 on hantavirus infection are important.
4. CCR5 gene editing and CCR5 modulation: what would be the effects of CCR5 absence on everyday life?
In 2019, the multiple roles of CCR5 in physiological and pathological conditions gained the attention of the scientific community and lay public due to CCR5 gene editing in human embryos, the “CRISPR babies”, performed by a Chinese biophysicist. The researcher claimed to have used CRISPR gene editing technology to edit the CCR5 of germline cells to mimic the effects of CCR5Δ32, aiming to generate babies resistant to HIV infection. The ethical, safety, and legal aspects related to this procedure have caused an intense and broad discussion in the media and scientific literature (Cohen, 2019; Daley et al., 2019; Greely, 2019; Rosenbaum, 2019), and this case culminated in a three years prison sentence for the biophysicist responsible for the procedure (Cyranoski, 2020). Also, the consequences of the CCR5 absence have brought many concerns to light, once the CCR5 protein participates in tissue development processes, control of immune responses, and several other physiological processes. Considering these concerns, our group and others described some undesirable effects associated to natural CCR5 absence (due to the CCR5Δ32 homozygous genotype) and CCR5 editing (Ellwanger et al., 2019; Wang and Yang, 2019; Xie et al., 2019).
Besides gene editing techniques, the expression of CCR5 can be artificially modulated through the use of pharmacological antagonists (e.g. maraviroc), chemokine ligands, and monoclonal antibodies (Fantuzzi et al., 2019). The use of CCR5 antagonists is well established for the treatment of HIV infection and is currently being tested for the treatment of many other diseases, such as cancer (Pervaiz et al., 2019; Huang et al., 2020a), stroke (Joy et al., 2019), and cocaine addiction-related disorders (Hall et al., 2020; Nayak et al., 2020).
Taking together, it is increasingly clear that the specific inhibition of CCR5 expression through different techniques is gaining pace in different clinical contexts. The pharmacological CCR5 blockade has many benefits in different clinical situations, particularly in the treatment of HIV infection. Also, the potential of gene editing (especially in somatic cells) for the treatment of genetic diseases is very promising. However, considering viral infections, two aspects must be considered, as follows:
I) The non-redundant characteristics of the CCR5 should be taken into consideration when studying the CCR5 protein and the effects of CCR5Δ32 on viral infections: The traditional concepts of redundancy and robustness of the chemokine system consider that the absence of a specific chemokine or chemokine receptor is adequately compensated by other molecules. Although these concepts are generally correct for some chemokines/receptors and for some physiological conditions, the lack of CCR5 expression may affect the host protection against some specific infectious diseases once the CCR5 absence is not perfectly compensated for by other receptors (Ellwanger et al., 2020a). The nonredundancy of CCR5 is relevant especially for infections by neuroinvasive flaviviruses (Bardina and Lim, 2012). For example, the absence of CCR5 due to CCR5Δ32 is considered deleterious in WNV infection (Glass et al., 2006; Lim et al., 2008) and in some aspects of TBEV infection (Ellwanger and Chies, 2019a). The non-redundant role of CCR5 is also likely in JEV infection (Larena et al., 2012).
II) Studies evaluating the actual impact of CCR5 antagonists on neuroinvasive infections are needed: It is possible that those individuals using CCR5 antagonists (e.g., for the treatment of HIV infection) and living in areas endemic for neuroinvasive viruses, especially WNV and TBEV, may be at increased risk of developing viral encephalitis-related problems. However, it is still necessary that studies consistently evaluate this assumption, since the available evidence does not support risks for the use of CCR5 antagonists in endemic areas of flaviviruses (Martin-Blondel et al., 2016). As mentioned in the topic addressing WNV in this review, it is prudent to recommend to individuals using CCR5 antagonists to adopt measures to minimize the risk of neuroinvasive infections, such as the use of mosquito repellents and mosquito nets (considering the risk of WNV infection), and to limit their exposure to tick-infested areas (considering the risk of TBEV infection). Concerns regarding the use of CCR5 antagonists in areas of JEV circulation have also been raised by other authors (Kim et al., 2016; Larena et al., 2012). If the connection between the use of CCR5 antagonists and increased risk of neuroinvasive infections is confirmed in methodologically well-controlled studies, these recommendations should be considered of essential importance for users of CCR5 antagonists.
5. The triad “extracellular vesicles, viruses, and CCR5”
“Extracellular vesicles” (EVs) is a general term used to designate different membranous vesicles released by various cell types. EVs include microparticles, microvesicles, nanovesicles, nanoparticles, ectosomes, exosomes, exovesicles, exosome-like vesicles, oncosomes, among other vesicle types. EVs act in the transport of different molecules (e.g. microRNAs, mRNAs, proteins) between cells and tissues in a regulated and precise manner. EVs promote the maintenance of physiological processes, also participating in the pathogenesis of various diseases, such as cancer and inflammatory diseases (Colombo et al., 2014; Théry et al., 2018). Besides, the regulation of multiple aspects of the immune system is strongly influenced by EVs (O’Neill and Quah, 2008; Colombo et al., 2014; Ellwanger et al., 2016).
The multiple roles of EVs in viral infections began to be studied more intensively in recent years, representing an emerging topic in the field of infectious diseases. Currently, the relationship between EVs and viral infections has already been explored (to a greater or lesser extent) in the context of the following viruses: Bunyaviruses, Cytomegalovirus, Dengue virus (DENV), Ebola virus, Epstein-Barr virus, Hepatitis A virus, Hepatitis C virus (HCV), Human papillomavirus, Herpes simplex virus, Human T cell lymphotropic virus, Kaposi’s sarcoma-associated herpesvirus, Zika virus (ZIKV), TBEV, HIV (Kaminski et al., 2019b), and Influenza virus (Zheng et al., 2020).
EVs participate in immune evasion processes, ultimately allowing viruses to bypass host defenses (Kaminski et al., 2019b). For example, HIV is likely to usurp the exosome biogenesis pathway in such a way that enhances its infectivity, while increasing its evading strategies from the host immune defenses (Ellwanger et al., 2017a). Regarding TBEV, exosomes were indicated as important participants in both viral infection and pathogenesis; in this case, tick-derived exosomes facilitate TBEV transmission to the host. Also, exosomes derived from TBEV-infected host neurons can facilitate the spread of the virus in the CNS (Zhou et al., 2018). Exosomes have shown multi-dimensional roles in DENV life cycle. They can modulate negatively or positively DENV pathogenesis, where they are suggested as instrumental for Dengue Hemorrhagic Fever development in reason of the transportation of specific microRNAs (Mishra et al., 2019). Since HCV can be found inside exosomes (Liu et al., 2014), it is not suprising that blood-derived exosomes from HCV-infected patients can transmit the virus to other cells (Bukong et al., 2014). These findings suggest that exosomes and other EVs assist in the transport of HCV particles/components between cells, ultimately facilitating viral infection. Of note, EVs can transport multiple viral components or molecules from virus-infected cells that end up facilitating the spread of the virus in the host. On the other hand, EVs can act in favor of the host, transporting molecules that assist host defenses in the elimination or control of infections (Kaminski et al., 2019b).
EVs can transport a range of cytokines and chemokines, such as IL-1, IL-2, IFN-α, IFN-β, CCL2, CCL3, CCL4, and CXCL10 (Chen et al., 2011; Konadu et al., 2015; Hosseinkhani et al., 2018; Kodidela et al., 2018; Gao et al., 2019a, b; Aiello et al., 2020; Chiantore et al., 2020). Besides, EVs and microparticles also transport chemokine receptors (Shen et al., 2016; Li et al., 2018; Liang et al., 2018), including CCR5 (Mack et al., 2000; Tokarz et al., 2019). Interestingly, EVs from CCR5 wild type cells can deliver CCR5 molecules to CCR5 Δ32/Δ32 cells, making them susceptible to HIV infection (Mack et al., 2000). A similar phenomenon has been reported involving microparticles, HIV, and CXCR4 (Rozmyslowicz et al., 2003). CCR5 expression is also influenced by the release of EVs, specifically microparticles (Renovato-Martins et al., 2017). Therefore, EVs have the potential to attribute greater complexity to CCR5 roles in viral infections. It is possible that the presence of CCR5 in cells is not only dependent on mechanisms of membrane expression and internalization of the receptor. It can also be dependent on CCR5 delivery mediated by EVs. However, the actual impact of EVs on CCR5-mediated immune response in infections remains to be determined and deserves further investigation.
Finally, a truncated CCR5 soluble form (Tsimanis et al., 2005), as well as soluble CXCR4, have also been reported in the plasma of humans (Malvoisin et al., 2011). Of note, the truncated CCR5 soluble form has ∼22 kDa (half the size of the CCR5 found on cell membranes) and is truncated at the end of the second extracellular loop (the third extracellular loop and the subsequent three transmembrane regions are absent) (Tsimanis et al., 2005). However, the existence of cell-free soluble CCR5 is still controversial and does not represent a consensus in the scientific community. Such doubts occur mainly in consideration of the structure of the receptor, which is highly characteristic of a cell membrane-associated molecule. We believe that EVs-containing CCR5 can explain potential (misleading) detections of soluble CCR5. The importance of circulating EVs-containing CCR5 on viral infections represents an additional and interesting open question to be investigated.
6. Neglected and emerging topics in CCR5 research: Rocio virus, Zika virus, Epstein-Barr virus, and rhinovirus
Chávez et al. (2013) demonstrated an involvement of the CCR5 protein in the infection by Rocio virus (ROCV), a mosquito-borne flavivirus that caused a major human encephalitis outbreak between 1975 and 1980, in the region of Ribeira Valley, São Paulo State, Brazil (Ellwanger et al., 2017b). Specifically, using a mouse model of ROCV-associated encephalitis, CCR5-deficient mice showed increased survival rate and reduced levels of brain inflammation compared to mice expressing CCR5, indicating the participation of CCR5 in ROCV-associated encephalitis (Chávez et al., 2013). Although other studies discussed in this review have shown an important involvement of CCR5 in infection by flaviviruses [especially as an important molecule for the resolution of neuroinfection, in opposition to results of Chávez et al. (2013)], the role of the CCR5 in ROCV infection represents a neglected topic. This knowledge gap should be addressed in further studies since the reemergence of ROCV in Brazil is a concern in terms of public health (Figueiredo, 2007; Ellwanger et al., 2017b). Although no other ROCV outbreaks have been reported in Brazil after the 1980s, there is evidence of ROCV circulation in animals (Casseb et al., 2014; Pauvolid-Corrêa et al., 2014; Silva et al., 2014).
Badolato-Corrêa et al. (2018) reported that Zika virus (ZIKV) infection induces an upregulation of CCR5 expression on T cells. Of note, ZIKV is another mosquito-borne flavivirus that recently reemerged in many countries, affecting Brazil in particular. ZIKV infection is associated with microcephaly and other human development problems (Baud et al., 2017). Zachova et al. (2019) showed that Epstein-Barr virus (EBV)-infected B cells have increased CCR5 expression compared to EBV-non-infected cells. Also, recent evidence points to a pivotal role of the CCR5 in the attenuation of rhinovirus-associated inflammation, by controlling the activity of CD4+Foxp3+ Treg cells (Hossain et al., 2020). Altered levels of cytokines and chemokines are associated with several aspects of ZIKV (Barros et al., 2018; Naveca et al., 2018; Khaiboullina et al., 2019; Lima et al., 2019), EBV (Piovan et al., 2005; Ehlin-Henriksson et al., 2009; Cohen et al., 2017), and rhinovirus infections (Rajan et al., 2014; Shelfoon et al., 2016; Hansel et al., 2017). However, the potential role of the chemokine receptor CCR5 in the pathogenesis of ZIKV, EBV, and rhinovirus represents open questions in the field of CCR5 research.
7. CCR5 and coronaviruses: is there a connection?
Coronaviruses are positive-sense RNA viruses that belong to the Coronaviridae family (Li et al., 2020). Coronaviruses infect a wide range of species, including humans. Until 2019, humans have faced two major outbreaks of high pathogenic coronaviruses, the severe acute respiratory syndrome coronavirus (SARS-CoV) outbreak and the Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak (Fung and Liu, 2019). In 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan (Hubei province, China) (Wu et al., 2020) and rapidly spread to more than 180 countries/regions (Interactive dashboard of global COVID-19 cases - Johns Hopkins University: https://arcg.is/0fHmTX; Dong et al., 2020). Of note, SARS-CoV-2 infection causes the “coronavirus disease 2019” and therefore is also called “COVID-19”. Considering the ongoing SARS-CoV-2 pandemic and the clinical variability observed in the affected individuals, ranging from mild to severe respiratory disease (Chen et al., 2020; Huang et al., 2020b), the following question arose: does CCR5 have any clinically relevant influence on SARS-CoV-2 or other coronaviruses infections?
Recent data indicated that SARS-CoV-2 binds to the ACE2 receptor (Andersen et al., 2020), using this receptor for entry into human cells. Therefore, it is unlikely that CCR5 or CCR5Δ32 have some significant effect on the susceptibility or resistance to SARS-CoV-2 infection. However, CCR5 may have some impact on the clinical course of SARS-CoV-2 infection.
The pattern and intensity of the human immune responses regulate the clinical progression and even the outcome of infections by coronaviruses (Li et al., 2020), including SARS-CoV-2 (Qin et al., 2020; Shi et al., 2020). SARS-CoV-infected mice showed increased expression of CCR5 mRNA in lungs along with the production of CCL2, CCL3 and CCL5 chemokines, the major CCR5 natural agonists (Chen et al., 2010). Intracranial infection of mice with Mouse Hepatitis Virus (MHV, a murine coronavirus) induces an increased CCR5 expression, which contributed to MHV-induced demyelination through CCR5-mediated macrophage recruitment to the CNS (Glass et al., 2001). Subsequent studies using MHV-infected mice showed that CCR5 participates in the regulation of CD4+ and CD8+ T cell activities against the virus in the CNS (Glass and Lane, 2003a, b). Also, SARS-CoV-infected human monocyte-derived dendritic cells showed increased CCR5 expression in vitro (Law et al., 2009).
Taking together, the findings mentioned above suggest that CCR5 could have some influence on the clinical course of SARS-CoV-2 infection. However, future studies addressing humans are needed to clarify this suggestion. In this sense, we emphasize that so far, there is no evidence showing a clear involvement of CCR5 in SARS-CoV-2 human infection.
8. Final considerations regarding the phenotypic effects of the CCR5
The CCR5Δ32 is a highly penetrating polymorphism, and exerts a robust phenotypic effect on CCR5. However, the expression of CCR5 is regulated by other polymorphisms in addition to CCR5Δ32 (Mehlotra, 2019). Also, the CCR5 expression is influenced by non-coding genetic elements. The effect of the genetic backround of the host can also be exacerbated or diminished depending on the environmental conditions to which the individual is exposed (Ellwanger et al., 2018c; Kulkarni et al., 2019). Taking together, these factors help to explain why many of the effects exerted by CCR5 on a given disease are specific to a certain population, ethnic group, or individual.
