Abstract
Coronaviruses (CoVs) are a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) disease (COVID-19) has caused major public health crises. There have been more than 4,400,000 reported cases of COVID-2019 and more than 300,000 reported deaths to date (16/05/2020). SARS-CoV, MERS-CoV and SARS-CoV-2 have attracted widespread global attention due to their high infectivity and pathogenicity. To date, there is no specific treatment proven effective against these viral infectious diseases. Vaccination is considered one of the most effective strategies to prevent viral infections. Therefore, the development of effective vaccines against highly pathogenic coronaviruses is essential. In this review, we will briefly describe coronavirus vaccine design targets, summarize recent advances in the development of coronavirus vaccines, and highlight current adjuvants for improving the efficacy of coronavirus vaccines.
Key words: Coronaviruses, SARS-CoV, MERS-CoV, SARS-CoV-2, Vaccine, Adjuvant
Graphical abstract
Vaccination is the most effective and economical way to prevent viral infections. This review describes coronavirus vaccine design targets, summarizes recent advances and potential strategies for coronavirus vaccine development, and highlights promising technological routes and adjuvants for improving the effectiveness of coronavirus vaccines.
1. Introduction
In 2019, a novel strain of coronavirus was found in humans1. On February 11, 2020, World Health Organization (WHO) announced a new name for the epidemic disease: Corona Virus Disease (COVID-19). Meanwhile, the International Committee on Taxonomy of Viruses named the novel coronavirus as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). As of May 16th, 2020, the epidemic of COVID-19 has caused more than 4,400,000 laboratory-confirmed cases and more than 300,000 reported deaths2.
COVID-19 is the third known zoonotic coronavirus disease3 after Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). SARS is a zoonosis caused by SARS-CoV, which has infected 8096 humans, including 774 deaths (mortality rate 9.6%), in at least 29 countries4. Another highly pathogenic coronavirus, MERS-CoV, has been reported in 27 countries with reported viral infection and 858 associated deaths (mortality rate 34.4%) 5. Research indicates that SARS-CoV was transmitted from civet cats to humans and MERS-CoV was transmitted from dromedary camels to humans. However, the intermediate host of SARS-CoV-2 has not been identified6, 7, 8.
SARS-CoV-2, together with SARS-CoV and MERS-CoV, has posed significant threats to international health due to theirs high pathogenicity and infectivity. Vaccination is an important strategy to provide protection from infectious diseases. However, to date, no vaccine has been approved to prevent coronavirus infection, indicating the need for further development of novel and effective vaccines against coronavirus infection.
In this review, we will illustrate vaccine design targets, review current advances and potential strategies for vaccine development based on the spike (S) protein of SARS-CoV and MERS-CoV, and focus on how to improve the efficacy of vaccines through adjuvant formulations. Overall, these strategies may provide useful guidance for vaccine development of SARS-CoV-2.
2. Spike protein: a key target for coronaviruses vaccine
Coronaviruses are widespread in nature. It can cause respiratory and intestinal infections in animals and humans. According to the phylogenetic relationships, coronavirus can be divided into four genera: Alpha, Beta, Gamma and Delta. Alpha and beta genera can infect mammals, while gamma and delta genera are mostly avian coronaviruses9. There are seven known coronavirus that can infect humans: 229E, OC43, NL63, HKU1, SARS-CoV, MERS-CoV and SARS-CoV-2. The first four viruses cause only mild minor respiratory illness. The other three strains—SARS-CoV, MERS-CoV and SARS-CoV-2—are zoonotic and lead to severe respiratory syndrome10, 11, 12.
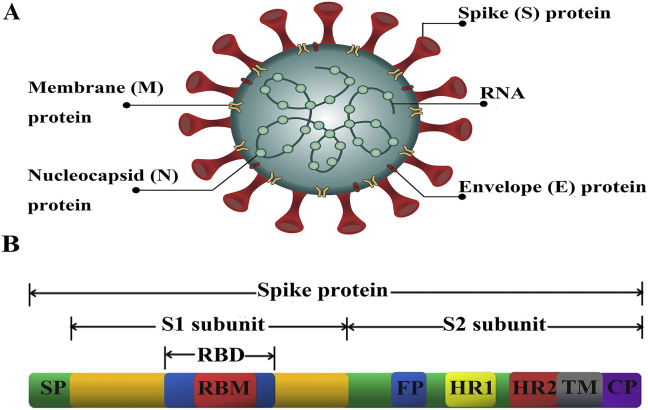
Coronaviruses are the largest single positive-strand RNA viruses with a genome of 27–32 kb13. It is named for its corona-like appearance (Fig. 1A). The virus genome mainly encodes four structural proteins: spike (S), nucleocapsid (N), membrane (M), envelope (E) proteins. S protein forms the spikes on the surface of coronaviruses and mediates adsorption and fusion of the virus and host cells. N protein forms a helical capsid, which locates inside the viral membrane to protect viral RNA. M and E proteins are important components of the viral envelope, and together mediate the assembly process of the virus14. Among the four structural proteins, S protein is the leading mediator of virus entry and is the main factor that determines the virulence and host range of the virus.
Figure 1.
Coronavirus and spike protein (S) structures. (A) Schematic structure of coronavirus and its key structural proteins, including spike (S), nucleocapsid (N), membrane (M), envelope (E) proteins. (B) Schematic structure of coronavirus S protein and its functional regions. S protein is composed of S1 and S2 subunits. SP, signal peptide. RBD, receptor-binding domain. RBM, receptor-binding motif. FP, fusion peptide. HR1 and HR2, heptad repeat one and two regions. TM, transmembrane. CP, cytoplasmic tail.
SARS-CoV, MERS-CoV, and SARS-CoV-2 have strong human-to-human characteristics, which is attributed to the interaction between S protein and host cell surface receptors. SARS-CoV and SARS-CoV-2 use the angiotensin-converting enzyme 2 (ACE2) as a receptor15,16, whereas MERS-CoV uses dipeptidyl peptidase 4 (DPP4; also known as CD26) as a receptor17. The distribution of receptors in humans and their affinity with S proteins determine the extent of tissue tropism and the intensity of transmission of coronavirus. The epidemiology and biological characteristics of SARS-CoV, MERS-CoV and SARS-CoV-2 are summarized in Table 12, 3, 4, 5, 6, 7, 8,12,15, 16, 17.
Table 1.
Epidemiology and biological characteristics of SARS-CoV, MERS-CoV and SARS-CoV-2, as of 16 May 2020.
| Characteristic | SARS-CoV | MERS-CoV | SARS-CoV-2 | |
|---|---|---|---|---|
| Clinical epidemiology | Total global number | 8096 | 2494 | 4,434,653 |
| Number of deaths | 774 | 858 | 302,169 | |
| Mortality | 9.6% | 34.4% | 6.8% | |
| Affected countries | 29 | 27 | 216 | |
| Transmission region | Globally | Regionally | Globally | |
| The predominant cell receptor | Human angiotensin-converting enzyme 2 (ACE2) | Human dipeptidyl peptidase 4 (DPP4 or CD26) | Human angiotensin-converting enzyme 2 (ACE2) | |
| Receptor binding affinity | High | High | Higher than SARS-CoV | |
| Pathogenic mechanism | Primarily infects ciliated bronchial epithelial cells and type II pneumocytes, resulting in massive viral replication and cell damage | Primarily infects unciliated bronchial epithelial cells and type II pneumocytes, resulting in massive viral replication and cell damage | Primarily infects ciliated bronchial epithelial cells and type II pneumocytes, resulting in massive viral replication and cell damage | |
S protein is a large type I transmembrane glycoprotein whose trimers constitute the spike structure on the surface of the virus. The S protein (Fig. 1B) can be divided into two functional subunits: an N-terminal S1 domain contains signal peptide and receptor binding domain (RBD), and a C-terminal S2 domain contains fusion peptide and two heptapeptide repeats (HR1 and HR2) to facilitate viral fusion. RBD mediates the binding of virus and cell receptor, which then triggers a conformational change of the S protein, exposing HR1 and HR2 to form a 6-helix bundle fusion core structure, further leading to membrane fusion and viral RNA release18, 19, 20, 21. Furthermore, S protein carries B-cell epitopes, which induces the body to produce neutralizing antibody and provides immune protection22. Because the S protein is involved in viral infection and is responsible for inducing host immune response and virus-neutralizing antibodies, it has been considered a key target for vaccine design.
Antigen-specific targets of S protein include full-length S protein, S1 subunits, RBD and S2 subunits. Viral vector vaccines encoding full-length S protein or S1 subunits have been demonstrated to induce high levels of neutralizing antibodies in various animal models23,24. However, some non-neutralizing epitopes on full-length S protein or S1 subunits may compete with neutralizing epitopes, leading to several safety concerns, including inflammatory and immunopathological effects such as pulmonary eosinophilic infiltration and antibody-dependent enhancement (ADE) following subsequent viral challenge of vaccinated animals22,25,26. ADE is a phenomenon in which non-neutralizing antibodies are produced following an infection or a vaccination leads to enhanced infection27. One approach to mitigate the adverse effects of ADE is to narrow the immune response to target only critical or beneficial epitopes25. Vaccines based on RBD elicited a robust protective immune response and neutralizing antibodies. At the same time, RBD does not contain non-neutralizing epitopes that may cause harmful immune responses, which is a hot spot for CoV vaccine development. It is worth mentioning that RBD has relatively low immunogenicity and often requires repeated doses and adjuvants28, 29, 30. Because the S2 subunit is highly conserved and not prone to mutation, S2 region has become an important target for the development of protective vaccines. However, reports regarding the presence of neutralizing epitopes in S2 and a protective role for antibodies to S2 have been inconsistent. Several studies demonstrated that S2 domain could induce specific cellular immune response and a high level of total IgG but little neutralizing antibodies against coronavirus infection31,32. On the contrary, there are also reports that showed that S2 domain contains neutralizing epitopes and could induce neutralizing antibodies33,34.
