Abstract
Introduction
The objective of this study was to evaluate the cell viability of layered cell sheets, irradiated with 222 nm UV light.
Methods
UV transmittance of 222 nm and 254 nm was evaluated when the cell sheets of NCTC Clone 929 cells were irradiated UV light. Cell viability was evaluated after irradiation of 222 nm using 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay. Following irradiation of two layered cell sheets at 500 mJ/cm2, the cell damage of lower layers was evaluated by a colony formation and MTT assays.
Results
The UV transmittance of 222 nm was 10 times less than that of 254 nm. A MTT assay revealed that cells of cell sheets irradiated at 222 nm was less damaged than those at 254 nm, when irradiated at 5 mJ/cm2. Cell colonies were formed for cells of lower layers irradiated at 222 nm whereas no colony formation was observed for those irradiated at 254 nm. Significantly higher MTT activity was observed for cells of lower layers irradiated at 222 nm than at 254 nm.
Conclusions
It is concluded that 222 nm irradiation is biologically safe for cell viability.
Keywords: Sterilization, UV, Cellular viability, Cell sheet
Abbreviations: MTT, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide; 3D, 3-dementional; PBS, phosphate-buffered saline; Kr–Cl, krypton-chloride; SD, standard deviation; H2O2, hydrogen peroxide; CPDs, cyclobutane-pyrimidine dimers
Highlights
-
•
The cell viability of two-layered cell sheets was evaluated after irradiation of UV light at 222 nm.
-
•
UV light at 222 nm is safer to the lower layer than the conventional UV light at 254 nm.
-
•
The reason can be attributed to the lower transmission of UV light at 222 nm through cell sheets.
-
•
UV light at 222 nm could be one of promising tools to be required for the sterilization in the field of regenerative therapy.
1. Introduction
It has been recently attracted considerable attention to culture cells of 3-dementional (3D) and apply them for drug screening or cell therapies. Patient cells derived 3D cell aggregates or spheroids and xenografts are one of the advanced drug screening models that reflect cancer heterogeneity [[1], [2], [3]]. There have been reported on 3D cell constructs based on cell sheet technology. It has been demonstrated that oral mucosal epithelial cell sheets are transported and transplanted on endoscopic submucosa [[4], [5], [6], [7], [8], [9], [10]].
It has been a considerable problem for the usage of 3D cell constructs that there exists abundant microorganism on the surface of the constructs. When cells derived from patients are cultured, it is quite important to confirm that the contamination is free. Bacterial contamination is a practical problem which cannot be escaped. There are three reasons. First, the end products are invalid. Second, the consequent cost is wasted. Last, the operators are often exposed to the risks of infection. Therefore, at cell processing centers, the contamination is carefully paid much attention to be prevented [11]. In addition, virus infection is also considered a serious problem because of the operators’ risks, and distortion of experimental results [12].
There are several conventional sterilization methods, but they have limitations. For example, anti-bacterial agents are not always effective for all types of microorganisms, although the effect depends on their sterilization mechanisms. Low-pressure mercury lamps of 254 nm UV-C can sterilize most of microbes without remaining agents. However, it is found that they have cytotoxic effects, such as damage at DNA levels. Recently, 207/222 nm UV-C are studied because they can sterilize almost all microbes and biologically safer to tissues [[13], [14], [15]]. Mammalian cells are composed of proteins. Most proteins show 10-fold more absorption coefficient at 222 nm than at 254 nm [16]. In case of spherical cells, nucleus and DNAs are covered with cytoplasm and protected [17]. A previous study demonstrates that UV irradiation of 222 nm induces no DNA mutagenesis on mice [15]. On the other hand, 222 nm UV irradiation can kill many species of microbes similarly to 254 nm [18]. However, little has been evaluated on the biological safety of 222 nm UV irradiation in a cellular level.
This study is undertaken to evaluate the cell damages of 222 nm UV irradiation for cell sheets. Following the irradiation to one or two-layered cell sheets, the cell damage of the one-layer sheet or the lower layer of the two-layered sheets (lower layer) was assessed by the conventional MTT and colony formation assays. The cell damage was compared with that of 254 nm UV irradiated. For the aseptic insurance, UV irradiation around 20–500 mJ/cm2 is practically required, although it depends on the type of microorganisms [18]. Based on this, the irradiation dose of 222 nm and 254 nm was selected in this study. First, we examined the UV transmittance of 222 nm and 254 nm through cell sheets. Second, the dose–response curve of UV lights was evaluated using 2D cultured cells. Third, the cell damages of lower cells when irradiated at 222 nm were evaluated. In addition, the viability assay of lower cells with high sensitivity was developed using layered cell sheets and confluent cells.
2. Materials and methods
2.1. Cell culture
NCTC Clone 929 cells (JCRB9003) were purchased from JCRB Cell Bank (Japanese Collection of Research Bioresources Cell Bank). The cells were cultured in MEM with Earle's Salts, L-Gln and Non-Essential Amino Acids, liquid with nonessential amino acids (Nacalai Tesque INC., 21,443–15), supplemented with 10 vol% fetal bovine serum (HyClone™, SH30910.03, GE Healthcare Life Sciences, England) and 1 vol % penicillin streptomycin (09367–34, Nacalai Tesque, Inc., Kyoto, Japan).
