Summary
Background
Our overall objective is to develop a single-stage in-theatre skin replacement by combining small explants of skin with a synthetic biodegradable dermal scaffold. The aim of the current study is to determine the concentration of fibrin constituents and their handling properties for both adhering skin explants to the scaffold and encouraging cellular outgrowth to achieve reepithelialization.
Methods
Small skin explants were combined with several concentrations of thrombin (2.5,4.5,and 6.5 I.U) and fibrinogen (18.75,67, and 86.5 mg/ml), cultured in Green's media for 14 days and cellular outgrowth was measured using Rose Bengal staining. They were also cultured on electrospun scaffolds for 14 and 21 days. Hematoxylin and eosin (H&E) staining was undertaken to visualize the interface between skin explants and scaffolds and metabolic activity and collagen production were assessed.
Results
A thrombin/fibrinogen combination of 2.5 I. U/ml /18.75 mg/ml showed significantly greater cell viability as assessed by Rose Bengal stained areas at days 7 and 14. This was also seen in DAPI images and H&E stains skin explant/scaffold constructs. Fibrin with a concentration of thrombin 2.5 I.U./ml took 5–6 min to set, which is convenient for distributing skin explants on the scaffold.
Conclusion
The study identified concentrations of thrombin (2.5 I.U/ml) and fibrinogen (18.75 mg/ml), which were easy to handle and aided the retention of skin explants and permitted cell outgrowth from explants.
Keywords: Meek grafting, Dermal substitute, Fibrin, Tissue-engineered skin
Introduction
While the development of tissue-engineered skin to provide barrier cover has made significant advances since the early 1980s1 there has been little adoption into routine clinical practice. Tissue-engineered skin substitutes require a dermal analogue and a cell source to populate it, consisting of autologous keratinocytes and fibroblasts. However, infrastructure costs (clean room facilities approved by Regulatory authorities and specialist cell culture staff) and production time (up to 3 weeks) have made adoption into routine clinical practice challenging.
To overcome this, we proposed a one-step in-theatre approach, using small squares of split thickness skin (STS) (called skin explants) as the cell source instead of isolated cells combined with an electrospun scaffold to act as a neodermis for skin replacement2.
The use of skin explants follows the principle of Meek grafting, which uses small squares of STS laid on excised wound beds in a predetermined expansion ratio for reepithelization.
Our dermal analogue is a templated trilayer electrospun scaffold made from Poly (D, L-lactide-co-glycolide) lactide: glycolide 75:25 (PLGA) and Polyhydroxybutyrate/Polyhydroxyvalerate (PHBV). The polymers used and the design of scaffolds mimic the microstructure of human skin and have been shown to be suitable for skin cell migration2, 3, 4. The upper and bottom layers of PLGA (75:25) facilitate cell migration from explants, ingrowth of fibroblasts, and collagen deposition on the wound bed, respectively. A templated PHBV middle layer mimics the rete ridges found between the epidermis and dermis.
Our initial study revealed that skin cells migrated from the cut edge of skin explants that are in contact with scaffold, but not within a suitable period for sustainable clinical translation (ideally 2–3 weeks). We also lost explants that failed to adhere to scaffolds. Failure to reepithelialize within a timely manner can lead to unpredictable and unfavorable scarring. Therefore, we hypothesized to optimize cellular migration, constant contact between skin explants and scaffolds that are required to be maintained throughout the culture period.
We proposed the use of fibrin to aid the adherence of skin explants onto the scaffold. The use of fibrin has previously been used in our laboratory to aid the retention of corneal explants on a human amniotic carrier for corneal regeneration5. This technique called simple limbal epithelial transplantation (SLET) has been clinically translated and is now adopted by more than twenty centers worldwide6,7.
