Abstract
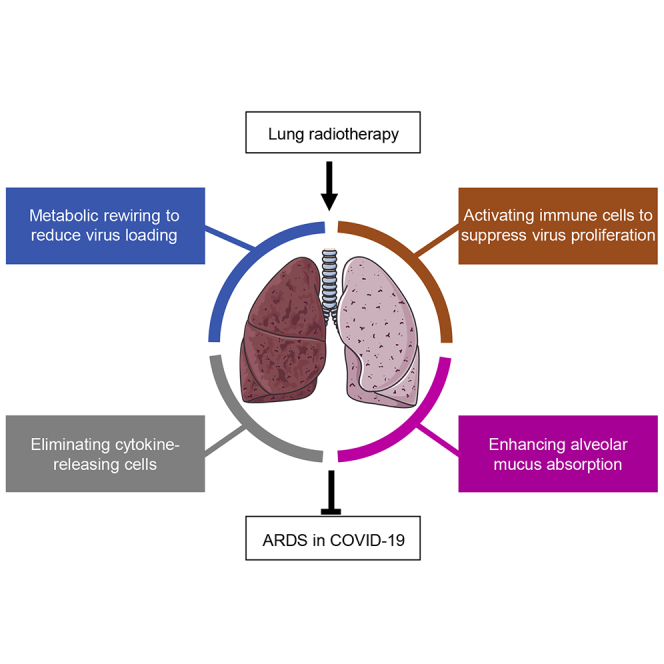
The acute respiratory distress syndrome (ARDS) induced by SARS-CoV-2-mediated cytokine storm (CS) in lungs leads to the high mortality in COVID-19 patients. To reduce ARDS, an ideal approach is to diminish virus loading by activating immune cells for CS prevention or to suppress the overactive cytokine-releasing immune cells for CS inhibition. Here, a potential radiation-mediated CS regulation is raised by reevaluating the radiation-mediated pneumonia control in the 1920s, with the following latent advantages of lung radiotherapy (LR) in treatment of COVID-19: (1) radiation accesses poorly circulated tissue more efficiently than blood-delivered medications; (2) low-dose radiation (LDR)-mediated metabolic rewiring and immune cell activation inhibit virus loading; (3) pre-consumption of immune reserves by LDR decreases CS severity; (4) higherdose radiation (HDR) within lung-tolerable doses relieves CS by eliminating in situ overactive cytokine-releasing cells. Thus, LDR and HDR or combined with antiviral and life-supporting modalities may mitigate SARS-CoV-2 and other virus-mediated ARDS.
Subject Areas: Health Sciences, Immunology, Therapy
Graphical Abstract
Health Sciences; Immunology; Therapy
Introduction
The SARS-CoV-2 virus that induces the pandemic of coronavirus disease 2019 (COVID-19) has infected more than 4 million people with over 320,000 casualties worldwide; the numbers are still increasing on a daily basis (WHO, 2020). Currently, effective treatments or preventive SARS-CoV-2 vaccination are not available, although beneficial effects are observed by using respiratory ventilation or extracorporeal membrane oxygenation (ECMO) as life-supporting measures (Matthay et al., 2020, Han et al., 2020, Ruan et al., 2020). Potential antiviral agents including remdesivir and favipiravir are still under investigation (Lu, 2020, Wu and McGoogan, 2020). Thus, although many infected people are able to self-recover or are cured with mild medication, inventing effective modalities or utilizing the existing therapies with proven safety to alleviate CS and ARDS remains as an urgent need (Mehta et al., 2020). The foremost life-threatening condition of ARDS is the highly elevated levels of cytokines and chemokines (termed the cytokine storm, CS) in the lungs caused by the heavy virus loading and the consequent overreaction of the host innate immune system. Thus, although a great effort is to be continued in pursuing of the anti-virus agents and vaccination (Li et al., 2020, Liu et al., 2020), new calls are made for inventing the host-directed approaches to block the virus-induced immune overreaction and CS in COVID-19 patients (Moore and June, 2020, Zumla et al., 2020). Therefore, the medications that can efficaciously prevent or reduce CS severity are to be urgently developed and/or tested.
Two major pathologic phases have been identified in the course of SARS-CoV-2-mediated lung dysfunction (Chan et al., 2020, Xu et al., 2020, Yao et al., 2020). The initial phase illustrates the primary antiviral inflammatory response, at which stage enhancing the host immune activity to diminish virus loading is suggested to benefit the prognosis. In contrast, the advanced phase is characterized by the severely CS-induced endotoxic lung damages due to the immune overreaction with noticeable alveolar mucus accumulation and limitation of gas exchange capacity. At this stage, immune suppressive approaches are strongly recommended (Huang et al., 2020, Matthay et al., 2020, Thevarajan et al., 2020, Wu et al., 2020). Thus, based on the radiation-mediated adaptive cell response (Alexandrou and Li, 2014) and radiation-induced immune regulation (Stoecklein et al., 2015), as well as the histological data on pneumonia control by radiotherapy (Calabrese and Dhawan, 2013), this perspective article is attempted to illustrate the potential effects of LR on CS in reducing ARDS in COVID-19 patients. The CS-preventive effects could be achieved by application of low-dose radiation (LDR) with the lung-safe dose range (equivalent to the dose received in a regular examination of computed tomography, CT). Without potential concern of major side effects, LDR could be repeated and precisely delivered to the infected area enhancing the anti-viral environment by rewiring epithelial cell metabolism and activating in situ immune cells. In addition, such a boosted host immune surveillance may also be beneficial in reducing the severity of CS due to LDR-mediated pre-consumption of immune reserves. On the another hand, when the symptoms of ARDS become obvious with the significant indications of CS in the lungs, a single dose of higher dose radiation (HDR) within the lung-tolerable dose range, could be an option for direct inhibition or elimination of the in situ overactive cytokine-releasing cells to suppress CS in the targeted local lung tissue. In addition, HDR to the CS tissue may restore a certain level of the alveolar gas exchange capacity via radiation-induced epithelial cell renewal and microvasculature modification. Because the radiation doses applied in LR will be in the lung-tolerable ranges, LR with LDR and HDR is proposed to serve as the alternative modalities to reduce ARDS in COVID-19 patients.