N protein serves multiple functions in viral replication, transcription, and assembly of the viral genome complex, which is more conservative than other proteins, such as S and M. Therefore, N protein has been also widely reported as a target antigen. N proteins have been shown to be highly immunogenic and capable of triggering T cell responses35. Remarkably, many studies indicated that the serum containing anti-N protein does not contain neutralizing antibodies against coronavirus infection36,37. In addition, vaccines based on N protein not only failed to protect from homologous or heterologous challenge, but resulted in enhanced immunopathology with eosinophilic infiltrates within the lungs of SARS-CoV-challenged mice38.
3. Advances in the development of SARS-CoV vaccines and MERS-CoV vaccines
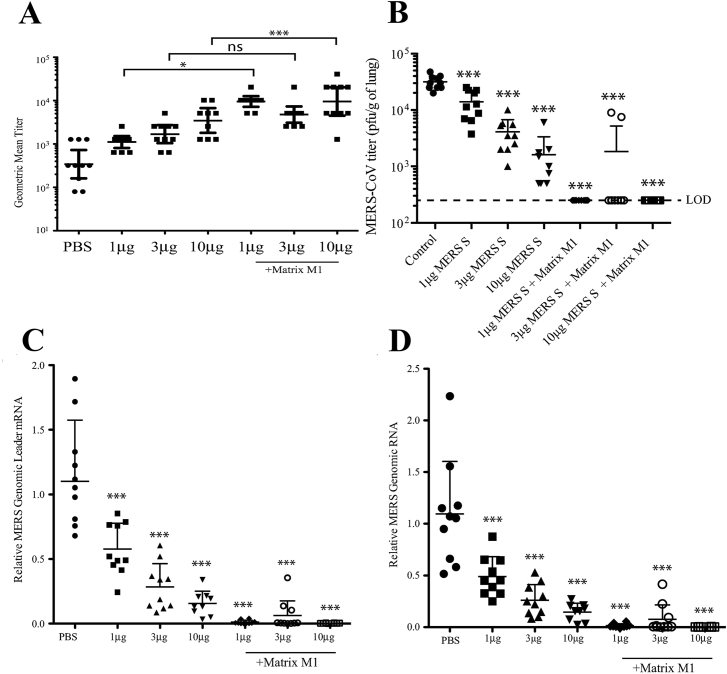
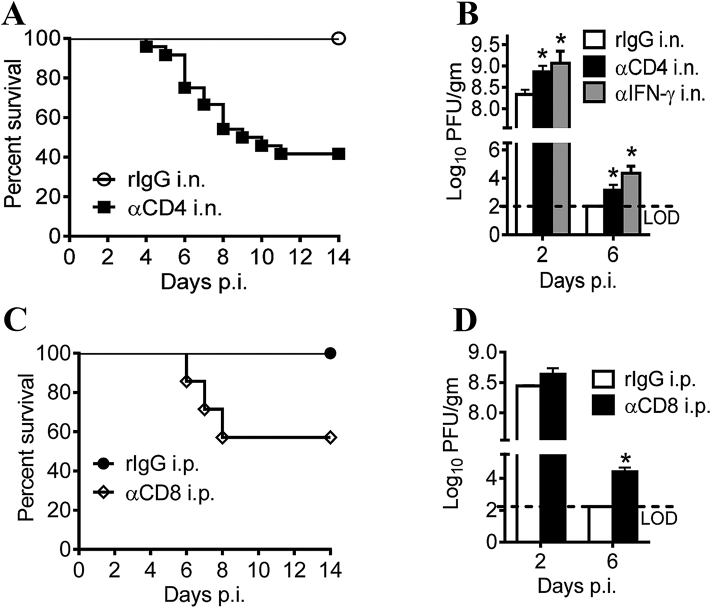
It is critical for CoV vaccines to induce robust humoral and cellular immunities. Previous studies indicated that the level of serum neutralizing antibody is correlated inversely with virus titers in the lungs, which effectively increased the survival rate of the vaccine host39, 40, 41. Production of high titer neutralizing antibodies can block MERS-CoV replication in the lungs of vaccinated mice (Fig. 2), confirming the importance of neutralizing antibody in fighting virus infection41. Similarly, the importance of T-cell responses against CoV infections was also highlighted in many studies42, 43, 44, 45. For example, depletion of CD4+ and CD8+ T cells in mice prior to challenge by SARS-CoV resulted in decreased survival rates to 35% and 45%, respectively (Fig. 3)44. Interestingly, virus-specific memory T cells but not neutralizing antibodies could be detected 6 years after infection in SARS survivors, suggesting memory T cell responses may provide broad and long-term protection against SARS-CoV infection45. In general, both neutralizing antibody levels and T cell responses should be considered in current CoV vaccination strategies.
Figure 2.
Vaccination of MERS S nanoparticle plus Matrix M1 protects mice from MERS-CoV challenge. (A) Neutralizing antibody levels against infections of live MERS-CoV. GMT ± standard deviation is graphed for each group of 10 mice. Dots represent individual mice. *P < 0.05, ***P < 0.001, ns means not significant. (B) Lung MERS-CoV replication was determined by plaque assay. (C) MERS-CoV specific Leader mRNA expression (D) MERS-CoV genomic RNA expression. Mean ± standard deviation are graphed for each group of 10 mice. Dots represent individual mice. LOD means limit of detection. ***P < 0.001. The figure was adapted with permission from Ref. 42. Copyright ©2017 Elsevier.
Figure 3.
Airway T cells are protective against SARS-CoV challenge. (A) Survival rate of SARS-CoV infected mice after depletion of airway CD4+ T cells. n = 5, rIgG i.n.; n = 24, aCD4 i.n. (B) Virus titers of SARS-CoV infected mice after depletion of airway CD4+ T cells. Titers are expressed as PFU/g tissue. n = 3 mice/group/time point. *P < 0.05. Data are representative of two independent experiments. (C) Survival rate of SARS-CoV infected mice after depletion of airway CD8+ T cells. n = 5, rIgG i.p.; n = 7, aCD8 i.p. (D) Virus titers of SARS-CoV infected mice after depletion of airway CD8+ T cells. n = 3 mice/group/time point. *P < 0.05. Data are representative of two independent experiments. The figure was adapted with permission from Ref. 45. Copyright © 2016 Elsevier.
No CoV vaccine is currently approved for use in humans. Most of the currently developed CoV vaccines are in the preclinical stage. In addition to the traditional inactivated and attenuated live vaccines, other development of candidate CoV vaccines was mainly focused on the S protein of coronaviruses. These types of S protein-based vaccines include nucleic acid-, viral vector-, virus-like particle (VLPs) and subunit vaccines. Because the research of SARS-CoV-2 vaccine is still in the early stages, the following review will discuss strategies for the development of SARS-CoV and MERS-CoV vaccines in the hope of providing useful guidance for the development of SARS-CoV-2 vaccines.
3.1. Inactivated and live-attenuated virus vaccines
SARS or MERS inactivated by physical (ultraviolet or radiation) or chemical (methanol, β-propiolactone and formalin) methods has been shown to cause high levels of neutralizing antibodies and protective immunity in several animal models including mice, rabbit and ferret46, 47, 48. However, eosinophil infiltration in the lungs of animals vaccinated with inactivated SARS-CoV vaccine or MERS-CoV vaccine has raised concerns about the safety and ultimate protective efficacy of inactivated vaccine49,50. In addition, a double-inactivated SARS-CoV (doubly inactivated by formalin and UV irradiation) vaccine provides poor protection against lethal disease in aged-animal models following heterologous challenge51. This result may be attributed to respiratory dendritic cells (rDCs) migration to draining lymph nodes (DLNs) progressively decreases as mice age, decreases in virus-specific CD8+ T cell responses in lungs and more severe disease in older mice infected with SARS-CoV52,53. Based on the above results, candidate vaccines against emerging coronaviruses should emphasize the efficacy in older animal with virus infection.
The live-attenuated virus vaccine, composed of recombinant SARS-CoV lacking the E gene (rSARS-CoV-E), produces significant neutralizing antibodies and virus-specific T cell responses54,55. More recently, a live attenuated SARS-CoV was generated through mutation of transcription regulatory networks (TRNs), where the attenuated virus effectively limits virulence reversal and protects mice against challenge56. Another study reported that rMERS-CoV-ΔE, a mutant of the MERS-CoV prepared using a new DNA cloning vector system, only replicates in a small number of cells, but can produce enough antigen to stimulate protective immunity in the host57. Although vaccine candidates based on the live-attenuated coronaviruses have the potential to induce a highly effective immune response and protection, it may present biosafety problems associated with virulence recovery54.
