Abstract
Background
Many neuroprotective approaches targeting neurons in animal models fail to provide benefits for the treatment of ischemic stroke in clinic and glial cells have become the targets in some basic studies. Baicalin has neuroprotective effects but the mechanisms related to glial cells are not revealed. This study investigated whether and how baicalin can combat excitotoxicity via protecting the functions of astrocytes in early stage of ischemia/reperfusion (I/R) insult by focusing on glutamine synthetase (GS).
Experimental approach
The role of baicalin was explored in primary astrocytes exposed to oxygen-glucose deprivation/reperfusion (OGD/R) and rats subjected to middle cerebral artery occlusion/reperfusion (MCAO/R).
Key results
Mitochondrial succinate dehydrogenase (SDH) activation led to an excessive production of reactive oxygen species (ROS) via reverse electron transport (RET) under conditions of OGD/R or I/R, which increased the carbonylation and proteasomal degradation of GS in astrocytes. Treatment of baicalin decreased the oxidative stress mediated by SDH and reduced the subsequent loss of GS. This effect increased the glutamate disposal by astrocytes and protected neurons from excitotoxicity in response to I/R insults.
Conclusions and implications
Baicalin inactivated SDH to suppress ROS production and protected GS protein stability against oxidative stress, contributing to the improvement of the glutamate disposal and decrease in excitotoxicity. These results suggest that protection of GS stability in astrocytes might be an effective strategy to prevent neuronal injury in acute ischemic stroke.
Keywords: Succinate dehydrogenase, Glutamine synthetase, Oxidative stress, Proteasomal degradation, Glutamate excitotoxicity, Baicalin
Graphical abstract
Highlights
-
•
SDH activation induced the excessive ROS production during early reperfusion.
-
•
Activated SDH-induced GS degradation by 20S proteasome impaired glutamate disposal.
-
•
Baicalin inactivated SDH, decreased GS loss and suppressed excitotoxicity.
No-standard abbreviations
- DMEM
Dulbecco's modified eagle medium
- FBS
fetal bovine serum
- GS
glutamine synthetase
- I/R
ischemia/reperfusion
- MCAO
middle cerebral artery occlusion
- MSO
methionine sulfoximine
- NAC
N-acetyl-L-cysteine
- OGD
oxygen-glucose deprivation
- OGD/R
oxygen-glucose deprivation/reperfusion
- ROS
reactive oxygen species
- SDH
succinate dehydrogenase
- TBST
Tris Buffered Saline with Tween-20
1. Introduction
Glutamate excitotoxicity is one of the facing challenges in the management of acute ischemic stroke and is a key target for increasing neuron survival [1]. Neuroprotection is an attractive strategy for the treatment of cerebral ischemic stroke, and many studies are focused on neurons to minimize the detrimental effects of brain ischemia [2]. Unfortunately, for decades, this strategy fails to provide benefits in clinical trials although it is promising in experimental studies [1,2]. In the brain, neurons structurally interact with glial cells and the cellular communication is essential for the functional integrity. Thus, considering cellular interaction into neuroprotection strategy may be more sufficient to salvage neurons and improve neurological outcome [3,4]. Astrocytes are the most abundant subtype of glial cells and maintain brain functions in multifaceted domains via the communication with neurons [5]. In response to neurotransmitter release from neurons, astrocytes integrate the synapse formation and modulate the synaptic signaling via gliotransmitter release [6]. As structural elements of gap junctions and the blood brain barrier, astrocytes maintain brain homeostasis [[7], [8], [9]]. Glutamate is the excitatory neurotransmitter released from neurons, while astrocytes take up glutamate and mediate the conversion to glutamine, ensuring glutamate-glutamine cycle between neurons and astrocytes [10,11].
Glutamine synthetase (GS, also called glutamate-ammonia ligase) is present primarily in astrocytes in the central nervous system (CNS) and acts as a key player in glutamate-glutamine cycle [12]. GS synthetizes glutamine using glutamate and ammonia in an ATP-dependent manner, contributing to the maintenance of neurotransmitter homeostasis by promoting glutamate clearance. For this, alterations in GS expression and activity are associated with neurological dysfunctions such as epilepsy and Alzheimer's disease [13]. GS activity is altered during ischemic stroke, but the cause and resulting consequence are poorly understood [14]. In Schwann cells and liver tissue GS protein is sensitive to proteasomal degradation in response to oxidative stress [[15], [16], [17]]. Because oxidative stress is associated with ischemic brain injury, these events suggest that reactive oxygen species (ROS) may be a cause for impaired GS activity and subsequent glutamate accumulation implicating in excitotoxicity. Reconstruction of blood supply is required to limit brain injury. However, reperfusion induces further damage due to excessive ROS production. Recently, it is found that the reversal succinate dehydrogenase (SDH)-mediated succinate accumulation is a common metabolic signature of ischemia, and accumulated succinate in turn triggers SDH activation during the early reperfusion to drive excessive mitochondrial ROS production owing to reverse electron transport (RET) [18]. In the context, astrocyte SDH deregulation and oxidative degradation of GS might converge in ischemic stroke, serving as potential targets for finding alternative approaches to combating glutamate excitotoxicity.
Baicalin is a flavonoid isolated from Scutellaria baicalensis Georgi, an important traditional Chinese medicine widely used for thousands of years [19]. It is also an active ingredient of several Chinese Traditional Medicine compounds ever used for the treatment of ischemic stroke in clinic [20,21]. A growing body of evidence has shown the beneficial roles of baicalin in stroke management, such as the anti-oxidant and anti-excitotoxic effects [19,20,22,23]. However, the mechanisms by which it provides neuroprotective benefits are still needed to addressed. In this study, we investigated the neuroprotective effects of baicalin with focus on the regulation of GS in astrocytes in the setting of ischemia-reperfusion insult. Our results show that protecting GS in astrocytes from 20S proteasomal degradation via suppression of SDH activity contributes to the anti-excitotoxic effect of baicalin, and GS-based neuroprotection might be a promising approach for the treatment of acute ischemic stroke.
2. Methods
2.1. Materials
Baicalin (purity≥98%) was obtained from Nanjing Zelang Biological Technology Co., Ltd (Nanjing, Jiangsu, China). Dimethyl succinate (hereafter referred as to succinate) and dimethyl malonate (hereafter referred as to malonate) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Glucose-free DMEM was purchased from Gibco (Grand Island, NY, USA). Mito-TEMPO and MG-132 were the products of MedChemExpress LLC (Monmouth Junction, NJ 08852, USA). N-acetylcysteine (NAC, MB1735) was purchased from Dalian Meilun Biotechnology (Dalian, China). Mouse monoclonal antibody anti-PSMC4 (G-4) (a component of 19S, sc-166115) was obtained from Santa Cruz Biotechnology, Inc (St. Paul, MN, USA). Anti-β-actin, anti-glutamine synthetase, Goat anti-Rabbit IgG (H + L) HRP (BS13278) were obtained from Bioworld Technology (St. Paul, MN, USA). Anti-Proteasome 20S LMP2 antibody (ab243556) was purchased from Abcam (Abcam, Shanghai, China). OxiSelect™ Protein Carbonyl Immunoblot Kit was the product of Cell BioLabs, Inc. (San Diego, CA, USA) and obtained from Shanghai office of Beijing XMJ Scientific Co., Ltd (Shanghai, China). RIPA lysis buffer (P0013), Protein A + G agarose (Fast Flow), DCFH-DA assay kit (S0033) and BCA protein assay kit (P0012) were purchased from Beyotime Institute of Biotechnology (Shanghai, China). MSO (Methionine sulfoximine) was obtained from Solarbio Biotechnology (Beijing, China).
