Abstract
Purpose
To explore the molecular mechanism of glycine in improving ischemic stroke.
Patients and Methods
The serum samples of patients with ischemic stroke and healthy people were compared. The ischemic stroke model of PC12 cells was established by oxygen-glucose deprivation (OGD). qPCR quantified miR-19a-3p and AMPK mRNA, and protein expression was detected by Western blot. MTT was used to detect cell activity. Flow cytometry was used to detect cells. Glucose metabolism kit was used to detect glucose intake and formation amount of lactic acid.
Results
Compared with the control group, OGD group (OGDG) showed lower cell activity and increased cell apoptosis. TNF-α, IL-1βI, L-6, Caspase 3, Caspase 9 and Bax were up-regulated, and Glut1, HK2, LDHA, PDK1, PKM2 and Bcl2 were down-regulated. At the same time, glucose intake, formation amount of lactic acid and cell apoptosis rate were reduced, and AMPK/GSK-3β/HO-1 pathway activity was down-regulated. Glycine could counteract the above phenomena in OGDG. miR-19a-3p and AMPK decreased and increased, respectively, during glycine therapy. AMPK was the target gene of miR-19a-3p. Rescue experiments demonstrated that glycine improved cell apoptosis, inflammatory response and glucose metabolism disorder of ischemic stroke through miR-19a-3p/AMPK/GSK-3β/HO-1 pathway.
Conclusion
Glycine improves ischemic stroke through miR-19a-3p/AMPK/GSK-3β/HO-1 pathway.
Keywords: ischemic stroke, AMPK/GSK-3β/HO-1 pathway, miR-19a-3p, glycine
Introduction
Cerebral artery occlusion leads to cerebral ischemia, which results in ischemic stroke.1 Ischemic stroke is a very dangerous cerebral ischemia disease, which easily leads to death or disability of patients.2 Age, marine n-3 polyunsaturated fatty acid, lipoprotein and cholesterol3–7 are all risk factors for ischemic stroke. Neuron injury and glucose metabolism disorder are common symptoms of ischemic stroke patients.8,9 If ischemic stroke patient is not treated in time, subsequent diseases may be induced.10 Therefore, it is one of the aims of the current research field to find accurate drug therapy strategies. The development of ischemic stroke is usually accompanied by changes in gene expression and protein level, which makes it possible to explore the therapeutic mechanism of drugs from the perspective of molecular level.
miR-19a-3p is a non-coding RNA with a length of about 82 bp located on human chromosome 14. miR-19a-3p is directly related to the development of various diseases.11–15 miR-19a-3p plays a dual role in ischemic diseases–it can not only improve myocardial ischemia16 by promoting angiogenesis and myocardial cell fibrosis, but also promote ischemic stroke17 by interfering glucose metabolism and inducing neuronal apoptosis. AMP-activated protein kinase (AMPK) protein is a protein18 that monitors cell energy balance. AMPK and its dominant signaling pathway are involved in the development and progression of various diseases.19–23 In the process of ischemic stroke, the role of AMPK is not single. On the one hand, activated AMPK inhibits cell oxidative stress and neuronal immunity24 through downstream proteins such as GSK-3β and HO-1. On the other hand, over-activation of AMPK leads to neuronal apoptosis.25
Glycine is an amino acid26 that improves ischemic stroke by promoting the survival of neuronal cells. However, its mechanism of neuronal protection is not perfect. In this paper, the ischemic stroke model was established on PC12 neuron cells by OGD, and glycine was applied to treat and analyze the effect of glycine on the ischemic stroke model. After glycine treatment, there were statistical changes in miR-19a-3p and AMPK/GSK-3β/HO-1 pathway levels associated with ischemic stroke. Accordingly, we speculated that glycine might improve ischemic stroke through miR-19a-3p and AMPK/GSK-3β/HO-1 pathway. In this article, a series of experiments were designed to verify the authenticity of this speculation, in order to provide a scientific basis for the development of drug therapy for ischemic stroke.
Materials and Methods
Ischemic Stroke Patients
From January 2015 to March 2016, the serum samples of 74 patients diagnosed with ischemic stroke in Dalian Municipal Central Hospital were collected, and the serum samples of 65 healthy people were collected as controls.
Inclusion criteria: Patients were diagnosed as ischemic stroke by pathology and they cooperated with the treatment.
Exclusion criteria were as follows: pulmonary fibrosis, endocrine and metabolic diseases, immune or inflammatory diseases, chronic cardiovascular disease, and chronic liver, kidney and lung function injury.
In Dalian Municipal Central Hospital, both patients and healthy volunteers were informed throughout the study in accordance with the Declaration of Helsinki and the written informed consents were acquired from them. This research has been approved by the Hospital Ethics Committee of Dalian Municipal Central Hospital. Serum samples were stored at −80°C for testing.
Oxygen-Glucose Deprivation (OGD) Model24
PC12 cells (CRL-1721) were purchased from the American type culture collection. This cell line was obtained through commercial purchase. PC12 cells were cultured at 37°C, 5%CO2 and 95%N2 in DMEM medium (Hyclone) containing sugar-free fetal bovine serum (FBS, Gibco) for 4 h. Subsequently, the culture medium was replaced with DMEM complete culture medium containing 10%FBS and 1% penicillin/streptomycin solution (100x, Solarbio), and the cells were transferred to 37°C, 5%CO2 animal cell incubator (German Binder Company). In control group (CG), cells were not stripped of oxygen-glucose. In OGDG, cells were stripped of oxygen-glucose only. In OGD+glycine group, 2 mmol/L glycine (sigma, USA) was added after OGD. miR-19a over-expression/inhibition vector (miR-19a mimics/inhibitor), AMPK over-expression vector (AMPK-ad) and negative control vector (NC mimics/NC-ad) were purchased from Sangon Biotech (Shanghai) Co., Ltd. The above vectors were, respectively, transfected into OGD-treated cell lines by lipofectamine 2000 transfection kit (Invitrogen, USA). The procedures referred to the kit instructions. After transfection for 8 h, fresh culture medium was replaced.
