Summary
The neuropathological feature of multiple system atrophy (MSA), a fatal adult-onset disorder without effective therapy, is the accumulation of pathological α-synuclein (α-Syn) in the central nervous system (CNS). Here we show that pathological α-Syn exists in nerve terminals in detrusor and external urethral sphincter (EUS) of patients with MSA. Furthermore, α-Syn-preformed fibrils (PFFs) injected in the EUS or detrusor in TgM83+/− mice initiated the transmission of pathological α-Syn from the urogenital tract to brain via micturition reflex pathways, and these mice developed widespread phosphorylated α-Syn inclusion pathology together with phenotypes. In addition, urinary dysfunction and denervation-reinnervation of external anal sphincter were detected earlier in the mouse models with α-Syn PFFs inoculation before the behavioral manifestations. These results suggest that pathological α-Syn spreading through the micturition reflex pathways retrogradely from the urogenital tract to CNS may lead to urinary dysfunction in patients with MSA, which is different from the etiology of idiopathic Parkinson disease.
Subject Areas: Biological Sciences, Neuroscience, Behavioral Neuroscience
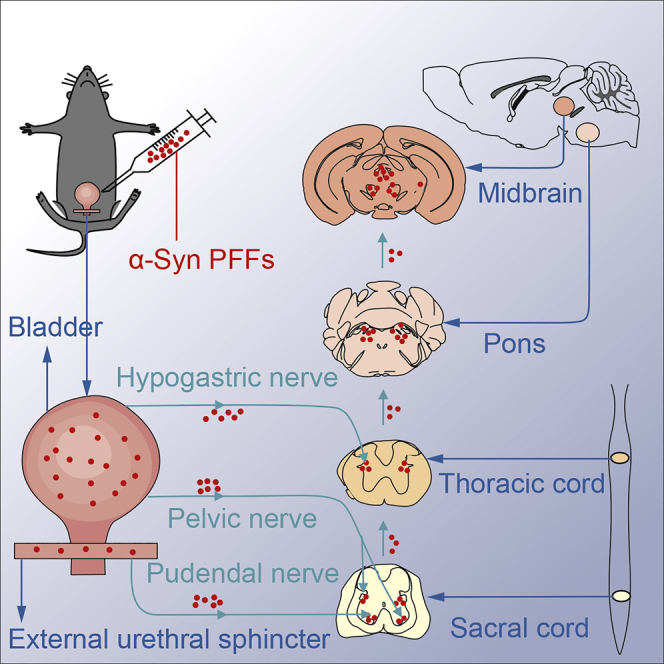
Graphical Abstract
Highlights
-
•
Pathological α-Syn exhibits in nerve terminals in DET and EUS of patients with MSA
-
•
Propagation of pathological α-Syn from urinary tract to CNS causes MSA-like syndrome
-
•
The mouse models show urinary dysfunction and abnormal EAS EMG before motor deficits
-
•
Lower urinary tract injection of α-Syn PFFs induces autonomic and motor dysfunctions
Biological Sciences; Neuroscience; Behavioral Neuroscience
Introduction
Multiple system atrophy (MSA) is a fatal, multisystem, neurodegenerative disorder characterized by a variable combination of rapidly progressive autonomic failures, ataxia, and parkinsonism.
According to the most recent guidelines, autonomic failures featuring urogenital dysfunction, orthostatic hypotension, and respiratory disorder are premonitory symptoms and necessary for the diagnosis of MSA (Gilman et al., 2008). Retrospective data indicate that among autonomic failures, urological symptoms occur several years before the neurological symptoms in the majority of patients with MSA (Beck et al., 1994, Jecmenica-Lukic et al., 2012, Sakakibara et al., 2000). Urogenital dysfunction in patients with extrapyramidal symptoms is thought to help differentiate between Parkinson disease (PD) and MSA in early disease stages (Wenning et al., 1999). Consistently, neuropathological studies reveal that widespread pathological lesions of micturition reflex pathways, including the periaqueductal gray (PAG), Barrington nucleus (BN), intermediolateral columns (IML), Onuf nucleus of the spinal cord, and so on, are present in the central nervous system (CNS) of patients with MSA (Stemberger et al., 2010, VanderHorst et al., 2015). To date, urological deficits have been reported in the transgenic PLP-SYN mice as a transgenic mouse model of MSA (Boudes et al., 2013). However, few animal models of MSA have been established to display MSA-like urinary dysfunction and denervation-reinnervation of external anal sphincter (EAS) simultaneously.
It has been acknowledged that the cellular hallmark lesion of MSA is misfolded α-Syn accumulation within glial cytoplasmic inclusions along with neuronal inclusions (NIs) in the CNS (Woerman et al., 2018). Moreover, Watts et al. reported that brain homogenates from MSA cases induced widespread deposits of phosphorylated α-Syn (pα-Syn) in the brains of MSA-inoculated mice, and it suggested that α-Syn aggregates in the brains of MSA are transmissible but with distinct characteristics from PD (Watts et al., 2013, Yamasaki et al., 2019).
Here, we show that misfolded α-Syn exists in nerve terminals in detrusor (DET) and external urethral sphincter (EUS) of patients with MSA. To determine whether the misfolded α-Syn can induce α-Syn inclusion pathology along with autonomic failure and motor impairments by transmitting from the autonomic control of the lower urinary tract to the brain via micturition reflex pathways, we injected α-Syn preformed fibrils (PFFs) to the lower urinary tract of hemizygous TgM83+/− mice, and then we observed the widespread pα-Syn pathology from the autonomic control of the lower urinary tract to the brain along the micturition reflex pathways along with urinary dysfunction and motor impairments. These data provide evidence that pathological α-Syn deposited in the lower urinary tract may have the potential to induce MSA.
Results
Clinical Characteristics of Patients
Forty-five patients were diagnosed as MSA, PD, or progressive supranuclear palsy (PSP) according to the consensus criteria (Gilman et al., 2008, Kalia and Lang, 2015, Litvan et al., 1996). Informed consent was obtained for each subject or their authorized surrogates on behalf of patients who lack decision-making ability. The clinical descriptions for each type of disease are summarized in Table S1. Among 32 patients (12 males and 20 females) with MSA, 13 patients had MSA with predominant parkinsonism (MSA-P), whereas 19 patients had MSA with predominant cerebellar ataxia (MSA-C). The mean ± SD of patients' ages for these two types of MSA at the time of being clinically diagnosed were 62.1 ± 7.1 years and 57.8 ± 6.6 years, respectively. The Unified Multiple System Atrophy Rating Scale (UMSARS) scores were utilized for the evaluation of patients' urological function. The score values (mean ± SD) of MSA-P and MSA-C were 44.4 ± 25.7 and 26.7 ± 15.3, respectively. In addition, these patients all had autonomic symptoms, including urological dysfunction, orthostatic dysregulation, or chronic constipation (Low et al., 2015, Stefanova et al., 2009, Wenning et al., 2004). Furthermore, the positive rates of urodynamic examination and perianal electromyography in MSA were 88.1% and 81.0%, respectively. Altogether, these data indicate that urological dysfunction is specific and common in patients with MSA.