3.2. Nucleic acid-based vaccines
Nucleic acid vaccines, including DNA and RNA vaccines, are based on plasmids or messenger RNA that encode vaccine antigens, and they are introduced into the host to produce immunological response to protect organisms against diseases58. The efficacy of DNA-based vaccines against SARS-CoV and MERS-CoV infections has been widely evaluated. In a phase I clinical trial, a SARS DNA vaccine produces cellular immune responses and neutralizing antibody in healthy adults59. Additionally, a DNA vaccine encoding the MERS-CoV S protein induces strong CD8+ and CD4+ T cell immunity and antigen-specific neutralizing antibodies in mice, camels and nonhuman primates (NHPs), and protects vaccinated rhesus macaques from infection by MERS-CoV60,61. GLS-5300, a DNA vaccine expressing MERS-CoV S-protein antigen, is the first MERS-CoV vaccine to advance into human trials. The vaccine induced durable immune responses, as most participants maintained detectable S1 binding antibodies and had cellular immune responses at almost 1 year after the last vaccination62. Nevertheless, CoV DNA vaccines based on full-length S protein may cause a Th2-related harmful immune response, leading to liver damage in vaccinated animals. One study comparing the immunogenicity of MERS-CoV DNA vaccines expressing S or S1 in mice showed that plasmids expressing the S1 (pS1) subunit triggered a balanced Th1/Th2 response, thereby avoiding the risk of immunopathological risk associated with Th2 response63. Moreover, immunization of mice with pS1 vaccine induced significantly higher levels of IFN-γ compared to pS vaccine63.
Messenger RNA (mRNA) vaccines carry transcripts encoding antigens, and use the host cell translational machinery to produce the antigens, which then stimulates an immune response64. Because of high yields of in vitro transcription reactions, mRNA has the potential for rapid, inexpensive and scalable manufacturing, which greatly shortens the development time and can respond quickly to epidemics. Compared to DNA vaccines, mRNA vaccines do not need to pass an additional membrane barrier (nuclear membrane), so it does not have safety concerns about integration into the host genome65. Due to the above advantages, mRNA vaccines are becoming a powerful tool against coronavirus infection.
However, their application has been restricted by the instability and inefficient in vivo delivery of nucleic acid (DNA or mRNA)66,67. To provide protection from degradation and facilitate their entry into targeted cells, efficient delivery systems for nucleic acid vaccines, particularly the nanocarriers, have been explored extensively.
3.3. Viral-vector vaccines
Viral vectors have a molecular mechanism that assists the target gene to enter cells and infect them, which is an important vector platform for CoV candidate vaccines. Viral vector-based vaccines encoding S protein of MERS-CoV and SARS-CoV have been widely studied. To date, adenovirus (Ad), modified vaccinia ankara (MVA), attenuated parainfluenza virus (BHPIV3) and rabies virus (RV) have been used as vaccine vector68, 69, 70, 71, 72. A previous report has indicated SARS-CoV S-specific neutralizing antibodies and mucosal responses are elicited in African green monkeys immunized with BHPIV3/SARS-S vector vaccines, protecting African green monkeys against SARS-CoV infection68. Another study reported that a single inoculation with the RV-based vaccine expressing SARS-CoV S protein can induce a strong SARS-CoV-neutralizing antibody response69. In addition, MERS-CoV S-specific neutralizing antibodies and antigen specific T cell response, are induced in mice after immunizing them with human adenovirus or MVA-based MERS-CoV S-expressing vaccines70,71. Furthermore, compared with MERS-CoV S-encoding Ad5 vaccines, MERS-CoV S1-encoding Ad5 vaccines might induce higher levels of neutralizing antibodies72. In a recent study, rAd5 constructs expressing CD40-targeted S1 fusion protein (rAd5-S1/F/CD40L) exhibited full protection against lethal MERS-CoV challenge, and prevented severe perivascular hemorrhage within the lungs as compared to non CD40-targeted vaccine (rAd5-S1)74. Currently, MERS-CoV S protein expressed by chimpanzee adenovirus (ChAdOx1) or modified vaccinia Ankara (MVA) vectors are at phase I clinical trial74,75. Indeed, viral vectors expressing S protein can induce viral neutralizing antibodies in vivo, providing an effective platform for the development of SARS-CoV-2 vaccine. However, some viral-vectors, such as certain serotypes of adenoviruses, may fail to induce effective immune responses owing to the high prevalence of virus-neutralizing antibodies in the human population resulting in elimination of viral vectors76. Thus, caution should be taken when developing CoV vaccines using viral vectors.
3.4. Virus-like particle (VLP) vaccines
Virus-like particles (VLPs) are multiprotein structures that mimic the organization and conformation of native viruses but devoid of infectious genetic materials. VLP is a potential candidate for the development of safe and effective CoV vaccines, which can efficiently stimulate innate and adaptive immune response functions. Bacterial, insect, yeast and mammalian cells expression systems have been widely used in the production of VLPs. One study indicated a chimeric VLPs that coexpression of SARS-CoV S protein and E, M and N proteins of mouse hepatitis virus resulted in the efficient production of neutralizing antibodies, thus inhibiting SARS-CoV replication in the lung77. The other chimeric VLPs, expressing SARS-CoV S protein and influenza M1 protein, can induce neutralizing antibodies and protect mice against deadly challenges78. Similar as SARS-CoV VLP vaccines that induce high titers of neutralizing antibodies against CoV infection, MERS-CoV VLP vaccines also elicit antigen specific cellular immune response against infections of MERS-CoV79. The VLP vaccine is a potential tool to provide protection against novel pandemic pathogens. However, VLPs as a preventive vaccine still have many problems to be considered. For example, viral mutations might allow the virus to evade antibody-mediated neutralization.
3.5. Subunit vaccines
Subunit vaccines are composed of highly purified antigens which require only a part of the pathogen to generate a protective immune response. Subunit vaccines are characterized by high security, controllable performance and easy production on a large scale, thereby gradually becoming the focus of more and more researchers. Compared to the full-length S protein, RBD contains several critical neutralizing epitopes and lacks non-neutralizing epitopes that may cause harmful pathological responses. Therefore, RBD-based subunit vaccines not only can induce effective neutralizing antibodies, but also avoid adverse immune responses. From the safety and effectiveness perspectives, the RBD-based CoV vaccines are more attractive candidates in the development of CoV vaccines.
Since the SARS and MERS outbreaks, subunit vaccines based on SARS-CoV and MERS-CoV RBD have been extensively studied and tested, showing sufficient effectiveness and strong protection against CoV infection in various animal models80, 81, 82, 83, 84. For example, RBD-Fc (RBD fused with human IgG1 Fc) elicits long-term humoral immune response, and produces neutralizing antibodies that protects the vaccinated mice from the SARS-CoV challenge without causing immunopathological damage80. Also, a newly designed RBD without the Fc tag induces robust humoral and T cell responses, particularly neutralizing antibodies in immunized mice, protecting mice against SARS-CoV infection81. It has been demonstrated that S377-588-Fc, which is a fusion protein of RBD fragment S377-588 (spike residues 377–588) and human IgG1 Fc, induces the higher-titer IgG antibodies and neutralizing antibodies among all RBD fragments in mice82. A study has also shown that i.n. vaccination of MERS-CoV RBD-Fc induces humoral IgG antibody response comparable to those induced by s.c. vaccination, including neutralizing antibodies, but more robust systemic cellular immune responses and higher local mucosal immune responses in mouse lungs83. In the rhesus macaque, a recombinant receptor-binding domain (rRBD) protein vaccine can also induce sustained and robust immunological responses84. These studies suggest that RBD-based CoV vaccines have potential for preventing respiratory infections caused by CoV, further enhancing beneficial strategies for emerging coronavirus infection. However, it is worth noting that highly purified proteins are generally low immunogenicity and often require the addition of vaccine adjuvants.
To summarize, an effective vaccine against coronavirus infection often needs to induce the body to produce strong humoral immune response and cellular immune response. The current advancements and vaccine strategies in the development of in the development of SARS-CoV vaccines and MERS-CoV vaccines are listed in Table 2 46, 47, 48,54, 55, 56, 57,59, 60, 61, 62,68, 69, 70, 71, 72, 73,77, 78, 79, 80, 81,84. Apart from inactivated and live-attenuated virus vaccines, nucleic acid-, viral vector- and VLPs-based vaccines, particularly subunit vaccines containing the RBD of CoV S protein, are critically important.
Table 2.