2.2. Preparation of cell sheets
UpCell 24 well plates were purchased from CellSeed Inc., Tokyo, Japan. The plate surface is covered with a temperature-responsible polymer [19]. Cells are seeded at the concentration of 8.3 × 104 cells/well and cultured at 37 °C in 5% CO2 - 95% atmospheric condition, while the culture medium was half changed every 1 or 2 days. L-ascorbic acid phosphate magnesium (013–19641, FUJIFILM Wako Pure Chemical Co., Osaka, Japan) was added into the culture medium as described below. As a stock solution, 10 mM of l-ascorbic acid phosphate magnesium was dissolved into sterilized phosphate-buffered saline solution (PBS) (pH 7.4, 1 wt%). PBS was purchased from Nissui Pharmaceutical Co. Ltd., Tokyo, Japan. The stock solution was dissolved by the culture medium at the volume ratio of 1:100. Further cell culture for 2 or 4 (for the lower layer), 7 (for UV transmittance measurement) or 10–11 days (for the upper layer for cell damage assay) was continued to obtain one cell sheets. Ascorbic acid stock solution was not added into culture medium post irradiation incubation.
To prepare the lower layers, cells were seeded on glass bottom dishes (D11140H, Matsunami Glass Ind. Ltd, Osaka, Japan), at the same concentration in cell sheets preparation. l-ascorbic acid phosphate magnesium was added into the culture medium as described above.
2.3. UV equipment
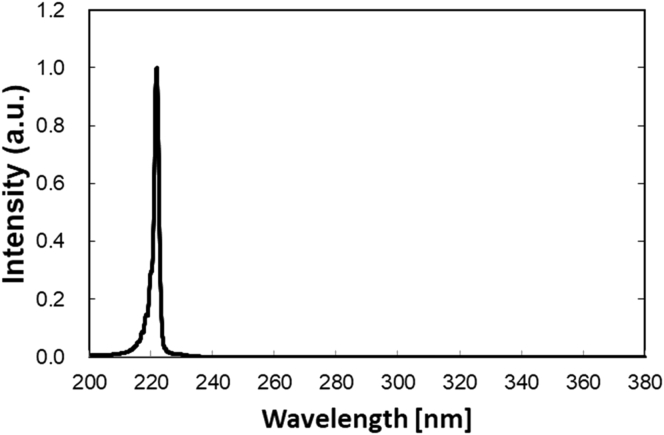
Two types of lamp devices were used for UVC light irradiation. One was a 222 nm-emitting device, which composes of a set of krypton-chloride (Kr–Cl) excimer lamp and other components. The other was a 254 nm light source, the conventional low-pressure mercury lamp (SUV-4, AS ONE co. Osaka, Japan). The 222-nm-emitting device (Ushio Inc. Tokyo, Japan) is composed of a Kr–Cl lamp, a custom band-pass filter, mirrors and air-cooling fan. Since a Kr–Cl excimer lamp emits other wavelength (around 230 nm–320 nm) slightly, a customized filter was used to block almost all wavelengths except for the dominant 222-nm (Fig. 1). Irradiance emitted by each device was measured using an S-172/UIT250 accumulated UV meter (Ushio Inc., Tokyo, Japan).
Fig. 1.
An emission spectrum of UV-C light of Kr–Cl excimer lamp.
2.4. Measurement of UV transmittance of cell sheets
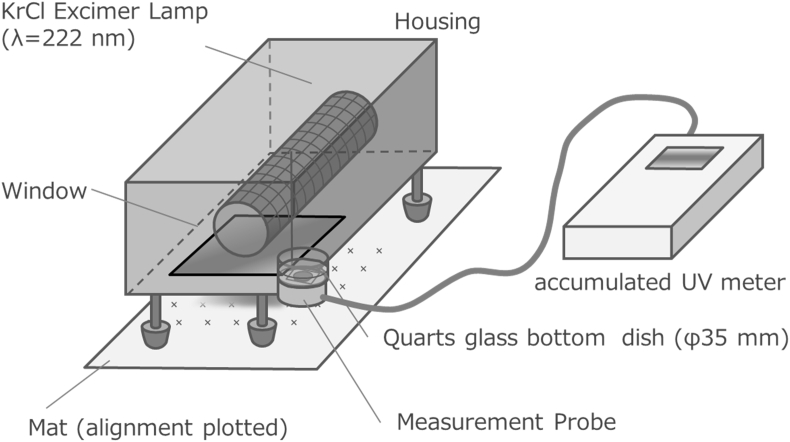
Cells were seeded on a quarts bottom dish (D11130S, Matsunami Glass Ind. Ltd, Osaka, Japan). A cell suspension of 135 μl, 3.0 × 105 cells/ml was seeded on the quartz surface of dishes. After 6 or 7 h of incubation, 2 ml/dish of culture medium with 100 μM of l-ascorbic acid was added into each dish. Culture medium was exchanged every 2 days. After 7 days of culture, the supernatant was discarded, and then cells were washed with 2 ml/dish of PBS for three times, following by addition of 1 ml fresh PBS. UV transmittance of one cell sheet was measured as shown in Fig. 2. To measure the baseline, a cell sheet-free blank culture dish with PBS was used. The baseline was measured every time before and after UV transmittance measurement for each sample. To increase the measurement accuracy, 1-min accumulated UV irradiance was measured. Experiments were independently performed 3 times for each sample unless otherwise mentioned.
Fig. 2.
An experimental set up to measure UV transmittance of cell sheets.