Detection of Pathological α-Syn in Patients' Samples
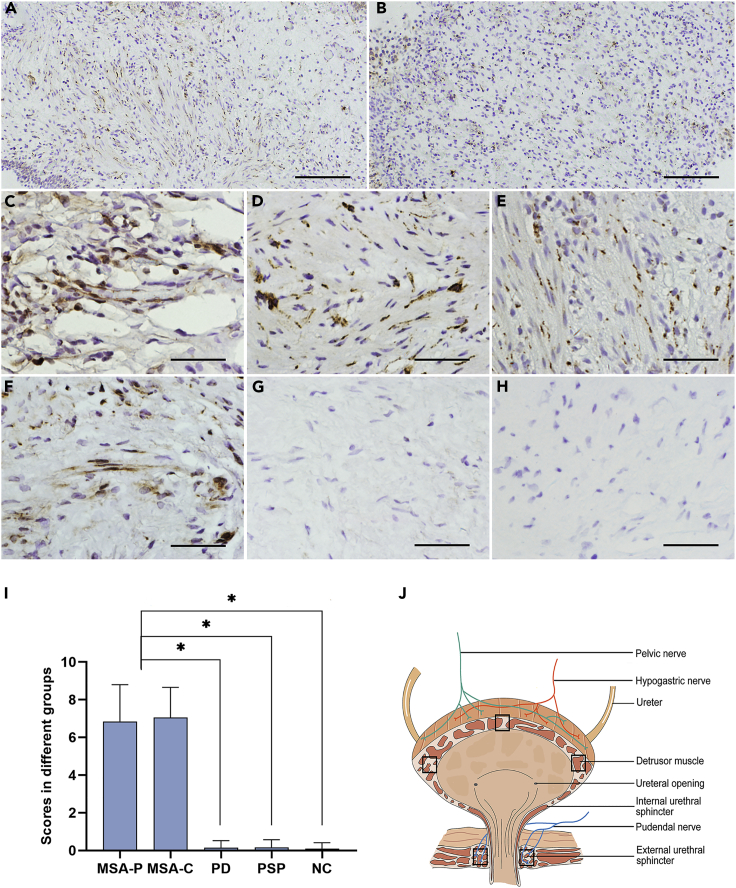
We then investigated the deposits of misfolded α-Syn in the DET or EUS of patients with MSA (Figure 1J), using anti-α-Syn filament (MJFR14), anti-pα-Syn (Ser129P), and anti-aggregated-α-Syn (5G4) antibodies. Patients with MSA exhibited deposits of misfolded α-Syn in biopsy tissues (Figures 1A–1F, S1E, and S1I and Table S1). Moreover, we assessed the pathology of three sites of DET (left wall, right wall, and triangle region) by scoring the immunohistochemical pictures. For nine immunohistochemical slides (using the three antibodies to detect three sites of DET) of a patient, if more than three of five views in one slide showed α-Syn-positive staining under the microscope, one point for the slide was scored. The patients with a cumulative score (≥six points) of the whole nine slides were considered α-Syn-positive. Then, we quantified the scores of α-Syn-positive staining in MSA-P, MSA-C, PD, PSP, and normal subjects. Compared with subjects with PD or PSP or normal subjects, patients with MSA-P and MSA-C scored significantly higher (p < 0.05) (Figure 1I). The scores of pathological α-Syn have no significant difference between MSA-P and MSA-C (p > 0.05) (Figure 1I). Among the patients examined, one had a urinary incontinence for 6 years before presence of movement deficits, and we made diagnosis for him as MSA after 8 months of movement deficits. A large amount of pathological α-Syn was found in his DET and EUS. Most remarkably, no PD cases, PSP cases, or normal controls tested show pathological α-Syn in bladder (Figures 1G–1I, S1F–S1H, and S1J–S1L). Taken together, these results show that pathological α-Syn exists in DET or EUS of the patients with MSA who were examined, whereas PD, PSP, and control subjects exhibit no detectable pathological α-Syn in their bladders.
Figure 1.
Immunohistochemical Results of the Sample Tissues from Different Subjects
(A–H) Representative images displayed misfolded α-Syn in DET of MSA-P (A and C–E) and MSA-C (B), and in EUS of MSA-P (F) stained with anti-α-Syn filament antibody (MJFR14), but not in PSP (G) and PD (H). (C–E) Representative images displayed the right wall of MSA-P (C), the left wall of MSA-P (D), and the triangle region of MSA-P (E).
(I) Histogram shows the scores of pathological α-Syn deposits in DET of different groups for anti-α-Syn filament (MJFR14), anti-pα-Syn (Ser129P), and anti-aggregated-α-Syn (5G4) antibodies. MSA-P, n = 13; MSA-C, n = 19; PD, n = 7; PSP, n = 6; NC, n = 20.
(J) Schematic displaying the anatomy of the lower urinary tract and sampling positions (DET and EUS).
Data are the means ± SD. Statistical significance was analyzed employing the one-way ANOVA. ∗p < 0.05. Scale bar, 400 μm in (A and B); 100 μm in (C–H).
Identification of the Micturition Reflex Pathways Controlling EUS or DET Using Fluoro-Gold
After we found that misfolded α-Syn proteins exist in the DET and EUS of the detected patients, we then used fluoro-gold (FG) injection to trace the micturition reflex pathways controlling EUS or DET in mice. FG was injected into both sides of EUS or DET in TgM83+/− and C57BL/6 mice; the sections from different parts of nervous system were detected at 14-day post-injection. FG-labeled neurons were detected in pelvic ganglia, spinal cord, pons, and midbrain bilaterally in both mouse models (Figure S2). In spinal cord, we found that FG-labeled neurons also existed at the T2 level, which has not been previously reported. At the T2 level, the FG-labeled neurons mostly appeared in the ventral horn and IML, which are closely associated with motor and autonomic functions. Among other levels of spinal cord, FG-labeled neurons gathered in the EUS motoneurons of lamina IX (ExU9) and sacral parasympathetic nucleus (SPSy) at S1 level; EAS motoneurons of lamina IX (ExA9), ExU9, gluteal motoneurons of lamina IX (Gl9), lamina VII of the spinal gray (7Sp), and lateral spinal nucleus (LSp) at L6 level; as well as psoas motoneurons of lamina IX (Ps9), quadriceps motoneurons of lamina IX (Q9), intercalated nucleus (ICL), IML, and lumbar dorsal commissural nucleus (LDCom) at L2 level. In brain, FG-labeled neurons appeared in BN, PAG, and locus coeruleus (LC), and these nuclei have been reported to participate in micturition reflex pathways (Fowler et al., 2008). In addition, FG-labeled neurons were found to be in parvocellular reticular nucleus alpha, mesencephalic trigeminal nucleus, and red nucleus (RN), which are involved in the general locomotion, postural control, and modulation of certain sensory and autonomic functions. Taken together, these results suggest that the micturition reflex pathways controlling EUS and DET are connected not only with the autonomic nervous system but also with the central motor pathways.
Spreading of Phosphorylated α-Syn from the Lower Urinary Tract to the Brain in TgM83+/− Mice via Micturition Reflex Pathways
To demonstrate whether spreading of misfolded α-Syn via the same pathways induces the MSA-like neuropathology, we next injected α-Syn PFFs to EUS or DET in TgM83+/− mice and evaluated pα-Syn in different sections at different time points.