2.2. Animals
Male Sprague-Dawley (SD) rats (250 ± 20 g), purchased from the Laboratory Animal Center of Qinglongshan (Nanjing, China), were housed in colony cages 12 h light/dark cycles with free access to food and water. The animal care and experimental procedures were approved by Animal Ethics Committee of China Pharmaceutical University.
2.3. Cell preparation and culture
The primary rat astrocytes were isolated from the cerebral cortices of 1-day-old Sprague-Dawley (SD) rats according to previously described procedures [24]. Briefly, cerebral cortices were removed, cleaned of meninges, cut into 1 mm3 pieces, and digested with 0.25% trypsin (Ameresco, USA) at 37 °C for 10 min prior to the addition of culture media (DMEM) (31600, Gibco, USA) containing 10% fetal bovine serum (FBS) (10270-106, Gibco, USA). Then, the suspension was filtered through a 75 μm cell strainer and centrifuged at 1000 rpm for 10 min. The pellet was resuspended in complete medium (DMEM containing 10% fetal bovine serum, 2 mmol/L l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin). The cells were seeded in culture flasks at a density of 3 × 104 cells/ml medium. After 9-11 days, when the primary culture reached confluence, the culture flasks were shaken at 250 rpm on an orbital shaker (ZQLY-180 N, Zhicu Instrument, Shanghai, China) at 37 °C for 12 h to remove microglia and oligodendrocytes. The attached cells were digested with 0.25% trypsin and transferred to new flasks at a density of 2 × 106/ml. After growing to 80% confluence, the cells were used for experiments. The astrocytes were identified with GFAP staining to ensure purity. Cultured cells were starved for 30 min in serum-free medium before experiments [24].
Primary cortical neurons were prepared from brains of newborn pups on postnatal day 1. Briefly, cerebral cortices were dissected and removed of the meninges. The tissue was minced into small pieces and digested in 10 ml 0.125% trypsin at 37 °C for 20 min. After addition of 20% FBS, the suspension was filtered through a 70 μm cell strainer and centrifuged at 1200 rpm for 5 min. The pellet was suspended in DMEM containing 10% FBS and filtered again through a 70 μm cell strainer before cells were seeded at 3 × 105/well into the upper chamber of transwell insert of 6-well culture plates. After 24 h and every 2 days thereafter, the medium was replaced by B27-Neurobasal medium (Gibco, Grand Island, USA) containing 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM glutamine. After 5-7 days, neurons were used for the co-culture experiments [25].
2.4. Astrocytes viability assay
Cell viability was determined using the CCK-8 assay kit (Dojindo Laboratories, Japan). In brief, astrocytes were grown in 96-well plates (1 × 104 cells/well) overnight, then astrocytes were pretreated with baicalin or malonate for 30 min, then subjected to OGD via incubation in glucose-free DMEM under hypoxia (1% O2, 5% CO2 and 94% N2) at 37 °C for 6 h, with or without baicalin (1 μmol/L, 10 μmol/L) or malonate (5 mmol/L). Cells were then reperfused (R) via incubation in normal DMEM containing baicalin or malonate under 95% air and 5% CO2 for 30 min prior to treatment with CCK-8 solution for 1 h at 37 °C. The absorbance at 450 nm was measured using a microplate reader (Thermo Varioskan LUX, MA, USA). Cell viability was expressed as absorbance values.
2.5. Assay of cytosolic ROS production
Astrocytes were grown in the 96-well plates (1 × 104 cells/well), then astrocytes were pretreated with baicalin or malonate for 30 min before treated with succinate (5 mmol/L) for 2 h or subjected to OGD/R (6 h/30 min) with or without baicalin (1 μmol/L, 10 μmol/L), malonate (5 mmol/L) or mito-TEMPO (15 nmol/L). The intracellular ROS generation was determined by measuring the oxidative conversion of cell permeable DCFH-DA (10 μmol/L) to fluorescent dichlorofluorescein. The fluorescence was detected by a microplate reader (Thermo Varioskan LUX, MA, USA) at an excitation wavelength 488 nm and an emission wavelength 525 nm.
2.6. Assay of mitochondrial ROS production
Astrocytes were seeded in the 4-well glass plates (2 × 105 cells/well) and pretreated with baicalin or malonate for 30 min, then exposed to succinate for 2 h or OGD/R for 6 h/30 min with or without baicalin (10 μmol/L), malonate (5 mmol/L) or mito-TEMPO (15 nmol/L) as described above. Cells were rinsed with PBS for three times and incubated with 5 μmol/L MitoSOX™ Red (Molecular Probes, Life Technologies, Carlsbad, CA, USA) and 50 nmol/L Mito -Green (Beyotime, Shanghai, China) for 30 min. The levels of mitochondrial ROS were viewed by a confocal scanning microscopy (Zeiss LSM 700, Jena, Germany) at an excitation wavelength 488 nm and an emission wavelength 525 nm.
2.7. Protein quantitative detection
Pierce Bicinchoninic Acid (BCA) protein assay was used to measure the protein levels of astrocytes and brain tissues. The absorbance was read at 562 nm using a microplate reader (Thermo Varioskan LUX, MA, USA).
2.8. Measurement of SDH and GS activity
Astrocytes were grown in 6-well plates (1 × 106 cells/well) and pretreated with baicalin or malonate for 30 min, then exposed to succinate for 2 h or subjected to OGD/R for 6 h/30 min with or without baicalin (0.1, 0.5, 1, 10, 100 μmol/L), malonate (5 mmol/L) or mito-TEMPO (15 nmol/L). For rats subjected to MCAO (2 h), the right brain cortices were dissected at 30 min after reperfusion. Cell or tissue proteins were extracted with RIPA lysis buffer. Lysates were centrifuged and supernatants were collected for next analysis. The activities of SDH and GS were measured by visible spectrophotometry using the SDH assay kit (Jiancheng Bioengineering Institute, Nanjing, China) and GS assay kit (Solarbio, Beijing, China) respectively, according to the manufacturer's instructions.
2.9. Measurement of succinate content
Astrocytes were grown in the 6-well plates (1 × 106 cells/well) and pretreated with baicalin (1 μmol/L, 10 μmol/L) or malonate (5 mmol/L) for 30 min followed by exposure to OGD in the presence of baicalin or malonate for 6 h. In vivo, rats were subjected to MCAO/R for 2 h/30 min and received intraperitoneal (i.p.) injection of baicalin (50 mg/kg) or malonate (80 mg/kg) 30 min before reperfusion. Then rats were killed and the right cerebral cortices were isolated. Cell or tissue proteins were extracted with RIPA lysis buffer and the lysates were centrifuged for collecting supernatants. Succinate contents were measured using the succinate assay kit (MB-5831A, Jiangsu Meibiao Company) according to the manufacturer's instructions.
