Abstract
The failure of insulin-producing β-cells is the underlying cause of hyperglycemia in diabetes mellitus. β-cell decay has been linked to hypoxia, chronic inflammation, and oxidative stress. Thioredoxin (Trx) proteins are major actors in redox signaling and essential for signal transduction and the cellular stress response. We have analyzed the cytosolic, mitochondrial, and extracellular Trx system proteins in hypoxic and cytokine-induced stress using β-cell culture, isolated pancreatic islets, and pancreatic islet transplantation modelling low oxygen supply.
Protein levels of cytosolic Trx1 and Trx reductase (TrxR) 1 significantly decreased, while mitochondrial Trx2 and TrxR2 increased upon hypoxia and reoxygenation. Interestingly, Trx1 was secreted by β-cells during hypoxia. Moreover, murine and human pancreatic islet grafts released Trx1 upon glucose stimulation. Survival of transplanted islets was substantially impaired by the TrxR inhibitor auranofin.
Since a release was prominent upon hypoxia, putative paracrine effects of Trx1 on β-cells were examined. In fact, exogenously added recombinant hTrx1 mitigated apoptosis and preserved glucose sensitivity in pancreatic islets subjected to hypoxia and inflammatory stimuli, dependent on its redox activity. Human subjects were studied, demonstrating a transient increase in extracellular Trx1 in serum after glucose challenge. This increase correlated with better pancreatic islet function. Moreover, hTrx1 inhibited the migration of primary murine macrophages.
In conclusion, our study offers evidence for paracrine functions of extracellular Trx1 that improve the survival and function of pancreatic β-cells.
Graphical abstract
1. Introduction
Inflammation and fluctuations in islet oxygen supply play a pivotal role in loss of insulin secreting β-cells of pancreatic islets during the onset and progression of diabetes mellitus [[1], [2], [3]]. Elevated levels of reactive oxygen species (ROS) and disturbed redox circuits, often referred to as oxidative stress, have been linked to this irreversible process [4]. Accordingly, it was shown that hyperglycemia creates a hypoxia-like condition in islets, which leads to activation of a hypoxic stress response [5]. β-cells are highly vulnerable to hypoxia resulting in functional impairment of insulin secretion and induction of apoptosis [[5], [6], [7]], accumulation of ROS [8], down-regulation of the unfolded protein response [7], up-regulation of NF-κB, and dys-regulation of cellular redox signaling [9,10].
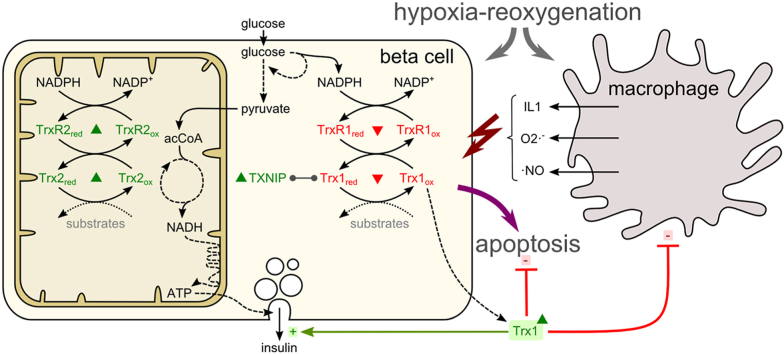
Interestingly, NF-κB-induced apoptosis is redox-regulated by proteins of the thioredoxin (Trx) family (for an overview see Ref. [11]). The Trx system, composed of Trx, the seleno protein Trx reductase (TrxR), and NADPH regulates specific proteins via reversible disulfide-dithiol exchange reactions and de-nitrosylation of cysteine (Cys) residues. Moreover, recent data indicate that Trx is also involved in hydrogen sulfide (H2S) signaling [12].
Mammalian genomes encode two forms of thioredoxin proteins: the cytosolic (Trx1, TrxR1) and the mitochondrial (Trx2, TrxR2) ones. Trx1 was furthermore shown to translocate into the nucleus [13] and to be secreted [14]. The activity of Trx is regulated by its potential inhibitor Txnip (Thioredoxin-interacting protein) which binds to and inhibits reduced Trx. Due to distinct substrate specificity, Thioredoxin proteins have a broad range of functions in the regulation of DNA synthesis [15], gene expression [16], regulation of intracellular hydrogen peroxide levels [17], proliferation and apoptosis [18,19], changes in oxygen supply, and the modulation of the immune response [20]. Modification of activity, expression levels, or localization of thioredoxins was associated with dys-regulation of proteins in distinct cellular pathways and various diseases, including diabetes [11].
The expression and anti-apoptotic functions of Trx1 were shown to improve islet survival following pancreatic islet transplantation, a therapeutic strategy to restore β-cell function [21], which inevitably involves hypoxia-reoxygenation injury as well as exposure of grafts to the host's inflammatory response. The general lack of oxygen and the generation of ROS, particularly during the reoxygenation period, may prevent long-term cell survival, cell regeneration, and restoration of insulin production and secretion. Moreover, the sensitive micro-milieu of pancreatic β-cells can further be disrupted by inflammatory processes, mediated by chemo- and cytokines, complement components, matrix metalloproteases, and infiltrating macrophages [[22], [23], [24]].
In the present study, evidence is provided that thioredoxin proteins of pancreatic β-cells are differentially regulated in cytosolic, mitochondrial, and extracellular compartments under hypoxic or inflammatory conditions. They are involved in the control of cellular processes facilitating cell survival, secretory function as well as extracellular signaling and cell communication of β-cells. Our data clearly confirm that distinct stimuli induce compartmentalized redox reactions and hold different effects on thioredoxin proteins. Moreover, this study shows that extracellular full-length hTrx1 possesses paracrine functions dependent on its redox activity, affecting β-cell survival and function.
2. Materials and methods
2.1. Materials and products
Mitochondrial and caspase activity kits of cells were purchased from Invitrogen (Karlsruhe, Germany) and Biozol (Eching, Germany), respectively. Insulin concentrations of isolated pancreatic islets or mouse β-cells were determined by specific sandwich ELISA assays from DRG Instruments (Marburg, Germany). hTrx1 activity was analyzed by Trx1-catalyzed reduction of insulin (Proteostat, Enzo, Lörrach, Germany). Primer sequences for qRT-PCR and antibodies are listed in Tables S1 and S2.
2.2. Protein expression, purification and activity
Recombinant human thioredoxin (hTrx1) was cloned into the pet20b plasmid and was expressed in E. coli and purified using HisTrap columns and the IMAC principle [25]. To produce endotoxin-free protein, expression was performed in ClearColi BL21(DE3) Electrocompetent Cells (Lucigen). In addition, hTrx1 with <1.0 endotoxin units/μg protein was purchased from Biocat GmbH (Heidelberg, Germany). As control, hTrx1 was denatured at 90 °C for 30 min (dhTrx1). In addition, a redox-inactive Cys32Ser mutant of hTrx1 (C32S) was generated by rolling-circle PCR using specific oligonucleotides (Fig. S1).
2.3. Pancreatic islet isolation and culture
Porcine islets were harvested from retired breeders as previously described [26]. Briefly, islets from a single pancreas were retrieved after vascular flush with University of Wisconsin solution (Du Pont Critical Care, IL, USA). The quality of the islets was evaluated by trypan blue exclusion, dithizone staining and glucose-stimulated insulin secretion (GSIS) to verify cell viability, purity, and function. Pig islets were cultured at non-CO2 air in CMRL 1066 (PAA, Pasching, Austria) supplemented with 25 mM HEPES, 20% heat-inactivated pig serum, 100 U/ml penicillin (Biochrom, Berlin, Germany), 100 μg/ml streptomycin, and 2 mg/ml glucose. Their insulin secretion was determined by calculating the ratio of stimulated and basal insulin concentrations measured at 22 and 2.8 mM glucose concentration in the medium, resulting in an insulin stimulation index (SI).
2.4. Cell culture and hypoxia
The murine β-cell line MIN6 was cultivated in high glucose DMEM medium (PAA, Pasching, Austria) supplemented with 20% heat-inactivated fetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin, 1 mM sodium pyruvate, and 71 μM mercaptoethanol at 37 °C in a 90% humidified atmosphere containing 5% CO2. Cells were grown under different oxygen concentrations (1%, 2% or 20%) using the New Brunswick Innova hypoxia chamber. Recombinant cytokines used in cell culture were 10 ng/ml TNF-α, 5 ng/ml IL-1β, 100 ng/ml IFN-γ purchased from R&D Systems (Marburg, Germany). Control cells were grown under standard cell culture conditions at 20% O2. Cells were harvested, washed with PBS, incubated in NP40-containing lysis buffer (10 mM Tris/HCl, 0.1% NP40, 10 mM NaCl, 3 mM MgCl2, and protease and phosphatase inhibitors, pH 7.4) for 15 min at room temperature, and were flash frozen in liquid nitrogen. Cell extracts were centrifuged at 13,000 rpm for 15 min at 4 °C. Protein levels were determined using Bradford reagent (Biorad, CA, USA) or BCA (Pierce, MA, USA). The cells’ constituent insulin secretion was determined by comparing the amount of insulin in their cell culture medium before and after the respective treatment.