9. Conclusions
Research involving CCR5 and HIV began in the mid-1990s. Since then, many achievements in the field of CCR5/HIV research have been made, such as the identification of CCR5Δ32 as a strong protective factor against HIV infection and the development of CCR5 antagonists for the treatment of HIV infection. Currently, these drugs are being tested for the treatment of other inflammatory and infectious diseases. In this context, the use of CCR5 antagonists has raised some concerns, since the blockade of CCR5 can affect or even weaken the host defenses against certain infections, especially neuroinvasive infections by flaviviruses. However, to date, there is no strong evidence indicating that the use of CCR5 antagonists has increased the susceptibility of individuals to problematic flaviviruses infections.
The frequency of CCR5Δ32 is quite varied among human populations, and therefore the effects of the allele in a particular population may not apply to another population. Moreover, the effects of CCR5 and CCR5Δ32 are disease-specific. For instance, the CCR5Δ32 does not significantly affect susceptibility to WNV infection. However, the effect of the CCR5 and CCR5Δ32 on WNV disease progression is very robust. Some animal-derived findings suggest the involvement of the CCR5 in the pathogenesis of DENV infection. However, the effects of CCR5 on DENV infection are very different between humans and rodents. Of note, CCR5Δ32 does not significantly affect susceptibility to DENV infection or disease progression. Moreover, the effect of CCR5Δ32 on HBV-related disease is population-specific. Interestingly, CMV release virokines, which are molecules that can manipulate the host immune response and affect the CCR5 function. The examples cited above highlight the varied impacts of CCR5 and CCR5Δ32 on viral infections.
The few studies involving CCHFV, EV, and Hantavirus infection have indicated important effects of CCR5Δ32 on these conditions. Based on these findings, further studies should investigate the role of CCR5Δ32 and CCR5 protein in such infections, considering populations with distinct genetic backgrounds. Some evidence suggested the participation of CCR5 in infections by ZIKV, EBV, and rhinovirus. Also, a mouse model of ROCV-associated encephalitis suggested an important role for CCR5 in host immune responses against the virus. However, the roles of CCR5 and CCR5Δ32 in infections by ZIKV, EBV, rhinovirus, and ROCV are still poorly understood and need to be investigated in future studies. The role of EVs in the transport of CCR5 between cells indicates that the expression of CCR5 on the cell surface may also depend on the release of EVs containing CCR5. Also, the transport of host molecules and viruses through EVs adds complexity to the topics covered in this review and should be taken into account in future studies that investigate the role of CCR5 in viral infections. Gene editing technologies have the potential to be used to treat different diseases, mainly when applied to somatic cells. However, CCR5 gene editing in human embryos presents a number of ethical problems. Besides, the absence of CCR5 can have deleterious effects in certain conditions, such as increased susceptibility to symptomatic WNV infection. Finally, this article showed that the participation of CCR5 in different viral infections is complex and varied and, therefore, cannot be generalized. This article also pointed out neglected gaps in knowledge involving CCR5 that should be addressed in future studies.
CRediT authorship contribution statement
Joel Henrique Ellwanger: Conceptualization, Investigation, Writing - original draft, Writing - review & editing, Visualization, Project administration. Bruna Kulmann-Leal: Investigation, Validation, Writing - review & editing. Valéria de Lima Kaminski: Investigation, Writing - review & editing. Andressa Gonçalves Rodrigues: Investigation, Writing - review & editing. Marcelo Alves de Souza Bragatte: Visualization, Writing - review & editing. José Artur Bogo Chies: Investigation, Resources, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgements and Funding
This work was supported by Brazilian funding agencies: JHE receives a postdoctoral fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil); BKL receives a master scholarship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil); VLK received a doctoral scholarship from CAPES (Brazil) and currently receives a postdoctoral fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Brazil); AGR received a scholarship from Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS, Brazil); MASB receives a doctoral scholarship from CAPES (Brazil); JABC receives a research fellowship from CNPq (Brazil).
References
- Abdolmohammadi R., Shahbazi Azar S., Khosravi A., Shahbazi M. CCR5 polymorphism as a protective factor for hepatocellular carcinoma in hepatitis B virus-infected Iranian patients. Asian Pac. J. Cancer Prev. 2016;17:4643–4646. doi: 10.22034/apjcp.2016.17.10.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achour L., Scott M.G., Shirvani H., Thuret A., Bismuth G., Labbé-Jullié C., Marullo S. CD4-CCR5 interaction in intracellular compartments contributes to receptor expression at the cell surface. Blood. 2009;113:1938–1947. doi: 10.1182/blood-2008-02-141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlenstiel G., Berg T., Woitas R.P., Grünhage F., Iwan A., Hess L., Brackmann H.H., Kupfer B., Schernick A., Sauerbruch T., Spengler U. Effects of the CCR5-Δ32 mutation on antiviral treatment in chronic hepatitis C. J. Hepatol. 2003;39:245–252. doi: 10.1016/s0168-8278(03)00193-4. [DOI] [PubMed] [Google Scholar]
- Ahlenstiel G., Woitas R.P., Rockstroh J., Spengler U. CC-chemokine receptor 5 (CCR5) in hepatitis C--at the crossroads of the antiviral immune response? J. Antimicrob. Chemother. 2004;53:895–898. doi: 10.1093/jac/dkh239. [DOI] [PubMed] [Google Scholar]
- Ahlenstiel G., Woitas R.P., Iwan A., Nattermann J., Feldmann G., Rockstroh J.K., Oldenburg J., Kupfer B., Sauerbruch T., Spengler U. Effects of the CCR5- Δ32 mutation on hepatitis C virus-specific immune responses in patients with haemophilia. Immunol. Invest. 2009;38:284–296. doi: 10.1080/08820130802307294. [DOI] [PubMed] [Google Scholar]
- Ahmadabadi B.N., Hassanshahi G., Khoramdelazad H., Mirzaei V., Sajadi S.M.A., Hajghani M., Khodadadi H., Pourali R., Arababadi M.K., Kennedy D. Downregulation of CCR5 expression on the peripheral blood CD8+ T cells of southeastern Iranian patients with chronic hepatitis B infection. Inflammation. 2013;36:136–140. doi: 10.1007/s10753-012-9528-4. [DOI] [PubMed] [Google Scholar]
- Ahn S.H., Kim D.Y., Chang H.Y., Hong S.P., Shin J.S., Kim D.Y.S., Kim H., Kim J.K., Paik Y.H., Lee K.S., Chon C.Y., Moon Y.M., Han K.H. Association of genetic variations in CCR5 and its ligand, RANTES with clearance of hepatitis B virus in Korea. J. Med. Virol. 2006;78:1564–1571. doi: 10.1002/jmv.20739. [DOI] [PubMed] [Google Scholar]
- Aiello A., Giannessi F., Percario Z.A., Affabris E. An emerging interplay between extracellular vesicles and cytokines. Cytokine Growth Factor Rev. 2020;51:49–60. doi: 10.1016/j.cytogfr.2019.12.003. [DOI] [PubMed] [Google Scholar]
- Ajuebor M.N., Aspinall A.I., Zhou F., Le T., Yang Y., Urbanski S.J., Sidobre S., Kronenberg M., Hogaboam C.M., Swain M.G. Lack of chemokine receptor CCR5 promotes murine fulminant liver failure by preventing the apoptosis of activated CD1d-restricted NKT cells. J. Immunol. 2005;174:8027–8037. doi: 10.4049/jimmunol.174.12.8027. [DOI] [PubMed] [Google Scholar]
- Ajuebor M.N., Carey J.A., Swain M.G. CCR5 in T cell-mediated liver diseases: what’s going on? J. Immunol. 2006;177:2039–2045. doi: 10.4049/jimmunol.177.4.2039. [DOI] [PubMed] [Google Scholar]
- Alkhatib G. The biology of CCR5 and CXCR4. Curr. Opin. HIV AIDS. 2009;4:96–103. doi: 10.1097/COH.0b013e328324bbec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A.G., Chung C.H., Atkins A., Dampier W., Khalili K., Nonnemacher M.R., Wigdahl B. Gene editing of HIV-1 co-receptors to prevent and/or cure virus infection. Front. Microbiol. 2018;9:2940. doi: 10.3389/fmicb.2018.02940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen N.B., Larsen S.M., Nissen S.K., Jørgensen S.E., Mardahl M., Christiansen M., Kay L., Mogensen T.H. Host genetics, innate immune responses, and cellular death pathways in poliomyelitis patients. Front. Microbiol. 2019;10:1495. doi: 10.3389/fmicb.2019.01495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arababadi M.K., Pourfathollah A.A., Jafarzadeh A., Hassanshahi G., Mohit M., Hajghani M., Shamsizadeh A. Peripheral blood CD8+ T cells CCR5 expression and its Δ32 mutation in Iranian patients with occult hepatitis B infections. Lab. Med. 2010;41:226–230. doi: 10.1309/LMVUKWROX0EBQR01. [DOI] [Google Scholar]
- Arasli M., Ozsurekci Y., Elaldi N., McAuley A.J., Karadag Oncel E., Tekin I.O., Gozel M.G., Kaya A., Icagasioglu F.D., Caglayik D.Y., Korukluoglu G., Kokturk F., Bakir M., Bente D.A., Ceyhan M. Elevated chemokine levels during adult but not pediatric Crimean-Congo hemorrhagic fever. J. Clin. Virol. 2015;66:76–82. doi: 10.1016/j.jcv.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Badolato-Corrêa J., Sánchez-Arcila J.C., Alves de Souza T.M., Santos Barbosa L., Conrado Guerra Nunes P., da Rocha Queiroz Lima M., Gandini M., de Filippis AM Bispo, Venâncio da Cunha R., Leal de Azeredo E., de-Oliveira-Pinto L.M. Human T cell responses to Dengue and Zika virus infection compared to Dengue/Zika coinfection. Immun. Inflamm. Dis. 2018;6:194–206. doi: 10.1002/iid3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badorff C., Lee G.H., Knowlton K.U. Enteroviral cardiomyopathy: bad news for the dystrophin-glycoprotein complex. Herz. 2000;25:227–232. doi: 10.1007/s000590050011. [DOI] [PubMed] [Google Scholar]
- Balistreri C.R., Carruba G., Calabrò M., Campisi I., Di Carlo D., Lio D., Colonna-Romano G., Candore G., Caruso C. CCR5 proinflammatory allele in prostate cancer risk: a pilot study in patients and centenarians from Sicily. Ann. N. Y. Acad. Sci. 2009;1155:289–292. doi: 10.1111/j.1749-6632.2008.03691.x. [DOI] [PubMed] [Google Scholar]
- Bardina S.V., Lim J.K. The role of chemokines in the pathogenesis of neurotropic flaviviruses. Immunol. Res. 2012;54:121–132. doi: 10.1007/s12026-012-8333-3. [DOI] [PubMed] [Google Scholar]
- Barros J.B.S., da Silva P.A.N., Koga R.C.R., Gonzalez-Dias P., Carmo Filho J.R., Nagib P.R.A., Coelho V., Nakaya H.I., Fonseca S.G., Pfrimer I.A.H. Acute Zika virus infection in an endemic area shows modest proinflammatory systemic immunoactivation and cytokine-symptom associations. Front. Immunol. 2018;9:821. doi: 10.3389/fimmu.2018.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud D., Gubler D.J., Schaub B., Lanteri M.C., Musso D. An update on Zika virus infection. Lancet. 2017;390:2099–2109. doi: 10.1016/S0140-6736(17)31450-2. [DOI] [PubMed] [Google Scholar]
- Bente D.A., Forrester N.L., Watts D.M., McAuley A.J., Whitehouse C.A., Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100:159–189. doi: 10.1016/j.antiviral.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., Myers M.F., George D.B., Jaenisch T., Wint G.R.W., Simmons C.P., Scott T.W., Farrar J.J., Hay S.I. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham A.W., Buckingham K.J., Husain S., Emond M.J., Bofferding K.M., Gildersleeve H., Rutherford A., Astakhova N.M., Perelygin A.A., Busch M.P., Murray K.O., Sejvar J.J., Green S., Kriesel J., Brinton M.A., Bamshad M. Host genetic risk factors for West Nile virus infection and disease progression. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bineshian F., Hosseini A., Sharifi Z., Aghaie A. A Study on the association between CCRΔ32 mutation and HCV Infection in Iranian patients. Avicenna J. Med. Biotechnol. 2018;10:261–264. [PMC free article] [PubMed] [Google Scholar]
- Blackard J.T., Kong L., Rouster S.D., Karns R., Horn P.S., Kottilil S., Shata M.T., Sherman K.E. CCR5 receptor antagonism inhibits hepatitis C virus (HCV) replication in vitro. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C., Migeotte I., Lee B., Vakili J., Doranz B.J., Govaerts C., Vassart G., Doms R.W., Parmentier M. CCR5 binds multiple CC-chemokines: MCP-3 acts as a natural antagonist. Blood. 1999;94:1899–1905. doi: 10.1182/blood.V94.6.1899. [DOI] [PubMed] [Google Scholar]
- Blanpain C., Lee B., Tackoen M., Puffer B., Boom A., Libert F., Sharron M., Wittamer V., Vassart G., Doms R.W., Parmentier M. Multiple nonfunctional alleles of CCR5 are frequent in various human populations. Blood. 2000;96:1638–1645. doi: 10.1182/blood.V96.5.1638.h8001638_1638_1645. [DOI] [PubMed] [Google Scholar]
- Boldt A.B.W., Culpi L., Tsuneto L.T., Souza I.R., Kun J.F.J., Petzl-Erler L.M. Analysis of the CCR5 gene coding region diversity in five South American populations reveals two new non-synonymous alleles in Amerindians and high CCR5*D32 frequency in Euro-Brazilians. Genet. Mol. Biol. 2009;32:12–19. doi: 10.1590/S1415-47572009005000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncompain G., Herit F., Tessier S., Lescure A., Del Nery E., Gestraud P., Staropoli I., Fukata Y., Fukata M., Brelot A., Niedergang F., Perez F. Targeting CCR5 trafficking to inhibit HIV-1 infection. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aax0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunersreuther V., Zernecke A., Arnaud C., Liehn E.A., Steffens S., Shagdarsuren E., Bidzhekov K., Burger F., Pelli G., Luckow B., Mach F., Weber C. Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2007;27:373–379. doi: 10.1161/01.ATV.0000253886.44609.ae. [DOI] [PubMed] [Google Scholar]