qPCR
Trypsin digested the adherent cells and prepared them into cell suspension. Total RNA of cells was extracted by Trizol, and serum RNA was extracted on the basis of the instructions of miRNeasy Serum/Plasma Kit (QIAGEN, Germany, 217184). The OD value of total RNA at 260–280nm was obtained by ultraviolet spectrophotometer, and OD260/OD280>1.8 was used for subsequent RT-PCR quantification. The FastKing one-step reverse transcription-fluorescence quantitative kit (Tiangen, Beijing) was used for reverse transcription and sequence amplification of RNA. The miR-19a-3p and AMPK primers were designed and synthesized by Sangon Biotech (Shanghai) Co., Ltd. The qPCR reaction system (50 μL) was as follows: 1.25 μL of upstream primers, 1.25 μL of downstream primers, 1.0 μL of probe, 10 pg/g of RNA template, 5 μL of 50×ROX Reference Dye ROX, plus RNase-Free ddH2O to the total reaction system of 50 μL. Reaction program was as follows: reverse transcription for 30 min at 50°C, 1 of cycle number, initial denaturation for 3 min at 95°C, 1 of cycle number, denaturation for 15 s at 95°C, annealing for 30 s at 60°C, with 40 of cycle number. Results were analyzed by ABI PRISM 7000 instrument (Applied Biosystems, USA). miR-19a-3p used U6 as the internal reference gene, AMPK mRNA used GAPDH as the internal reference gene, and the relative expression was standardized by 2–ΔΔCt. miR-19a-3p forward: 5ʹ-TGT GCA AAT CTA TGC AAA-3ʹ, miR-19a-3p reserve: 5ʹ-CAG TGC GTG TCG TGG AGT-3ʹ, AMPK (PRKAA1) forward: 5ʹ-AAT TCG CAG GGA GAT TCA GA-3ʹ, AMPK (PRKAA1) reserve: 5ʹ- ACA GCT CTC CTC CAG AAA CG-3ʹ. GAPDH forward: 5ʹ- CCC ACT AAC ATC AAA TGG GG-3ʹ, GAPDH reserve: 5ʹ- ATC CAC AGT CTT CTG GGT GG-3ʹ.
Western Blot
1 mL of protein extract was added to the cell suspension to extract the protein, and the solution was blown repeatedly to facilitate the cell lysis. This solution was centrifuged at 4°C at 1.6×104 ×g for 20 min. The supernatant was taken and the protein concentration was detected by BCA kit (Thermo Fisher). The protein was separated by SDS-PAGE electrophoresis and transferred to polyvinylidene fluoride membrane (EMD millipore). The electrophoretic sample quantity was 20–30 μg. The protein was sealed by 5% skim milk-PBS solution for 1 h at room temperature. Then, the protein to be tested and β-actin primary antibody were added and stood overnight at 4°C. Polyvinylidene fluoride membrane was washed for three times with PBS solution. Then, goat anti-rabbit secondary antibody (HRP cross-linking) was added, and stood at room temperature for 1 h. Finally, PBS solution was used to wash polyvinylidene fluoride membrane and ECL luminescent solution was used for visualization. The internal reference proteins were β-actin and lamine B. The relative expression level of the protein to be tested = the gray value of the band to be measured/the gray value of the β-actin band. The protein to be tested, β-actin and lamine B primary antibody, goat anti-rabbit secondary antibody (HRP cross-linked) were all purchased from Abcam. The protein extract contained 20 mmol/L Tris-HCl solution (pH7.5, Solarbio) and protein inhibitor (Solarbio). The proteins to be tested were as follows: Glut1, HK2, LDHA, PDK1, PKM2, TNF-α, IL-1β, IL-6, Caspase 3, Caspase 9, Bax,Bcl2, AMPK,p-AMPK,GSK-3β,p-GSK-3β,HO-1, Nrf2.
Cell Activity
Four 96-well plates were taken, and any 3 wells of each well plate were inoculated with 100 μL transfected cells (including 5×103 cells). One well plate was taken out for 24 h, 48 h, 72 h and 96 h after inoculation, respectively. MTT solution (5 mg/mL, Sigma-Aldrich) was added to the well plate on the basis of 10 μL/well, and cells were cultured at 37°C/5% CO2 for 4 h. Subsequently, the culture medium was removed. 150 μL DMSO (Solarbio) was added to each well, and it was oscillated at low speed for 10 min. The OD value at 570 nm was analyzed by enzyme-labeling instrument.
Cell Apoptosis
The cell suspension with cell number 1×106 was prepared. Cells were immobilized in 70% ethanol ice-cold solution at 4°C for 30 min. Then, the ethanol solution was removed. Annexin V-FITC/PI-A mixed solution was added to the cells, and the cells were incubated at room temperature for 30 min. FACScan flow cytometer (Becton Dickinson, USA) was used to analyze the apoptosis. The number of cells in each quadrant was recorded.
Glucose Metabolism
Glucose intake and formation amount of lactic acid of PC12 cells were detected by Glucose Uptake Colorimetric Assay Kit (sigma, USA) and Lactate Colorimetric Assay Kit (sigma, USA). The operation was in strict accordance with the kit instructions.
Dual-Luciferase Reporter Gene
Cells were cultured on 12-well plates until they grew well. Targetscan7.2 was used to predict the pairing sites of miR-19a and AMPK. AMPK mutant was constructed according to the pairing site. The empty plasmid vector (GLO), plasmid +AMPK wild-type vector (GLO-AMPK-wt), plasmid +AMPK mutant vector (GLO-AMPK-mut) were constructed to co-transfect PC12 cells in NC mimics and miR-19a mimics respectively. After transfection for 48 h, the medium was removed and PBS buffer was carefully used to clean the cell surface. The double luciferase reporter gene detection system (Promega) was used to detect luciferase intensity per well. Relative luciferase intensity = firefly luciferase intensity/marine coelenterate luciferase intensity.
