Abstract
Management of a difficult airway caused by pathology below the glottis is high-risk and requires a shared approach to airway planning and surgical treatment. Access to the trachea requires a careful assessment of the airway since the end-point of laryngoscopy for infraglottic airway management is not visualization of the larynx for tube placement, but access to the laryngotracheal complex in cases where intubation may not be feasible or may preclude surgical access.
This work provides a common framework for creating multidisciplinary shared-airway management plans and presents devices and strategies that have in recent years improved airway management safety in this difficult patient group and may prove useful in the setting of the novel Coronavirus Disease 2019 (COVID-19).
Introduction
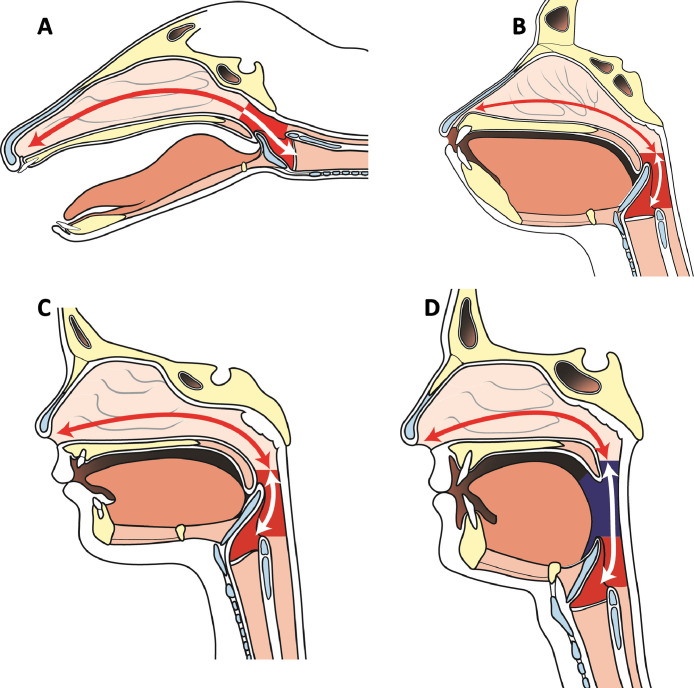
The majority of laryngoscopies performed under general anesthesia aim to establish a ventilatory conduit to permit gaseous exchange and/or secretions management during surgical intervention or during critical illness. The human upper aerodigestive tract's major evolutionary pivot toward speech (Figure 1 ) makes the “path to larynx” hyperangulated and over-reliant on neuromuscular tone .1 Successful laryngoscopy therefore relies on reducing this acute curvature into a line of sight. This is achieved, on the one hand, by positioning the patient's head and neck to extend the atlanto-occipital joint and flex the cervical spine, and on the other hand, by applying controlled pressure with a laryngoscope to compress and displace oral and pharyngeal tissues. These interventions achieve the net effect of exposing the larynx (Figure 2 ).2 , 3
Figure 1.
Supralaryngeal tract angulation in the barking deer (A), ape (B), human infant (C), and adult human (D). The adult human's supralaryngeal tract assumes a tortuous path due to species-specific descent of the larynx which divides the supralaryngeal tract into two roughly equal parts and in so doing facilitates speech. Images areredrawn from pathological specimens printed in the Comparative Anatomy and Physiology of the Larynx.1
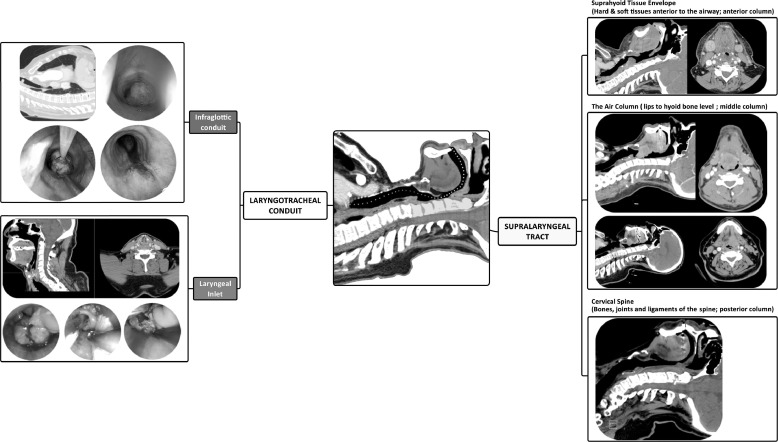
Figure 2.
A representation of the anatomical “path to intubation.” The supralaryngeal tract spans from the lips to the level of the hyoid bone (yellow path). It corresponds to the supralaryngeal tract as the term has been understood in the context of speech articulation. From the perspective of laryngoscopy, the supralaryngeal tract can be considered as three columns. The suprahyoid tissue envelope (anterior column) consists of all of the soft and bony tissues in front of the air column including the maxillary arch; the air column (middle column), is the air passageway itself; and the cervical spine (posterior column) consists of the bones, joines and ligaments of the cervical spine and the cranio-cervical articulation.2 The CT scan images of the suprahyoid tissue envelope denote retrognathia and an adipose neck. These will interfere with the laryngoscopist's ability to compress and displace soft tissues into the neck. The CT scan representing the air column shows a base of tongue tumor which can obscure and distort laryngeal exposure and increase the risk of contact bleeding. The CT scan corresponding to the cervical spine is of a hangman's fracture which prevents spinal manipulation.28 The laryngotracheal conduit can be divided into the laryngeal inlet and the infraglottic conduit. The CT scan and endoscopic images representing laryngeal inlet are of a patient with an advanced laryngeal cancer who presented with acute airway obstruction. The CT scan and endoscopic images representing the infraglottic conduit are of a patient who presented with acute airway obstruction due to a pedunculated tracheal squamous cell carcinoma.
