Highlights
-
•
Variant2 is the dominant IBV genotype in the Middle East and recently have been reported from Europe.
-
•
Current IB vaccines and using cross protection strategies doesn’t work well against Variant2.
-
•
It is the report about using autogenous Variante2 inactive vaccine for evaluation against the virus.
-
•
According to the results, the application of the autogenous vaccine can be very useful.
Keywords: Avian infectious bronchitis, Variants 2, Cross-protection, Vaccination
Abstract
The infectious bronchitis virus (IBV) is the cause of avian infectious bronchitis (IB). IB is one of the most highly contagious diseases, which results in many economic losses in the poultry industry worldwide. The nature of this virus is such that it generates new genotypes continuously. Proper vaccination is the most suitable way of combatting IB. One of the novel genotypes of IBV, which has been circulating in the Middle Eastern countries, is the variant 2 (IS-1494/GI-23) genotype. This study aims to design and produce an autogenous variant 2 vaccines. After isolation and characterization of the Iranian variant 2, the inactivated vaccine was formulated according to the OIE guidelines, and its different aspects (Purity, titration, inactivation, immunization) were evaluated. The designed vaccine passed all of OIE quality control standards. In the assessment process, the protection rate in the groups receiving the variant 2 and commercial vaccines was 67 % and 60 %, respectively. Although the differences were not significant, they indicated better protection, and the viral load in the feces and the kidney of the group receiving the variant 2 vaccine was lower than that in the commercial vaccine. It is suggested that the variant2 strain should be added as one of the local strains to the commercial inactivated vaccines in areas affected by this genotype. The use of this vaccine in layer and breeder flocks can help to protect them against variant 2 during the production phase. Also, the transfer of maternal antibodies to offspring can provide strain-specific immunity for one-day-old chickens.
1. Introduction
Avian infectious bronchitis (IB) is one of the important and economically-impactful diseases in the poultry industry that affects broiler chickens, pullets, layers, breeders. The cause of IB is a Gamma coronavirus that has a single-stranded RNA genome with a length of ∼27.6 kb [1]. The genome consists of 5-pol-S-E-M-N-3 components, the most important of which is the spike gene (S). This gene plays a major role in the production of neutralizing antibodies and determines the genotype of the virus based on the S gene sequencing. According to the high mutations and recombination events in the genome of the IBV, especially the S gene, new genotypes of the IBV are constantly emerging. One of these emerging genotypes is variant 2 (GI-23/IS-1494) which originally emerged in Israel, 1994, as a nephropathogenic genotype [2,3] and is currently reported in different countries in the Middle East including Iran, Afghanistan, Iraq, Oman, Turkey, Jordan, and Europe [[4], [5], [6], [7], [8]]. Cross-protection studies have revealed that once a Massachusetts type vaccine is used, the protection level is about 25 %. However, the combined usage of Mass type and 793/B vaccines in the vaccination program increases the protection rate by up to 80 % [9,10]. Different studies indicate that there is no proper protection against this genotype through routine IB vaccination programs. One of the best strategies in controlling IBV is the preparation of autogenous vaccines from the dominant circulating strain and its use in breeders, which will transfer maternal antibodies to offspring and protect them during the production phase. This study aims to develop and provide the autogenous variant 2 vaccine and to determine the immunogenicity of this vaccine.
2. Materials and methods
2.1. Strain selection, characterization, and virus titration
The vaccine strain was selected based on molecular epidemiology studies as well as molecular characterization from the Faculty of Veterinary Medicine, University of Tehran. The virus was isolated from broiler flocks in 2015 and was confirmed by the sequencing of spike gene and full genome characterization [7]. The tenth passage of this virus was selected. 50 % of embryo infectious doses (EID50) was calculated using the Spearman-Karber method [11].
2.2. Sterility and purity tests
The harvested allantois fluid was cultivated in aerobic and anaerobic bacterial and fungal growth media and was examined daily for a week in terms of any microbial growth. To determine the purity of the virus, a PCR test was conducted for Avian Influenza, Newcastle Disease, in addition to Mycoplasma spp., and Avian adenovirus [12].