Statistics and Analysis
The experiments were repeated for 3 times in each group. The measurement results were expressed by Mean±SD (standard deviation). The data were collected and analyzed by SPSS 20.0 (Asia Analytics Formerly SPSS China). The pictures were drawn by Graphpad 8.0. The differences between the two groups were compared by independent sample T test. One-way ANOVA was used to analyze the differences among groups. Afterwards, pairwise comparison was conducted by LSD-t test. All comparisons were two-sided tests. 95% was taken as the confidence interval and the difference was statistically significant with all P<0.05.
Results
Glycine Inhibited Cell Apoptosis, Glucose Metabolism Disorder and Inflammatory Response
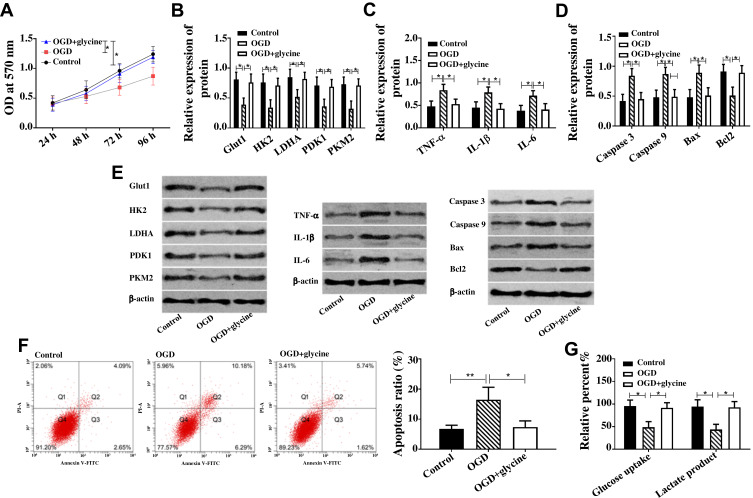
Apoptosis, glucose metabolism disorder and inflammatory reaction were important manifestations of ischemic stroke. In this article, the effect of glycine on ischemic stroke was evaluated through the above three factors. In this paper, cell apoptosis rate and apoptosis-related proteins (Caspase 3, Caspase 9, Bax, Bcl-2) were used to evaluate cell apoptosis. Pro-inflammatory factors TNF-α, IL-1β, and IL-6 were used to evaluate inflammatory reaction. Glucose metabolism was evaluated by glucose intake, formation amount of lactic acid, and glucose metabolism-related proteins (Glut1, HK2, LDHA, PDK1, PKM2). Figure 1 indicates that compared with CG, pro-inflammatory factors (TNF-α, IL-1βI, L-6) and pro-apoptotic proteins (Caspase 3, Caspase 9, Bax) in OGDG were up-regulated, and glucose metabolic proteins (Glut1, HK2, LDHA, PDK1, PKM2) and anti-apoptotic proteins Bcl-2 were down-regulated, while glucose intake, formation amount of lactic acid and apoptosis rate were reduced. Compared with OGDG, the pro-inflammatory factors (TNF-α, IL-1βI, L-6) and pro-apoptotic proteins (Caspase 3, Caspase 9, Bax) in OGD+glycine group were down-regulated, while glucose metabolic proteins (Glut1, HK2, LDHA, PDK1, PKM2) and anti-apoptotic proteins Bcl-2 were up-regulated, and glucose intake, formation amount of lactic acid and apoptosis rate were reduced. In addition, the cell activity test indicated that the cell activity of OGDG was lower than that of CG, while the cell activity of OGD+glycin group was higher than that of OGDG. The above results indicated that glycine inhibited cell apoptosis, glucose metabolism disorder and inflammatory response of ischemic stroke.
Figure 1.
Glycine inhibited cell apoptosis, glucose metabolism disorder and inflammatory response.
Notes: (A) Glycine improved cell activity of ischemic stroke when * was 96 h, P<0.05. (B) Glycine up-regulated Glut1, HK2, LDHA, PDK1, PKM2 in ischemic stroke. (C) Glycine down-regulated TNF-α, IL-1βI and L-6 in ischemic stroke. (D) Glycine down-regulated Caspase 3, Caspase 9, Bax and up-regulated Bcl2 in ischemic stroke. (E) Western blot band of protein; Glycine up-regulated Caspase 3, Caspase 9, Bax and Bcl2 in ischemic stroke. (F) Glycine reduced apoptosis of ischemic stroke. (G) Glycine increased glucose intake and formation amount of lactic acid in ischemic stroke. *P<0.05, **P<0.01. The experiments were repeated for three times.
Abbreviations: OD, optical density; Glut1, Glucose transporter type 1; PKM2, Pyruvate Kinase M2; HK2, Hexokinase 2; LDHA, Lactate Dehydrogenase A; PDK1/2, Pyruvate Dehydrogenase Kinase ½; TNF-α, Tumor Necrosis Factor α; IL-1β, interleukin-1β; IL-6, interleukin-6; Bax, BCL2 Associated X, Apoptosis Regulator; Bcl2, BCL2 Apoptosis Regulator; PI-A, propidiumiodide; Annexin V-FIFC, Fluorescein isothiocyanate; OGD, glycose-oxygen deprivation.
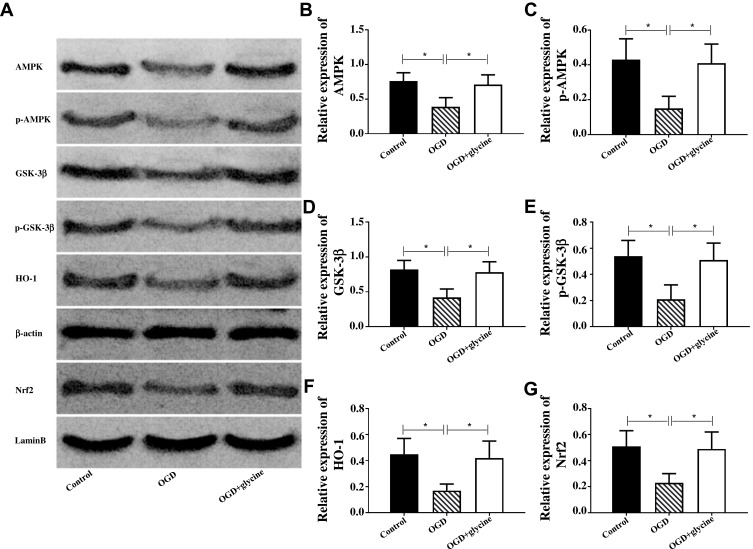
Glycine Activated AMPK/GSK-3β/HO-1 Pathway
AMPK/GSK-3β/HO-1 pathway was closely related to ischemic stroke, while HO-1 protein was positively correlated with Nrf2 protein. Therefore, this article was designed to explore the mechanism of glycine on ischemic stroke with AMPK, p-AMPK (phosphorylated AMPK), GSK-3β, p- GSK-3β (phosphorylated GSK-3β), Nrf2, HO-1. Figure 2 indicates that compared with CG, AMPK, p-AMPK, GSK-3β, p- GSK-3β, Nrf2, HO-1 were down-regulated in OGDG. Compared with OGDG, AMPK, p-AMPK, GSK-3β, p- GSK-3β, Nrf2, HO-1 in OGD+glycine group were up-regulated. These results indicated that glycine could activate AMPK/GSK-3β/HO-1 pathway in ischemic stroke.