2.5. Macrophage migration
Peritoneal macrophages were retrieved following thioglycollate challenge as described [24]. They were added to the insert of 12-well plates (Greiner bio-one, Kremsmünster, Austria) to give a volume of 250 μl with or without Trx1. Wells contained 600 μl of supernatant from pancreatic islet free floating tissue culture. Macrophages were allowed to migrate for 48 h at 37 °C in a 5% CO2 humidified atmosphere and labelled adding 8 μM calcein AM. Cells migrating to the bottom surface of the filter were removed by using trypsin EDTA and detected using a fluorescence plate reader (Berthold Technologies-Mithras LB940, Germany).
2.6. Immunoblot
Samples (20 μg of total protein) were combined with sample loading buffer (0.3 M Tris-HCl, pH 7, 50% glycerol, 5% SDS, 0.1% bromphenol blue). Proteins were reduced by a 20 min incubation at RT with 100 mM DTT, followed by an additional incubation for 10 min at 94 °C. Proteins were separated on a reducing SDS-PAGE (Pierce, MA, USA) and transferred to a PVDF membrane, which was blocked with 5% milk powder and 1% BSA in Tris-buffered saline containing 0.05% Tween20. Proteins were detected using specific primary antibodies, a horseradish peroxidase-coupled secondary antibody and the electrogenerated chemiluminescence method. Images were obtained using the Chemostar system. ImageJ was employed for the densitometric quantification.
2.7. Enzyme linked immunosorbent assays (ELISA)
Detection of human or porcine Trx1 in plasma was performed customizing a sandwich procedure described by Lundberg [27]. Capture antibody (mouse monoclonal (mAb) MT17R6) was diluted in PBS (1.0 μg/ml) and absorbed to 96-well Maxisorp plates (Nunc) for 24 h at 4 °C. After washing steps with PBS/0.1% Tween20, a dilution buffer (ELISA diluent, Mabtech 3652-D2) was applied to prevent interference by heterophilic antibodies. Accordingly, assays were designed for quantification of Trx1, Trx2, TrxR1, TrxR2, and Txnip in MIN6 cell culture. Supernatants or lyzed cells taken from MIN6 cell culture were prepared as described for immunoblotting. Capture antibodies with reactivity to the respective mouse target proteins were coated to microtiter plates. These stationary phase antibodies bound sample or purified recombinant target proteins while non-bound proteins were removed by washing. Samples or standard proteins were added in appropriate dilution in triplicates and incubated at 4 °C overnight. After washing (PBS, 0.05% v/v Tween 20) three times, 100 μl of biotin-conjugated goat-anti-rabbit (Abcam, UK) detection antibody was added and incubated for 2 h at room temperature. 0.1 μg/ml streptavidin-horseradish peroxidase conjugate (SA-HRP, 50 ng/ml, Mabtech) was added to each well and incubated for 1 h. The assay was developed by addition of substrate 3,3′,5,5’ tetramethylbenzidine (TMB, Sigma) and stopped after 15 min using 1 M H2SO4. Absorbance at 450 nm was determined using the Multimode reader Mithras LB940 from Berthold, Bad Wildbad, Germany.
2.8. LDH activity
Extracellular LDH activity was analyzed in triplicates using the CytoTox 96-non- radioactive cytotoxicity assay (Promega) according to manufacturer's instructions. Plain medium was used as negative control. Absorbance was measured at 490 nm.
2.9. Immunohistochemistry
Consecutive frozen sections (7 μm) were prepared from liver transplanted with isolated pancreatic islets. Cryosections from mouse pancreas including islets were used as controls. Slides were incubated with polyclonal anti-insulin antibody (DAKO) from guinea pig for immunohistochemistry. Administration of primary antibodies against Trx1, TrxR1, Trx2, TrxR2, and Txnip from rabbit (Table S1) was customized to porcine tissue using the protocol of Godoy et al. [28]. Insulin co-staining was used to identify β-cells. Pancreata were used as control tissue. Sections were incubated with secondary antibodies, FITC-donkey anti-guinea pig and appropriate PE (phycoerythricin)-donkey anti-rabbit antibody (Jackson ImmunoResearch, Germany) for 1 h at 22 °C. Sections were washed, counterstained with DAPI and analyzed under the fluorescent microscope LB30T equipped with the digital camera DFC420C (Leica, Wetzlar, Germany). Images were taken at 400× magnification as islets were too small for analysis at 100× and 200x. Frames with pancreatic and transplanted islets were examined with ImageJ. Pixels were converted into mm2 pre-programmed in a macro, and a threshold was set between 40 and 45 to adjust for unspecific background staining. The area stained by the antibody directed against the respective thioredoxin was expressed as the relation to the area stained with insulin. This resulted in a dimensionless quantity equivalent to the expression of the protein in β-cells.
2.10. Gene expression
Porcine pancreatic islets were lyzed and total RNA was extracted using the RNAeasy kit (Qiagen, Hilden, Germany). A sample of RNA (300 ng) was used as a template for the generation of cDNAs from Oligo (dT) 20 primers using the Superscript III first strand synthesis system (Invitrogen, Karlsruhe, Germany). Real-time PCR was performed with cDNA using specific primers (Table S2) and the Power SYBR Green PCR Master Mix reagents (Applied Biosystems, Darmstadt, Germany) using a StepOnePlus™ Real-Time PCR System. The expression of selected genes was assessed independently, and expression of ribosomal protein L13a (RPL13A) was used as a control for normalization. The effects of different treatments on individual gene expression were calculated as fold induction with the gene expression level in the control samples as fold one, with normoxic samples or hypoxic samples calculated separately and respectively to show the difference between these two environments.
2.11. Streptozotocin-induced diabetes and pancreatic islet transplantation
Animal research was approved by the Regional Administrative Council Giessen, Veterinary Department under the code GI20/11Nr.15/2006 and performed in accordance with the German Animal Welfare law and the ARRIVE guidelines. Diabetes was induced in NRMI nu/nu mice at the age of 12 weeks by a single injection of 180 mg/kg streptozotocin (Sigma, MS, USA) intraperitoneally. Blood glucose levels were monitored using the glucometer Elite (Bayer, Leverkusen, Germany). Mice with a non-fasting blood glucose concentration of more than 16.7 mM for three consecutive days were selected for transplantation (n = 16). Mice were divided into two groups and treated i.p. with 0.12 mg/kg/day thioredoxin reductase inhibitor auranofin (0.2 mg/ml in 0,9% saline, dissolved in 0.25 ml ethanol and 0.2 ml Cremophor EL) or vehicle (n = 8 per group) throughout the study period. Recipients were anaesthetized with avertine and maintained with isoflurane. 2000 pancreatic pig islet equivalents were transplanted into the liver via the portal vein with a 27-gauge needle as previously described [29]. Grafted livers and portal vein blood were recovered 30 min after transplantation of pancreatic islets. Healthy pancreata were used as controls. Grafted livers were embedded in TissueTek optimal cutting temperature compound (VWR International GmbH, Darmstadt, Germany) and snap-frozen in liquid nitrogen.
2.12. Trx1 secretion in patients transplanted with pancreatic islets
Trial activities were acknowledged by the local Ethics Committee of the Faculty of Medicine, Justus Liebig University, Giessen, Germany under Reg.-No. 54/01. Intravenous glucose tolerance tests (IVGTT) were performed in patients before and after one year of pancreatic islet transplantation and in non-diabetic subjects as controls matched for age, sex and body weight.
Pancreatic islets were transplanted to type 1 diabetic patients with a history of hypoglycemia unawareness as part of the Edmonton trial at Giessen center [30]. Patients had a mean age of 41 ± 3 years, the mean time of diabetes since diagnosis was 28 ± 4 years, and the mean insulin requirement per day was 0.55 ± 0.06 U/kg. They received 14.743 ± 1278 (mean ± SD) total islet equivalents per kg body weight. Intravenous glucose tolerance test (IVGTT) was carried out as described [31,32]. Briefly, probands were advised to ingest >150 g/day carbohydrates three days and remain fasting 10–16 h before the test. 0.3 g/kg glucose was injected in 1 min into a peripheral vein catheter, blood sampling times were −10, 0, 3, 4, 5, 7, 10, 15, 20, 25, and 30 min. Blood was collected into tubes containing EDTA. The samples were centrifuged immediately at 3000 rpm at 4 °C for 15 min and were stored in liquid nitrogen until analysis of thioredoxin concentration by ELISA. Acute thioredoxin (ATRg) response to glucose bolus injection was calculated as the mean concentration at 3, 4, and 5 normalized by basal level at 0 min.
