Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused by a novel coronavirus, SARS-CoV-2, has infected more than 22 million individuals and resulted in over 780,000 deaths globally. The rapid spread of the virus and the precipitously increasing numbers of cases necessitate the urgent development of accurate diagnostic methods, effective treatments, and vaccines. Here, we review the progress of developing diagnostic methods, therapies, and vaccines for SARS-CoV-2 with a focus on current clinical trials and their challenges. For diagnosis, nucleic acid amplification tests remain the mainstay diagnostics for laboratory confirmation of SARS-CoV-2 infection, while serological antibody tests are used to aid contact tracing, epidemiological, and vaccine evaluation studies. Viral isolation is not recommended for routine diagnostic procedures due to safety concerns. Currently, no single effective drug or specific vaccine is available against SARS-CoV-2. Some candidate drugs targeting different levels and stages of human responses against COVID-19 such as cell membrane fusion, RNA-dependent RNA polymerase, viral protease inhibitor, interleukin 6 blocker, and convalescent plasma may improve the clinical outcomes of critical COVID-19 patients. Other supportive care measures for critical patients are still necessary. Advances in genetic sequencing and other technological developments have sped up the establishment of a variety of vaccine platforms. Accordingly, numerous vaccines are under development. Vaccine candidates against SARS-CoV-2 are mainly based upon the viral spike protein due to its vital role in viral infectivity, and most of these candidates have recently moved into clinical trials. Before the efficacy of such vaccines in humans is demonstrated, strong international coordination and collaboration among studies, pharmaceutical companies, regulators, and governments are needed to limit further damage due the emerging SARS-CoV-2 virus.
Keywords: COVID-19, SARS-CoV-2, Clinical trials, NAATs, Spike protein, Vaccine
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which emerged in Wuhan, China in late 2019, has rapidly spread throughout China and globally. Although belonging to the same family, SARS-CoV-2 has different clinical and epidemiological characteristics from SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV). Its high transmissibility resembles that observed for pandemic influenza viruses; however, tools for diagnosis, treatment, and vaccines still need tremendous work to achieve the levels needed to respond to a pandemic flu.
Although other measures designed to respond to and control a pandemic such as surveillance, quarantine, and social distancing work efficiently to flatten the curve at a major cost to the economy, the development and deployment of effective tests, drugs, and vaccines to protect lives and limit disease spread are still urgent. Emergency Use Authorizations (EUA) expedite the availability of drugs to prevent serious or life-threatening diseases or conditions when there are no adequate, approved, and available alternatives. For many drugs that are already marketed for other conditions, off-label use can increase access for patients who need them. Currently, thousands of clinical trials are ongoing to test clinical outcomes. Adequate clinical trials will soon confirm or refute the usefulness of several candidate drugs and vaccines in treating and preventing COVID-19. Here, we review the state of diagnostic tests, initial clinical experience on accessible drugs and convalescent plasma administered to patients with COVID-19, and updated information on vaccine development against COVID-19.
Diagnosis
For COVID-19 patients, fever and cough are the two most common symptoms, and some patients might also suffer from sputum production, sore throat, headache, myalgia/arthralgia, rhinorrhea, and diarrhea [1]. Shortness of breath and dyspnea occur in cases that have progressed to pneumonia. Of note, a substantial proportion of patients reported olfactory and gustatory disorders, and thus sudden anosmia or ageusia may represent a clinical screening tool to identify COVID-19 patients [2]. Most patients had normal or decreased leukocyte count, lymphopenia, and elevated C-reactive protein, and some also had thrombocytopenia and elevated D-dimer, lactate dehydrogenase, and alanine aminotransferase [1]. In patients with pneumonia, ground-glass opacity is the typical radiological finding on chest computed tomography (CT) scan, and it may be obscure on chest X-ray; in patients with severe pneumonia, local or bilateral patchy consolidation has also been seen on CT images [1]. However, these clinical, laboratory, and imaging findings are nonspecific and cannot differentiate COVID-19 from other viral respiratory infections; viral diagnostic methods specific for SARS-CoV-2 should be applied for disease confirmation.
Nucleic-acid-based molecular methods
The current standard diagnostics for COVID-19 are based on detection of the SARS-CoV-2 RNA by nucleic acid amplification tests (NAATs), usually through real-time reverse transcription polymerase chain reaction (RT-PCR) with conformation by sequence analysis when necessary [3]. Reverse transcription loop-mediated isothermal amplification (LAMP) assays also appear to be a simple and sensitive diagnostic tool without a requirement high-level facilities and instruments [4]. Samples recommended for testing are those from the lower respiratory tract, including sputum, bronchoalveolar lavage (BAL), and endotracheal aspirates when possible [5]. Sputum, nasopharyngeal swab (NP), and oropharyngeal swabs (OP) are the most common sample types taken from patients with mild to moderate illness. If both NP and OP are collected, they can be placed in the same tube and tested simultaneously to save reagents [5,6]. In general, BAL showed the highest positive rates, followed by sputum, NP, and OP in order of decreasing sensitivity [[7], [8], [9]]. Throat gargling samples are an alternative specimen, although they are less sensitive than sputum [9].
The laboratory confirmation of cases in regions without COVID-19 virus circulation requires detection of two different genetic targets of the COVID-19 viral genome, while in regions with established COVID-19 virus circulation, confirmation through detection of a single genetic target is considered sufficient [3,5]. Many national laboratories have established and published their diagnostic protocols, which are summarized on the World Health Organization (WHO) website [10]. For example, the Charité protocol from Germany recommended detecting the E (envelope) gene for screening, followed by confirmation of E gene-positive samples through detection of the RNA-dependent RNA polymerase (RdRp) gene, where the E assay is specific for all SARS-CoV related viruses (i.e., SARS-CoV, SARS-CoV-2, and bat-derived SARS-related CoV) and the RdRP assay using the P2 probe only detects SARS-CoV-2 [5].
Pharyngeal virus shedding is very high during the first week of symptoms, and viral RNA shedding from sputum persists even after resolution of symptoms and seroconversion [9,11]. In a study with most samples (>90%) taken from the lower respiratory tract, the median duration of RNA detection was 17 days (interquartile range, 13–22 days) after illness onset, and independent risk factors for prolonged SARS-CoV-2 RNA shedding (>15 days) included male sex, delayed hospital admission, and invasive mechanical ventilation [12]. However, viral RNA does not equate to a live virus, and more data are needed to realize whether viral RNA load correlates with infectivity [6].
It has also been noted that a negative NAAT result does not mean that COVID-19 is absent, since several factors can lead to false-negative results. These factors include inappropriate sample collection or transportation, sample collection at time when the patient was not shedding sufficient virus, and technical reasons [3,5]. Periodically sequencing the evolving viruses is also suggested to monitor any mutations in the regions targeted by the assays that might affect test performance [3,6]. Moreover, the presence of a non-SARS-CoV-2 pathogen does not preclude the possibility of COVID-19; approximately one-fifth of specimens positive for SARS-CoV-2 were positive for one or more additional common respiratory viruses [13].