Vaccine strategies of SARS-CoV and MERS-CoV.
| Vaccine strategy | Process of production | Result and reference |
|
|---|---|---|---|
| SARS-CoV | MERS-CoV | ||
| Inactivated virus vaccines | Virus particles are inactivated by physical or chemical methods |
|
Induces S-specific antibody responses and neutralizing antibodies in mice (>1:103); neutralizes pseudotyped MERS-CoV48. |
| Live-attenuated virus vaccines | Genomes are mutated by mutagenesis or targeted deletions |
|
rMERS-CoV-E generated by reverse genetics system is a replication-competent, propagation-defective virus57. |
| Nucleic acid-based vaccines | Genetically engineered DNA/mRNA encode antigenic compounds | Induces S-specific antibody responses and neutralizing antibodies in 80% subjects, neutralizes pseudotyped SARS-CoV; elicits T-cell responses in all subjects59. |
|
| Viral-vector vaccines | Inserting foreign gene units into the viral genome by homologous recombination |
|
|
| Virus-like particle (VLPs) vaccines | Genes clone viral structural proteins into expression system |
|
Induced RBD-specific antibody responses and neutralizing antibodies in mice (1: 320), neutralizes pseudotyped MERS-CoV; elicit T-cell responses79. |
| Subunit vaccines | Antigenic components including immunogenic pathogen fragment without nucleic acid |
|
Induced RBD-specific antibody responses and neutralizing antibodies in rhesus monkey (1:1600), neutralizes pseudotyped MERS-CoV; elicits T-cell responses and reduces virus titers84. |
4. Adjuvant systems for improving the immunogenicity of coronavirus subunit vaccines
Highly purified proteins in subunit vaccines are usually not inherently immunogenic, as they generally do not directly stimulate the innate immune system. However, the development of effective CoV vaccines requires the activation of powerful humoral and cellular immunity to induce protective immunity and virus clearance in the body. Therefore, adjuvants are needed to be incorporated in subunit vaccines to enhance the immunogenicity of these weaker antigens and evoke the required antigen-specific immune response phenotype, thus improving the overall potency of poorly immunogenic subunit vaccines. The following review will discuss adjuvants commonly used in subunit vaccines against coronavirus infection.
4.1. Aluminum-based adjuvants
Aluminum (Alum) adjuvant is the longest and most frequently used adjuvant in licensed vaccines, with an extensive safety record. Alum is a Th2-type adjuvant that induces strong humoral immune response, including the production of neutralizing antibodies85. Therefore, Alum is incorporated into a range of vaccines against viral infection where neutralizing antibodies to viral antigens are required for protection, including human papillomavirus, rabies and hepatitis B86.
Aluminum adjuvant has been widely used in the development of CoV vaccine due to a variety of advantages noted above. Several studies have indicated that RBD-based subunit vaccines in the presence of alum induce powerful serum-specific and neutralizing antibodies, providing a degree of protection against viral challenges84,87. It is noteworthy to mention that eliciting powerful cellular and humoral immunity is critical for a potential CoV vaccine. Virus-specific T cells can secrete IFN-γ and promote virus clearance. Meanwhile, effector T cells can further differentiate into memory T cells, which is expected to respond quickly and effectively to subsequent CoV infection88,89. Although alum successfully induces antibody-mediated protective immunity, its ability to induce cellular immune responses is limited. One approach to overcome the limitations of alum is to use it in combination with other adjuvants to enhance cellular immune responses.
4.2. Emulsions
Another approach that has an extensive history of use as CoV vaccine adjuvants are emulsions. Freund's adjuvant is a water-in-oil emulsion, divided into complete Freund's adjuvant (CFA) and incomplete Freund's adjuvant (IFA). As a powerful agonist for Th1 cells, CFA can induce Th1 cytokines and enhance cellular and humoral immune responses. While IFA generally induce Th2 cytokines90, 91, 92. Mice immunized with SARS-CoV rRBD antigen together with Freund's adjuvant induce not just high titer of neutralizing antibodies, but relatively high levels of CTL and Th responses93. Freund's adjuvant induces a more balanced Th1 and Th2 immune response, providing more comprehensive protection against coronavirus infection. Freund's adjuvant is not approved for use in human vaccines due to its toxicity94. Despite this, Montanide ISA-51, also known as incomplete Freund's adjuvant (IFA), has been approved for human use in 201295,96. Similar as classic Freund's water emulsion, the addition of Montanide ISA-51 in RBD-based vaccines can produce strong antigen-specific neutralizing antibodies response against CoV infection82,97.
The toxicity of Freund's adjuvant mainly comes from the non-degradable oil in the ingredients. To avoid the toxicity problem of Freund's adjuvant, oil-in-water emulsions prepared from biocompatible oils such as squalene (e.g., SAS and MF59) were developed98, 99, 100. The Sigma adjuvant system (SAS) is a stable oil-in-water emulsion containing monophosphoryl lipid A, which has been used in animal experiments. One study indicated that the SARS-CoV rRBD protein with SAS protect mouse against lethal SARS-CoV challenge, where the protection correlated well with the high titer of neutralizing antibodies101. In addition, MERS-CoV S1 protein together with SAS was identified to elicit robust serum neutralizing activity against several MERS-CoV strains in immunized mice102.
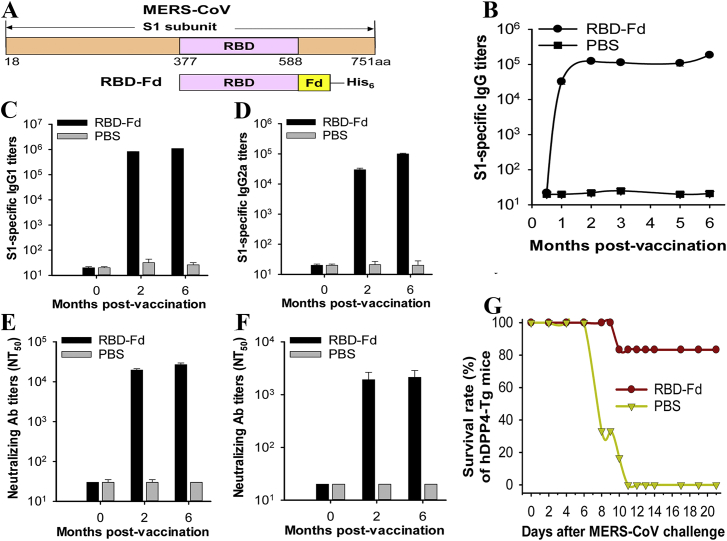
MF59, another squalene-based oil-in-water emulsion, has been licensed in Europe for adjuvanted influenza vaccines. It is revealed that MERS-CoV RBD in trimeric form with MF59 elicits highly efficacious Th2-based IgG1 and Th1-based IgG2 antibody responses, as well as neutralizing antibodies against pseudotyped and live MERS-CoV, protecting 83% of hDPP4-Tg mice from lethal MERS-CoV infection (Fig. 4)103. Similarly, the activation of IgG subtype antibody response and production of neutralizing antibodies following immunization with RBD-Fc and MF59, resulting in the fully protection against MERS-CoV infection104. Additionally, MF59 induces stronger and broader IgG subtype antibody response than several other commercial adjuvants, including Freund's adjuvant, aluminum, Monophosphoryl lipid A and Montanide ISA5128. Based on the security and effectiveness of MF59, it becomes a promising candidate adjuvant for the development of coronavirus subunit vaccine.
Figure 4.
MERS-CoV RBD in trimeric form with MF59 protects human dipeptidyl peptidase 4 (hDPP4) transgenic mice from MERS-CoV infection. (A) Schematic structure of MERS-CoV S1 subunit and construction of RBD-Fd. A His6 tag was added at the C-terminus of RBD-Fd (B) MERS-CoV S1-specific IgG antibody titers. (C) and (D) MERS-CoV S1-specific IgG1 and IgG2a antibody titers. (E) and (F) neutralizing antibody levels against infections of pseudotyped and live MERS-CoV of EMC2012 strain. (G) Survival rate of MERS-CoV infected mice after vaccination. Fd: foldon. The figure was adapted with permission from Ref. 104. Copyright © 2017 Elsevier.
4.3. Toll-like receptors (TLRs) agonist
The innate immune system recognizes pathogen-associated molecular patterns (PAMPS) mainly through pattern recognition receptors (PRRs). As one of the best characterized PRRs, Toll-like receptors (TLRs), which are widely distributed in antigen presenting cells and play an important role in triggering innate immunity and priming the adaptive immune response105. Therefore, several TLR agonists have been investigating as virus-specific vaccine adjuvants to induce strong and sustained immune responses106,107.
The Toll-like receptor (TLR) families have been characterized as key players in RNA virus detection and antiviral immunity. Polyinosinic acid‒polycytidylic acid (PolyI:C) is a synthetic double-stranded RNA (dsRNA) analogue that acts as a TLR3 receptor agonist, inducing the production of type I IFN and inflammatory cytokines through a TRIF-dependent pathway108. Previous reports have indicated mice deficient in the TLR3 signaling way are extremely susceptible to SARS-CoV infection, showing increased lung pathology and higher viral titers109. Additionally, intranasal treatment with PolyI:C induces both innate and T cell immune responses against viral infections, protecting aged animals from infection by IAV or SARS-CoV, as well as providing more rapid virus clearance110. Furthermore, MERS-CoV S protein together with poly I:C effectively trigger CD8 T-cells response to accelerate MERS-CoV clearance without immunopathological effects111. These results suggest that PolyI:C, a potent type I IFN inducer, should be evaluated as a promising adjuvant in CoV subunit vaccines.