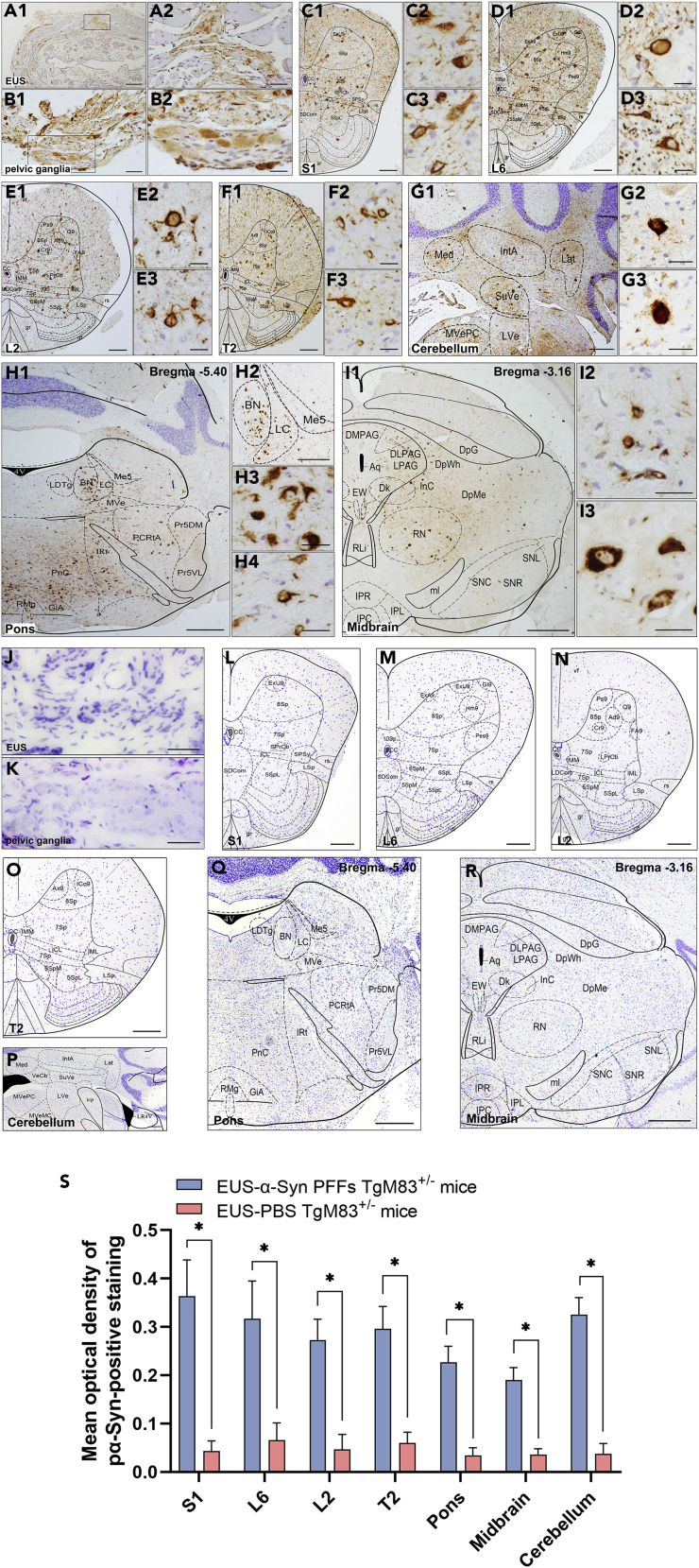
Immunohistochemical results show that pα-Syn, stained with the anti-pα-Syn antibody, was detected at 5 months post-injection (mpi) in both EUS- and DET-α-Syn PFFs TgM83+/− mice (Figures 2A–2I, 2S). In contrast, pα-Syn has not been detected in EUS-phosphate buffer saline (PBS) (Figures 2J–2S), DET-PBS TgM83+/−, and C57BL/6 mice post-injection. In EUS-α-Syn PFFs TgM83+/− mice, small numbers of pα-Syn were detected in EUS and pelvic ganglia (Figures 2A and 2B). Furthermore, pα-Syn was detected at the S1, L6, L2, and T2 levels of spinal cord, and they mostly existed in laminae V-VII and IX of these levels (Figures 2C–2F). In brain, pα-Syn existed in pons and midbrain (Figures 2H and 2I). These observations are consistent with the results of the above-mentioned FG study. In addition, using immunohistochemical approach, pα-Syn was also found in the cerebellar nuclei (Figure 2G). The diseased EUS-α-Syn PFFs TgM83+/− mice showed a distinct loss of calbindin-D28k and microtubule-associated protein-2 (MAP-2) (Figures 3A–3D). The number and optical density of neurons of EUS-α-Syn PFFs TgM83+/− mice are significantly less than PBS control groups (p < 0.05) (Figures 3A3–3D3). The neuropathological findings in DET-α-Syn PFFs TgM83+/− mice were similar to those in EUS-α-Syn PFFs TgM83+/− mice. In conclusion, transmission of pathological α-Syn in these mice invades not only the autonomic nervous system associated with urinary function but also the extrapyramidal system via the micturition reflex pathways, and these findings are consistent with MSA pathology found in patient autopsy (Cykowski et al., 2015, Stemberger et al., 2010, VanderHorst et al., 2015, Yoshida, 2007). Thus, these findings suggest that pathological α-Syn spreads from the autonomic innervation of the lower urinary tract to extrapyramidal system via the micturition reflex pathways, leading to widespread α-Syn pathology.
Figure 2.
Representative Immunohistochemical Results of Different Segments from EUS-α-Syn PFFs and PBS TgM83+/− Mice at 6 mpi
(A–R) Pathological α-Syn was stained with anti-phospho-α-Syn (Ser 129) antibody for EUS-α-Syn PFFs TgM83+/− mice (A–I) and EUS-PBS TgM83+/− mice (J–R). Representative images displayed the distribution of pα-Syn in EUS (A1, J), pelvic ganglia (B1, K), S1 (C1, L), L6 (D1, M), L2 (E1, N), T2 (F1, O), cerebellum (G1, P), pons (H1, Q), and midbrain (I1, R). (A2–I2, C3–I3, H4) Higher-magnification views relative to the main image.
(S) Quantification of pα-Syn immunoreactivity of different segments from EUS-α-Syn PFFs and EUS-PBS TgM83+/− mice at 6 mpi. EUS-α-Syn PFFs TgM83+/− mice, n = 20; EUS-PBS TgM83+/− mice, n = 10.
Data are the means ± SD. Statistics were analyzed employing the Student's t test and Mann-Whitney test. ∗p < 0.05 indicates a significant difference between α-Syn PFFs groups and PBS groups. Scale bars, 500 μm in (A1, G1, H2, P); 250 μm in (C1–F1, L–O); 100 μm in (A2, B1); 50 μm in (B2, G2, I2, G3–I3, H4); 40 μm in (J, K); 25 μm in (C2–F2, C3–F3); 1 mm (H1, I1, Q, R).] DLPAG, dorsolateral periaqueductal gray; DMPAG, dorsomedial periaqueductal gray; DpG, deep gray layer of the superior colliculus; DpMe, deep mesencephalic nucleus; DpWh, deep white layer of the superior colliculus; EW, Edinger-Westphal nucleus; GiA, gigantocellular reticular nucleus; IntA, interposed cerebellar nucleus, anterior part; IPC, interpeduncular nucleus, caudal subnucleus; IPL, internal plexiform layer of the olfactory bulb; IPR, interpeduncular nucleus, rostral subnucleus; IRt, intermediate reticular nucleus; Lat, lateral (dentate) cerebellar nucleus; LDTg, tegmental nucleus; LPAG, lateral periaqueductal gray; LVe, lateral vestibular nucleus; Med, medial (fastigial) cerebellar nucleus; MVePC, medial vestibular nucleus; parvocellular part; MVe, medial vestibular nucleus; PnC, pontine reticular nucleus, caudal part; Pr5DM, principal sensory trigeminal nucleus, dorsomedial part; Pr5VL, principal sensory trigeminal nucleus, ventrolateral part; RLi, rostral linear nucleus of the raphe; RMg, raphe magnus nucleus; SuVe, superior vestibular nucleus.
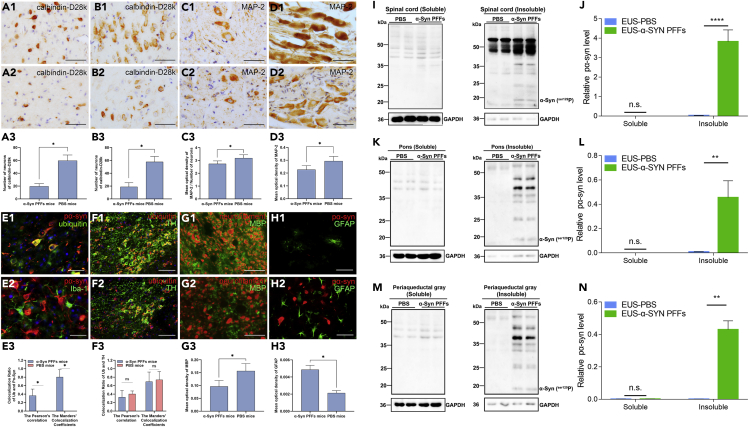
Figure 3.
Immunostaining and Western Blot Analyses of Different Segments from Diseased EUS-α-Syn PFFs TgM83+/− Mice and Age-Matched EUS-PBS TgM83+/− Mice
(A–D) Immunohistochemical results from EUS-PBS TgM83+/− mice (A1–D1) and diseased EUS-α-Syn PFFs TgM83+/− mice (A2-D2) for calbindin-D28k in the periaqueductal gray (A) and Barrington nucleus (B) and MAP-2 in the intermediolateral columns of L2 (C) and pelvic ganglia (D). (A3–D3) Quantification of the number (A3, B3) and optical density (C3, D3) of neurons shown in (A1–D1 and A2–D2).