2.5. MTT assay of monolayer cultured cells
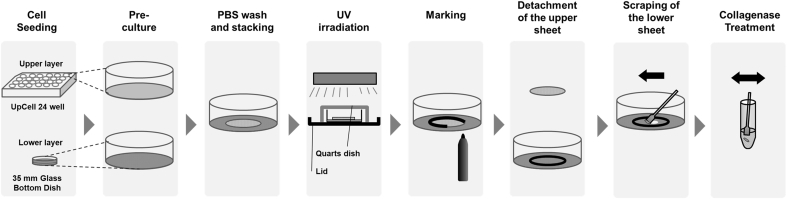
Cells were seeded on glass bottom dishes (D11140H, Matsunami Glass Ind. Ltd, Osaka, Japan), similarly to the procedure described above. l-ascorbic acid phosphate magnesium was added into the culture medium. After 2 days of incubation, cells were washed with 2 ml/dish of PBS three times, the lid of culture dish was exchanged with a quarts dish (3-2445-02, AS ONE Co., Osaka, Japan), and monolayer cultured cells were irradiated by UV light as shown in Fig. 3. PBS was added to keep the cells wet. Next, PBS was discarded, and the fresh culture medium was added, following by additional incubation for 3 days.
Fig. 3.
A schematic illustration of colony formation assay of cells in the lower cell layer.
MTT assay was performed as described below. As a stock solution, MTT reagent (23,547–05, Nacalai Tesque, Inc., Kyoto, Japan) was dissolved into PBS to give the concentration 12 mM, and then the resultant solution was sterilized through the filtration. As a working solution, the MTT stock solution was dissolved into the culture medium at the volume ratio of 1:10.
The excessive culture medium was discarded, and 2 ml of MTT working solution was added into each sample. The cells were incubated at 37 °C for 2.5 h. After macroscopic and microscopic observation of each sample, the MTT working solution was removed. 300 μl of 2-propanol was added into each sample and left for 3.5 h to extract formazan from the cells. The formazan extract was diluted if needed in a 96-well plate and its absorbance was measured by a Microplate Reader (SpectraMax i3x, Molecular Devices Japan Co., Ltd., Tokyo, Japan).
2.6. UV irradiation to two-layered cell sheets
UV irradiation experiments were performed as Fig. 3. Cell sheets were rinsed three times with PBS before the UV exposure because the remaining culture medium might absorb UV lights. Cell sheets were cultured for 10 or 11 days, and then detached from UpCell by leaving at room temperature. The cell sheet (an upper layer) was carefully transferred by pipetting, and stacked on another cell layer (a lower layer). The lower layer was confluent cells, cultured for 4 days (MTT assay) or 2 days (colony formation assay), respectively. To exclude the medium remaining, the cell sheets were washed with PBS (1 ml for the upper sheets, 2 ml for the lower sheets, respectively) for 3 times before stacked. The upper cell sheets were stacked upon the lower cell sheets, and immediately after that, UV light was irradiated. The lid of culture dish was exchanged with a quarts dish (3-2445-02, AS ONE Co., Osaka, Japan), and the cell sheets were irradiated by UV lights as shown in Fig. 3. After irradiation, the outline of the upper cell sheet was marked using a pen. Next, PBS was added, and only the upper layer was carefully peeled off. The fresh culture medium was added the lower layer, and then the lower layer were cultured for MTT and colony formation assays.
2.7. MTT and colony formation assays of the lower layers
MTT assay was performed as described above, with some modification.
After MTT working solution was removed, and the center of the lower layer was cut into 7 mm diameter shape using cell scrapers and 200 μl pipette chips. The cells around the cut circles were removed using cell scrapers, and washed with 2 ml of PBS twice.
Colony formation assay was performed according to the procedure previously described with some modification [13,20]. A single cell suspension was prepared by the method previously reported with some modification [21].
A collagenase working solution was prepared immediately before use. Collagenase (S1746501, Nordmark, Cat.) was dissolved into serum-free culture medium at the concentration of 3 mg/ml, and kept at 4 °C until used. PBS was removed and the center 8 mm of the lower layer was scraped using mini-cell scraper (MCS 200, United Biosystems Inc., USA). The scraped cells were dipped into 220 μl of collagenase working solution in a 1.5 ml tube, and agitated at 37 °C 400 rpm using a shaker. Cells were agitated by pipetting, and 200 μl of 2.5 g/l tripsin- 1 mmol/l-EDTA solution was added into each tube. Samples were agitated at 37 °C 400 rpm for 5 min with a shaker, and 600 μl of PBS containing 10 vol % FCS was added. Samples were centrifuged at 1000 rpm for 5 min, the supernatant was removed carefully, and the pellets were again suspended in 100 μl of PBS containing 10 vol % FCS. The cell suspension of 100, 1,000, and 10,000 cells/well (including both live and dead cells evaluated by trypan-blue exclusion assay) was seeded into 6-well plates, and incubated for 6 days.
After the medium change, further 3 days incubation was performed. Then, the medium was discarded, and 5 ml of PBS was added for cells rinsing. The cells were stained with 2 ml mixed 6 vol/vol % glutaraldehyde and 0.5 w/vol % crystal violet aqueous solution for 30 min. After rinsing with tap water and dried, the number of colonies in each well was counted and macroscopic images were taken.
2.8. Statistical analysis
All the experimental data were described as the mean ± standard deviation (SD). Student t-test or Tukey–Kramer paired comparison test was used to statistical analysis. Statistical significance was accepted at the p value of less than 0.05.