Fibrin is a meshwork of insoluble protein fibers formed when thrombin (a serine protease) converts the soluble fibrinogen to protofibrils, which aggregate into insoluble fibrin8,9. It results in the release of cytokines and growth factors that promote reepithelialization, angiogenesis, and collagen deposition10. Furthermore, fibroblasts are stimulated to proliferate and produce extracellular matrix replacing the fibrin on the neodermis. Keratinocytes are also stimulated by the fibrin meshwork to detach from unaffected wound edges (in this case skin explants) and start migration10,11 . The thrombin concentration has a significant influence on fibrin structure12. Low concentrations of thrombin (< 1 nM, < 0.1 U/mL) produce thick, loosely woven fibrils of fibrin, while higher concentrations produce relatively thinner, more tightly packed fibrils13. Accordingly, it is important to determine the range of thrombin and fibrinogen concentrations that will produce fibrin, which is easy to handle, facilitates adherence of skin explants on scaffolds and has a microstructure to allow for cellular migration from explants onto the scaffold. One such fibrin product in clinical use is TISSEEL™ (Baxter Healthcare Corp, Deerfield, IL, USA). This has a thrombin concentration ranging from 400 to 625 units/mL and a fibrinogen concentration ranging from 67 to 106 mg/mL14. As this is FDA approved, widely available, and used routinely, it was felt that it would be beneficial to test its suitability in our in vitro constructs, as this would make clinical translation using fibrin more realistic.
This study has two related aims:1 to determine the optimum concentration of thrombin and fibrinogen to achieve a fibrin meshwork that will allow for the adhesion of skin explants.2 Also to facilitate the outgrowth of skin cells from skin explants and to assess handling properties of thrombin and fibrinogen combinations with respect to the placing of skin explants on the electrospun scaffold.
Methods
Preparation of skin explants
STS was obtained under a Human Tissue Authority research tissue bank licence:15/YH/0017 anonymously from patients undergoing breast reconstruction using excess unwanted abdominal skin. It was harvested manually under sterile conditions by using a Watson knife set at 2 mm. Square pieces measuring 5 × 5 mm2 were cut and placed in phosphate-buffered saline (PBS). They were then finely minced into 16–20 squares to create skin explants, which were kept in Green's media. Green's media consists of a 3:1 v/v of Dulbecco's modified Eagle's medium and Ham's F12 (Sigma-Aldrich, Dorset UK) supplemented with 10% v/v fetal calf serum (Biosera Sussex, UK), 10 ng mL−1 epidermal growth factor (human recombinant - R&D systems, Biotechne, Minnesota, USA), 0.4 μg mL−1 hydrocortisone, 1 × 10−10 mol mL−1 cholera toxin, 1.8 × 10−4 mol L−1 adenine, 5 μg mL−1 insulin, 2 × 10−3 mol L−1 glutamine, 0.625 μg mL−1 amphotericin B, 100 IU mL−1 penicillin, and 100 μg mL−1 streptomycin (all from Sigma Aldrich, Dorset, UK).
Fibrin preparation
Fibrinogen from human plasma (Sigma- Aldrich, UK) (Table 1) was added to 1 ml of 0.9% sodium chloride (w/v) and dissolved for 4 h at 37 °C, then filter sterilized. Human thrombin (Sigma-Aldrich, UK; 1KU) was dissolved in 4 ml of distilled water (dH2O) to obtain a concentration of 250 I.U/ml and filter sterilized. Various concentrations were obtained by diluting the thrombin (250 IU/ml) with dH2O (Table 1).
Table 1.
Various concentrations of fibrinogen and thrombin.
