Abstract
Basic research on SARS-CoV-2 is essential to understand its detailed pathophysiology and identify best drug targets. Models that can faithfully reproduce the viral life cycle and reproduce the pathology of COVID-19 are required. Here, we briefly review the cell lines, organoids, and animal models that are currently being used in COVID-19 research.
Keywords: SARS-CoV-2, COVID-19, cell models, animal models, organoids
Overview of SARS-CoV-2
In December 2019, pneumonia of an unknown etiology was confirmed in China [1]. The Chinese Center for Disease Control and Prevention (CCDC) identified a novel coronavirus infection as the cause of this pneumonia [2]. The World Health Organization (WHO) named the disease ‘2019-new coronavirus disease’ (COVID-19)i and the International Committee on Taxonomy of Viruses named the virus ‘severe acute respiratory syndrome coronavirus 2’ (SARS-CoV-2)ii. The WHO soon declared that COVID-19 was a fast-evolving pandemiciii. As of 26 May 2020, it is estimated that 5 406 282 people have been infected with COVID-19 and 343 562 people have died globallyiii. Multiple clinical trials are currently underway for prevention or intervention in the disease progression [3]. In parallel, it is also equally essential to carry out basic research on SARS-CoV-2 to support the efficient development of therapeutic agents. For this, models that can faithfully reproduce the behavior of the virus and reproduce the pathology of COVID-19 are required. Here, we briefly review relevant cell lines, organoids, and animal model animals.
Cell Lines and Organoids for SARS-CoV-2 Research
An in vitro cell model for SARS-CoV-2 research is essential for understanding the viral life cycle, for amplifying and isolating the virus for further research, and for preclinical evaluation of therapeutic molecules. This section lays out the cell lines used to replicate and isolate SARS-CoV-2, as well as organoids that can be used to examine the effects of SARS-CoV-2 infection on specific human tissues (Table 1 and Figure 1 ).
Table 1.
Cell Lines and Organoids and Animal Models Currently Being Used in COVID-19 Research
| Cell lines and organoids | ||||
|---|---|---|---|---|
| Type | Origin | Key points | Refs | |
| Human airway epithelial cells | Commercially available from various vendors (Lonza, PromoCell, etc.) | Human airway epithelial cells can isolate SARS-CoV-2 and mimic infected human lung cells. After SARS-CoV-2 infection, cytopathic effects were observed. | [5] | |
| Vero E6 cells | Wild type cells | Isolated from kidney epithelial cells of an African green monkey | Vero E6 cells are the most widely used clone used to replicate and isolate the SARS-CoV-2. | [11] |
| TMPRSS2-overexpressing cells | Viral RNA copies in the culture supernatants of these cells were >100 times higher than those of wild type Vero E6 cells. | [12] | ||
| Caco-2 cells | Isolated from human colon adenocarcinoma | SARS-CoV-2 could replicate in Caco-2 cells (data not shown). | [6] | |
| Calu-3 cells | Isolated from non-small cell lung cancer | Compared with mock control, SARS-CoV-2 S pseudovirions showed an over 500-fold increase in luciferase activities in Calu3 cells. | [7] | |