(E–H) Double immunofluorescence analysis of the L2 level of the spinal cord (E), substantia nigra (F), and pons (G, H) from EUS-PBS TgM83+/− mice (F1–H1) and diseased EUS-α-Syn PFFs TgM83+/− mice (E, F2–H2) for pα-Syn (red, E, H) with ubiquitin (Ub) (green, E1), Iba-1 (green, E2), and glial fibrillary acidic protein (GFAP) (green, H); Ub (red, F) with tyrosine hydroxylase (TH) (green, F); and neurofilament (red, G) with MBP (green, G). (E3, F3) Quantification of colocalization of Ub with pα-Syn (E3) and TH (F3) shown in (E1, F1, F2). (G3, H3) Quantification of optical density of MBP (G3) and GFAP (H3) shown in (G1, G2, H1, H2). Co-immunolabeling is represented by signal in yellow. Cell nuclei were counterstained with Hoechst 33258 (blue). EUS-α-Syn PFFs TgM83+/− mice, n = 20; EUS-PBS TgM83+/−, mice n = 10. Scale bars: 200 μm in (F1, F2), 50 μm in (A1–D1, A2–D2), and 40 μm in (E1, E2, G1, G2, H1, H2).
(I–N) Representative immunoblots (I, K, M) and quantification (J, L, N) of pα-Syn in the sarkosyl-soluble and insoluble fractions of spinal cord (I, J), pons (K, L), and PAG (M, N). Blots were probed for GAPDH as a loading control (bottom). Molecular weight markers of migrated protein standards are expressed in kDa. n = 3 animals per group.
Data are the means ± SD. Statistical significance was analyzed by using the Student's t test and Mann-Whitney test, ∗∗∗∗p < 0.0001, ∗∗p < 0.01, ∗p < 0.05; n.s., non-significant.
To further characterize the nature of α-Syn-positive deposits in diseased EUS- or DET-α-Syn PFFs TgM83+/− mice and the distribution of pathological α-Syn in various cells, double immunofluorescence staining was then employed. First, the result revealed the ubiquitin-positive pα-Syn deposits in spinal cord of diseased EUS- or DET-α-Syn PFFs TgM83+/− mice (Figures 3E1 and 3E3). Then, we observed the distribution of pα-Syn and Iba-1 (microglia marker) in spinal cord (Figure 3E2), which indicates that microglia are recruited around neurons containing pα-Syn. In addition, the diseased EUS-α-Syn PFFs TgM83+/− mice showed a distinct loss of myelin basic protein and increase of glial fibrillary acidic protein (p < 0.05) (Figures 3G and 3H). No pα-Syn was detected in substantia nigra pars compacta (SNc). We noticed high level of ubiquitin protein in tyrosine hydroxylase-positive dopamine neurons in SNc of both EUS- or DET-α-Syn PFFs TgM83+/− mice and PBS control groups (Figure 3F). Taken together, these results suggest that the injection of α-Syn PFFs into EUS or DET of TgM83+/− mice initiates pathological α-Syn transmission through micturition reflex pathways, which could be associated with neuronal loss, microglia recruitment, demyelination, and astrogliosis.
An immunoblot of spinal cord, pons, and PAG homogenate probed with pα-Syn (Ser129P) antibody was conducted to confirm that injection of α-Syn PFFs into EUS or DET of TgM83+/− mice initiates MSA-like neuropathology. In the radio immunoprecipitation assay (RIPA) buffer-insoluble and sarkosyl-insoluble fractions, immunoblots of pα-Syn reveal that bands around 15 kDa were detected in all examined diseased EUS-α-Syn TgM83+/− mice, whereas they were faintly detected in EUS-PBS TgM83+/− mice (Figures 3I–3N). Statistical analysis shows that pα-Syn is significantly increased in diseased EUS-α-Syn TgM83+/− mice (Figures 3J, 3L, and 3N). These data support that the injection of α-Syn PFFs into EUS or DET can induce the spreading of pathological α-Syn in CNS.
Early-Onset Denervation-Reinnervation of EAS in EUS- or DET-α-Syn PFFs TgM83+/−
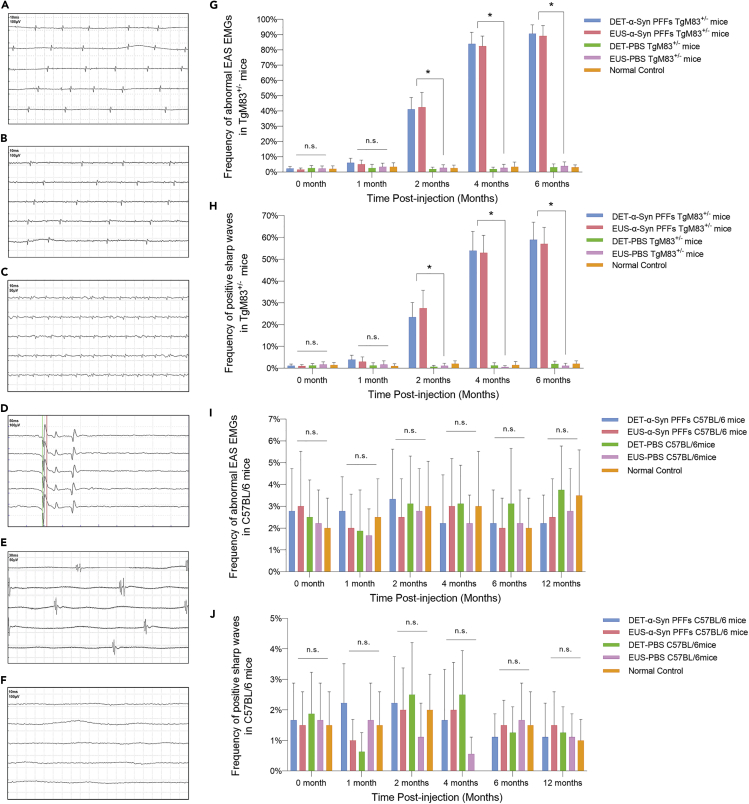
Twenty age-matched healthy TgM83+/− and C57BL/6 male mice were used respectively; no abnormal EAS electromyography (EMG) in these mice was detected. Based on the EMGs of the previous literature (Daube and Rubin, 2009, Palace et al., 1997, Schwarz et al., 1997), abnormal EAS EMG would be defined if the EMG findings satisfied any one of the following six conditions: (1) fibrillation potentials, (2) positive sharp waves, (3) complex repetitive discharge, (4) fasciculation potentials, (5) myokymic discharges, and (6) satellite potential.
EUS- and DET-α-Syn PFFs TgM83+/− mice show abnormal EAS EMGs at 2 mpi (p < 0.05), whereas no abnormality of EAS EMG was detected in PBS groups. Representative abnormal and normal EAS EMGs are shown in Figures 4A–4F, respectively. The mean frequency of abnormal EAS EMGs in EUS-α-Syn PFFs TgM83+/− mice was 42.50%, 82.50%, and 89.00% at 2, 4, and 6 mpi, respectively, versus 41.11%, 83.89%, and 90.56% in DET-α-Syn PFFs TgM83+/− mice, respectively (Figure 4G). The frequency of positive sharp waves for α-Syn PFFs-injected TgM83+/− mice started to be significantly higher than that of PBS-injected TgM83+/− mice and normal control at 2 mpi (Figure 4H). In C57BL/6 mice, the data show no significant difference between EUS- or DET-α-Syn PFFs groups and PBS groups (Figures 4I and 4J). The results suggest that the frequency of abnormal EAS EMGs increases along with the progression of neural lesions caused by α-Syn PFFs. We also injected α-Syn PFFs into the intestine wall of stomach and duodenum of TgM83+/− mice. However, the TgM83+/− mice with intestine-α-Syn PFFs did not develop abnormal EAS EMG, whereas TgM83+/− mice did. Taken together, the denervation-reinnervation of EAS occurs in the early stage of neuropathological process in a time-dependent manner and may be caused by spreading of α-Syn PFFs from the lower urinary tract through micturition reflex pathways.
Figure 4.
EAS EMG Analysis of TgM83+/− Mice and C57BL/6 Mice
(A–E) Representative abnormal EAS EMGs from diseased EUS- or DET-α-Syn PFFs TgM83+/− mice. Abnormal EAS EMGs show as fibrillation potential (A), positive sharp waves (B), complex repetitive discharge (C), satellite potential (D), and myokymic discharges (E).