3. Results
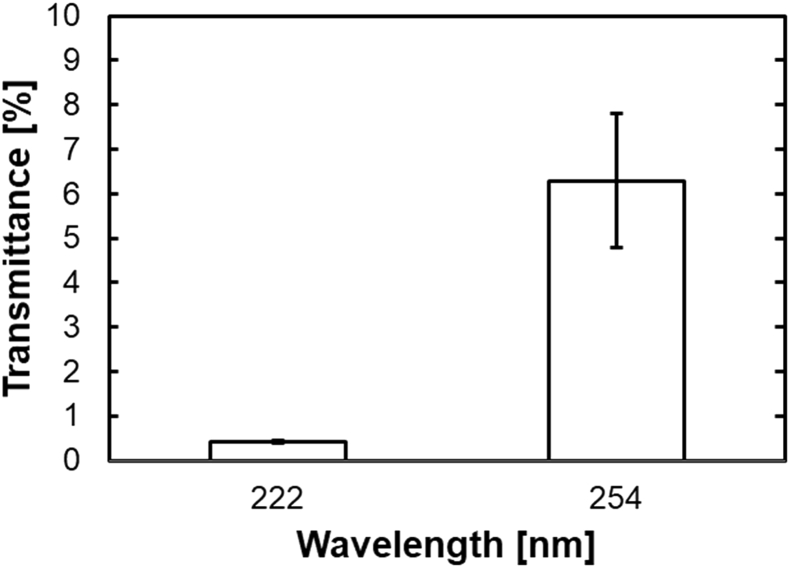
3.1. UV transmittance of cell sheets
Fig. 4 shows the UV transmittance of adherent cell sheets, which are cultured on the quartz bottom dishes. The transmittance was 0.41 ± 0.03% and 6.29 ± 1.51%, for 222 nm and 254 nm UV irradiation, respectively.
Fig. 4.
UV transmittance for cell sheets seeded on quartz bottom dishes.
3.2. MTT assay of only one layered cells
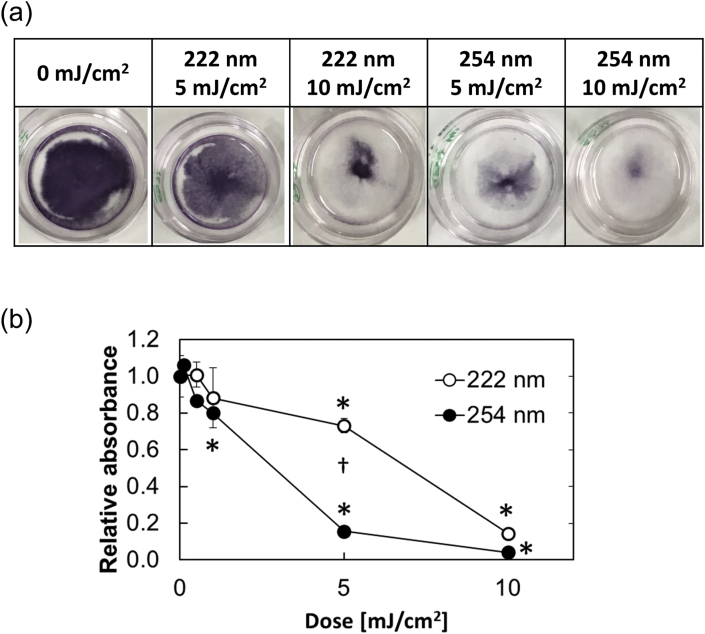
Fig. 5a shows the macroscopic images, and Fig. 5b shows MTT dose–response curve of cells irradiated by 222 nm or 254 nm. The IC50 values of cells were about 8 and 4 mJ/cm2 for 222 and 254 nm UV irradiation, respectively.
Fig. 5.
MTT assay of cell sheets 3 days after incubation. The cells were irradiated at 5 or 10 mJ/cm2 of 222 or 254 nm UV light. (a) Macroscopic images of cells with or without UV irradiation. (b) Dose–response of MTT assay; (〇) 222 nm and (●) 254 nm ∗, p < 0.05, significant difference between the negative controls and the irradiated groups. †, p < 0.05, significant difference between 222 and 254 nm UV light irradiated groups at the corresponding dose.
3.3. MTT assay of lower cell sheets
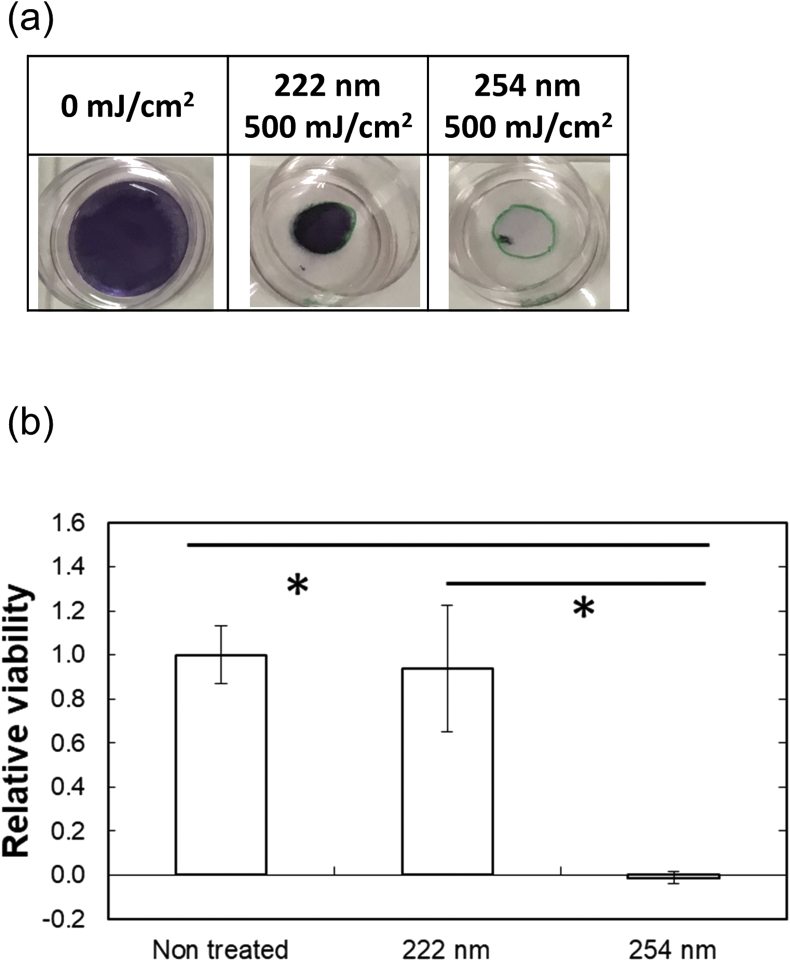
Fig. 6a shows the macroscopic images of bottom cell sheets, stained with the MTT reagents. Green circles at the center of dishes show the outline of the upper sheets. The violet area of MTT activity completely disappeared for the area without covered by the upper cell sheets by the irradiation of 222 and 254 nm. On the contrary, at the area covered by the upper cell sheets, the violet area remained after irradiation of 222 nm, in a remarked contrast to that of 254 nm irradiation.