2.13. Statistical analysis
Statistical analysis was performed using Graph Pad Prism 6 (GraphPad Software, San Diego, USA) using ANOVA with post hoc Tukey tests. Gene expressions without recombinant hTrx1 were normalized to the internal reference gene, genes that changed in the presence of hTrx1 to the expression level without hTrx1 (2ΔΔCT-method).
The graft rejection curve was generated according to Kaplan-Meier and analyzed by the Chi square test. A p-value <0.05 was considered significant. Data were expressed as mean ± SD unless otherwise stated.
3. Results
3.1. Thioredoxin proteins in β-cells exposed to hypoxia in culture
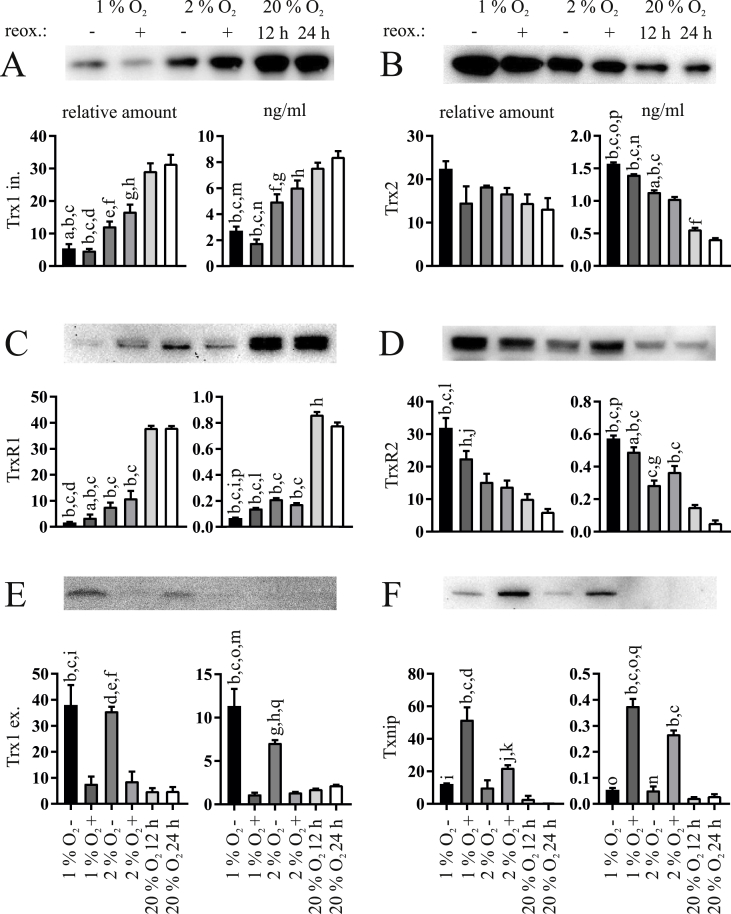
MIN6 β-cells cultured for 12 h at 1% and 2% O2 with and without subsequent reoxygenation for 12 h at 20% O2 were analyzed for protein levels using Western Blot and ELISA (Fig. 1). Interestingly, the cytosolic and mitochondrial thioredoxin proteins showed opposite responses to the respective growth conditions. Protein amounts of cytosolic Trx1 (Fig. 1A) and TrxR1 (Fig. 1C) were significantly decreased upon hypoxia as compared to normoxia and were maintained during reoxygenation. By contrast, protein amounts of mitochondrial Trx2 (Fig. 1B) and TrxR2 (Fig. 1D) were enhanced during low oxygen and reoxygenation periods compared to the normoxic controls. Txnip (Fig. 1F) was detectable at hypoxia only and further accumulated in the reoxygenation period.
Fig. 1.
Effects of hypoxia and reoxygenation on thioredoxin proteins in MIN6 cells. Each panel shows a representative western blot, the respective densitometric quantification data on the left, and the respective ELISA data on the right. (A) Intracellular Trx1, (B) Trx2, (C) TrxR1, (D) TrxR2, (E) extracellular Trx1, and (F) Txnip were analyzed in MIN6 cells cultured at 1% or 2% O2 for 12 h with (+) or without (−) reoxygenation (reox.) for another 12 h. Normoxic cells cultured at 20% O2 for 12 or 24 h were used as controls. Extracellular concentrations of Trx1 were determined in the supernatant of the cultivated cells. Actin or tubulin were not detected in these samples excluding the presence of cell lysis as potential source of extracellular Trx1. Images and densitometric quantifications are representative of n = 3–5 independent experiments. All ELISA data are normalized to 20 μg of protein lysate per well and given in ng/ml (n = 5). ap<0.05 vs. 2% O2 +; bp < 0.0001 vs. 20% O2 12 h; , cp < 0.0001 vs. 20% O2 24 h; dp < 0.01 vs. 2% O2 +; ep < 0.001 vs. 20% O2 12 h; fp < 0.001 vs. 20% O2 24 h; gp < 0.01 vs. 20% O2 12 h; hp < 0.01 vs. 20% O2 24 h; ip < 0.001 vs. 1% O2 +; jp < 0.05 vs. 20% O2 12 h, kp < 0.05 vs. 20% O2 24 h; lp < 0.01 vs. 2% O2 -; mp < 0.05 vs. 2% O2 -; np < 0.0001 vs. 2% O2 +; op < 0.0001 vs. 1% O2 +; pp < 0.0001 vs. 2% O2 –; qp < 0.001 vs. 2% O2 +.
Because Trx1 is known to be secreted, we also analyzed the medium of cultivated cells for extracellular Trx1. Indeed, MIN6 cells secreted a significant quantity of Trx1 during hypoxia (Fig. 1E). We measured the extracellular protein levels of actin and tubulin to exclude a passive release of Trx1, for example due to cell death, and could detect neither of the two using Western Blot (data not shown). In addition, lactate dehydrogenase (LDH) activity was measured in the respective samples to analyze cell lysis/viability. It was significantly increased only at 1% O2 without reoxygenation as well as after 24 h of normoxic culture when compared to 12 h of normoxic culture (Fig. S2).
3.2. Thioredoxin protein expression and secretion in transplanted pancreatic islets
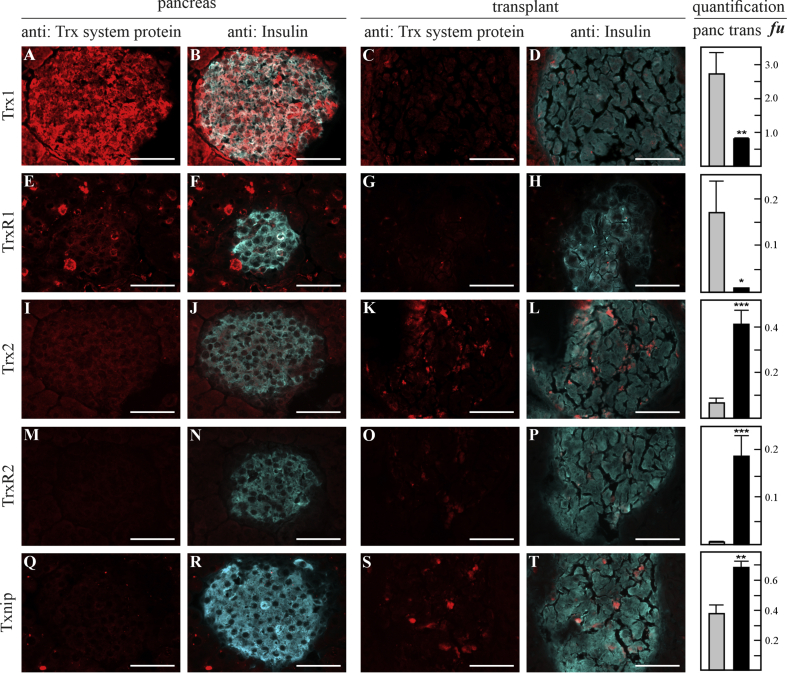
Next, we analyzed if these dramatic changes could also be detected in vivo by employing a pig-to-mouse model of islet transplantation. Thioredoxin proteins were examined by immunohistochemistry in islet grafts recovered 30 min after transplantation. Indeed, compared to native islets (Fig. 2A, B and E, F), immunofluorescent signals representing Trx1 and TrxR1 were significantly reduced by 70% (p < 0.005) and 95% (p < 0.05) in islets transplanted to the liver (Fig. 2C, D and G, H), respectively. Immunofluorescence of Trx2 (Fig. 2I, J and K, L), TrxR2 (Figure 2M, N and O, P), and Txnip (Figure 2Q, R and S, T) was significantly elevated in grafts compared to pancreatic controls: 80% for Trx2 (p < 0.0001), 95% for TrxR2 (p < 0.0001), and 45% for Txnip (p < 0.005).