- Bravo D., Clari M.A., Aguilar G., Belda J., Giménez E., Carbonell J.A., Henao L., Navarro D. Looking for biological factors to predict the risk of active cytomegalovirus infection in non-immunosuppressed critically ill patients. J. Med. Virol. 2014;86:827–833. doi: 10.1002/jmv.23838. [DOI] [PubMed] [Google Scholar]
- Brelot A., Chakrabarti L.A. CCR5 revisited: how mechanisms of HIV entry govern AIDS pathogenesis. J. Mol. Biol. 2018;430:2557–2589. doi: 10.1016/j.jmb.2018.06.027. [DOI] [PubMed] [Google Scholar]
- Brestovac B., Halicki L.A., Harris R.P., Sampson I., Speers D.J., Mamotte C., Williams D. Primary acute dengue and the deletion in chemokine receptor 5 (CCR5Δ32) Microbes Infect. 2014;16:518–521. doi: 10.1016/j.micinf.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Bukong T.N., Momen-Heravi F., Kodys K., Bala S., Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill M.E., Conley S., DeWan A.T., Montgomery R.R. Identification of genetic variants associated with dengue or West Nile virus disease: a systematic review and meta-analysis. BMC Infect. Dis. 2018;18:282. doi: 10.1186/s12879-018-3186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D.J. Control of regulatory T cell migration, function, and homeostasis. J. Immunol. 2015;195:2507–2513. doi: 10.4049/jimmunol.1500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin L.E., Hemann E.A., Zacharias Z.R., Heusel J.W., Legge K.L. Natural killer cell recruitment to the lung during Influenza A virus infection is dependent on CXCR3, CCR5, and virus exposure dose. Front. Immunol. 2018;9:781. doi: 10.3389/fimmu.2018.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casseb A.R., Cruz A.V., Jesus I.S., Chiang J.O., Martins L.C., Silva S.P., Henriques D.F., Casseb L.M.N., Vasconcelos P.F.C. Seroprevalence of flaviviruses antibodies in water buffaloes (Bubalus bubalis) in Brazilian Amazon. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014;20:9. doi: 10.1186/1678-9199-20-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC - Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Vector-Borne Diseases (DVBD) 2018. West Nile virus. Transmission. Available at: https://www.cdc.gov/westnile/transmission/index.html Acessed on: January 27, 2020. [Google Scholar]
- Chávez J.H., França R.F.O., Oliveira C.J.F., de Aquino M.T.P., Farias K.J.S., Machado P.R.L., de Oliveira T.F.M., Yokosawa J., Soares E.G., da Silva J.S., da Fonseca B.A.L., Figueiredo L.T.M. Influence of the CCR-5/MIP-1 α axis in the pathogenesis of Rocio virus encephalitis in a mouse model. Am. J. Trop. Med. Hyg. 2013;89:1013–1018. doi: 10.4269/ajtmh.12-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che L.F., Shao S.F., Wang L.X. Downregulation of CCR5 inhibits the proliferation and invasion of cervical cancer cells and is regulated by microRNA-107. Exp. Ther. Med. 2016;11:503–509. doi: 10.3892/etm.2015.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R., Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010;84:1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Guo J., Yang M., Zhu X., Cao X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J. Immunol. 2011;186:2219–2228. doi: 10.4049/jimmunol.1002991. [DOI] [PubMed] [Google Scholar]
- Chen Q., Liu Y., Lu A., Ni K., Xiang Z., Wen K., Tu W. Influenza virus infection exacerbates experimental autoimmune encephalomyelitis disease by promoting type I T cells infiltration into central nervous system. J. Autoimmun. 2017;77:1–10. doi: 10.1016/j.jaut.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiantore M.V., Mangino G., Iuliano M., Capriotti L., Di Bonito P., Fiorucci G., Romeo G. Human Papillomavirus and carcinogenesis: novel mechanisms of cell communication involving extracellular vesicles. Cytokine Growth Factor Rev. 2020;51:92–98. doi: 10.1016/j.cytogfr.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chies J.A.B., Nardi N.B. Sickle cell disease: a chronic inflammatory condition. Med. Hypotheses. 2001;57:46–50. doi: 10.1054/mehy.2000.1310. [DOI] [PubMed] [Google Scholar]
- Choo Q.L., Kuo G., Weiner A.J., Overby L.R., Bradley D.W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Chowdhury P., Khan S.A. Differential expression levels of inflammatory chemokines and TLRs in patients suffering from mild and severe Japanese encephalitis. Viral Immunol. 2019;32:68–74. doi: 10.1089/vim.2018.0103. [DOI] [PubMed] [Google Scholar]
- Christmann B.S., Moran J.M., McGraw J.A., Buller R.M., Corbett J.A. Ccr5 regulates inflammatory gene expression in response to encephalomyocarditis virus infection. Am. J. Pathol. 2011;179:2941–2951. doi: 10.1016/j.ajpath.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciota A.T. West Nile virus and its vectors. Curr. Opin. Insect Sci. 2017;28-36 doi: 10.1016/j.cois.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Coenen M., Nattermann J. The role of CCR5 in HCV infection. Eur. J. Med. Res. 2010;15:97–101. doi: 10.1186/2047-783x-15-3-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. The untold story of the ‘circle of trust’ behind the world’s first gene-edited babies. Science. 2019 doi: 10.1126/science.aay9400. [DOI] [Google Scholar]
- Cohen M., Vistarop A.G., Huaman F., Narbaitz M., Metrebian F., De Matteo E., Preciado M.V., Chabay P.A. Cytotoxic response against Epstein Barr virus coexists with diffuse large B-cell lymphoma tolerogenic microenvironment: clinical features and survival impact. Sci. Rep. 2017;7:10813. doi: 10.1038/s41598-017-11052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Cooper L.T.Jr. Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales I., Giménez E., Solano C., Amat P., de la Cámara R., Nieto J., Garcia-Noblejas A., Navarro D. Incidence and dynamics of active cytomegalovirus infection in allogeneic stem cell transplant patients according to single nucleotide polymorphisms in donor and recipient CCR5, MCP-1, IL-10, and TLR9 genes. J. Med. Virol. 2015;87:248–255. doi: 10.1002/jmv.24050. [DOI] [PubMed] [Google Scholar]
- Crough T., Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin. Microbiol. Rev. 2009;22:76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranoski D. What CRISPR-baby prison sentences mean for research. Nature. 2020;577:154–155. doi: 10.1038/d41586-020-00001-y. [DOI] [PubMed] [Google Scholar]
- Daley G.Q., Lovell-Badge R., Steffann J. After the storm - A responsible path for genome editing. N. Engl. J. Med. 2019;380:897–899. doi: 10.1056/NEJMp1900504. [DOI] [PubMed] [Google Scholar]
- Danial-Farran N., Eghbaria S., Schwartz N., Kra-Oz Z., Bisharat N. Genetic variants associated with susceptibility of Ashkenazi Jews to West Nile virus infection. Epidemiol. Infect. 2015;143:857–863. doi: 10.1017/S0950268814001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A.S.W., Roozen H.N., Dufort M.J., DeBerg H.A., Delaney M.A., Mair F., Erickson J.R., Slichter C.K., Berkson J.D., Klock A.M., Mack M., Lwo Y., Ko A., Brand R.M., McGowan I., Linsley P.S., Dixon D.R., Prlic M. The human tissue-resident CCR5+ T cell compartment maintains protective and functional properties during inflammation. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aaw8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T.C., Beck M.A., Kuziel W.A., Henderson F., Maeda N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am. J. Pathol. 2000;156:1951–1959. doi: 10.1016/S0002-9440(10)65068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Liu R., Ellmeier W., Choe S., Unutmaz D., Burkhart M., Di Marzio P., Marmon S., Sutton R.E., Hill C.M., Davis C.B., Peiper S.C., Schall T.J., Littman D.R., Landau N.R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- de-Oliveira-Pinto L.M., Marinho C.F., Povoa T.F., de Azeredo E.L., de Souza L.A., Barbosa L.D.R., Motta-Castro A.R., Alves A.M.B., Ávila C.A.L., de Souza L.A.J., da Cunha R.V., Damasco P.V., Paes M.V., Kubelka C.F. Regulation of inflammatory chemokine receptors on blood T cells associated to the circulating versus liver chemokines in dengue fever. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval H., Alagarasu K., Mittal M., Srivastava N., Bachal R., Gondhalekar A., Chaudhary U., Chowdhary D., Bondre V.P. Association of single nucleotide polymorphisms in TNFA and CCR5 genes with Japanese Encephalitis: a study from an endemic region of North India. J. Neuroimmunol. 2019;336 doi: 10.1016/j.jneuroim.2019.577043. [DOI] [PubMed] [Google Scholar]
- Diamond M.S. Virus and host determinants of West Nile virus pathogenesis. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobaczewski M., Xia Y., Bujak M., Gonzalez-Quesada C., Frangogiannis N.G. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am. J. Pathol. 2010;176:2177–2187. doi: 10.2353/ajpath.2010.090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doodes P.D., Cao Y., Hamel K.M., Wang Y., Rodeghero R.L., Kobezda T., Finnegan A. CCR5 is involved in resolution of inflammation in proteoglycan-induced arthritis. Arthritis Rheum. 2009;60:2945–2953. doi: 10.1002/art.24842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T., Litwin V., Allaway G.P., Martin S.R., Huang Y., Nagashima K.A., Cayanan C., Maddon P.J., Koup R.A., Moore J.P., Paxton W.A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Durrant D.M., Daniels B.P., Pasieka T., Dorsey D., Klein R.S. CCR5 limits cortical viral loads during West Nile virus infection of the central nervous system. J. Neuroinflammation. 2015;12:233. doi: 10.1186/s12974-015-0447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlin-Henriksson B., Liang W., Cagigi A., Mowafi F., Klein G., Nilsson A. Changes in chemokines and chemokine receptor expression on tonsillar B cells upon Epstein-Barr virus infection. Immunology. 2009;127:549–557. doi: 10.1111/j.1365-2567.2008.03029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwanger J.H., Chies J.A.B. Host immunogenetics in tick-borne encephalitis virus infection-The CCR5 crossroad. Ticks Tick. Dis. 2019;10:729–741. doi: 10.1016/j.ttbdis.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Ellwanger J.H., Chies J.A.B. Host genetic factors can impact vaccine immunogenicity and effectiveness. Lancet Infect. Dis. 2019;19:359–360. doi: 10.1016/S1473-3099(19)30121-5. [DOI] [PubMed] [Google Scholar]
- Ellwanger J.H., Crovella S., Dos Reis E.C., Pontillo A., Chies J.A.B. Exosomes are possibly used as a tool of immune regulation during the dendritic cell-based immune therapy against HIV-I. Med. Hypotheses. 2016;95:67–70. doi: 10.1016/j.mehy.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Ellwanger J.H., Veit T.D., Chies J.A.B. Exosomes in HIV infection: a review and critical look. Infect. Genet. Evol. 2017;53:146–154. doi: 10.1016/j.meegid.2017.05.021. [DOI] [PubMed] [Google Scholar]
- Ellwanger J.H., Kaminski V.L., Chies J.A.B. Rocio virus: an overview. RDGBM. 2017;1:14–20. [Google Scholar]
- Ellwanger J.H., Kaminski V.L., Valverde-Villegas J.M., Simon D., Lunge V.R., Chies J.A.B. Immunogenetic studies of the hepatitis C virus infection in an era of pan-genotype antiviral therapies - Effective treatment is coming. Infect. Genet. Evol. 2018;66:376–391. doi: 10.1016/j.meegid.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Ellwanger J.H., Leal B.K., Valverde-Villegas J.M., Simon D., Marangon C.G., Mattevi V.S., Lazzaretti R.K., Sprinz E., Kuhmmer R., Chies J.A.B. CCR5Δ32 in HCV infection, HCV/HIV co-infection, and HCV-related diseases. Infect. Genet. Evol. 2018;59:163–166. doi: 10.1016/j.meegid.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Ellwanger J.H., Zambra F.M.B., Guimarães R.L., Chies J.A.B. MicroRNA-related polymorphisms in infectious diseases-Tiny changes with a huge impact on viral infections and potential clinical applications. Front. Immunol. 2018;9:1316. doi: 10.3389/fimmu.2018.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwanger J.H., Kaminski V.L., Chies J.A.B. CCR5 gene editing - revisiting pros and cons of CCR5 absence. Infect. Genet. Evol. 2019;68:218–220. doi: 10.1016/j.meegid.2018.12.027. [DOI] [PubMed] [Google Scholar]
- Ellwanger J.H., Kaminski V.L., Chies J.A.B. What we say and what we mean when we say redundancy and robustness of the chemokine system - how CCR5 challenges these concepts. Immunol. Cell Biol. 2020;98:22–27. doi: 10.1111/imcb.12291. [DOI] [PubMed] [Google Scholar]
- Ellwanger J.H., Kulmann-Leal B., Wolf J.M., Michita R.T., Simon D., Lunge V.R., Chies J.A.B. Role of the genetic variant CCR5Δ32 in HBV infection and HBV/HIV co-infection. Virus Res. 2020;277 doi: 10.1016/j.virusres.2019.197838. [DOI] [PubMed] [Google Scholar]
- El-Moamly A.A., El-Sweify M.A., Rashad R.M., Abdalla E.M., Ragheb M.M., Awad M.M. Role of CCR5Δ32 mutation in protecting patients with Schistosoma mansoni infection against hepatitis C viral infection or progression. Parasitol. Res. 2013;112:2745–2752. doi: 10.1007/s00436-013-3380-9. [DOI] [PubMed] [Google Scholar]
- Engin A., Köksal B., Doğan ÖT., Elaldi N., Dökmetaş İ, Bakir M., Özdemir Ö. Cytochrome P450 2D6 and MDR1 gene mutation in relation to mortality in patients with Crimean-Congo hemorrhagic fever: a preliminary study. Turkiye Klinikleri J Med Sci. 2009;29:905–910. [Google Scholar]
- Ergönül O. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 2006;6:203–214. doi: 10.1016/S1473-3099(06)70435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel S.A., Bromley S.K., Medoff B.D., Luster A.D. CXCR3-deficiency protects influenza-infected CCR5-deficient mice from mortality. Eur. J. Immunol. 2008;38:3376–3387. doi: 10.1002/eji.200838628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon A., Cuevas M.T., Rodriguez-Frandsen A., Reyes N., Pozo F., Moreno S., Ledesma J., Martínez-Alarcón J., Nieto A., Casas I. CCR5 deficiency predisposes to fatal outcome in influenza virus infection. J. Gen. Virol. 2015;96:2074–2078. doi: 10.1099/vir.0.000165. [DOI] [PubMed] [Google Scholar]