| Experiment | Concentration of thrombin (I.U)/ml | Concentration of fibrinogen mg/ml | ||
|---|---|---|---|---|
| 1 | 2.5 | 18.75 | 67 | 86.5 |
| 2 | 4.5 | 18.75 | 67 | 86.5 |
| 3 | 6.5 | 18.75 | 67 | 86.5 |
Electrospinning of PLGA–PHBV– PLGA trilayer scaffolds
10 wt.% PLGA (75:25) was made by dissolving PLGA (Sigma-Aldrich, Dorset, UK) in dichloromethane (DCM, Sigma Aldrich, Dorset, UK). 10 wt.% PHBV, 12% biopolymer solutions were made by dissolving PHBV (Goodfellow, Cambridge limited, Huntingdon, England) in 10 wt.% methanol and 80 wt.% DCM (solvent ratio 88.8: 11.1 DCM–MeOH, (Sigma Aldrich)). These solutions were loaded into four separate 5 ml syringes (20 ml in total) fitted with blunt-tip needles (0.6 mm ID), and loaded into a syringe pump (Genie Plus, Kent Scientific, Connecticut, USA). Metal templates were attached (using carbon conductive tape (Agar Scientific, Stanstead, UK)) to the center of an earthed 10 × 10 cm copper board wrapped in aluminum foil to create the templated PHBV- that mimics the rete ridges. Nontemplated scaffolds simply did not have the metal template, so the PBHV layer was flat. A working distance of 17 cm from the needle tip was used for PLGA and 10 cm for PHBV. A potential of +17,000 V was applied to the needle tips (73030P, Genvolt, Shropshire UK), and a flow rate of 40 μl min−1 per syringe was used. Trilayer membranes were electrospun by consecutively spinning a PHBV–PLGA bilayer as above. The bilayer was peeled off the backing foil, turned over to reveal the uncoated PHBV side, and reattached using autoclave tape to the aluminum foil. A final coating of PLGA was spun on the exposed PHBV face using the above-mentioned conditions.
Assessment of cell outgrowth from explants onto tissue culture plastic using rose bengal staining
STS explants in Green's media were placed in the center of polystyrene (with a cell culture coating (proprietary)) six well plates (CostarT, Corning, ME, USA) – referred to as tissue culture plastic to which varying concentrations of thrombin and fibrinogen were added (Table 1). They were incubated at 37 °C, 5% CO2 for 14 days. After removing culture media, 1 ml of 1% Rose Bengal (Sigma–Aldrich, UK) was added and incubated for 5 min at room temperature. Samples were washed with PBS to remove excess Rose Bengal, and photographed against a white background to allow the quantitation of the stained region surrounding the explant using ImageJ (NIH, USA) data expressed as mean ± SEM. Statistical analysis was performed by using Graphpad Prism 7.0 and a two-sample t-test with no assumption of equal variance.
Detection of cellular migration using fluorescence microscopy
Constructs were fixed using 3.7% formaldehyde (Sigma-Aldrich, Dorset, UK). Media were discarded and constructs were washed three times with sterile PBS. To each well plate, 3.7% formaldehyde was added and incubated for at least 2 h at 4°C. DAPI (4′,6-diamidino-2-phenylindole, 1 ng/ml in PBS) of 1 ml was added to each fixed sample and incubated in the dark at 37°C for 30 min. Samples were washed with PBS and imaged using an Axon ImageXpress microscope (AxonCorp, USA) at excitation and emission wavelengths of 360 nm and 480 nm, respectively. DAPI-stained cell nuclei adjacent to skin explants indicate migration.
Culture of skin explants on trilayer electrospun scaffolds and fibrin or TISSEELTM
Sterilized 1.5 × 1.5 cm scaffolds were placed in a 6 well plate. To this, skin explants suspended in Greens media, followed by either 30 µl of fibrinogen and 30 µl of thrombin (concentrations and units as described) or TISSEEL™ were added and cultured for 14 and 21 days, respectively (37 °C, 5% v/v CO2).
Detection of cellular migration onto scaffold fibers using H&E staining
Formalin (Sigma-Aldrich, Dorset, UK) fixed samples were embedded in OCT compound, snap frozen in liquid nitrogen, and 7 μm cryosections cut using a cryotome (Leica, Germany). Sections were washed in dH2O for 2 min before incubation in Harris's Hematoxylin (Sigma Aldrich, Dorset, UK) for 2 min. Samples were then washed in dH2O for 1 min, followed by incubation in Eosin Y (water based, Sigma Aldrich, Dorset UK) for 5 min. Samples were then rinsed with dH2O and mounted in glycerol before imaging on a light microscope.