TLR4 mainly recognizes lipopolysaccharide (LPS) derived from the cell wall of gram-negative bacteria. Unlike other TLRs, TLR4 agonists mediate the production of inflammatory cytokines and type I IFN via MyD88 as well as TRIF signaling pathway112. It has been demonstrated that pretreatment with TLR4 ligands provides protective immunity against infections by SARS-CoV110. Monophosphoryl lipid A (MPLA)—a TLR4 agonist—is an attenuated version of lipopolysaccharide (LPS). MPLA has proven its safety and effectiveness in licensed vaccines, including human papillomavirus and hepatitis B vaccines113,114. Previous work has shown that inclusion of MPLA as an adjuvant in influenza vaccine promotes mucosal and systemic (Th1-skewed) immune responses after pulmonary vaccination115. In addition, SARS-CoV S protein with adjuvant TLR3 and TLR4 agonists successfully induce high expression of antigen-specific IgG and neutralizing antibodies without eosinophilic infiltrations and elicit Th1/17 cytokine responses in the lungs after the SARS-CoV challenge infection in the mouse model116. The above results indicated that TLR4 agonist, as a powerful Th1-type adjuvant, can effectively improve the immunogenicity of the CoV subunit vaccines and reduce the Th2-related eosinophilic pathological response.
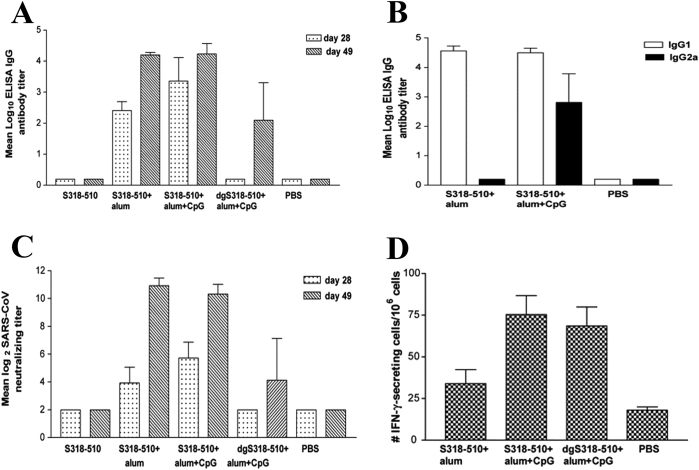
TLR9 recognizes unmethylated cytosine-phosphate-guanine (CpG) motifs that are commonly found in bacterial and viral DNA, and then mediates the antiviral response of type I IFN via the TLR9-MyD88 pathway117. CpG-containing oligodeoxynucleotides (CpG ODN) sequence 1018, as an adjuvant for immunization against hepatitis B virus (HBV), has been proven to significantly increase neutralizing antibody titers in clinical trials118. Recently it has been approved for adults in the United States119. A novel CpG ODN (BW001) has been shown to effectively activate B and NK cells and stimulate the body to secrete high level of IFN-a and IFN-γ, thereby producing strong anti-SARS-CoV activity120. Furthermore, CpG have been identified to be superior to other TLR agonists (TLR3 agonists: PolyI:C; TLR7/8 agonists: R848) in the secretion of inflammatory cytokines and activation of SARS-CoV S peptide specific CD8+ T cell response121. Due to the strong ability of CpG to induce cellular immunity, it is often used in combination with alum to supplement the defects of alum in inducing cellular immunity. For instance, mice immunized with SARS-CoV S protein amino acids 318–510 (S318–510) together with alum and CpG ODN show higher humoral and cellular immune responses than those immunized with S318–510 antigen and alum alone (Fig. 5)29,122. The above findings suggest that CpG can be used as a potent adjuvant for coronavirus vaccines.
Figure 5.
Mice immunized with SARS-CoV spike protein amino acids 318–510 (S318–510) with alum plus CpG elicited strong antibody and cellular immune responses. (A) SARS-CoV-specific IgG antibody titers. (B) SARS-CoV-specific IgG1 and IgG2a antibody titers. (C) Neutralizing antibody levels against infections of SARS-CoV of Tor-2 strain. The figure was adapted with permission from Ref. 124. Copyright © 2007 Elsevier.
4.4. Stimulator of interferon genes (STING) agonists
STING (stimulator of interferon genes), a central component in the innate immune response, plays an important role in defense against viral and intracellular bacterial infections. STING is a transmembrane protein localized to the endoplasmic reticulum. Following stimulation by cytosolic cyclic dinucleotides (CDNs), STING undergoes a conformational change, resulting in a downstream signaling cascade involving the activation of NF-κB signaling pathway and the production of type I interferon123,125. STING agonists are potent adjuvants capable of eliciting robust humoral and CD8+ T cell immune responses in mice by simulating the early phase of viral infection without concomitant excess inflammation126. Meanwhile, recombinant MERS-CoV RBD antigens with cyclic diguanylate monophosphate (cdGMP), a canonical STING agonist, have shown to effectively elicit neutralization antibody and antigen-specific T cell responses126.
To summarize, the application of vaccine adjuvant requires a thorough understanding of the effect of adjuvants on immune response and mechanisms of action. The application of adjuvants in subunit vaccines are listed in Table 3. In addition to safety considerations, the design of adjuvants must also pay attention to the ability to selectively induce and regulate the types of immune responses in the body, so as to effectively promote the humoral and cellular immunity to combat coronavirus infection. It is also noteworthy to mention that an existing well-established adjuvant could be combined with new immunostimulants (e.g., TLRs agonist) to improve the breadth and intensity of the immune responses, which has become a potential strategy for exploring efficient adjuvant systems.
Table 3.
Application of adjuvants in subunit vaccines.
| Adjuvant | Composition | Mechanism | Antibody responses and neutralizing antibody | Cellular immune response |
|---|---|---|---|---|
| Alum | Aluminum hydroxide/Aluminum phosphate |
|
No-report | |
| Emulsions | ||||
| Freund's adjuvant | IFA: an water-in-oil emulsion formed by mixing mineral oil with an emulsifier CFA: killed bacteria M. tuberculosis added to IFA |
Induces SARS-CoV RBD-specific antibody responses and neutralizing antibodies (>1:104/1:102–103) in mice; neutralizes pseudotyped and live SARS-CoV93. | Elicits SARS-CoV RBD-specific T cell responses in mice93. | |
| Montanide ISA51 | IFA | No-report | ||
| Sigma adjuvant system (SAS) | Oil-in-water emulsion containing monophosphoryl lipid A | No-report | ||
| MF59 | Squalene-based oil-in-water emulsion |
|
No significant increase in T-cell response28. | |
| Toll-like receptors (TLRs) agonists | ||||
| TLR3 agonist | Double-stranded RNA (dsRNA) analogue | No-report | ||
| TLR4 agonist | LPS/MPLA | Induces SARS-CoV S-specific antibody and virus-specific antibody (>1:104)116. | Induces the production of Th1 cytokines, elicits T cell responses116. | |
| TLR9 agonist | CpG DNA | No-report | ||
| Stimulator of interferon genes (STING) agonists | ||||
| STING agonist | cdGMP | Induces MERS-CoV RBD-specific IgG subtype antibody (IgG1 and IgG2a) and neutralizing antibodies (1:40–320) in mice; neutralizes live MERS-CoV126. | Induces the production of IFN-γ and memory T cells response126. | |
5. Conclusions and perspectives
SARS-CoV-2 has spread rapidly since its outbreak and has now posed a risk to countries worldwide, making it urgent to develop a safe and effective vaccine against the infection. This review introduces the structure and functions of coronavirus S protein and summarizes the advancements and potential strategies of SARS-CoV and MERS-CoV vaccines based on CoV S protein. The CoV S protein-based vaccines are further classified into different types, including viral vector, nucleic acid (DNA and RNA), VLP and protein-based vaccines. Subunit vaccines have become the focus of current research due to their numerous advantages, but it often requires appropriate adjuvants to enhance their immunogenicity. In the USA, aluminum, MF59, and CpG are adjuvants included in licensed vaccines. These three adjuvants have shown induction of serum neutralizing antibodies and protection against infection in mice challenged with an infectious virus, which might be used for CoV subunit vaccine administration28,84,87,103,120,121. However, alum alone cannot induce a potent Th1 response unless combined with another adjuvant, such as CpG. This adjuvant combination will improve the effectiveness of the CoV subunit vaccines122. Similarly, in order to effectively activate the complex and orderly natural immune system and produce the expected acquired immune response, it is sometimes necessary to use an adjuvant combination29. Here, we believe that adjuvant formulations that induce a balanced Th1 and Th2 immune response can more effectively improve the immunogenicity of the antigen72,83,103, becoming a new trend in the development of coronavirus adjuvants. Ideally, an effective CoV vaccine is required to induce both robust humoral and cell-mediated immunities. Even though many promising vaccine candidates have been reported, there are still no commercial vaccines available against SARS-CoV and MERS-CoV. The comprehensive lessons and experiences brought by the outbreak of SARS and MERS provide valuable insights and progress on how to respond to COVID-19.