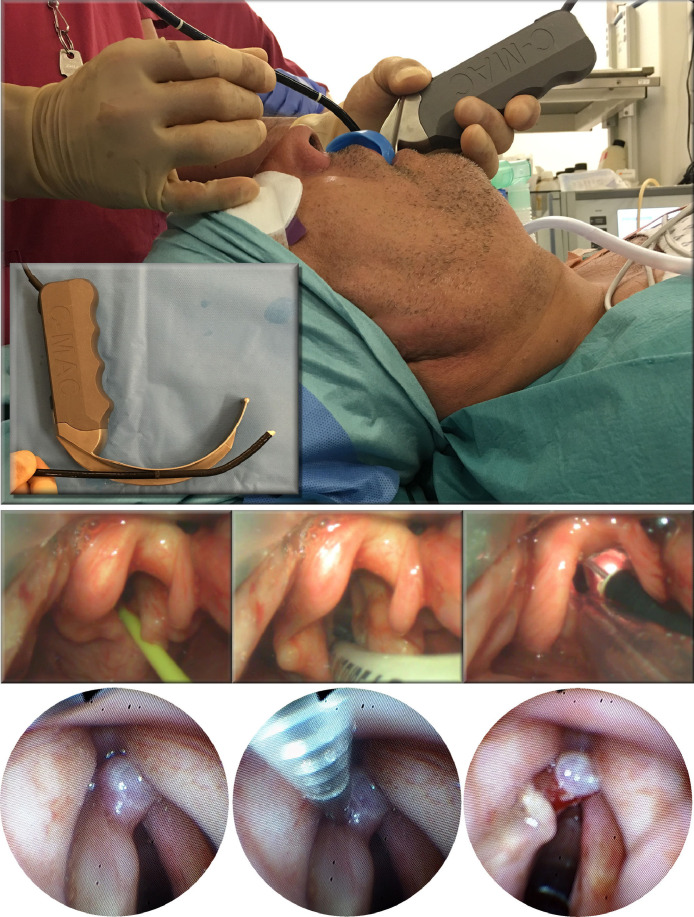
The majority of the laryngoscopies performed for “difficult airways” involve navigating or bypassing a tortuous supralaryngeal tract that is often also lined by friable tissues or pathology. In these cases, successful laryngoscopy and intubation may still be achieved transorally but it may require the use of hyperangulated instruments like the C-MAC blade to negotiate rather than to flatten the supralaryngeal tract (Figure 3 ). The airway may also be secured either transnasally or transtracheally (Figure 4 ). This work describes a multidisciplinary approach to managing a difficult airway when airway pathology arises in the infraglottic ventilatory conduit either in isolation or in combination with supralaryngeal tract pathologies.
Figure 3.
A case of “navigating” an unfavorable supralaryngeal tract. Clinical presentation was of new-onset hoarseness with flexible laryngoscopic finding of a left anterior vocal lesion in a patient whose T1 glottic cancer had been treated with radiotherapy some years previously. The patient was intubated with video laryngoscopy using a gum-elastic bougie and a C-MAC video laryngoscope (Karl Storz Endoscopy, Tuttlingen, Germany). However, line-of-sight surgical access to the lesion could not be established due to the presence of retrognathia and fibrotic neck tissues (i.e. pathologies within the suprahyoid tissue envelope of the supralaryngeal tract). The lesion was accessed and biopsied using a combination of C-MAC laryngoscopy to negotiate and retract tissues, and flexible endoscopy to access and biopsy the lesion.
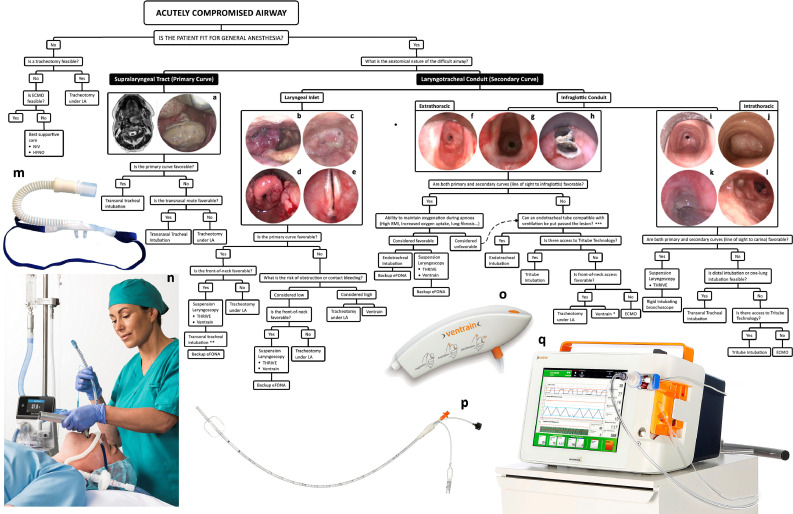
Figure 4.