(F) Representative normal EAS EMG referring to resting potential from EUS-PBS TgM83+/− mice at 5 mpi.
(G–J) Frequencies of abnormal EAS EMGs (G, I) and positive sharp waves (H, J) in different groups of TgM83+/− mice (G, H) and C57BL/6 mice (I and J). DET-α-Syn PFFs mice, n = 18; EUS-α-Syn PFFs mice, n = 20; DET-PBS mice, n = 16; EUS-PBS mice, n = 18; normal control, n = 20.
Data are the means ± SEM. Statistics was analyzed employing the one-way ANOVA. ∗p < 0.05 relative to the corresponding PBS groups and normal control groups.
Urinary Dysfunction in EUS- or DET-α-Syn PFFs TgM83+/− Mice
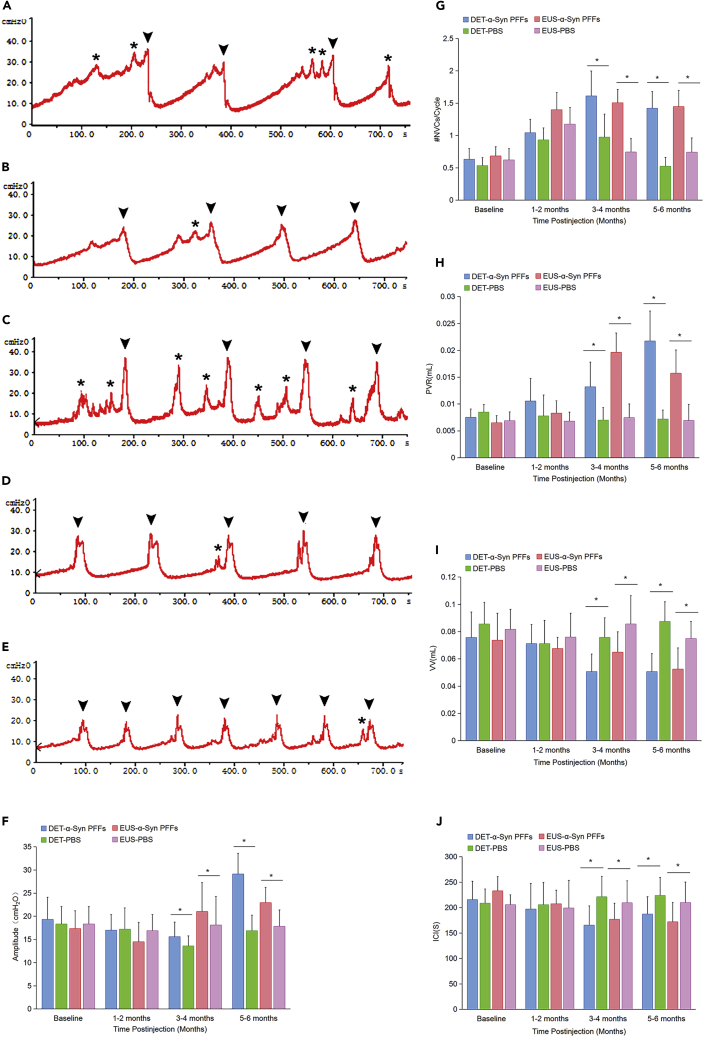
The urodynamic baseline is determined by cystometry results of 2-month-old male TgM83+/− and C57BL/6 mice before treatments. Urinary dysfunction was observed in EUS- and DET-α-Syn PFFs TgM83+/− mice between 3 and 4 mpi and persisted to the last stage examined. At 4 mpi, both EUS- and DET-α-Syn PFFs TgM83+/− mice exhibited a significant increase in amplitude, postvoid residual volume (PVR), and nonvoiding contractions (NVCs) during the filling phase compared with PBS groups (p < 0.05). Meanwhile, voided volume (VV) and intercontraction interval (ICI) in EUS- or DET-α-Syn PFFs TgM83+/− mice were found less and shorter, respectively (Figure 5). The body mass of EUS- or DET-α-Syn PFFs TgM83+/− mice was mostly lighter than that of EUS- or DET-PBS TgM83+/− mice; however, the bladder of EUS- or DET-α-Syn PFFs TgM83+/− mice exhibited overtly greater size compared with EUS- or DET-PBS mice (Figure S3D), which was probably due to progressive urothelium and DET hyperplasia in α-Syn PFFs TgM83+/− mice. By 14 mpi, EUS- or DET-α-Syn PFFs C57BL/6 mice did not show any urinary dysfunction. The intestine-α-Syn PFFs TgM83+/− mice did not show any urinary dysfunction at 3.5 mpi even when EUS- and DET-α-Syn PFFs TgM83+/− mice did already. All aforementioned results suggest that urodynamic assessment in EUS- or DET-α-Syn PFFs TgM83+/− mice was characterized by an overactive, less-stable, and inefficient bladder. In addition, α-Syn PFFs injection into EUS or DET of TgM83+/− mice caused potential dyssynergia between DET and EUS, leading to hyperactive bladder and DET hyperreflexia (Boudes et al., 2013, Hamill et al., 2012), which resembles urinary dysfunction in patients with MSA. Thus, we developed an animal model to replicate MSA-like urinary disorders and abnormal EAS EMGs, which has not been previously reported.
Figure 5.
Urinary Function Analysis of TgM83+/− Mice
(A–E) Representative cystometry traces in DET-α-Syn PFFs (A), DET-PBS (B), EUS-α-Syn PFFs (C), and EUS-PBS (D) TgM83+/− mice at 5 mpi and baseline group (E). Arrowheads indicate void events and asterisks indicate NVCs.
(F–J) Summary bar graphs from urodynamic evaluation for EUS and DET TgM83+/− mice including amplitude (F), #NVCs/cycle (G), PVR (H), VV (I), and ICI (J). EUS-α-Syn PFFs TgM83+/− mice, n = 18; DET-α-Syn PFFs TgM83+/− mice, n = 16; EUS-PBS TgM83+/− mice, n = 22; DET-PBS TgM83+/−, mice n = 20.
Data are the means ± SD. Statistics was analyzed employing the Student's t test and Mann-Whitney test. ∗p < 0.05 indicates a significant difference between EUS- or DET-α-Syn PFFs groups and EUS- or DET-PBS groups.
Motor Impairments in EUS- or DET-α-Syn PFFs TgM83+/− Mice
Both EUS- and DET-α-Syn PFFs TgM83+/− mice began to exhibit motor impairments from 5 mpi. Most diseased mice presented an arched back initially and then progressed with weight loss, ataxia, paralysis, and a moribund state requiring euthanasia within 3 weeks (Figure S4A). Compared with DET-α-Syn PFFs TgM83+/− mice, the behavioral deficiency was more obvious in EUS-α-Syn PFFs TgM83+/− mice. At 5 mpi, α-Syn PFFs TgM83+/− mice showed significantly increased motor behavioral scale (MBS) score compared with EUS- or DET-PBS TgM83+/− mice, which is considered as a semi-quantitative assessment for MBS rating (Figure S4B). The rotarod test was carried out to assess coordination capability. The performance on the rotating rod was significantly impaired in EUS- and DET-α-Syn PFFs TgM83+/− mice compared with PBS controls, as their latency to fall was markedly reduced (Figure S4C). In an open field test, EUS- and DET-α-Syn PFFs TgM83+/− mice showed significantly reduced spontaneous activities when compared with PBS-injected mice (Figures S4J and S4K). Footprint analysis indicates that EUS- and DET-α-Syn PFFs TgM83+/− mice have shorter stride length and wider base width compared with PBS-injected mice (Figures S4D and S4E). Moreover, EUS- and DET-α-Syn PFFs TgM83+/− mice also showed significant motor dysfunction in the beam walking test (Figures S4F and S4G) and pole test (Figures S4H and S4I). EUS- or DET-PBS TgM83+/− mice did not show any phenotype until they were 22 months old, consistent with our spontaneously sick TgM83+/− mice in timeline. As the previous study reported, spontaneously sick TgM83+/− mice develop series of phenotypes between 22 and 28 months of age (Giasson et al., 2002). Nevertheless, EUS- and DET-α-Syn PFFs C57BL/6 mice failed to exhibit behavioral abnormalities up to 420 days post-injection (Figure S5).