| HEK293T cells | Isolated from human embryonic kidney (HEK) cells grown in tissue culture | Cells showed only modest viral replication. | [8] | |
| Huh7 cells | Isolated from hepatocyte-derived cellular carcinoma cells | Cells showed about a tenfold increase in luciferase activity when transduced by SARS-CoV-2 S pseudovirions. | [7] | |
| Human bronchial organoids | Generated from commercially available human bronchial epithelial cells | After SARS-CoV-2 infection, not only the intracellular viral genome, but also progeny virus, cytotoxicity, pyknotic cells, and moderate increases of the type I interferon signal can be observed. | [17] | |
| Human lung organoids | Generated from human embryonic stem cells | The lung organoids, particularly alveolar type II cells, are permissive to SARS-CoV-2 infection. | [18] | |
| Human kidney organoids | Generated from human embryonic stem cells | Human kidney organoids produce infectious progeny virus. | [19] | |
| Human liver ductal organoids | Generated from primary bile ducts isolated from human liver biopsies | Human liver ductal organoids are permissive to SARS-CoV-2 infection, and SARS-CoV-2 infection impairs the bile acid transporting functions of cholangiocytes. | [20] | |
| Human intestinal organoids | Generated from primary gut epithelial stem cells | Human intestinal organoids were readily infected by SARS-CoV-2, as demonstrated by confocal and electron microscopy. Significant titers of infectious viral particles were detected. | [22,23] | |
| Human blood vessel organoids | Generated from human induced pluripotent stem cells | SARS-CoV-2 can directly infect human blood vessel organoids. | [19] | |
| Animal models | |||
|---|---|---|---|
| Animal species | Key points | Refs | |
| Mice | Wild type mice | SARS-CoV-2 cannot invade cells through mouse Ace2. | [11] |
| Human ACE2 transgenic mice | After SARS-CoV-2 infection, the mice show weight loss, virus replication in the lungs, and interstitial pneumonia. | [25] | |
| Syrian hamster | After SARS-CoV-2 infection, the hamsters show rapid breathing, weight loss, and diffuse alveolar damage with extensive apoptosis. | [26] | |
| Ferrets | After SARS-CoV-2 infection, acute bronchiolitis was observed in the lungs. | [27] | |
| Cats | After SARS-CoV-2 infection, intra-alveolar edema and congestion in the interalveolar septa were observed. Abnormal arrangement of the epithelium with loss of cilia and lymphocytic infiltration into the lamina propria were also observed. | [28] | |
| Cynomolgus macaques | SARS-CoV-2 can infect both type I and type II pneumocytes. After SARS-CoV-2 infection, pulmonary consolidation, pneumonia, and edema fluid in alveolar lumina were observed. | [29] | |
| Rhesus macaques | Infected macaques had high viral loads in the upper and lower respiratory tract, humoral and cellular immune responses, and pathologic evidence of viral pneumonia. The therapeutic effects of adenovirus-vectored vaccine, DNA vaccine candidates expressing S protein, and remdesivir treatment could be evaluated. | [30., 31., 32., 33.] | |
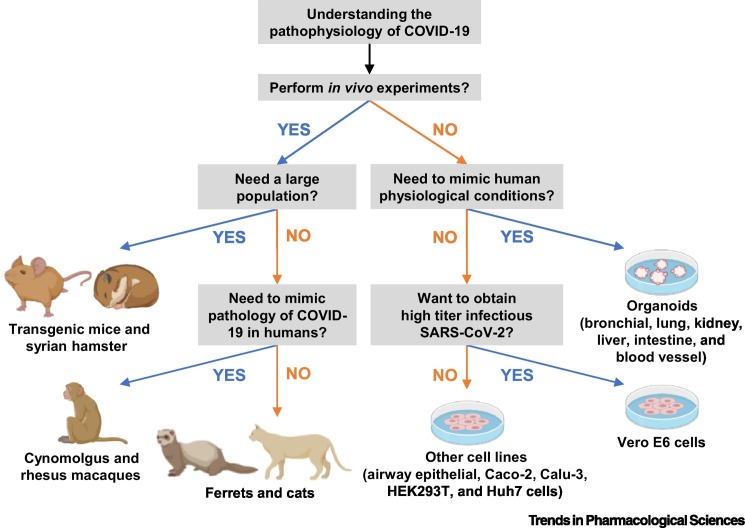
Figure 1.
Schematic Showing a Decision Flowchart of Cell Lines, Organoids, and Animal Models in Studying COVID-19 Pathophysiology.
Figure created with Biorender (https://biorender.com).