A global view of managing difficult airways. The first question in any difficult airway situation is whether it is safe to undertake general anesthesia. This principally rests on hemodynamic stability and likely adequacy of oxygenation after induction of anesthesia. The question in terms of managing supralaryngeal tract pathologies is whether they can be navigated transorally or whether they need to be bypassed. The answer to this question, as with others in this decision-tree, is not absolute and is based on the experience of, and equipment available to, the team. Laryngotracheal conduit pathologies may be divided into those that obstruct and obscure the laryngeal inlet, principally oropharyngeal, supraglottic, and transglottic lesions, and those that arise below the laryngeal inlet. The standard of care for managing airway compromise due to laryngeal inlet pathology is a tracheotomy under local anesthesia, but in highly-selected proportion of these patients, they may be managed with suspension laryngoscopy and THRIVE10. Management of difficult airways due to infraglottic conduit pathologies is described in the text. (A) Case of a pedunculated tumor arising from the posterior pharyngeal wall and causing obstruction of the air column; (B) A hemangioma arising from the aryepiglottic fold and obstructing the laryngeal inlet; (C) a hypopharyngeal tumor causing severe distortion and obstruction of the laryngeal inlet; (D) laryngeal sarcoidosis; (E) airway compromise due to bilateral vocal fold immobility caused by a posterior glottic scar; (F, g) Two cases of intubation-related extrathoracic tracheal stenosis causing airway compromise; h. acute airway obstruction due to in-situ tracheal stent fracturing; (I) circatricial intrathoracic tracheal stenosis caused by tracheostomy tube cuff injury; (J) An intrathoracic tracheal tumor; (K) Acute airway obstruction caused by tracheostomy tube tip injury in a patient with long-term tracheotomy due to bilateral vocal immobility. The lesion is fully wrapped around the endotracheal tube which has been introduced through the tracheotomy stoma; (L) Complete left-sided and 60% right-sided main bronchial obstructions in a patient with a history of mediastinal lymphoma whose acute airway obstruction was treated some years previously with an uncovered metal stent. The lymphoma fully responded to oncological treatment but subsequent clinical presentation was with stent migration, fracturing, and incorporation into the distal tracheal and bronchial walls with recurrent granulation tissue formation and distal tracheal and bronchial airway obstruction; (M) Nasal high-flow cannula (OptiFlow, Fisher and Paykel Healthcare, Auckland, New Zealand); (N) Nasal high-flow being used for perioxygenation and intubation (images M and N were provided courtesy of Fisher and Paykel Healthcare); (O) Ventrain system; (P) The Tritube; (Q) The Evone ventilator delivering flow-controlled ventilation (images O-Q were provided courtesy of Ventinova Medical, Eindhoven, Netherlands)). LA, local anesthesia; NIV, noninvasive ventilation; HFNO, high-flow nasal oxygen; eFONA, emergency front-of-neck access; THRIVE, transnasal humidified rapid-insufflation ventilatory exchange; ECMO, extracorporeal membrane oxygenation. * Use of Cricath with Ventrain (Ventinova Medical, Eindhoven, Netherlands) should be considered with caution given the risk of total outflow obstruction and barotrauma. ** The expectation with these cases will be that intubation attempts will be performed under suspension laryngoscopy and with line-of-sight instruments, but in highly selected cases the team may elect to perform translaryngeal intubation with anesthetic instruments. An example would be image b where the supraglottic hemangioma obscures but does not obstruct laryngeal inlet and the airway may be amenable to transoral or transnasal intubation. *** These procedures are expected to be performed in an awake patient receiving high-flow nasal oxygen.
Clinical presentation and initial assessment
A difficult airway due to infraglottic pathology may present acutely in the settings of foreign-body inhalation, postbronchoscopy hemorrhage and edema of a tracheobronchial lesion, or postextubation stridor. It can also present as chronic exertional dyspnea, effort intolerance and stridor in patients with benign or malignant tracheobronchial stenosis, compression, or malacia.4 In all cases of a known or suspected diagnosis of infraglottic obstruction, initial assessment follows an ABC approach (Table 1 ). Note should be made of clinical features such as oxygen saturations, degree of respiratory effort, tracheal tugging, and the use of accessory muscles. Arterial blood gases analysis can identify carbon dioxide accumulation signifying impending fatigue.
Table 1.
Life support algorithm in the context of a compromised airway
| Abbreviation | Description | Adjunct |
|---|---|---|
| A | Airway | …with cervical spine protection |
| B | Breathing | …with high-flow nasal oxygen |
| C | Circulation | …with hemorrhage control |
| D | Disability | …with neurological assessment |
| E | Exposure | …with environment control |
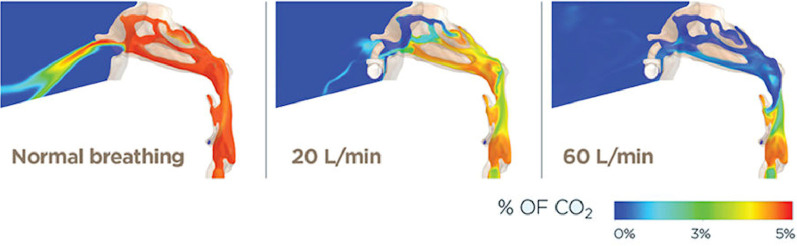
When managing such cases priority should be given to oxygenating the patient and reducing the work of breathing and in our practice, warmed and humidified high-flow nasal oxygen is immediately applied unless there is a known or strong suspicion of a base of skull fracture, or if there is active upper airway bleeding. An initial flow rate of 30 L/min is given and is increased over a course of 2-3 minutes to a maximum of 70 L/min as tolerated by the patient (Figure 5 ). All adult patients are also given an initial 5mL of nebulized 1:1,000 epinephrine and 4-8 mg of intravenous dexamethasone in order to reduce tissue edema which may be exacerbating or may indeed be the main cause of, airway obstruction. High-flow nasal oxygen effectively “bypasses'” the supralaryngeal tract and the glottis, which between them account for approximately 50% of the total resistance of the respiratory system (Figure 6 ).5., 6., 7. As a possible corollary, application of nasal high-flow has been shown to reduce the work of breathing by approximately 50%.8
Figure 5.