Assessment of metabolic activity and collagen deposition of TISSEEL™/Skin explant constructs
Rezazurin (5 µg/ml in PBS) was used to assess cellular viability. Media were removed from constructs followed by incubation in 2.5 ml of Rezazurin for 1 h at 37°C. Rezazurin (200 µl) was taken from each sample and absorbance at 570 nm measured (Bio-tek ELx800). For collagen deposition, 2 ml of 1 mg/ml Sirius red (F3B (C.I. 35,780, Direct Red 80, Sigma–Aldrich, Dorset, UK) dissolved in saturated picric acid was added to each fixed construct and shaken for 18 h at room temperature. After prolonged washing with PBS (no further red color was eluted), the remaining stain from scaffolds was eluted by incubating constructs in 500 μl of destaining solution (0.2 M NaOH/methanol (1:1)) for 30 min. Samples (100 μl) were taken and absorbance was measured at 490 nm (Bio-tek ELx800).
Results
Characterization of electrospun scaffolds
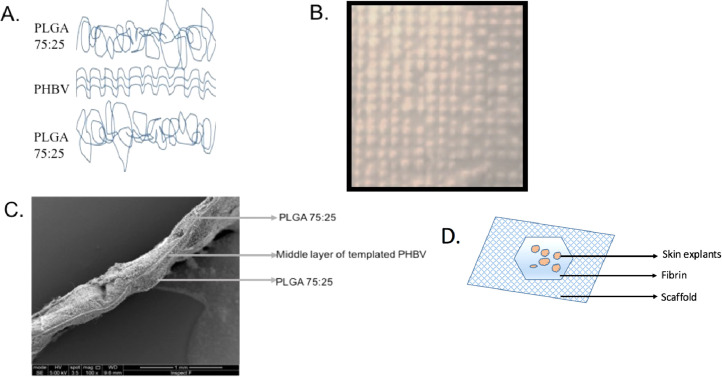
Figure 1 shows that PLGA fibers formed a random microfibrous mesh on either side of the thinner PHBV random layer to create a “trilayer” structure. The average overall thickness of scaffolds was 0.178 (range: 0.155–0.22) mm. Pore and fiber diameters were measured for two polymers from 100 fibers of 3 different magnifications (x 500, x2000, and x5000; n = 3). The PHBV component of scaffolds consisted of randomly oriented fibers with a mean diameter of 1.89 µm ± 0.072, and pore size of 4.16 µm ± 0.2. PLGA layers consisted of randomly oriented fibers with a mean diameter of 2.22 µm ± 0.074 and pore size of 8.062 µm ± 0.304.
Figure 1.
A: Templated trilayer scaffold comprising upper and lower layers of randomly arranged PLGA75:25 microfibers and a central templated PHBV layer. B: Photograph of templated PHBV scaffold. C: SEM image of trilayer scaffold, the same showing templates and strands of PGLA on the surface. D: Illustration depicting the experimental set up of scaffold with fibrin and skin explants on top of the fibrin.