Pneumonia Radiotherapy
Historically, LR and antiserum treatment were the only two choices for the control of bacteria- and virus-induced pneumonia. In fact, X-ray was used to treat pertussis/whooping cough from 1923 to 1936 in North America and Europe (Calabrese et al., 2017). Surprisingly, LR was reported to reduce the pneumonia mortality to a similar rate as treated by immune serum and sulfonamide. In 2013, Calabreses and Dhawan published a milestone review on this historic period regarding control of different types of pneumonia by radiation (Calabrese and Dhawan, 2013). Among a total of 863 pneumonia patients treated by X-ray therapy, including 85 virus-induced pneumonia and 36 interstitial pneumonia cases, the reported total cure rate was 83.1% (717 out of 863 cases), including 67 of 85 (78.8%) cases of virus-induced pneumonia and 22 of 29 (75.9%) cases of interstitial pneumonia (Calabrese and Dhawan, 2013). In addition, animal model using feline-virus-induced cat pneumonia, which mimicked the human atypical pneumonia, showed that the incidence of pneumonia was reduced by 50% and 25%, respectively by radiation delivered 24 h and 48 h after the virus infection (Baylin et al., 1946, Dubin et al., 1946). Impressively, the same group also demonstrated the survival advantage of LR in a mouse pneumonia model induced with high-risk swine influenza virus (Baylin et al., 1946, Dubin et al., 1946). It is remarkable that the radiation-mediated pneumonia control was believed by then to be linked to the number and responsiveness of immune cells (Glenn, 1946). However, as noted by Calabrese and Dhawan, due to limitations, the control groups were not validated or well described in these studies, and little LR research has been conducted since 1946 (Calabrese and Dhawan, 2013), which apparently is due to the emergence of more effective vaccines and antibiotics. Additional reasons are the well-documented radiation-associated complications such as lung fibrosis induced by thoracic irradiation (average dose range 45–65 Gy) (Beach et al., 2020, Ding et al., 2013). Nevertheless, in the light of enormous scientific advances in the past century, especially in the fields of molecular biology, radiation biology, and immunology, and with the urgent situation of mitigating ARDS in COVID-19 patients, the potential benefits of LR should be reanalyzed. The latent LR therapeutic effects could be validated with the incredible advances in the methodologies that are able to precisely deliver the dose to the target tissue under sophisticated imaging-guided approaches. In addition, together with the modern biological technologies, more details of virus-induced pneumonia such as the virus genomic identification, virus loading, and cytokine increments can be quickly obtained in clinic. Thus, radiation-mediated host immune surveillance is worthy to be investigated in the control of CS with updated information of SARS-CoV-2, CS level, and the pathologic progression of ARDS. Radiation with LDR is well defined to induce an adaptive homeostatic function in mammalian cells, which fits into the need of boosting immune cells to reduce virus loading, whereas HDR may be considered to inhibit or eliminate the in situ overactive cytokine-generating immune cells to suppress CS.
Compared with the vanished LR, the antiserum modality of convalescent plasma used 100 years ago for pneumonia treatment remains as a modality in control of SARS-CoV-2. However, although clinical benefits have been reported in patients with SARS-CoV, H5N1, H1N1 influenza, Ebola virus, and MERS (Middle East Respiratory Syndrome), and recently SARS-CoV-2 (Chen et al., 2020a, Florescu et al., 2015, Hung et al., 2011, Shen et al., 2020, Van Griensven et al., 2016, Zhou et al., 2007), convalescent plasma will face the same challenges of the mono-targeting of virus and the limited serum resource. LR does not have such weaknesses and holds the non-invasive advantage. With its broad impact on host cells, exceptional source availability, uncostly procedure, and adjustable doses, radiation is an ideal approach to regulate CS in prevention and/or mitigation of ARDS. The dose ranges applicable in LDR and HDR are to be carefully tested and optimized to precisely fit into the purpose of CS inhibition to minimize the risk of side effects. Based on the data from in vitro cell and in vivo animal experiments, and human data of radiation exposure, the dose range of LDR to a local lung area in the initial phase of virus infection is recommended as 0.1–0.5 Gy; and the range of HDR useful for eliminating in situ cytokine-generating cells is recommended as 3–8 Gy. Both dose ranges of radiation are tolerable by normal lung tissues and are below the clinical dosage used in clinic thoracic radiotherapy, thus minimizing the potential adverse effects.
LDR-Rewired Epithelial Cell Metabolism Limits Virus Replication
It is known that virus can hijack the metabolism in the host cells to meet the increased energy consumption for the fast virus replication. Both DNA and RNA viruses are able to reprogram the carbon metabolic pathways in the host cell by accelerating glycolysis and pentose phosphate activity to increase the energy demands for virus proliferation. Thaker et al. has demonstrated that viruses are depending on consuming the major nutrients including glucose and glutamine for anabolic augmentation (Thaker et al., 2019). Thus, LDR-enhanced mitochondrial-dominated oxygen metabolism in the lung epithelial cells will compete and/or inhibit such virus-mediated rewiring of bioenergetics to slow down virus replication and loading (Figure 1). LDR-induced biological homeosis in mammals has been extensively studied (Morgan, 2006, Olivieri et al., 1984, Wiencke et al., 1986, Cai and Liu, 1992a). Such LDR-induced metabolism is demonstrated in an array of normal cells with increased ability of defending cells against genotoxic stress and cell transformation (Azzam et al., 1994, Bosi and Olivieri, 1989, BOYDEN, 1999, Cai et al., 1993, Cai and Liu, 1992b, Gourabi and Mozdarani, 1998, Morgan, 2006, Redpath and Antoniono, 1998). The LDR-mediated homeostasis is further identified with enhanced cellular capacity to detoxify damages of reactive oxygen species (ROS) by increasing mitochondrial antioxidant enzyme MnSOD and mitochondrial metabolic functions (Candas et al., 2013, Eldridge et al., 2012, Grdina et al., 2012). The adaptive cellular protection with enhanced metabolic function is linked with the large scale of genes upregulated by LDR, including the fundamental factors involved in DNA repair and pro-survival signaling pathways (Boothman et al., 1989, Ikushima et al., 1996, Stecca and Gerber, 1998), reduction of ROS (Schaue et al., 2002, Spitz and Hauer-Jensen, 2014), as well as enhancing mitochondrial function and antioxidant activity (Fan et al., 2007). Mice treated with whole-body LDR (10 cGy) were protective against radiation-induced cell death and inflammatory response (Jin et al., 2015). Thus, application of LDR will help the host cells to reduce the rate of viral replication by a potential metabolic competition to deprive the virus energy dependence. In addition, LDR applied to the non-infected lung tissue may also be beneficial by increasing energy metabolism in the healthy cells to limit the scale of virus infection rate. However, the dose range for LDR in blocking virus loading in human lung epithelial cells is to be tested and optimized by tests with animal models.
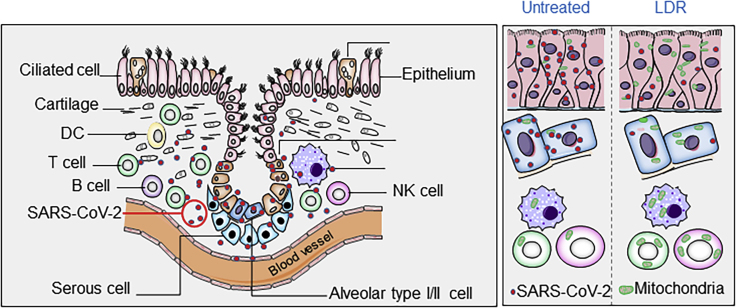
Figure 1.
LDR-Induced Metabolic Rewiring in Lung Epithelial and In Situ Immune Cells Enhances the Anti-Virus Environment
LDR-induced adaptive metabolic enhancement in lung epithalamium (including ciliated cells and alveolar type I and II cells) may limit virus replication via energy competition. In addition, LDR-enhanced mitochondrial function in the immune cells (e.g., T cell, B cell, macrophages) is able to increase the immune cell activity to release cytokines to attack virus. Thus, LDR-mediated epithelial metabolism and enhanced immune cell activity coordinatively increase the anti-virus environment in the infected lungs.