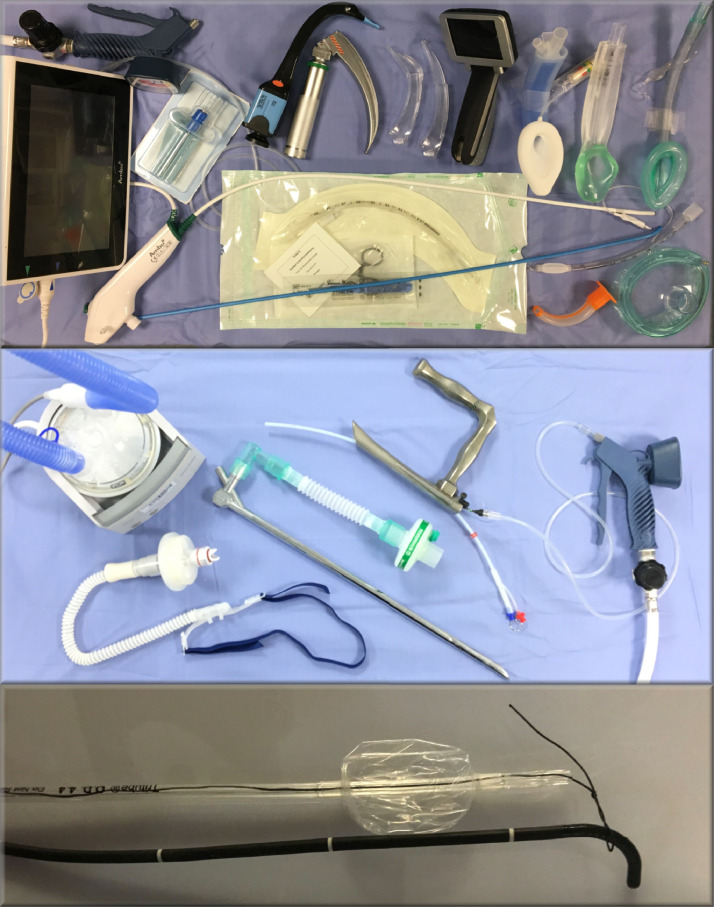
A range of instruments used to manage pathologies of the supralaryngeal tract (primary curve; top panel) and the laryngotracheal conduit (secondary curve; middle panel). The bottom image shows the set up for awake tracheal intubation using the Tritube. The manual jet ventilator showed in the middle panel should only be used in emergency situations (for trained experts only) and elective or semi-elective cases should be ventilated using a jet ventilator with in-built safety monitoring features like shown in Figure 8.
Figure 6.
Computational Fluid Dynamic (CFD) models of end-expiration awake spontaneous ventilation without nasal flow (left), with 20 L/min of nasal high-flow (middle) and with 60 L/min of nasal high-flow (right). At 60 L/min there is near-total washout of carbon dioxide at the end of expiration which equates to presence of 100% oxygen through a flow vortex at the glottis. Images supplied courtesy of Fisher and Paykel Anaesthesia Research Group.
The use of nasal high-flow in the context of the Severe Acute Respiratory Syndrome caused by the novel Coronavirus-2 (SARS-Cov2) is more nuanced. Clinical practice and learned opinion has ranged from nasal high-flow being the mainstay of treating COVID-19 pneumonia9 to its use being recommended against10. The use of nasal high-flow in the settings of awake tracheal intubation in COVID-19 patients has been shown to reduce the risk of desaturation and reduce the duration of intubation11. The exact risk of cross-infection from the use of nasal high-flow in comparison to other interventions is unclear12 and13 and in our opinion, provided that the patient is placed in isolation and all staff use full Personal Protective equipment at all times a patient receives nasal high-flow, as a temporary holding intervention the benefits of its use outweigh the risks. An additional specific consideration with infraglottic airway obstruction is the risk of aerosol generation from air turbulence due to normal tidal breathing through a tight stricture compared with the use of nasal high flow. We do and have on this basis therefore use nasal high-flow in the settings of awake tracheal intubation and as a temporary holding intervention in the specific setting of acute airway compromise due to an infraglottic obstruction just prior to definitive management14. [xx7]
The principal method of assessing the suprahyoid tissue envelop and the cervical spine are bedside clinical evaluation. In selected cases, this may be supplemented by specialist investigations like flexion-extension x-rays of the cervical spine. Note should be made of the presence of prominent maxillary teeth, shape of the jaw and in particular presence of retrognathia, and the volume and compliance of the submandibular tissues.