Fig. 2.
Immunohistochemical analysis of thioredoxin proteins in native and transplanted pancreatic islets. Porcine islet grafts recovered 30 min after pig-to-mouse transplantation were stained with respective antibodies against the thioredoxin proteins and co-stained against insulin to identify islets. Thioredoxin proteins are represented by panels A, E, I, M, and Q in native pancreatic islets and C, G, K, O, and S in islets transplanted to the liver. Expression was compared between native pancreas and transplants for Trx1 (A–D), TrxR1 (E–H), Trx2 (I–L), TrxR2 (M–P), and Txnip (Q–T). White scale bars indicate 50 μm. Data presented as mean ± SEM. *p < 0.05, **p < 0.005, ***p < 0.0001 (t-test), n = 6–12 islet sections were evaluated for each condition.
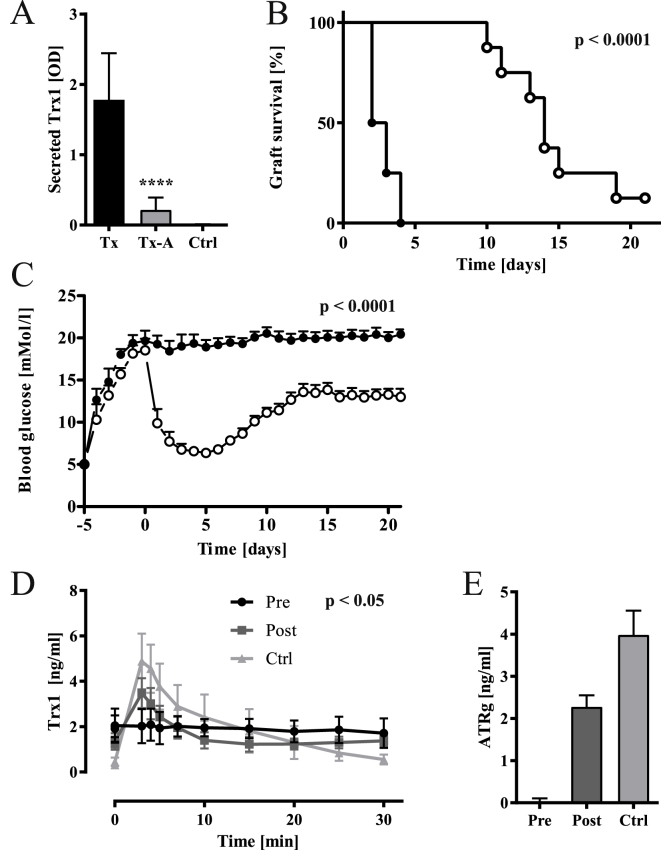
In correspondence with the secretion of Trx1 in MIN6 cells, Trx1 protein was found in portal vein blood 30 min after islet transplantation as opposed to non-transplanted mice (Ctrl, Fig. 3A). To gain deeper insight into the functional significance of the thioredoxin proteins, particularly the increase in mitochondrial Trx2 and TrxR2 protein levels for β-cell survival and function, a subgroup of mice transplanted with pancreatic islets was administered 0.12 mg/kg/day of the TrxR-inhibitor auranofin for 21 days. 50% of grafts failed within 2.5 days as opposed to 14 days in control mice receiving vehicle (p < 0.0001, Fig. 3B). Blood glucose levels normalized in control mice immediately after transplantation before slowly rising again later. Total mean blood glucose levels during the 21-day observation period were elevated for auranofin-as compared with vehicle-treated mice (18.7 ± 3.2 vs. 11.3 ± 3.5 mM, p < 0.0001, Fig. 3C). Secreted Trx1 was decreased by 85% in auranofin-as opposed to vehicle-treated mice (Tx vs. Tx-A, p < 0.0001, Fig. 3A).
Fig. 3.
Glucose-induced Trx secretion in pig and human islet grafts and the effect of TrxR inhibitor auranofin on Trx secretion and graft survival. (A) Trx1 protein release in transplanted mouse liver. Porcine Trx1 protein was specifically measured in portal vein blood retrieved 30 min after transplantation from transplanted mice (Tx), animals transplanted and treated with auranofin (Tx-A), and control mice (Ctrl) without transplanted pancreatic islets. Treatment with auranofin significantly inhibited Trx1 release from porcine islets. p < 0.0001 (one-way ANOVA), n = 8. (B, C) Auranofin treatment affected graft function in streptozotocin-induced diabetes. (B) Graft failure was characterized by recurrence of diabetes mellitus with elevated blood glucose >11.1 mM. The overall percentage of dysfunctional grafts in auranofin-treated mice (filled circles) and controls (open circles) was significant during the observation period of 21 days. p < 0.0001 (Chi square), n = 8. (C) Daily blood glucose levels in each of the two experimental groups given as mean ± SEM. The overall mean concentrations over the 21 days were significantly different between groups. p < 0.0001 (two-way ANOVA), n = 8. (D, E) Glucose stimulated Trx1 in type 1 diabetic patients before (Pre), 12 months after (Post) pancreatic islet transplantation, and matched non-diabetic healthy probands (Ctrl). Prior to transplantation, patients completely lacked pancreatic islet cells as demonstrated by failing insulin release in Pre-Tx condition (not shown). The acute Thioredoxin (ATRg) response upon an intravenous glucose tolerance test (IVGTT) to 0.3 g/kg glucose was calculated by determining the mean value normalized by basal concentration from time points 3, 4, and 5 min after intravenous injection of glucose. Analysis of variance showed significant overall difference (p = 0.0131) of Pre-Tx, Post-Tx, and Ctrl groups for ATRg. Post-hoc analysis demonstrated significant differences of Trx1 responses to the glucose challenge. p < 0.05 (two-way ANOVA) n = 8.
Interestingly, when human probands were challenged with glucose bolus injection, an acute Trx1 (ATRg) response was triggered in the presence of functional pancreatic islets (Fig. 3D and E). In diabetic subjects eligible for pancreatic islet transplantation and completely lacking residual insulin (Pre) secretion of Trx1 was poor compared to both post transplantation (Post) and non-diabetic, non-transplanted individuals (Ctrl), respectively (ATRg Post 2.3 ± 0.5, Ctrl 4.0 ±.1.1 ng/ml, p = 0.0131). Hence, transplantation of pancreatic islets reconstituted a significant rise of Trx1 following the intravenous glucose challenge.
3.3. Addition of human recombinant Trx1 maintained cell viability and preserved insulin secretion in cultured β-cells
Transplantation of pancreatic islets has two major drawbacks, the low survival of transplanted cells due to hypoxia and an instant blood mediated immune response. Trx1 was shown to be involved in the regulation of apoptosis, specific transcription factors such as HIF-1α and NF-κB, and immunomodulation. Since we observed that the cytosolic Trx1 was significantly decreased in vitro and in vivo in favor of secretion, we aimed to analyze the impact of extracellular Trx1 on β -cell viability and function.
Therefore, porcine pancreatic islet cells were analyzed by staining with annexin V and propidium iodide (PI) (Fig. 4). Cytometric analysis showed a reduction of the percentage of A/PI double positive cells under normoxic conditions (20% oxygen) in the presence of 30 and 60 μg/ml hTrx1. 30 μg/ml hTrx1 reduced A/PI cells from 9.8 ± 1.3 to 5.7 ± 0.5% and 60 μg/ml hTrx1 to 2.0 ± 1.6% (p < 0.0001) in hypoxic (2% oxygen) culture. 60 μg/ml thermally inactivated, i.e. denatured, dhTrx1 and mutated redox-inactive hTrx1 C32S did not change the annexin/PI ratio under the analyzed culture conditions. Furthermore, MIN6 cells were subjected to 2% or to 20% oxygen in the absence or presence of a cytokine mix containing 100 ng/ml IFN-γ, 5 ng/ml IL-1β, and 10 ng/ml TNF-α. The local levels of these cytokines rise at the transplantation site due to tissue injury and remodeling [33]. Cell viability was analyzed using the MTT assay. The conversion of MTT was amplified when 30 and 60 μg/ml hTrx1 was added, 25 and 56% upon hypoxia, 29 and 40% in the presence of cytokines, and even 93 and 174% at the combination of hypoxia and cytokines (p = 0.0001, Fig. S3). Concordantly, 30 and 60 μg/ml hTrx1 reduced caspase 3/7 activity measured with luminescence upon hypoxia by 21 and 39%, in the presence of the cytokines by 13 and 54%, and upon both hypoxia and cytokine treatment by 22 and 49%, respectively (p < 0.0001, Fig. S4). 60 μg/ml hTrx1 suppressed p65 phosphorylation at four and 8 h of hypoxia by 50 and 66% (p = 0.0001, Fig. S5). Again, dhTrx1 and Trx1 C32S did not induce any changes and showed comparable results as the untreated control.