LDR-Activated In Situ Immune Cells Reinforce the Antiviral Condition
Effective modalities capable of activating the in situ immune cell are urgently needed to reduce SARS-CoV-2-mediated CS. A weak immune force in the host tissue is well evidenced to cause CS via the increased viral loading in the lungs as well as in the circulation (Mehta et al., 2020). In addition to the protective function of LDR in lung epithelial cells, LDR-mediated immune activation has been observed by in vitro and in vivo models (Arenas et al., 2012, Trott and Kamprad, 1999). LDR-associated anti-inflammatory effects could be achieved by irradiation of either a locally infected area (Arenas et al., 2006) or the whole body (Calabrese and Dhawan, 2013, Frey et al., 2009). Radiation-induced nitric oxide is shown to raise the function of macrophages (Hildebrandt et al., 1998), and human lymphocytes pre-exposed to LDR exhibited an adaptive homeostasis, with reduced apoptosis and susceptibility to subsequent cytotoxic stress (Cregan et al., 1999, Olivieri et al., 1984, Shadley and Wolff, 1987, Wiencke et al., 1986). Additional human data demonstrated the LDR-induced adaptive activation in the lymphocytes isolated from the workers who received low levels of environmental or occupational radiation (Barquinero et al., 1995, Gourabi and Mozdarani, 1998, Monsieurs et al., 2000, Tedeschi et al., 1996). LDR-induced lymphocyte activation can enhance the local level of cytokines to enhance anti-viral force, thus reducing SARS-CoV-2 proliferation. Furthermore, it is shown that a constant immunogenic stimulation can cause lymphocyte exhaustion (Yi et al., 2010). Thus, the immune cell activation by LDR before the onset of the CS may have a preventing effect to limit the coming wave of virus-induced CS. LDR may consume a critical portion of the “immune reserve,” which is otherwise required to induce a full bursting of CS. Thus, in addition to LDR-induced metabolic advantage in lung epithelial cells described above, LDR-induced in situ immune cell activation and immune consumption may effectively reduce the CS severity and ARDS (Figure 1). The local anti-viral enhancement induced by LDR could be further increased via a potential radiation-induced immunogenicity in the infected lung tissue, which is described below.
Radiation-Mediated Immunogenicity Attracts Immune Cells to the Infected Area
Accumulating evidences indicate that radiation can increase the immune cell infiltration into a solid tumor via generation of immunogenic DNA and cellular damages. Such radiation-mediated immune priming effects have long been observed, termed as the “abscopal effect” due to radiation-induced tumor immunogenicity (Brooks and Chang, 2019, Golden et al., 2015, Postow et al., 2012). Radiation-induced damage-associated molecular patterns (DAMPs), including the high-mobility group box 1 protein (HMGB1), can enhance antigen presentation by dendritic cells (DCs) to T cells, thereby activating immune attack on irradiated tumor cells (Barker et al., 2015, Gameiro et al., 2014, Hu et al., 2017, Derer et al., 2015, Zhang et al., 2007). In addition, HMGB1 is shown to enhance the ability of immune cells to produce tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), IL-6, and IL-8 (Andersson et al., 2000). Currently, it is unknown whether such radiation-associated immunogenicity in tumor cells could be strongly induced by radiation in normal human lung tissue. Importantly, it could be a study with high priority that LDR-induced lung tissue immunogenicity is a cross-priming of radiation-induced immunogenicity with a potential specific viral antigen. The first-needed supportive evidence could be an observation that circulating immune cells are significantly augmented into the irradiated areas as illustrated in Figure 2. The priming function of radiation was actually implicated in the early studies of virus-induced pneumonia in human and animal models (Calabrese and Dhawan, 2013). The LDR-boosted recruitment of circulating immune cells will increase the immune cell presentation in the infected area to further block virus loading. Indeed, a single dose of 0.5 Gy was shown to cause the recruitment of NOS2-expressing macrophages and T cells into the irradiated area (Klug et al., 2013). Stoecklein et al. have further demonstrated that a cluster of active immune cells, including macrophages, DCs, natural killer cells, T cells, and B cells, is dose-dependently activated in mice treated with whole-body irradiation with the dose range 1–4 Gy (Stoecklein et al., 2015). However, a remaining key question to be addressed is how effectively LDR can induce such immune priming function, which could be validated by experiments with soon available animal models generated with SARS-CoV-2-mediated CS and ARDS. Such in vivo tests may be able to identify a specific immunogenicity in the SARS-CoV-2-infected lungs that matches the potential immunogenicity in human lung tissue infected with SARS-CoV-2. It could be expected that the LDR-induced SARS-CoV-2 specific antigens efficaciously mobilize more host innate immune cells to the irradiated area. Thus, together with LDR-induced in situ immune cell activation, LDR-enhanced recruitment of circulating immune cells will further strengthen the antiviral environment.
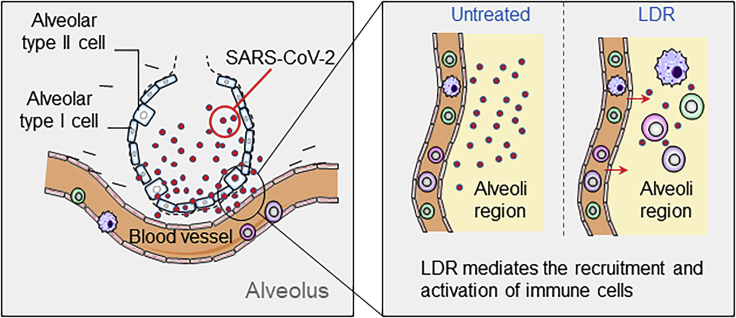
Figure 2.
LDR Potentially Induces a Radiation-Associated Cellular Immunogenicity in the Virus-Infected Lung Tissue Which Attracts Immune Cells to the Infected Area
Radiation-induced immunogenicity of tumor cells has been well demonstrated by enhanced immune cell infiltration into the solid tumors via radiation-enhanced antigen transfer by immune cells. It is thus assumed that LDR-treated lung tissue with virus infection may generate potential specific immunogenicity capable of attracting the circulating immune cells to the infected area to reduce the virus loading and the CS. The postulated specific antigens induced by different doses of radiation in the infected lung tissue are to be identified with the soon available animal models with the SARS-CoV-2-mediated ARDS.
Suppressing CS by HDR Elimination of In Situ Overactive Cytokine-Releasing Cells
The lethal course of ARDS is related to the levels of CS-mediated lung dysfunction (Mehta et al., 2020, Zhang et al., 2020). Contrasted to the need of activating immune cells to block virus loading for preventing CS, the major task during CS-medated ARDS is proposed to timely inhibit or eliminate the overactive cytokine/chemokine-releasing cells that include immune cells and the endothelial cells as demonstrated in Figure 3. The increased levels of cytokines and chemokines cause hyperinflammation, and the clinical symptoms of ARDS have been observed in the cases of ARDS induced by SARS-CoV-2 and other viruses including SARS, MERS, H1N1, and H5N1 (Channappanavar and Perlman, 2017, Mehta et al., 2020, Ruan et al., 2020, Teijaro et al., 2014). At the onset of CS, the overreactive immune cells, including T cells, B cells, macrophages, natural killer cells, DCs are mobilized to infected areas. These overactive immune cells together with the endothelial cells (Teijaro et al., 2011) are the major cells releasing cytokines and chemokines, including IL-2, IL-7, granulocyte colony-stimulating factor (GCSF), interferon-γ-inducible protein 10 (IP-10, CXCL10), monocyte chemoattractant protein 1 (MCP-1, CCL2), macrophage inflammatory protein 1-α (MIP-1α), TNF-α, and ferritin. A cluster of 14 cytokines has been specified in COVID-19 patients, and three of them, CXCL10, CCL7, and the IL-1 receptor antagonist, have been linked with the poor prognosis of COVID-19 due to increased virus loading and lung dysfunction (Vaninov, 2020, Yang et al., 2020). Thus, effective inhibition of the overactive cytokine-releasing cells in the CS tissue is required to reduce the severity of CS and ARDS. However, the doses of HDR that can instantly eliminate the in situ cytokine-generating cells for suppressing CS with minimal adverse effect is to be determined.