Taken together, EUS- and DET-α-Syn PFFs TgM83+/− mice developed distinct motor signs including weight loss, bradykinesia, ataxia, and paralysis at 5 mpi. We conclude that injection with α-Syn PFFs into EUS or DET in TgM83+/− mice initiates MSA-like motor deficits.
Discussion
In MSA, autonomic dysfunctions, especially urinary dysfunction (Kirby et al., 1986), are premonitory and prominent, which are different from the other main synucleinopathies, e.g., PD. A recent review suggests that peripheral microbial infections could be potential triggers of α-synucleinopathies (Tulisiak et al., 2019). When the physiological integrity of the urinary tract is breached, the urinary tract can be at a heightened risk or even have recurrent episodes of microbial infections (Hickling et al., 2015). Based on our long-term observations of clinical subjects with these diseases, we reckoned that pathological α-Syn might exist in the lower urinary tract at the early stage of MSA instead of the gut as shown in PD (Holmqvist et al., 2014, Kim et al., 2019). To test this hypothesis, we first performed bladder biopsy in participants. The findings show that pα-Syn pathology indeed exists in DET or EUS in the included patients with MSA. The subsequent results from immunohistochemistry studies in experimental mice show that α-Syn aggregates invade the micturition reflex pathways. In addition, we found widely positive staining of pα-Syn in ventral white matter of spinal cord, possibly due to nerve tracts from brain and comprehensively longitudinal connections by synapses of numerous nerve fibers. Moreover, we detected overt pα-Syn in cerebellar nucleus, which indicates that α-Syn aggregates transmit to cerebellum via rubro-cerebello-rubrospinal circuit (Larson-Prior and Cruce, 1992). α-Syn pathology in EUS- or DET-α-Syn PFFs C57BL/6 mice was not detected at 14 mpi. The results of double immunofluorescence analysis further demonstrate the pathological lesions in the CNS of the two mouse models. There was apparent demyelination in the CNS of EUS-α-Syn PFFs TgM83+/− mice, which is a major pathological feature of MSA (Ettle et al., 2016, Woerman et al., 2019). The immunostaining results validate the hypothesis that pathological α-Syn transmits initially from urogenital autonomic nerves to extrapyramidal system, inducing pα-Syn pathology. EAS EMG has been previously proposed as a diagnostic method for MSA (Lee et al., 2002). Abnormalities of EAS EMG in MSA indicate the denervation-reinnervation of EAS caused by neuronal loss of Onuf nucleus in the anterior horn of the spinal cord (Lee et al., 2002, Libelius and Johansson, 2000). In this study, we conducted EAS EMG in mouse models to assess denervation-reinnervation of EAS. Remarkably, abnormal EAS EMGs emerged at 2 mpi in EUS- or DET-α-Syn PFFs TgM83+/− mice. Furthermore, the mean frequency reached around 90% in EUS- or DET-α-Syn PFFs TgM83+/− mice at 6 mpi, whereas no abnormality was detected in PBS-injected TgM83+/− mice. We demonstrate that EMG experimental results in the mouse models are similar to EAS EMG feature of patients with MSA preceding urinary dysfunction and movement disorders. Previous studies (Yamamoto et al., 2005) presented a view that selective neuronal loss of Onuf nucleus, which innervates EAS, results in abnormal EAS EMGs in patients with MSA. We found that pα-Syn aggregates are present in Onuf nucleus in both EUS- and DET-α-Syn PFFs TgM83+/− mice.
In this study, we implemented urodynamic assessment in different time points to evaluate urinary function in experimental mice. Consequently, EUS- or DET-α-Syn PFFs TgM83+/− mice exhibited urodynamic changes after 3 mpi, before motor impairments, versus no changes in PBS groups. Here, we identified that urinary dysfunction, characterized by urinary bladder hyperreflexia of α-Syn PFFs TgM83+/− mice, replicates the altered bladder function in patients with MSA including urinary incontinence, frequency, urgency, and retention (Fowler et al., 2010, Ragab and Mohammed, 2011). Previous study (Libelius and Johansson, 2000) showed that the spontaneous TgM83+/− mice developed urinary bladder dysfunction before motor dysfunction due to A53T mutant α-Syn. In our study, EUS- or DET-α-Syn PFFs TgM83+/− mice started to perform urinary dysfunction at 3.5 mpi, whereas EUS- or DET-PBS and non-inoculated TgM83+/− mice did not show any urinary dysfunction until they were 22 months old. The occurrence of urinary dysfunction in EUS- or DET-α-Syn PFFs TgM83+/− mice is earlier than that in PBS control groups and non-inoculated TgM83+/− mice. In our α-Syn PFFs TgM83+/− mice, the micturition reflex pathways, including EUS and DET, pelvic ganglia, Onuf nucleus, IML, PAG, BN, and LC, exhibited pα-Syn pathology, revealing pathological mechanisms of the urinary dysfunction. However, we did not observe appreciable levels of misfolded α-Syn deposition in oligodendrocytes within the TgM83+/− mice. This may be related to the transgenic background of TgM83+/− mice we used. As previous study reported, α-Syn in TgM83 mice is expressed under the control of the prion promoter (Giasson et al., 2002). Thus, the pathology in TgM83 mice remains essentially neuronal. This observation could be also explained by misfolded α-Syn originating from different parts of peripheral nervous system (PNS) to CNS via neuronal projections transsynaptically. In spontaneously ill TgM83+/− mice, α-Syn aggregates have not been detected in ExU9, ICL, ExA9, Gl9, sacral dorsal commissural nucleus, IML, LDCom, BN, and PAG, which is different from the diseased EUS-α-Syn PFFs TgM83+/− mice. As these spared areas are involved in controlling the urinary bladder (Fowler et al., 2008), these data support that the preceding autonomic dysfunction of EUS- or DET-α-Syn PFFs TgM83+/− mice results from exogenously injected α-Syn PFFs instead of A53T mutant α-Syn. Besides, we injected synthetic mouse α-Syn PFFs into the EUS of C57BL/6 mice. By 6 mpi, the EUS-α-Syn PFFs C57BL/6 mice exhibited sparse pathological pα-Syn deposits at the S1, L6, L2, and T2 levels of spinal cord, and in pons and midbrain of brain (Figure S6). Moreover, they showed abnormal EAS EMG by 4 mpi and urinary dysfunction by 6 mpi (Figure S6). Together, our results suggest that misfolded α-Syn spreading through the micturition reflex pathways retrogradely may lead to urinary dysfunction.
Previous studies indicate that pathological α-Syn spreads from PNS to CNS through retrograde axonal transport, in a stereotypical and topographical pattern (Bernis et al., 2015, Braak et al., 2003, Holmqvist et al., 2014, Luk et al., 2012). Experimental studies suggest that PD pathology may originate in the vagal nerves from the gut and gradually propagate to the brain (Holmqvist et al., 2014, Kim et al., 2019). These findings support the hypothesis that different synucleinopathies may originate from different part of the PNS and gradually propagate to the CNS. Hence, we speculate that pathological α-Syn originating from the autonomic innervation of the lower urinary tract has the potential to propagate to CNS and induce MSA. Besides that, different strains of α-Syn could account for differences between α-synucleinopathies according to previous studies (Melki, 2015). Bousset et al. found that α-Syn PFFs have higher toxicity and more seeding and propagation properties compared with α-Syn ribbons (Bousset et al., 2013). Our data demonstrate that misfolded α-Syn PFFs can induce α-Syn pathology along with autonomic failure and motor impairments by transmitting from the autonomic control of the lower urinary tract to the brain via micturition reflex pathways.
As previously reported by others, peripheral injection of α-Syn PFFs into multiple sites could promote the development of α-Syn pathology in the CNS of TgM83+/− mice (Ayers et al., 2017, Breid et al., 2016, Holmqvist et al., 2014, Krejciova et al., 2019, Luk et al., 2012, Sacino et al., 2014a, Sacino et al., 2014b, Watts et al., 2013, Woerman et al., 2019). We injected α-Syn PFFs into the striatum of TgM83+/− mice in initial studies. The results show that the lower urinary tract pathology cannot be obtained at the sixth month after intracerebral α-Syn PFFs injection. We also injected α-Syn PFFs into the intestine wall of stomach and duodenum of TgM83+/− mice. However, the intestine-α-Syn PFFs TgM83+/− mice did not develop abnormal EAS EMG and urinary dysfunction at 5 mpi when they had α-Syn pathology in the CNS and motor impairments already. Thus, α-Syn injection into intestine wall alone could not induce the denervation-reinnervation of EAS and urinary dysfunction before motor impairments in TgM83+/− mice. Again, this further indicates that α-Syn of PD and MSA may start in different places.