Cell Lines
In humans, airway epithelial cells highly express the putative SARS-CoV-2 entry receptor, angiotensin-converting enzyme 2 (ACE2) and transmembrane serine proteinase 2 (TMPRSS2), the receptor that the virus uses to prime the S protein (spike protein of SARS-CoV-2) [4]. SARS-CoV-2 infection experiments using primary human airway epithelial cells have been found to have cytopathic effects 96 h after the infection [5]. However, primary human airway epithelial cells are expensive and do not proliferate indefinitelyiv. Several infinitely proliferating cell lines, such as Caco-2 [6], Calu-3 [7], HEK293T [8], and Huh7 [7] have been utilized in SARS-CoV-2 infection experiments. These cell lines do not accurately mimic human physiological conditions and generate low titer of infectious SARS-CoV-2 [6., 7., 8.]. Despite this limitation, valuable information about the virus infection and replication can be learned from studies using these cell lines.
However, Vero cells have given high titer of viral particles [8]. For efficient SARS-CoV-2 research, a cell line, such as Vero cells, that can easily replicate and isolate the virus is essential. These cells were isolated from the kidney epithelial cells of an African green monkey in 1963 and have been shown to not produce interferon (IFN) when infected with Newcastle disease virus, rubella virus, and other viruses [9]. A homozygous ~9 Mbp deletion on chromosome 12 causes the loss of the type I interferon (IFN-I) gene cluster and of cyclin-dependent kinase inhibitor genes [10]. The IFN deficiency allows SARS-CoV-2 to replicate in Vero cells. Among the several Vero cell clones, Vero E6 is the most widely used to replicate and isolate SARS-CoV-2 [11], because these cells highly express ACE2 on the apical membrane domain. However, the expression level of TMPRSS2, the receptor that the virus uses to prime the S protein (spike protein of SARS-CoV-2) [4], is quite low in this clone. To enhance the replication and isolation efficiencies of SARS-CoV-2 in Vero E6 cells, Matsuyama et al. have used TMPRSS2-overexpressing Vero E6 cells [12]. They reported that the viral RNA copies in the culture supernatants of these cells were >100 times higher than those of Vero E6 cells, suggesting that it would be possible to isolate higher titer virus using TMPRSS2-overexpressing Vero E6 cells.
Organoids
Organoids are composed of multiple cell types and model the physiological conditions of human organs. Because organoids have the ability to self-replicate, they are also suitable models for large-scale screening in drug discovery and disease research [13]. Besides the lung damage caused by pneumonia, SARS-CoV-2 affects several organs like the kidney [14], liver [15], and the cardiovascular system [16]. Suzuki et al. and Han et al. generated human bronchial organoids [17] or human lung organoids [18], respectively, for SARS-CoV-2 research. They showed that their organoids were permissive to the SARS-CoV-2 infection and could evaluate antiviral effects of COVID-19 candidate therapeutic compounds, including camostat [17]. Besides the lung damage caused by pneumonia, SARS-CoV-2 affects several organs like the kidney [14], liver [15], and the cardiovascular system [16]. Monteil et al. have shown that the supernatant of SARS-CoV-2 infected kidney organoids differentiated from human embryonic stem cells can efficiently infect Vero E6 cells, showing that the kidney organoids produce infectious progeny virus [19]. In addition, Zhao et al. have demonstrated that human liver ductal organoids are permissive to SARS-CoV-2 infection and support replication [20]. Interestingly, virus infection impaired the bile acid transporting functions of cholangiocytes [20]. This effect might be the reason for the bile acid accumulation and consequent liver damage in patients with COVID-19. Furthermore, it is expected that the intestine is another viral target organ [21]. Lamers et al. and Zhou et al. have reported that human intestinal organoids, which were established from primary gut epithelial stem cells, support SARS-CoV-2 replication [22,23]. Moreover, Monteil et al. have also demonstrated that SARS-CoV-2 can directly infect human blood vessel organoids differentiated from human induced pluripotent stem cells [19]. Consistently, Varga et al. confirmed the presence of viral elements within endothelial cells and an accumulation of inflammatory cells [24]. Taken together, the two studies suggest that SARS-CoV-2 infection induces endotheliitis in several organs as a direct consequence of virus involvement. However, while organoids can reproduce the pathology of COVID-19 in specific tissues on which they are modeled, they cannot reproduce the systemic symptoms associated with whole body responses to the viral infection.