- Fantuzzi L., Tagliamonte M., Gauzzi M.C., Lopalco L. Dual CCR5/CCR2 targeting: opportunities for the cure of complex disorders. Cell. Mol. Life Sci. 2019;76:4869–4886. doi: 10.1007/s00018-019-03255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fätkenheuer G., Hoffmann C., Slim J., Rouzier R., Keung A., Li J., Treitel M., Sansone-Parsons A., Kasserra C., O’Mara E., Schürmann D. Short-term administration of the CCR5 antagonist vicriviroc to patients with HIV and HCV coinfection is safe and tolerable. J. Acquir. Immune Defic. Syndr. 2010;53:78–85. doi: 10.1097/QAI.0b013e3181bb28dc. [DOI] [PubMed] [Google Scholar]
- Figueiredo L.T. Emergent arboviruses in Brazil. Rev. Soc. Bras. Med. Trop. 2007;40:224–229. doi: 10.1590/s0037-86822007000200016. [DOI] [PubMed] [Google Scholar]
- Froberg M.K. Review: CMV escapes! Ann. Clin. Lab. Sci. 2004;34:123–130. [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- Ganczak M., Skonieczna-Żydecka K., Drozd-Dąbrowska M., Adler G. Possible impact of 190G & A CCR2 and Δ32 CCR5 mutations on decrease of the HBV vaccine immunogenicity-A preliminary report. Int. J. Environ. Res. Public Health. 2017;14:166. doi: 10.3390/ijerph14020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K., Jin J., Huang C., Li J., Luo H., Li L., Huang Y., Jiang Y. Exosomes derived from septic mouse serum modulate immune responses via exosome-associated cytokines. Front. Immunol. 2019;10:1560. doi: 10.3389/fimmu.2019.01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K., Zhu W., Li H., Ma D., Liu W., Yu W., Wang L., Cao Y., Jiang Y. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod. Rheumatol. 2019 doi: 10.1080/14397595.2019.1651445. in press. [DOI] [PubMed] [Google Scholar]
- Garrison A.R., Smith D.R., Golden J.W. Animal models for Crimean-Congo hemorrhagic fever human disease. Viruses. 2019;11:590. doi: 10.3390/v11070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinto C., Mawela M.P., Chokephaibulkit K., Negra M.D., Mitha I.H., Fourie J., Fang A., van der Ryst E., Valluri S.R., Vourvahis M., Zhang-Roper R.Y., Craig C., McFadyen L., Clark A., Heera J. Pharmacokinetics, safety and efficacy of maraviroc in treatment-experienced pediatric patients infected with CCR5-tropic HIV-1. Pediatr. Infect. Dis. J. 2018;37:459–465. doi: 10.1097/INF.0000000000001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas J., Török H.P., Simperl C., König A., Martin K., Schmidt F., Schaefer M., Schiemann U., Folwaczny C. The Δ32 mutation of the chemokine-receptor 5 gene neither is correlated with chronic hepatitis C nor does it predict response to therapy with interferon-α and ribavirin. Clin. Immunol. 2003;108:46–50. doi: 10.1016/S1521-6616(03)00059-7. [DOI] [PubMed] [Google Scholar]
- Glass W.G., Lane T.E. Functional expression of chemokine receptor CCR5 on CD4+ T cells during virus-induced central nervous system disease. J. Virol. 2003;77:191–198. doi: 10.1128/jvi.77.1.191-198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass W.G., Lane T.E. Functional analysis of the CC chemokine receptor 5 (CCR5) on virus-specific CD8+ T cells following coronavirus infection of the central nervous system. Virology. 2003;312:407–414. doi: 10.1016/s0042-6822(03)00237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass W.G., Liu M.T., Kuziel W.A., Lane T.E. Reduced macrophage infiltration and demyelination in mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology. 2001;288:8–17. doi: 10.1006/viro.2001.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass W.G., Rosenberg H.F., Murphy P.M. Chemokine regulation of inflammation during acute viral infection. Curr. Opin. Allergy Clin. Immunol. 2003;3:467–473. doi: 10.1097/01.all.0000104448.09202.91. [DOI] [PubMed] [Google Scholar]
- Glass W.G., Lim J.K., Cholera R., Pletnev A.G., Gao J.L., Murphy P.M. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J. Exp. Med. 2005;202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass W.G., McDermott D.H., Lim J.K., Lekhong S., Yu S.F., Frank W.A., Pape J., Cheshier R.C., Murphy P.M. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J. Exp. Med. 2006;203:35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding C., McManus R., Murphy A., MacDonald G., Barrett S., Crowe J., Hegarty J., McKiernan S., Kelleher D. The CCR5-Δ32 mutation: impact on disease outcome in individuals with hepatitis C infection from a single source. Gut. 2005;54:1157–1161. doi: 10.1136/gut.2004.055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govaerts C., Bondue A., Springael J.Y., Olivella M., Deupi X., Le Poul E., Wodak S.J., Parmentier M., Pardo L., Blanpain C. Activation of CCR5 by chemokines involves an aromatic cluster between transmembrane helices 2 and 3. J. Biol. Chem. 2003;278:1892–1903. doi: 10.1074/jbc.M205685200. [DOI] [PubMed] [Google Scholar]
- Goyal A., Suneetha P.V., Kumar G.T., Shukla D.K., Arora N., Sarin S.K. CCR5Δ32 mutation does not influence the susceptibility to HCV infection, severity of liver disease and response to therapy in patients with chronic hepatitis C. World J. Gastroenterol. 2006;12:4721–4726. doi: 10.3748/wjg.v12.i29.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson M.H., Ramos M.S., Rohlfing M.M., Kitchens R., Wang H.D., Gould A., Agapov E., Holtzman M.J. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J. Immunol. 2007;179:1438–1448. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- Greely H.T. CRISPR’d babies: human germline genome editing in the ‘He Jiankui affair’. J. Law Biosci. 2019;6:111–183. doi: 10.1093/jlb/lsz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregianini T.S., Varella IRS Fisch P., Martins L.G., Veiga A.B.G. Dual and triple infections with influenza A and B viruses: a case-control study in Southern Brazil. J. Infect. Dis. 2019;220:961–968. doi: 10.1093/infdis/jiz221. [DOI] [PubMed] [Google Scholar]
- Griffiths P., Baraniak I., Reeves M. The pathogenesis of human cytomegalovirus. J. Pathol. 2015;235:288–297. doi: 10.1002/path.4437. [DOI] [PubMed] [Google Scholar]
- Guglielmi L., Gimenez S., Larroque M., Tong X., Portalès P., Corbeau P. Circulating human CD4+ T cells have intracellular pools of CCR5 molecules. Blood. 2011;118:1177–1178. doi: 10.1182/blood-2011-03-339754. [DOI] [PubMed] [Google Scholar]
- Gulick R.M., Fatkenheuer G., Burnside R., Hardy W.D., Nelson M.R., Goodrich J., Mukwaya G., Portsmouth S., Heera J.R. Five-year safety evaluation of maraviroc in HIV-1-infected treatment-experienced patients. J. Acquir. Immune Defic. Syndr. 2014;65:78–81. doi: 10.1097/QAI.0b013e3182a7a97a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N., Rao P.V. Transcriptomic profile of host response in Japanese encephalitis virus infection. Virol. J. 2011;8:92. doi: 10.1186/1743-422X-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R.K., Abdul-Jawad S., McCoy L.E., Mok H.P., Peppa D., Salgado M., Martinez-Picado J., Nijhuis M., Wensing A.M.J., Lee H., Grant P., Nastouli E., Lambert J., Pace M., Salasc F., Monit C., Innes A.J., Muir L., Waters L., Frater J., Lever A.M.L., Edwards S.G., Gabriel I.H., Olavarria E. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature. 2019;568:244–248. doi: 10.1038/s41586-019-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R.K., Peppa D., Hill A.L., Gálvez C., Salgado M., Pace M., McCoy L.E., Griffith S.A., Thornhill J., Alrubayyi A., Huyveneers L.E.P., Nastouli E., Grant P., Edwards S.G., Innes A.J., Frater J., Nijhuis M., Wensing A.M.J., Martinez-Picado J., Olavarria E. Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV. 2020;7:e340–e347. doi: 10.1016/S2352-3018(20)30069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M.G., Gubler D.J., Izquierdo A., Martinez E., Halstead S.B. Dengue infection. Nat. Rev. Dis. Primers. 2016;2:16055. doi: 10.1038/nrdp.2016.55. [DOI] [PubMed] [Google Scholar]
- Haïm-Boukobza S., Balabanian K., Teicher E., Bourgeade M., Perlemuter G., Roque-Afonso A.M., Duclos-Vallee J.C. Blockade of CCR5 to protect the liver graft in HIV/HCV co-infected patients. J. Hepatol. 2013;59:613–615. doi: 10.1016/j.jhep.2013.03.034. [DOI] [PubMed] [Google Scholar]
- Hall F.S., Raman D., Tiwari A.K. CCR5 and responses to cocaine: addiction is not just about the brain. Brain Behav. Immun. 2020;84:8–9. doi: 10.1016/j.bbi.2019.12.008. [DOI] [PubMed] [Google Scholar]
- Hansel T.T., Tunstall T., Trujillo-Torralbo M.B., Shamji B., Del-Rosario A., Dhariwal J., Kirk P.D.W., Stumpf M.P.H., Koopmann J., Telcian A., Aniscenko J., Gogsadze L., Bakhsoliani E., Stanciu L., Bartlett N., Edwards M., Walton R., Mallia P., Hunt T.M., Hunt T.M.L., Hunt D.G., Westwick J., Edwards M., Kon O.M., Jackson D.J., Johnston S.L. A comprehensive evaluation of nasal and bronchial cytokines and chemokines following experimental rhinovirus infection in allergic asthma: increased interferons (IFN-γ and IFN-λ) and type 2 inflammation (IL-5 and IL-13) EBioMedicine. 2017;19:128–138. doi: 10.1016/j.ebiom.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier S., Frodsham A.J., Hennig B.J.W., Klenerman P., Knapp S., Ramaley P., Satsangi J., Wright M., Zhang L., Thomas H.C., Thursz M., Hill A.V.S. Association of genetic variants of the chemokine receptor CCR5 and its ligands, RANTES and MCP-2, with outcome of HCV infection. Hepatology. 2003;38:1468–1476. doi: 10.1016/j.hep.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Herbein G. The human cytomegalovirus, from oncomodulation to oncogenesis. Viruses. 2018;10:408. doi: 10.3390/v10080408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover K.C. Intragenus (Homo) variation in a chemokine receptor gene (CCR5) PLoS One. 2018;13 doi: 10.1371/journal.pone.0204989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain F.M.A., Park S.O., Kim H.J., Eo J.C., Choi J.Y., Uyangaa E., Kim B., Kim K., Eo S.K. CCR5 attenuates neutrophilic airway inflammation exacerbated by infection with rhinovirus. Cell. Immunol. 2020 doi: 10.1016/j.cellimm.2020.104066. in press. [DOI] [PubMed] [Google Scholar]
- Hosseinkhani B., Kuypers S., van den Akker N.M.S., Molin D.G.M., Michiels L. Extracellular vesicles work as a functional inflammatory mediator between vascular endothelial cells and immune cells. Front. Immunol. 2018;9:1789. doi: 10.3389/fimmu.2018.01789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Paxton W.A., Wolinsky S.M., Neumann A.U., Zhang L., He T., Kang S., Ceradini D., Jin Z., Yazdanbakhsh K., Kunstman K., Erickson D., Dragon E., Landau N.R., Phair J., Ho D.D., Koup R.A. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- Huang H., Zepp M., Georges R.B., Jarahian M., Kazemi M., Eyol E., Berger M.R. The CCR5 antagonist maraviroc causes remission of pancreatic cancer liver metastasis in nude rats based on cell cycle inhibition and apoptosis induction. Cancer Lett. 2020;474:82–93. doi: 10.1016/j.canlet.2020.01.009. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huik K., Avi R., Carrillo A., Harper N., Pauskar M., Sadam M., Karki T., Krispin T., Kongo U.K., Jermilova T., Rüütel K., Talu A., Abel-Ollo K., Uusküla A., Ahuja S.K., He W., Lutsar I. CCR5 haplotypes influence HCV serostatus in Caucasian intravenous drug users. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter G., Nowak D., Mossner M., Ganepola S., Müssig A., Allers K., Schneider T., Hofmann J., Kücherer C., Blau O., Blau I.W., Hofmann W.K., Thiel E. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- Hütter G., Neumann M., Nowak D., Klein S., Klüter H., Hofmann W.K. The effect of the CCR5-delta32 deletion on global gene expression considering immune response and inflammation. J. Inflamm. Lond. 2011;8:29. doi: 10.1186/1476-9255-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M., Kalita T., Saikia A.K., Begum A., Baruah V., Singh N., Borkotoky R., Bose S. Significance of RANTES-CCR5 axis and linked downstream immunomodulation in Dengue pathogenesis: A study from Guwahati, India. J. Med. Virol. 2019;91:2066–2073. doi: 10.1002/jmv.25561. [DOI] [PubMed] [Google Scholar]
- Johnson E.L., Howard C.L., Thurman J., Pontiff K., Johnson E.L.S., Chakraborty R. Cytomegalovirus upregulates expression of CCR5 in central memory cord blood mononuclear cells, which may facilitate in utero HIV type 1 transmission. J. Infect. Dis. 2015;211:187–196. doi: 10.1093/infdis/jiu424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.L., Boggavarapu S., Johnson E.L.S., Lal A.A., Agrawal P., Bhaumik S.K., Murali-Krishna K., Chakraborty R. Human cytomegalovirus enhances placental susceptibility and replication of Human Immunodeficiency Virus type 1 (HIV-1), which may facilitate in utero HIV-1 transmission. J. Infect. Dis. 2018;218:1464–1473. doi: 10.1093/infdis/jiy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy M.T., Ben Assayag E., Shabashov-Stone D., Liraz-Zaltsman S., Mazzitelli J., Arenas M., Abduljawad N., Kliper E., Korczyn A.D., Thareja N.S., Kesner E.L., Zhou M., Huang S., Silva T.K., Katz N., Bornstein N.M., Silva A.J., Shohami E., Carmichael S.T. CCR5 is a therapeutic target for recovery after stroke and traumatic brain injury. Cell. 2019;176:1143–1157. doi: 10.1016/j.cell.2019.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski V.L., Ellwanger J.H., Sandrim V., Pontillo A., Chies J.A.B. Influence of NKG2C gene deletion and CCR5Δ32 in pre-eclampsia-Approaching the effect of innate immune gene variants in pregnancy. Int. J. Immunogenet. 2019;46:82–87. doi: 10.1111/iji.12416. [DOI] [PubMed] [Google Scholar]