Figure 2.
Glycine activated AMPK/GSK-3β/HO-1 pathway in ischemic stroke.
Notes: (A) The Western blot band of each protein, and the internal reference genes were β-actin and lamine B. (B-G) Relative expression of each protein. *P<0.05. The experiments were repeated for three times.
Abbreviations: AMPK, Adenosine 5‘-monophosphate (AMP)-activated protein kinase; p-AMPK, AMPK phosphorylation; GSK-3β, Glycogen Synthase Kinase 3β; p-GSK-3β, GSK-3β phosphorylation; HO-1, Heme Oxygenase 1; Nrf2, Nuclear Factor Erythroid 2-Related Factor 2; OGD, glycose-oxygen deprivation.
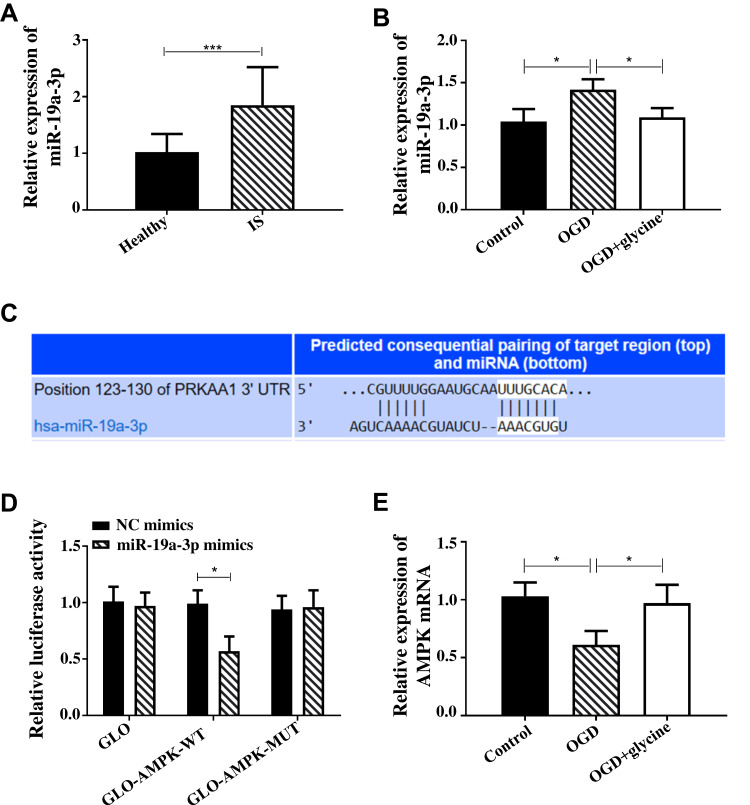
Glycine Regulated the Effects of AMPK on Ischemic Stroke Through miR-19a-3p
In this paper, serum samples of 74 patients with ischemic stroke and 65 healthy people were collected. qPCR quantified miR-19a-3p and found that it was up-regulated in the serum of patients with ischemic stroke (Figure 3A). During the research process, miR-19a-3p and AMPK showed completely different changing trends. Figure 3B indicates that miR-19a-3p in OGDG was up-regulated and AMPK was down-regulated compared with CG. Compared with OGDG, miR-19a-3p in OGD+glycine group was down-regulated and AMPK was up-regulated. Targetscan7.2 predicted that miR-19a-3p and AMPK had binding sites (Figure 3C). The double luciferase reporter gene indicated that miR-19a-3p can bind to AMPK (Figure 3D). These results revealed that glycine regulated the effects of AMPK on ischemic stroke through miR-19a-3p. Figure 3E shows that OGD induced the down-regulation of AMPK mRNA and glycine induced the up-regulation of AMPK mRNA.
Figure 3.
Glycine regulated the effects of AMPK on ischemic stroke through miR-19a-3p.
Notes: (A) miR-19a-3p was up-regulated in patients with ischemic stroke. There were 65 cases in Healthy group and 74 cases in IS group. (B) The effect of glycine on miR-19a. (C) miR-19a-3p and AMPK had binding sites, and the white bottom part was the predicted binding site. (D) The double luciferase reporter gene indicated that miR-19a-3p can bind to AMPK. (E) The effect of glycine on AMPK mRNA. *P<0.05 and ***P<0.001. The cell experiments were repeated for three times.
Abbreviations: IS, ischemic stroke; AMPK, Adenosine 5‘-monophosphate (AMP)-activated protein kinase; OGD, glycose-oxygen deprivation; NC, negative control; WT, wild type; MUT, mutant; GLO, pmirGLO vector.
