Abstract
Urinary tract infections (UTIs) belong to the most common community-acquired and nosocomial infections. A main etiological factor of UTIs is uropathogenic Escherichia coli (UPEC). This review describes the current state of knowledge on the resistance of UPEC to antibiotics recommended for the treatment of UTIs based on the available literature data. Nitrofurantoin and fosfomycin are recommended as first-line therapy in the treatment of uncomplicated cystitis, and the resistance to these antimicrobial agents remains low between UPEC. Recently, in many countries, the increasing resistance is observed to trimethoprim-sulfamethoxazole, which is widely used as the first-line antimicrobial in the treatment of uncomplicated UTIs. In European countries, the resistance of UPEC to this antimicrobial agent ranges from 14.6% to 60%. The widespread use of fluoroquinolones (FQs), especially ciprofloxacin, in the outpatients is the cause of a continuous increase in resistance to these drugs. The resistance of UPEC to FQs is significantly higher in developing countries (55.5–85.5%) than in developed countries (5.1–32.0%). Amoxicillin-clavulanic acid is recommended as first line-therapy for pyelonephritis or complicated UTI. Resistance rates of UPEC to amoxicillin-clavulanic acid are regionally variable. In European countries the level of resistance to this antimicrobial ranges from 5.3% (Germany) to 37.6% (France). Increasing rates of UPEC resistance to antimicrobials indicate that careful monitoring of their use for UTI treatment is necessary.
Key words: uropathogenic Escherichia coli, antibiotic resistance, antibiotic therapy, urinary tract infections, treatment of UTIs
Introduction
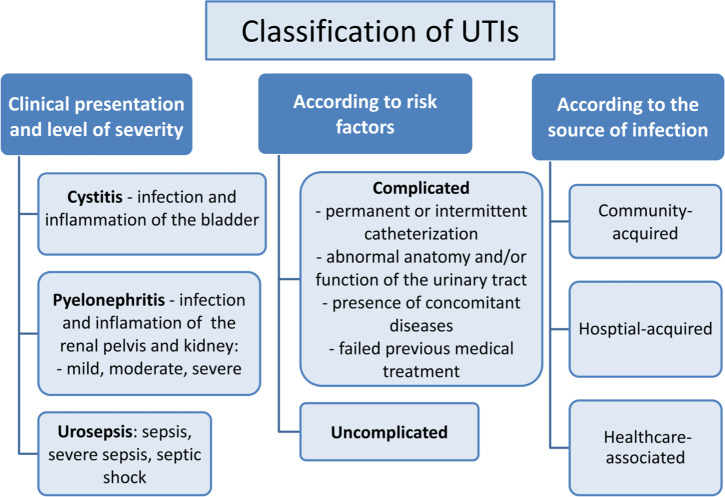
Urinary tract infections (UTIs) are among the most common bacterial infections in humans (Bischoff et al. 2018). It is estimated that 40% of women and 12% of men experience a minimum one symptomatic UTI episode during their lifetimes, and 27 to 48% of the affected women suffer from recurrent UTIs (Braumbaugh et al. 2013; Micali et al. 2014). UTIs comprise about 40% of all hospital-acquired infections and 50% of bacterial infections that contribute to increased morbidity causing prolonged hospitalization (Asadi Karam et al. 2019). UTIs are also an economic problem. In the United States, about 11 million people per year have been treated due to UTIs, generating the cost of about $6 billion (Mann et al. 2017). Healthcare-associated infections (HAI) are a serious threat for patients in terms of morbidity and mortality, with the healthcare-associated urinary tract infections (HAUTI) being among the most frequent HAI. In Europe, HAUTI account for 19.0% of all HAI (ECDCP 2013). Community- or healthcare-acquired UTIs are clinically divided into complicated or uncomplicated, and among many other factors, this classification determines what antimicrobial agents can be applied for treatment (Zacchè and Giarenis 2016). Complicated UTIs require prolonged therapy and occur in patients with renal failure, anatomical urinary tract abnormalities such as urinary obstruction and retention or in patients that use medical devices such as a catheter. Complicated UTIs are also associated with immunosuppression and previous antibiotic exposure. This category of UTIs increases the risk of chronic and/or recurrent infections. Uncomplicated UTIs are found in patients who have no anatomical urinary tract abnormalities and do not use the urinary tract instrumentation. In uncomplicated UTIs, host immune response may successfully fight infection without antibiotic therapy (Mann et al. 2017). The symptomatic UTIs are classified as urosepsis, pyelonephritis (infection of the upper UTI) or cystitis (infection of the lower UTI) (Terlizzi et al. 2017) (Fig. 1). The presence of numerous UPEC cells in the urine (≥ 105 CFU/ml) without the clinical symptoms is called asymptomatic bacteriuria (ABU) and in healthy non-pregnant women is not treated in 20–80% of cases (Schneeberger et al. 2014).