Virus isolation
Virus isolation is essential to obtain isolates for characterization and to support the development of antivirals and vaccines. However, although SARS-CoV-2 can be cultured in selected cell lines, such as Vero cells and LLC-MK2 cells, in a biosafety level-3 laboratory (BSL-3), viral isolation is not recommended as a routine diagnostic procedure due to biosafety concerns and time constraints [3,9,11]. Studies have indicated that infectious virus can be readily isolated during the first week of symptoms, with sputum having a higher culture yield than NP or OP; however, infectious virus could not be isolated from samples taken 8 days after onset despite ongoing high viral load detected by RT-PCR [11]. It appears that virus isolation success depends on viral load, as culture failed to yield virus when samples contained <106 copies per mL or per sample [11]. Further studies elucidating the duration of culture-positivity would provide a rationale for proposing strategies of isolation of infected patients.
Serological antibody tests
Serological antibody detection is the other broad category of tests to diagnose COVID-19, and this method detects IgM, IgG, or total antibodies (typically in the blood) against SARS-CoV-2. Techniques used for antibody detection include virus neutralization assay, enzyme-linked immunosorbent assay (ELISA), immunochromatographic assay, chemiluminescent immunoassay, etc. [11,[14], [15], [16], [17]]. Most tests are designed to capture antibodies, which recognize the nucleocapsid (N) protein and the S1 subunit and receptor biding domain (RBD) of Spike (S) proteins, as N and S proteins are the two major coronavirus immunogens [15,16]. RBD-specific monoclonal antibodies derived from two B cell clones of one COVID-19 patient have demonstrated impressive binding and neutralizing activity against live SARS-CoV-2 [18]. Of importance, these serologic tests should not cross-react with other seasonal coronaviruses.
Nevertheless, the use of antibody tests is limited to settings of acute illness because it takes time for hosts to mount an adequate immune response [3]. Studies indicate that the majority of patients have seroconversion 2 weeks after symptom onset [11,16,19]. Less than 40% of patients had detectable antibodies within 1 week of onset, but this percentage rapidly increased to 94.3% (IgM) and 79.8% (IgG) 15 days after onset, with the median seroconversion time of 12 and 14 days, respectively [16]. IgM began to decline 4 weeks after symptom onset, while IgG remained at high levels after 7 weeks [17,19]. Based on the time course of seroconversion, serological tests could be used as complementary tools to identify patients presenting late in their illness [16]. As mentioned earlier, seroconversion has not usually been followed by a rapid decline in viral RNA load [11].
Serological tests may also aid in (i) contact tracing, (ii) assessment of prior infection and immunity to SARS–CoV-2 (if there is protective immunity), (iii) determining the extent of the pandemic with seroprevalence data, and (iv) vaccine evaluation studies [3,6]. To date, it is not known whether antibodies elicited by SARS–CoV-2 provide protective immunity against reinfection and how long the protective immunity lasts. A rhesus macaque study does suggest protective immunity after recovery from primary infection, since reinfection did not occur in convalescent monkeys rechallenged with the same dose of SARS-CoV-2 strains [20]. Further studies are necessary to elucidate the situation in humans.
Rapid antigen-detection methods using immunoassays targeted at N or S proteins are under development, although with the same challenge of low sensitivity observed in influenza virus antigen tests. To date, a number of laboratory-developed assays and commercially available kits (mostly NAATs and serological antibody tests) have been granted an EUA by the US Food and Drug Administration (FDA), which greatly strengthens the diagnostic capability of frontline clinical laboratories [21].
Therapies
Currently, there are no drugs or other therapies approved by the US FDA to treat COVID-19. The major clinical treatment and management approaches emphasize the importance of life supportive care and relief of complications. Oxygen-based therapy has been applied when patients experience dyspnea, and advanced sepsis management has been warranted for patients who progressed to severe sepsis and acute respiratory distress syndrome.
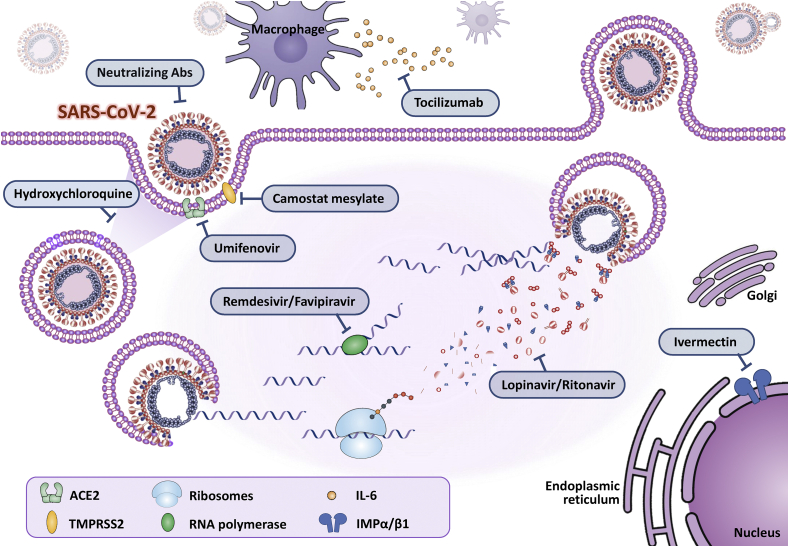
No existing antiviral drugs have sufficient evidence that they efficaciously treat COVID-19 pneumonia. Some drugs have been selected to treat COVID-19 pneumonia patients [Table 1]; most of these drugs were designed for other purpose such as Ebola, influenza, parasites, human immunodeficiency virus (HIV) infections, and immune therapy for some autoimmune and inflammatory diseases. Clinical trials have been conducted in which potential antiviral therapy targets were tested, such as blocking viral entry to human cells, inhibiting viral enzymes that were responsible for genome replication. Others focus on the human immune system to boost the innate response and inhibit the inflammatory process to relieve rapid progressed acute lung injuries. Global, large-scale, randomized clinical trials are still ongoing to test the safety and clinical outcomes of these drugs. Here, we summarize the mechanisms of potential therapeutic options that may combat the emerging SARS-CoV-2 [Fig. 1].
Table 1.
Drugs to be potentially used for SARS-CoV-2.