2.10. Measurement of glutamate, glutamine and carbonyls content
Astrocytes were pretreated with baicalin or malonate for 30 min, then exposed to succinate for 2 h or subjected to OGD/R for 6 h/30 min with or without baicalin, malonate or MSO. Cells were washed with PBS and then stimulated with 2 mmol/L glutamate for 2 h. The culture media were harvested for the measurement of glutamate using the commercial assay kit (Jiancheng Bioengineering Institute, Nanjing, China). Cells were washed twice, lyzed with RIPA lysis buffer, and centrifuged at 12000 g for 10 min. Glutamine contents in the supernatant was measured using the glutamine assay kit (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
For the culture of brain tissues, the frontal cortices separated from normal adult rats were cut into several pieces (0.4 mm) and cultured in 12-well plates. The tissues were pretreated with baicalin or malonate for 30 min before subjected to OGD in an incubator with 1% O2, 5% CO2 and 94% N2, at 37 °C for 2 h and re-oxygenized with baicalin or malonate under 5% CO2 and 95% air at 37 °C for 30 min. The glutamate level in media was measured with the glutamic acid assay kit as described above. The tissues were homogenized with RIPA lysis buffer and centrifuged at 12000 g for 10 min. The total carbonyls in supernatants were measured using the commercial kits for carbonyls assay (Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer's instructions.
2.11. Neuron viability assay
Cell viability was determined using the CCK-8 assay kit. Before co-culture, astrocytes were grown in 6-well plates (5 × 106 cells/well) and neurons cultured on the transwell insert of 6-well culture plates (5 × 105 cells/well) independently. Astrocytes were first exposed to OGD for 4 h with or without baicalin or MSO, and finally co-cultured with neurons seeded on the inserts. The solo or co-cultured neurons were subjected to OGD/R (2 h/0.5 h) or incubated in DMEM under 95% air and 5% CO2 for 2.5 h. Neurons on inserts were then incubated with CCK-8 solution for 1 h at 37 °C. The absorbance at 450 nm was measured using a microplate reader (Thermo Varioskan LUX, MA, USA).
To assess the role of astrocytes on glutamate-induced impairment in neurons, astrocytes were first treated with 5 mmol/L succinate for 2 h or exposed to OGD/R for 6 h/30 min in 6-well plates (5 × 106 cells/well), with or without baicalin or MSO, then washed with PBS prior to co-culture with neurons seeded on the inserts of upper chamber (5 × 105 cells/well). All the co-cultured systems were then treated with 2 mmol/L glutamate for 2 h. The survival of neurons cultured on inserts was measured by CCK-8 method as above.
2.12. Ischemia/reperfusion (I/R) model and drug treatment
Middle cerebral artery occlusion (MCAO) is currently the most common stroke model [26]. The procedure was carried out as previously reported with minor modifications [27]. Briefly, rats were divided into 4 groups: sham, model, model group received baicalin (50 mg/kg) or malonate (80 mg/kg). After anaesthesia, the right common carotid artery (CCA) was exposed and the external carotid artery (ECA) and internal carotid artery (ICA) were isolated. A monofilament nylon suture (No. 2636-A4, Beijing Xinontech Co., Ltd., Beijing, China) was introduced into the right ICA through the ECA and then advanced into ICA for approximately 20 mm until mild resistance was encountered. Body temperature of rats was maintained at 37 ± 0.5 °C throughout the experiment. After 2 h, the suture was removed from ICA for reperfusion (for 30 min or 24 h) and the ECA stump was tied off with a short suture kept in it. For rats in sham group, the procedure was similar except that the monofilament nylon suture was not inserted into ICA. 30 min before reperfusion, rats in sham and model groups were i. p. injected with normal saline (5 ml/kg), and the other two groups received an i. p. injection of 50 mg/kg baicalin and 80 mg/kg malonate respectively.
2.13. Longa's 5-point score
After MCAO (2 h) and reperfusion for 24 h, neurological functions were evaluated according to Longa's 5-point scoring method [28]. The scoring criteria were as follows: 0, no neurological deficits; 1, failure to fully extend the contralateral forepaw; 2, circling to the opposite side; 3, falling to the opposite side; 4, failure to walk spontaneously, loss of consciousness.
2.14. Infarct volume evaluation
2,3,5-Triphenyltetrazolium chloride (TTC, Yeasen, Shanghai, China) was utilized to quantify the infarct volume [27]. Brains were isolated after 24 h-reperfusion, frozen for 20 min, and carefully cut into 2-mm coronal slices. The brain slices were immersed in 1% TTC at 37 °C for 30 min in the dark and fixed in 4% paraformaldehyde for 2 h. The TTC staining was recorded by a blind observer using a digit camera, infarct area fraction was quantified with Image J software.
2.15. Measurement of H2O2 content in brain tissue
Frontal brain cortical tissues were isolated after reperfusion for 30 min and the proteins were extracted with RIPA lysis buffer. Lysates were centrifuged for the collection of supernatants. The content of H2O2 in the supernatant was measured with H2O2 assay kit (Jiancheng Bioengineering Institute, Nanjing, China) and normalized to the protein level.
2.16. RNA extraction and qPCR
RNA was isolated from astrocytes using the Trizol Total (Invitrogen, CA, USA) and transcribed into cDNA using the PrimeScript™ RT reagent Kit (Yeasen, Shanghai, China) with gDNA Eraser. The quality and quantity of the extracted RNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). The qPCR was performed using the Stratagene Mx3000P system (Agilent Technologies) with the SYBR® Green PCR Core Reagents kit (Yeasen, Shanghai, China) in accordance with the manufacturer's instructions. The relative mRNA expression levels of GS were measured by qPCR and standardized to that of β-actin. The primers for β-actin (F: 5′-GCAAGTGCTTCTAGGCGGAC-3'; R: 5′-AAGAAAGGGTGTAAAACGCAGC-3′) and GS (F: 5′-GTTCCCACTTGAACAAAGGCA-3'; R: 5′-ACCCAGATATACATGGCTTGGA-3′) were used for PCR amplification by the following steps: enzyme activation at 95 °C for 5 min, 95 °C for 10 s of denaturing, annealing and extending for 30 s at 60 °C and the last two steps were repeated 40 cycles. The 2−ΔΔCt method was used for relative quantification of the target gene.
2.17. Western blot analysis and immunoprecipitation
Brain tissue and cell protein were extracted with RIPA lysis buffer. The lysates were centrifuged and the supernatants were collected. After quantification, the protein was resolved by 10-12% SDS-PAGE, transferred onto PVDF membranes. The membranes were blocked with 5% non-fat dry milk prepared in TBST, incubated with primary antibodies and then with the corresponding secondary antibodies. The relative expression level of the target protein was normalized to β-actin. The antibody reactivity was then detected by ECL (Yeasen, Shanghai, China) and quantified with Image J software.
For immunoprecipitation, lysates of primary rat astrocytes or rat brain tissues were centrifuged, the supernatants were collected and incubated with the anti-GS antibody or the anti-19S antibody overnight at 4 °C before being mixed with protein A + G agarose beads. The mixture was incubated at 4 °C under rotary agitation for 4 h. After washing with lysis buffer, the Ag-Ab complex was eluted from the beads by boiling samples in loading buffer with denaturant SDS according to the protocols. Target protein expressions of 19S, 20S, GS and β-actin were analyzed by Western blotting as described above. The total carbonyls and the carbonylated GS were also measured by Western blotting using 2,4-dinitrophenylhydrazine (DNPH) and anti-DNP system in OxiSelect™Protein Carbonyl Immunoblot Kits. The values of band intensities were developed with ECL enhanced chemiluminescence (Yeasen, Shanghai, China) and quantized by Image-ProPlus 6.0 software (Rockville, MD, USA).