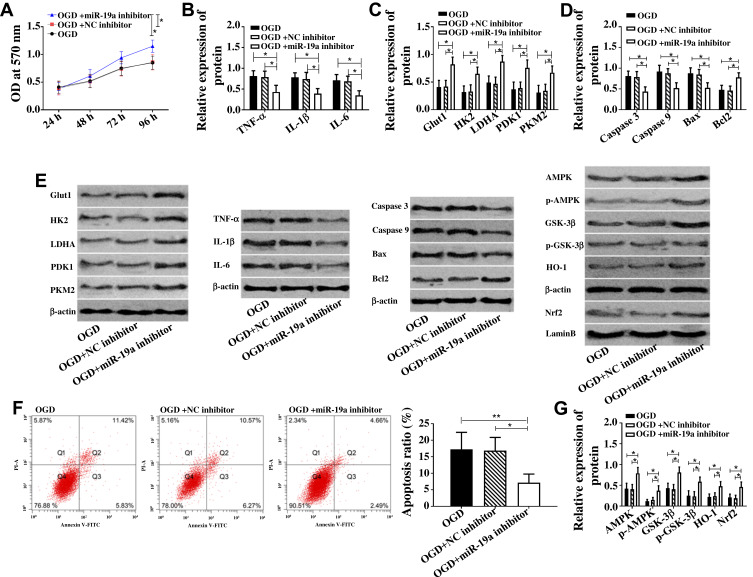
Down-Regulation of miR-19a-3p Caused by Glycine Inhibited Cell Apoptosis, Glucose Metabolism Disorder and Inflammatory Response
In view of the down-regulation of miR-19a-3p by glycine and the up-regulation of miR-19a-3p in patients with ischemic stroke, the effect of glycine on miR-19a-3p was simulated with miR-19a-3p inhibitor. Figure 4 indicates that compared with OGDG and OGD+NC inhibitor group, the pro-inflammatory factors (TNF-α, IL-1βI, L-6) and pro-apoptotic proteins (Caspase 3, Caspase 9, Bax) in OGD+miR-19a-3p inhibitor group were down-regulated, and glucose metabolic proteins (Glut1, HK2, LDHA, PDK1, PKM2), anti-apoptotic proteins Bcl-2, AMPK/GSK-3β/HO-1 pathway proteins were up-regulated, while cell apoptosis rate was reduced and cell activity was up-regulated. The above results revealed that the down-regulation of miR-19a-3p caused by glycine promoted AMPK/GSK-3β/HO-1 pathway and inhibited cell apoptosis, glucose metabolism disorder and inflammatory response.
Figure 4.
Down-regulation of miR-19a-3p caused by glycine inhibited cell apoptosis, glucose metabolism disorder and inflammatory response.
Notes: (A) Down-regulation of miR-19a-3p enhanced cell activity when * was 96 h, P<0.05. (B) Down-regulation of miR-19a-3p reduced TNF-α, IL-1β and IL-6. (C) Down-regulation of miR-19a-3p up-regulated Glut1, HK2, LDHA, PDK1, PKM2. (D) Down-regulation of miR-19a-3p inhibited Caspase 3, Caspase 9, Bax and promoted Bcl2. (E) Western blot band of each protein. (F) Down-regulation of miR-19a-3p reduced the apoptosis rate. (G) Down-regulation of miR-19a-3p up-regulated AMPK, p-AMPK, GSK-3β, p- GSK-3β, Nrf2, Ho-1. *P<0.05, **P<0.01. The experiments were repeated for three times.
Abbreviations: OD, optical density; Glut1, Glucose transporter type 1; PKM2, Pyruvate Kinase M2; HK2, Hexokinase 2; LDHA, Lactate Dehydrogenase A; PDK1/2, Pyruvate Dehydrogenase Kinase 1/2; TNF-α, Tumor Necrosis Factor α; IL-1β, interleukin-1β; IL-6, interleukin-6; Bax, BCL2 Associated X, Apoptosis Regulator; Bcl2, BCL2 Apoptosis Regulator; PI-A, propidiumiodide; Annexin V-FIFC, Fluorescein isothiocyanate; AMPK, Adenosine 5‘-monophosphate (AMP)-activated protein kinase; p-AMPK, AMPK phosphorylation; GSK-3β, Glycogen Synthase Kinase 3β; p-GSK-3β, GSK-3β, phosphorylation; HO-1, Heme Oxygenase 1; Nrf2, Nuclear Factor Erythroid 2-Related Factor 2; OGD, glycose-oxygen deprivation; NC, negative control.
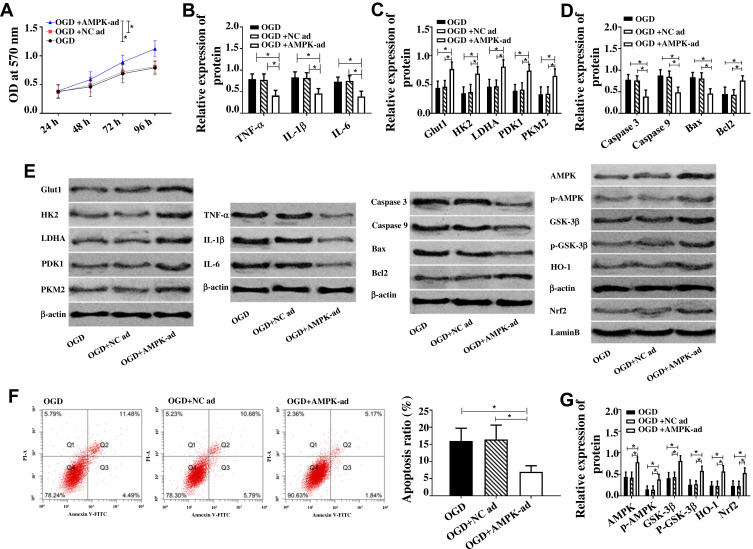
Up-Regulation of AMPK Caused by Glycine Inhibited Cell Apoptosis, Glucose Metabolism Disorder and Inflammatory Reaction
Since glycine could up-regulate AMPK, the effect of glycine on AMPK was simulated by AMPK over-expression vector. Figure 5 indicates that compared with OGDG and OGD+NC inhibitor group, the pro-inflammatory factors (TNF-α, IL-1βI, L-6) and pro-apoptotic proteins (Caspase 3, Caspase 9, Bax) in OGD+AMPK-ad group were down-regulated, and glucose metabolic proteins (Glut1, HK2, LDHA, PDK1, PKM2), anti-apoptotic proteins Bcl-2, AMPK/GSK-3β/HO-1 pathway proteins were up-regulated, while cell apoptosis rate was reduced and cell activity was up-regulated. The above results revealed that the up-regulation of AMPK caused by glycine inhibited cell apoptosis, glucose metabolism disorder and inflammatory response.