| Mechanism | Dose | Adverse Effects/Drug to Drug Interactions (DDI) | |
|---|---|---|---|
| Blocking Virus–Cell Membrane Fusion | |||
| Chloroquine/hydroxychloroquine | Block viral entry into cells by inhibiting glycosylation of host receptors, proteolytic processing, and endosomal acidification | Adult: 1. 400 mg Q12H*2 doses, followed by 200 mg Q12H*5 days 2. 200 mg Q8H Children: 6.5 mg/kg Q12H on day 1 (maximum initial dose: 400 mg Q12H), followed by 3.25 mg/kg Q12H on days 2–5 (maximum dose: 200 mg every 12 h). |
1. Prolongs PR, QRS, and QTc intervals, especially in patients with underlying risk factors or use in combination with other QT-prolonging drugs 2. Substrate of CYP2C8 and CYP3A4; co-administration with moderate and strong CYP2C8 and CYP3A4 inhibitors may result in increased plasma concentrations of hydroxychloroquine |
| Camostat mesylate | Inhibitor of the cellular serine protease TMPRSS2, human cell surface serine protease, resulting in membrane fusion | 400 mg tid; no pediatric usage is suggested currently | Mild adverse effects |
| Umifenovir | Targeting the S protein/ACE2 interaction and inhibiting membrane fusion of the viral envelope | 200 mg orally every 8 h | Mild adverse effects |
| RNA-dependent RNA polymerase inhibitor | |||
| Remdesivir | A nucleotide analogue prodrug that inhibits viral RNA-dependent RNA polymerase (RdRp), results in premature termination of the viral RNA chain, and consequently halts the replication of the viral genome | Adult: 200 mg intravenously on day 1, followed by 100 mg daily for the remaining 9 days | The most common adverse events were increased hepatic enzymes, diarrhea, rash, renal impairment |
| Favipiravir | Competes with purine nucleosides and interferes with viral replication by incorporation into the virus RNA and thus potentially inhibits the RNA dependent RNA polymerase (RdRp) | Adult 3200 mg (1600 mg twice daily) loading dose on day-1 followed by 1200 mg maintenance dose (600 mg twice daily) on day-2 to day-14 |
1. Few adverse effects were reported during treatment 2. Undergoes metabolism in the liver by aldehyde oxidase (AO) and xanthine oxidase (XO), producing an inactive oxidative metabolite and is excreted by the kidney 3. No hepatic or kidney adjustments, but initiation is not recommended in patients with an estimated glomerular filtration rate less than 30 mL/min |
| Viral Protease Inhibitor | |||
| Ivermectin | Inhibiting viral RNA activity by binding to IMP α/β1 mediated transport of proteins and RNA during infection | Single dose 3 mg (200 μg/kg) for people more than 15 kg | Extensively metabolized by human liver microsomes by cytochrome P450 3A4; Monitor liver function |
| Lopinavir/Ritonavir | Inhibiting protease inhibitor | Lopinavir–Ritonavir (400/100) mg bid for 14 days | CYP3A inhibitors, significant drug-drug interactions were reported |
| Acting on the Immune System | |||
| IL6 blocker | |||
| Tocilizumab | A recombinant humanized monoclonal anti-IL-6 receptor, antibody, reduce the effects of cytokine release syndrome (CRS) | 4 to 8 mg/kg (usual dose: 400 mg/dose; maximum: 800 mg/dose) intravenous use as a single dose; may consider repeat dose in ≥12 h | Interferes with serum concentration of CYP3A4 substrates; adverse hepatic effects were reported |
| SARS-CoV-2-Specific Neutralizing Antibodies | Direct spike-binding antibodies targeting virus S1, RBD, and S2 regions | 2 doses of 200 mL of convalescent plasma (CP) derived from recently recovered donors with neutralizing antibody titers above 1:640 | Transfusion-associated circulatory overload (TACO) and transfusion-associated acute lung injury (TRALI) |
| Dexamethasone | Mitigate inflammatory organ injury in viral pneumonia | 6 mg per day for up to 10 days | Interfere CYP3A4 metabolic pathway, be aware with drug-drug interactions |
Fig. 1.
The mechanisms of potential therapeutic options that may combat the emerging SARS-CoV-2.
Blocking virus–cell membrane fusion
Chloroquine/hydroxychloroquine
Chloroquine and hydroxychloroquine have a long-standing history in the prevention and treatment of malaria and the treatment of chronic inflammatory diseases such as systemic lupus erythematosus and rheumatoid arthritis. Chloroquine and hydroxychloroquine block viral entry into cells by inhibiting the glycosylation of host receptors, proteolytic processing, and endosomal acidification. Immunomodulatory effects through attenuation of cytokine production and inhibition of autophagy and lysosomal activity in host cells have also been reported [22,23]. The experiences of the US, China, and Europe have indicated clinical effects of chloroquine and hydroxychloroquine in the early phase but limited effects in the late phase [24]. An earlier study demonstrated that hydroxychloroquine was significantly associated with viral load reduction/disappearance in COVID-19 patients and its effect was strengthened by azithromycin [25]. Nevertheless, a recent meta-analysis indicated hydroxychloroquine alone, or in combination with azithromycin had no significant benefits in positive-to-negative conversion of SARS-CoV-2 and reduction of progression rate [26]. US study which evaluated hospitalized patients with COVID-19 in metropolitan New York showed no significant difference in in-hospital mortality among patients treated with hydroxychloroquine, azithromycin, both, and neither of them [27]. Safety data and data from larger, randomized, placebo-controlled high-quality trials with longer follow-up are urgently needed. Hydroxychloroquine can prolong the PR, QRS and QTc intervals, especially in patients with underlying risk factors or use in combination with other QT-prolonging drugs; cautious monitoring of ECG changes during treatment is important. Chloroquine and hydroxychloroquine are substrates of CYP2C8 and CYP3A4; therefore, co-administration with moderate and strong CYP2C8 and CYP3A4 inhibitors may result in increased plasma concentrations of hydroxychloroquine. Experiences and Interim Guidelines for Clinical Management of SARS-CoV-2 Infection in Taiwan advised that early administration of hydroxychloroquine may be considered to be given for 7 days after thoroughly evaluating potential risks/benefits and ethical issues [28].
Viral protease inhibitor
Lopinavir/ritonavir
Lopinavir, an HIV type 1 aspartate protease inhibitor, has in vitro inhibitory activity against SARS-CoV [29]. Ritonavir is combined with lopinavir to increase its plasma half-life through the inhibition of cytochrome P450. An open-label study published in 2004 suggested, by comparison with a control group that received only ribavirin, that the addition of lopinavir–ritonavir (400 mg and 100 mg, respectively) to ribavirin reduced the risk of adverse clinical outcomes (acute respiratory distress syndrome or death) and viral load among patients with SARS [29]. Lopinavir also has activity, both in vitro [30] and in an animal model [31], against MERS-CoV, and case reports have suggested that the combination of lopinavir–ritonavir with ribavirin and interferon alfa resulted in virologic clearance and survival [32]. A randomized controlled trial enrolled COVID-19 patient with dyspnea and desaturation in China and suggested that treatment with lopinavir–ritonavir was similar to standard care in the time to clinical improvement. Gastrointestinal adverse events were more common in the lopinavir–ritonavir group, but serious adverse events were more common in the standard-care group. Lopinavir–ritonavir treatment was stopped early because of adverse events such as nausea, diarrhea and hepatotoxicity [33]. The latest update of NIH COVID-19 treatment guidelines published on July 17, 2020 did not recommend Lopinavir–ritonavir or other HIV protease inhibitors as the treatment of COVID-19, except in a clinical trial [34]. Additionally, as a CYP3A inhibitor, significant drug–drug interactions have been reported before.
Ivermectin
Ivermectin is an FDA-approved broad spectrum anti-parasitic agent. It was identified as an inhibitor in vitro and has been demonstrated to limit infection by a broad spectrum of RNA viruses such as Dengue virus (DENV), West Nile Virus, Venezuelan equine encephalitis virus, and influenza by binding to inhibitors of importin α/β-mediated transports of proteins and RNA during infection. In the Phase III clinical trial in Thailand in 2014–2017 against DENV infection with a single daily oral dose, ivermectin was observed to be safe and resulted in a significant reduction in serum levels of viral NS1 protein, but no change in viremia or clinical benefit was observed [35]. Ivermectin has been shown to reduce viral RNA up to 5000-fold after 48 h of infection with SARS-CoV-2 [36]. This drug is extensively metabolized in human liver microsomes by CYP3A4. For patients with liver function abnormality, drug effects and interactions should be closely monitored during treatment.