2.3. Inactivation of the virus and vaccine formulation
The virus was inactivated with 37 % formaldehyde (concentration of 0.1 %) for 24 h at 37 °C. To confirm the inactivation of the virus, samples from each batch were randomly taken and injected into eggs and passaged at least three times. After complete inactivation, inactivated viruses were emulsified at a ratio of 30–70 (w/w) in an aqueous phase from the ISA-70 mineral emulsion (SEPPIC, Cosmetics/Pharmacy Division, France) according to manufacturer’s instructions. Each dose of the vaccine contained 0.5 mL (106.5 EID50) of vaccine emulsion, similar to the commercial vaccine [12].
2.4. Safety test
One dose of the vaccine was subcutaneously injected into each of the ten 21-days-old white specific pathogen-free (SPF) chickens. These chickens were examined for abnormal reactions for 21 days after injection [12].
2.5. Vaccine efficacy and Cross protection study
One hundred SPF chickens were divided into 5 groups (Table 1 ) of 20, including the H120+variant 2 vaccine group, the H120+ inactive commercial vaccine (M41) group, the H120 group, and the two control groups. In order to prime the immune system, all groups (except control groups) were vaccinated with live attenuated H120 vaccine on day one. Then they received an inactivated subcutaneous vaccine three weeks after priming. The control group received 0.5 mL of PBS. Three weeks after vaccination, the chickens were sampled for antibody titer (IDEXX) and challenged with variant 2 viruses (103 EID50) oculonasal. On the 5th day after the challenge, chickens of each group were euthanized. Their trachea was carefully removed and examined for the activity of the tracheal respiratory cilia. Moreover, the viral load in the kidney and the feces (15 samples/each group) was evaluated using Real-Time quantitative RT-PCR [13].
Table 1.
The evaluation of Autogenic Variant 2 Vaccine and Commercial Vaccine against Challenge with Variant 2 Infectious Bronchitis Avian.
| H120 Ocular- One day | Injectable Vaccine- 21 day | Challenge Ocular Variant 2 42 days |
|
|---|---|---|---|
| 1 (Variant 2 Inac Vaccine) | + | + | + |
| 2 (Commercial Inac Vaccine) | + | + | + |
| 3 (H120 Vaccine) | + | – | + |
| 4 (No Vaccine + Challenge) | – | – | + |
| 5 (No Vaccine) | – | – | – |
2.6. Ciliostasis test