Detection of cellular migration through a fibrin meshwork using rose bengal staining
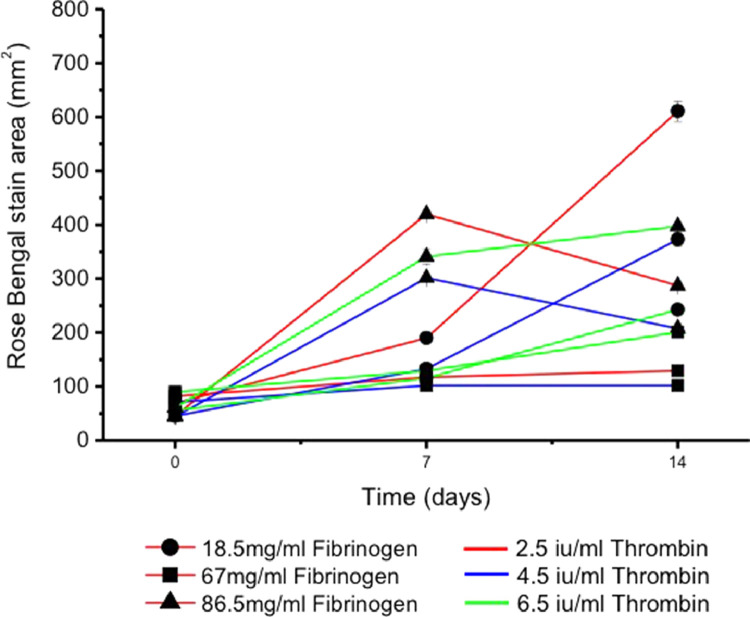
Rose Bengal stains by localizing primarily in cellular nuclei and, to a lesser degree other organelles15. It allowed us to measure the extent of cellular outgrowth throughout the 14-day culture period as the outward spread of the stain (pink against a white background) could be easily measured as an indicator of outward cellular migration without the need to fix constructs, which would result in cell death5. Figure 2 shows a progressive increase in the Rose Bengal stained area for the thrombin groups 2.5 I. U /ml and 4.5 I. U/ml with all concentrations of fibrinogen from day 0 to day 14. Constructs containing thrombin/fibrinogen 2.5 I. U /ml /18.75 mg/ml showed significantly greater stained areas at day 7 and 14 compared to the other two fibrinogen concentrations (p = 0.0041 at day 7 and p = 0.0005 at day 14) (Figure 2). Constructs with a thrombin concentration of 6.5 I. U/ml showed a decrease in the stained area from day 7 to 14 at all fibrinogen concentrations apart from 86.5 mg/ml, which had a small but insignificant increase compared to the other fibrinogen concentrations (p = 0.436).
Figure 2.
Area of Rose Bengal Staining over a 14-day culture period using constructs containing various combinations of thrombin (Red line = 2.5 IU/ml, blue line = 4.5 IU/ml, and green line = 6.5 IU/ml) and fibrinogen (Circle = 18.5 mg/ml, square = 7 mg/ml, and triangle = 86.5 mg/ml) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Detection of cellular migration through a fibrin meshwork onto tissue culture plastic using fluorescence microscopy
A range of thrombin and fibrinogen concentrations were examined for their effect on cellular migration from skin explants onto tissue culture plastic. The results as shown in Figure 3, indicate that cellular migration surrounding skin explants was greatest using the lowest concentration of thrombin and fibrinogen. The migration of skin cells seemed to decrease as the fibrinogen concentration increased with negligible migration of cells from skin explants in the other thrombin/fibrinogen group combinations.
Figure 3.
Identification of cell outgrowth from skin explants onto tissue culture plastic with explants attached, using varying concentrations of thrombin and fibrinogen, and assessed using DAPI fluorescence staining to identify cells. Scale bars represent 50 µm.
The use of various concentrations of thrombin and fibrinogen to secure explants onto electrospun trilayer scaffolds
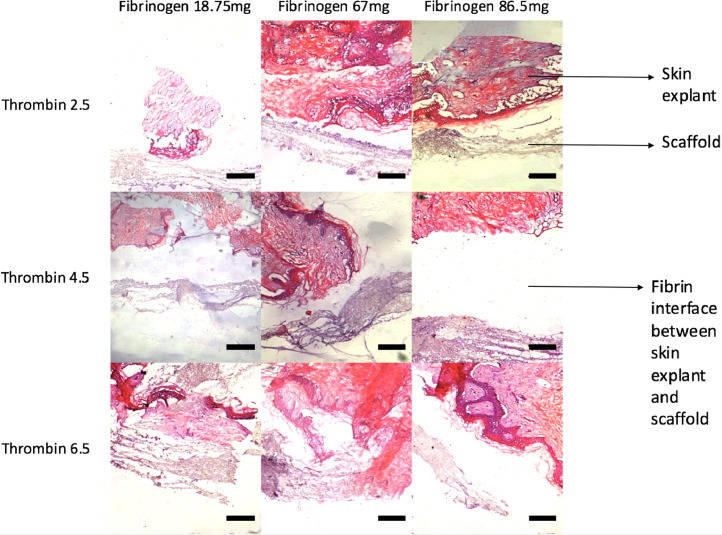