RNA-dependent RNA polymerase inhibitor
Remdesivir
Remdesivir, a nucleotide analogue prodrug that inhibits RdRp, results in premature termination of the viral RNA chain and consequently halts replication of the viral genome. It has shown broad spectrum activity against members of several virus families, including filoviruses (e.g., Ebola) and coronaviruses (e.g., SARS-CoV and MERS-CoV), and has shown prophylactic and therapeutic efficacy in nonclinical models of these coronaviruses [[39], [37], [38]]. Remdesivir in vitro testing has also shown that remdesivir has activity against SARS-CoV-2 [37]. The latest study suggested remdesivir could reduce the time to clinical recovery in patients with severe COVID-19. The benefit of remdesivir was most apparent in hospitalized patients who only required supplemental oxygen. No observed benefit of remdesivir in those who were on high-flow oxygen, noninvasive ventilation, mechanical ventilation, or ECMO have been reported in some studies, but the present study was not powered to detect differences within subgroups [34]. The most common adverse events were increased hepatic enzymes, diarrhea, rash, renal impairment, and hypotension, and some of the patients discontinued remdesivir treatment prematurely.
Favipiravir
Favipiravir is a pyrazine carboxamide derivative (6-fluoro-3-hydroxy-2-pyrazinecarboxamide) and a broad-spectrum antiviral drug approved in Japan for the treatment of influenza. Favipiravir competes with purine nucleosides and interferes with viral replication by incorporation into viral RNA and potential inhibition of RdRp. During the 2014–2015 Ebola virus outbreak, which initiated in West Africa, a proof-of-concept trial with favipiravir was carried out in Guinea, and patients treated with favipiravir showed a trend towards improved survival. A clinical trial conducted in China demonstrated significantly shorter viral clearance time (median 4 days vs. 11 days) and higher improvement rate in chest imaging in the favipiravir arm (91.43% versus 62%) [40]. Another multicentered randomized clinical study also suggested that the 7 day clinical recovery rate increased in the favipiravir treatment group, along with shorter defervescence time and cough in patients with hypertension and/or diabetes [41]. Although few adverse effects were reported during treatment, potential drug and drug interactions should be kept in mind because favipiravir undergoes metabolism in the liver by aldehyde oxidase and xanthine oxidase to produce an inactive oxidative metabolite and is excreted by the kidney [42]. No hepatic or kidney adjustments are recommended at this time, but initiation is not recommended in patients with an estimated glomerular filtration rate less than 30 mL/min.
SARS-CoV-2-specific neutralizing antibodies
The humoral immune response mediated by antibodies is crucial for preventing viral infections. Therefore, the development of specific surface epitope-targeting neutralizing antibodies is a more long-term, albeit more specific, approach to target COVID-19 [43]. SARS-CoV-2-specific neutralizing antibodies (NAb) have been detected in patients from day 10–15 after the onset of the disease and remained thereafter. The titers of NAb among these patients correlated with the spike-binding antibodies targeting the S1, RBD, and S2 regions. Studies in China indicated that one or two doses of 200 mL of convalescent plasma derived from recently recovered donors with neutralizing antibody titers above 1:640 were well tolerated and could significantly increase or maintain the neutralizing antibodies at a high level; this outcome leads to disappearance of viremia, improvement of clinical symptoms, and absorption of lung lesions in radiological examination when administered, in addition to supportive care and antiviral agents such as lopinavir/ritonavir and favipiravir. The donors must be symptom-free for at least 14 days, have a negative SARS-CoV-2 PCR test after recovery to provide ABO-compatible and test negative plasma for major blood-borne diseases at the time of blood donation. Known general reactions such as transfusion-associated circulatory overload and transfusion-associated acute lung injury in patients with already severe lung damage still exist and need to be closely monitored [[47], [44], [45], [46]].
Blocking the interleukin (IL)-6 pathway
Evidence suggests that cytokine release syndrome (CRS) might play a major role in severe COVID-19 [23,48]. Inflammatory cytokines and chemokines, including IL-6, IL-1β, induced protein 10 and monocyte chemoattractant protein-1, are significantly elevated in COVID-19 patients, especially in severe patients with life-threatening multiple organ dysfunction. In COVID-19 patients with elevated inflammatory cytokines, postmortem pathology has revealed tissue necrosis and interstitial macrophage and monocyte infiltrations in the lung, heart, and gastrointestinal mucosa [49,50]. Moreover, severe lymphopenia with hyperactivated proinflammatory T cells [49] and decreased regulatory T cells [50] is commonly seen in critically ill patients, suggesting dysregulated immune responses. Tocilizumab is a recombinant humanized monoclonal anti-IL-6 receptor antibody. It binds both soluble and membrane-bound IL-6R to inhibit IL-6-mediated cis- and trans-signaling [51]. Given the efficacy of tocilizumab in CRS and the pivotal role of IL-6 in COVID-19, clinical use should consider evaluation of patients with the following criteria: (i) H score, a diagnostic score for hemophagocytic lymphohistiocytosis (HLH), to discriminate patients with CRS; (ii) Chinese guidelines for COVID-19 grade patients into mild, moderate, severe, and critical by vital signs, radiographic findings, and complications; (iii) IL-6 measurement, as IL-6 levels are significantly elevated in COVID-19 patients, especially in ICU patients [52]. CRP, an acute-phase inflammatory protein synthesized by IL-6-dependent hepatic biosynthesis, is a reliable marker of IL-6 bioactivity and is used to predict CRS severity and monitor IL-6 blockade efficacy [53]. Most studies suggested that elevated CRP levels are associated with severe COVID-19 [52]. Clinicians should add antivirals and cautiously evaluate the possibility of secondary infection thereafter [54]. Adverse hepatic effects have been reported previously, and clinicians should consider discontinuation of drug in cases of marked elevation of liver enzymes and hyperbilirubinemia. Other drug–drug interactions should be monitored because tocilizumab interferes with the serum concentration of CYP3A4 substrates.
Other possibly effective drugs in clinical trials
Arbidol hydrochloride (umifenovir)
Umifenovir targets the S protein/ACE2 interaction and inhibits membrane fusion of the viral envelope [55]. The agent is currently approved in Russia and China for the treatment and prophylaxis of influenza and is being tested in some clinical trials treating COVID-19 based on in vitro data suggesting activity against SARS [56].
The current dose of 200 mg orally every 8 h for influenza is being studied for COVID-19 treatment (NCT04260594). Limited clinical experience with umifenovir for COVID-19 in China in a nonrandomized study of 67 patients with COVID-19 showed that treatment with umifenovir for a median duration of 9 days was associated with lower mortality rates (0% [0/36] vs 16% [5/31]) and higher discharge rates compared with patients who did not receive the agent [57]. Another randomized Chinese study compared umifenovir (Arbidol) (200 mg∗3/day) and favipiravir (1600 mg∗2/first day followed by 600 mg∗2/day) and indicated that favipiravir, compared to umifenovir, did not significantly improve the clinically recovery rate at Day 7 [40].