Assessment of protection against challenge in each experiment was evaluated using ciliostasis test [14]. Five days post-challenge, all of the chickens in each group were euthanized. The tracheas were carefully removed and examined for ciliary's activity, as described previously [15]. Briefly, each of 10 tracheal rings (3 rings of upper, 4 rings of middle and 3 rings of the lower trachea) prepared from each trachea was examined by low-power microscopy and ciliary activity scored as follows: 0=all cilia beating; 1 = 75 % beating; 2 = 50 % beating; 3 = 25 % beating; and 4 = none beating (100 % ciliostasis). This gave a maximum possible ciliostasis score for a trachea of 40 in case of complete ciliostasis (total lack of protection).
2.7. Statistical analysis
In this experiment, GraphPad Prism software was used for statistical analysis (One Way ANOVA). The p-value lower than 0.05 was considered significant.
3. Results
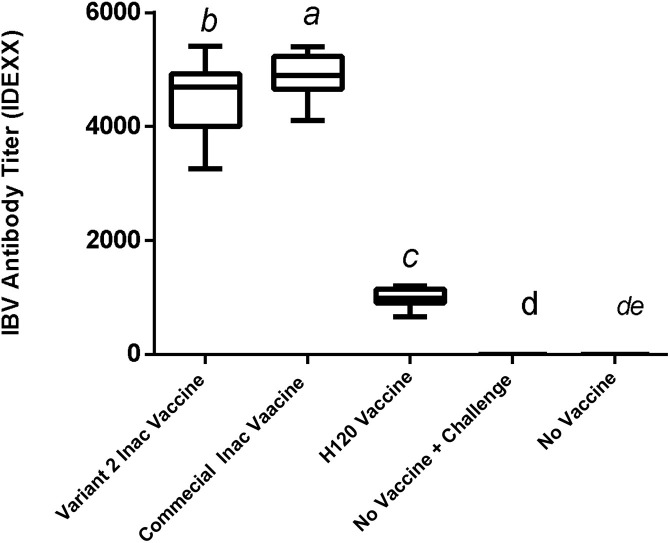
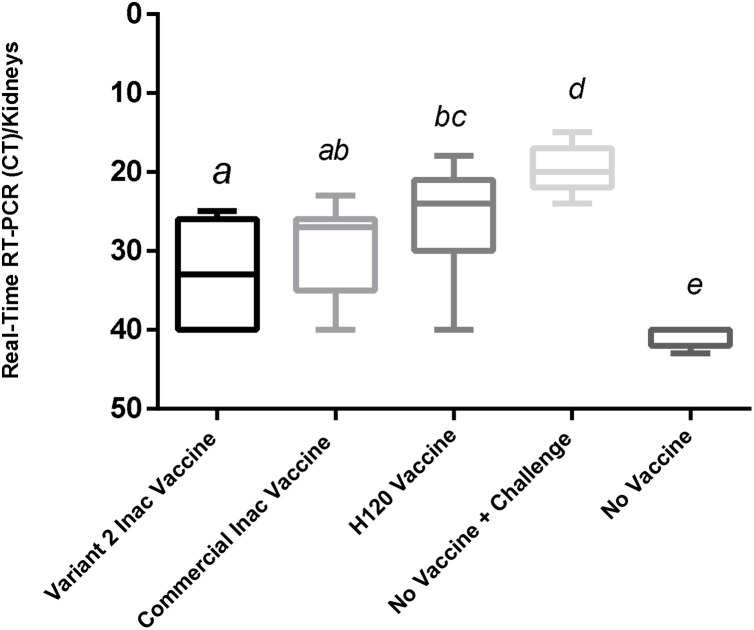
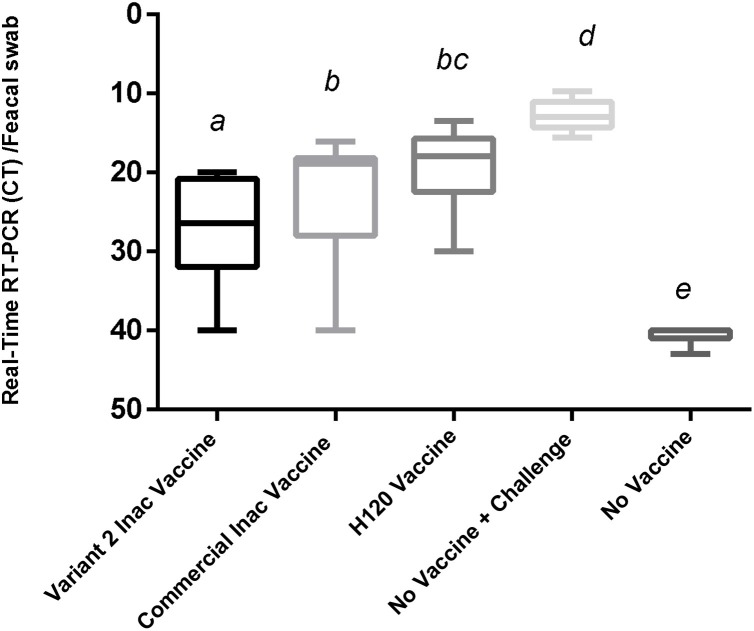
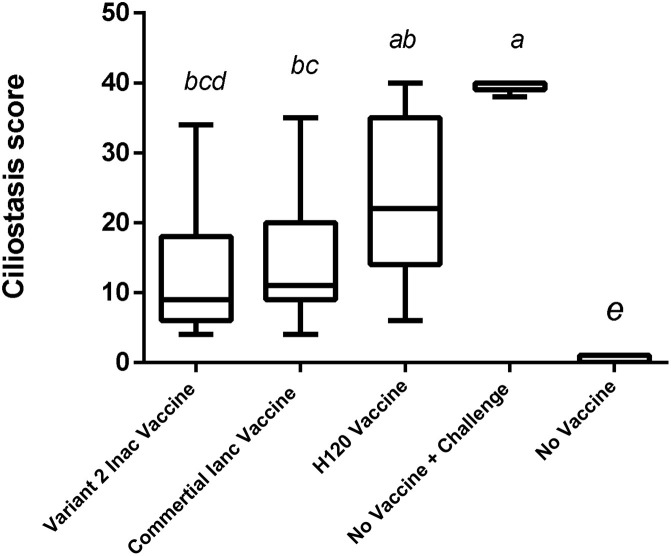
Inactivation of the virus was confirmed. Inoculated embryos showed no specific IBV symptoms such as fatality, dwarfism, stunting, and torsion after at least three passages. The PCR test results were negative for viruses (AI, ND, and Adenovirus) and Mycoplasma spp. In a stability test after a period, there was no physical change in vaccine suspension, and, with a slight shake, the vaccine regained its former emulsion status. Therefore, the vaccine will be stable for a week at 37 °C and for least 3 months at 4 °C without any physical changes. The results of the immunization test revealed that these vaccines were safe for chickens, and none of the inoculated chickens showed local or systemic abnormal reactions. The titer results from the ELISA test showed that the ELISA titer derived from the commercial vaccine (M41) was 4882 ± 91.64, and the autogenous vaccine was 4503 ± 160. This difference in antibody titers was significant (p < 0.05). The group that received only one H120 vaccine also had a titer of 996.08 ± 38.4 (Fig. 1 ).In the Real-Time RT-PCR test of the kidneys, the average CT in the varniat2 group was (33.1 ± 1.5), while in the commercial vaccine group, it was (30.13 ± 1.51), and in H120 vaccine group was (25.86 ± 1.72) (non-significant) (Fig. 2 ). Also, the average CT of the Real-Time RT-PCR test on fecal swabs is (28.2 ±1.6) for the group that received the variant2 inactive vaccine and (22.68± 1.82) for commercial vaccine group was (non-significant) (Fig. 3 ). These results indicate the proper functioning of the autogenous vaccine protection of the kidneys. Although the difference was non-significant (p > 0.05), the ciliostasis score in the autogenous vaccine group was (13 ± 2.2), which was less than that in the commercial vaccine (16.13 ± 2.64) (non-significant) (p > 0.05) (Fig. 4 ).