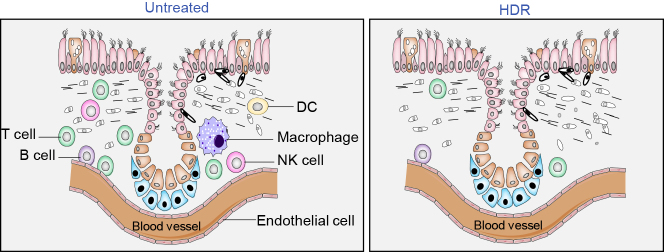
Figure 3.
Eliminating the In Situ Overactive Cytokine-Releasing Immune Cells by HDR to Reduce the Severity of CS
HDR-mediated immune suppression via the direct cytotoxicity on active immune cells may serve as an applicable tool in eliminating the local in situ overactive cytokine-releasing cells to reduce CS. Due to a relatively high radiosensitivity of lymphocytes, HDR may effectively suppress the cytokine-releasing immune cells and endothelial cells in the infected lung tissue. Thus, a treatment with HDR within the lung tolerable radiation doses may serve as a choice to instantly suppress CS for relieving the severity of ARDS by removing the cytokine-releasing cells.
HDR-mediated immune suppression has long been applied to the treatments of many human diseases, including patients receiving bone marrow transplantation. Due to the feature of multiple cytokines involved in the CS, blocking or inhibiting a limited number of cytokines may be insufficient to suppress the CS or ARDS (Channappanavar and Perlman, 2017, Li et al., 2010, Wonderlich et al., 2017). In addition, endothelial cells in the lungs have a similar radiosensitivity with immune cells (Hei et al., 1987) and are also actively involved in influenza-induced cytokine generation and the CS (Teijaro et al., 2011). Therefore, it is reasonable to assume that LR with HDR is able to at least temporally suppress CS by targeting the cytokine-generating immune cells and endothelial cells (Chen et al., 2014, Kobayashi et al., 2013, Teijaro et al., 2014). Because lung epithelial cells are relatively resistant to radiation, the lung epithelial cells may be relatively less damaged by HDR, thus remaining a potential ability in restoring alveolar gas exchange under CS-inhibited condition. This suggestion is encouraged by the facts that human lymphocytes are known to belong to the most radiosensitive mammalian cells (Huang et al., 1987, Slavin, 1987, Trowell, 1952). A single dose of 1.5-Gy radiation can kill 50% of lymphocytes in the lymph nodes (Trowell, 1952), and their nuclear degradation occurred as early as 1 h post-irradiation (Schrek, 1947, Schrek, 1948, Trowell, 1952). Such interphase cell death is mainly mediated via p53-dependent apoptosis (Anderson and Warner, 1976, Seki et al., 1994), although some activated lymphocytes, such as CD4+ regulatory T cells and antigen-experienced and memory T cells, are shown to be slightly radioresistant compared with naive T cells (Brent and Medawar, 1966, Dunn and North, 1991, Grayson et al., 2002, Qu et al., 2010). In contrast, CD8+ T cells are the most radiosensitive lymphocyte population (Crompton and Ozsahin, 1997, Wilkins et al., 2002). Data from Fornace lab have further identified that radiation with 3 Gy can effectively block T cell activation by impairing metabolic pathways (Li et al., 2015). Therefore, based on above radiobiologic evidences, the goal of CS inhibition by HDR may be achievable by application of a dose in the HDR range 3–8 Gy that is still below the standard total dosage of thoracic radiotherapy (Fairchild et al., 2008). Such potential HDR benefits could be enhanced by precisely targeting radiation with imaging guidance to the CS area or a lobe in the infected lungs enriched with images of CS. However, unquestionably, the HDR-mediated CS suppression will be carefully tested and evaluated with the details of basal health conditions including overall lung function, severity of CS, as well as serum levels of cytokines.
Improving Alveolar Gas Exchange by HDR-Induced Vasculature Modification
It has been well documented that HDR induces reoxygenation in the hypoxic area in the poor-circulated solid tumor (Hong et al., 2016, Moon et al., 2010, Okamoto et al., 2016). The respiratory system in humans is co-developed with the cardiovascular system via crosstalk and integration of multiple cellular populations (Morrisey and Hogan, 2010). Adult human lungs have functional gas exchange surfaces of about 70 m2 with a tremendous compensation ability (Basil et al., 2020). Under conditions of repairing cellular loss or lung function deficiency, the alveolar cells are able to self-renew and proliferate to restore normal alveolar structure and gas exchange function, which are regulated via an integrated network of mesenchymal, endothelial, and epithelial cells and other components (Barkauskas et al., 2013, Lee et al., 2017). The direct lethal course of SARS-CoV-2 is due to the severely damaged alveolar gas exchange resulted from CS, leading to lower blood oxygenation (Chen et al., 2020b, Guan et al., 2020). The associated major pathologic alterations related to the reduced gas exchange capacity are identified as proteinaceous exudate with globules, epithelial cell proliferation with inflammatory cell infiltration, formation of multinucleated giant cells, and hyaline membrane formation in the alveolus (Tian et al., 2020). Such pathological features in the lungs of COVID-19 patients are also found to be shared with other virus-induced pneumonia.
The defective circulation induced by CS makes it difficult to rescue the dysfunctional lung tissue in COVID-19 patients. The drainage of mucus from the alveoli under CS is more challenging due to inflammation-mediated congestion, which further worsens the circulation and mucus accumulation, damaging the gas exchanging efficacy. Such a “locked area” may severely limit the access by medications using drugs and/or antiviral agents. As postulated by the scheme shown in Figure 4, HDR-mediated modifications in the microvasculature in the poor-circulated CS tissue may help to improve the absorption of alveolar mucus due to HDR-induced cell apoptosis and the potential modification of microvasculature via endothelial cell renewal. Thus, the proposed HDR-mediated effects in alveolar clearance may be achievable based on the relative radiosensitivity of endothelial cells that are radiosensitive epithelial cells. Teijaro et al. have revealed that blocking S1P(1) receptor that is expressed on both endothelial cells and lymphocytes in the lung can suppress CS with reduced mortality in mice infected with human influenza virus (Teijaro et al., 2011). In addition, the lung microvasculature is shown to contain heterogenic endothelial subpopulations with various vasculogenic capacities coordinately contributing to lung injury response (Niethamer et al., 2020, Stevens et al., 2008). Notably, proliferation of the lung endothelial cell subpopulation is enhanced during influenza-mediated lung injury (Basil et al., 2020, Niethamer et al., 2020). On the other hand, cutaneous low-dose radiation has been shown to increase tissue vascularity through upregulation of angiogenic and vasculogenic pathways (Thanik et al., 2010). Thus, an optimized stimulus by a proper radiation dose of HDR to the CS tissue may effectively improve the local circulation and alveolar gas exchange function. However, this point of view on HDR-mediated alveolar mucus absorption is to be debated and needs to be further evaluated and approved by experimental data. It is assumed that although HDR-induced adverse effects such as lung fibrosis is a potential concern, the weighting on LR potential function could be favored to the urgent need of restoring the alveolar function to at least temporally relieving the severe situation of the acute respiratory failure in ARDS.