With the spread of COVID-19, scientific institutions and pharmaceutical companies around the world are racing against time to develop SARS-CoV-2 vaccine. According to WHO, there are at least 100 research projects on SARS-CoV-2 vaccines in the world127. In addition to traditional inactivated virus and live-attenuated virus vaccines, the developments of most SARS-CoV-2 vaccines are also based on some new technological routes, such as mRNA vaccines, subunit vaccines, viral vector vaccines, and DNA vaccines. Up to now, eight candidate vaccines have entered clinical trials, including three inactivated vaccines from Wuhan Institute of Biological Products128, Beijing Institute of Biological Products129 and Sinovac130, two adenovirus vector vaccines (Ad5-nCoV from CanSino Biologicals131 and COV001 from Inovio132), two mRNA vaccines (mRNA-1273 from Moderna133 and BNT162 from BioNTech134) and one DNA vaccine (INO-4800 from Inovio135). Although some progress has been made in the development of SARS-CoV-2 vaccines, it is important to realize that vaccine development is a rigorous scientific exploration process. Due to many challenges of verifying the effectiveness of vaccines, it is still very likely that no SARS-CoV-2 vaccine will be available in the market for human in near future. Therefore, SARS-CoV-2 vaccine development still requires the unremitting efforts of researchers in the world.
Acknowledgments
We acknowledge the financial support of the National Natural Science Foundation of China (No. 81925036), Special Funds for Prevention and Control of COVID-19 of Sichuan University (No. 0082604151018, China) and Zhejiang University special scientific research fund for COVID-19 prevention and control (No. 2020XG2X018, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Author contributions
Chunting He and Ming Qin collected and reorganized the literature material. Chunting He wrote the manuscript. Xun Sun revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
There are no conflicts of interest to declare.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019;2020:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2020. Coronavirus disease (COVID-2019) situation reports.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [cited 2020 May 16th]. Available from: [Google Scholar]
- 3.Ahmad T., Khan M., Haroon Musa TH., Nasir S., Hui J. COVID-19: zoonotic aspects. Trav Med Infect Dis. 2020:101607. doi: 10.1016/j.tmaid.2020.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Cumulative number of reported probable cases of severe acute respiratory syndrome (SARS) [cited 2020 March 30]. Available from: http://www.who.int/csr/sars/country/en/.
- 5.World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) [cited 2020 March 30]. Available from: https://www.who.int/emergencies/mers-cov/en/
- 6.Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 7.Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., Burbelo P.D. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio. 2014;5:e00884–14. doi: 10.1128/mBio.00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corman V.M., Muth D., Niemeyer D., Drosten C. Hosts and sources of endemic human coronaviruses. Adv Virus Res. 2018;100:163–188. doi: 10.1016/bs.aivir.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corman V.M., Lienau J., Witzenrath M. Coronaviren als Ursache respiratorischer Infektionen [Coronaviruses as the cause of respiratory infections] Internist (Berl) 2019;60:1136–1145. doi: 10.1007/s00108-019-00671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92:548–551. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beniac D.R., deVarennes S.L., Andonov A., He R., Booth T.F. Conformational reorganization of the SARS coronavirus spike following receptor binding: implications for membrane fusion. PLoS One. 2007;2 doi: 10.1371/journal.pone.0001082. e1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q., Wong G., Lu G., Yan J., Gao G.F. MERS-CoV spike protein: targets for vaccines and therapeutics. Antivir Res. 2016;133:165–177. doi: 10.1016/j.antiviral.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q., Zhang L., Kuwahara K., Li L., Liu Z., Li T. Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect Dis. 2016;2:361–376. doi: 10.1021/acsinfecdis.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haagmans B.L., van den Brand J.M., Raj V.S., Volz A., Wohlsein P., Smits S.L. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2016;351:77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- 24.Alharbi N.K., Padron-Regalado E., Thompson C.P., Kupke A., Wells D., Sloan M.A. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine. 2017;35:3780–3788. doi: 10.1016/j.vaccine.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okba N.M., Raj V.S., Haagmans B.L. Middle East respiratory syndrome coronavirus vaccines: current status and novel approaches. Curr Opin Virol. 2017;23:49–58. doi: 10.1016/j.coviro.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol. 2004;78:12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S.F., Tseng S.P., Yen C.H., Yang J.Y., Tsao C.H., Shen C.W. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451:208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang N., Channappanavar R., Ma C., Wang L., Tang J., Garron T. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell Mol Immunol. 2016;13:180–190. doi: 10.1038/cmi.2015.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan J., Deng Y., Chen H., Lu G., Wang W., Guo X. Tailoring subunit vaccine immunity with adjuvant combinations and delivery routes using the Middle East respiratory coronavirus (MERS-CoV) receptor-binding domain as an antigen. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyon M.P., Du L., Tseng C.K., Seid C.A., Pollet J., Naceanceno K.S. Engineering a stable CHO cell line for the expression of a MERS-coronavirus vaccine antigen. Vaccine. 2018;36:1853–1862. doi: 10.1016/j.vaccine.2018.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y., Sun S., Wang K., Zhang S., Zhu W., Chen Z. Elicitation of immunity in mice after immunization with the S2 subunit of the severe acute respiratory syndrome coronavirus. DNA Cell Biol. 2005;24:510–515. doi: 10.1089/dna.2005.24.510. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Ulitzky L., Silberstein E., Taylor D.R., Viscidi R. Immunogenicity and protection efficacy of monomeric and trimeric recombinant SARS coronavirus spike protein subunit vaccine candidates. Viral Immunol. 2013;26:126–132. doi: 10.1089/vim.2012.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keng C.T., Zhang A., Shen S., Lip K.M., Fielding B.C., Tan T.H. Amino acids 1055 to 1192 in the S2 region of severe acute respiratory syndrome coronavirus S protein induce neutralizing antibodies: implications for the development of vaccines and antiviral agents. J Virol. 2005;79:3289–3296. doi: 10.1128/JVI.79.6.3289-3296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H., Wang G., Li J., Nie Y., Shi X., Lian G. Identification of an antigenic determinant on the S2 domain of the severe acute respiratory syndrome coronavirus spike glycoprotein capable of inducing neutralizing antibodies. J Virol. 2004;78:6938–6945. doi: 10.1128/JVI.78.13.6938-6945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng H., Yang L.T., Wang L.Y., Li J., Huang J., Lu Z.Q. Long-lived memory T lymphocyte responses against SARS coronavirus nucleocapsid protein in SARS-recovered patients. Virology. 2006;351:466–475. doi: 10.1016/j.virol.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yong C.Y., Ong H.K., Yeap S.K., Ho K.L., Tan W.S. Recent advances in the vaccine development against Middle East respiratory syndrome-coronavirus. Front Microbiol. 2019;10:1781. doi: 10.3389/fmicb.2019.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchholz U.J., Bukreyev A., Yang L. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci U S A. 2004;101:9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasui F., Kai C., Kitabatake M., Inoue S., Yoneda M., Yokochi S. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol. 2008;181:6337–6348. doi: 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- 39.Ho M.S., Chen W.J., Chen H.Y., Lin S.F., Wang M.C., Di J. Neutralizing antibody response and SARS severity. Emerg Infect Dis. 2005;11:1730–1737. doi: 10.3201/eid1111.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry J.D., Hay K., Rini J.M., Yu M., Wang L., Plummer F.A. Neutralizing epitopes of the SARS-CoV S-protein cluster independent of repertoire, antigen structure or mAb technology. mAbs. 2010;2:53–66. doi: 10.4161/mabs.2.1.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coleman C.M., Venkataraman T., Liu Y.V., Glenn G.M., Smith G.E., Flyer D.C. MERS-CoV spike nanoparticles protect mice from MERS-CoV infection. Vaccine. 2017;35:1586–1589. doi: 10.1016/j.vaccine.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao J., Zhao J., Van Rooijen N., Perlman S. Evasion by stealth: inefficient immune activation underlies poor T cell response and severe disease in SARS-CoV-infected mice. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao J., Alshukairi A.N., Baharoon S.A., Ahmed W.A., Bokhari A.A., Nehdi A.M. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aan5393. eaan5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao J., Zhao J., Mangalam A.K., Channappanavar R., Fett C., Meyerholz D.K. Airway memory CD4+ T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang F., Quan Y., Xin Z.T., Wrammert J., Ma M.J., Lv H. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186:7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- 46.He Y., Zhou Y., Siddiqui P., Jiang S. Inactivated SARS-CoV vaccine elicits high titers of spike protein-specific antibodies that block receptor binding and virus entry. Biochem Biophys Res Commun. 2004;325:445–452. doi: 10.1016/j.bbrc.2004.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.See R.H., Petric M., Lawrence D.J., Mok C.P.Y., Rowe T., Zitzow L.A. Severe acute respiratory syndrome vaccine efficacy in ferrets: whole killed virus and adenovirus-vectored vaccines. J Gen Virol. 2008;89:2136–2146. doi: 10.1099/vir.0.2008/001891-0. [DOI] [PubMed] [Google Scholar]