Fig. 1.
Infectious Bronchitis ELISA Titer (IDEXX) in Target Groups in Evaluation of Autogenic Variant 2 and Commercial Vaccines against Challenge with Variants 2 Infectious Bronchitis.
Fig. 2.
Viral load in Kidney Using Real-Time RT-PCR in Target Group for Evaluation of Autogenous Variant 2 and Commercial Vaccines against Challenge with Variants 2 of Avian Infectious Bronchitis.
Fig. 3.
Viral load in Feces Using Real-Time RT-PCR in the Target group for Evaluation of Autogenous Variant 2 and Commercial Vaccines against Challenge with Variants 2 of Avian Infectious Bronchitis.
Fig. 4.
Ciliostasis score for Evaluation of Autogenous Variant 2 and Commercial Vaccines against Challenge with Variants 2 of Avian Infectious Bronchitis.
4. Discussion
Infectious Bronchitis (IB) is a contagious viral infection that can infect chickens of all ages. The pathogenicity of this virus is related to chickens’ respiratory, reproductive, and urinary system that leads to weight loss, decrease in egg production, and deformation, ultimately leading to increased bird losses, especially in industrial production. As previously described, the virus tends to mutate easily, and its recombination property results in antigenic changes [16]. Vaccination is one of the main and established strategies for controlling IB. The fact that many of the IBV serotypes and genotypes are circulating the world makes it difficult to control the IBV [1]
The protection studies have revealed that the use of homologous strains vaccine can provide better protection against the IBV. Also, efforts to extend the protective effect of IB vaccines via the experimental compounds of live IB vaccines have provided a successful strategy for protecting chickens against acute heterologous strains of the IBV [13]. Different serotypes such as Massachusetts, 793/B, etc. have been used to control the pathogenicity of IBV in poultry farms.
Nevertheless, there have been reports of disease from across the country due to the failure to vaccinate. The reason for this is the impossibility of inducing cross-protection from the vaccine strains with the pathogenic strains that are constantly emerging. Therefore, determining all strains, especially the dominant strains in the country, is necessary for a proper vaccination program [13].