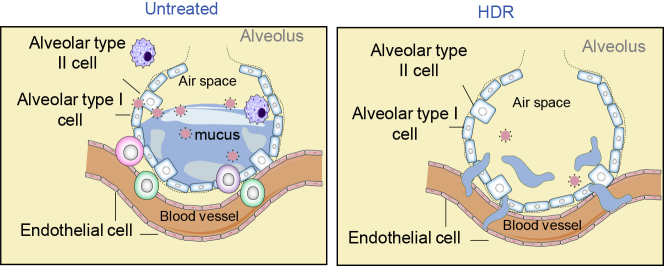
Figure 4.
Potential Alveolar Mucus Absorption by Radiation-Induced Vascular Modification
The major lethal course in COVID-19 patients is associated with the dropped blood oxygenation level due to CS-induced damages in alveoli gas exchange capacity. The drainage of alveolar mucus in the inflammation-mediated congestion has been a challenge in the treatment of COVID-19 patients due to limited access of medications using blood-delivered drugs and/or antiviral agents. Radiation has the potential ability of tissue re-oxygenation by medication of microvasculature in the hypoxic area in solid tumor. Thus, with the unique capacity of deep tissue penetration, LR of HDR may be able to improve gas exchange capacity by effectively relieving the alveolar mucus accumulation via radiation-mediated microvasculature alternations that includes endothelial cell apoptosis and replace. The potential benefits and effectiveness of HDR-accelerated mucus clearance are to be tested by animal models.
Perspectives
Further research on LR-mediated potential therapeutic benefits is urgently needed for enhancing the control of the CS and ARDS in COVID-19 patients. Based on the pathological progression, early lung injuries are caused by direct virus-induced cytopathic changes, followed by an advanced phase of CS-induced massive lung injury and ARDS. Thus, a potential effective window time for RL should be validated by monitoring the progress of virus infection including blood cytokine levels, lung CT images, and clinical symptoms. For the purpose of preventing CS by LDR, animal tests are to be conducted to determine the effects of LDR in treatments of both infected and uninfected lung areas for enhancing the immune surveillance in blocking or reducing virus loading. Data from animal studies have showed that LDR treatment 24 h after virus infection reduced the duration of the acute inflammatory phase from 10 days to 5 days (Arenas et al., 2006, Calabrese and Dhawan, 2013). Accordingly, LDR in the very low-dose range (e.g., 0.1–0.5 Gy) could be safely repeated to the infected lung areas within 24–72 h after the appearance of virus-inducted inflammatory response. Instead, for instant elimination of the overactive cytokine-releasing cells by HDR, both in vitro and in vivo models are to be applied to ascertain whether a dose of 2 Gy or above to the local area or a lobe with significant CS could effectively inhibit cytokine levels. In addition, improved quality of radiation sources, such as high-energy particles, has been shown to induce tumor immunogenicity (Gridley et al., 2006, Janiak et al., 2017, Onishi et al., 2018), which is also worthy to be investigated for enhancing the potential effects of LR with LDR and HDR. The potential HDR-induced side effects could be further reduced by application of such high-energy radiation sources within the standard range of lung tolerable doses as set by the European Organization for Research and Treatment of Cancer (EORTC).
Conclusions
This perspective is attempted to reevaluate a potential benefit of lung radiotherapy with varied radiation doses to fit into the needs of CS control at different stages of SARS-CoV-2 infection. To prevent CS, LDR is suggested to apply in the phase of virus infection to reduce virus loading, whereas HDR could be a choice to instantly eliminate the overactive cytokine-releasing cells to suppress CS thus relieving ARDS. It is expected that with the soon available animal models of SARS-CoV-2-induced pneumonia, the timing and dosage of LDR and HDR could be investigated and more precisely validated. Meanwhile, clinical trials could be conducted with LDR that is relatively safe by application of local radiation via a careful selection of patients with confirmed phase of viral loading. Most importantly, the proposed LR may be considered to synergize the effects of antiviral and life-supportive modalities to improve lung functions in COVID-19 patients. LDR may especially enhance the effectiveness of drugs that can reduce the production of mucus by alveolar cells. Nevertheless, in order to efficaciously reduce the onset and duration of the CS without severe lung adverse effects, LR needs to be precisely evaluated on its potential effects in control of CS and ARDS. All of these tasks and potential clinical benefits may only be achieved by overcoming the challenges in experimental models and via pre-clinical trials. Regardless, it is hoped that LR may shed light on the way out of the current darkness.
Acknowledgment
The author likes to thank Somona Fiogoie at iSience and the anonymous reviewers for their critical review and insightful suggestions that have significantly improved this work for publication. I acknowledge Ji Ming Wang for review of the manuscript; Joe Chang for suggestion on clinical radiotherapy; Bowen Xie and Yixin Duan for preparing the figures; Douglas Spitz and David Gius for collaboration on mitochondrial research; William Murphy, Ralph Weichselbaum, and Yang-Xin Fu for discussion on radiation-associated immune regulation; Gayle Woloschak and David Grdina for collaboration in research of low-dose radiation. The research in J.J.L.'s lab has been supported by NIH National Cancer Institute Grant R01 CA213830. The funding source did not influence the design of experiments and data explanation or the related concepts presented in this article. This work is dedicated to the memory of Dr. Nancy H. Colburn, a great mentor and friend, and to the people who recently passed away due to virus-induced pneumonia. The author apoligizes to the people whose valuable works could not be cited to this article due to limited space.