Camostat mesylate
Camostat mesylate is an inhibitor of the cellular serine protease TMPRSS2 [58], which is used by SARS-CoV-2 for S protein priming [59]. It was developed to treat chronic pancreatitis (600 mg daily in three divided doses) and reflux esophagitis (300 mg daily in three divided doses after each meal). SARS-CoV-2 is surrounded by an envelope composed of a lipid bilayer and envelope proteins. SARS-CoV-2 initiates human cell entry after the S protein present on the envelope binds to a cell membrane receptor called ACE2. The S protein is cleaved into two subunits, S1 and S2, by a human cell-derived protease, which is thought to be furin. S1 then binds to its receptor, ACE2. The other fragment, S2, is cleaved by TMPRSS2, a human cell surface serine protease, resulting in membrane fusion. Both ACE2 and TMPRSS2 are therefore thought to be essential in airway cells for SARS-CoV-2 infection. An in vitro study found that nafamostat and camostat suppressed SARS-CoV-2 S protein-initiated fusion in 293FT cells (derived from the human fetal kidney) ectopically expressing ACE2 and TMPRSS2 [59]. Nafamostat, a new developed IV form drug, was found to inhibit SARS-CoV-2 S protein-initiated fusion at a concentration less than one-tenth of that needed by camostat. Adverse effects included mild gastrointestinal upset, dizziness, skin rash, thrombocytopenia, and elevated liver enzymes [60].
Dexamethasone
Inflammatory organ injury may occur in severe Covid-19, but the value of glucocorticoids has been widely debated. The Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, the Panel recommends using dexamethasone 6 mg per day for up to 10 days for the treatment of COVID-19 in patients who are mechanically ventilated. In patients who do not require supplemental oxygen, dexamethasone for the treatment of COVID-19 might be not substantial [61].
Vaccines
Vaccination probably offers the best option for blocking infectious disease circulation. Vaccine-induced humoral immune responses, especially involving the production of neutralizing antibodies, are crucial to limiting infection and preventing reinfection. No coronavirus vaccines to prevent respiratory infections in humans have been licensed. Only a few promising vaccines for SARS-CoV made it to Phase I clinical trials, and vaccine development was shelved because of the cessation of the SARS epidemic. SARS-CoV and SARS-CoV-2 bind the same host cell receptor (ACE2) and may share similar disease pathogeneses and limited cross-neutralizing antibodies [23]. Current approaches for the development of SARS-CoV-2 vaccines are mostly based on methods used for the development of SARS-CoV vaccines. Knowledge from these vaccines has enabled great progress for SARS-CoV-2 vaccines within a few weeks of the outbreak onset.
With the genome sequence of SARS-CoV-2 published on January 11, 2020, multiple vaccine candidates have been proposed or are in various stages of development. The most advanced candidates have recently moved into clinical trials. Numerous other vaccines still remain in the pre-clinical stage. The majority of candidate vaccines aim to induce neutralizing antibodies against the viral S protein or RBD, preventing uptake via the human ACE2 receptor. Multiple platforms are under development. Here, we summarized the vaccine candidates that are progressing into clinical trials [Table 2]; they are further discussed in the following section.
Table 2.
Overview of ongoing clinical trials of vaccines for COVID-19 (assess at ClinicalTrials.gov as of Aug. 12, 2020).
| Platform | Vaccine candidate | Study identifier/Phase | Immunogen | Sponsor | Subject |
|---|---|---|---|---|---|
| mRNA-based | mRNA1273 |
NCT04470427 Phase III |
S protein | ModernaTX, Inc. | ≥18 years |
| BNT162b1/2 |
NCT04368728 Phase II/III |
receptor-binding domain antigen/ S protein | BioNTech SE/ Pfizer | 18–85 Years | |
| DNA-based | INO-4800 |
NCT04336410 Phase II |
S protein | Inovio Pharmaceuticals | 18–50 years |
| Inactivated whole-virus vaccine | inactivated SARS-CoV-2 vaccine (CoronaVac) |
NCT04456595 Phase III |
Whole virus | Butantan Institute/ Sinovac | ≥18 years |
| Adenovirus viral vector | Ad5-nCoV |
NCT04341389 Phase II |
S protein | Insitute of Biotechnology/ Academy of Military Medical Sciences/ PLA of China | 18–60 years |
| ChAdOx1 nCoV-19 |
NCT04400838 Phase II/III |
S protein | University of Oxford/AstraZeneca | ≥5 years | |
| Lentivirus vector | Covid-19/aAPC (modified aAPCs) |
NCT04299724 Phase I |
Structural proteins and a polyprotein protease | Shenzhen Geno-Immune Medical Institute | 6 months–80 years |
| LV-SMENP-DC (modified DCs) |
NCT04276896 Phase I/II |
Structural proteins and a polyprotein protease | Shenzhen Geno-Immune Medical Institute | 6 months–80 years | |
| Bifidobacterium vector | bacTRL-Spike |
NCT04334980 Phase I |
S protein | Symvivo Corporation | 19 to 45 years |
| Live attenuated vaccines | BCG |
NCT04328441 Phase III |
? | UMC Utrecht | Healthcare workers (≥18 years) |
|
NCT04327206 Phase III |
Danish strain 1331 | Murdoch Children’s Research Institute | Healthcare workers (≥18 years) |
||
|
NCT04350931 Phase III |
Danish Strain 1331 | Ain Shams University | Healthcare workers (≥18 years) |
||
|
NCT04348370 Phase IV |
Tice strain | Texas A&M University | Healthcare workers (18–74 years) |
Platform
mRNA-based vaccines comprise mRNA that encodes a protein antigen. Conventional mRNA-based vaccines encode the antigen of interest and contain 5′ and 3′ untranslated regions, whereas the virally derived, self-amplifying RNAs encode not only the antigen but also the viral replication machinery that enables intracellular RNA amplification and abundant protein expression [62]. Recent mRNA vaccine designs have improved the stability and protein translation efficiency for enhanced innate and adaptive immunogenicity [62]. Delivery of the mRNA vaccine has been optimized by use of lipid nanoparticles for intramuscular or intradermal administration [63]. Additionally, unlike conventional vaccines, which are made from either inactivated pathogens or the small subunit of live pathogens, no infectious virus needs to be handled for mRNA vaccines. Therefore, testing is relatively safe, efficient, cost effective, and rapid. Three mRNA vaccine candidates for COVID-19, mRNA-1273, BNT162b1 and BNT162b2, are currently being evaluated in ongoing Phase II/III clinical trials, which are recruiting volunteers aged 18 years and older to assess the efficacy, safety, reactogenicity, and immunogenicity.
DNA vaccines, another type of nucleic acid-based vaccines, consist of plasmid-DNA encoding one or several antigens that will be expressed in host cells. DNA vaccines can be produced rapidly and at low cost. However, the need for specific delivery systems to achieve good immunogenicity and possible genomic integration and persistence in host cells is a remaining concern [63]. DNA vaccines encoding the S protein of the SARS-CoV and MERS-CoV have been shown to elicit T cell and neutralizing antibody responses, as well as protective immunity in mouse model and human studies [64,65]. INO-4800 is a DNA vaccine candidate targeting the S protein of SARS-CoV-2. A Phase I open-label clinical trial is underway, and the estimated completion date is November 2020.
Generation of an inactivated whole-virus (IWV) vaccine is the quickest approach for vaccine production following a new outbreak. Such vaccines have successfully been developed for influenza virus and enterovirus 71 [66,67]. IWV vaccines are usually made by exposure of a virulent virus to chemical or physical agents, e.g., formaldehyde or gamma irradiation, to destroy infectivity while retaining immunogenicity. The need to use large amounts of antigen to elicit an adequate antibody response and the possibility of causing Th2-bias hypersensitivity are major concerns for IWV vaccines [68]. One inactivated vaccine candidate that displayed good cross-neutralization to different COVID-19 strains has received approval for testing in human trials [69].