Figure 5.
Up-regulation of AMPK caused by glycine inhibited cell apoptosis, glucose metabolism disorder and inflammatory reaction.
Notes: (A) Up-regulation of AMPK enhanced cell activity when * was 96 h, P<0.05. (B) Up-regulation of AMPK down-regulated TNF-α, IL-1β, IL-6. (C) Up-regulation of AMPK up-regulated Glut1, HK2, LDHA, PDK1, PKM2. (D) Up-regulation of AMPK inhibited Caspase 3, Caspase 9, Bax and promoted Bcl2. (E) The band diagram of each protein. (F) Up-regulation of AMPK reduced apoptosis rate. (G) Up-regulation of AMPK up-regulated AMPK, p-AMPK, GSK-3β, p- GSK-3β, Nrf2, HO-1. *P<0.05. The experiments were repeated for three times.
Abbreviations: OD, optical density; Glut1, Glucose transporter type 1; PKM2, Pyruvate Kinase M2; HK2, Hexokinase 2; LDHA, Lactate Dehydrogenase A; PDK1/2, Pyruvate Dehydrogenase Kinase 1/2; TNF-α, Tumor Necrosis Factor α; IL-1β, interleukin-1β; IL-6, interleukin-6; Bax, BCL2 Associated X, Apoptosis Regulator; Bcl2, BCL2 Apoptosis Regulator; PI-A, propidiumiodide; Annexin V-FIFC, Fluorescein isothiocyanate; AMPK, Adenosine 5‘-monophosphate (AMP)-activated protein kinase; p-AMPK, AMPK phosphorylation; GSK-3β, Glycogen Synthase Kinase 3β; p-GSK-3β, GSK-3β phosphorylation; HO-1, Heme Oxygenase 1; Nrf2, Nuclear Factor Erythroid 2-Related Factor 2; OGD, glycose-oxygen deprivation; NC, negative control.
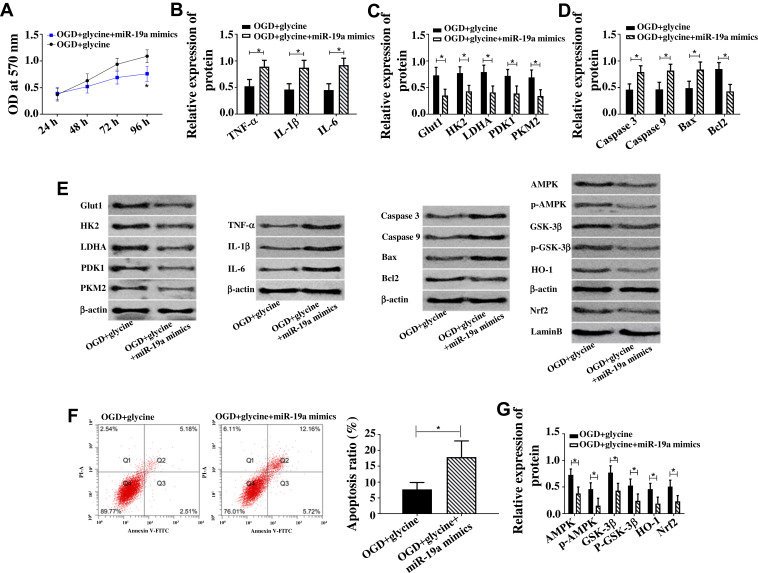
Rescue Experiment
In order to verify that glycine improves ischemic stroke through miR-19a-3p/AMPK/GSK-3β/HO-1 pathway, miR-19a-3p over-expression vector (miR-19a mimics) was constructed to counteract the down-regulation of miR-19a-3p caused by glycine, and cell changes were observed during this process. Figure 6 indicates that up-regulation of miR-19a-3p could offset the decrease of cell apoptosis, increase of cell activity, down-regulation of proinflammatory factors, up-regulation of glucose metabolic protein and AMPK/GSK-3β/HO-1 pathway protein caused by glycine. The above results indicated that glycine improved cell apoptosis, inflammatory response and glucose metabolism disorder of ischemic stroke through miR-19a-3p/AMPK/GSK-3β/HO-1 pathway.
Figure 6.
Rescue experiment.
Notes: (A) Up-regulation of AMPK enhanced cell activity, *P<0.05. (B) Up-regulation of AMPK down-regulated TNF-α, IL-1β, IL-6. (C) Up-regulation of AMPK enhanced Glut1, HK2, LDHA, PDK1, PKM2. (D) Up-regulation of AMPK inhibited Caspase 3, Caspase 9, Bax and promoted Bcl2. (E) Western blot band of each protein. (F) Up-regulation of AMPK reduced apoptosis rate. (G) Up-regulation of AMPK up-regulated AMPK, p-AMPK, GSK-3β, p- GSK-3β, Nrf2, HO-1. *P<0.05. The experiments were repeated for three times.
Abbreviations: OD, optical density; HK2, Hexokinase 2; LDHA, Lactate Dehydrogenase A; PDK1/2, Pyruvate Dehydrogenase Kinase 1/2; TNF-α, Tumor Necrosis Factor α; IL-1β, interleukin-1β; IL-6, interleukin-6; Bax, BCL2 Associated X, Apoptosis Regulator; Bcl2, BCL2 Apoptosis Regulator; PI-A, propidiumiodide; Annexin V-FIFC, Fluorescein isothiocyanate; AMPK, Adenosine 5‘-monophosphate (AMP)-activated protein kinase; p-AMPK, AMPK phosphorylation; GSK-3β, Glycogen Synthase Kinase 3β; p-GSK-3β, GSK-3β phosphorylation; HO-1, Heme Oxygenase 1; Nrf2, Nuclear Factor Erythroid 2-Related Factor 2; OGD, glycose-oxygen deprivation.