- Kaminski V.L., Ellwanger J.H., Chies J.A.B. Extracellular vesicles in host-pathogen interactions and immune regulation - exosomes as emerging actors in the immunological theater of pregnancy. Heliyon. 2019;5:e02355. doi: 10.1016/j.heliyon.2019.e02355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasztelewicz B., Czech-Kowalska J., Lipka B., Milewska-Bobula B., Borszewska-Kornacka M.K., Romańska J., Dzierżanowska-Fangrat K. Cytokine gene polymorphism associations with congenital cytomegalovirus infection and sensorineural hearing loss. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:1811–1818. doi: 10.1007/s10096-017-2996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohane C., Chimelli L., Ryan A. Poliovirus infection and postpolio syndrome. In: Chrétien F., Wong K.T., Sharer L.R., Keohane C., Gray F., editors. Infections of the Central Nervous System: Pathology and Genetics. John Wiley & Sons Ltd.; 2020. pp. 195–204. [Google Scholar]
- Kew O., Pallansch M. Breaking the last chains of poliovirus transmission: progress and challenges in global polio eradication. Annu. Rev. Virol. 2018;5:427–451. doi: 10.1146/annurev-virology-101416-041749. [DOI] [PubMed] [Google Scholar]
- Keynan Y., Juno J., Meyers A., Ball T.B., Kumar A., Rubinstein E., Fowke K.R. Chemokine receptor 5 Δ32 allele in patients with severe pandemic (H1N1) 2009. Emerg Infect Dis. 2010;16:1621–1622. doi: 10.3201/eid1610.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaiboullina S.F., Lopes P., de Carvalho T.G., Real A.L.C.V., Souza D.G., Costa V.V., Teixeira M.M., Bloise E., Verma S.C., Ribeiro F.M. Host immune response to ZIKV in an immunocompetent embryonic mouse model of intravaginal infection. Viruses. 2019;11:558. doi: 10.3390/v11060558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I.A., Thomas S.Y., Moretto M.M., Lee F.S., Islam S.A., Combe C., Schwartzman J.D., Luster A.D. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2006;2:e49. doi: 10.1371/journal.ppat.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorramdelazad H., Hakimizadeh E., Hassanshahi G., Rezayati M., Sendi H., Arababadi M.K. CCR5 Δ 32 mutation is not prevalent in Iranians with chronic HBV infection. J. Med. Virol. 2013;85:964–968. doi: 10.1002/jmv.23510. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Patil A.M., Choi J.Y., Kim S.B., Uyangaa E., Hossain FMA Park S.Y., Lee J.H., Eo S.K. CCR5 ameliorates Japanese encephalitis via dictating the equilibrium of regulatory CD4+Foxp3+ T and IL-17+CD4+ Th17 cells. J. Neuroinflammation. 2016;13:223. doi: 10.1186/s12974-016-0656-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.A., Baillie J., Sinclair J.H. Human cytomegalovirus modulation of CCR5 expression on myeloid cells affects susceptibility to human immunodeficiency virus type 1 infection. J. Gen. Virol. 2006;87:2171–2180. doi: 10.1099/vir.0.81452-0. [DOI] [PubMed] [Google Scholar]
- Klein R.S. Discussion on frequency of the HIV-protective CC chemokine receptor 5-Δ32/Δ32 genotype is increased in hepatitis C. Gastroenterology. 2003;124:1558. doi: 10.1016/s0016-5085(03)00348-2. [DOI] [PubMed] [Google Scholar]
- Kletenkov K., Martynova E., Davidyuk Y., Kabwe E., Shamsutdinov A., Garanina E., Shakirova V., Khaertynova I., Anokhin V., Tarlinton R., Rizvanov A., Khaiboullina S., Morzunov S. Δccr5 genotype is associated with mild form of nephropathia epidemica. Viruses. 2019;11:675. doi: 10.3390/v11070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodidela S., Ranjit S., Sinha N., McArthur C., Kumar A., Kumar S. Cytokine profiling of exosomes derived from the plasma of HIV-infected alcohol drinkers and cigarette smokers. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier J.E., Miller S.C., Smith J., Lu B., Gerard C., Cookenham T., Roberts A.D., Woodland D.L. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29:101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konadu K.A., Chu J., Huang M.B., Amancha P.K., Armstrong W., Powell M.D., Villinger F., Bond V.C. Association of cytokines with exosomes in the plasma of HIV-1-seropositive individuals. J. Infect. Dis. 2015;211:1712–1716. doi: 10.1093/infdis/jiu676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi I., Horiike N., Hiasa Y., Michitaka K., Onji M. CCR5 promoter polymorphism influences the interferon response of patients with chronic hepatitis C in Japan. Intervirology. 2004;47:114–120. doi: 10.1159/000077835. [DOI] [PubMed] [Google Scholar]
- Kramer L.D., Styer L.M., Ebel G.D. A global perspective on the epidemiology of West Nile virus. Annu. Rev. Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- Krammer F., Smith G.J.D., Fouchier R.A.M., Peiris M., Kedzierska K., Doherty P.C., Palese P., Shaw M.L., Treanor J., Webster R.G., García-Sastre A. Influenza. Nat. Rev. Dis. Primers. 2018;4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl U., Pauschinger M., Seeberg B., Lassner D., Noutsias M., Poller W., Schultheiss H.P. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- Kühl U., Lassner D., von Schlippenbach J., Poller W., Schultheiss H.P. Interferon-Beta improves survival in enterovirus-associated cardiomyopathy. J. Am. Coll. Cardiol. 2012;60:1295–1296. doi: 10.1016/j.jacc.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Kulkarni S., Lied A., Kulkarni V., Rucevic M., Martin M.P., Walker-Sperling V., Anderson S.K., Ewy R., Singh S., Nguyen H., McLaren P.J., Viard M., Naranbhai V., Zou C., Lin Z., Gatanaga H., Oka S., Takiguchi M., Thio C.L., Margolick J., Kirk G.D., Goedert J.J., Hoots W.K., Deeks S.G., Haas D.W., Michael N., Walker B., Le Gall S., Chowdhury F.Z., Yu X.G., Carrington M. CCR5AS lncRNA variation differentially regulates CCR5, influencing HIV disease outcome. Nat. Immunol. 2019;20:824–834. doi: 10.1038/s41590-019-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulmann-Leal B., Ellwanger J.H., Chies J.A.B. A functional interaction between the CCR5 and CD34 molecules expressed in hematopoietic cells can support (or even promote) the development of cancer. Hematol Transfus Cell Ther. 2020;42:70–76. doi: 10.1016/j.htct.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacalle R.A., Blanco R., Carmona-Rodríguez L., Martín-Leal A., Mira E., Mañes S. Chemokine receptor signaling and the hallmarks of cancer. Int. Rev. Cell Mol. Biol. 2017;331:181–244. doi: 10.1016/bs.ircmb.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Lang J., Neumann-Haefelin C., Thimme R. Immunological cure of HBV infection. Hepatol. Int. 2019;13:113–124. doi: 10.1007/s12072-018-9912-8. [DOI] [PubMed] [Google Scholar]
- Larena M., Regner M., Lobigs M. The chemokine receptor CCR5, a therapeutic target for HIV/AIDS antagonists, is critical for recovery in a mouse model of Japanese encephalitis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrubia J.R., Calvino M., Benito S., Sanz-de-Villalobos E., Perna C., Pérez-Hornedo J., González-Mateos F., García-Garzón S., Bienvenido A., Parra T. The role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infection. J. Hepatol. 2007;47:632–641. doi: 10.1016/j.jhep.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Lassner D., Siegismund C.S., Kühl U., Rohde M., Stroux A., Escher F., Schultheiss H.P. CCR5del32 genotype in human enteroviral cardiomyopathy leads to spontaneous virus clearance and improved outcome compared to wildtype CCR5. J. Transl. Med. 2018;16:249. doi: 10.1186/s12967-018-1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latinovic O.S., Reitz M., Heredia A. CCR5 inhibitors and HIV-1 infection. J AIDS HIV Treat. 2019;1:1–5. doi: 10.33696/AIDS.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law H.K., Cheung C.Y., Sia S.F., Chan Y.O., Peiris J.S., Lau Y.L. Toll-like receptors, chemokine receptors and death receptor ligands responses in SARS coronavirus infected human monocyte derived dendritic cells. BMC Immunol. 2009;10:35. doi: 10.1186/1471-2172-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Flohic G., Porphyre V., Barbazan P., Gonzalez J.P. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Negl. Trop. Dis. 2013;7:e2208. doi: 10.1371/journal.pntd.0002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebecque O., Dupont M. Puumala hantavirus: an imaging review. Acta Radiol. 2019 doi: 10.1177/0284185119889564. in press. [DOI] [PubMed] [Google Scholar]
- Lecointe D., Dugas N., Leclerc P., Hery C., Delfraissy J.F., Tardieu M. Human cytomegalovirus infection reduces surface CCR5 expression in human microglial cells, astrocytes and monocyte-derived macrophages. Microbes Infect. 2002;4:1401–1408. doi: 10.1016/s1286-4579(02)00022-9. [DOI] [PubMed] [Google Scholar]
- Lee H.M., Banini B.A. Updates on chronic HBV: current challenges and future goals. Curr. Treat. Options Gastroenterol. 2019;17:271–291. doi: 10.1007/s11938-019-00236-3. [DOI] [PubMed] [Google Scholar]
- Li H., Xie H.Y., Zhou L., Wang W.L., Liang T.B., Zhang M., Zheng S.S. Polymorphisms of CCL3L1/CCR5 genes and recurrence of hepatitis B in liver transplant recipients. HBPD INT. 2011;10:593–598. doi: 10.1016/S1499-3872(11)60101-X. [DOI] [PubMed] [Google Scholar]
- Li M., Lu Y., Xu Y., Wang J., Zhang C., Du Y., Wang L., Li L., Wang B., Shen J., Tang J., Song B. Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene. 2018;676:101–109. doi: 10.1016/j.gene.2018.07.018. [DOI] [PubMed] [Google Scholar]
- Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Qiao L., Peng X., Cui Z., Yin Y., Liao H., Jiang M., Li L. The chemokine receptor CCR1 is identified in mast cell-derived exosomes. Am. J. Transl. Res. 2018;10:352–367. [PMC free article] [PubMed] [Google Scholar]
- Lichterfeld M., Leifeld L., Nischalke H.D., Rockstroh J.K., Hess L., Sauerbruch T., Spengler U. Reduced CC chemokine receptor (CCR) 1 and CCR5 surface expression on peripheral blood T lymphocytes from patients with chronic hepatitis C infection. J. Infect. Dis. 2002;185:1803–1807. doi: 10.1086/340829. [DOI] [PubMed] [Google Scholar]
- Lim J.K., Murphy P.M. Chemokine control of West Nile virus infection. Exp. Cell Res. 2011;317:569–574. doi: 10.1016/j.yexcr.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.K., Glass W.G., McDermott D.H., Murphy P.M. CCR5: no longer a ‘good for nothing’ gene--chemokine control of West Nile virus infection. Trends Immunol. 2006;27:308–312. doi: 10.1016/j.it.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Lim J.K., Louie C.Y., Glaser C., Jean C., Johnson B., Johnson H., McDermott D.H., Murphy P.M. Genetic deficiency of chemokine receptor CCR5 is a strong risk factor for symptomatic West Nile virus infection: a meta-analysis of 4 cohorts in the US epidemic. J. Infect. Dis. 2008;197:262–265. doi: 10.1086/524691. [DOI] [PubMed] [Google Scholar]
- Lim J.K., McDermott D.H., Lisco A., Foster G.A., Krysztof D., Follmann D., Stramer S.L., Murphy P.M. CCR5 deficiency is a risk factor for early clinical manifestations of West Nile virus infection but not for viral transmission. J. Infect. Dis. 2010;201:178–185. doi: 10.1086/649426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M.C., de Mendonça L.R., Rezende A.M., Carrera R.M., Aníbal-Silva C.E., Demers M., D’Aiuto L., Wood J., Chowdari K.V., Griffiths M., Lucena-Araujo A.R., Barral-Netto M., Azevedo E.A.N., Alves R.W., Farias P.C.S., Marques E.T.A., Castanha PMS Donald C.L., Kohl A., Nimgaonkar V.L., Franca R.F.O. The transcriptional and protein profile from human infected neuroprogenitor cells is strongly correlated to Zika virus microcephaly cytokines phenotype evidencing a persistent inflammation in the CNS. Front. Immunol. 2019;10:1928. doi: 10.3389/fimmu.2019.01928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingala S., Ghany M.G. Natural history of hepatitis C. Gastroenterol. Clin. North Am. 2015;44:717–734. doi: 10.1016/j.gtc.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Zhang X., Yu Q., He Jj. Exosome-associated hepatitis C virus in cell cultures and patient plasma. Biochem. Biophys. Res. Commun. 2014;455:218–222. doi: 10.1016/j.bbrc.2014.10.146. [DOI] [PubMed] [Google Scholar]
- Liu Q., Zhou Y.H., Yang Z.Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016;13:3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Xiao C., Wang F., Xiang X., Ou A., Wei J., Li B., Shao D., Miao D., Zhao F., Long G., Qiu Y., Zhu H., Ma Z. Chemokine receptor antagonist block inflammation and therapy Japanese encephalitis virus infection in mouse model. Cytokine. 2018;110:70–77. doi: 10.1016/j.cyto.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Loeb M., Eskandarian S., Rupp M., Fishman N., Gasink L., Patterson J., Bramson J., Hudson T.J., Lemire M. Genetic variants and susceptibility to neurological complications following West Nile virus infection. J. Infect. Dis. 2011;204:1031–1037. doi: 10.1093/infdis/jir493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler J., Steffens M., Arlt E.M., Toliat M.R., Mezger M., Suk A., Wienker T.F., Hebart H., Nürnberg P., Boeckh M., Ljungman P., Trenschel R., Einsele H. Polymorphisms in the genes encoding chemokine receptor 5, interleukin-10, and monocyte chemoattractant protein 1 contribute to cytomegalovirus reactivation and disease after allogeneic stem cell transplantation. J. Clin. Microbiol. 2006;44:1847–1850. doi: 10.1128/JCM.44.5.1847-1850.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M., Karrer U., Lucas A., Klenerman P. Viral escape mechanisms - escapology taught by viruses. Int. J. Exp. Pathol. 2001;82:269–286. doi: 10.1046/j.1365-2613.2001.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M., Kleinschmidt A., Brühl H., Klier C., Nelson P.J., Cihak J., Plachý J., Stangassinger M., Erfle V., Schlöndorff D. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 2000;6:769–775. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- Maestri A., dos Santos M.C., Ribeiro-Rodrigues E.M., de Mello W.A., Sousa R.C.M., dos Santos S.E., Sortica V.A. The CCR5Δ32 (rs333) polymorphism is not a predisposing factor for severe pandemic influenza in the Brazilian admixed population. BMC Res. Notes. 2015;8:326. doi: 10.1186/s13104-015-1299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvoisin E., Livrozet J.M., Makloufi D., Vincent N. Soluble chemokine receptor CXCR4 is present in human sera. Anal. Biochem. 2011;414:202–207. doi: 10.1016/j.ab.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Mangia A., Santoro R., D’agruma L., Andriulli A. HCV chronic infection and CCR5-Δ32/Δ32. Gastroenterology. 2003;124:868–869. doi: 10.1053/gast.2003.50134. [DOI] [PubMed] [Google Scholar]