- 48.Deng Y., Lan J., Bao L., Huang B., Ye F., Chen Y. Enhanced protection in mice induced by immunization with inactivated whole viruses compare to spike protein of middle east respiratory syndrome coronavirus. Emerg Microb Infect. 2018;7:60. doi: 10.1038/s41426-018-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tseng C.T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agrawal A.S., Tao X., Algaissi A., Garron T., Narayanan K., Peng B.H. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccines Immunother. 2016;12:2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spruth M., Kistner O., Savidis-Dacho H., Hitter E., Crowe B., Gerencer M. A double-inactivated whole virus candidate SARS coronavirus vaccine stimulates neutralising and protective antibody responses. Vaccine. 2006;24:652–661. doi: 10.1016/j.vaccine.2005.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao J., Zhao J., Legge K., Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frieman M., Yount B., Agnihothram S., Page C., Donaldson E., Roberts A. Molecular determinants of severe acute respiratory syndrome coronavirus pathogenesis and virulence in young and aged mouse models of human disease. J Virol. 2012;86:884–897. doi: 10.1128/JVI.05957-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fett C., DeDiego M.L., Regla-Nava J.A., Enjuanes L., Perlman S. Complete protection against severe acute respiratory syndrome coronavirus-mediated lethal respiratory disease in aged mice by immunization with a mouse-adapted virus lacking E protein. J Virol. 2013;87:6551–6559. doi: 10.1128/JVI.00087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Netland J., DeDiego M.L., Zhao J., Fett C., Álvarez E., Nieto-Torres J.L. Immunization with an attenuated severe acute respiratory syndrome coronavirus deleted in E protein protects against lethal respiratory disease. Virology. 2010;399:120–128. doi: 10.1016/j.virol.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham R.L., Deming D.J., Deming M.E., Yount B.L., Baric R.S. Evaluation of a recombination-resistant coronavirus as a broadly applicable, rapidly implementable vaccine platform. Commun Biol. 2018;1:179. doi: 10.1038/s42003-018-0175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Almazán F., DeDiego M.L., Sola I., Zuñiga S., Nieto-Torres J.L., Marquez-Jurado S. Engineering a replication-competent, propagation-defective Middle East respiratory syndrome coronavirus as a vaccine candidate. mBio. 2013;4:e00650–13. doi: 10.1128/mBio.00650-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rauch S., Jasny E., Schmidt K.E., Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin J.E., Louder M.K., Holman L.A., Gordon I.J., Enama M.E., Larkin B.D. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26:6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muthumani K., Falzarano D., Reuschel E.L., Tingey C., Flingai S., Villarreal D.O. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med. 2015;7:301ra132. doi: 10.1126/scitranslmed.aac7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cockrell A.S., Baric R.S. An effective DNA vaccine platform for Middle East respiratory syndrome coronavirus. Ann Transl Med. 2016;4:499. doi: 10.21037/atm.2016.11.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Modjarrad K., Roberts C.C., Mills K.T., Castellano A.R., Paolino K., Muthumani K. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019;19:1013–1022. doi: 10.1016/S1473-3099(19)30266-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Amri S.S., Abbas A.T., Siddiq L.A., Alghamdi A., Sanki M.A., Al-Muhanna M.K. Immunogenicity of candidate MERS-CoV DNA vaccines based on the spike protein. Sci Rep. 2017;7:44875. doi: 10.1038/srep44875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan L., Sun X. Recent advances in mRNA vaccine delivery. Nano Res. 2018;11:5338–5354. [Google Scholar]
- 65.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geall A.J., Mandl C.W., Ulmer J.B. RNA: the new revolution in nucleic acid vaccines. Semin Immunol. 2013;25:152–159. doi: 10.1016/j.smim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 67.Hobernik D., Bros M. DNA vaccines-how far from clinical use?. Int J Mol Sci. 2018;19:3605. doi: 10.3390/ijms19113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bukreyev A., Lamirande E.W., Buchholz U.J., Vogel L.N., Elkins W.R., St Claire M. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363:2122–2127. doi: 10.1016/S0140-6736(04)16501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faber M., Lamirande E.W., Roberts A., Rice A.B., Koprowski H., Dietzschold B. A single immunization with a rhabdovirus-based vector expressing severe acute respiratory syndrome coronavirus (SARS-CoV) S protein results in the production of high levels of SARS-CoV-neutralizing antibodies. J Gen Virol. 2005;86:1435–1440. doi: 10.1099/vir.0.80844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Volz A., Kupke A., Song F., Jany S., Fux R., Shams-Eldin H. Protective efficacy of recombinant modified vaccinia virus Ankara delivering Middle East Respiratory Syndrome coronavirus spike glycoprotein. J Virol. 2015;89:8651–8656. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo X., Deng Y., Chen H., Lan J., Wang W., Zou X. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology. 2015;145:476–484. doi: 10.1111/imm.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim E., Okada K., Kenniston T., Raj V.S., AlHajri M.M., Farag E.A. Immunogenicity of an adenoviral-based Middle East respiratory syndrome coronavirus vaccine in BALB/c mice. Vaccine. 2014;32:5975–5982. doi: 10.1016/j.vaccine.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hashem A.M., Algaissi A., Agrawal A.S., Al-Amri S.S., Alhabbab R.Y., Sohrab S.S. A highly immunogenic, protective, and safe Adenovirus-based vaccine expressing Middle East respiratory syndrome coronavirus S1-CD40L fusion protein in a transgenic human dipeptidyl peptidase 4 mouse model. J Infect Dis. 2019;220:1558–1567. doi: 10.1093/infdis/jiz137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.National Institutes of Health [NIH]. Safety and immunogenicity of a candidate MERS-CoV vaccine (MERS001). Available from: https://clinicaltrials.gov/ct2/show/study/NCT03399578.
- 75.National Institutes of Health [NIH]. Safety, tolerability and immunogenicity of vaccine candidate MVA-MERS-S. Available from: https://clinicaltrials.gov/ct2/show/NCT03615911#outcomemeasures.
- 76.Lasaro M.O., Ertl H.C. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lokugamage K.G., Yoshikawa-Iwata N., Ito N., Watts D.M., Wyde P.R., Wang N. Chimeric coronavirus-like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protect mice against challenge with SCoV. Vaccine. 2008;26:797–808. doi: 10.1016/j.vaccine.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y.V., Massare M.J., Barnard D.L., Kort T., Nathan M., Wang L. Chimeric severe acute respiratory syndrome coronavirus (SARS-CoV) S glycoprotein and influenza matrix 1 efficiently form virus-like particles (VLPs) that protect mice against challenge with SARS-CoV. Vaccine. 2011;29:6606–6613. doi: 10.1016/j.vaccine.2011.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang C., Zheng X., Gai W., Wong G., Wang H., Jin H. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antivir Res. 2017;140:55–61. doi: 10.1016/j.antiviral.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Du L., Zhao G., He Y., Guo Y., Zheng B.J., Jiang S. Receptor-binding domain of SARS-CoV spike protein induces long-term protective immunity in an animal model. Vaccine. 2007;25:2832–2838. doi: 10.1016/j.vaccine.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Du L., Zhao G., Chan C.C., Li L., He Y., Zhou Y. A 219-mer CHO-expressing receptor-binding domain of SARS-CoV S protein induces potent immune responses and protective immunity. Viral Immunol. 2010;23:211–219. doi: 10.1089/vim.2009.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma C., Wang L., Tao X., Zhang N., Yang Y., Tseng C.K. Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments—the importance of immunofocusing in subunit vaccine design. Vaccine. 2014;32:6170–6176. doi: 10.1016/j.vaccine.2014.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma C., Li Y., Wang L., Zhao G., Tao X., Tseng C.T. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: implication for designing novel mucosal MERS vaccines. Vaccine. 2014;32:2100–2108. doi: 10.1016/j.vaccine.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lan J., Yao Y., Deng Y., Chen H., Lu G., Wang W. Recombinant receptor binding domain protein induces partial protective immunity in Rhesus macaques against Middle East respiratory syndrome coronavirus challenge. EBioMedicine. 2015;2:1438–1446. doi: 10.1016/j.ebiom.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He P., Zou Y., Hu Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccines Immunother. 2015;11:477–488. doi: 10.1080/21645515.2014.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baylor N.W., Egan W., Richman P. Aluminum salts in vaccines—US perspective. Vaccine. 2002;20:S18–S23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 87.Kim Y.S., Son A., Kim J., Kwon S.B., Kim M.H., Kim P. Chaperna-mediated assembly of ferritin-based Middle East respiratory syndrome-coronavirus nanoparticles. Front Immunol. 2018;9:1093. doi: 10.3389/fimmu.2018.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mubarak A., Alturaiki W., Hemida M.G. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J Immunol Res. 2019;2019:6491738. doi: 10.1155/2019/6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takamura S. Persistence in temporary lung niches: a survival strategy of lung resident memory CD8+ T cells. Viral Immunol. 2017;30:438–450. doi: 10.1089/vim.2017.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jensen F.C., Savary J.R., Diveley J.P., Chang J.C. Adjuvant activity of incomplete Freund's adjuvant. Adv Drug Deliv Rev. 1998;32:173–186. doi: 10.1016/s0169-409x(98)00009-x. [DOI] [PubMed] [Google Scholar]