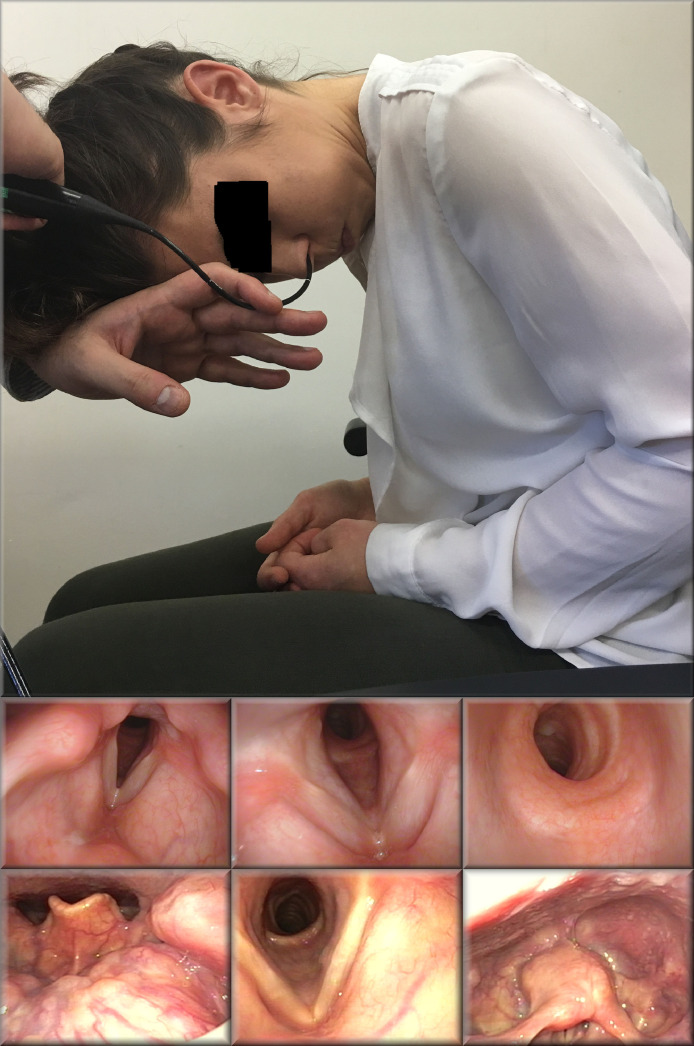
The air column of the supralaryngeal tract is evaluated by flexible nasendoscopy. A standard flexible nasendoscopy evaluates the nasal passages which may need to be used for transnasal intubation and examines the nasopharynx, oropharynx, hypopharynx, and larynx. The subglottis and the proximal trachea may be viewed, without the need to administer local anesthesia, using the Laryngeal Distraction Maneuver (Figure 7 ).15 Distal tracheal and main bronchi may be assessed via a tracheotomy, if present, or, in a tracheotomy-free patient, following administration of 2-3 mL of local anesthesia either through the suction channel of the endoscope, a mucosal atomisation device, or through a cricothyroid injection. Administration of local anesthesia reduces the patient's perception of their breathing through action on laryngeal and tracheal flow and pressure sensory receptors and this can cause some patients to become anxious and hyperventilate. This may in turn exacerbate the obstruction. At the same time, direct visualization of the distal trachea (Figure 4j) provides valuable information about lesion consistency and whether it is likely to be bypassed with an endotracheal tube. In the patient who is presented in Figure 4K for instance, performing a tracheoscopy through the tracheotomy tube identified a soft pedunculated lesion that was judged to permit the passage of a small endotracheal tube and subsequently would be accessible for surgical excision. This allowed an initial attempt at suspension laryngoscopy and jet ventilation which, when failed due to loss of airway tone and shrink-wrapping of the trachea around the lesion leading to complete airway obstruction, was then uneventfully managed by placing a bougie through the tracheotomy and bypassing the lesion with a microlaryngoscopy tube, from around which the lesion was then excised. Had the lesion been identified as a craggy and friable carinal tumor, greater consideration toward primary use of extracorporeal membrane oxygenation (ECMO) would have been given. As such, the value of additional information gained from direct visualization of the distal trachea must be set against the risk of aggravating anxiety or traumatizing the distal airway, and therefore exacerbating the airway obstruction.
Figure 7.
Flexible nasendoscopy in combination with the Laryngeal Distraction Maneuvre. This maneuver brings the tracheal into the birds-eye view of the endoscope and distracts the epiglottis. In combination with slow deep inspiration it allows visualization of the proximal, and in some cases the distal trachea. In combination with a Valsalva maneuver, it permits visualization of the postcricoid larynx that may in turn identify difficulties with placing a Supraglottic Airway Device. Reproduced, with permission from Laryngology: A Case-Based Approach by Allen, Nouraei, and Sandhu.15
Additional anatomical and physiological investigations
The objective of airway assessment is to construct a plan with a number of contingencies that are tailored both to the patient and to the team in order to avoid a Cannot Intubate Cannot Oxygenate (CICO) scenario upon induction of anesthesia and to expeditiously rescue it if it arises. In the settings of the global COVID-19 pandemic, these objectives need to be achieved whilst also minimizing the risk of viral aerosolization leading to cross-infections. We have found that in an emergency, amelioration of patient distress and improving oxygenation through the application of high-flow nasal oxygen buys valuable time to construct and discuss a safe airway plan and in some cases, to allow for a more experienced and airway-focused team to be assembled and with appropriate precautions, it is a strategy which we believe continues to be justified in this specific patient population.