Fig. 6.
MTT assay of lower cell layers 3 days after incubation. (a) Macroscopic images of cells with or without UV irradiation. (b) (〇) 222 nm and (●) 254 nm ∗, p < 0.05, significant difference between the two groups.
The quantitative result of MTT assay (Fig. 6b) also confirmed the significantly higher MTT activity for the 222 nm group than for 254 one. The MTT activity was similar to that of no treatment.
3.4. Colony formation assay of lower cell sheets
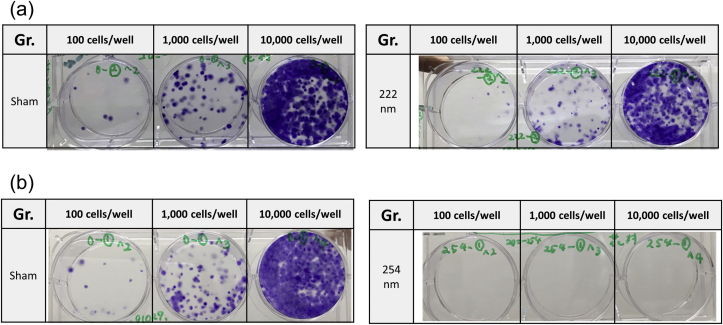
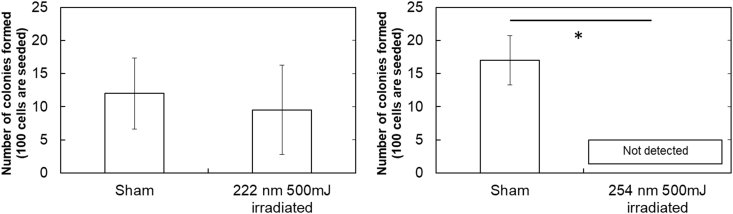
Fig. 7a and b shows the representative images of colonies. Fig. 8 shows the quantitative results of cell colonies formed. The surviving fractions of cells were 0.79 ± 0.56 and 0.0 ± 0.0 for 222 and 254 nm irradiation, respectively.
Fig. 7.
Colony formation assay 9 days after incubation. The cells were irradiated at 500 mJ/cm2of 222 or 254 nm UV light. (a) Macroscopic images of colonies with or without UV irradiation of 222 (a) or 254 nm (b).
Fig. 8.
Dose–response of colony formation assay; (〇) 222 nm and (●) 254 nm ∗, p < 0.05, significant difference between the two groups.
4. Discussion
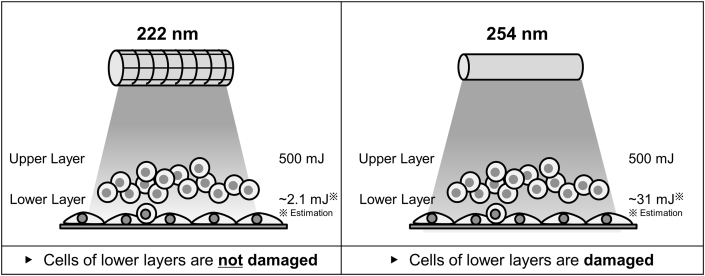
This study demonstrates that cell damages for 222 nm irradiation was significantly less than those of 254 nm. This strongly indicates the biological safety of 222 nm UV light for cells applicable for regenerative therapy. Following the UV irradiation to two-layered cells, the cell damages the lower layer cells with or without the coverage of the upper cell sheets were evaluated. The dose of 500 mJ/cm2 for 222 nm and 254 nm irradiation was used, because the dose can decrease almost all representative species of microbes to – 3 logs [18]. The UV transmittance experiment (Fig. 4) indicates that the lower layer was irradiated around 2.1 mJ (222 nm) or 31 mJ (254 nm), when the two-layered cells were irradiated at 500 mJ/cm2. Our result shows the similar tendency to previous studies. A previous study reported that 222 nm is absorbed by proteins 10 times more than 254 nm [16]. In addition, the UV transmittance of 254 nm is found to be 10 times more than 220 nm [22].
MTT dose–response assay of 222 nm or 254 nm demonstrates that the 222 nm irradiation gave cells less damage than the 254 nm one (Fig. 5a and b). The IC90 of a shorter wavelength UV was reported to be higher than that of 254 nm [23]. It is suggested that the difference might be due to the shielding of nucleus by cytoplasm and membrane [24]. This study results are similar to those of researches previously reported. When irradiated at 500 mJ/cm2, the cells of layered cells would be damaged by the 254 nm irradiation, but not by the 222 nm. It is apparent from Fig. 5a that cells at the center of culture dishes were less damaged than those at the peripheral. That might be due to the morphological difference of cells. It is possible that the cells at the center tended to pile up to form a multi-layer, resulting in less cell damage. The MTT dose–response of 254 nm is experimentally confirmed by several researches previously reported [[25], [26], [27]]. We can say with certainty that 254 nm irradiation at higher than 10 mJ/cm2 gave cells damages heavy enough to lose their viability.