A range of thrombin and fibrinogen concentrations were used to assess the ease of handling of the various fibrin compositions and their effect on the attachment of explants to the trilayer scaffolds. Figure 4 shows H&E histology from various concentrations of thrombin and fibrinogen cultured with skin explants on scaffolds for 21 days. Gaps between skin explants and scaffolds at higher thrombin/fibrinogen concentrations were larger compared to explants incubated with lower concentrations of thrombin and fibrinogen. At the lowest thrombin concentration, 2.5 I.U /ml, explants were found to be directly attached to scaffolds, seen for all three concentrations of fibrinogen. We also wanted to have sufficient time to position the skin explants onto the scaffold before the fibrin sets, and a less dense clot is desirable as it allows explants to be spread evenly on the surface rather than clumping into a mass. As expected, the time to set was influenced by the concentration of thrombin and fibrinogen as summarized in Table 2. Higher concentrations of thrombin and fibrinogen resulted in dense clots, which set within 1–2 min and did not allow the even spread of explants, whereas lower concentrations set within 5–6 min.
Figure 4.
H&E slides of varying combinations of thrombin and fibrinogen illustrating the resultant fibrin clots, which have developed between skin explants and scaffolds. Scale bars represent 50 µm.
Table 2.
Relationship between thrombin concentration and time taken for fibrin to set.
| Thrombin concentration (I.U/ml) | Time taken for fibrin clot to set (min) |
|---|---|
| 2.5 | 5–6 |
| 4.5 | 3–5 |
| 6.5 | 1–2 |
| TISSEEL™:500 | 0.16–0.33 |
The use of commercially available fibrin (TISSEEL™) to secure explants onto electrospun trilayer scaffolds
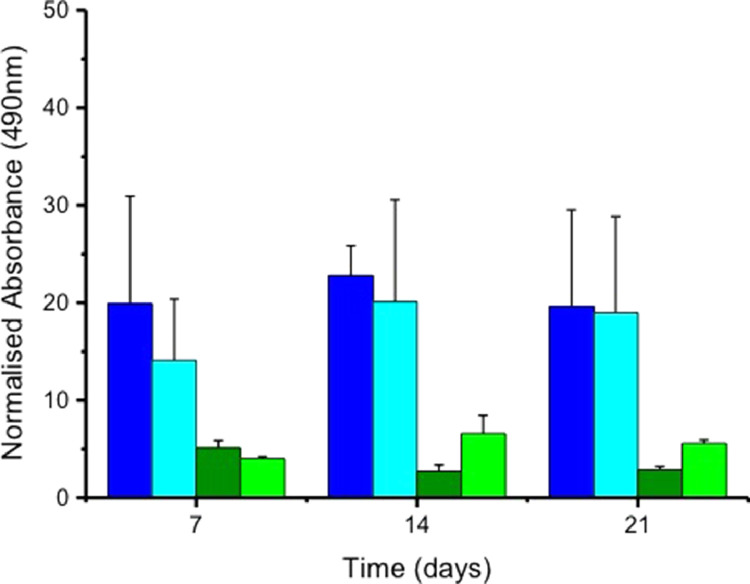
We assessed the use of TISSEEL™ to secure skin explants onto electrospun scaffolds and cultured samples for 21 days. The metabolic activity and total collagen production of constructs with and without TISSEEL™ were measured at 7,14, and 21 days using Rezazurin and Sirius red, respectively. Figure 5 illustrates that constructs with TISSEEL™ were viable throughout the culture period, with a gradual increase in metabolic activity at 3 weeks. Samples without fibrin were shown to have lower metabolic activity rates throughout the culture period. The addition of TISSEEL™ to constructs resulted in an overall increase in collagen production compared to samples without fibrin (Figure 6). However, TISSEEL™ in keeping with its intended use as a hemostat, set into a solid dense clot in under a minute. This gave little time for skin explants to be spread on scaffolds and there was a tendency for skin explants to clump.