Here we further show that injection with α-Syn PFFs into EUS or DET induces a rapid progression of motor dysfunctions. Our study shows that injection with α-Syn PFFs into EUS or DET in TgM83+/− mice causes not only seeding of α-Syn aggregation in the CNS but also rapid progressive motor dysfunctions evaluated using a spectrum of behavioral tests. From our findings, the occurrence of motor impairments in our EUS- or DET-α-Syn PFFs TgM83+/− mice was much earlier than spontaneously ill TgM83+/− mice (Giasson et al., 2002). According to previous studies (Breid et al., 2016, Holmqvist et al., 2014, Prusiner et al., 2015, Sacino et al., 2014a, Sacino et al., 2014b), the animal models of synucleinopathy induced by exogenous inoculation involve different inocula and inoculation positions, developing variable α-Syn pathology and motor impairments without autonomic dysfunction. Furthermore, pathology of α-Syn inclusions observed in motor neuron of ventral horn, cerebellum, and RN in α-Syn PFFs TgM83+/− mice provides neuropathological evidence for the motor impairments.
In summary, we observe that pathological α-Syn exists in nerve terminals in DET and EUS of patients with MSA, which has not been recognized before. Also, this study suggests one possible pathogenic mechanism of MSA, which is the spreading of α-Syn pathology from the autonomic control of the lower urinary tract to the brain. Finally, our data support the view that pathological α-Syn may originate from different parts of PNS among different disorders of synucleinopathies. However, the full mechanisms of MSA warrant further explanation as other possibilities exist at the same time.
Limitations of the Study
MSA is a sporadic disease rather than a genetic disease; the conclusions accordingly are constrained by the transgenic mice used for the research, and further investigation on wild-type mice might provide more evidence. In addition, the essentially neuronal pathology and the absence of oligodendroglial inclusion in TgM83+/− mice may provide an imperfect model for MSA.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Xuejing Wang (fccwangxj2@zzu.edu.cn).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
All data supporting the findings of this study are included in the article and its Supplemental Information, or are available from the corresponding authors on request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank all our collaborators at the First Affiliated Hospital of Zhengzhou University for their assistance with patient sample collection. Specially, we thank Dr. Jianping Wang for his material support and Dr. Shuyan Feng for her excellent technical support. This work was supported by National Natural Science Foundation of China (No. 81873791, 81471307) to X.W.; Natural Science Foundation of Henan Province of China (182300410373) to X.D.; National Natural Science Foundation of China (No. 81671267) and Natural Science Foundation of Henan Province of China (182300410320) to J.T.; and Baylor Scott & White Health Funds to E.W.
Author Contributions
X.W. and X.D. designed the study; X.W., J.T., and M.M. organized the research project; L.Z., X.J., H.L., J.Y., R.Z., and D.L. conducted the experiments; E.W. and M.M. designed the statistical analysis; X.D., F.W., and X.J. executed the statistical analysis; X.W., J.T., and B.T. did review and critique of the statistical analysis; X.W. wrote the first draft; E.W., L.Z., and H.L. did review and critique of the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: June 26, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101166.
Contributor Information
Xuebing Ding, Email: fccdingxb@zzu.edu.cn.
Mingming Ma, Email: macklon12@zzu.edu.cn.
Beisha Tang, Email: bstang7398@163.com.
Erxi Wu, Email: erxi.wu@bswhealth.org.
Junfang Teng, Email: 13838210077@163.com.
Xuejing Wang, Email: fccwangxj2@zzu.edu.cn.
Supplemental Information
References
- Ayers J.I., Brooks M.M., Rutherford N.J., Howard J.K., Sorrentino Z.A., Riffe C.J., Giasson B.I. Robust central nervous system pathology in transgenic mice following peripheral injection of alpha-synuclein fibrils. J. Virol. 2017;91 doi: 10.1128/JVI.02095-16. e02095–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R.O., Betts C.D., Fowler C.J. Genitourinary dysfunction in multiple system atrophy: clinical features and treatment in 62 cases. J. Urol. 1994;151:1336–1341. doi: 10.1016/s0022-5347(17)35246-1. [DOI] [PubMed] [Google Scholar]
- Bernis M.E., Babila J.T., Breid S., Wusten K.A., Wullner U., Tamguney G. Prion-like propagation of human brain-derived alpha-synuclein in transgenic mice expressing human wild-type alpha-synuclein. Acta Neuropathol. Commun. 2015;3:75. doi: 10.1186/s40478-015-0254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudes M., Uvin P., Pinto S., Voets T., Fowler C.J., Wenning G.K., De Ridder D., Stefanova N. Bladder dysfunction in a transgenic mouse model of multiple system atrophy. Mov. Disord. 2013;28:347–355. doi: 10.1002/mds.25336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousset L., Pieri L., Ruiz-Arlandis G., Gath J., Jensen P.H., Habenstein B., Madiona K., Olieric V., Bockmann A., Meier B.H. Structural and functional characterization of two alpha-synuclein strains. Nat. Commun. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Del Tredici K., Rub U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Breid S., Bernis M.E., Babila J.T., Garza M.C., Wille H., Tamguney G. Neuroinvasion of alpha-synuclein prionoids after intraperitoneal and intraglossal inoculation. J. Virol. 2016;90:9182–9193. doi: 10.1128/JVI.01399-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cykowski M.D., Coon E.A., Powell S.Z., Jenkins S.M., Benarroch E.E., Low P.A., Schmeichel A.M., Parisi J.E. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain. 2015;138:2293–2309. doi: 10.1093/brain/awv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daube J.R., Rubin D.I. Needle electromyography. Muscle Nerve. 2009;39:244–270. doi: 10.1002/mus.21180. [DOI] [PubMed] [Google Scholar]
- Ettle B., Kerman B.E., Valera E., Gillmann C., Schlachetzki J.C., Reiprich S., Buttner C., Ekici A.B., Reis A., Wegner M. alpha-Synuclein-induced myelination deficit defines a novel interventional target for multiple system atrophy. Acta Neuropathol. 2016;132:59–75. doi: 10.1007/s00401-016-1572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler C.J., Dalton C., Panicker J.N. Review of neurologic diseases for the urologist. Urol. Clin. North Am. 2010;37:517–526. doi: 10.1016/j.ucl.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Fowler C.J., Griffiths D., de Groat W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson B.I., Duda J.E., Quinn S.M., Zhang B., Trojanowski J.Q., Lee V.M. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Gilman S., Wenning G.K., Low P.A., Brooks D.J., Mathias C.J., Trojanowski J.Q., Wood N.W., Colosimo C., Durr A., Fowler C.J. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill R.W., Tompkins J.D., Girard B.M., Kershen R.T., Parsons R.L., Vizzard M.A. Autonomic dysfunction and plasticity in micturition reflexes in human alpha-synuclein mice. Dev. Neurobiol. 2012;72:918–936. doi: 10.1002/dneu.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickling D.R., Sun T.T., Wu X.R. Anatomy and physiology of the urinary tract: relation to host defense and microbial infection. Microbiol. Spectr. 2015;3:10.1128. doi: 10.1128/microbiolspec.UTI-0016-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist S., Chutna O., Bousset L., Aldrin-Kirk P., Li W., Bjorklund T., Wang Z.Y., Roybon L., Melki R., Li J.Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- Jecmenica-Lukic M., Poewe W., Tolosa E., Wenning G.K. Premotor signs and symptoms of multiple system atrophy. Lancet Neurol. 2012;11:361–368. doi: 10.1016/S1474-4422(12)70022-4. [DOI] [PubMed] [Google Scholar]