- 91.Aucouturier J., Dupuis L., Ganne V. Adjuvants designed for veterinary and human vaccines. Vaccine. 2001;19:2666–2672. doi: 10.1016/s0264-410x(00)00498-9. [DOI] [PubMed] [Google Scholar]
- 92.Stills H.F., Jr. Adjuvants and antibody production: dispelling the myths associated with Freund's complete and other adjuvants. ILAR J. 2005;46:280–293. doi: 10.1093/ilar.46.3.280. [DOI] [PubMed] [Google Scholar]
- 93.Du L., Zhao G., Li L., He Y., Zhou Y., Zheng B.J. Antigenicity and immunogenicity of SARS-CoV S protein receptor-binding domain stably expressed in CHO cells. Biochem Biophys Res Commun. 2009;384:486–490. doi: 10.1016/j.bbrc.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stuewart-Tull D.E., Shimono T., Kotani S., Knights B.A. Immunosuppressive effect in mycobacterial adjuvant emulsions of mineral oils containing low molecular weight hydrocarbons. Int Arch Allergy Appl Immunol. 1976;52:118–128. doi: 10.1159/000231673. [DOI] [PubMed] [Google Scholar]
- 95.Aucouturier J., Dupuis L., Deville S., Ascarateil S., Ganne V., Montanide I.S.A. 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1:111–118. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 96.Sesardic D., Dobbelaer R. European Union regulatory developments for new vaccine adjuvants and delivery systems. Vaccine. 2004;22:2452–2456. doi: 10.1016/j.vaccine.2003.11.071. [DOI] [PubMed] [Google Scholar]
- 97.Du L., Zhao G., Kou Z., Ma C., Sun S., Poon V.K. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J Virol. 2013;87:9939–9942. doi: 10.1128/JVI.01048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sastry M., Zhang B., Chen M., Joyce M.G., Kong W.P., Chuang G.Y. Adjuvants and the vaccine response to the DS-Cav1-stabilized fusion glycoprotein of respiratory syncytial virus. PloS One. 2017;12 doi: 10.1371/journal.pone.0186854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gavin A.L., Hoebe K., Duong B., Ota T., Martin C. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O'Hagan D.T., Ott G.S., De Gregorio E., Seubert A. The mechanism of action of MF59—an innately attractive adjuvant formulation. Vaccine. 2012;30:4341–4348. doi: 10.1016/j.vaccine.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 101.Du L., Zhao G., Chan C.C., Sun S., Chen M., Liu Z. Recombinant receptor-binding domain of SARS-CoV spike protein expressed in mammalian, insect and E. coli cells elicits potent neutralizing antibody and protective immunity. Virology. 2009;393:144–150. doi: 10.1016/j.virol.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang L., Shi W., Joyce M.G., Modjarrad K., Zhang Y., Leung K. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun. 2015;6:7712. doi: 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tai W., Zhao G., Sun S., Guo Y., Wang Y., Tao X. A recombinant receptor-binding domain of MERS-CoV in trimeric form protects human dipeptidyl peptidase 4 (hDPP4) transgenic mice from MERS-CoV infection. Virology. 2016;499:375–382. doi: 10.1016/j.virol.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Y., Tai W., Yang J., Zhao G., Sun S., Tseng C.K. Receptor-binding domain of MERS-CoV with optimal immunogen dosage and immunization interval protects human transgenic mice from MERS-CoV infection. Hum Vaccines Immunother. 2017;13:1615–1624. doi: 10.1080/21645515.2017.1296994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kawai T., Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 106.Lester S.N., Li K. Toll-like receptors in antiviral innate immunity. J Mol Biol. 2014;426:1246–1264. doi: 10.1016/j.jmb.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baum A., García-Sastre A. Induction of type I interferon by RNA viruses: cellular receptors and their substrates. Amino Acids. 2010;38:1283–1299. doi: 10.1007/s00726-009-0374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oshiumi H., Matsumoto M., Funami K., Akazawa T., Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 109.Totura A.L., Whitmore A., Agnihothram S., Schäfer A., Katze M.G., Heise M.T. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio. 2015;6 doi: 10.1128/mBio.00638-15. e00638–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao J., Wohlford-Lenane C., Zhao J., Fleming E., Lane T.E., McCray P.B., Jr. Intranasal treatment with poly(I·C) protects aged mice from lethal respiratory virus infections. J Virol. 2012;86:11416–11424. doi: 10.1128/JVI.01410-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao J., Li K., Wohlford-Lenane C., Agnihothram S.S., Fett C., Zhao J., Gale M.J., Jr. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tanimura N., Saitoh S., Matsumoto F., Akashi-Takamura S., Miyake K. Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling. Biochem Biophys Res Commun. 2008;368:94–99. doi: 10.1016/j.bbrc.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 113.Didierlaurent A.M., Morel S., Lockman L., Giannini S.L., Bisteau M., Carlsen H. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 114.Garçon N., Chomez P., Van Mechelen M. GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007;6:723–739. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 115.Patil H.P., Murugappan S., ter Veer W., Meijerhof T., de Haan A., Frijlink H.W. Evaluation of monophosphoryl lipid A as adjuvant for pulmonary delivered influenza vaccine. J Contr Release. 2014;174:51–62. doi: 10.1016/j.jconrel.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 116.Sekimukai H., Iwata-Yoshikawa N., Fukushi S., Tani H., Kataoka M., Suzuki T. Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol Immunol. 2020;64:33–51. doi: 10.1111/1348-0421.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Latz E., Verma A., Visintin A., Gong M., Sirois C.M., Klein D.C. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol. 2007;8:772–779. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- 118.Eng N.F., Bhardwaj N., Mulligan R., Diaz-Mitoma F. The potential of 1018 ISS adjuvant in hepatitis B vaccines: HEPLISAV™ review. Hum Vaccines Immunother. 2013;9:1661–1672. doi: 10.4161/hv.24715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Splawn L.M., Bailey C.A., Medina J.P., Cho J.C. Heplisav-B vaccination for the prevention of hepatitis B virus infection in adults in the United States. Drugs Today (Barc) 2018;54:399–405. doi: 10.1358/dot.2018.54.7.2833984. [DOI] [PubMed] [Google Scholar]
- 120.Bao M., Zhang Y., Wan M., Dai L., Hu X., Wu X. Anti-SARS-CoV immunity induced by a novel CpG oligodeoxynucleotide. Clin Immunol. 2006;118:180–187. doi: 10.1016/j.clim.2005.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao K., Wang H., Wu C. The immune responses of HLA-A∗0201 restricted SARS-CoV S peptide-specific CD8⁺ T cells are augmented in varying degrees by CpG ODN, PolyI: C and R848. Vaccine. 2011;29:6670–6678. doi: 10.1016/j.vaccine.2011.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zakhartchouk A.N., Sharon C., Satkunarajah M., Auperin T., Viswanathan S., Mutwiri G. Immunogenicity of a receptor-binding domain of SARS coronavirus spike protein in mice: implications for a subunit vaccine. Vaccine. 2007;25:136–143. doi: 10.1016/j.vaccine.2006.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ahn J., Barber G.N. STING signaling and host defense against microbial infection. Exp Mol Med. 2019;51:1–10. doi: 10.1038/s12276-019-0333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abe T., Marutani Y., Shoji I. Cytosolic DNA-sensing immune response and viral infection. Microbiol Immunol. 2019;63:51–64. doi: 10.1111/1348-0421.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Ji, Li Peiyu, Yu Yang, Fu Yuhong, Jiang Hongye, Lu Min. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science. 2020;367:869. doi: 10.1126/science.aau0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin L.C., Huang C.Y., Yao B.Y., Lin J.C., Agrawal A., Algaissi A. Viromimetic STING agonist-loaded hollow polymeric nanoparticles for safe and effective vaccination against Middle East Respiratory Syndrome Coronavirus. Adv Funct Mater. 2019;29:1807616. doi: 10.1002/adfm.201807616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.World Health Organization. Draft landscape of COVID-19 candidate vaccines [cited 2020 May 16th]. Available from: https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines.
- 128.Chinese Clinical Trial Registry. A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated Novel Coronavirus Pneumonia vaccine (Vero cells) [cited 2020May 16th]. Available from: http://www.chictr.org.cn/showprojen.aspx?proj=52227.
- 129.Chinese Clinical Trial Registry. Evaluation of the safety and immunogenicity of inactivated novel coronavirus (2019-CoV) vaccine (Vero cells) in healthy population aged 3 years and above: a randomized, double-blind, placebo parallel-controlled phase I/II clinical trial [cited 2020 May 16th]. Available from: http://www.chictr.org.cn/showproj.aspx?proj=53003.
- 130.National Institutes of Health. Safety and immunogenicity study of inactivated vaccine for prophylaxis of SARS CoV-2 infection (COVID-19) [cited 2020 May 16th]. Available from: https://clinicaltrials.gov/ct2/show/NCT04352608.
- 131.National Institutes of Health. A phase I clinical trial in 18‒60 adults (APICTH) [cited 2020 May 16th]. Available from: https://clinicaltrials.gov/ct2/show/NCT04313127.
- 132.National Institutes of Health. A study of a candidate COVID-19 vaccine (COV001) [cited 2020 May 16th]. Available from: https://clinicaltrials.gov/ct2/show/NCT04324606.
- 133.National Institutes of Health. Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) to prevent SARS-CoV-2 infection [cited 2020 May 16th]. Available from: https://clinicaltrials.gov/ct2/show/NCT04283461.
- 134.National Institutes of Health. Study to describe the safety, tolerability, Immunogenicity, and potential efficacy of RNA vaccine candidates against COVID-19 in healthy adults [cited 2020 May 16th]. Available from: https://clinicaltrials.gov/ct2/show/NCT04368728.
- 135.National Institutes of Health. Safety, Tolerability and immunogenicity of INO-4800 for COVID-19 in healthy volunteers [cited 2020 May 16th]. Available from: https://clinicaltrials.gov/ct2/show/NCT04336410.