Furthermore, in this study, the cell damage of two-layered cells was conducted. When irradiated at 500 mJ/cm2 of 222 nm, the lower layer is estimated to be irradiated at 2.1 mJ/cm2. It is expected considering the dose–response result (Fig. 5b), the lower layer is not damaged similarly to the negative control. On the other hand, when irradiated at 500 mJ/cm2 of 254 nm, the lower cell layer will be estimated to be irradiated at 31 mJ/cm2. As the result, the lower cell layer is not viable. At least - 4 log reduction was observed for the colony forming ability. Taken together, it is highly conceivable that the lowest transmittance of 222 nm through cells resulted in the less cell damage of the lower cell layer (Fig. 9).
Fig. 9.
Result and illustration of cell viability irradiated at 222 and 254 nm.
The colony formation assay has the highest sensitivity to detect the cell damage. MTT assay is also highly sensitive compared with other cell damage assays [28]. In addition, in this study, the cell damages of 2D-culture cells were evaluated using a novel method as shown in Fig. 3. This method enables a simpler cell damage assay, compared with other types of 3D-cultured viability assays. Generally, to evaluate the cell damage of 3D cultured cells, combined fluorescence staining and confocal laser microscopy is used [[29], [30], [31]]. However, there are several difficulties to evaluate the cell damages of 3D cultured cells. Since it takes time to allow fluorescent reagents to penetrate inside 3D cultured cells, it is likely that the superficial layers are stained more strongly than inside areas. Based on that, it is experimentally difficult to understand whether or not strongly stained areas are highly viable because of the concentration gradient of reagents. In this study, to avoid the concern, two-layered cell sheets were used. Following the two layered cell sheets were UV irradiated, the cell damage of lower cell layer covered with the upper layer was examined by the colony formation and MTT assays to evaluate the biological activity loss of cells in a depth direction. The sensitivity of colony formation assay to evaluate the cell viability was high when compared with other assays, such as MTT and sulforhodamine B assays [32].
Materials applicable for regenerative therapy products are usually obtained from easily accessible cell sources, such as skins [33] or oral mucosa [34]. The tissues anatomically position on the outer surface of bodies, and the structure is of stratified epithelium to protect bodies. When cell products are prepared from the types of tissues, it is important to protect their basal layers, which have biological activities to be expected. In the case of oral mucosal epithelial cell sheets, punch biopsy samples of oral mucosal tissue are used [34]. Stem cells which exist in the basal layer, are essential and important to prepare the cell sheets in terms of the therapeutic effect expected [35]. Cytokeratin 4, which is expressed in the middle and superficial layer of naïve oral mucosal epithelium, was often hardly expressed in the final products of cell sheets [36]. The UV irradiation at 222 nm, which has an ability to kill cells only on the surface layer, would be a promising tool to easily sterilize the surface of skin and oral tissues. Antibacterial agents are one of the most commonly used sterilization methods for cell processing. However, they sometimes give some damages to some types of cells [37]. It is reported to use amphotericin-B at a lower concentration than that commercially recommended to avoid the cytotoxic effects [38]. This study clearly indicates that the irradiation of 222 nm maintained the cell viability of lower layers of two-layered cells, even at the high dose. The 222 nm irradiation could be a promising option for novel sterilization method. Another sterilization method of hydrogen peroxide (H2O2) exposure is also cytotoxic [39] and the H2O2 sometimes remains on the surface of the materials exposed [40,41]. On the contrary, 222 nm UV light irradiation does not have such a remaining problem and is a convenient method to switch ON/OFF quickly.
In this study, there are three limitations to evaluate the cell viability. First, all the experiments are conducted for NCTC Clone 929 cells. The NCTC Clone 929 is easy to prepare cell sheets, and it is well known as one of often used cell lines for the cytotoxicity assay [42,43]. Based on the findings, in this study, the cell was selected as a model cell. For further study and applications, other types of cells used for practical applications, such as mesenchymal stem cells and matured cells, should be used.
Second, only two types of viability assays are conducted in this study, such as MTT and colony formation assays. There are so many types of cell damage or viability assays, and none of them can prove the cell death directly. Among them, MTT and colony formation assays are as one of the most sensitive viability assay of cells [32]. The sensitive assays are demonstrated to accurately detect the cell damage of lower cell layers which are covered with the upper cell layers, irradiated at 222 nm. However, there is one of the most major DNA damages, such as cyclobutane-pyrimidine dimers (CPDs) [44]. Further evaluation at the DNA level would give us further understanding on the safety of 222 nm irradiation.
Third, the biological function of cells is important for cell applications. The influence of 222 nm irradiation on the cellular biological functions should be investigated.
It is concluded that UV light at 222 nm is safer to the lower layer than the conventional UV light at 254 nm, which showed a high cytotoxicity to the lower layer. UV light at 222 nm could be one of promising tools to be required for the sterilization of cell products in the future.