Viral vector vaccines are also potential tools for vaccine development. These vaccines can specifically deliver genes to target cells, enhance immunogenicity without an adjuvant, and induce a robust cytotoxic T cell response to eliminate virus-infected cells. Although the results of viral vector-based vaccines have been encouraging in animal models, some obstacles need to be overcome before use in humans. These obstacles include genetic stability, ability to evade pre-existing immunity, and genotoxicity. Adenovirus serotype 5 (Ad5) is the most widely used vector because this vector can be easily produced and has high levels of transgene expression and a broad range of viral tropism [70]. The ability to enhance mucosal immunity through targeting epithelial cells of the upper respiratory tract and gut, two main sites that express high levels of the ACE2 receptor for SARS-CoV-2, makes Ad5 an advantageous viral vector against COVID-19. Recombinant Ad5 vector-based vaccines have been examined in clinical trials against infectious diseases [71,72]. The work for COVID-19 has been accelerated based on the experiences of previous trials. A candidate vaccine known as Ad5-nCoV, which encodes a full-length S protein of SARS-CoV-2, is the first demonstrated to be safe for humans and to proceed to a Phase II clinical trial in China. Another viral vector vaccine, ChAdOx1 nCoV-19, is composed of a nonreplicating chimpanzee adenovirus vector and genetic sequence of S protein. The vector represents an attractive alternative to the human adenoviral vector due to its good safety profile and lack of pre-existing immunity in human population [73]. The vaccine candidate has entered a Phase II/III clinical trial.
Lentivirus vector (LV) systems represent an attractive technology for vaccine development. In addition to their ability to effectively deliver genes or antigens of interest into cells and to generate humoral and cellular mediated immune response against the encoded transgenes [74], LVs can transduce antigen presenting cells (APCs), the main cell types mediating the immune response, at high efficiencies with little to no cytotoxicity [75]. Through up or downregulation of immune modulatory genes in APCs by LVs, the genetically modified APCs may potentially activate a strong protective immunity against infections [75]. Two vaccine candidates, Covid-19/aAPC vaccine and LV-SMENP-DC vaccine, which were made by modifying artificial APCs and dendritic cells with LVs expressing multiple viral genes and immune modulatory genes, act as ‘Trojan horses’ against SARS-CoV-2 virus. Clinical trials are currently underway to evaluate their safety and immune reactivity.
Bifidobacterium is one of the domestic, nonpathogenic anaerobic bacteria found in the intestine of humans. These organisms are believed to have health-promoting properties for their host, including increasing the immune response and protecting the host against viral infection [76]. As vaccine vectors, they offer several advantages including low cost, low resistance to antibiotics, noninvasive administration, and high safety levels. The most attractive feature is that bifidobacterium tends to elicit high levels of mucosal antibodies against the expressed foreign antigen following uptake via the mucosal immune system [77]. Some strains of bifidobacterium have been used as a delivery vector for the development of vaccines against Hepatitis C virus and enterovirus 71 [78,79]. The bacTRL-Spike vaccine candidate contains live Bifidobacterium longum, which contains synthetic plasmid DNA encoding the S protein of SARS-CoV-2. The ongoing trial is designed to evaluate the safety and tolerability of orally delivered bacTRL-Spike vaccine in healthy adults.
Antibody-dependent enhancement
Antibody-dependent enhancement (ADE) is a condition in which subneutralizing or nonneutralizing antibodies are produced following primary infection or vaccination, and they enhance the infectivity of subsequent infections [80]. ADE modulates the immune response and elicits sustained inflammation, lymphopenia, and/or cytokine storm [81]. ADE has been observed for a variety of viruses, most notably flaviviruses (e.g., DNEV) [82]. ADE also occurs in SARS-CoV infection [83,84]. Diluted anti-sera against SARS-CoV promotes SARS-CoV infection, and this enhancement is significantly mediated by anti-S protein antibodies [83,84]. Similarly, vaccination with recombinant S protein of SARS-CoV elicits both neutralizing and ADE-inducing IgG antibodies [85]. As described above, many potential vaccine candidates against SARS-CoV-2 have focused on the full-length S protein. Attributed to the taxonomic and structural similarities between SARS-CoV and SARS-CoV-2, ADE is a critical issue that should be considered seriously during the practical application of SARS-CoV-2 vaccines.
Three approaches have been suggested to mitigate the adverse effects of ADE. The first one is shielding the nonneutralizing epitopes of the S proteins by glycosylation. The second approach is immunofocusing, which aims to direct the adaptive immune responses to target only the critical neutralizing epitope to elicit a more robust protective immunity. Supporting evidence for the latter is that a vaccine candidate based on the shorter RBD induced higher neutralizing activity than based the full-length S protein [10,86]. The third approach is eliminating epitope sequences that mediate enhancement of infection. Protein sequences responsible for ADE have been identified at S597−603 of the SARS-CoV S protein [85], a region that is also conserved in SARS-CoV-2 [40]. Thus, vaccines against COVID-19 could be engineered to minimize ADE via elimination of the epitope.
BCG vaccination
Bacillus Calmette-Guérin (BCG), the most commonly administered vaccine worldwide, contains a live attenuated strain of Mycobacterium bovis to protect against tuberculosis (TB). Universal vaccination at birth with a single dose of BCG is recommended in many countries where TB is highly endemic or where there is high risk of exposure to TB, such as Japan, China, and Taiwan. Other countries, such as Spain, France, and Switzerland, have discontinued their universal vaccine policies because of the declining incidence of TB infection and the proven variable effectiveness in preventing adult TB. Countries such as the United States, Italy, and the Netherlands have yet to adopt universal vaccine policies [87].
Although developed to prevent severe forms of tuberculosis in children, BCG vaccination has been shown to induce heterologous or nonspecific immune effects against nonmycobacterial pathogens, a phenomenon termed ‘trained immunity’. Trained immunity refers to the ability of innate immune memory to mount an enhanced subsequent response to diverse microbes [88]. Favorable effects of BCG have been observed in mouse and human studies for distinct viral pathogens [89,90]. Epidemiological studies have also linked BCG vaccination to the reduction in all-cause mortality in neonates and respiratory infections in elderly [91,92]. NOD2-and mTOR-mediated changes in the epigenetic landscape of immune cells is proposed to underly such protection to increase the secretion of pro-inflammatory cytokines, particularly IL-1β, and enhance anti-viral immunity [88,93].
Recent preprint studies suggested significant associations of BCG vaccination with prevalence, progression of disease, and mortality due to COVID-19 [94,95]. The authors indicated that countries without universal policies for BCG vaccination have been more severely affected compared to countries with routine use of the vaccine in neonates. The National Immunization Program in Taiwan has included neonatal BCG vaccination since 1965, and the coverage rate has remained at 97% since 2001 [96]. As of May 20, 2020, a cumulative total of 440 COVID-19 cases were confirmed in Taiwan with a case fatality rate was of 1.6%. The low morbidity and mortality rate are attributed to the government's quick response, border control, case identification, containment, and resource allocation to protect public health [97]. It is not known whether BCG vaccination plays a protective role against COVID-19 infection in Taiwan.