Figure 5.
Metabolic activity of skin explants with and without Tisseel on templated and nontemplated trilayer electrospun scaffolds over a 21-day period (mean ± SEM). In all cases, explants were placed on the scaffolds, which were either templated or nontemplated and in half of the experiments explants were kept in place using Tisseel fibrin glue.
Figure 6.
Collagen production of skin explants with and without Tisseel on templated and nontemplated trilayer electrospun scaffolds over a 21-day period determined by staining for collagen with Sirius red. Results shown are mean ±SEM, (n = 3). In all cases, explants were placed on scaffolds, which were either templated or nontemplated and in half of the experiments explants were kept in place using Tisseel. █ Templated Scaffolds with Tisseel, █ Nontemplated Scaffolds with Tisseel, █ Templated Scaffolds, and █ Nontemplated Scaffolds.
Discussion
Our aim was to identify a fibrin composition that could be used to adhere skin explants onto a synthetic dermal substitute and promote cellular outgrowth from explants.
This idea of using tissue explants rather than cultured cells is based on the MEEK technique for skin expansion and on our laboratories’ experience of introducing tissue explants rather than cultured cells for achieving corneal regeneration5, 6, 7. SLET removes the need for specialized facilities and trained staff to culture cells for clinical use. Scar tissue is debrided from the affected eye, a limbal sample from the unaffected eye is minced into small explants and placed on a biodegradable human amniotic membrane attached to the debrided eye using fibrin. Corneal cells migrate out of the limbal explants to form a new cornea for 6–8 weeks and results using this technique have proven slightly better than using laboratory expanded corneal cells7.
With respect to resurfacing a full thickness skin defect, we suggest a synthetic biodegradable scaffold as a proto-synthetic dermal substitute instead of human donor tissue. These electrospun scaffolds can be made in large quantities from biodegradable, FDA approved, synthetic polymers, which can be tailored to degrade very slowly (100% PLLA) or rapidly (50/50 PLGA)3 and sterilized using gamma radiation, which is nontoxic16. We suggest these polymers are suitable for providing a synthetic dermal scaffold to be repopulated in situ on the patient using tissue explants.
The use of fibrin is a logical choice to adhere explants to the scaffold but depending on the constituent concentrations of thrombin and fibrinogen, fibrin can promote or inhibit cell outgrowth. In this study, we wished to establish a working composition of fibrin glue to use for this purpose. Thus, we looked at the effect of a range of concentrations of thrombin and fibrinogen on cellular outgrowth from explants onto tissue culture plastic, the ease of handling of fibrin including the time taken to set on scaffolds and the ability of the fibrin to attach explants to scaffolds.
The first concentration tested was based on our previous work for corneal tissue engineering where a 1:1 mixture of fibrinogen (18.75 mg/ml) and thrombin (2.5 IU/ml), was found to maintain limbal explants on the scaffold and also facilitate outward cellular migration. A wider range of thrombin and fibrinogen were included based on concentrations found in commercial fibrin glue preparations. Thrombin/fibrinogen concentrations of 2.5–6.5 IU/67–86.5 mg/ml are based on Artiss™ a fibrin adhesive for skin graft attachment17. The relatively low thrombin concentration allows up to 6 min to position grafts or flaps before setting. The fibrinogen concentrations that we chose (67 mg and 86.5 mg) reflects the lower and average fibrinogen concentration of Artiss™.
We found that the thrombin/fibrinogen concentrations that most facilitated our requirements were 2.5 I. U/ml /18.75 mg/ml, respectively. This resulted in the greatest cell outgrowth from skin explants (confirmed by Rose Bengal, DAPI, and H&E staining) and resulted in the smallest gap between skin explants and scaffolds. This handled well and allowed an even spread of skin explants.