2.18. Statistical analysis
Data were expressed as mean ± SD (standard deviation) of at least five independent experiments except that having been indicated. Statistical analysis was performed using GraphPad Prism 6.0 software. One-way ANOVA followed by Tukey test were used to compare the statistical significance of differences between the samples and their respective controls. A value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. Baicalin suppresses mitochondrial ROS production in astrocytes by SDH inactivation
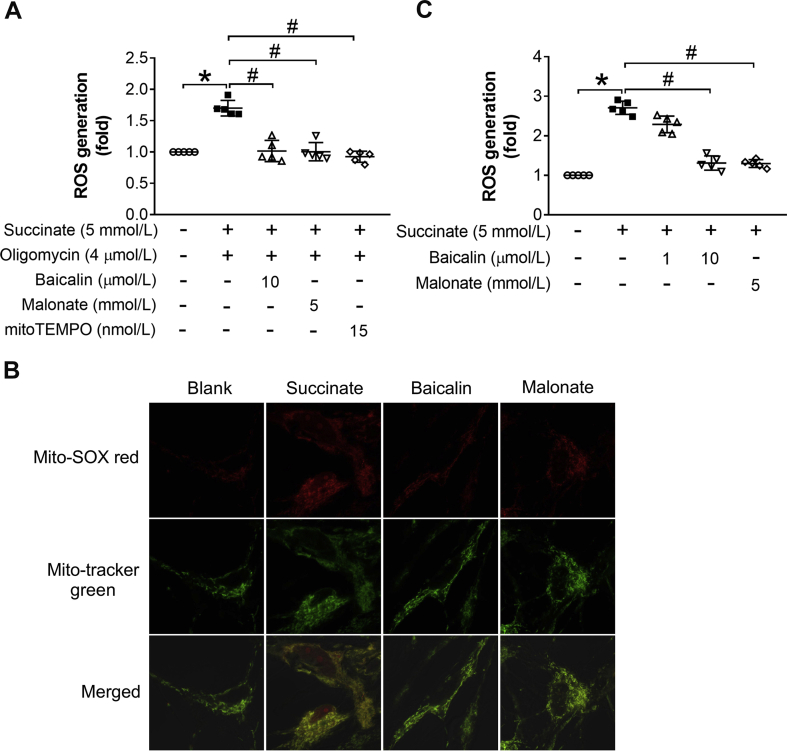
We first examined the potential role of baicalin in primary rat astrocytes and found that baicalin protected astrocyte survival from OGD/R insult in a concentration-dependent manner (Fig. 1A). By using Mito-SOX red probe, we found baicalin effectively suppressed mitochondrial ROS production in response to reoxygenation (Fig. 1B). In addition, intracellular ROS was also reduced by baicalin and mito-TEMPO (Fig. 1C). SDH activation is a driving force for ROS production upon reoxygenation owing to RET [18]. When membrane-permeable succinate was added as a substrate to activate SDH in astrocytes, baicalin significantly inhibited SDH activity and the IC50 was 0.9125 μmol/L (Fig. 1D). During ischemia, SDH activation in reverse induces succinate accumulation, which promotes ROS production upon reperfusion owing to RET [18]. As expected, baicalin prevented succinate accumulation in astrocytes subjected to OGD (Fig. 1E). To mimic ROS production from RET, we treated astrocytes with succinate to activate SDH when ATP synthase was inhibited by oligomycin A. We observed that baicalin markedly suppressed the mitochondrial and intracellular ROS production (Fig. 1F, Suppl. Fig. 1A). Baicalin also effectively reduced the mitochondrial and intracellular ROS levels when cells were stimulated with succinate alone (Suppl. Fig. 1B and C). Like baicalin, SDH inhibitor malonate protected cell survival and suppressed ROS production. Together, these results indicate that baicalin, likely by inactivating SDH, reduced succinate accumulation during ischemia and suppressed ROS production upon reoxygenation.
Fig. 1.
Baicalin protects astrocytes from OGD/R injury by SDH inactivation. (A) Astrocyte survival after OGD/R (n = 5 each group); (B) The fluorescence intensity of mitochondrial ROS in astrocytes exposed to OGD/R (n = 5 each group); (C) ROS production in the cytosol in response to OGD/R (n = 5 each group); (D) SDH inhibition by baicalin and the IC50 (n = 5 each group); (E) Succinate accumulation in astrocytes after OGD (n = 5 each group); (F) The fluorescence intensity of mitochondrial ROS in response to combination treatment with succinate and oligomycin A (n = 5 each group). Data were showed as mean ± SD. OGD/R, oxygen glucose deprivation/reperfusion; ROS, reactive oxygen species; SDH, succinate dehydrogenase. *p < 0.05 vs blank group (without any treatment), #p < 0.05 vs control group (with OGD or OGD/R).
3.2. Baicalin promotes glutamate uptake by astrocytes
Intact astrocytes can attenuate excitotoxicity by taking up the released glutamate to prevent extracellular glutamate accumulation. During cerebral ischemia, excessive glutamate is released from neurons and even astrocytes and triggers excitotoxicity because glutamate released cannot be removed effectively [29,30]. Here, OGD/R insult increased the extracellular glutamate content in the media of cultured brain tissues, which was blunted by baicalin (Fig. 2A). The effect of baicalin was also evidenced by the increased protein expression of GS (Fig. 2B). To confirm the role of astrocytes, we added exogenous glutamate in culture medium and observed that OGD/R impaired the clearance of glutamate by astrocytes; however, baicalin, as well as SDH inhibitor malonate, reduced extracellular glutamate contents with an increased glutamine level in astrocytes (Fig. 2C and D). Similar effects were also seen in cultured astrocytes when succinate was added to activate SDH (Fig. 2E and F). These results suggest that baicalin facilitated glutamate uptake by astrocytes to prevent extracellular accumulation of glutamate.
Fig. 2.
Baicalin promotes glutamate disposal by astrocytes. (A) Glutamate release in cultured brain tissues exposed to OGD/R (n = 5 each group). (B) Protein expression of GS in brain tissues (n = 5 each group); (C) Extracellular glutamate after astrocytes were exposed to OGD/R (n = 5 each group); (D) Intracellular glutamine after astrocytes were exposed to OGD/R (n = 5 each group); (E) Extracellular glutamate after astrocytes were treated with succinate (n = 5 each group); (F) Intracellular glutamine after astrocytes were treated with succinate (n = 5 each group). Data were showed as mean ± SD. GS, glutamine synthetase, OGD/R, oxygen glucose deprivation/reperfusion. *p < 0.05 vs blank group (astrocytes or brain slice without any treatment), #p < 0.05 vs control group (brain slice treated with OGD/R or astrocytes treated with OGD/R or succinate).
3.3. Baicalin improves GS activity in astrocytes and attenuates glutamate excitotoxicity
We next examined the effect of baicalin on GS in cultured astrocytes. OGD/R insult attenuated GS protein expression and impaired GS activity, but these alterations were reversed by baicalin and malonate, respectively (Fig. 3A). Similar regulation was also observed in astrocytes when exposed to succinate (Fig. 3B). Intracellular ROS scavenger N-acetylcysteine (NAC) protected GS expression and activity under both OGD/R and succinate-stimulated conditions, indicative of the involvement of ROS in the impairment of GS (Suppl. Fig. 2A and B). GS mediates the synthesis of glutamine by using glutamate as a substrate and thus promotes glutamate uptake. This was also demonstrated here by using GS inhibitor methionine sulfoximine (MSO). MSO remarkedly increased extracellular glutamate and decreased intracellular glutamine (Fig. 3C and D), which was not relieved by baicalin. In contrast, baicalin showed the ability to counteract the impairment of glutamate disposal caused by OGD/R (Fig. 3C and D). These data suggested baicalin promoted glutamate disposal by improving GS activity, and the increase in GS activity was likely due to the increased expression of GS, not the direct activation of GS by baicalin.