In the acute settings, acquiring every item of information about the airway, for example a computed tomography (CT) scan, is a risk-benefit analysis of value in improving the airway plan set against the risk of patient fatigue with respiratory failure from definitive treatment delay, and/or a change in the underlying nature of the obstruction, such as may occur by additional swelling or from displacement of a foreign body. In practice, many patients with what turns out to have been respiratory compromise due to infraglottic airway obstruction, will have already been investigated for more common causes like a pulmonary embolism and an airway CT scan will in many cases already be available as a byproduct of previous investigations. If not, a risk-benefit analysis should be performed and should take into account the benefits of performing a CT scan against the likely change in the underlying nature of the lesion during transfer, the risk of respiratory failure due to fatigue, tolerability of lying flat during a multislice CT scan, response to application of nasal high-flow in terms of ameliorating work of breathing against the risk of aerosol-generation in environments that may not be well isolated, and feasibility of performing an emergency intubation or front-of-neck access in the event of airway obstruction. Only if deemed safe should a CT scan of the neck and chest be obtained prior to induction of anesthesia. In the patient shown in Figure 2 under infraglottic conduit, the CT scan identified a pedunculated extrathoracic obstructive lesion. This knowledge, in combination with a clinical assessment which confirmed a favorable supralaryngeal tract for suspension laryngoscopy, and information obtained from direct visualization of the trachea using flexible laryngoscopy with the laryngeal distraction maneuver, allowed for the construction of the airway plan. In that particular case, plan A was transnasal humidified rapid-insufflation ventilatory exchange (THRIVE)16 perioxygenation and apnoeic ventilation with suspension laryngoscopy, plan B was to commence jet ventilation in combination with THRIVE, and, in the event of failure of suspension laryngoscopy, plan C was to attempt transoral or transnasal microlaryngoscopy tube tracheal intubation followed by open surgical exploration of the neck. In the event, the airway was managed with suspension laryngoscopy, ventilation was maintained using THRIVE, and the lesion was secured with a ureteric stone retrieval basket and excised.
A flow-volume loop17 is a standard of care for assessing and monitoring patients with known laryngotracheal stenosis and in many cases will already be available. In the acute setting, flow-volume loops are generally not recommended since the maximum-effort breath may alter the nature of the obstruction and a maximum-effort flow-volume loop is likely aerosol-generating.
Patient managemement
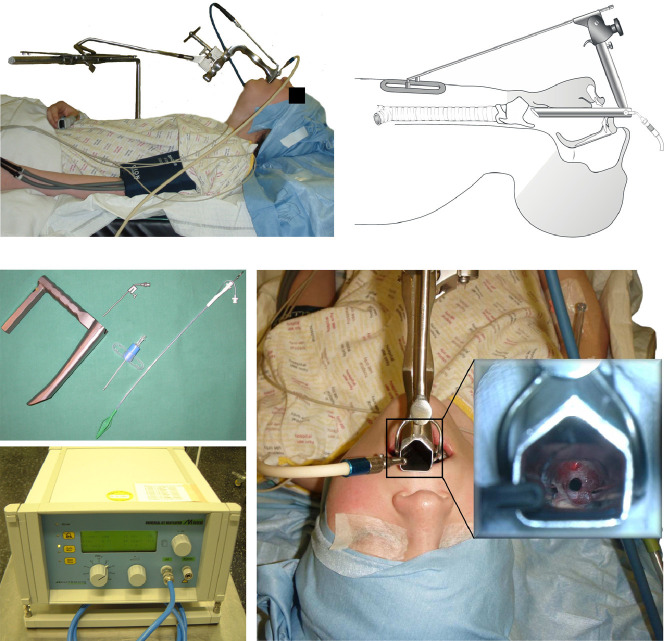
Surgical management of the full spectrum of benign and malignant laryngotracheal conditions is beyond the scope of the work and has been presented elsewhere.4 Where skills and expertise exist, managing infraglottic difficult airway is accomplished through shared-airway surgery both to ventilate the patient and to treat the obstruction. Where local expertise are not available, the most appropriate management would be to secure the airway and to arrange for safe patient transfer to the nearest airway unit. In selected cases, airway management may be accomplished through deployment of temporary airway stents under bronchoscopic or radiological control with care being exercised not to deploy uncovered metal stents except as an end-of-life palliative procedure,18 but in most cases relief of infraglottic airway obstruction will require general anesthesia. Once anesthetized, the Difficult Airway Society failed intubation guidelines apply.19 Awake Tracheal Intubation should be performed in accordance with the Difficult Airway Society guidelines.20 Different considerations apply when managing extrathoracic and intrathoracic airways. Lesions of the subglottis and the cervical trachea may be accessed via suspension laryngoscopy and are amenable to jet ventilation or THRIVE. Loss of airway tone can be managed by the effective application of jaw-thrust and proximity of the lesions to the cricoid ring makes shrink wrapping of the airway around the lesion less likely. Moreover, once the airway has been accessed through suspension laryngoscopy (Figure 8 ), 4-handed line-of-sight instrumentation becomes possible, lasers may be deployed, and persistent obstruction and/or malacia may be supported by an intraluminal stent that may be secured through the neck.4 If suspension laryngoscopy proves difficult, the airway may be managed by placing the smallest possible endotracheal tube or a laryngeal mask airway.21 Of note, the introduction of the Evone ventilator and the Tritube (Figure 4) increase the safety of endotracheal intubation in the settings of an acutely obstructed airway (Figure 5). In the event of failure of suspension laryngoscopy and with a secure airway, in highly selected cases, an open procedure like a tracheal resection (Figure 9 ) may be considered. By contrast, the intrathoracic trachealis and tracheal ring can readily shrink-wrap around a lesion due to the loss of airway tone (Figure 4J and K) and cause total airway obstruction due to loss of airway tone upon induction of anesthesia. The ability to transorally bypass these lesions with rigid instrumentation, namely a rigid bronchoscope (Figure 5) requires favorable supralaryngeal tract as well as laryngotracheal conduit anatomy in order to allow access as far down as the carina. If made possible by lesion consistency and location, endotracheal intubation distal to the lesion, either above the carina or into one lung would secure the airway but this could also make shared airway surgery considerably more challenging. These types of lesions were historically considered for cardiopulmonary bypass or ECMO.22 With the advent of the Evone ventilator and the global SARS-CoV-2 pandemic with its attending risk of viral aerosolization, the use of tubeless ventilation methods such as jet ventilation has been limited (Figure 5). In response, we have begun to use the Tritube system more widely and in conjunction with a systematic approach to reduce the risk of aerosolization at different stepts of shared-airway management (Figure 10 ). ECMO or cardiopulmonary bypass remain options that need to be explored early when managing this small but very challenging group of patients. Management options include lesion removal using a variety of energy devices such as laser, diathermy or cryotherapy, although all attempts are made to avoid using laser or other particle-dispersing devices in the settings of COVID-19. Airway dilation using standard and/or cutting-balloon devices are encouraged,23 and use of distal or Y-shaped stents,24 and in very highly selected cases, consideration of primary bronchoplastic surgery may be considered.25
Figure 8.