In addition to BCG, live attenuated influenza vaccine has been shown to promote NK cell-mediated heterologous immunity [98]. Previous studies also suggest that the heterologous beneficial effects of BCG vaccination may vary by BCG formulation, age, and route of administration [91,99]. Although these vaccines may bridge the gap until a vaccine specifically for SARS-CoV-2 is available, their protective effects and clinical relevance need to be further characterized. Clinical trials have been initiated to study the effects of BCG vaccination given to healthcare workers who are at the frontline of the COVID-19 pandemic [Table 2]. Before the evidence is available, the WHO is not likely to recommend BCG vaccination for the prevention of COVID-19 [100].
Conclusions
In the face of a pandemic, the rapid development, production, and deployment of diagnostic tools, drugs and vaccines are critical. Scientific advancements since the SARS and MERS pandemics have accelerated our understanding of the epidemiology, pathogenesis, and diagnosis of SARS-CoV-2, as well as the development of therapies to treat viral infection.
Rigorous and adequate clinical trials for drug safety and effectiveness in randomized, controlled trials remain fundamental measures to protect the public from drugs that are ineffective, unsafe, or both. Some available candidate drugs targeting different levels of human responses to COVID-19, such as cell membrane fusion, RNA-dependent RNA polymerase, viral protease inhibitor, IL-6 blocker and convalescent plasma, may improve the clinical outcomes of critical COVID-19 patients.
As no effective treatment against SARS-CoV-2 is currently available, the best action is to develop vaccines to prevent the infection. Some potential vaccine candidates have progressed to Phase I and II clinical trials, but a year and a half are likely to pass before an effective vaccine is vetted through trials and is ready for marketing for humans. Therefore, considerable efforts should be given to limit or hinder the spread of the virus. In addition, pandemics will generate simultaneous demand for drugs and vaccines around the world. The elderly and those with underlying diseases or chronic comorbidities are at greater risk of severe disease or mortality. Clinical and serologic studies will be needed to confirm which populations remain at the highest risk once effective treatments or vaccines are available. Strong international coordination and collaboration among studies, pharmaceutical companies, regulators, and governments are needed to ensure that promising therapies or vaccines can be manufactured and supplied successfully.
The first wave of this pandemic has created devastating social, economic, and political threats. It is time for us to work together, share experiences, and move forward to fight COVID-19. Although this virus persists, there is light at the end of the tunnel.
Conflicts of interest
The authors declare that there are no competing interests.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020;383:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol. 2020 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 19 March 2020. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases, Interium guidance. assessed 24 April. [Google Scholar]
- 4.Yan C., Cui J., Huang L., Du B., Chen L., Xue G. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin Microbiol Infect. 2020;26:773–779. doi: 10.1016/j.cmi.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan American Health Organization and World Health Organization . 20 March 2020. Laboratory guidelines for the detection and diagnosis of COVID-19 virus infection. [Google Scholar]
- 6.Patel R., Babady E., Theel E.S., Storch G.A., Pinsky B.A., St George K. Report from the American society for microbiology COVID-19 international summit, 23 march 2020: value of diagnostic testing for SARS-CoV-2/COVID-19. mBio. 2020;11 doi: 10.1128/mBio.00722-20. e00722-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 Viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W.D., Chang S.Y., Wang J.T., Tsai M.J., Hung C.C., Hsu C.L. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Coronavirus disease (COVID-19) technical guidance: Laboratory testing for 2019-nCoV in humans. https://www.who.int/docs/default-source/coronaviruse/whoinhouseassays.pdf?sfvrsn=de3a76aa_2
- 11.Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 12.Xu K., Chen Y., Yuan J., Yi P., Ding C., Wu W. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020 doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan Y., Li X., Yang G., Fan J., Tang Y., Zhao J. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okba N.M.A., Muller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020 doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao D.A.T., Gao D.C., Zhang D.S. Profile of specific antibodies to SARS-CoV-2: the first report. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju B., Zhang Q., Ge X., Wang R., Yu J., Shan S. Potent human neutralizing antibodies elicited by SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 19.Liu W., Liu L., Kou G., Zheng Y., Ding Y., Ni W. Evaluation of nucleocapsid and spike protein-based ELISAs for detecting antibodies against SARS-CoV-2. J Clin Microbiol. 2020 doi: 10.1128/JCM.00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao L., Deng W., Gao H., Xiao C., Liu J., Xue J. Reinfection could not occur in SARS-CoV-2 infected rhesus macaques. bioRxiv. 2020 [Google Scholar]
- 21.Cheng M.P., Papenburg J., Desjardins M., Kanjilal S., Quach C., Libman M. Diagnostic testing for severe acute respiratory syndrome–related coronavirus-2: a narrative review. Ann Intern Med. 2020;172:726–734. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou D., Dai S.M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020 doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahévas M., Tran V.T., Roumier M., Chabrol A., Paule R., Guillaud C. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang T.H., Chou C.Y., Yang Y.F., Yang Y.P., Chien C.S., Yarmishyn A.A. Systematic review and meta-analysis of the effectiveness and safety of hydroxychloroquine in COVID-19. medRxiv. 2020 [Google Scholar]
- 27.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020 doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Interim guidelines for clinical management of SARS-CoV-2 infection. 5th ed. 2020. https://www.cdc.gov.tw/File/Get/-ewtg9-RCAetCPKR4_rnCw [Google Scholar]
- 29.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan J.F., Yao Y., Yeung M.L., Deng W., Bao L., Jia L. Treatment with lopinavir/ritonavir or interferon-beta1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim U.J., Won E.J., Kee S.J., Jung S.I., Jang H.C. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-alpha for Middle East respiratory syndrome. Antivir Ther. 2016;21:455–459. doi: 10.3851/IMP3002. [DOI] [PubMed] [Google Scholar]
- 33.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institute of Health . 2020. NIH COVID-19 Treatment Guidelines.https://www.covid19treatmentguidelines.nih.gov [Google Scholar]
- 35.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheahan T.P., Sims A.C., Leist S.R., Schafer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci Unit States Am. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen C., Zhang Y., Huang J., Yin P., Cheng Z., Wu J. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020 [Google Scholar]
- 41.Madelain V., Nguyen T.H., Olivo A., de Lamballerie X., Guedj J., Taburet A.M. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin Pharmacokinet. 2016;55:907–923. doi: 10.1007/s40262-015-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou G., Zhao Q. Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int J Biol Sci. 2020;16:1718–1723. doi: 10.7150/ijbs.45123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci Unit States Am. 2020:202004168. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen C., Huang J., Cheng Z., Wu J., Chen S., Zhang Y. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020 [Google Scholar]
- 47.Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020 03.30.20047365. [Google Scholar]