Fig. 3.
Baicalin preserves GS activity in astrocytes and decreases excitotoxicity. (A) Baicalin restored GS protein expression and activity in astrocytes under OGD/R (n = 5 each group); (B) Baicalin increased GS protein expression and activity in astrocytes treated with succinate (n = 5 each group); (C) Extracellular glutamate under OGD/R (n = 5 each group); (D) Intracellular glutamine under OGD/R (n = 5 each group); (E) Neuronal survival in co-cultured system exposed to OGD/R (n = 5 each group); (F) Neurons survival in co-cultured system exposed to glutamate (n = 5 each group). Data were showed as mean ± SD. GS, glutamine synthetase; MSO, methionine sulfoximine; OGD/R, oxygen glucose deprivation/reperfusion. *p < 0.05 vs blank group (astrocytes without any treatment), #p < 0.05 vs control group (astrocytes with OGD/R or succinate).
Given that baicalin promoted glutamate disposal by preserving GS protein and activity in astrocytes, we examined the potential in the protection of neuron survival when neurons were co-cultured with astrocytes. Neurons were sensitive to OGD/R insult, while co-culture with intact astrocytes protected neurons survival (Fig. 3E). However, when astrocytes were pretreated with GS inhibitor MSO or pre-subjected to OGD for 4 h, the protective effect was lost (Fig. 3E), indicating that preserved GS activity in astrocytes was required for neuroprotection, likely due to enhanced glutamate disposal. In contrast, when astrocytes were pretreated with baicalin for 4 h during OGD, the lost neuroprotective function of astrocytes in co-culture was restored (Fig. 3E). In addition, we co-cultured primary neurons with pretreated astrocytes before addition of exogenous glutamate to further demonstrate the protective role of baicalin. The survival of neurons co-cultured with MSO-pretreated astrocytes was decreased dramatically compared with that co-cultured with intact astrocytes (Fig. 3F), again demonstrating the protective role of GS. Succinate or OGD/R-pretreated astrocytes remarkedly decreased neuron survival, while this was rescued by pretreatment of astrocytes with baicalin (Fig. 3F). Together, these results demonstrate that baicalin preserved GS activity in astrocytes was responsible for the neuronal protection.
3.4. Baicalin protects GS by improving the protein stability
Protein levels are generally regulated at transcriptional or post-transcriptional levels. Although OGD/R and succinate reduced GS protein expression, these treatments did not show an obvious impact on the gene expression (Fig. 4A). Baicalin restored GS protein expression (Fig. 3A) without affecting gene expression (Fig. 4A), suggesting the possibility that it protected GS protein at the post-transcriptional levels. Proteasome inhibitor MG-132, but not lysosomal V-ATPase inhibitor bafilomycin A1, prevented the loss of GS protein in astrocytes (Fig. 4B), indicating that OGD/R reduced GS protein expression through proteasomal degradation. For further confirmation, we investigated the GS degradation in succinate-treated astrocytes when protein synthesis was inhibited by cycloheximide. Succinate impaired the stability of GS protein, evidenced by continuous degradation from 2 to 8 h after treatment (Fig. 4C). In contrast, baicalin treatment improved GS stability (Fig. 4C). Because we have shown that the antioxidant NAC could reverse the loss of GS protein, the enhanced GS protein degradation should be a result from ROS production. Consistently, H2O2 treatment concentration-dependently impaired GS protein stability and this was also blocked by MG132 (Suppl. Fig. 3A and B), providing evidence to support our speculation.
Fig. 4.
Baicalin protects GS protein expression by preventing protein degradation. (A) Gene expression (mRNA) for GS in astrocytes under OGD/R (n = 5 each group); (B) GS protein expression in astrocytes under OGD/R (n = 5 each group); (C) GS protein expression in astrocytes treated with succinate (n = 5 each group). Data were showed as mean ± SD. GS, glutamine synthetase; OGD/R, oxygen glucose deprivation/reperfusion. *p < 0.05 vs blank group (without any treatment), #p < 0.05 vs control group (with OGD/R or succinate).
3.5. Baicalin protects GS protein from 20S proteasomal degradation
Because oxidative modification can impair protein stability [14,15], we next examined the influence of baicalin on protein oxidation. Baicalin reduced carbonyl contents in brain tissues subjected to OGD/R insult (Fig. 5A) and attenuated the total protein carbonyls and GS carbonylation in astrocytes exposed to OGD/R (Fig. 5B and C). Malonate and mito-TEMPO also decreased GS carbonylation in astrocytes (Fig. 5B and C). Although it is well known that proteasomal degradation is mainly mediated by the ubiquitin-26S proteasome system, 20S proteasome is proposed to act as a guarder in oxidative stress owing to its ability to degrade oxidized protein [31]. For this, we examined the affinity of GS protein with proteasomes in the setting of oxidative stress. When GS was immunoprecipitated and the association with proteasomes was detected using 20S and 19S antibodies, OGD/R treatment increased the binding of 20S to GS protein, while 19S was barely detected (Fig. 5D). When 19S proteasome was immunoprecipitated, despite that there was quite a few 20S coupled to 19S (Fig. 5D), less GS was observed. Baicalin, as well as malonate and mito-TEMPO, attenuated the binding of 20S to GS without affecting 19S (Fig. 5D), indicating that it combated oxidative stress to keep GS protein stability from 20S-mediated proteasomal degradation. Different from 26S proteasomal degradation, 20S-mediated degradation of oxidized protein is independent of ATP. ATP synthase inhibitor oligomycin A failed to affect H2O2-mediated GS degradation (Suppl. Fig. 4), supporting that 20S proteasome, but not 26S proteasome, mediated GS protein degradation in the context of oxidative stress. Similarly, baicalin, malonate and mito-TEMPO decreased the carbonylation of GS and the binding of 20S to GS when the astrocytes were exposed to combination treatment of succinate and oligomycin A (Fig. 5E). These results showed that baicalin counteracted the 20S-mediated degradation of GS in response to OGD/R, which was likely attributed to reducing the ROS generation via SDH inhibition.
Fig. 5.
Baicalin protects GS protein from 20S proteasomal degradation. (A) Carbonyl content in the brain tissues exposed to OGD/R (n = 5 each group); (B) Carbonyl protein expression in astrocytes under OGD/R (n = 5 each group); (C) GS carbonylation in astrocytes under OGD/R (n = 5 each group); (D) GS binding to 20S or 19S subunit in astrocytes exposed to OGD/R (n = 5 each group); (E) GS carbonylation and GS binding to 20S proteasome when astrocytes were stimulated with succinate plus oligomycin A (n = 5 each group). Data were showed as mean ± SD. GS, glutamine synthetase; OGD/R, oxygen glucose deprivation/reperfusion. *p < 0.05 vs blank group (astrocytes or brain slice without any treatment), #p < 0.05 vs control group (brain slice treated with OGD/R or astrocytes treated with OGD/R or succinate).