Suspension laryngoscopy using a Dedo-Pilling laryngoscope. Tubeless ventilation may be achieved using supraglottic jet ventilation or THRIVE.
Figure 9.
Tracheal Resection.
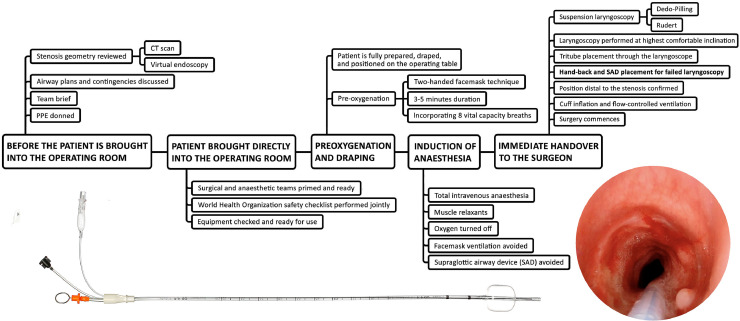
Figure 10.
A process for minimising aerosol generation when managing patients with airway compromise due to laryngotracheal stenosis. Unless absolutely contraindicated due to impending airway obstruction, a pre-operative computed tomography (CT) scan must be performed and if available, virtual endoscopy views must be reconstructed (tinyurl.com/virtualendoscopy). The sequence of donning, draping and pre-oxygenation, along with available rescue manoeuvres have been adjusted to minimise aerosol generation. A rapid sequence induction dose of rocuronium is given in all cases to optimise laryngoscopy conditions with minimal apnoea time. Laryngoscopy is then performed to visualise the glottis and subglottis. The the Tritube® is then placed through the surgical laryngoscope. Therefore, the surgical laryngoscope needs to provide access to the glottis and subglotis and be of sufficient diameter to allow the Tritube to pass through it once it has been placed. An additional consideration is that the initial laryngoscopy must be performed in head-up position at the highest possible angle of inclination in order to reduce the speed of compression atelectasis development and early desaturation. The bottom images show the Tritube and the subglottis and the cervical trachea of a patient with Myer-Cotton grade 2 stenosis whose trachea has been intubated with the Tritube.
Conclusions
Managing a difficult airway due to infraglottic obstruction is a specialist and potentially high-risk procedure and the global Coronavirus pandemic has further compounded airway management challenges in this patient group. This is a shared airway intervention and its safe performance requires a shared understanding and clear communication of patient and lesion information, and involves complex technical and nontechnical factors within the assembled airway team. In larger institutions, this role is becoming increasingly specialized within a Difficult Airway Response Team (DART) model,26 and specific team building and rehearsals of difficult airway scenarios are possible,27 but in most centers, acute presentation of a patient with an obstructed airway often brings together a diverse group of practitioners who may not regularly work together and whose regular practices may not encompass a large volume of difficult airway work. In these settings, preservation of the patient's life, prevention of hypoxic brain injury and other complications, and minimizing the risk of viral transmission to members of the team, should be the priorities. This is dependent on good communication and obtaining the most appropriate airway management plan, taking into consideration the particular blend of skills available to the team and the clinical condition of the patient. This work presents a framework around which this shared understanding may develop.
Disclosures
Authors have received support from Fisher and Paykel Healthcare Ltd.
References
- 1.Negus V.E. Hafner; New York: 1949. The Comparative Anatomy and Physiology of the Larynx. [Google Scholar]
- 2.Greenland K.B., Edwards M.J., Hutton N.J. Changes in airway configuration with different head and neck positions using magnetic resonance imaging of normal airways: A new concept with possible clinical applications. Br J Anaesth. 2010;105:683–690. doi: 10.1093/bja/aeq239. [DOI] [PubMed] [Google Scholar]
- 3.Hochman I.I., Zeitels S.M., Heaton J.T. Analysis of the forces and position required for direct laryngoscopic exposure of the anterior vocal folds. Ann Otol Rhinol Laryngol. 1999;108:715–724. doi: 10.1177/000348949910800801. [DOI] [PubMed] [Google Scholar]
- 4.Sandhu G.S., Nouraei S.A.R. Plural Publishing; San Diego: 2016. Laryngeal and Tracheobronchial Stenosis. [Google Scholar]
- 5.England S.J., Bartlett D., Jr., Daubenspeck J.A. Influence of human vocal cord movements on airflow and resistance during eupnea. J Appl Physiol Respir Environ Exerc Physiol. 1982;52:773–779. doi: 10.1152/jappl.1982.52.3.773. [DOI] [PubMed] [Google Scholar]
- 6.Schiratzki H. The oral and laryngeal components of the upper airway resistance during mouth breathing. Acta Otolaryngol. 1965;60:71–82. doi: 10.3109/00016486509126989. [DOI] [PubMed] [Google Scholar]
- 7.Ferris B.G., Jr., Mead J., Opie L.H. Partitioning of respiratory flow resistance in man. J Appl Physiol. 1964;19:653–658. doi: 10.1152/jappl.1964.19.4.653. [DOI] [PubMed] [Google Scholar]
- 8.Delorme M., Bouchard P.A., Simon M. Effects of high-flow nasal cannula on the work of breathing in patients recovering from acute respiratory failure. Crit Care Med. 2017;45:1981–1988. doi: 10.1097/CCM.0000000000002693. [DOI] [PubMed] [Google Scholar]
- 9.Wang K., Zhao W., Li J., Shu W., Duan J. The Experience of High-Flow Nasal Cannula in Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Two Hospitals of Chongqing China. Ann Intensive Care. 2020 doi: 10.1186/s13613-020-00653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://icmanaesthesiacovid-19.org/critical-care-preparation-and-management-in-the-covid-19-pandemic. Accessed 13 May 2020..