Fig. 4.
Influence of hTrx1 on β-cell viability. Dissociated porcine pancreatic islet cells were analyzed for apoptosis and necrosis by fluorescence-activated cytometry (FACSCalibur and FACSuite Software, BD Biosciences, Heidelberg, Germany) probing them with annexin V and propidium iodide (PI). Cytometric analysis of islet cells treated with two concentrations of hTrx1 showed a significant (p < 0.001) reduction of Annexin V and PI (A/PI) double positive cells under 12 h normoxia (20% oxygen, 4.3 ± 0.3% to 2.5 ± 1.3%) with 60 μg/ml thioredoxin. Under 12 h hypoxia (2% oxygen), 30 μg/ml hTrx1 reduced A/PI cells also in significant way (p < 0.0001, one-way ANOVA) from 9.8 ± 1.3% to 5.7 ± 0.5% and 60 μg/ml hTrx1 to 2.0 ± 1.6%. As control for potential endotoxin contamination, we incubated hTrx1 for 30 min at 90 °C (dhTrx1). Thereby, we denatured and inactivated the protein. This process, however, does not affect endotoxins. Therefore, we tested the function of the redox-active domain of Trx1 using a redox-inactive Trx1 C32S mutant that lacks the N-terminal active site Cys. Both controls, 60 μg/ml denatured dhTrx1 and the redox-inactive C32S, did not affect the cells' viability. ****p < 0.0001, **p < 0.001 (one-way ANOVA), n = 6.
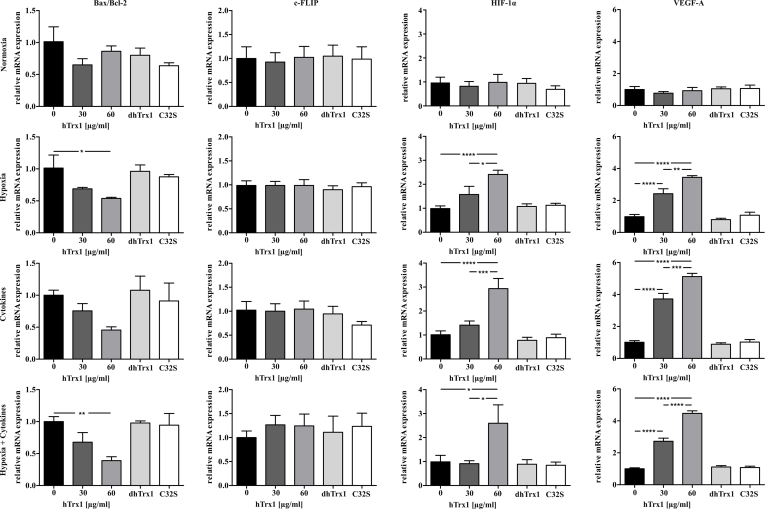
To further examine the impact of hTrx1 on cell viability, levels of pro-apoptotic gene Bax/Bcl-2 and anti-apoptotic gene c-FLIP were measured in porcine islets (Fig. 5). hTrx1 decreased transcription of Bax/Bcl-2 during hypoxia and hypoxic culture enriched with cytokines. C-FLIP gene expression was not altered by hTrx1. The expression of the pro-survival gene VEGF-A displayed a significant increase with the presence of hTrx1. Moreover, hTrx1 amplified the expression of the HIF-1α gene. Thus, hTrx1 interfered with target genes, cytosolic, and membrane proteins involved in early and late stages of the apoptotic pathway in a specific pro-survival mode for pancreatic islets. None of the tested genes were altered following the treatment with dhTrx1 or Trx1 C32S. Taken together hTrx1 reduced apoptotic processes while increasing mitochondrial oxidative function. An overview of the observed effects of hTrx1 on β-cell viability is given in Table S3.
Fig. 5.
Expression of genes in pancreatic islets at different oxygen levels and in the presence of cytokines. Target gene mRNA expression of Bax, Bcl2, c-FLIP, HIF1α and VEGF-A in porcine pancreatic islets cultured for 24 h under normoxia (20% oxygen) or hypoxia (2% oxygen) and in the presence of IFN-γ (100 ng/ml), IL-1β (5 ng/ml), and TNF-α (10 ng/ml) and 30 or 60 μg/ml hTrx1 or as control 60 μg/ml denatured hTrx1 (dhTrx1) or redox inactive hTrx1 C32S (C32S), respectively. The ratio of the mRNA expression of the proapoptotic gene Bax and antiapoptotic gene Bcl2 is given instead of the respective sole expression. Upon hypoxia, treatment with 60 μg/ml hTrx1 significantly reduced the relative mRNA expression ratio of Bax/Bcl-2 by 46%. Upon cytokine treatment during normoxia and hypoxia, as well as upon hypoxia without cytokine treatment, HIF-1α expression increased 2.9-fold, 2.6-fold and 2.4-fold, respectively. 60 μg/ml denatured dhTrx1 and the redox-inactive C32S served as controls and did not show any significant effects. Treatment time was 24 h ****p < 0.0001, ***p < 0.001, **p < 0.005, *p < 0.05 (one-way ANOVA), n = 9.
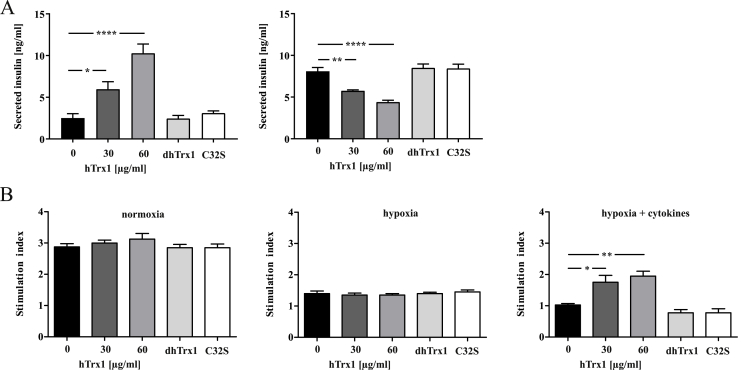
The significance of extracellular Trx1 for the constituent insulin secretion from MIN6 cells cultivated after 12 h at 2% oxygen was examined. Basal cell culture medium contained 2.5 ± 1 ng/mg insulin; when 30 or 60 μg/ml hTrx1 were added a concentration-dependent increase of secreted insulin 6.0 ± 1.8 and 10.3 ± 2.2 ng/mg (p < 0.005 compared with basal) was observed. The intracellular insulin content of cells without hTrx1 was 8.9 ± 0.9 ng/mg protein and 5.7 ± 0.2 or 4.4 ± 0.4 ng/mg with 30 or 60 μg/ml hTrx1, respectively (Fig. 6A). In isolated pancreatic islets, glucose stimulated insulin secretion given as stimulation index (SI) was measured. No significant increase of SI in the presence of hTrx1 was observed upon normoxia or hypoxia with 2% oxygen. However, with the combined hypoxia and inflammatory cytokine treatment, hTrx1 significantly (p < 0.01) increased the insulin release up to two-fold as compared to control (Fig. 6B).
Fig. 6.
Influence of hTrx1 on insulin secretion. The effect of reduced hTrx1 in tissue culture was studied at concentrations 0, 30, 60, and 90 μg/ml. As control for potential endotoxin contamination, we incubated the protein for 30 min at 90 °C (dhTrx1). Thereby, we denatured and inactivated the protein. This process, however, does not affect endotoxins. Therefore, we tested the function of the redox-active domain of Trx1 using a redox-inactive Trx1 C32S mutant that lacks the N-terminal active site Cys. (A) MIN6 cell lysates and supernatants with secreted insulin were collected following 12 h hypoxic (2% oxygen) cell culture in the absence or presence of 30 μg/ml or 60 μg/ml reduced hTrx1. Mouse insulin was analyzed using a specific ELISA. Intracellular insulin was normalized to the total protein amount. hTrx1 stimulated insulin secretion and likewise reduced intracellular insulin dose-dependently, whereas denatured or mutated hTrx1 had no effect. ****p < 0.0001, **p < 0.01, *p < 0.05 (one-way ANOVA), n = 4. (B) Porcine islets were cultured for 24 h under normoxic (20% oxygen) and hypoxic (2% oxygen) conditions in the absence or presence of cytokines IFN-γ (100 ng/ml), IL-1β (5 ng/ml), and TNF-α (10 ng/ml). The insulin stimulation index (SI) was calculated as the ratio of stimulated and basal insulin concentrations measured at 22 and 2.8 mM glucose concentrations in the medium. hTrx1 significantly restituted SI up to two-fold in combined hypoxic and inflammatory, but not in hypoxic condition alone. **p < 0.01, *p < 0.05 (one-way ANOVA), n = 4.