A study conducted by De Wit et al. revealed the greater similarity between amino acids in the S1 gene sequence of the vaccine strain, and the wild strain generally results in the lower chance of occurrence of genetic mutations. Besides, it can lead to stronger cross-protection immunity between the two strains of the virus. The difference for homology between vaccine strains and field virus is one of the most important reasons for the failure of the vaccination program. Variant 2 (IS-1494) was first reported in Israel in 1994. In Iran, Variant 2 of IBV was detected in 2010 for the first time and was reported as the most dominant type in the molecular study conducted between 2014 and 2017. In spite of the implementation of different biosecurity measurements, including combination use of Mass and 793/B type vaccines, variant 2 is still further circulating in Iranian chicken farms. This may indicate that current vaccines and vaccination strategies do not protect completely against variant 2. The inclusion of variant 2 in the vaccination program may help to induce strain-specific immunity and decrease the infection of variant 2. Different types of live and inactivated vaccines against the disease have been designed, produced, and used. Live vaccines, according to their nature, contribute to the strengthening of the mucosal and cellular immunity, and inactivate vaccines play a role in the development of humoral immunity. The inactivated vaccines for IB in layer and breeder flocks are used before they are primed with live vaccines. The purpose of the administration of an inactive vaccine is to protect the flock during the production stage as well as to increase the level of maternal antibodies in the one-day-old chicks. Different tests have been performed by researchers to evaluate cross-protection of vaccines on variant 2. Elhady et al. (2018) investigated a field live attenuated variant 2 vaccine. They concluded that the combination of the variant 2 and H120 vaccines could result in better protection against variant 2. Habibi et al. obtained a 69 % of protection against variant 2 using the Massachusetts vaccine in one-day-old chicks and 793/B when they were 14 days of age [9].
Awad et al. (2015) determined the ability of live attenuated H120 (a Massachusetts strain), and CR88 (a 793B strain) vaccine in protection against two Middle East IBV isolates, IS/885 and IS/1494, in broiler chicks. Results showed that administering combined live H120 and CR88 vaccines simultaneously in day-old chicks followed by CR88 vaccine at the age of 14 days gave more than 80 percent tracheal ciliary protection from both Middle East isolates. Also, this program conferred 100 percent protection from clinical signs and trachea and kidney lesions. The other vaccination program, H120 in day-old chicks followed by CR88 when they were 14 days old, the tracheal ciliary protection was 60 % and 80 % from IS-885-like and IS-1494-like Isolates, respectively [10]. Bru et al. found in their study that H120 + D274 (1-day-old) and live QX strain provided 70 % protection in 14-day-old chicks. This finding confirmed findings by Habibi et al. at the time when H120 and 1/96 were prescribed at days 1 and 14 of age [17]. DeWitt et al. have proven that prescribing two inactivated vaccines in weeks 16 and 17 of age in comparison to a single shot immunization (in the16th week) would protect flocks better during the production period (improving the quantity and quality of production as well) when used in chickens with different field viruses including QX/Q1 and variant 2 [18].
One of the problems in inactivated vaccines’ production is field trial evaluation due to the high costs of testing and the lack of appropriate facilities. The test is time-consuming and costly, depending on which production cycle the birds are in (the nature of the work is in the isolator). There are various types of strains of IB in inactivated vaccines worldwide. The most famous genotype used is M41. Some commercial vaccines have different variants in addition to the standard M41 strain, including the Dutch strains (D1466/D274). In an unpublished study conducted by the authors, it was shown that the addition of these Dutch strains could provide a high level of protection (non-significant).
According to the present study, if the strain of variant 2 is added to the vaccine as a strain of the variant with M41 standard strain, the flock will be better protected. The study results indicated that since the autogenous vaccine was produced to prevent respiratory disease in chickens, the vaccine produced from Variants 2 and M41 had better protection against variant 2 in comparison to H120. These results were confirmed by investigating the antibodies titers in chickens’ blood sera. Although the protection difference between M41 and variant 2 vaccine was not statistically significant, even a 7% increase in protection rate in the farms could be considered significant. According to the results, the application of the autogenous vaccine can be very useful, especially in combination with the M41 strain. With this combination, we can expect flock protection during the production phase (Layers and breeders) and, more importantly, the protection of chicks to whom the maternal antibodies will be transferred.
Ethical statement
The procedure has been conducted according to the University of Tehran’s instructor’s guide and ethical standards for treating animals.
Declaration of Competing Interest
No conflict of interest is declared.
Acknowledgments
The authors gratefully acknowledge the support of the Ghalyanchi lab experts, especially Mr. Behrooz Asadi, for his extensive technical support. The Research and Technology deputy of the Academic Center for Education, Culture, and Research (ACECR) in Iran supported this study.