References
- Alexandrou A.T., Li J.J. Cell cycle regulators guide mitochondrial activity in radiation-induced adaptive response. Antioxid. Redox Signal. 2014;20:1463–1480. doi: 10.1089/ars.2013.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.E., Warner N.L. Ionizing radiation and the immune response. Adv. Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- Andersson U., Wang H., Palmblad K., Aveberger A.C., Bloom O., Erlandsson-Harris H., Janson A., Kokkola R., Zhang M., Yang H. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas M., Gil F., Gironella M., Hernandez V., Jorcano S., Biete A., Pique J.M., Panes J. Anti-inflammatory effects of low-dose radiotherapy in an experimental model of systemic inflammation in mice. Int. J. Radiat. Oncol. Biol. Phys. 2006;66:560–567. doi: 10.1016/j.ijrobp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Arenas M., Sabater S., Hernandez V., Rovirosa A., Lara P.C., Biete A., Panes J. Anti-inflammatory effects of low-dose radiotherapy. Indications, dose, and radiobiological mechanisms involved. Strahlenther Onkol. 2012;188:975–981. doi: 10.1007/s00066-012-0170-8. [DOI] [PubMed] [Google Scholar]
- Azzam E., Raaphorst G., Mitchel R. Radiation-induced adaptive response for protection against micronucleus formation and neoplastic transformation in C3H 10T1/2 mouse embryo cells. Radiat. Res. 1994;138:S28–S31. [PubMed] [Google Scholar]
- Barkauskas C.E., Cronce M.J., Rackley C.R., Bowie E.J., Keene D.R., Stripp B.R., Randell S.H., Noble P.W., Hogan B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H.E., Paget J.T., Khan A.A., Harrington K.J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquinero J., Barrios L., Caballin M., Miro R., Ribas M., Subias A., Egozcue J. Occupational exposure to radiation induces an adaptive response in human lymphocytes. Int. J. Radiat. Biol. 1995;67:187–191. doi: 10.1080/09553009514550231. [DOI] [PubMed] [Google Scholar]
- Basil M.C., Katzen J., Engler A.E., Guo M., Herriges M.J., Kathiriya J.J., Windmueller R., Ysasi A.B., Zacharias W.J., Chapman H.A. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell. 2020;26:482–502. doi: 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin G.J., Dubin I.N., Gobbel W.G., Jr. The effect of roentgen therapy on experimental virus pneumonia; on feline virus pneumonia. Am. J. Roentgenol. Radium. Ther. 1946;55:473–477. [PubMed] [Google Scholar]
- Beach T.A., Groves A.M., Williams J.P., Finkelstein J.N. Modeling radiation-induced lung injury: lessons learned from whole thorax irradiation. Int. J. Radiat. Biol. 2020;96:129–144. doi: 10.1080/09553002.2018.1532619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothman D.A., Bouvard I., Hughes E.N. Identification and characterization of X-ray-induced proteins in human cells. Cancer Res. 1989;49:2871–2878. [PubMed] [Google Scholar]
- Bosi A., Olivieri G. Variability of the adaptive response to ionizing radiations in humans. Mut. Res. Fund. Mol. Mech. Mut. 1989;211:13–17. doi: 10.1016/0027-5107(89)90102-4. [DOI] [PubMed] [Google Scholar]
- BOYDEN G.R.S. Adaptive response and its variation in human normal and tumour cells. Int. J. Radiat. Biol. 1999;75:865–873. doi: 10.1080/095530099139926. [DOI] [PubMed] [Google Scholar]
- Brent L., Medawar P. Quantitative studies on tissue transplantation immunity. 8. The effects of irradiation. Proc. R. Soc. Lond. B Biol. Sci. 1966;165:413–423. doi: 10.1098/rspb.1966.0074. [DOI] [PubMed] [Google Scholar]
- Brooks E.D., Chang J.Y. Time to abandon single-site irradiation for inducing abscopal effects. Nat. Rev. Clin. Oncol. 2019;16:123–135. doi: 10.1038/s41571-018-0119-7. [DOI] [PubMed] [Google Scholar]
- Cai L., Jiang J., Wang B., Yao H., Wang X. Induction of an adaptive response to dominant lethality and to chromosome damage of mouse germ cells by low dose radiation. Mutat. Res. 1993;303:157–161. doi: 10.1016/0165-7992(93)90017-p. [DOI] [PubMed] [Google Scholar]
- Cai L., Liu S. Effect of cycloheximide on the adaptive response induced by low dose radiation. Biomed. Environ. Sci. 1992;5:46–52. [PubMed] [Google Scholar]
- Cai L., Liu S.Z. Study on the mechanism of cytogenetic adaptive response induced by low dose radiation. Chin. Med. J. (Engl). 1992;105:277–283. [PubMed] [Google Scholar]
- Calabrese E.J., Dhawan G. How radiotherapy was historically used to treat pneumonia: could it Be useful today? Yale. J. Biol. Med. 2013;86:555–570. [PMC free article] [PubMed] [Google Scholar]
- Calabrese E.J., Dhawan G., Kapoor R. Radiotherapy for pertussis: an historical assessment. Dose Response. 2017;15 doi: 10.1177/1559325817704760. 1559325817704760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candas D., Fan M., Nantajit D., Vaughan A.T., Murley J.S., Woloschak G.E., Grdina D.J., Li J.J. CyclinB1/Cdk1 phosphorylates mitochondrial antioxidant MnSOD in cell adaptive response to radiation stress. J. Mol. Cell Biol. 2013;5:166–175. doi: 10.1093/jmcb/mjs062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Zhang A.J., Yuan S., Poon V.K., Chan C.C., Lee A.C., Chan W.M., Fan Z., Tsoi H.W., Wen L. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li X., Tian L., Zheng S., Yang S., Dong Y., Wang Y., Cui D., Liu X., Liang W. Dynamic behavior of lymphocyte subgroups correlates with clinical outcomes in human H7N9 infection. J. Infect. 2014;69:358–365. doi: 10.1016/j.jinf.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Cregan S.P., Brown D.L., Mitchel R.E. Apoptosis and the adaptive response in human lymphocytes. Int. J. Radiat. Biol. 1999;75:1087–1094. doi: 10.1080/095530099139548. [DOI] [PubMed] [Google Scholar]
- Crompton N.E., Ozsahin M. A versatile and rapid assay of radiosensitivity of peripheral blood leukocytes based on DNA and surface-marker assessment of cytotoxicity. Radiat. Res. 1997;147:55–60. [PubMed] [Google Scholar]
- Derer A., Deloch L., Rubner Y., Fietkau R., Frey B., Gaipl U.S. Radio-Immunotherapy-induced immunogenic cancer cells as basis for induction of systemic anti-tumor immune responses – pre-clinical evidence and ongoing clinical applications. Front. Immunol. 2015;6:505. doi: 10.3389/fimmu.2015.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N.H., Li J.J., Sun L.Q. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr. Drug Targets. 2013;14:1347–1356. doi: 10.2174/13894501113149990198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin I.N., Baylin G.J., Gobble W.G., Jr. The effect of roentgen therapy on experimental virus pneumonia; on pneumonia produced in white mice by swine influenza virus. Am. J. Roentgenol. Radium. Ther. 1946;55:478–481. [PubMed] [Google Scholar]
- Dunn P.L., North R.J. Selective radiation resistance of immunologically induced T cells as the basis for irradiation-induced T-cell-mediated regression of immunogenic tumor. J. Leukoc. Biol. 1991;49:388–396. doi: 10.1002/jlb.49.4.388. [DOI] [PubMed] [Google Scholar]
- Eldridge A., Fan M., Woloschak G., Grdina D.J., Chromy B.A., Li J.J. Manganese superoxide dismutase interacts with a large scale of cellular and mitochondrial proteins in low-dose radiation-induced adaptive radioprotection. Free Radic. Biol. Med. 2012;53:1838–1847. doi: 10.1016/j.freeradbiomed.2012.08.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild A., Harris K., Barnes E., Wong R., Lutz S., Bezjak A., Cheung P., Chow E. Palliative thoracic radiotherapy for lung cancer: a systematic review. J. Clin. Oncol. 2008;26:4001–4011. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- Fan M., Ahmed K.M., Coleman M.C., Spitz D.R., Li J.J. Nuclear factor-kappaB and manganese superoxide dismutase mediate adaptive radioresistance in low-dose irradiated mouse skin epithelial cells. Cancer Res. 2007;67:3220–3228. doi: 10.1158/0008-5472.CAN-06-2728. [DOI] [PubMed] [Google Scholar]