Declaration of competing interests
The authors declare no competing interests.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Hidalgo M., Amant F., Biankin A.V., Budinska E., Byrne A.T., Caldas C. Patient-derived xenograft models: an emerging platform for translational cancer research. Canc Discov. 2014;4:998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izumchenko E., Paz K., Ciznadija D., Sloma I., Katz A., Vasquez-Dunddel D. Patient-derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann Oncol. 2017;28:2595–2605. doi: 10.1093/annonc/mdx416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo J., Endo H., Okuyama H., Ishikawa O., Iishi H., Tsujii M. Retaining cell-cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci U S A. 2011;108:6235–6240. doi: 10.1073/pnas.1015938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi N., Isomoto H., Kobayashi S., Kanai N., Kanetaka K., Sakai Y. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci Rep. 2017;7:17460. doi: 10.1038/s41598-017-17663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerqueira M.T., Pirraco R.P., Martins A.R., Santos T.C., Reis R.L., Marques A.P. Cell sheet technology-driven re-epithelialization and neovascularization of skin wounds. Acta Biomater. 2014;10:3145–3155. doi: 10.1016/j.actbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Kirby G.T.S., Michelmore A., Smith L.E., Whittle J.D., Short R.D. Cell sheets in cell therapies. Cytotherapy. 2018;20:169–180. doi: 10.1016/j.jcyt.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Masumoto H., Yamashita J.K. Human iPS cell-engineered three-dimensional cardiac tissues perfused by capillary networks between host and graft. Inflamm Regen. 2018;38:26. doi: 10.1186/s41232-018-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuura K., Utoh R., Nagase K., Okano T. Cell sheet approach for tissue engineering and regenerative medicine. J Contr Release. 2014;190:228–239. doi: 10.1016/j.jconrel.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto R., Miyagawa S., Toda K., Kainuma S., Yoshioka D., Yoshikawa Y. Long-term outcome of ischemic cardiomyopathy after autologous myoblast cell-sheet implantation. Ann Thorac Surg. 2019;108:e303–e306. doi: 10.1016/j.athoracsur.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Mizutani M., Samejima H., Terunuma H., Kino-Oka M. Experience of contamination during autologous cell manufacturing in cell processing facility under the Japanese Medical Practitioners Act and the Medical Care Act. Regen Ther. 2016;5:25–30. doi: 10.1016/j.reth.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shioda S., Kasai F., Watanabe K., Kawakami K., Ohtani A., Iemura M. Screening for 15 pathogenic viruses in human cell lines registered at the JCRB Cell Bank: characterization of in vitro human cells by viral infection. Roy Soc Open Sci. 2018;5 doi: 10.1098/rsos.172472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buonanno M., Randers-Pehrson G., Bigelow A.W., Trivedi S., Lowy F.D., Spotnitz H.M. 207-nm UV light - a promising tool for safe low-cost reduction of surgical site infections. I: in vitro studies. PloS One. 2013;8 doi: 10.1371/journal.pone.0076968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buonanno M., Stanislauskas M., Ponnaiya B., Bigelow A.W., Randers-Pehrson G., Xu Y. 207-nm UV light-A promising tool for safe low-cost reduction of surgical site infections. II: in-vivo safety studies. PloS One. 2016;11 doi: 10.1371/journal.pone.0138418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narita K., Asano K., Morimoto Y., Igarashi T., Nakane A. Chronic irradiation with 222-nm UVC light induces neither DNA damage nor epidermal lesions in mouse skin, even at high doses. PloS One. 2018;13 doi: 10.1371/journal.pone.0201259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreusch S., Schwedler S., Tautkus B., Cumme G.A., Horn A. UV measurements in microplates suitable for high-throughput protein determination. Anal Biochem. 2003;313:208–215. doi: 10.1016/s0003-2697(02)00460-8. [DOI] [PubMed] [Google Scholar]
- 17.Coohill T.P., Knauer D.J., Fry D.G. The effect of changes in cell geometry on the sensitivity to ultraviolet radiation of mammalian cellular capacity. Photochem Photobiol. 1979;30:565–572. doi: 10.1111/j.1751-1097.1979.tb07181.x. [DOI] [PubMed] [Google Scholar]
- 18.Clauss M. Higher effectiveness of photoinactivation of bacterial spores, UV resistant vegetative bacteria and mold spores with 222 nm compared to 254 nm wavelength. Acta Hydrochim Hydrobiol. 2006;34:525–532. [Google Scholar]
- 19.Haraguchi Y., Shimizu T., Sasagawa T., Sekine H., Sakaguchi K., Kikuchi T. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat Protoc. 2012;7:850–858. doi: 10.1038/nprot.2012.027. [DOI] [PubMed] [Google Scholar]
- 20.Franken N.A., Rodermond H.M., Stap J., Haveman J., van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 21.Washio K., Kuroda H., Iwata T., Yoshida T., Yamato M., Okano T. Improved enzymatic treatment for accurate cell counting from extracellular matrix-rich periodontal ligament cell sheets. Int J Oral Maxillofac Implants. 2014;29:e117–e121. doi: 10.11607/jomi.te50. [DOI] [PubMed] [Google Scholar]
- 22.Coohill T.P. Virus-cell interactions as probes for vacuum ultraviolet radiation-damage and repair. Photochem Photobiol. 1986;44:359–363. doi: 10.1111/j.1751-1097.1986.tb04676.x. [DOI] [PubMed] [Google Scholar]
- 23.Kochevar I.E., Walsh A.A., Green H.A., Sherwood M., Shih A.G., Sutherland B.M. DNA damage induced by 193-nm radiation in mammalian-cells. Canc Res. 1991;51:288–293. [PubMed] [Google Scholar]