- Kalia L.V., Lang A.E. Parkinson's disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- Kim S., Kwon S.H., Kam T.I., Panicker N., Karuppagounder S.S., Lee S., Lee J.H., Kim W.R., Kook M., Foss C.A. Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson's disease. Neuron. 2019;103:627–641.e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby R., Fowler C., Gosling J., Bannister R. Urethro-vesical dysfunction in progressive autonomic failure with multiple system atrophy. J. Neurol. Neurosurg. Psychiatry. 1986;49:554–562. doi: 10.1136/jnnp.49.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejciova Z., Carlson G.A., Giles K., Prusiner S.B. Replication of multiple system atrophy prions in primary astrocyte cultures from transgenic mice expressing human alpha-synuclein. Acta Neuropathol. Commun. 2019;7:81. doi: 10.1186/s40478-019-0703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Prior L.J., Cruce W.L. The red nucleus and mesencephalic tegmentum in a ranid amphibian: a cytoarchitectonic and HRP connectional study. Brain Behav. Evol. 1992;40:273–286. doi: 10.1159/000113918. [DOI] [PubMed] [Google Scholar]
- Lee E.A., Kim B.J., Lee W.Y. Diagnosing multiple system atrophy with greater accuracy: combined analysis of the clonidine-growth hormone test and external anal sphincter electromyography. Mov. Disord. 2002;17:1242–1247. doi: 10.1002/mds.10225. [DOI] [PubMed] [Google Scholar]
- Libelius R., Johansson F. Quantitative electromyography of the external anal sphincter in Parkinson's disease and multiple system atrophy. Muscle Nerve. 2000;23:1250–1256. doi: 10.1002/1097-4598(200008)23:8<1250::aid-mus14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Litvan I., Agid Y., Calne D., Campbell G., Dubois B., Duvoisin R.C., Goetz C.G., Golbe L.I., Grafman J., Growdon J.H. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Low P.A., Reich S.G., Jankovic J., Shults C.W., Stern M.B., Novak P., Tanner C.M., Gilman S., Marshall F.J., Wooten F. Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol. 2015;14:710–719. doi: 10.1016/S1474-4422(15)00058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk K.C., Kehm V., Carroll J., Zhang B., O'Brien P., Trojanowski J.Q., Lee V.M. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki R. Role of different alpha-synuclein strains in synucleinopathies, similarities with other neurodegenerative diseases. J. Parkinsons Dis. 2015;5:217–227. doi: 10.3233/JPD-150543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palace J., Chandiramani V.A., Fowler C.J. Value of sphincter electromyography in the diagnosis of multiple system atrophy. Muscle Nerve. 1997;20:1396–1403. doi: 10.1002/(sici)1097-4598(199711)20:11<1396::aid-mus7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Prusiner S.B., Woerman A.L., Mordes D.A., Watts J.C., Rampersaud R., Berry D.B., Patel S., Oehler A., Lowe J.K., Kravitz S.N. Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl. Acad. Sci. U S A. 2015;112:E5308–E5317. doi: 10.1073/pnas.1514475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab M.M., Mohammed E.S. Idiopathic Parkinson's disease patients at the urologic clinic. Neurourol Urodyn. 2011;30:1258–1261. doi: 10.1002/nau.20983. [DOI] [PubMed] [Google Scholar]
- Sacino A.N., Brooks M., Thomas M.A., McKinney A.B., Lee S., Regenhardt R.W., McGarvey N.H., Ayers J.I., Notterpek L., Borchelt D.R. Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc. Natl. Acad. Sci. U S A. 2014;111:10732–10737. doi: 10.1073/pnas.1321785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacino A.N., Brooks M., Thomas M.A., McKinney A.B., McGarvey N.H., Rutherford N.J., Ceballos-Diaz C., Robertson J., Golde T.E., Giasson B.I. Amyloidogenic alpha-synuclein seeds do not invariably induce rapid, widespread pathology in mice. Acta Neuropathol. 2014;127:645–665. doi: 10.1007/s00401-014-1268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara R., Hattori T., Uchiyama T., Kita K., Asahina M., Suzuki A., Yamanishi T. Urinary dysfunction and orthostatic hypotension in multiple system atrophy: which is the more common and earlier manifestation? J. Neurol. Neurosurg. Psychiatry. 2000;68:65–69. doi: 10.1136/jnnp.68.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J., Kornhuber M., Bischoff C., Straube A. Electromyography of the external anal sphincter in patients with Parkinson's disease and multiple system atrophy: frequency of abnormal spontaneous activity and polyphasic motor unit potentials. Muscle Nerve. 1997;20:1167–1172. doi: 10.1002/(sici)1097-4598(199709)20:9<1167::aid-mus12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Stefanova N., Bucke P., Duerr S., Wenning G.K. Multiple system atrophy: an update. Lancet Neurol. 2009;8:1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- Stemberger S., Poewe W., Wenning G.K., Stefanova N. Targeted overexpression of human alpha-synuclein in oligodendroglia induces lesions linked to MSA-like progressive autonomic failure. Exp. Neurol. 2010;224:459–464. doi: 10.1016/j.expneurol.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulisiak C.T., Mercado G., Peelaerts W., Brundin L., Brundin P. Can infections trigger alpha-synucleinopathies? Prog. Mol. Biol. Transl. Sci. 2019;168:299–322. doi: 10.1016/bs.pmbts.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderHorst V.G., Samardzic T., Saper C.B., Anderson M.P., Nag S., Schneider J.A., Bennett D.A., Buchman A.S. alpha-Synuclein pathology accumulates in sacral spinal visceral sensory pathways. Ann. Neurol. 2015;78:142–149. doi: 10.1002/ana.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J.C., Giles K., Oehler A., Middleton L., Dexter D.T., Gentleman S.M., DeArmond S.J., Prusiner S.B. Transmission of multiple system atrophy prions to transgenic mice. Proc. Natl. Acad. Sci. U S A. 2013;110:19555–19560. doi: 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning G.K., Scherfler C., Granata R., Bosch S., Verny M., Chaudhuri K.R., Jellinger K., Poewe W., Litvan I. Time course of symptomatic orthostatic hypotension and urinary incontinence in patients with postmortem confirmed parkinsonian syndromes: a clinicopathological study. J. Neurol. Neurosurg. Psychiatry. 1999;67:620–623. doi: 10.1136/jnnp.67.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning G.K., Tison F., Seppi K., Sampaio C., Diem A., Yekhlef F., Ghorayeb I., Ory F., Galitzky M., Scaravilli T. Development and validation of the unified multiple system Atrophy rating scale (UMSARS) Mov. Disord. 2004;19:1391–1402. doi: 10.1002/mds.20255. [DOI] [PubMed] [Google Scholar]
- Woerman A.L., Oehler A., Kazmi S.A., Lee J., Halliday G.M., Middleton L.T., Gentleman S.M., Mordes D.A., Spina S., Grinberg L.T. Multiple system atrophy prions retain strain specificity after serial propagation in two different Tg(SNCA∗A53T) mouse lines. Acta Neuropathol. 2019;137:437–454. doi: 10.1007/s00401-019-01959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerman A.L., Watts J.C., Aoyagi A., Giles K., Middleton L.T., Prusiner S.B. Alpha-synuclein: multiple system Atrophy prions. Cold Spring Harb. Perspect. Med. 2018;8:a024588. doi: 10.1101/cshperspect.a024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Sakakibara R., Uchiyama T., Liu Z., Ito T., Awa Y., Yamamoto K., Kinou M., Yamanishi T., Hattori T. When is Onuf's nucleus involved in multiple system atrophy? A sphincter electromyography study. J. Neurol. Neurosurg. Psychiatry. 2005;76:1645–1648. doi: 10.1136/jnnp.2004.061036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T.R., Holmes B.B., Furman J.L., Dhavale D.D., Su B.W., Song E.S., Cairns N.J., Kotzbauer P.T., Diamond M.I. Parkinson's disease and multiple system atrophy have distinct alpha-synuclein seed characteristics. J. Biol. Chem. 2019;294:1045–1058. doi: 10.1074/jbc.RA118.004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M. Multiple system atrophy: alpha-synuclein and neuronal degeneration. Neuropathology. 2007;27:484–493. doi: 10.1111/j.1440-1789.2007.00841.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are included in the article and its Supplemental Information, or are available from the corresponding authors on request.