- Florescu D.F., Kalil A.C., Hewlett A.L., Schuh A.J., Stroher U., Uyeki T.M., Smith P.W. Administration of brincidofovir and convalescent plasma in a patient with Ebola virus disease. Clin. Infect. Dis. 2015;61:969–973. doi: 10.1093/cid/civ395. [DOI] [PubMed] [Google Scholar]
- Frey B., Gaipl U.S., Sarter K., Zaiss M.M., Stillkrieg W., Rodel F., Schett G., Herrmann M., Fietkau R., Keilholz L. Whole body low dose irradiation improves the course of beginning polyarthritis in human TNF-transgenic mice. Autoimmunity. 2009;42:346–348. doi: 10.1080/08916930902831738. [DOI] [PubMed] [Google Scholar]
- Gameiro S.R., Jammeh M.L., Wattenberg M.M., Tsang K.Y., Ferrone S., Hodge J.W. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–416. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn G.C. Further studies on the influence of X-rays on the phagocytic indices of healthy rabbits. J. Immunol. 1946;53:95–100. [PubMed] [Google Scholar]
- Golden E.B., Chhabra A., Chachoua A., Adams S., Donach M., Fenton-Kerimian M., Friedman K., Ponzo F., Babb J.S., Goldberg J. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16:795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- Gourabi H., Mozdarani H. A cytokinesis-blocked micronucleus study of the radioadaptive response of lymphocytes of individuals occupationally exposed to chronic doses of radiation. Mutagenesis. 1998;13:475–480. doi: 10.1093/mutage/13.5.475. [DOI] [PubMed] [Google Scholar]
- Grayson J.M., Harrington L.E., Lanier J.G., Wherry E.J., Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J. Immunol. 2002;169:3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- Grdina D.J., Murley J.S., Miller R.C., Mauceri H.J., Sutton H.G., Thirman M.J., Li J.J., Woloschak G.E., Weichselbaum R.R. A manganese superoxide dismutase (SOD2)-Mediated adaptive response. Radiat. Res. 2012 doi: 10.1667/RR3126.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley D.S., Dutta-Roy R., Andres M.L., Nelson G.A., Pecaut M.J. Acute effects of iron-particle radiation on immunity. Part II: leukocyte activation, cytokines and adhesion. Radiat. Res. 2006;165:78–87. doi: 10.1667/rr3490.1. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q., Lin Q., Jin S., You L. Recent insights into 2019-nCoV: a brief but comprehensive review. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.010. [DOI] [Google Scholar]
- Hei T.K., Marchese M.J., Hall E.J. Radiosensitivity and sublethal damage repair in human umbilical cord vein endothelial cells. Int. J. Radiat. Oncol. Biol. Phys. 1987;13:879–884. doi: 10.1016/0360-3016(87)90103-9. [DOI] [PubMed] [Google Scholar]
- Hildebrandt G., Seed M.P., Freemantle C.N., Alam C.A., Colville-Nash P.R., Trott K.R. Mechanisms of the anti-inflammatory activity of low-dose radiation therapy. Int. J. Radiat. Biol. 1998;74:367–378. doi: 10.1080/095530098141500. [DOI] [PubMed] [Google Scholar]
- Hong B.J., Kim J., Jeong H., Bok S., Kim Y.E., Ahn G.O. Tumor hypoxia and reoxygenation: the yin and yang for radiotherapy. Radiat. Oncol. J. 2016;34:239–249. doi: 10.3857/roj.2016.02012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z.I., McArthur H.L., Ho A.Y. The abscopal effect of radiation therapy: what is it and how can we use it in breast cancer? Curr. Breast Cancer Rep. 2017;9:45–51. doi: 10.1007/s12609-017-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A.T., Hunter R.F., Roth P.A., Mold N.G. Immune lymphocyte survival after chemotherapy and radiation. Blood. 1987;69:1269. [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F., To K.K., Lee C.-K., Lee K.-L., Chan K., Yan W.-W., Liu R., Watt C.-L., Chan W.-M., Lai K.-Y. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikushima T., Aritomi H., Morisita J. Radioadaptive response: efficient repair of radiation-induced DNA damage in adapted cells. Mut. Res. Fund. Mol. Mech. Mut. 1996;358:193–198. doi: 10.1016/s0027-5107(96)00120-0. [DOI] [PubMed] [Google Scholar]
- Janiak M.K., Wincenciak M., Cheda A., Nowosielska E.M., Calabrese E.J. Cancer immunotherapy: how low-level ionizing radiation can play a key role. Cancer Immunol. Immunother. 2017;66:819–832. doi: 10.1007/s00262-017-1993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Qin L., Shi Y., Candas D., Fan M., Lu C.L., Vaughan A.T., Shen R., Wu L.S., Liu R. CDK4-mediated MnSOD activation and mitochondrial homeostasis in radioadaptive protection. Free Radic. Biol. Med. 2015;81:77–87. doi: 10.1016/j.freeradbiomed.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug F., Prakash H., Huber P.E., Seibel T., Bender N., Halama N., Pfirschke C., Voss R.H., Timke C., Umansky L. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Iwata A., Suzuki K., Suto A., Kawashima S., Saito Y., Owada T., Kobayashi M., Watanabe N., Nakajima H. B and T lymphocyte attenuator inhibits LPS-induced endotoxic shock by suppressing Toll-like receptor 4 signaling in innate immune cells. Proc. Natl. Acad. Sci. U S A. 2013;110:5121–5126. doi: 10.1073/pnas.1222093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Tammela T., Hofree M., Choi J., Marjanovic N.D., Han S., Canner D., Wu K., Paschini M., Bhang D.H. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell. 2017;170:1149–1163 e1112. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.H., Wang Y.W., Chen R., Zhou B., Ashwell J.D., Fornace A.J., Jr. Ionizing radiation impairs T cell activation by affecting metabolic reprogramming. Int. J. Biol. Sci. 2015;11:726–736. doi: 10.7150/ijbs.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Lim A., Phoon M.C., Narasaraju T., Ng J.K., Poh W.P., Sim M.K., Chow V.T., Locht C., Alonso S. Attenuated Bordetella pertussis protects against highly pathogenic influenza A viruses by dampening the cytokine storm. J. Virol. 2010;84:7105–7113. doi: 10.1128/JVI.02542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang C., Liu L., Gu M. Existing bitter medicines for fighting 2019-nCoV-associated infectious diseases. FASEB J. 2020;34:6008–6016. doi: 10.1096/fj.202000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimm. 2020 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- Matthay M.A., Aldrich J.M., Gotts J.E. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir. Med. 2020;8:433–434. doi: 10.1016/S2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Hlh Across Speciality Collaboration U.K. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsieurs M.A., Thierens H.M., Vral A.M., Van De Wiele C., De Ridder L.I., Dierckx R.A. Adaptive response in patients treated with 131I. J. Nucl. Med. 2000;41:17–22. [PubMed] [Google Scholar]
- Moon E.J., Sonveaux P., Porporato P.E., Danhier P., Gallez B., Batinic-Haberle I., Nien Y.C., Schroeder T., Dewhirst M.W. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc. Natl. Acad. Sci. U S A. 2010;107:20477–20482. doi: 10.1073/pnas.1006646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- Morgan W.F. Will radiation-induced bystander effects or adaptive responses impact on the shape of the dose response relationships at low doses of ionizing radiation? Dose Response. 2006;4:257–262. doi: 10.2203/dose-response.06-110.Morgan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey E.E., Hogan B.L. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethamer T.K., Stabler C.T., Leach J.P., Zepp J.A., Morley M.P., Babu A., Zhou S., Morrisey E.E. Defining the role of pulmonary endothelial cell heterogeneity in the response to acute lung injury. Elife. 2020;9:e53072. doi: 10.7554/eLife.53072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S., Shiga T., Yasuda K., Watanabe S., Hirata K., Nishijima K.I., Magota K., Kasai K., Onimaru R., Tuchiya K. The reoxygenation of hypoxia and the reduction of glucose metabolism in head and neck cancer by fractionated radiotherapy with intensity-modulated radiation therapy. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:2147–2154. doi: 10.1007/s00259-016-3431-4. [DOI] [PubMed] [Google Scholar]