- 11.Wu C.N., Xia L.Z., Li K.H., Ma W.H., Yu D.N., Qu B., Li B.X., Cao Y. High-flow nasal-oxygenation-assisted fibreoptic tracheal intubation in critically ill patients with COVID-19 pneumonia: a prospective randomised controlled trial. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loh N.W., Tan Y., Taculod J., Gorospe B., Teope A.S., Somani J., Tan A.Y.H. The impact of high-flow nasal cannula (HFNC) on coughing distance: implications on its use during the novel coronavirus disease outbreak. Can J Anaesth. 2020 doi: 10.1007/s12630-020-01634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Fink J.B., Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J. 2020 doi: 10.1183/13993003.00892-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad I., Wade S., Langdon A., Chamarette H., Walsh M., Surda P. Awake tracheal intubation in a suspected COVID-19 patient with critical airway obstruction. Anaesth Rep. 2020;8(1):28–31. doi: 10.1002/anr3.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nouraei S.A.R. Intubation-related tracheal stenosis. In: Allen J., Nouraei S.A.R., Sandhu G.S., editors. Laryngology: A Case-based Approach. Plural Publishing; San Diego: 2020. pp. 399–424. [Google Scholar]
- 16.Patel A., Nouraei S.A. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): A physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015;70:323–329. doi: 10.1111/anae.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyatt R.E., Black L.F. The flow-volume curve. A current perspective. Am Rev Respir Dis. 1973;107:191–199. doi: 10.1164/arrd.1973.107.2.191. [DOI] [PubMed] [Google Scholar]
- 18.Asopa S., Moorjani N., Saad R.A. Rare and fatal complication of Gianturco tracheobronchial stent. Ann Thorac Surg. 2007;84:1758–1760. doi: 10.1016/j.athoracsur.2007.03.092. [DOI] [PubMed] [Google Scholar]
- 19.Frerk C., Mitchell V.S., McNarry A.F. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. 2015;115:827–848. doi: 10.1093/bja/aev371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad I., El-Boghdadly K., Bhagrath R. Difficult Airway Society guidelines for awake tracheal intubation (ATI) in adults. Anaesthesia. 2020;75:509–528. doi: 10.1111/anae.14904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nouraei S.A., Giussani D.A., Howard D.J. Physiological comparison of spontaneous and positive-pressure ventilation in laryngotracheal stenosis. Br J Anaesth. 2008;101:419–423. doi: 10.1093/bja/aen171. [DOI] [PubMed] [Google Scholar]
- 22.Malpas G., Hung O., Gilchrist A. The use of extracorporeal membrane oxygenation in the anticipated difficult airway: A case report and systematic review. Can J Anaesth. 2018;65:685–697. doi: 10.1007/s12630-018-1099-x. [DOI] [PubMed] [Google Scholar]
- 23.Nouraei S.A., Mills H., Butler C.R. Outcome of treating airway compromise due to bronchial stenosis with intralesional corticosteroids and cutting-balloon bronchoplasty. Otolaryngol Head Neck Surg. 2011;145:623–627. doi: 10.1177/0194599811413683. [DOI] [PubMed] [Google Scholar]
- 24.Himeji D., Tanaka G.I., Fukuyama C. Severe tracheal stenosis after first administration of pembrolizumab rescued by Dumon Y-stent in a lung cancer patient. Respir Med Case Rep. 2019;28 doi: 10.1016/j.rmcr.2019.100923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato R., Kakizaki T., Hangai N. Bronchoplastic procedures for tuberculous bronchial stenosis. J Thorac Cardiovasc Surg. 1993;106:1118–1121. [PubMed] [Google Scholar]
- 26.Mark L., Lester L., Cover R. A decade of difficult airway response team: Lessons learned from a hospital-wide difficult airway response team program. Crit Care Clin. 2018;34:239–251. doi: 10.1016/j.ccc.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Coyle M., Martin D., McCutcheon K. Interprofessional simulation training in difficult airway management: A narrative review. Br J Nurs. 2020;29:36–43. doi: 10.12968/bjon.2020.29.1.36. [DOI] [PubMed] [Google Scholar]
- 28.Takenaka I., Aoyama K., Iwagaki T. The sniffing position provides greater occipito-atlanto-axial angulation than simple head extension: A radiological study. Can J Anaesth. 2007;54:129–133. doi: 10.1007/BF03022009. [DOI] [PubMed] [Google Scholar]