3.6. Baicalin protects the brain from I/R injury
Finally, we confirmed the effect of baicalin in rats when baicalin was administered by i. p. injection 1.5 h after the artery was occluded (0.5 h before reperfusion). TTC is a proton receptor of the pyridine-nucleoside structural enzyme system in the respiratory chain. Dehydrogenase in normal brain tissue can reduce TTC to insoluble red stable triphenyl formazan, while damaged brain tissue is resistant to TTC staining. I/R insult induced cerebral infarction, indicated by a decreased area of TTC staining (appeared white) in the brain after reperfusion for 24 h; however, i. p. injection of baicalin and malonate reduced the brain infarction area by 57% and 61%, respectively, and improved neurological function (Fig. 6A and B), demonstrating the role in neuroprotection. Baicalin and malonate inactivated SDH with limited succinate accumulation in the cortex during the early stage of reperfusion (reperfusion for 30 min) (Fig. 6C and D). In line with the SDH inactivation, baicalin reduced the production of H2O2 (Fig. 6E). Moreover, baicalin prevented the binding of 20S proteasome to GS and restored GS protein expression with improved activity in the brain of rats suffered to I/R (Fig. 6F–H). These results provide in-vivo evidence to support that baicalin combated oxidative stress to protect GS from 20S-mediated proteasomal degradation via inhibiting SDH.
Fig. 6.
Baicalin protects the brain from I/R injury. (A) Cerebral infarction area measured by TTC staining (appears white) in rats subjected to unilateral MCAO (2 h) and reperfusion (24 h) (n = 5 each group); (B) Neurological score in rats subjected to I/R (2 h/24 h) (n = 5 each group); (C) SDH activity in brain cortices after I/R (2 h/0.5 h) (n = 5 each group); (D) Succinate content in brain cortices after I/R for 2 h/0.5 h (n = 5 each group); (E) H2O2 content in brain cortices after I/R (2 h/0.5 h) (n = 5 each group); (F) GS activity in brain cortices after I/R (2 h/0.5 h) (n = 5 each group); (G) GS protein expression in brain cortices after I/R (n = 5 each group); (H) GS binding to 20S proteasome after I/R (2 h/24 h) (n = 5 each group). Data were showed as mean ± SD. GS, glutamine synthetase; I/R, ischemia and reperfusion. *p < 0.05 vs blank group (rats without any treatment), #p < 0.05 vs control group (rats with ischemia or I/R).
4. Discussion
Astrocytes are the predominant type of glial cells tightly interacting with surrounding neurons to maintain the integrity of neuron function [4,5,32]. Given the failure to successfully translate neuroprotective approaches in animal models of ischemic stroke to clinical therapies, increasing studies turn to targeting astrocytes. Here we showed that baicalin combated excitotoxicity via protecting GS in astrocytes from oxidative stress-induced proteasomal degradation.
4.1. The alteration and role of GS after I/R
GS is a key player in glutamate-glutamine cycle and impacts the glutamate disposal by astrocytes. Although a series of studies have indicated the different changes of GS expression and activity in brain injury, the consequences and underlying mechanisms are not fully elucidated [15,33,34]. Different from neurons which are sensitive to oxygen and glucose depletion, astrocytes are more tolerant to this insult, partially because they can use stored glycogen to provide glucose to meet the need of energy via glycolysis [[35], [36], [37], [38]]. Thus, in this study, astrocytes were exposed to a 6 h-OGD before 30 min-reoxygenation other than a 2 h period of OGD usually applied in neuronal experiments. Interestingly, we found that GS protein expression dynamically changed in cultured cells exposed to OGD/R. GS protein was decreased sharply at the early stage of 0-60 min after reoxygenation, and then gradually returned to normal levels from 4 h to 8 h. A more elevated levels could be observed at the time after 12 h–24 h (Suppl. Fig. 2C). Impaired GS protein at the early stage was also observed in MCAO/R rats (Fig. 6G), consistent with the published study which showed that loss of GS was obvious 1 h post 30 min-hypoxic insult in piglets [39]. These findings suggest a potential therapeutic window for pharmacological intervention to prevent glutamate accumulation, Upon ischemic attack, extracellular glutamate accumulation is mainly due to the increased release from neurons and the disruption of uptake by astrocytes. It is proposed that glutamate generation from glutamine has a contribution to glutamate accumulation [40]. In this context, increased GS activity might exacerbate the excitotoxicity. In contrast, other studies indicate that GS-mediated glutamine synthesis helps astrocytes to normalize extracellular glutamate and protects neurons from I/R insult [40]. Here, by using the GS inhibitor MSO and co-culture system, we further demonstrated that restoration of GS expression and activity provided benefits for glutamate uptake and neuronal protection. This result was in line with those of other two studies, which revealed the potential role of GS in ischemic conditioning [41,42]. There are several possible explanations for neuroprotection by the glutamate-glutamine cycle. One is that glutamine synthesis by GS consumes the extracellular glutamate to prevent subsequent excitotoxicity. Second, when glutamine is transferred in neurons, glutaminolysis-derived glutamate can serve as an alternative energy substrate to fuel the tricarboxylic acid cycle in the form ɑ-ketoglutarate [40]. Besides, ɑ-ketoglutarate is reported to regulate autophagy and thus keep the metabolic integrity [43]. Third, glutamine is a precursor of glutathione protecting neurons from oxidative stress.
4.2. The relationship between GS and SDH in I/R and the mechanism of GS loss
Oxidative stress mediates ischemic injury and mitochondria are the main source of ROS production upon reperfusion [44]. Chouchani ET and co-workers revealed that succinate accumulates during ischemia due to reversal of SDH and serves as reducing equivalent to drive SDH activation at the early stage of reperfusion, leading to a burst of ROS production at complex I owing RET. This finding elucidates the critical role of SDH activation in I/R injury [18]. Although this conclusion was questioned by a recent study [45], we demonstrated that SDH inhibitor malonate reduced succinate accumulation during ischemia and suppressed mitochondrial ROS production in early reperfusion, providing evidence to support the conclusion. GS is vulnerable to oxidative stress [[15], [16], [17]], and SDH inhibition is shown to preserve GS activity in the cerebral cortex and striatum and increase the tolerance against MCAO insult [41].
In the study, we revealed that SDH was responsible for the loss of GS, likely owing to oxidation-associated proteasomal degradation. Protein homeostasis is tightly controlled by the balance between protein synthesis and degradation [46]. Exposure to OGD/R failed to influence gene expression of GS, proteasome inhibitor MG-132, but not lysosomal V-ATPase inhibitor bafilomycin A1, prevented the reduction of GS protein expression, validating that protein degradation was the primary cause for the loss of GS. ROS scavenger NAC mitigated OGD/R- or succinate-elicited loss of GS, and MG-132 also alleviated H2O2-induced GS reduction. These events further confirmed the involvement of ROS in GS degradation.
Oxidation and reduction occurring on amino acid residues of proteins comprise of a part of redox regulation [47]. Protein carbonylation is a common post translational modification in response to different oxidative stresses including ROS, lipid oxidation products or advanced glycation end products [47,48], resultantly impairing protein function [48]. To combat this damage, cells tend to repair the slightly carbonylated proteins via reduction reactions and degrade the seriously damaged proteins via proteasomes and/or lysosomes. In immunoprecipitation experiments, we observed an obvious increase in carbonylated GS level in OGD/R group, and the molecular weight of carbonylated GS ranged from about 30 KD to 70 KD. This change might be due to the cleavage of the carbonylated peptides and the cross-linking occurring between oxidized proteins, such as the formation of disulphied bonds and Schiff base [47]. The increased total carbonyls including carbonylated GS could impairs cellular homeostasis, having a contribution to astrocyte injury in the setting of OGD/R insult.