3.4. hTrx1 mitigated macrophage migration
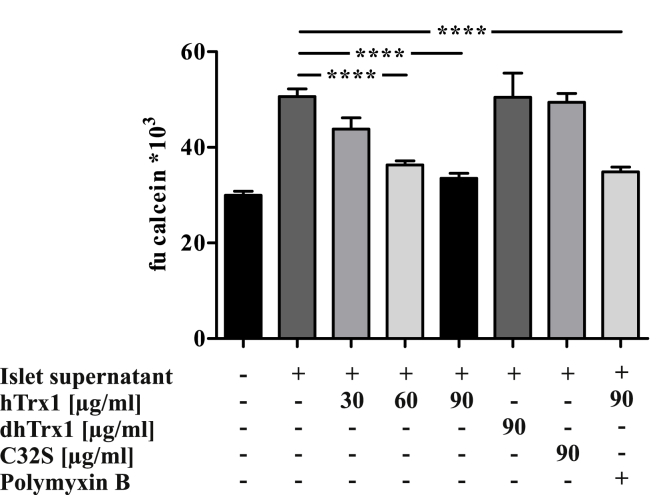
To assess a possible immunomodulatory effect under inflammatory conditions, the impact of hTrx1 on macrophage migration was studied. Spontaneous movements of macrophages were responsive to supernatant taken from pancreatic islet culture. Total migratory activity of macrophages stimulated by supernatant was suppressed 29 and 34% by 60 and 90 μg/ml hTrx1, respectively, as opposed to control wells without hTrx1 (p < 0.0001, Fig. 7). This inhibition was specific and persisted when polymyxin B, an inhibitor for endotoxins, was added. Moreover, denatured dhTrx1 and mutated redox-inactive hTrx1 C32S only had 0.3 and 2.5% inhibitory effect on overall macrophage migration induced by pancreatic islets.
Fig. 7.
Effects of hTrx1 on macrophage migration towards pancreatic islets. Cell migration assays were performed with primary murine macrophages cultured for 48 h in transwell plates using pancreatic islet culture medium as chemotactic stimulus in the presence or absence of reduced hTrx1 (final concentration 30, 60, 90 μg/ml), calcein-labelled migrated cells were counted. After 48 h of culture, islets were collected and washed three times with serum-free CMRL 1066 (PAA, Pasching, Austria). They were subsequently cultured for 48 h in serum-free CMRL 1066 supplemented with 2,5 mM l-Glutamine, 1 mM sodium pyruvate, 2 mg/ml glucose solution, 10 mM nicotinamide, Penicillin-Streptomycin and 200 mg Ciprofloxacin. The supernatant was collected for migration assay. As negative control, culture medium without islets was used. In addition, cells moving towards the islets' supernatant in the presence of 90 μg/ml denatured Trx1 (dhTrx1), redox-inactive hTrx1 C32S (C32S), or hTrx1 with 100 μM Polymyxin B were analyzed. The presence of 60 and 90 μg/ml hTrx1 significantly inhibited macrophage migration in contrast to dhTrx1 and C32S, which had no effect. Addition of Polymyxin B did not affect migration, ruling out LPS-mediated effects. ***p < 0.0001 (one-way ANOVA), n = 6.
4. Discussion
As we and others have demonstrated previously, hypoxia and inflammation cause damage to pancreatic islets [1,7,9,24]. In particular, pancreatic islet transplantation is associated with acute injury as islets are deprived of their vascularization and encounter an instant blood-mediated inflammatory response. Overcoming conditions of hypoxia and reoxygenation is vital to maintain cell viability and insulin secretion capacity. Thioredoxin proteins as key enzymes of rapid, reversible, and specific redox reactions show varying expression patterns and activity in different compartments when β-cells are challenged by metabolic stress [34]. Moreover, their expression and activity are altered in models of ischemia/reperfusion [11].
Our findings clearly demonstrate a scenario of distinct changes in both the cytoplasmic and the mitochondrial systems along with the secretion of Trx1 in response to hypoxia and inflammation. Upon hypoxic stress, an up-regulation in mitochondrial Trx2/TrxR2 was accompanied by decreased protein levels of cytosolic Trx1/TrxR1. This opposite response to the same stimuli ex vivo and in vivo emphasizes the concept of compartmentalized redox signaling and specific functions of members of the Trx family of proteins. Interestingly, the potential endogenous inhibitor of Trx, Txnip, is upregulated by high glucose levels [8] and proinflammatory cytokines [35], while Txnip-deficient β-cells were characterized by augmented GSIS [36]. We found elevated Txnip levels ex vivo and in vivo as a response to hypoxia and inflammation. Increased Txnip protein levels were shown to correlate with an increase in caspase-3 cleavage and the induction of apoptosis [10]. Txnip was furthermore identified as an activator of the inflammasome in a ROS-dependent manner [22], and an opponent of Trx1 in having detrimental effects on the β-cell.
In phases of high metabolic activity islets suffer from a hypoxia-like condition [5] and thus depend highly on coping strategies in order to overcome deprivation of oxygen and to preserve secretory function [6]. A central actor linking hypoxia with thioredoxins is the Hypoxia-inducible factor-1α (HIF-1α). Overexpression of cytosolic Trx1 supports the stabilization and transactivation of HIF-1α upon hypoxia, whereas mitochondrial Trx2 opposes its activation [37]. Thus, decreased Trx1 and enhanced Trx2 intracellular concentrations observed in transplanted islets might jointly contribute to inhibition of HIF1α.
In contradiction to its generally accepted role as controlling element in conditions of nutritional and oxygen deprivation, the transcription factor HIF-1α was associated with a shift in glucose metabolism, switching from aerobic oxidative phosphorylation to anaerobic glycolysis and impaired glucose-stimulated insulin secretion [38]. It is tempting to speculate that HIF-1α might also be involved in the secretion of Trx1 and the up-regulation of Trx2 upon hypoxia. Interestingly, addition of hTrx1 protein increased the expression of hypoxia-induced HIF-1α when isolated pancreatic islets were compromised with hypoxia in the presence or absence of cytokines.
Extracellular Trx1 seemed to have a paracrine and autocrine influence that is vital for islet protection. As recently demonstrated, injection of recombinant Trx1 to mice transplanted with pancreatic islets significantly improved blood glucose control and islet viability, while overexpression of cellular Trx1 in grafted islet cells was not effective [39], supporting an essential role for extracellular Trx1.
Interestingly, Trx1 was originally described as T-cell leukemia-derived factor secreted by various cell types via an ER- and Golgi-independent pathway [11,13] and was later detected in blood of patients diagnosed with different diseases such as rheumatoid arthritis [40] or diabetes mellitus [41]. A secretion of Trx1 was reported in macrophages stimulated with lipopolysaccharide (LPS) [42]. However, the exact secretory mechanisms remained indistinct and extracellular functions of thioredoxin proteins are not well understood. A few examples for redox regulation of membrane proteins by Trx1 have been described that show the induction of conformational changes and modulation of ligand binding and/or activity. Xu et al. suggested a direct interaction of Trx1 with transient receptor potential (TRP) channels [43]. Multiple subtypes of TRP channels, among others TRPM5 and TRPV4, exist on the β-cell and were shown to enhance insulin production upon activation [44,45]. Other examples comprise for instance tumor necrosis factor receptor superfamily member 8 (TNFRSF8) [46], HIV-1 gp120 [47], and transglutaminase 2 [48]. A receptor-agonist-like redox regulation of membrane channels and receptors might well account for the effects of extracellular Trx1 and their dose-dependency on insulin synthesis, protection of apoptosis, and macrophage migration, which we observed in our experiments. Furthermore, Kondo et al. showed that extracellular Trx1 can be transported into cells, indicating that it shuttles between the cytoplasm and the extracellular space [49]. Again, the exact mechanism is not fully understood. One suggested way of Trx1 uptake is internalization by lipid rafts [50]. Such specialized membrane domains contribute to exocytosis of insulin as part of the β-cell's surface proteome [51]. It is possible that re-internalization of secreted Trx1 is one mechanism in the restoration of intracellular Trx1 levels which we observed in β-cells during reoxygenation after exposure with 2%, but not 1% hypoxia (Fig. 1). In addition, we performed a macrophage migration assay to support the suggestion that extracellular, redox-active Trx1 is indeed functional in the inhibition of macrophage migration towards pancreatic islets. In line with our observation in peritoneal macrophages, extracellular Trx1 reduces the chemotaxis of human eosinophils [52], macrophage inflammatory protein 1 and 2-induced leukocyte chemotaxis [53], and LPS-induced neutrophil chemotaxis [54]. Further experiments are needed to confirm these characteristics of Trx1 as co-chemokine. Interestingly, Ro et al. reported that pancreatic islets subjected to hypoxia secreted chemoattractants for macrophages [55], and cytosolic Trx1 was shown to act as an inhibitor of this secretion process [56]. Exogenously administered Trx1 was shown to prolong cell survival during myocardial reperfusion injury [57] and expression of Trx1 in a redox-active form at the surface of endothelial cells inhibited complement deposition as well as neutrophil chemotaxis [58]. Migrant macrophages maintained local inflammation by secretion of IL-1β and other cytokines, which were shown to be counteracted by thioredoxin [39].