References
- 1.De Wit J., Cook J.K., Van Der Heijden H.M. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40(3):223–235. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelb J., Jr., Weisman Y., Ladman B., Meir R. S1 gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996 to 2000) Avian Pathol. 2005;34(3):194–203. doi: 10.1080/03079450500096539. [DOI] [PubMed] [Google Scholar]
- 3.Valastro V., Holmes E.C., Britton P., Fusaro A., Jackwood M.W., Cattoli G., Monne I. S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification, Infection. Genetics and Evolution. 2016;39:349–364. doi: 10.1016/j.meegid.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seger W., GhalyanchiLangeroudi A., Karimi V., Madadgar O., Marandi M.V., Hashemzadeh M. Genotyping of infectious bronchitis viruses from broiler farms in Iraq during 2014-2015. Arch. Virol. 2016;161(5):1229–1237. doi: 10.1007/s00705-016-2790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamadan A.M., Ghalyanchilangeroudi A., Hashemzadeh M., Hosseini H., Karimi V., Yahyaraeyat R., Najafi H. Genotyping of Avian infectious bronchitis viruses in Iran (2015–2017) reveals domination of IS-1494 like virus. Virus Res. 2017;240:101–106. doi: 10.1016/j.virusres.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Shekaili T., Baylis M., Ganapathy K. Molecular detection of infectious bronchitis and avian metapneumoviruses in Oman backyard poultry. Res. Vet. Sci. 2015;99:46–52. doi: 10.1016/j.rvsc.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mousavi F.S., Ghalyanchilangeroudi A., Hosseini H., Fasaei B.N., Ghafouri S.A., Abdollahi H., Fallah-Mehrabadi M.H., Sadri N. Complete genome analysis of Iranian IS-1494 like avian infectious bronchitis virus. Virus Dis. 2018:1–5. doi: 10.1007/s13337-018-0462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lisowska A., Sajewicz-Krukowska J., Fusaro A., Pikula A., Domanska-Blicharz K. First characterization of a Middle-East GI-23 lineage (Var2-like) of infectious bronchitis virus in Europe. Virus Res. 2017;242:43–48. doi: 10.1016/j.virusres.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habibi M., Karimi V., Langeroudi A., Ghafouri S., Hashemzadeh M., Farahani R., Maghsoudloo H., Abdollahi H., Seifouri P. Combination of H120 and 1/96 avian infectious bronchitis virus vaccine strains protect chickens against challenge with IS/1494/06 (variant 2)-like infectious bronchitis virus. Acta Virol. 2017;61(2):150–160. doi: 10.4149/av_2017_02_04. [DOI] [PubMed] [Google Scholar]
- 10.Awad F., Forrester A., Baylis M., Lemiere S., Ganapathy K. Protection conferred by live infectious bronchitis vaccine viruses against variant Middle East IS/885/00-like and IS/1494/06-like isolates in commercial broiler chicks. Vet. Rec. Open. 2015;2(2) doi: 10.1136/vetreco-2014-000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epizooties O.Id. OIE; 2004. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals:(Mammals, Birds and Bees) [PubMed] [Google Scholar]
- 12.Manual O.T. 2018. Newcaslte Disease. [Google Scholar]
- 13.De Wit J., Cook J.K. Factors influencing the outcome of infectious bronchitis vaccination and challenge experiments. Avian Pathol. 2014;43(6):485–497. doi: 10.1080/03079457.2014.974504. [DOI] [PubMed] [Google Scholar]
- 14.Cook J.K., Darbyshire J., Peters R. The use of chicken tracheal organ cultures for the isolation and assay of avian infectious bronchitis virus. Arch. Virol. 1976;50(1–2):109–118. doi: 10.1007/BF01318005. [DOI] [PubMed] [Google Scholar]
- 15.Cavanagh D., Elus M.M., Cook J.K. Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection in vivo. Avian Pathol. 1997;26(1):63–74. doi: 10.1080/03079459708419194. [DOI] [PubMed] [Google Scholar]
- 16.Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38(2):281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- 17.Bru T., Vila R., Cabana M., Geerligs H. Protection of chickens vaccinated with combinations of commercial live infectious bronchitis vaccines containing Massachusetts, Dutch and QX-like serotypes against challenge with virulent infectious bronchitis viruses 793B and IS/1494/06 Israel variant 2. Avian Pathol. 2017;46(1):52–58. doi: 10.1080/03079457.2016.1203393. [DOI] [PubMed] [Google Scholar]
- 18.de Wit J., Malo A., Cook J.K. Induction of IBV strain-specific neutralizing antibodies and broad spectrum protection in layer pullets primed with IBV Massachusetts (mass) and 793B vaccines prior to injection of inactivated vaccine containing mass antigen. Avian Pathol. 2019:1–13. doi: 10.1080/03079457.2018.1556778. [DOI] [PubMed] [Google Scholar]