One important function of proteasome system is to remove incorrectly synthesized and oxidatively damaged proteins via degradation [31,49]. In eukaryotic cells, 26S holoenzymes are the primary form of proteasomes [50]. However, acute oxidative stress causes the rapid disassembly of 26S proteasomes into 20S core and 19S regulatory subunits, leading to the inactivation of 26S proteasomes [31,50,51]. Under the conditions, 20S proteasome is in charge of the degradation of oxidized proteins. It is usually considered that 26S mediates protein degradation in a manner dependent on ubiquitin and ATP, while 20S-mediated protein degradation is independent of ubiquitin and ATP [31,50]. Although previous studies have indicated GS is sensitive to oxidative stress, little is known of the details. To further reveal the exact role of proteasomes in the degradation of GS, we assessed the interaction between GS and 20S or 19S proteasome. GS bound to the 20S core but not 19S regulatory particle in early reperfusion, and H2O2-mediated GS protein degradation was not affected by oligomycin A, further confirming that 20S proteasome was responsible for GS degradation under oxidative conditions.
4.3. The role of baicalin on GS in astrocytes
It is documented that baicalin is potential for the treatment of ischemic stroke via anti-excitotoxicity and anti-oxidative stress [3,4,22]. Given the glutamate-glutamine cycle between astrocytes and neurons, we investigated the effect of baicalin on glutamate disposal from the aspect of GS function in astrocytes. Because of the reported role of SDH in I/R injury and the sensitivity of GS to oxidative stress, we speculated that baicalin might protect GS from SDH-mediated oxidative stress in early reperfusion. The results in this study confirmed the suppression of SDH activity by baicalin in vivo and ex vivo, and demonstrated that the anti-oxidant and anti-excitotoxic effects of baicalin converged in astrocytes during I/R. We reason that inactivation of SDH should be the main mechanism for baicalin to protect GS activity. During the ischemic state, anaerobic metabolism activates SDH in reverse to reduce fumarate to succinate [18], and thus inactivation of SDH by baicalin should contribute to preventing succinate accumulation. Upon reperfusion, SDH activation is a driving force for mitochondrial ROS production, and we demonstrated the inhibitory effects of baicalin in astrocytes when succinate and ATP synthase inhibitor oligomycin A were added to minic ROS production from RET (Fig. 1F, Suppl. Fig. 1A). Although we showed that baicalin suppressed mitochondrial ROS to prevent GS degradation, we cannot say with certainty that protection of GS by SDH inactivation is the only reason. In fact, baicalin is a multifunctional compound. Its pharmacological activities, including anti-inflammation, mitochondrial protection and anti-apoptotic effects, should contribute to neuroprotection from different aspects [22]. These findings reveal the specific mechanisms of baicalin in neuroprotection and extent the cognition of the anti-oxidant properties, which will help to prepare a legitimate therapy for ischemic stroke with baicalin or Scutellaria baicalensis Georgi.
5. Conclusion
The study revealed that SDH activation is a driving force for mitochondrial ROS production in brain ischemic injury, and 20S proteasome-mediated GS protein degradation impairs glutamate disposal. Baicalin inactivated SDH to suppress ROS production and protected GS protein stability against oxidative stress to promote glutamate disposal, having a contribution to combating excitotoxicity. These results suggest that protecting astrocyte function to clear released glutamate might be potential to prevent ischemic neuronal injury.
Author contributions
Xianrui Song performed most of the experiments and analyses involved, analyzed the data and wrote the paper. Zixuan Gong and Kaili Liu performed some of the cellular experiments. Kang Liu and Baolin Liu conceived, designed, supervised the study, and wrote the paper. Junping Kou supervised the study.
Declaration of competing interest
We declared that we have no conflicts of interest to this work, and we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Baicalin combats glutamate excitotoxicity via protecting glutamine synthetase from ROS-induced 20S proteasomal degradation” (REDOX_2020_219_R1)”.
Acknowledgements
The work was supported by “Double First-Class” University project (CPU2018GF07).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101559.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
References
- 1.Chamorro Á., Dirnagl U., Urra X., Planas A.M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 2.Patel Rajan A.G., McMullen P.W. Neuroprotection in the treatment of acute ischemic stroke. Prog. Cardiovasc. Dis. 2017;59:542–548. doi: 10.1016/j.pcad.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Li Y., Liu Z., Xin H., Chopp M. The role of astrocytes in mediating exogenous cell-based restorative therapy for stroke. Glia. 2014;62:1–16. doi: 10.1002/glia.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stary C.M., Giffard R.G. Advances in astrocyte-targeted approaches for stroke therapy: an emerging role for mitochondria and microRNAS. Neurochem. Res. 2015;40:301–307. doi: 10.1007/s11064-014-1373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barreto G.E., Gonzalez J., Torres Y., Morales L. Astrocytic-neuronal crosstalk: implications for neuroprotection from brain injury. Neurosci. Res. 2011;71:107–113. doi: 10.1016/j.neures.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Karve I.P., Taylor J.M., Crack P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016;173:692–702. doi: 10.1111/bph.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasile F., Dossi E., Rouach N. Human astrocytes: structure and functions in the healthy brain. Brain Struct. Funct. 2017;222:2017–2029. doi: 10.1007/s00429-017-1383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z., Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 2016;144:103–120. doi: 10.1016/j.pneurobio.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson J.G., Robinson M.B. Regulation of mitochondrial dynamics in astrocytes: mechanisms, consequences, and unknowns. Glia. 2018;66:1213–1234. doi: 10.1002/glia.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy Z., Nardai S. Cerebral ischemia/reperfusion injury: from bench space to bedside. Brain Res. Bull. 2017;134:30–37. doi: 10.1016/j.brainresbull.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Sonnewald U., Qu H., Aschner M. Pharmacology and toxicology of astrocyte-neuron glutamate transport and cycling. J. Pharmacol. Exp. Therapeut. 2002;301:1–6. doi: 10.1124/jpet.301.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Zwingmann C., Leibfritz D. Regulation of glial metabolism studied by 13C-NMR. NMR Biomed. 2003;16:370–399. doi: 10.1002/nbm.850. [DOI] [PubMed] [Google Scholar]
- 13.Rose C.F., Verkhratsky A., Parpura V. Astrocyte glutamine synthetase: pivotal in health and disease. Biochem. Soc. Trans. 2013;41:1518–1524. doi: 10.1042/BST20130237. [DOI] [PubMed] [Google Scholar]
- 14.Jeitner T.M., Battaile K., Cooper A.J. Critical evaluation of the changes in glutamine synthetase activity in models of cerebral stroke. Neurochem. Res. 2015;40:2544–2556. doi: 10.1007/s11064-015-1667-1. [DOI] [PubMed] [Google Scholar]
- 15.Oliver C.N., Starke-Reed P.E., Stadtman E.R., Liu G.J., Carney J.M., Floyd R.A. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5144–5147. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Görg B., Qvartskhava N., Voss P., Grune T., Häussinger D., Schliess F. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2007;581:84–90. doi: 10.1016/j.febslet.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 17.Sahakian J.A., Szweda L.I., Friguet B., Kitani K., Levine R.L. Aging of the liver: proteolysis of oxidatively modified glutamine synthetase. Arch. Biochem. Biophys. 1995;318:411–417. doi: 10.1006/abbi.1995.1248. [DOI] [PubMed] [Google Scholar]