- Mangieri L.F.L., Sena M.M., Cezar-Dos-Santos F., Trugilo K.P., Okuyama N.C.M., Pereira ÉR., Maria G.C.Q., Watanabe M.A.E., de Oliveira K.B. CCR5 genetic variants and epidemiological determinants for HPV infection and cervical premalignant lesions. Int. J. Immunogenet. 2019;46:331–338. doi: 10.1111/iji.12444. [DOI] [PubMed] [Google Scholar]
- Markotić A., Hensley L., Daddario K., Spik K., Anderson K., Schmaljohn C. Pathogenic hantaviruses elicit different immunoreactions in THP-1 cells and primary monocytes and induce differentiation of human monocytes to dendritic-like cells. Coll. Antropol. 2007;31:1159–1167. [PubMed] [Google Scholar]
- Marques R.E., Guabiraba R., Del Sarto J.L., Rocha R.F., Queiroz A.L., Cisalpino D., Marques P.E., Pacca C.C., Fagundes C.T., Menezes G.B., Nogueira M.L., Souza D.G., Teixeira M.M. Dengue virus requires the CC-chemokine receptor CCR5 for replication and infection development. Immunology. 2015;145:583–596. doi: 10.1111/imm.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blondel G., Brassat D., Bauer J., Lassmann H., Liblau R.S. CCR5 blockade for neuroinflammatory diseases-beyond control of HIV. Nat. Rev. Neurol. 2016;12:95–105. doi: 10.1038/nrneurol.2015.248. [DOI] [PubMed] [Google Scholar]
- Mascheretti S., Hinrichsen H., Ross S., Buggisch P., Hampe J., Foelsch U.R., Schreiber S. Genetic variants in the CCR gene cluster and spontaneous viral elimination in hepatitis C-infected patients. Clin. Exp. Immunol. 2004;136:328–333. doi: 10.1111/j.1365-2249.2004.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos A.R., Martins J.S.C.C., Oliveira M.L.A., Garcia C.C., Siqueira M.M. Human CCR5Δ32 (rs333) polymorphism has no influence on severity and mortality of influenza A(H1N1)pdm09 infection in Brazilian patients from the post pandemic period. Infect. Genet. Evol. 2019;67:55–59. doi: 10.1016/j.meegid.2018.10.024. [DOI] [PubMed] [Google Scholar]
- Matsukura S., Kokubu F., Kubo H., Tomita T., Tokunaga H., Kadokura M., Yamamoto T., Kuroiwa Y., Ohno T., Suzaki H., Adachi M. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. Am. J. Respir. Cell Mol. Biol. 1998;18:255–264. doi: 10.1165/ajrcmb.18.2.2822. [DOI] [PubMed] [Google Scholar]
- Mehlotra R.K. CCR5 promoter polymorphism -2459G & A: forgotten or ignored? Cells. 2019;8:651. doi: 10.3390/cells8070651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michlmayr D., Lim J.K. Chemokine receptors as important regulators of pathogenesis during arboviral encephalitis. Front. Cell. Neurosci. 2014;8:264. doi: 10.3389/fncel.2014.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzabekov T., Bannert N., Farzan M., Hofmann W., Kolchinsky P., Wu L., Wyatt R., Sodroski J. Enhanced expression, native purification, and characterization of CCR5, a principal HIV-1 coreceptor. J. Biol. Chem. 1999;274:28745–28750. doi: 10.1074/jbc.274.40.28745. [DOI] [PubMed] [Google Scholar]
- Mishra R., Lata S., Ali A., Banerjea A.C. Dengue haemorrhagic fever: a job done via exosomes? Emerg. Microbes Infect. 2019;8:1626–1635. doi: 10.1080/22221751.2019.1685913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morard I., Clément S., Calmy A., Mangia A., Cerny A., De Gottardi A., Gorgievski M., Heim M., Malinverni R., Moradpour D., Müllhaupt B., Semela D., Pascarella S., Bochud P.Y., Negro F., Swiss Hepatitis C Cohort Study Group Clinical significance of the CCR5delta32 allele in hepatitis C. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Gustot T., Nicaise C., Quertinmont E., Nagy N., Parmentier M., Le Moine O., Devière J., Louis H. CCR5 deficiency exacerbates T-cell-mediated hepatitis in mice. Hepatology. 2005;42:854–862. doi: 10.1002/hep.20865. [DOI] [PubMed] [Google Scholar]
- Moudi B., Heidari Z., Mahmoudzadeh-Sagheb H. CCR5, MCP-1 and VDR gene polymorphisms are associated with the susceptibility to HBV infection. Indian J. Clin. Biochem. 2019;34:407–417. doi: 10.1007/s12291-018-0772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehling L.M., Turner R.B., Brown K.B., Wright P.W., Patrie J.T., Lahtinen S.J., Lehtinen M.J., Kwok W.W., Woodfolk J.A. Single-cell tracking reveals a role for pre-existing CCR5+ memory Th1 cells in the control of Rhinovirus-A39 after experimental challenge in humans. J. Infect. Dis. 2018;217:381–392. doi: 10.1093/infdis/jix514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A., Kelly E., Strange P.G. Pathways for internalization and recycling of the chemokine receptor CCR5. Blood. 2002;99:785–791. doi: 10.1182/blood.v99.3.785. [DOI] [PubMed] [Google Scholar]
- Muntinghe F.L.H., Verduijn M., Zuurman M.W., Grootendorst D.C., Carrero J.J., Qureshi A.R., Luttropp K., Nordfors L., Lindholm B., Brandenburg V., Schalling M., Stenvinkel P., Boeschoten E.W., Krediet R.T., Navis G., Dekker F.W. CCR5 deletion protects against inflammation-associated mortality in dialysis patients. J. Am. Soc. Nephrol. 2009;20:1641–1649. doi: 10.1681/ASN.2008040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntinghe F.L., Abdulahad W.H., Huitema M.G., Damman J., Seelen M.A., Lems S.P., Hepkema B.G., Navis G., Westra J. CCR5Δ32 genotype leads to a Th2 type directed immune response in ESRD patients. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B.R., Whitehead S.S. Immune response to dengue virus and prospects for a vaccine. Annu. Rev. Immunol. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- Mustonen J., Mäkelä S., Outinen T., Laine O., Jylhävä J., Arstila P.T., Hurme M., Vaheri A. The pathogenesis of nephropathia epidemica: new knowledge and unanswered questions. Antiviral Res. 2013;100:589–604. doi: 10.1016/j.antiviral.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Nattermann J., Timm J., Nischalke H.D., Olbrich A., Michalk M., Tillmann H.L., Berg T., Wedemeyer H., Tenckhoff H., Wiese M., Kullig U., Göbel U., Capka E., Schiefke I., Güthof W., Grüngreiff K., König I., Roggendorf M., Sauerbruch T., Spengler U., East German HCV Study Group The predictive value of IL28B gene polymorphism for spontaneous clearance in a single source outbreak cohort is limited in patients carrying the CCR5Δ32 mutation. J. Hepatol. 2011;55:1201–1206. doi: 10.1016/j.jhep.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Naveca F.G., Pontes G.S., Chang A.Y., da Silva G.A.V., do Nascimento V.A. Analysis of the immunological biomarker profile during acute Zika virus infection reveals the overexpression of CXCL10, a chemokine linked to neuronal damage. Mem. Inst. Oswaldo Cruz. 2018;113 doi: 10.1590/0074-02760170542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S.U., Cicalese S., Tallarida C., Oliver C.F., Rawls S.M. Chemokine CCR5 and cocaine interactions in the brain: cocaine enhances mesolimbic CCR5 mRNA levels and produces place preference and locomotor activation that are reduced by a CCR5 antagonist. Brain Behav. Immun. 2020;83:288–292. doi: 10.1016/j.bbi.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndung’u T., McCune J.M., Deeks S.G. Why and where an HIV cure is needed and how it might be achieved. Nature. 2019;576:397–405. doi: 10.1038/s41586-019-1841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill H.C., Quah B.J.C. Exosomes secreted by bacterially infected macrophages are proinflammatory. Sci. Signal. 2008;1:pe8. doi: 10.1126/stke.16pe8. [DOI] [PubMed] [Google Scholar]
- Omran M.H., Khamis M., Nasr N., Massoud A.A., Youssef S.S., Bader El Din N.G., Dawood R.M., Atef K., Moustafa R.I., Nabil W., Tabll A.A., El Awady M.K. A study of CC-Chemokine Receptor 5 (CCR5) polymorphism on the outcome of HCV therapy in Egyptian patients. Hepat. Mon. 2013;13 doi: 10.5812/hepatmon.13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelas A.M.M., Xavier-de-Carvalho C., Alvarado-Arnez L.E., Ribeiro-Alves M., Rossi ÁD., Tanuri A., de Aguiar R.S., Moraes M.O., Cardoso C.C. Association between MBL2 haplotypes and dengue severity in children from Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2019;114 doi: 10.1590/0074-02760190004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslund K.L., Baumgarth N. Influenza-induced innate immunity: regulators of viral replication, respiratory tract pathology & adaptive immunity. Future Virol. 2011;6:951–962. doi: 10.2217/fvl.11.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek S., Roy S., Kumari B., Jain P., Banerjee A., Vrati S. MiR-155 induction in microglial cells suppresses Japanese encephalitis virus replication and negatively modulates innate immune responses. J. Neuroinflammation. 2014;2014(11):97. doi: 10.1186/1742-2094-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier M. CCR5 and HIV infection, a view from Brussels. Front. Immunol. 2015;6:295. doi: 10.3389/fimmu.2015.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauvolid-Corrêa A., Campos Z., Juliano R., Velez J., Nogueira R.M.R., Komar N. Serological evidence of widespread circulation of West Nile virus and other flaviviruses in equines of the Pantanal. Brazil. PLoS Negl Trop Dis. 2014;8:e2706. doi: 10.1371/journal.pntd.0002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena S.D.J., Di Pietro G., Fuchshuber-Moraes M., Genro J.P., Hutz M.H. The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz A., Zepp M., Mahmood S., Ali D.M., Berger M.R., Adwan H. CCR5 blockage by maraviroc: a potential therapeutic option for metastatic breast cancer. Cell. Oncol. Dordr. (Dordr) 2019;42:93–106. doi: 10.1007/s13402-018-0415-3. [DOI] [PubMed] [Google Scholar]
- Petersen L.R., Brault A.C., Nasci R.S. West Nile virus: review of the literature. JAMA. 2013;310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova V.N., Russell C.A. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 2018;16:60. doi: 10.1038/nrmicro.2017.146. [DOI] [PubMed] [Google Scholar]
- Picton A.C.P., Shalekoff S., Paximadis M., Tiemessen C.T. Marked differences in CCR5 expression and activation levels in two South African populations. Immunology. 2012;136:397–407. doi: 10.1111/j.1365-2567.2012.03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschmann T., Brown R.J.P. Hepatitis C virus. Trends Microbiol. 2019;27:379–380. doi: 10.1016/j.tim.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Piovan E., Tosello V., Indraccolo S., Cabrelle A., Baesso I., Trentin L., Zamarchi R., Tamamura H., Fujii N., Semenzato G., Chieco-Bianchi L., Amadori A. Chemokine receptor expression in EBV-associated lymphoproliferation in hu/SCID mice: implications for CXCL12/CXCR4 axis in lymphoma generation. Blood. 2005;105:931–939. doi: 10.1182/blood-2004-03-0799. [DOI] [PubMed] [Google Scholar]
- Pirozyan M.R., Nguyen N., Cameron B., Luciani F., Bull R.A., Zekry A., Lloyd A.R. Chemokine-regulated recruitment of antigen-specific T-cell subpopulations to the liver in acute and chronic hepatitis C infection. J. Infect. Dis. 2019;219:1430–1438. doi: 10.1093/infdis/jiy679. [DOI] [PubMed] [Google Scholar]
- Poljak M., Seme K., Marin I.J., Babič D.Z., Matčič M., Meglič J. Frequency of the 32-base pair deletion in the chemokine receptor CCR5 gene is not increased in hepatitis C patients. Gastroenterology. 2003;124:1558–1560. doi: 10.1016/s0016-5085(03)00349-4. [DOI] [PubMed] [Google Scholar]
- Promrat K., McDermott D.H., Gonzalez C.M., Kleiner D.E., Koziol D.E., Lessie M., Merrell M., Soza A., Heller T., Ghany M., Park Y., Alter H.J., Hoofnagle J.H., Murphy P.M., Liang T.J. Associations of chemokine system polymorphisms with clinical outcomes and treatment responses of chronic hepatitis C. Gastroenterology. 2003;124:352–360. doi: 10.1053/gast.2003.50061. [DOI] [PubMed] [Google Scholar]
- Proudfoot A.E. Chemokine receptors: multifaceted therapeutic targets. Nat. Rev. Immunol. 2002;2:106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V.R. One hundred years of poliovirus pathogenesis. Virology. 2006;344:9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Rajan D., McCracken C.E., Kopleman H.B., Kyu S.Y., Lee F.E., Lu X., Anderson L.J. Human rhinovirus induced cytokine/chemokine responses in human airway epithelial and immune cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautenbach A., Williams A.A. Metabolomics as an approach to characterise the contrasting roles of CCR5 in the presence and absence of disease. Int. J. Mol. Sci. 2020;21:1472. doi: 10.3390/ijms21041472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renovato-Martins M., Matheus M.E., de Andrade I.R., Moraes J.A., da Silva S.V., Citelli Dos Reis M., de Souza A.A.P., da Silva C.C., Bouskela E., Barja-Fidalgo C. Microparticles derived from obese adipose tissue elicit a pro-inflammatory phenotype of CD16+, CCR5+ and TLR8+ monocytes. Biochim Biophys Acta Mol Basis Dis. 2017;1863:139–151. doi: 10.1016/j.bbadis.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Rodriguez A., Falcon A., Cuevas M.T., Pozo F., Guerra S., García-Barreno B., Martinez-Orellana P., Pérez-Breña P., Montoya M., Melero J.A., Pizarro M., Ortin J., Casas I., Nieto A. Characterization in vitro and in vivo of a pandemic H1N1 influenza virus from a fatal case. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C., Cuomo G., Ferrara R., Del Mastro A., Esposito S., Sellitto A., Adinolfi L.E. Uncommon immune-mediated extrahepatic manifestations of HCV infection. Expert Rev. Clin. Immunol. 2018;14:1089–1099. doi: 10.1080/1744666X.2018.1538790. [DOI] [PubMed] [Google Scholar]