Besides the full-length Trx1, its secreted, truncated form Trx80 was described as an eosinophilic cytotoxicity enhancing factor and mitogenic cytokine [59,60]. Both proteins were shown to have different expression patterns [61], and whereas Trx1 is an oxidoreductase, the truncated Trx80 functions as a redox-inactive dimer [59,60]. Our experiments show that the paracrine functions of the full-length Trx1 on β-cell survival and function depend on a catalytically active enzyme, because the redox-inactive Cys32Ser mutant does not show significant changes compared to the control cells. Since pancreatic β-cells express the Toll-like receptor 4 (TLR4) and it is known that lipopolysaccharide affects cell viability and insulin release [[62], [63], [64], [65]], we included denaturated protein and used polymyxin b as endotoxin-controls. Indeed, all our experiments rule out potential effects due to endotoxin contamination, attributing the observed experimental outcomes to redox-active full-length Trx.
In conclusion, we have shown that the cytosolic and mitochondrial Trx systems responded differentially to hypoxia- and cytokine-mediated stress ex vivo and in vivo using freshly transplanted β-cells. Cytosolic Trx1 was secreted. Chemical depletion of the activity of the mitochondrial Trx system by inhibition of TrxR2 in the absence of the cytosolic Trx1 and TrxR1 resulted in rapid islet decay, emphasizing important functions of these Trx proteins. Addition of extracellular Trx1 counteracted apoptosis and preserved insulin secretion. Notably, these paracrine effects are mediated by the redox-active, full-length Trx1. A graphical summary of the results was designed in Fig. S6.
Author contributions
Conceptualization: CHL, TL, EMH, Data Curation: EMH, SFP, SE, CCM, NL, CHL, TL, Formal Analysis: EMH, SFP, SE, CCM, NL, CHL, TL, Investigation: EMH, SFP, SE, NL, Writing, Review & Editing: EMH, SFP, SE, CCM, NL, CHL, TL, Guarantor of the research: TL.
Declaration of competing interest
None.
Acknowledgments
TL is the guarantor of this work and as such had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Support from Klaus T. Preissner's lab, Biochemical Institute, Faculty of Medicine, Justus-Liebig University regarding design and establishment of the immunosorbent assays is highly acknowledged. The authors thank Gundula Hertl, Birte Hussmann, Doris Erb, and Divya Rawat for superb technical work.
This work was fully supported by grants of the Von Behring-Röntgen-Stiftung, Marburg, Germany to CHL and TL. It has been partially supported by current grants from Forschungscampus Mittelhessen and Federal Ministry of Education and Research (BMBF) to TL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101570.
Contributor Information
Sebastian Friedrich Petry, Email: sebastian.petry@innere.med.uni-giessen.de.
Christopher Horst Lillig, Email: horst@lillig.de.
Thomas Linn, Email: thomas.linn@innere.med.uni-giessen.de.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gunton J.E. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122(3):337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 2.Gerber P.A. Hypoxia lowers SLC30A8/ZnT8 expression and free cyto-solic Zn2+ in pancreatic beta cells. Diabetologia. 2014;57(8):1635–1644. doi: 10.1007/s00125-014-3266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell-Thompson M. Insulitis and beta-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719–731. doi: 10.2337/db15-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenzen S. Chemistry and biology of reactive species with special reference to the antioxidative defence status in pancreatic β-cells. Biochim. Biophys. Acta. 2017;1861(8):1929–1942. doi: 10.1016/j.bbagen.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Sato Y. Cellular hypoxia of pancreatic beta-cells due to high levels of oxygen consumption for insulin secretion in vitro. J. Biol. Chem. 2011;286(14):12525–12532. doi: 10.1074/jbc.M110.194738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dionne K.E., Colton C.K., Yarmush M.L. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42(1):12–21. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 7.Bensellam M. Hypoxia reduces ER-to-Golgi protein trafficking and increases cell death by inhibiting the adaptive unfolded pro-tein response in mouse beta cells. Diabetologia. 2016;59(7):1492–1502. doi: 10.1007/s00125-016-3947-y. [DOI] [PubMed] [Google Scholar]
- 8.Schulze P.C., Yoshioka J., Takahashi T., He Z., King G.L., Lee R.T. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J. Biol. Chem. 2004;279(29):30369–30374. doi: 10.1074/jbc.M400549200. [DOI] [PubMed] [Google Scholar]
- 9.Lai Y. Activation of NFkB depend-ent apoptotic pathway in pancreatic islet cells by hypoxia. Islets. 2009;1(1):19–25. doi: 10.4161/isl.1.1.8530. [DOI] [PubMed] [Google Scholar]
- 10.Chen J., Saxena G., Mungrue I.N., Lusis A.J., Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes. 2008;57(4):938–944. doi: 10.2337/db07-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanschmann E.M., Godoy J.R., Berndt C., Hudemann C., Lillig C.H. Thioredoxins, glutaredoxins, and peroxiredoxins–molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxidants Redox Signal. 2013;19(13):1539–1605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikami Y., Shibuya N., Kimura Y., Nagahara N., Ogasawara Y., Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 2011;439(3):479–485. doi: 10.1042/BJ20110841. [DOI] [PubMed] [Google Scholar]
- 13.Hirota K. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J. Biol. Chem. 1999;274(39):27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 14.Rubartelli A., Bajetto A., Allavena G., Wollman E., Sitia R. Secretion of thioredoxin by normal and neo-plastic cells through a leaderless secretory pathway. J. Biol. Chem. 1992;267(34):24161–24164. [PubMed] [Google Scholar]
- 15.Laurent T.C., Moore E.C., Reichard P. Enzymatic synthesis of deoxyribonucleotides. IV. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli B. J. Biol. Chem. 1964;239:3436–3444. [PubMed] [Google Scholar]
- 16.Abate C., Patel L., Rauscher F.J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 17.Berggren M.I., Husbeck B., Samulitis B., Baker A.F., Gallegos A., Powis G. Thioredoxin peroxidase-1 (peroxiredoxin-1) is increased in thioredoxin-1 transfected cells and results in enhanced protection against apoptosis caused by hydrogen peroxide but not by other agents including dexamethasone, etoposide, and doxorubicin. Arch. Biochem. Biophys. 2001;392(1):103–109. doi: 10.1006/abbi.2001.2435. [DOI] [PubMed] [Google Scholar]
- 18.Saitoh M. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17(9):2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powis G., Kirkpatrick D.L., Angulo M., Baker A. Thioredoxin redox control of cell growth and death and the effects of inhibitors. Chem. Biol. Interact. 1998;111–112:23–34. doi: 10.1016/s0009-2797(97)00148-8. [DOI] [PubMed] [Google Scholar]
- 20.Steinbrenner H., Speckmann B., Klotz L.O. Selenoproteins: antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016;595:113–119. doi: 10.1016/j.abb.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Chou F.C., Sytwu H.K. Overexpression of thioredoxin in islets transduced by a lentiviral vector pro-longs graft survival in autoimmune diabetic NOD mice. J. Biomed. Sci. 2009;16:71. doi: 10.1186/1423-0127-16-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 23.Lee K.U. Preferential infiltration of macrophages during early stages of insulitis in diabetes-prone BB rats. Diabetes. 1988;37(8):1053–1058. doi: 10.2337/diab.37.8.1053. [DOI] [PubMed] [Google Scholar]
- 24.Lingwal N., Padmasekar M., Samikannu B., Bretzel R.G., Preissner K.T., Linn T. Inhibition of gelatinase B (matrix metalloprotease-9) activity reduces cellular inflammation and restores function of transplanted pancreatic islets. Diabetes. 2012;61(8):2045–2053. doi: 10.2337/db11-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banaei-Bouchareb L. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J. Leukoc. Biol. 2004;76(2):359–367. doi: 10.1189/jlb.1103591. [DOI] [PubMed] [Google Scholar]
- 26.Brandhorst H., Brandhorst D., Hering B.J., Bretzel R.G. Significant progress in porcine islet mass isola-tion utilizing liberase HI for enzymatic low-temperature pancreas digestion. Transplantation. 1999;68(3):355–361. doi: 10.1097/00007890-199908150-00006. [DOI] [PubMed] [Google Scholar]