Animal Models for SARS-CoV-2 Research
The complex pathophysiology of the disease will only be understood by reproducing tissue-specific and systemic virus–host interactions. While cell lines and organoids are faster systems to study the virus and its interactions inside host cells, these can only reproduce the symptoms of COVID-19 in a specific cell type or organ, respectively. However, the pathology of COVID-19 can be reproduced and observed in a tissue-specific and systemic manner in animal models. Several different animals are being used to study the disease and to test candidate therapeutic compounds (Table 1 and Figure 1).
Small Animals
One of the works that set the pace for discovery of animal models was by Zhou et al. who conducted SARS-CoV-2 infection experiments using HeLa cells that expressed ACE2 proteins taken from multiple animal species, from mice to humans [11]. Interestingly, SARS-CoV-2 could use all ACE2 proteins, except for mouse ACE2. Therefore, Bao et al. used transgenic mice that express human ACE2 [25]. The team found that such mice, after SARS-CoV-2 infection, showed weight loss, virus replication in the lungs, and interstitial pneumonia [25]. In the search of alternative small animal models, molecular docking studies were performed on the binding between ACE2 of various mammals and the S protein of SARS-CoV-2, with the finding that the Syrian hamster might be suitable [26]. After infection, these hamsters show rapid breathing, weight loss, and alveolar damage with extensive apoptosis [26].
Large Animals
Small animals like mice and Syrian hamster are advantageous to study SARS-CoV-2, as they reproduce faster; however, to faithfully reproduce COVID-19 pathology in humans, larger animal models are preferred. Kim et al. reported nonlethal acute bronchiolitis in the lungs of a ferret model [27]. Another study showed that SARS-CoV-2 can replicate in ferrets and cats, but not in pigs, chickens, and ducks [28]. Based on these findings, it is recommended to use ferrets and cats when selecting large experimental animals rather than rodents.
Another model that can be used for COVID-19 studies and is currently the closest to humans in pathophysiology, is the primate cynomolgus macaques. Rockx et al. used cynomolgus macaques to compare MERS-CoV, SARS-CoV, and SARS-CoV-2 [29]. Although MERS-CoV mainly infected type II pneumocytes, both SARS-CoV and SARS-CoV-2 infect type I and II pneumocytes. After SARS-CoV-2 infection, damage on type I pneumocytes led to pulmonary edema and the formation of hyaline membranes. Thus, cynomolgus macaques can be infected with SARS-CoV-2 and reproduce some of the human pathologies of COVID-19.
Rhesus macaques have also been used in COVID-19 studies [30] where the therapeutic effects of adenovirus-vectored vaccine [31], DNA vaccine candidates expressing S protein [32], and remdesivir treatment [33] were confirmed. While these models probably are best in replicating virus–human host interactions, a major limitation is that the reproduction rate in cynomolgus and rhesus monkeys is less and slower. Hence this can be preceded by experiments with transgenic mice and Syrian hamsters.
Concluding Remarks
COVID-19 has spread rapidly all over the world in the past 5 months. Even now, the number of infected people and deaths continues to rise. At this time, there are no therapeutic prevention or intervention methods available. The only ways to control the pandemic and reduce associated loss of lives has been to change peoples’ behavior, like quarantine and social distancing. Therapeutic strategies for prevention and/or intervention is the need of the hour. While multiple clinical trials are currently underway, in parallel, preclinical research on in vitro and model organisms is also needed, both to understand the virus and to test therapeutic agents for safety and efficacy. We believe that this overview will help researchers select suitable cell and animal models for SARS-CoV-2 research (Table 1 and Figure 1) and help assess the advantages and disadvantages of each towards discovery of better models.