- 48.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A. FDA approval summary: tocilizumab for treatment of Chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncol. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci Unit States Am. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alattar R., Ibrahim T.B.H., Shaar S.H., Abdalla S., Shukri K., Daghfal J.N. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol. 2020 doi: 10.1002/jmv.25964. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kadam R.U., Wilson I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc Natl Acad Sci Unit States Am. 2017;114:206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khamitov R.A., Loginova S., Shchukina V.N., Borisevich S.V., Maksimov V.A., Shuster A.M. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr Virusol. 2008;53:9–13. [PubMed] [Google Scholar]
- 57.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical Features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J., Xue J., Dong X., Yu Q., Baker S.N., Wang M. Antimicrobial properties of benzalkonium chloride derived polymerizable deep eutectic solvent. Int J Pharm. 2020;575:119005. doi: 10.1016/j.ijpharm.2019.119005. [DOI] [PubMed] [Google Scholar]
- 59.Kawase M., Shirato K., van der Hoek L., Taguchi F., Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L. Dexamethasone in hospitalized patients with Covid-19-preliminary report. N Engl J Med. 2020 NEJMoa2021436. [Google Scholar]
- 62.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rauch S., Jasny E., Schmidt K.E., Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Modjarrad K., Roberts C.C., Mills K.T., Castellano A.R., Paolino K., Muthumani K. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019;19:1013–1022. doi: 10.1016/S1473-3099(19)30266-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vajo Z., Kosa L., Visontay I., Jankovics M., Jankovics I. Inactivated whole virus influenza A (H5N1) vaccine. Emerg Infect Dis. 2007;13:807–808. doi: 10.3201/eid1305.061248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ong K.C., Devi S., Cardosa M.J., Wong K.T. Formaldehyde-inactivated whole-virus vaccine protects a murine model of enterovirus 71 encephalomyelitis against disease. J Virol. 2010;84:661–665. doi: 10.1128/JVI.00999-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agrawal A.S., Tao X., Algaissi A., Garron T., Narayanan K., Peng B.H. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccines Immunother. 2016;12:2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M. Rapid development of an inactivated vaccine for SARS-CoV-2. Science. 2020 doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ura T., Okuda K., Shimada M. Developments in viral vector-based vaccines. Vaccines. 2014;2:624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hammer S.M., Sobieszczyk M.E., Janes H., Karuna S.T., Mulligan M.J., Grove D. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013;369:2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smaill F., Jeyanathan M., Smieja M., Medina M.F., Thanthrige-Don N., Zganiacz A. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci Transl Med. 2013;5:205ra134. doi: 10.1126/scitranslmed.3006843. [DOI] [PubMed] [Google Scholar]
- 73.Dicks M.D., Spencer A.J., Edwards N.J., Wadell G., Bojang K., Gilbert S.C. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gallinaro A., Borghi M., Bona R., Grasso F., Calzoletti L., Palladino L. Integrase defective lentiviral vector as a vaccine platform for delivering influenza antigens. Front Immunol. 2018;9:171. doi: 10.3389/fimmu.2018.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen X., He J., Chang L.J. Alteration of T cell immunity by lentiviral transduction of human monocyte-derived dendritic cells. Retrovirology. 2004;1:37. doi: 10.1186/1742-4690-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saavedra J.M., Bauman N.A., Oung I., Perman J.A., Yolken R.H. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. 1994;344:1046–1049. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- 77.Kandasamy S., Chattha K.S., Vlasova A.N., Rajashekara G., Saif L.J. Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model. Gut Microb. 2014;5:639–651. doi: 10.4161/19490976.2014.969972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takei S., Omoto C., Kitagawa K., Morishita N., Katayama T., Shigemura K. Oral administration of genetically modified Bifidobacterium displaying HCV-NS3 multi-epitope fusion protein could induce an HCV-NS3-specific systemic immune response in mice. Vaccine. 2014;32:3066–3074. doi: 10.1016/j.vaccine.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 79.Yu Z., Huang Z., Sao C., Huang Y., Zhang F., Ma G. Oral immunization of mice using Bifidobacterium longum expressing VP1 protein from enterovirus 71. Arch Virol. 2013;158:1071–1077. doi: 10.1007/s00705-012-1589-z. [DOI] [PubMed] [Google Scholar]
- 80.Kuzmina N.A., Younan P., Gilchuk P., Santos R.I., Flyak A.I., Ilinykh P.A. Antibody-dependent enhancement of Ebola virus infection by human antibodies isolated from survivors. Cell Rep. 2018;24:1802–1815. doi: 10.1016/j.celrep.2018.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rothman A.L. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 82.Katzelnick L.C., Gresh L., Halloran M.E., Mercado J.C., Kuan G., Gordon A. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Z.Y., Werner H.C., Kong W.P., Leung K., Traggiai E., Lanzavecchia A. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc Natl Acad Sci Unit States Am. 2005;102:797–801. doi: 10.1073/pnas.0409065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yip M.S., Leung N.H., Cheung C.Y., Li P.H., Lee H.H., Daeron M. Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol J. 2014;11:82. doi: 10.1186/1743-422X-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Q., Zhang L., Kuwahara K., Li L., Liu Z., Li T. Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect Dis. 2016;2:361–376. doi: 10.1021/acsinfecdis.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang B., He J., Liu C., Chang L.J. An effective cancer vaccine modality: lentiviral modification of dendritic cells expressing multiple cancer-specific antigens. Vaccine. 2006;24:3477–3489. doi: 10.1016/j.vaccine.2006.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zwerling A., Behr M.A., Verma A., Brewer T.F., Menzies D., Pai M. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Netea M.G., Joosten L.A., Latz E., Mills K.H., Natoli G., Stunnenberg H.G. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arts R.J.W., Moorlag S., Novakovic B., Li Y., Wang S.Y., Oosting M. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 90.Moorlag S., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 91.Jensen K.J., Larsen N., Biering-Sorensen S., Andersen A., Eriksen H.B., Monteiro I. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial. J Infect Dis. 2015;211:956–967. doi: 10.1093/infdis/jiu508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wardhana, Datau E.A., Sultana A., Mandang V.V., Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011;43:185–190. [PubMed] [Google Scholar]
- 93.Kleinnijenhuis J., Quintin J., Preijers F., Benn C.S., Joosten L.A., Jacobs C. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun. 2014;6:152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller A., Reandelar M.J., Fasciglione K., Roumenova V., Li Y., Otazu G.H. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv. 2020 [Google Scholar]
- 95.Sala G., Miyakawa T. Association of BCG vaccination policy with prevalence and mortality of COVID-19. medRxiv. 2020 [Google Scholar]
- 96.Jou R., Huang W.L., Su W.J. Tokyo-172 BCG vaccination complications, Taiwan. Emerg Infect Dis. 2009;15:1525–1526. doi: 10.3201/eid1509.081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang C.J., Ng C.Y., Brook R.H. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020;323:1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- 98.Goodier M.R., Rodriguez-Galan A., Lusa C., Nielsen C.M., Darboe A., Moldoveanu A.L. Influenza vaccination generates cytokine-induced memory-like NK cells: impact of human Cytomegalovirus infection. J Immunol. 2016;197:313–325. doi: 10.4049/jimmunol.1502049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kiravu A., Osawe S., Happel A.U., Nundalall T., Wendoh J., Beer S. Bacille Calmette-guerin vaccine strain modulates the ontogeny of both mycobacterial-specific and heterologous T cell immunity to vaccination in infants. Front Immunol. 2019;10:2307. doi: 10.3389/fimmu.2019.02307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kam Y.W., Kien F., Roberts A., Cheung Y.C., Lamirande E.W., Vogel L. Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcgammaRII-dependent entry into B cells in vitro. Vaccine. 2007;25:729–740. doi: 10.1016/j.vaccine.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]