- 27.Lundberg M. Methodological aspects of ELISA analysis of thioredoxin 1 in human plasma and cerebrospinal fluid. PloS One. 2014;9(7) doi: 10.1371/journal.pone.0103554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godoy J.R. Redox atlas of the mouse. Immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochim. Biophys. Acta. 2011;1810(1):2–92. doi: 10.1016/j.bbagen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Chen C., Kuehn R., Bretzel R.G., Linn T. Anti-inflammatory thalidomide improves islet grafts survival and functions in a xenogenic environment. PloS One. 2009;4(7) doi: 10.1371/journal.pone.0006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro A.M. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 31.Bingley P.J. Standardization of the IVGTT to predict IDDM. Diabetes Care. 1992;15(10):1313–1316. doi: 10.2337/diacare.15.10.1313. [DOI] [PubMed] [Google Scholar]
- 32.Marcelli-Tourvieille S., Hubert T., Pattou F., Vantyghem M.C. Acute insulin response (AIR): review of protocols and clinical interest in islet transplantation. Diabetes Metab. 2005;32(4):295–303. doi: 10.1016/s1262-3636(07)70283-5. [DOI] [PubMed] [Google Scholar]
- 33.Xenos E.S. The role of nitric oxide in IL-1 beta-mediated dysfunction of rodent islets of Langerhans. Implications for the function of intrahepatic islet grafts. Transplantation. 1994;57(8):1208–1212. doi: 10.1097/00007890-199404270-00012. [DOI] [PubMed] [Google Scholar]
- 34.Lenzen S., Drinkgern J., Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996;20(3):463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 35.Hong K., Xu G., Grayson T.B., Shalev A. Cytokines regulate β-cell thioredoxin-interacting protein (TXNIP) via distinct mechanisms and pathways. J. Biol. Chem. 2016;291(16):8428–8439. doi: 10.1074/jbc.M115.698365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rani S. Decreasing Txnip mRNA and protein levels in pancreatic MIN6 cells reduces reactive oxygen species and restores glucose regulated insulin secretion. Cell. Physiol. Biochem. 2010;25(6):667–674. doi: 10.1159/000315086. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J., Damdimopoulos A.E., Spyrou G., Brüne B. Thioredoxin 1 and thioredoxin 2 have opposed regulatory functions on hypoxia-inducible factor-1alpha. J. Biol. Chem. 2007;282(10):7482–7490. doi: 10.1074/jbc.M608289200. [DOI] [PubMed] [Google Scholar]
- 38.Puri S., Cano D.A., Hebrok M. A role for von Hippel-Lindau protein in pancreatic beta-cell function. Diabetes. 2009;58(2):433–441. doi: 10.2337/db08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asami K. Thioredoxin-1 attenuates early graft loss after intraportal islet transplantation in mice. PloS One. 2013;8(8) doi: 10.1371/journal.pone.0070259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jikimoto T., Nishikubo Y., Koshiba M. Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Mol. Immunol. 2002;38(10):765–772. doi: 10.1016/s0161-5890(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 41.Kakisaka Y. Elevation of serum thioredoxin levels in patients with type 2 diabetes. Horm. Metab. Res. 2002;34(3):160–164. doi: 10.1055/s-2002-23201. [DOI] [PubMed] [Google Scholar]
- 42.Checconi P. Redox proteomics of the inflammatory secretome identifies a common set of redoxins and other glutathionylated proteins released in inflammation, influenza virus infection and oxidative stress. PloS One. 2015;10(5) doi: 10.1371/journal.pone.0127086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu S.Z. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451(7174):69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brixel L.R. TRPM5 regulates glucose-stimulated insulin secretion. Pflügers Archiv. 2010;460(1):69–76. doi: 10.1007/s00424-010-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skrzypski M. Activation of TRPV4 channel in pancreatic INS-1E beta cells enhances glucose-stimulated insulin secretion via calcium-dependent mechanisms. FEBS Lett. 2013;587(19):3281–3287. doi: 10.1016/j.febslet.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 46.Schwertassek U. Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin-1. EMBO J. 2007;26(13):3086–3097. doi: 10.1038/sj.emboj.7601746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azimi I., Matthias L.J., Center R.J., Wong J.W., Hogg P.J. Disulfide bond that constrains the HIV-1 gp120 V3 domain is cleaved by thioredoxin. J. Biol. Chem. 2010;285(51):40072–40080. doi: 10.1074/jbc.M110.185371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plugis N.M., Palanski B.A., Weng C.H., Albertelli M., Khosla C. Thioredoxin-1 selectively activates transglutaminase 2 in the extracellular matrix of the small intestine: implications for celiac disease. J. Biol. Chem. 2017;292(5):2000–2008. doi: 10.1074/jbc.M116.767988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kondo N. Redox sens-ing release of human thioredoxin from T lymphocytes with negative feedback loops. J. Immunol. 2004;172(1):442–448. doi: 10.4049/jimmunol.172.1.442. [DOI] [PubMed] [Google Scholar]
- 50.Kondo N. Lipid raft-mediated uptake of cysteine-modified thiore-doxin-1: apoptosis enhancement by inhibiting the endogenous thioredoxin-1. Antioxidants Redox Signal. 2007;9(9) doi: 10.1089/ars.2007.1665. 1439-1348. [DOI] [PubMed] [Google Scholar]
- 51.Xia F. Disruption of pancreatic beta-cell lipid rafts modifies Kv2.1 channel gating and insulin exocytosis. J. Biol. Chem. 2004;279(23):24685–24691. doi: 10.1074/jbc.M314314200. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi N. Thioredoxin reduces C-C chemokine-induced chemotaxis of human eosinophils. Allergy. 2009;64(8):1130–1135. doi: 10.1111/j.1398-9995.2009.01969.x. [DOI] [PubMed] [Google Scholar]
- 53.Liu W. Thioredoxin-1 ameliorates myosin-induced autoimmune myocarditis by suppressing chemokine expressions and leukocyte chemotaxis in mice. Circulation. 2004;110(10):1276–1283. doi: 10.1161/01.CIR.0000141803.41217.B6. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura H. Circulating thioredoxin suppresses lipopolysaccharide-induced neutrophil chemotaxis. Proc. Natl. Acad. Sci. U. S. A. 2001;98(26):15143–15148. doi: 10.1073/pnas.191498798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ro H., Lee E.W., Hong J.H. Roles of islet Toll-like receptors in pig to mouse islet xenotransplantation. Cell Transplant. 2013;22(9):1709–1722. doi: 10.3727/096368912X657684. [DOI] [PubMed] [Google Scholar]
- 56.Pagliei S. Thioredoxin specifically cross-desensitizes monocytes to MCP-1. Eur. Cytokine Netw. 2002;13(2):261–267. [PubMed] [Google Scholar]
- 57.Tao L. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation. Proc. Natl. Acad. Sci. U. S. A. 2004;101(31):11471–11476. doi: 10.1073/pnas.0402941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King B.C., Nowakowska J., Karsten C.M., Köhl J., Renström E., Blom A.M. Truncated and full-length thioredoxin-1 have opposing activating and inhibitory properties for human complement with relevance to endothelial surfaces. J. Immunol. 2012;188(8):4103–4112. doi: 10.4049/jimmunol.1101295. [DOI] [PubMed] [Google Scholar]
- 59.Pekkari K., Gurunath R., Arner E.S., Holmgren A. Truncated thioredoxin is a mitogenic cytokine for resting human peripheral blood mononuclear cells and is present in human plasma. J. Biol. Chem. 2000;275(48):37474–37480. doi: 10.1074/jbc.M001012200. [DOI] [PubMed] [Google Scholar]
- 60.Pekkari K., Holmgren A. Truncated thioredoxin: physiological functions and mechanism. Antioxidants Redox Signal. 2004;6(1):53–61. doi: 10.1089/152308604771978345. [DOI] [PubMed] [Google Scholar]
- 61.Sahaf B. Thioredoxin expression and localization in human cell lines: detection of full-length and truncated species. Exp. Cell Res. 1997;236(1):181–192. doi: 10.1006/excr.1997.3699. [DOI] [PubMed] [Google Scholar]
- 62.Garay-Malpartida H.M., Mourão R.F., Mantovani M., Santos I.A., Sogayar M.C., Goldberg A.C. Toll-like receptor 4 (TLR4) expression in human and murine pancreatic beta-cells affects cell viability and insulin homeostasis. BMC Immunol. 2011;12:18. doi: 10.1186/1471-2172-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vives-Pi M. Evidence of expression of endotoxin receptors CD14, toll-like receptors TLR4 and TLR2 and associated molecule MD-2 and of sensitivity to endotoxin (LPS) in islet beta cells. Clin. Exp. Immunol. 2003;133(2):208–218. doi: 10.1046/j.1365-2249.2003.02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Witek-Janusek L., Filkins J.P. Insulin-like action of endotoxin: antagonism by steroidal and nonsteroidal anti-inflammatory agents. Circ. Shock. 1981;8(5):573–583. [PubMed] [Google Scholar]
- 65.Acosta-Montaño P. Fatty acid and lipopolysaccharide effect on beta cells proteostasis and its impact on insulin secretion. Cells. 2019;8(8) doi: 10.3390/cells8080884. Pii: E884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.