Fig. 1.
Classification of urinary tract infections (Bartoletti et al. 2016).
The increase of antibiotic resistance and appearance of multi-drug resistant (MDR) pathogens in the course of UTI is related to high rates of inadequate antibiotic empirical therapies prescribed without the antibiotic susceptibility testing and finally result in an ineffective UTI treatment (Adamus-Białek 2018). Wagenlehner et al. (2016) showed that among 27 542 patients from 856 urology units in 70 countries, 56% of the hospitalized patients were treated with antimicrobials. Among them, 46% received prophylactic antibiotic treatment, 26% of them had the antimicrobials prescribed for the microbiologically proven UTI, 21% − for suspected UTI, and 7% for other infections. The above study also has revealed that broad-spectrum antibiotics were applied such as fluoroquinolones (35%), cephalosporins (27%), and penicillins (16%). The results obtained by Cek et al. (2014) showed the correlation between the increased use of broad-spectrum antibiotics and increased antimicrobial resistance and multi-resistance of bacteria. These authors also observed that prophylactic antibiotic treatment of urological patients occurred most frequently in Asia, Africa, and Latin America (86%, 85%, and 84%, respectively), followed by Europe (67%). The increasing number of MDR isolates from UTIs of outpatients makes treatment more difficult. Risk factors of MDR isolated from UTIs include prior use of antimicrobials, hospitalization, genitourinary disturbances, age, and recurrent UTIs (Walker et al. 2016). Tenney et al. (2018) have recently analyzed the published data (25 studies including 31 284 patients with the confirmed UTI) to determine the risk factors for MDR isolated from UTIs and revealed that previous antibiotic treatment applied from 2 to 365 days earlier was the most commonly identified risk factor. The analysis by Tenney et al. (2018) showed also that urinary catheterization, previous hospitalization, and residence in a nursing home were strong risk factors of MDR isolated from UTI. Present work aimed to review the available literature published in 2016–2019 to investigate the prevalence of UPEC resistant to antibiotics recommended for the treatment of UTIs. The resistance to antibiotics of UPEC isolated in different regions of the world (European countries, North America, Asia, and some countries of Africa) was compared. The increase of resistance to fluoroquinolones, which was significantly higher in developing countries than in developed countries, has been revealed. Also, the resistance mechanisms identified in these pathogens were discussed.
Escherichia coli as a main etiological agent of UTIs
The bacteria belonging to the Enterobacteriaceae such as Klebsiella pneumoniae (about 7%), Proteus mirabilis (about 5%), Citrobacter, Enterobacter, and other bacteria such as Pseudomonas aeruginosa, Acinetobacter baumannii, Staphylococcus aureus, Staphylococcus saprophiticus, Enterococcus faecalis, Streptococcus bovis, and the fungus Candida albicans can cause UTIs (Hof 2017; Mann et al. 2017). However, among the bacterial species involved in UTIs, uropathogenic Escherichia coli strains (UPEC) are the most common. UPEC account for about 80% of uncomplicated UTIs, 95% of community-acquired infections, and 50% of hospital-acquired infections (Tabasi et al. 2016). UPEC also remains the most frequent pathogen in complicated UTIs (Bartoletti et al. 2016).