- 24.Green H., Boll J., Parrish J.A., Kochevar I.E., Oseroff A.R. Cytotoxicity and mutagenicity of low intensity, 248 and 193 nm excimer laser radiation in mammalian cells. Canc Res. 1987;47:410–413. [PubMed] [Google Scholar]
- 25.Suzuki F., Han A., Lankas G.R., Utsumi H., Elkind M.M. Spectral dependencies of killing, mutation, and transformation in mammalian cells and their relevance to hazards caused by solar ultraviolet radiation. Canc Res. 1981;41:4916–4924. [PubMed] [Google Scholar]
- 26.Lackinger D., Eichhorn U., Kaina B. Effect of ultraviolet light, methyl methanesulfonate and ionizing radiation on the genotoxic response and apoptosis of mouse fibroblasts lacking c-Fos, p53 or both. Mutagenesis. 2001;16:233–241. doi: 10.1093/mutage/16.3.233. [DOI] [PubMed] [Google Scholar]
- 27.Tyrrell R.M., Pidoux M. Action spectra for human skin cells: estimates of the relative cytotoxicity of the middle ultraviolet, near ultraviolet, and violet regions of sunlight on epidermal keratinocytes. Canc Res. 1987;47:1825–1829. [PubMed] [Google Scholar]
- 28.Fotakis G., Timbrell J.A. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;160:171–177. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y., Gao D., Liu H., Lin S., Jiang Y. Drug cytotoxicity and signaling pathway analysis with three-dimensional tumor spheroids in a microwell-based microfluidic chip for drug screening. Anal Chim Acta. 2015;898:85–92. doi: 10.1016/j.aca.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Garcia I., Henriksen-Lacey M., Calvo J., de Aberasturi D.J., Paz M.M., Liz-Marzan L.M. Size-dependent transport and cytotoxicity of mitomycin-gold nanoparticle conjugates in 2D and 3D mammalian cell models. Bioconjugate Chem. 2019;30:242–252. doi: 10.1021/acs.bioconjchem.8b00898. [DOI] [PubMed] [Google Scholar]
- 31.Theumer A., Grafe C., Bahring F., Bergemann C., Hochhaus A., Clement J.H. Superparamagnetic iron oxide nanoparticles exert different cytotoxic effects on cells grown in monolayer cell culture versus as multicellular spheroids. J Magn Magn Mater. 2015;380:27–33. [Google Scholar]
- 32.Banasiak D., Barnetson A.R., Odell R.A., Mameghan H., Russell P.J. Comparison between the clonogenic, MTT, and SRB assays for determining radiosensitivity in a panel of human bladder cancer cell lines and a ureteral cell line. Radiat Oncol Invest. 1999;7:77–85. doi: 10.1002/(SICI)1520-6823(1999)7:2<77::AID-ROI3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 33.Liu W., Chen B., Deng D., Xu F., Cui L., Cao Y. Repair of tendon defect with dermal fibroblast engineered tendon in a porcine model. Tissue Eng. 2006;12:775–788. doi: 10.1089/ten.2006.12.775. [DOI] [PubMed] [Google Scholar]
- 34.Takagi R., Murakami D., Kondo M., Ohki T., Sasaki R., Mizutani M. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:1253–1259. doi: 10.1016/j.gie.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Kasai Y., Morino T., Kikuchi S., Mitsuyoshi R., Takahashi M., Yamamoto K. Analysis of human nasal mucosal cell sheets fabricated using transported tissue and blood specimens. Regen Ther. 2019;11:88–94. doi: 10.1016/j.reth.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami D., Yamato M., Nishida K., Ohki T., Takagi R., Yang J. Fabrication of transplantable human oral mucosal epithelial cell sheets using temperature-responsive culture inserts without feeder layer cells. J Artif Organs. 2006;9:185–191. doi: 10.1007/s10047-006-0342-3. [DOI] [PubMed] [Google Scholar]
- 37.Uribe C.C., Dos Santos de Oliveira F., Grossmann B., Kretzmann N.A., Reverbel da Silveira T., Giugliani R. Cytotoxic effect of amphotericin B in a myofibroblast cell line. Toxicol Vitro. 2013;27:2105–2109. doi: 10.1016/j.tiv.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Takagi R., Kobayashi S., Yamato M., Owaki T., Kasai Y., Hosoi T. How to prevent contamination with Candida albicans during the fabrication of transplantable oral mucosal epithelial cell sheets. Regenerative Therapy. 2015;1:1–4. doi: 10.1016/j.reth.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asada S., Fukuda K., Nishisaka F., Matsukawa M., Hamanisi C. Hydrogen peroxide induces apoptosis of chondrocytes; involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm Res. 2001;50:19–23. doi: 10.1007/s000110050719. [DOI] [PubMed] [Google Scholar]
- 40.Ikarashi Y., Tsuchiya T., Nakamura A. Cytotoxicity of medical materials sterilized with vapour-phase hydrogen peroxide. Biomaterials. 1995;16:177–183. doi: 10.1016/0142-9612(95)92115-m. [DOI] [PubMed] [Google Scholar]
- 41.Chihara R., Kitajima H., Ogawa Y., Nakamura H., Tsutsui S., Mizutani M. Effects of residual H2O2 on the growth of MSCs after decontamination. Regen Ther. 2018;9:111–115. doi: 10.1016/j.reth.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hexig B., Nakaoka R., Tsuchiya T. Safety evaluation of surgical materials by cytotoxicity testing. J Artif Organs. 2008;11:204–211. doi: 10.1007/s10047-008-0429-0. [DOI] [PubMed] [Google Scholar]
- 43.Isama K., Matsuoka A., Haishima Y., Tsuchiya T. Proliferation and differentiation of normal human osteoblasts on dental Au-Ag-Pd casting alloy: comparison with cytotoxicity to fibroblast L929 and V79 cells. Mater Trans. 2002;43:3155–3159. [Google Scholar]
- 44.Rastogi R.P., Richa Kumar A., Tyagi M.B., Sinha R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids. 2010;2010:592980. doi: 10.4061/2010/592980. [DOI] [PMC free article] [PubMed] [Google Scholar]