Discussion
The brain is an important central system of the human body. Central system damage will inevitably cause human body dysfunction. Ischemic stroke caused by cerebral blood supply insufficiency not only causes serious functional damage, but also has obvious influence on patients’ psychology. Early intervention or timely treatment with drugs is one of the main ideas against ischemic stroke. In this paper, OGD model of PC12 cells was established to simulate the effect of ischemic stroke on neuronal cells, and patients were given glycine to observe the protective mechanism of glycine on PC12 cells.
Figure 1A indicates that ischemic stroke causes cellular inflammation, while glycine can alleviate inflammatory reaction by down-regulating inflammatory factors TNF-α, IL-1β and IL-6. PC12 cells need sufficient energy supply in the process of life. Therefore, if glucose metabolism and other energy supply disorders, the cell process will be directly disturbed. In this paper, glucose metabolism of PC12 cells was evaluated by glucose metabolism kit, and glucose metabolism related proteins (Glut1/HK2/LDHA/PDK1/PKM2) were detected by Western blot. Figure 1B and G indicated that PC12 cells induced by OGD showed a trend of decreased glucose intake and lactic acid production, accompanied by down-regulation of metabolic related proteins (Glut1, HK2, LDHA, PDK1, PKM2, etc). However, after glycine treatment, glucose intake and lactic acid production of cells increased, and metabolism-related proteins were up-regulated. This indicated that in the process of ischemic stroke, the glucose metabolism of neuron cells was disordered, and glycine could improve this disorder. Figure 1A, D and F indicated that ischemic stroke caused a decrease in PC cell activity and an increase in cell apoptosis, while glycine inhibited cell apoptosis and up-regulated cell activity by down-regulating pro-apoptotic proteins Caspase 3, Caspase 9 and Bax and up-regulating anti-apoptotic protein Bcl2. The above results indicated that glycine could effectively improve cell apoptosis, glucose disorder and inflammatory response caused by ischemic stroke.
It is worth mentioning that miR-19a-3p was down-regulated and AMPK/GSK-3β/HO-1 pathway activity was up-regulated after treatment of PC21 cells with glycine (Figures 2 and 3). Although previous studies have shown that27 glycine inhibited autophagy and apoptosis of cells by down-regulating the over-activated AMPK pathway, the results in this paper showed that glycine had the function in up-regulating AMPK pathway. This seemingly contradictory phenomenon may be caused by the two sides of AMPK and its dominant pathway. Glycine can not only improve cell energy disorder and apoptosis by activating AMPK/GSK-3β/HO-1 pathway, but also inhibit cell autophagy by down-regulating the over-activation of AMPK/GSK-3β/HO-1 pathway. It is through this dynamic regulation that glycine maintains the role of AMPK/GSK-3β/HO-1 pathway in the process of ischemic stroke.
Comparing the expression levels of miR-19a-3p between patients with ischemic stroke and normal people, it was concluded that miR-19a-3p was up-regulated in patients with ischemic stroke (Figure 3A). However, does miR-19a-3p participate in ischemic stroke through AMPK/GSK-3β/HO-1 pathway? In order to solve this problem, we focused on the relationship between miR-19a-3p and AMPK gene in this paper. First, the Targetscan7.2 database predicted that miR-19a-3p can bind to AMPK gene sequence 3ʹUTR (Figure 3C), and then the double luciferase reporter gene verified that miR-19a-3p could inhibit its expression by binding to AMPK1. The above results revealed that glycine might regulate the life process of PC12 cells through miR-19a-3p/AMPK/GSK-3β/HO-1 pathway, thus improving ischemic stroke.
In this paper, miR-19a-3p inhibitor and AMPK-ad vectors were constructed to verify whether glycine improves ischemic stroke through miR-19a-3p/AMPK/GSK-3β/HO-1 pathway. The results showed that down-regulation of miR-19a-3p caused up-regulation of AMPK. Down-regulation of miR-19a-3p or up-regulation of AMPK resulted in the decrease of cell activity, down-regulation of pro-apoptotic protein, glucose metabolism protein and inflammatory factor, and up-regulation of anti-apoptotic protein and AMPK/GSK-3β/HO-1 pathway. Previous study17 pointed out that miR-19a-3p regulated ischemic stroke through glucose metabolism and apoptosis. In addition, AMPK and its dominant signaling pathway constantly monitor the intake and metabolism of glucose by cells.28 According to the above research and the results of this article, miR-19a-3p inhibited AMPK/GSK-3β/HO-1 pathway activity by down-regulating AMPK, thus interfering with the life process of nerve cells and promoting the development and progression of ischemic stroke. However, glycine counteracted the inhibition of miR-19a-3p on AMPK/GSK-3β/HO-1 pathway by down-regulating miR-19a-3p, and ultimately played a role in improving ischemic stroke.