- 18.Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L. Ischemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaire B.P., Moon S.K., Kim H. Scutellaria baicalensis in stroke management: nature's blessing in traditional eastern medicine. Chin. J. Integr. Med. 2014;20:712–720. doi: 10.1007/s11655-014-1347-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q., Fu X., Wang J., Yang M., Kong L. Treatment effects of ischemic stroke by berberine, baicalin, and jasminoidin from Huang-Lian-Jie-Du-Decoction (HLJDD) explored by an integrated metabolomics approach. Oxid Med Cell Longev. 2017;2017:9848594. doi: 10.1155/2017/9848594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng F., Wang X., Lu Y., Zhong X., Zhao Y., Wang Q. Chinese medicine injection Qingkailing for treatment of acute ischemia stroke: a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2012;2012:213172. doi: 10.1155/2012/213172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang W., Huang X., Chen W. The Effects of baicalin and baicalein on cerebral ischemia: a review. Aging Dis. 2017;8:850–867. doi: 10.14336/AD.2017.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu T., Ma C., Fan S., Deng N., Lian Y., Tan L. Systematic understanding of the mechanism of baicalin against ischemic stroke through a network pharmacology approach. Evid Based Complement Alternat Med. 2018;2018:2582843. doi: 10.1155/2018/2582843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Z., Zhang Q., Yu Z., Zhang L., Tian D., Zhu S. Inhibiting cell cycle progression reduces reactive astrogliosis initiated by scratch injury in vitro and by cerebral ischemia in vivo. Glia. 2007;55:546–558. doi: 10.1002/glia.20476. [DOI] [PubMed] [Google Scholar]
- 25.Li S., Jiang Z., Chai W., Xu Y., Wang Y. Autophagy activation alleviates nonylphenol-induced apoptosis in cultured cortical neurons. Neurochem. Int. 2019;122:73–84. doi: 10.1016/j.neuint.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Gao J., Long L., Xu F., Feng L., Liu Y., Shi J. Icariside II, a phosphodiesterase 5 inhibitor, attenuates cerebral ischemia/reperfusion injury by inhibiting GSK-3β-mediated activation of autophagy. Br. J. Pharmacol. 2019 doi: 10.1111/bph.14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X., Li R., Wang X., Fu Q., Ma S. Umbelliferone ameliorates cerebral ischemia -reperfusion injury via upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3 inflammasome. Neurosci. Lett. 2015;600:182–187. doi: 10.1016/j.neulet.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Longa E.Z., Weinstein P.R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 29.Rossi D.J., Brady J.D., Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat. Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmoud S., Gharagozloo M., Simard C., Gris D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells. 2019;8:184. doi: 10.3390/cells8020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raynes R., Pomatto L.C., Davies K.J. Degradation of oxidized proteins by the proteasome: distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways. Mol. Aspect. Med. 2016;50:41–55. doi: 10.1016/j.mam.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih E.K., Robinson M.B. Role of astrocytic mitochondria in limiting ischemic brain injury? Physiology. 2018;33:99–112. doi: 10.1152/physiol.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou J., Wang Y.X., Dou F.F., Lu H.Z., Ma Z.W., Lu P.H. Glutamine synthetase downregulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem. Int. 2010;56:577–584. doi: 10.1016/j.neuint.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayakumar A.R., Norenberg M.D. Glutamine synthetase: role in neurological disorders. Adv Neurobiol. 2016;13:327–350. doi: 10.1007/978-3-319-45096-4_13. [DOI] [PubMed] [Google Scholar]
- 35.Niitsu Y., Hori O., Yamaguchi A., Bando Y., Ozawa K., Tamatani M. Exposure of cultured primary rat astrocytes to hypoxia results in intracellular glucose depletion and induction of glycolytic enzymes. Brain Res Mol Brain Res. 1999;74:26–34. doi: 10.1016/s0169-328x(99)00245-4. [DOI] [PubMed] [Google Scholar]
- 36.Rossi D.J., Brady J.D., Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat. Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dienel G.A., Hertz L. Astrocytic contributions to bioenergetics of cerebral ischemia. Glia. 2005;50:362–388. doi: 10.1002/glia.20157. [DOI] [PubMed] [Google Scholar]
- 38.Falkowska A., Gutowska I., Goschorska M., Nowacki P., Chlubek D., Baranowska-Bosiacka I. Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism. Int. J. Mol. Sci. 2015;16:25959–25981. doi: 10.3390/ijms161125939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee A., Lingwood B.E., Bjorkman S.T., Miller S.M., Poronnik P., Barnett N.L. Rapid loss of glutamine synthetase from astrocytes in response to hypoxia: implications for excitotoxicity. J. Chem. Neuroanat. 2010;39:211–220. doi: 10.1016/j.jchemneu.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Stelmashook E.V., Isaev N.K., Lozier E.R., Goryacheva E.S., Khaspekov L.G. Role of glutamine in neuronal survival and death during brain ischemia and hypoglycemia. Int. J. Neurosci. 2011;121:415–422. doi: 10.3109/00207454.2011.570464. [DOI] [PubMed] [Google Scholar]
- 41.Hoshi A., Nakahara T., Kayama H., Yamamoto T. Ischemic tolerance in chemical preconditioning: possible role of astrocytic glutamine synthetase buffering glutamate-mediated neurotoxicity. J. Neurosci. Res. 2006;84:130–141. doi: 10.1002/jnr.20869. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W., Miao Y., Zhou S., Jiang J., Luo Q., Qiu Y. Neuroprotective effects of ischemic postconditioning on global brain ischemia in rats through upregulation of hippocampal glutamine synthetase. J. Clin. Neurosci. 2011;18:685–689. doi: 10.1016/j.jocn.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 43.Villar V.H., Merhi F., Djavaheri-Mergny M., Durán R.V. Glutaminolysis and autophagy in cancer. Autophagy. 2015;11:1198–1208. doi: 10.1080/15548627.2015.1053680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keil U., Scherping I., Hauptmann S., Schuessel K., Eckert A., Müller W.E. Piracetam improves mitochondrial dysfunction following oxidative stress. Br. J. Pharmacol. 2006;147:199–208. doi: 10.1038/sj.bjp.0706459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrienko T.N., Pasdois P., Pereira G.C., Ovens M.J., Halestrap A.P. The role of succinate and ROS in reperfusion injury-A critical appraisal. J. Mol. Cell. Cardiol. 2017;110:1–14. doi: 10.1016/j.yjmcc.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savitski M.M., Zinn N., Faelth-Savitski M., Poeckel D., Gade S., Becher I. Multiplexed proteome dynamics profiling reveals mechanisms controlling protein homeostasis. Cell. 2018;173:260–274. doi: 10.1016/j.cell.2018.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madian A.G., Regnier F.E. Proteomic identification of carbonylated proteins and their oxidation sites. J. Proteome Res. 2010;9:3766–3780. doi: 10.1021/pr1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong C.M., Bansal G., Marcocci L., Suzuki Y.J. Proposed role of primary protein carbonylation in cell signaling. Redox Rep. 2012;17:90–94. doi: 10.1179/1351000212Y.0000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newton T.M., Duce J.A., Bayle E.D. The proteostasis network provides targets for neurodegeneration. Br. J. Pharmacol. 2019;176:3508–3514. doi: 10.1111/bph.14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Livnat-Levanon N., Kevei É., Kleifeld O., Krutauz D., Segref A., Rinaldi T. Reversible 26S proteasome disassembly upon mitochondrial stress. Cell Rep. 2014;7:1371–1380. doi: 10.1016/j.celrep.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 51.Wang X., Yen J., Kaiser P., Huang L. Regulation of the 26S proteasome complex during oxidative stress. Sci. Signal. 2010;3(151) doi: 10.1126/scisignal.2001232. ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.