- Olivieri G., Bodycote J., Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223:594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- Onishi M., Okonogi N., Oike T., Yoshimoto Y., Sato H., Suzuki Y., Kamada T., Nakano T. High linear energy transfer carbon-ion irradiation increases the release of the immune mediator high mobility group box 1 from human cancer cells. J. Radiat. Res. 2018;59:541–546. doi: 10.1093/jrr/rry049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow M.A., Callahan M.K., Barker C.A., Yamada Y., Yuan J., Kitano S., Mu Z., Rasalan T., Adamow M., Ritter E. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y., Jin S., Zhang A., Zhang B., Shi X., Wang J., Zhao Y. Gamma-ray resistance of regulatory CD4+CD25+Foxp3+ T cells in mice. Radiat. Res. 2010;173:148–157. doi: 10.1667/RR0978.1. [DOI] [PubMed] [Google Scholar]
- Redpath J.L., Antoniono R.J. Induction of an adaptive response against spontaneous neoplastic transformation in vitro by low-dose gamma radiation. Radiat. Res. 1998;149:517–520. [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaue D., Marples B., Trott K.R. The effects of low-dose X-irradiation on the oxidative burst in stimulated macrophages. Int. J. Radiat. Biol. 2002;78:567–576. doi: 10.1080/09553000210126457. [DOI] [PubMed] [Google Scholar]
- Schrek R. Primary and secondary vacuoles in thymic cells exposed in vitro to X-rays. J. Cell Comp. Physiol. 1947;30:203–224. doi: 10.1002/jcp.1030300302. [DOI] [PubMed] [Google Scholar]
- Schrek R. Cytologic changes in thymic glands exposed in vivo to X-rays. Am. J. Pathol. 1948;24:1055–1065. [PMC free article] [PubMed] [Google Scholar]
- Seki H., Kanegane H., Iwai K., Konno A., Ohta K., Yachie A., Taniguchi N., Miyawaki T. Ionizing radiation induces apoptotic cell death in human TcR-gamma/delta+ T and natural killer cells without detectable p53 protein. Eur. J. Immunol. 1994;24:2914–2917. doi: 10.1002/eji.1830241150. [DOI] [PubMed] [Google Scholar]
- Shadley J.D., Wolff S. Very low doses of X-rays can cause human lymphocytes to become less susceptible to ionizing radiation. Mutagenesis. 1987;2:95–96. doi: 10.1093/mutage/2.2.95. [DOI] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin S. Total lymphoid irradiation. Immunol. Today. 1987;8:88–92. doi: 10.1016/0167-5699(87)90852-8. [DOI] [PubMed] [Google Scholar]
- Spitz D.R., Hauer-Jensen M. Ionizing radiation-induced responses: where free radical chemistry meets redox biology and medicine. Antioxid. Redox Signal. 2014;20:1407–1409. doi: 10.1089/ars.2013.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecca C., Gerber G.B. Adaptive response to DNA-damaging agents: a review of potential mechanisms. Biochem. Pharmacol. 1998;55:941–951. doi: 10.1016/s0006-2952(97)00448-6. [DOI] [PubMed] [Google Scholar]
- Stevens T., Phan S., Frid M.G., Alvarez D., Herzog E., Stenmark K.R. Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc. Am. Thorac. Soc. 2008;5:783–791. doi: 10.1513/pats.200803-027HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoecklein V.M., Osuka A., Ishikawa S., Lederer M.R., Wanke-Jellinek L., Lederer J.A. Radiation exposure induces inflammasome pathway activation in immune cells. J. Immunol. 2015;194:1178–1189. doi: 10.4049/jimmunol.1303051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi B., Caporossi D., Vernole P., Padovani L., Mauro F. Do human lymphocytes exposed to the fallout of the Chernobyl accident exhibit an adaptive response? III. Challenge with bleomycin in lymphocytes from children hit by the initial acute dose of ionizing radiation. Mut. Res. Fund. Mol. Mech. Mut. 1996;354:77–80. doi: 10.1016/0027-5107(96)00039-5. [DOI] [PubMed] [Google Scholar]
- Teijaro J.R., Walsh K.B., Cahalan S., Fremgen D.M., Roberts E., Scott F., Martinborough E., Peach R., Oldstone M.B., Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R., Walsh K.B., Rice S., Rosen H., Oldstone M.B. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc. Natl. Acad. Sci. U S A. 2014;111:3799–3804. doi: 10.1073/pnas.1400593111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker S.K., Ch'ng J., Christofk H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019;17:59. doi: 10.1186/s12915-019-0678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanik V.D., Chang C.C., Lerman O.Z., Greives M.R., Le H., Warren S.M., Schneider R.J., Formenti S.C., Saadeh P.B., Levine J.P. Cutaneous low-dose radiation increases tissue vascularity through upregulation of angiogenic and vasculogenic pathways. J. Vasc. Res. 2010;47:472–480. doi: 10.1159/000313875. [DOI] [PubMed] [Google Scholar]
- Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott K.R., Kamprad F. Radiobiological mechanisms of anti-inflammatory radiotherapy. Radiother. Oncol. 1999;51:197–203. doi: 10.1016/s0167-8140(99)00066-3. [DOI] [PubMed] [Google Scholar]
- Trowell O.A. The sensitivity of lymphocytes to ionising radiation. J. Pathol. Bacteriol. 1952;64:687–704. doi: 10.1002/path.1700640403. [DOI] [PubMed] [Google Scholar]
- Van Griensven J., Edwards T., de Lamballerie X., Semple M.G., Gallian P., Baize S., Horby P.W., Raoul H., Magassouba N.F., Antierens A. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N. Engl. J. Med. 2016;374:33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaninov N. In the eye of the COVID-19 cytokine storm. Nat. Rev. Immunol. 2020;20:277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2020). Novel Coronavirus (COVID-19) Situation. May 23.
- Wiencke J.K., Afzal V., Olivieri G., Wolff S. Evidence that the [3H] thymidine-induced adaptive response of human lymphocytes to subsequent doses of X-rays involves the induction of a chromosomal repair mechanism. Mutagenesis. 1986;1:375–380. doi: 10.1093/mutage/1.5.375. [DOI] [PubMed] [Google Scholar]
- Wilkins R.C., Wilkinson D., Maharaj H.P., Bellier P.V., Cybulski M.B., McLean J.R. Differential apoptotic response to ionizing radiation in subpopulations of human white blood cells. Mutat. Res. 2002;513:27–36. doi: 10.1016/s1383-5718(01)00290-x. [DOI] [PubMed] [Google Scholar]
- Wonderlich E.R., Swan Z.D., Bissel S.J., Hartman A.L., Carney J.P., O'Malley K.J., Obadan A.O., Santos J., Walker R., Sturgeon T.J. Widespread virus replication in alveoli drives acute respiratory distress syndrome in aerosolized H5N1 influenza infection of macaques. J. Immunol. 2017;198:1616–1626. doi: 10.4049/jimmunol.1601770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Shen C., Li J., Yuan J., Yang M., Wang F., Li G., Li Y., Xing L., Peng L. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv. 2020 doi: 10.1101/2020.03.02.20029975. [DOI] [Google Scholar]
- Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., Mou H.M., Wang L.H., Zhang H.R., Fu W.J. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- Yi J.S., Cox M.A., Zajac A.J. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Bowerman N.A., Salama J.K., Schmidt H., Spiotto M.T., Schietinger A., Yu P., Fu Y.X., Weichselbaum R.R., Rowley D.A. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J. Exp. Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Zhong N., Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N. Engl. J. Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- Zumla A., Hui D.S., Azhar E.I., Memish Z.A., Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395:e35–e36. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]