Acknowledgments
We thank Dr Peter Karagiannis (Kyoto University), Dr Yoichi Miyamoto (National Institutes of Biomedical Innovation, Health and Nutrition), Dr Sachiyo Yoshio (National Center for Global Health and Medicine), Dr Kohji Moriishi (University of Yamanashi), and Dr Toru Okamoto (Osaka University) for critical reading of the manuscript. We also thank Dr Eiri Ono (Kyoto University) for creating the figure.
Resources
ihttps://apps.who.int/iris/handle/10665/330893iihttps://talk.ictvonline.org/information/w/news/1300/pageiiiwww.who.int/emergencies/diseases/novel-coronavirus-2019ivwww.fishersci.se/shop/products/human-bronchial-epithelial-cells-nhbe/13499079References
- 1.Lu H. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J. Med. Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lythgoe M.P., Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol. Sci. 2020;41:363–382. doi: 10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J-M. Identification of coronavirus isolated from a patient in Korea with COVID-19. Osong Public Health Res. Perspect. 2020;11:3. doi: 10.24171/j.phrp.2020.11.1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ou X. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1–12. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harcourt J. Isolation and characterization of SARS-CoV-2 from the first US COVID-19 patient. bioRxiv. 2020 doi: 10.1101/2020.03.02.972935. Published online March 3, 2020. [DOI] [Google Scholar]
- 9.Desmyter J. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero) J. Virol. 1968;2:955–961. doi: 10.1128/jvi.2.10.955-961.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osada N. The genome landscape of the African green monkey kidney-derived Vero cell line. DNA Res. 2014;21:673–683. doi: 10.1093/dnares/dsu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou P. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuyama S. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. U. S. A. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranga A. Drug discovery through stem cell-based organoid models. Adv. Drug Deliv. Rev. 2014;69:19–28. doi: 10.1016/j.addr.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Li Z. Caution on kidney dysfunctions of COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.02.08.20021212. Published online March 27, 2020. [DOI] [Google Scholar]
- 15.Fan Z. Clinical features of COVID-19-related liver damage. Clin. Gastroenterol. Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Y-Y. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki T. Generation of human bronchial organoids for SARS-CoV-2 research. bioRxiv. 2020 doi: 10.1101/2020.05.25.115600. Published online May 26, 2020. [DOI] [Google Scholar]
- 18.Han Y. Identification of candidate COVID-19 therapeutics using hPSC-derived lung organoids. bioRxiv. 2020 doi: 10.1101/2020.05.05.079095. Published online May 5, 2020. [DOI] [Google Scholar]
- 19.Monteil V. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao B. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020 doi: 10.1007/s13238-020-00718-6. Published online April 17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Z. Effect of gastrointestinal symptoms on patients infected with COVID-19. Gastroenterology. 2020;158:2294–2297. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamers M.M. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020 doi: 10.1126/science.abc1669. Published online May 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 2020 doi: 10.1038/s41591-020-0912-6. Published online May 13, 2020. [DOI] [PubMed] [Google Scholar]
- 24.Varga Z. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bao L. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020 doi: 10.1038/s41586-020-2312-y. Published online May 7, 2020. [DOI] [PubMed] [Google Scholar]
- 26.Chan J.F-W. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa325. Published online March 26, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y-I. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27:704–709.e2. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi J. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science. 2020 doi: 10.1126/science.abb7015. Published online April 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockx B. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020 doi: 10.1126/science.abb7314. Published online April 17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandrashekar A. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020 doi: 10.1126/science.abc4776. Published online May 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Doremalen N. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. 2020 doi: 10.1101/2020.05.13.093195. Published online May 13, 2020. [DOI] [PubMed] [Google Scholar]
- 32.Yu J. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020 doi: 10.1126/science.abc6284. Published online May 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson B.N. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.04.15.043166. Published online April 22, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]