TISSEEL™ was also assessed for its ability to fix skin explants onto the electrospun scaffold14. We thought that if it proved suitable, it would make clinical translation using fibrin more realistic. However, it sets too quickly, and was too firm in texture, tending to clump explants preventing an even distribution on scaffolds. One possibility is to use TISSEEL™ kit itself but dilute the concentration of thrombin from 500 I.U to 2.5 I.U with normal saline. In conclusion, we have established a suitable fibrin composition that can be used to allow skin explants to adhere to electrospun scaffolds and facilitate outward cellular migration.
Declaration of Competing Interest
None.
Acknowledgments
The Maurice Wohl Fellowship, Royal College of Surgeons Edinburgh for funding Miss Sharma's work.
Dr. Ilida Ortega Asencio for providing metallic collectors for the manufacture of electrospun scaffolds.
Footnotes
This work was presented at the British Burns Association Meeting, Swansea Apr 11–13th 2018.
References
- 1.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445:874–880. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 2.Sharma K., Bullock A., Ralston D. Development of a one-step approach for the reconstruction of full thickness skin defects using minced split thickness skin grafts and biodegradable synthetic scaffolds as a dermal substitute. Burns. 2014;40:957–965. doi: 10.1016/j.burns.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Blackwood K.A., McKean R., Canton I. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials. 2008;29:3091–3104. doi: 10.1016/j.biomaterials.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Bye F.J., Bissoli J., Black L. Development of bilayer and trilayer nanofibrous/microfibrous scaffolds for regenerative medicine. Biomater Sci. 2013;1:942–951. doi: 10.1039/c3bm60074b. [DOI] [PubMed] [Google Scholar]
- 5.Deshpande P., Ramachandran C., Sefat F. Simplifying corneal surface regeneration using a biodegradable synthetic membrane and limbal tissue explants. Biomaterials. 2013;34:5088–5106. doi: 10.1016/j.biomaterials.2013.03.064. [DOI] [PubMed] [Google Scholar]
- 6.Basu S., Sureka S.P., Shanbhag S.S. Simple limbal epithelial transplantation: long-term clinical outcomes in 125 cases of unilateral chronic ocular surface burns. Ophthalmology. 2016;123:1000–1010. doi: 10.1016/j.ophtha.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Sangwan V.S., Basu S., MacNeil S. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. British Journal of Ophthalmology. 2012;96:931–934. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 8.Janmey P., Winer J., Weisel J. Fibrin gels and their clinical and bioengineering applications. Journal of the Royal Society, Interface. 2009;6:1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed T.A.E., Dare E.V., Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 10.Laurens N., Koolwijk P., De Maat M. Fibrin structure and wound healing. Journal of Thrombosis and Haemostasis. 2006;4:932–939. doi: 10.1111/j.1538-7836.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 11.Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 12.Blomback B., Carlsson K., Fatah B. Fibrin in human plasma: gel architectures governed by rate and nature of fibrinogen activation. Thrombosis Research. 1994;75:521–538. doi: 10.1016/0049-3848(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 13.Wolberg A.S. Thrombin generation and fibrin clot structure. Blood Reviews. 2007;21:131–142. doi: 10.1016/j.blre.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Baxter. Tisseelhttps://www.fda.gov/downloads/biologicsbloodvaccines/bloodbloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm072968.pdf (Accessibility verified January 27, 2019).
- 15.Feenstra R.P., Tseng S.C. Comparison of fluorescein and rose bengal staining. Ophthalmology. 1992;99:605–617. doi: 10.1016/s0161-6420(92)31947-5. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran C., Sangwan V.S., Ortega I. Synthetic biodegradable alternatives to the use of the amniotic membrane for corneal regeneration: assessment of local and systemic toxicity in rabbits. British Journal of Ophthalmology. 2019 Feb;103:286–292. doi: 10.1136/bjophthalmol-2018-312055. [DOI] [PubMed] [Google Scholar]
- 17.Baxter. Artiss. https://www.fda.gov/downloads/biologicsbloodvaccines/bloodbloodproducts/approvedproducts/licensedproductsblas/fractionatedplasmaproducts/ucm073054.pdf (Accessibility verified January 27, 2019).