To sum up, it was concluded that glycine improved ischemic stroke by regulating glucose metabolism, inflammatory response and cell apoptosis through miR-19a-3p/AMPK/GSK-3β/HO-1 pathway.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38(7):1167–1186. [DOI] [PubMed] [Google Scholar]
- 2.Gallo A, Galliazzo S, Grazioli S, Guasti L, Ageno W, Squizzato A. Epidemiology and secondary prevention of ischemic stroke in patients on antiplatelet drug: a retrospective cohort study. J Thromb Thrombolysis. 2019;48(2):336–344. doi: 10.1007/s11239-019-01893-y [DOI] [PubMed] [Google Scholar]
- 3.Navis A, Garcia-Santibanez R, Skliut M. Epidemiology and outcomes of ischemic stroke and transient ischemic attack in the adult and geriatric population. J Stroke Cerebrovasc Dis. 2019;28(1):84–89. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.013 [DOI] [PubMed] [Google Scholar]
- 4.Veno SK, Bork CS, Jakobsen MU, et al. Marine n-3 polyunsaturated fatty acids and the risk of ischemic stroke. Stroke. 2019;50(2):274–282. doi: 10.1161/STROKEAHA.118.023384 [DOI] [PubMed] [Google Scholar]
- 5.Sun L, Clarke R, Bennett D, et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med. 2019;25(4):569–574. doi: 10.1038/s41591-019-0366-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nave AH, von Eckardstein A. Is lipoprotein(a) a risk factor for ischemic stroke and venous thromboembolism? Clin Res Cardiol Suppl. 2019;14(Suppl 1):28–32. doi: 10.1007/s11789-019-00101-8 [DOI] [PubMed] [Google Scholar]
- 7.Varbo A, Nordestgaard BG. Remnant cholesterol and risk of ischemic stroke in 112,512 individuals from the general population. Ann Neurol. 2019;85(4):550–559. doi: 10.1002/ana.25432 [DOI] [PubMed] [Google Scholar]
- 8.Shakib N, Khadem Ansari MH, Karimi P, Rasmi Y. Neuroprotective mechanism of low-dose sodium nitrite in oxygen-glucose deprivation model of cerebral ischemic stroke in PC12 cells. EXCLI J. 2019;18:229–242. doi: 10.17179/excli2018-1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urabe T, Watada H, Okuma Y, et al. Prevalence of abnormal glucose metabolism and insulin resistance among subtypes of ischemic stroke in Japanese patients. Stroke. 2009;40(4):1289–1295. doi: 10.1161/STROKEAHA.108.522557 [DOI] [PubMed] [Google Scholar]
- 10.Bjerkreim AT, Khanevski AN, Thomassen L, et al. Five-year readmission and mortality differ by ischemic stroke subtype. J Neurol Sci. 2019;403:31–37. doi: 10.1016/j.jns.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 11.Li X, Yan X, Wang F, et al. Down-regulated lncRNA SLC25A5-AS1 facilitates cell growth and inhibits apoptosis via miR-19a-3p/PTEN/PI3K/AKT signalling pathway in gastric cancer. J Cell Mol Med. 2019;23(4):2920–2932. doi: 10.1111/jcmm.14200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai R, Cui Z, Ma Y, et al. The NF-kappaB-modulated miR-19a-3p enhances malignancy of human ovarian cancer cells through inhibition of IGFBP-3 expression. Mol Carcinog. 2019;58(12):2254–2265. doi: 10.1002/mc.23113 [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Lee H, Bae H, Choi EH, Kim SJ. Epigenetic silencing of miR-19a-3p by cold atmospheric plasma contributes to proliferation inhibition of the MCF-7 breast cancer cell. Sci Rep. 2016;6:30005. doi: 10.1038/srep30005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Liu Y, Zhang J. Silencing of miR-19a-3p enhances osteosarcoma cells chemosensitivity by elevating the expression of tumor suppressor PTEN. Oncol Lett. 2019;17(1):414–421. doi: 10.3892/ol.2018.9592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang XM, Yu XN, Liu TT, et al. microRNA-19a-3p promotes tumor metastasis and chemoresistance through the PTEN/Akt pathway in hepatocellular carcinoma. Biomed Pharmacother. 2018;105:1147–1154. doi: 10.1016/j.biopha.2018.06.097 [DOI] [PubMed] [Google Scholar]
- 16.Gollmann-Tepekoylu C, Polzl L, Graber M, et al. miR-19a-3p containing exosomes improve function of ischemic myocardium upon shock wave therapy. Cardiovasc Res. 2019. [DOI] [PubMed] [Google Scholar]
- 17.Ge XL, Wang JL, Liu X, Zhang J, Liu C, Guo L. Inhibition of miR-19a protects neurons against ischemic stroke through modulating glucose metabolism and neuronal apoptosis. Cell Mol Biol Lett. 2019;24:37. doi: 10.1186/s11658-019-0160-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol. 2017;45:31–37. doi: 10.1016/j.ceb.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 19.Gupta AP, Singh P, Garg R, et al. Pancreastatin inhibitor activates AMPK pathway via GRP78 and ameliorates dexamethasone induced fatty liver disease in C57BL/6 mice. Biomed Pharmacother. 2019;116:108959. doi: 10.1016/j.biopha.2019.108959 [DOI] [PubMed] [Google Scholar]
- 20.Sun P, Yin JB, Liu LH, et al. Protective role of Dihydromyricetin in Alzheimer’s disease rat model associated with activating AMPK/SIRT1 signaling pathway. Biosci Rep. 2019;39:1. doi: 10.1042/BSR20180902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Q, Li X, Liu J, et al. AMPK is associated with the beneficial effects of antidiabetic agents on cardiovascular diseases. Biosci Rep. 2019;39:2. doi: 10.1042/BSR20181995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day EA, Ford RJ, Steinberg GR. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol Metab. 2017;28(8):545–560. doi: 10.1016/j.tem.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 23.Bayliss JA, Lemus MB, Stark R, et al. Ghrelin-AMPK signaling mediates the neuroprotective effects of calorie restriction in Parkinson’s disease. J Neurosci. 2016;36(10):3049–3063. doi: 10.1523/JNEUROSCI.4373-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan J, Cui J, Yang Z, et al. Neuroprotective effect of Apelin 13 on ischemic stroke by activating AMPK/GSK-3beta/Nrf2 signaling. J Neuroinflammation. 2019;16(1):24. doi: 10.1186/s12974-019-1406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagon Y, Avraham Y, Magen I, Gertler A, Ben-Hur T, Berry EM. Nutritional status, cognition, and survival: a new role for leptin and AMP kinase. J Biol Chem. 2005;280(51):42142–42148. doi: 10.1074/jbc.M507607200 [DOI] [PubMed] [Google Scholar]
- 26.Liu R, Liao XY, Pan MX, et al. Glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of M1 microglial polarization via the NF-kappaB p65/Hif-1alpha signaling pathway. J Immunol. 2019;202(6):1704–1714. doi: 10.4049/jimmunol.1801166 [DOI] [PubMed] [Google Scholar]
- 27.Cai CC, Zhu JH, Ye LX, et al. Glycine protects against hypoxic-ischemic brain injury by regulating mitochondria-mediated autophagy via the AMPK pathway. Oxid Med Cell Longev. 2019;2019:4248529. doi: 10.1155/2019/4248529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018;27(2):299–313. doi: 10.1016/j.cmet.2017.10.009 [DOI] [PubMed] [Google Scholar]