- Rosenbaum L. The future of gene editing - toward scientific and social consensus. N. Engl. J. Med. 2019;380:971–975. doi: 10.1056/NEJMms1817082. [DOI] [PubMed] [Google Scholar]
- Rosenberg A.S., Roivainen M., Hovi T., Liu Q., Murphy P.M. CCR5 deficiency and severe polio infection in the 1984 outbreak in Finland. J. Med. Virol. 2013;85:2139–2140. doi: 10.1002/jmv.23739. [DOI] [PubMed] [Google Scholar]
- Rozmyslowicz T., Majka M., Kijowski J., Murphy S.L., Conover D.O., Poncz M., Ratajczak J., Gaulton G.N., Ratajczak M.Z. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17:33–42. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- Rudd J.M., Pulavendran S., Ashar H.K., Ritchey J.W., Snider T.A., Malayer J.R., Marie M., Chow V.T.K., Narasaraju T. Neutrophils induce a novel chemokine receptors repertoire during Influenza pneumonia. Front. Cell. Infect. Microbiol. 2019;9:108. doi: 10.3389/fcimb.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ferrer M., Barroso N., Antiñolo G., Aguilar-Reina J. Analysis of CCR5-Δ32 and CCR2-V64I polymorphisms in a cohort of Spanish HCV patients using real-time polymerase chain reaction and fluorescence resonance energy transfer technologies. J. Viral Hepat. 2004;11:319–323. doi: 10.1111/j.1365-2893.2004.00510.x. [DOI] [PubMed] [Google Scholar]
- Rustemoglu A., Ekinci D., Nursal A.F., Barut S., Duygu F., Günal Ö. The possible role of CCR5Δ32 mutation in Crimean-Congo hemorrhagic fever infection. J. Med. Virol. 2017;89:1714–1719. doi: 10.1002/jmv.24865. [DOI] [PubMed] [Google Scholar]
- Safari H., Sarab G.A., Fereidouni M., Ziaee M., Mahavar N., Naghizadeh M.S., Taene A., Mahdavi R., Naseri M. The CCR5-Δ32 mutation: impact on disease outcome in individuals with Hepatitis B infection in the Southern Khorasan population (East of Iran) Hepat. Mon. 2017;17 doi: 10.5812/hepatmon.55014. [DOI] [Google Scholar]
- Sagar S., Liu P.P., Cooper L.T.Jr. Myocarditis. Lancet. 2012;379:738–747. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksida A., Duh D., Wraber B., Dedushaj I., Ahmeti S., Avšič-Županc T. Interacting roles of immune mechanisms and viral load in the pathogenesis of crimean-congo hemorrhagic fever. Clin. Vaccine Immunol. 2010;17:1086–1093. doi: 10.1128/CVI.00530-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salentin R., Gemsa D., Sprenger H., Kaufmann A. Chemokine receptor expression and chemotactic responsiveness of human monocytes after influenza A virus infection. J. Leukoc. Biol. 2003;74:252–259. doi: 10.1189/jlb.1102565. [DOI] [PubMed] [Google Scholar]
- Sales K.J., Adefuye A., Nicholson L., Katz A.A. CCR5 expression is elevated in cervical cancer cells and is up-regulated by seminal plasma. Mol. Hum. Reprod. 2014;20:1144–1157. doi: 10.1093/molehr/gau063. [DOI] [PubMed] [Google Scholar]
- Salnikova L.E., Smelaya T.V., Moroz V.V., Golubev A.M., Rubanovich A.V. Host genetic risk factors for community-acquired pneumonia. Gene. 2013;518:449–456. doi: 10.1016/j.gene.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Samson M., Libert F., Doranz B.J., Rucker J., Liesnard C., Farber C.M., Saragosti S., Lapouméroulie C., Cognaux J., Forceille C., Muyldermans G., Verhofstede C., Burtonboy G., Georges M., Imai T., Rana S., Yi Y., Smyth R.J., Collman R.G., Doms R.W., Vassart G., Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Samson M., LaRosa G., Libert F., Paindavoine P., Detheux M., Vassart G., Parmentier M. The second extracellular loop of CCR5 is the major determinant of ligand specificity. J. Biol. Chem. 1997;272:24934–24941. doi: 10.1074/jbc.272.40.24934. [DOI] [PubMed] [Google Scholar]
- Sanchooli J., Sanadgol N., Kazemi Arababadi M., Kennedy D. CCR5 plays important roles in hepatitis B infection. Viral Immunol. 2014;27:2–6. doi: 10.1089/vim.2013.0067. [DOI] [PubMed] [Google Scholar]
- Sanjosé S., Brotons M., Pavón M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;47:2–13. doi: 10.1016/j.bpobgyn.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Santos E.U.D., Lima G.D.C., Oliveira M.L., Heráclio S.A., Silva HDA Crovella S., Maia M.M.D., Souza P.R.E. CCR2 and CCR5 genes polymorphisms in women with cervical lesions from Pernambuco, Northeast Region of Brazil: a case-control study. Mem. Inst. Oswaldo Cruz. 2016;111:174–180. doi: 10.1590/0074-02760150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauren J.S., Marasca J.A., Veit T.D., Monticielo O.A., Xavier R.M., Brenol J.C.T., Chies J.A.B. CCR5delta32 in systemic lupus erythematosus: implications for disease susceptibility and outcome in a Brazilian population. Lupus. 2013;22:802–809. doi: 10.1177/0961203313491848. [DOI] [PubMed] [Google Scholar]
- Schiffman M., Doorbar J., Wentzensen N., de Sanjosé S., Fakhry C., Monk B.J., Stanley M.A., Franceschi S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers. 2016;2:16086. doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- Schlabe S., Rockstroh J.K. Advances in the treatment of HIV/HCV coinfection in adults. Expert Opin. Pharmacother. 2018;19:49–64. doi: 10.1080/14656566.2017.1419185. [DOI] [PubMed] [Google Scholar]
- Sejvar J.J. West Nile virus infection. Microbiol. Spectr. 2016;4 doi: 10.1128/microbiolspec.EI10-0021-2016. [DOI] [PubMed] [Google Scholar]
- Settergren B. Clinical aspects of nephropathia epidemica (Puumala virus infection) in Europe: a review. Scand. J. Infect. Dis. 2000;32:125–132. doi: 10.1080/003655400750045204. [DOI] [PubMed] [Google Scholar]
- Sezgin E., van Natta M.L., Ahuja A., Lyon A., Srivastava S., Troyer J.L., O’Brien S.J., Jabs D.A., Studies of the Ocular Complications of AIDS Research Group Association of host genetic risk factors with the course of cytomegalovirus retinitis in patients infected with human immunodeficiency virus. Am. J. Ophthalmol. 2011;151:999–1006. doi: 10.1016/j.ajo.2010.11.029. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen Z.R., Naatz A., Corbett J.A. CCR5-dependent activation of mTORC1 regulates translation of inducible NO synthase and COX-2 during Encephalomyocarditis virus infection. J. Immunol. 2015;195:4406–4414. doi: 10.4049/jimmunol.1500704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaik M.M., Peng H., Lu J., Rits-Volloch S., Xu C., Liao M., Chen B. Structural basis of coreceptor recognition by HIV-1 envelope spike. Nature. 2019;565:318–323. doi: 10.1038/s41586-018-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelfoon C., Shariff S., Traves S.L., Kooi C., Leigh R., Proud D. Chemokine release from human rhinovirus-infected airway epithelial cells promotes fibroblast migration. J. Allergy Clin. Immunol. 2016;138:114. doi: 10.1016/j.jaci.2015.12.1308. e4-122.e4. [DOI] [PubMed] [Google Scholar]
- Shen B., Liu J., Zhang F., Wang Y., Qin Y., Zhou Z., Qiu J., Fan Y. CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016;2016 doi: 10.1155/2016/1240301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvani H., Achour L., Scott M.G., Thuret A., Labbé-Jullié C., Bismuth G., Marullo S. Evidence for internal stores of CCR5 in blood cells. Blood. 2011;118:1175–1176. doi: 10.1182/blood-2011-02-330886. [DOI] [PubMed] [Google Scholar]
- Sierra B., Perez A.B., Garcia G., Aguirre E., Alvarez M., Gonzalez D., Guzman M.G. Role of CC chemokine receptor 1 and two of its ligands in human dengue infection. Three approaches under the Cuban situation. Microbes Infect. 2014;16:40–50. doi: 10.1016/j.micinf.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Signoret N., Pelchen-Matthews A., Mack M., Proudfoot A.E., Marsh M. Endocytosis and recycling of the HIV coreceptor CCR5. J. Cell Biol. 2000;151:1281–1294. doi: 10.1083/jcb.151.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J.R., Romeiro M.F., Souza W.M., Munhoz T.D., Borges G.P., Soares O.A.B., Campos C.H.C., Machado R.Z., Silva M.L.C.R., Faria J.L.M., Chávez J.H., Figueiredo L.T.M. A Saint Louis encephalitis and Rocio virus serosurvey in Brazilian horses. Rev. Soc. Bras. Med. Trop. 2014;47:414–417. doi: 10.1590/0037-8682-0117-2014. [DOI] [PubMed] [Google Scholar]
- Silva-Carvalho W.H.V., de Moura R.R., Coelho A.V.C., Crovella S., Guimarães R.L. Frequency of the CCR5-delta32 allele in Brazilian populations: a systematic literature review and meta-analysis. Infect. Genet. Evol. 2016;43:101–107. doi: 10.1016/j.meegid.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Simonetta F., Bourgeois C. CD4+FOXP3+ regulatory T-cell subsets in Human immunodeficiency virus infection. Front. Immunol. 2013;4:215. doi: 10.3389/fimmu.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sachan R., Jain M., Mittal B. CCR5-Δ32 polymorphism and susceptibility to cervical cancer: association with early stage of cervical cancer. Oncol. Res. 2008;17:87–91. doi: 10.3727/096504008784523667. [DOI] [PubMed] [Google Scholar]
- Sironi M., Cagliani R., Pontremoli C., Rossi M., Migliorino G., Clerici M., Gori A. The CCR5Δ32 allele is not a major predisposing factor for severe H1N1pdm09 infection. BMC Res. Notes. 2014;7:504. doi: 10.1186/1756-0500-7-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sládková T., Kostolanský F. The role of cytokines in the immune response to influenza A virus infection. Acta Virol. 2006;50:151–162. [PubMed] [Google Scholar]
- Solloch U.V., Lang K., Lange V., Böhme I., Schmidt A.H., Sauter J. Frequencies of gene variant CCR5-Δ32 in 87 countries based on next-generation sequencing of 1.3 million individuals sampled from 3 national DKMS donor centers. Hum. Immunol. 2017;78:710–717. doi: 10.1016/j.humimm.2017.10.001. [DOI] [PubMed] [Google Scholar]
- St John A.L., Rathore A.P.S. Adaptive immune responses to primary and secondary dengue virus infections. Nat. Rev. Immunol. 2019;19:218–230. doi: 10.1038/s41577-019-0123-x. [DOI] [PubMed] [Google Scholar]
- Stanaway J.D., Shepard D.S., Undurraga E.A., Halasa Y.A., Coffeng L.E., Brady O.J., Hay S.I., Bedi N., Bensenor I.M., Castañeda-Orjuela C.A., Chuang T.W., Gibney K.B., Memish Z.A., Rafay A., Ukwaja K.N., Yonemoto N., Murray C.J.L. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016;16:712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens K.E., Thio C.L., Osburn W.O. CCR5 deficiency enhances hepatic innate immune cell recruitment and inflammation in a murine model of acute hepatitis B infection. Immunol. Cell Biol. 2019;97:317–325. doi: 10.1111/imcb.12221. [DOI] [PubMed] [Google Scholar]
- Stumbrytė-Kaminskienė A., Gudlevičienė Ž, Dabkevičienė D., Mackevičienė I. Combined effect of HPV and several gene SNPs in laryngeal cancer. Medicina Kaunas (Kaunas) 2020;56:81. doi: 10.3390/medicina56020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suneetha P.V., Sarin S.K., Goyal A., Kumar G.T., Shukla D.K., Hissar S. Association between vitamin D receptor, CCR5, TNF-α and TNF-β gene polymorphisms and HBV infection and severity of liver disease. J. Hepatol. 2006;44:856–863. doi: 10.1016/j.jhep.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Suppiah V., Armstrong N.J., O’Connor K.S., Berg T., Weltman M., Abate M.L., Spengler U., Bassendine M., Dore G.J., Irving W.L., Powell E., Nattermann J., Mueller T., Riordan S., Stewart G.J., George J., Booth D.R., Ahlenstiel G., International Hepatitis C Genetics Consortium (IHCGC) CCR5-Δ32 genotype does not improve predictive value of IL28B polymorphisms for treatment response in chronic HCV infection. Genes Immun. 2013;14:286–290. doi: 10.1038/gene.2013.15. [DOI] [PubMed] [Google Scholar]
- Suthar M.S., Diamond M.S., Gale M.Jr. West Nile virus infection and immunity. Nat. Rev. Microbiol. 2013;11:115–128. doi: 10.1038/nrmicro2950. [DOI] [PubMed] [Google Scholar]
- Suzuki P.S., de Oliveira K.B., Aoki M.N., Tatakihara VLH Borelli S.D., Watanabe M.A.E. Investigating the association of chemokine receptor 5 (CCR5) polymorphism with cervical cancer in human papillomavirus (HPV) positive patients. Acta Sci Health Sci. 2008;30:95–100. doi: 10.4025/actascihealthsci.v30i2.944. [DOI] [Google Scholar]
- Tadagaki K., Tudor D., Gbahou F., Tschische P., Waldhoer M., Bomsel M., Jockers R., Kamal M. Human cytomegalovirus-encoded UL33 and UL78 heteromerize with host CCR5 and CXCR4 impairing their HIV coreceptor activity. Blood. 2012;119:4908–4918. doi: 10.1182/blood-2011-08-372516. [DOI] [PubMed] [Google Scholar]
- Tan M.C.B., Goedegebuure P.S., Belt B.A., Flaherty B., Sankpal N., Gillanders W.E., Eberlein T.J., Hsieh C.S., Linehan D.C. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J. Immunol. 2009;182:1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q., Zhu Y., Li J., Chen Z., Han G.W., Kufareva I., Li T., Ma L., Fenalti G., Li J., Zhang W.W., Xie X., Yang H., Jiang H., Cherezov V., Liu H., Stevens R.C., Zhao Q., Wu B. Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science. 2013;341:1387–1390. doi: 10.1126/science.1241475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapparel C., Siegrist F., Petty T.J., Kaiser L. Picornavirus and enterovirus diversity with associated human diseases. Infect. Genet. Evol. 2013;14:282–293. doi: 10.1016/j.meegid.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Tavares L.P., Garcia C.C., Gonçalves A.P.F., Kraemer L.R., Melo E.M., Oliveira F.M.S., Freitas C.S., Lopes G.A.O., Reis D.C., Cassali G.D., Machado A.M., Mantovani A., Locati M., Teixeira M.M., Russo R.C. ACKR2 contributes to pulmonary dysfunction by shaping CCL5:CCR5-dependent recruitment of lymphocytes during Influenza A infection in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;318:L655–L670. doi: 10.1152/ajplung.00134.2019. [DOI] [PubMed] [Google Scholar]
- Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7 doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thio C.L., Astemborski J., Bashirova A., Mosbruger T., Greer S., Witt M.D., Goedert J.J., Hilgartner M., Majeske A., O’Brien S.J., Thomas D.L., Carrington M. Genetic protection against hepatitis B virus conferred by CCR5Δ32: evidence that CCR5 contributes to viral persistence. J. Virol. 2007;81:441–445. doi: 10.1128/JVI.01897-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thio C.L., Astemborski J., Thomas R., Mosbruger T., Witt M.D., Goedert J.J., Hoots K., Winkler C., Thomas D.L., Carrington M. Interaction between RANTES promoter variant and CCR5Δ32 favors recovery from hepatitis B. J. Immunol. 2008;181:7944–7947. doi: 10.4049/jimmunol.181.11.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoelen I., Verbeeck J., Wollants E., Maes P., Robaeys G., Matheï C., Buntinx F., Nevens F., Van Ranst M. Frequency of the CCR5-Δ32 mutant allele is not increased in Belgian hepatitis C virus-infected patients. Viral Immunol. 2005;18:232–235. doi: 10.1089/vim.2005.18.232. [DOI] [PubMed] [Google Scholar]
- Tiwari S., Singh R.K., Tiwari R., Dhole T.N. Japanese encephalitis: a review of the Indian perspective. Braz. J. Infect. Dis. 2012;16:564–573. doi: 10.1016/j.bjid.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Tokarz A., Konkolewska M., Kuśnierz-Cabala B., Maziarz B., Hanarz P., Żurakowski A., Szuścik I., Stępień E.Ł. Retinopathy severity correlates with RANTES concentrations and CCR 5-positive microvesicles in diabetes. Folia Med. Cracov. 2019;59:95–112. doi: 10.24425/fmc.2019.131139. [DOI] [PubMed] [Google Scholar]
- TrehanPati N., Geffers R., Sukriti Hissar S., Riese P., Toepfer T., Buer J., Kumar M., Guzman C.A., Sarin S.K. Gene expression signatures of peripheral CD4+ T cells clearly discriminate between patients with acute and chronic hepatitis B infection. Hepatology. 2009;49:781–790. doi: 10.1002/hep.22696. [DOI] [PubMed] [Google Scholar]
- Tsimanis A., Kalinkovich A., Bentwich Z. Soluble chemokine CCR5 receptor is present in human plasma. Immunol. Lett. 2005;96:55–61. doi: 10.1016/j.imlet.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Tyner J.W., Uchida O., Kajiwara N., Kim E.Y., Patel A.C., O’Sullivan M.P., Walter M.J., Schwendener R.A., Cook D.N., Danoff T.M., Holtzman M.J. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat. Med. 2005;11:1180–1187. doi: 10.1038/nm1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvári I., Tölgyesi G., Semsei A.F., Nagy A., Radosits K., Keszei M., Kozma G.T., Falus A., Szalai C. CCR5Δ32 mutation, Mycoplasma pneumoniae infection, and asthma. J. Allergy Clin. Immunol. 2007;119:1545–1547. doi: 10.1016/j.jaci.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Valaperti A., Nishii M., Liu Y., Naito K., Chan M., Zhang L., Skurk C., Schultheiss H.P., Wells G.A., Eriksson U., Liu P.P. Innate immune interleukin-1 receptor-associated kinase 4 exacerbates viral myocarditis by reducing CCR5+CD11b+ monocyte migration and impairing interferon production. Circulation. 2013;128:1542–1554. doi: 10.1161/CIRCULATIONAHA.113.002275. [DOI] [PubMed] [Google Scholar]
- van den Hurk A.F., Ritchie S.A., Mackenzie J.S. Ecology and geographical expansion of Japanese encephalitis virus. Annu. Rev. Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- Van Der Ryst E. Maraviroc - A CCR5 antagonist for the treatment of HIV-1 infection. Front. Immunol. 2015;6:277. doi: 10.3389/fimmu.2015.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani S., Frascaroli G., Homman-Loudiyi M., Feld S., Landini M.P., Söderberg-Nauclér C. Human cytomegalovirus inhibits the migration of immature dendritic cells by down-regulating cell-surface CCR1 and CCR5. J. Leukoc. Biol. 2005;77:219–228. doi: 10.1189/jlb.0504301. [DOI] [PubMed] [Google Scholar]
- Veiga-Parga T., Sehrawat S., Rouse B.T. Role of regulatory T cells during virus infection. Immunol. Rev. 2013;255:182–196. doi: 10.1111/imr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Petrovic A., Van Ryk D.I., Locati M., Weissman D., Murphy P.M. Reduced cell surface expression of CCR5 in CCR5Δ32 heterozygotes is mediated by gene dosage, rather than by receptor sequestration. J. Biol. Chem. 2002;277:2287–2301. doi: 10.1074/jbc.M108321200. [DOI] [PubMed] [Google Scholar]
- Venuti A., Pastori C., Siracusano G., Riva A., Sciortino M.T., Lopalco L. ERK1-based pathway as a new selective mechanism to modulate CCR5 with natural antibodies. J. Immunol. 2015;195:3045–3057. doi: 10.4049/jimmunol.1500708. [DOI] [PubMed] [Google Scholar]
- Venuti A., Pastori C., Pennisi R., Riva A., Sciortino M.T., Lopalco L. Class B β-arrestin2-dependent CCR5 signalosome retention with natural antibodies to CCR5. Sci. Rep. 2016;6:39382. doi: 10.1038/srep39382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuti A., Pastori C., Lopalco L. The role of natural antibodies to CC chemokine receptor 5 in HIV infection. Front. Immunol. 2017;8:1358. doi: 10.3389/fimmu.2017.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuti A., Pastori C., Siracusano G., Pennisi R., Riva A., Tommasino M., Sciortino M.T., Lopalco L. The abrogation of phosphorylation plays a relevant role in the CCR5 signalosome formation with natural antibodies to CCR5. Viruses. 2018;10:9. doi: 10.3390/v10010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viganò M., Andreoni M., Perno C.F., Craxì A., Aghemo A., Alberti A., Andreone P., Babudieri S., Bonora S., Brunetto M.R., Bruno R., Bruno S., Calvaruso V., Caporaso N., Cartabellotta F., Ceccherini-Silberstein F., Cento V., Ciancio A., Colombatto P., Coppola N., Di Marco V., Di Perri G., Fagiuoli S., Gaeta G.B., Gasbarrini A., Lampertico P., Pellicelli A., Prestileo T., Puoti M., Raimondo G., Rizzardini G., Taliani G., Zignego A.L. Real life experiences in HCV management in 2018. Expert Rev. Anti. Ther. 2019;17:117–128. doi: 10.1080/14787210.2019.1563755. [DOI] [PubMed] [Google Scholar]
- Vincent T., Portales P., Baillat V., de Boever C.M., Le Moing V., Vidal M., Ducos J., Clot J., Reynes J., Corbeau P. T-Cell surface CCR5 density is not correlated with hepatitis severity in hepatitis C virus/HIV-coinfected individuals: implications for the therapeutic use of CCR5 antagonists. J. Acquir. Immune Defic. Syndr. 2005;38:305–309. doi: 10.1097/01.qai.0000149793.14334.6c. [DOI] [PubMed] [Google Scholar]
- Vomaske J., Denton M., Kreklywich C., Andoh T., Osborn J.M., Chen D., Messaoudi I., Orloff S.L., Streblow D.N. Cytomegalovirus CC chemokine promotes immune cell migration. J. Virol. 2012;86:11833–11844. doi: 10.1128/JVI.00452-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald O., Pappo O., Ari Z.B., Azzaria E., Wiess I.D., Gafnovitch I., Wald H., Spengler U., Galun E., Peled A. The CCR5Δ32 allele is associated with reduced liver inflammation in hepatitis C virus infection. Eur. J. Immunogenet. 2004;31:249–252. doi: 10.1111/j.1365-2370.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- Wang H., Yang H. Gene-edited babies: what went wrong and what could go wrong. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth H.E., Werth A., Mueller T., Berg T., Dietrich C.G., Geier A., Schirin-Sokhan R., Gartung C., Lorenzen J., Matern S., Lammert F. CC chemokine receptor 5 Δ32 polymorphism in two independent cohorts of hepatitis C virus infected patients without hemophilia. J. Mol. Med. 2004;82:64–69. doi: 10.1007/s00109-003-0505-0. [DOI] [PubMed] [Google Scholar]
- Weintraub R.G., Semsarian C., Macdonald P. Dilated cardiomyopathy. Lancet. 2017;390:400–414. doi: 10.1016/S0140-6736(16)31713-5. [DOI] [PubMed] [Google Scholar]
- Weiss I.D., Shoham H., Wald O., Wald H., Beider K., Abraham M., Barashi N., Galun E., Nagler A., Peled A. Ccr5 deficiency regulates the proliferation and trafficking of natural killer cells under physiological conditions. Cytokine. 2011;54:249–257. doi: 10.1016/j.cyto.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Woitas R.P., Ahlenstiel G., Iwan A., Rockstroh J.K., Brackmann H.H., Kupfer B., Matz B., Offergeld R., Sauerbruch T., Spengler U. Frequency of the HIV-protective CC chemokine receptor 5-Δ32/Δ32 genotype is increased in hepatitis C. Gastroenterology. 2002;122:1721–1728. doi: 10.1053/gast.2002.33660. [DOI] [PubMed] [Google Scholar]
- Wu L., Paxton W.A., Kassam N., Ruffing N., Rottman J.B., Sullivan N., Choe H., Sodroski J., Newman W., Koup R.A., Mackay C.R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J. Exp. Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.L.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki C.A., Jiang Q., Panoskaltsis-Mortari A., Taylor P.A., McKinnon K.P., Su L., Blazar B.R., Serody J.S. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier-Carvalho C., Gibson G., Brasil P., Ferreira R.X., de Souza Santos R., Gonçalves Cruz O., de Oliveira S.A., de Sá Carvalho M., Pacheco A.G., Kubelka C.F., Moraes M.O. Single nucleotide polymorphisms in candidate genes and dengue severity in children: a case-control, functional and meta-analysis study. Infect. Genet. Evol. 2013;20:197–205. doi: 10.1016/j.meegid.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Xie Y., Zhan S., Ge W., Tang P. The potential risks of C-C chemokine receptor 5-edited babies in bone development. Bone Res. 2019;7:4. doi: 10.1038/s41413-019-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhong Z., Ding Y., Zhang W., Ma Y., Zhou L. Bioinformatic identification of key genes and pathways that maY. be involved in the pathogenesis of HBV-associated acute liver failure. Genes Dis. 2018;5:349–357. doi: 10.1016/j.gendis.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A., Alagozlu H., Ozdemir O., Arici S. Effects of the chemokine receptor 5 (CCR5)-delta32 mutation on hepatitis C virus-specific immune responses and liver tissue pathology in HCV infected patients. Hepat. Mon. 2014;14 doi: 10.5812/hepatmon.11283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen M.F., Chen D.S., Dusheiko G.M., Janssen H.L.A., Lau D.T.Y., Locarnini S.A., Peters M.G., Lai C.L. Hepatitis B virus infection. Nat. Rev. Dis. Primers. 2018;4:18035. doi: 10.1038/nrdp.2018.35. [DOI] [PubMed] [Google Scholar]
- Zachova K., Kosztyu P., Zadrazil J., Matousovic K., Vondrak K., Hubacek P., Kostovcikova K., Tlaskalova Hogenova H., Mestecky J., Raska M. Multiparametric flow cytometry analysis of peripheral blood B cell trafficking differences among Epstein-Barr virus infected and uninfected subpopulations. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. 2019 doi: 10.5507/bp.2019.052. in press. [DOI] [PubMed] [Google Scholar]
- Zahran A.M., Hetta H.F., Rayan A., Eldin A.S., Hassan E.A., Fakhry H., Soliman A., El‑Badawy O. Differential expression of Tim‑3, PD‑1, and CCR5 on peripheral T and B lymphocytes in hepatitis C virus‑related hepatocellular carcinoma and their impact on treatment outcomes. Cancer Immunol. Immunother. 2020 doi: 10.1007/s00262-019-02465-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Goedert J.J., O’Brien T.R. High frequency of CCR5-Δ32 homozygosity in HCV-infected, HIV-1-uninfected hemophiliacs results from resistance to HIV-1. Gastroenterology. 2003;124:867–868. doi: 10.1053/gast.2003.50132. [DOI] [PubMed] [Google Scholar]
- Zhang C., He Y., Shan K.R., Tan K., Zhang T., Wang C.J., Guan Z.Z. Correlations between polymorphisms in the uridine diphosphate-glucuronosyltransferase 1A and C-C motif chemokine receptor 5 genes and infection with the hepatitis B virus in three ethnic groups in China. J. Int. Med. Res. 2018;46:739–751. doi: 10.1177/0300060517730174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Qi L., Li T., Li X., Yang D., Cao S., Ye J., Wei B. PD1+CCR2+CD8+ T cells infiltrate the central nervous system during acute Japanese encephalitis virus infection. Virol. Sin. 2019;34:538–548. doi: 10.1007/s12250-019-00134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Wiklund F., Gharizadeh B., Sadat M., Gambelunghe G., Hallmans G., Dillner J., Wallin K.L., Ghaderi M. Genetic polymorphism of chemokine receptors CCR2 and CCR5 in Swedish cervical cancer patients. Anticancer Res. 2006;26:3669–3674. [PubMed] [Google Scholar]
- Zheng Y., Han G.W., Abagyan R., Wu B., Stevens R.C., Cherezov V., Kufareva I., Handel T.M. Structure of CC chemokine receptor 5 with a potent chemokine antagonist reveals mechanisms of chemokine recognition and molecular mimicry by HIV. Immunity. 2017;46:1005–1017. doi: 10.1016/j.immuni.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Zhou J., Wang H. Host microRNAs and exosomes that modulate influenza virus infection. Virus Res. 2020;279 doi: 10.1016/j.virusres.2020.197885. [DOI] [PubMed] [Google Scholar]
- Zhou W., Woodson M., Neupane B., Bai F., Sherman M.B., Choi K.H., Neelakanta G., Sultana H. Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A., Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Zwolińska K., Knysz B., Rybka K., Pazgan-Simon M., Gąsiorowski J., Sobczyński M., Gładysz A., Piasecki E. Protective effect of CCR5-Δ32 against HIV infection by the heterosexual mode of transmission in a Polish population. AIDS Res. Hum. Retroviruses. 2013;29:54–60. doi: 10.1089/AID.2011.0362. [DOI] [PubMed] [Google Scholar]