UPEC is a heterogeneous group of extraintestinal pathogenic E. coli (ExPEC) that seem to originate from the gut. Many studies suggest that farm animals may be reservoirs of E. coli strains carrying virulence genes responsible for UTI in humans. Comparison of the antimicrobial resistance profiles and genetic virulence determinants in the E. coli strains isolated from UTI patients, farm animals or meat (particularly chicken) showed high similarity (Jakobsen et al. 2010; Mellata et al. 2018). Food-borne urinary tract infections (FUTI) include UTIs acquired from bacteria-contaminated food (Nordstrom et al. 2013). ExPEC can survive in the alimentary tract but do not cause diseases of the digestive system. However, ExPEC strains present in other sites such as central nervous system, blood or the urinary tract may cause serious illness. Four main UPEC phylogroups (A, B1, B2, and D) were identified based on the genomic Pathogenicity Islands (PAI) occurrence and strains belonging to B2 and D groups are isolated most frequently from UTI (Kot et al. 2016; Asadi Karam et al. 2019). The intestinal E. coli enters the urinary tract system and colonizes the periurethral and vaginal areas and the urethra. After that, bacteria reach the bladder and attach to the surface epithelium using fimbrial and non-fimbrial adhesins (Mann et al. 2017). Adhering bacteria may be internalized into the uroepithelial facet cells, and then they can enter the cytoplasm, replicate and form intracellular bacterial communities (IBCs) being a source of quiescent intracellular reservoirs (QIRs) (Asadi Karam et al. 2019). The host immune system may remove some of the IBCs by exfoliation of bladder surface facet cells and excreting them with the urine but the remaining bacteria can grow as a biofilm resistant to immune mechanisms and antibacterial agents (McLellan and Hunstad 2016). Some of these bacteria escape from the biofilm, convert to motile form and disseminate into the bladder lumen or even ascend into the kidneys, causing pyelonephritis (Flores-Mireles et al. 2015). UPEC may also spread from the urinary tract to the bloodstream causing bacteremia. For 15% to 23% of episodes of UTI positive blood cultures could be obtained (Velasco et al. 2003; Bahagon et al. 2007; van Nieuwkoop et al. 2010). The results presented by Abernethy et al. (2017) suggest that treatment failure in UTIs, as well as an inappropriate use and management of urinary catheters in the hospital and community, are important risk factors for the development of E. coli bacteremia.
Treatment of UTIs
Based on the general European susceptibility patterns and according to the European Association of Urology guidelines, the following antimicrobial agents are recommended for treatment of uncomplicated cystitis in premenopausal women and uncomplicated pyelonephritis in all European countries: nitrofurantoin, fosfomycin trometamol and trimethoprim-sulfamethoxazole (TMP-SMZ) (Bartoletti et al. 2016). Nitrofurantoin and fosfomycin trometamol are recommended as first-line therapy for uncomplicated cystitis (Bonkat et al. 2017; Asadi Karam et al. 2019). TMP-SMZ is not indicated as the empirical treatment due to the high prevalence of bacterial resistance and can be considered only for the patients with a low prevalence of resistant E. coli (< 20%) (Bartoletti et al. 2016; Bonkat et al. 2017). Fluoroquinolones (ciprofloxacin and levofloxacin) play an important role in the treatment of more severe infections and septicemia, and thus, ciprofloxacin should be considered as an alternative, not as a first-line antibiotic, in the treatment of uncomplicated cystitis (Bartoletti et al. 2016). Ciprofloxacin could be used as second-line empiric therapy in cases of mild and moderate pyelonephritis or complicated UTI treatment, and as third-line empiric treatment for uncomplicated cystitis. Amoxicillin-clavulanic acid is recommended as first line-therapy for mild and moderate pyelonephritis or complicated UTI, as well as alternative empiric therapy for uncomplicated cystitis. For complicated UTI (high fever, sepsis, vomiting) or severe pyelonephritis, amoxicillin with gentamicin or a second-generation cephalosporin with an aminoglycoside are recommended as first-line empiric therapy, and third-generation cephalosporin applied intravenously as alternative empiric therapy (Bonkat et al. 2017; Cheung et al. 2017). The choice of antimicrobials for the treatment of UTI is also based on local resistance profiles of the pathogen.
Antimicrobial resistance of UPEC
UTIs are associated with significant use of antibiotics that cause implications for bacterial ecology and spread of resistance to antibiotics, especially when it stems from the empirical antimicrobial treatment of recurrent UTIs. Antimicrobial resistance in UPEC and the spreading of MDR UPEC in recent decades is a clinical problem, particularly in women with recurrent UTIs. The increasing frequency of MDR UPEC, especially in developing countries, results in excessive use of broad-spectrum antibiotics such as fluoroquinolones, cephalosporins, and aminoglycosides that raise the cost of treatment and hospitalization (Bartoletti et al. 2016; Sanchez et al. 2016). Antimicrobial resistance between UPEC is increasing in many countries and shows the time- and area-related variability (Fig. 2).
Fig. 2.
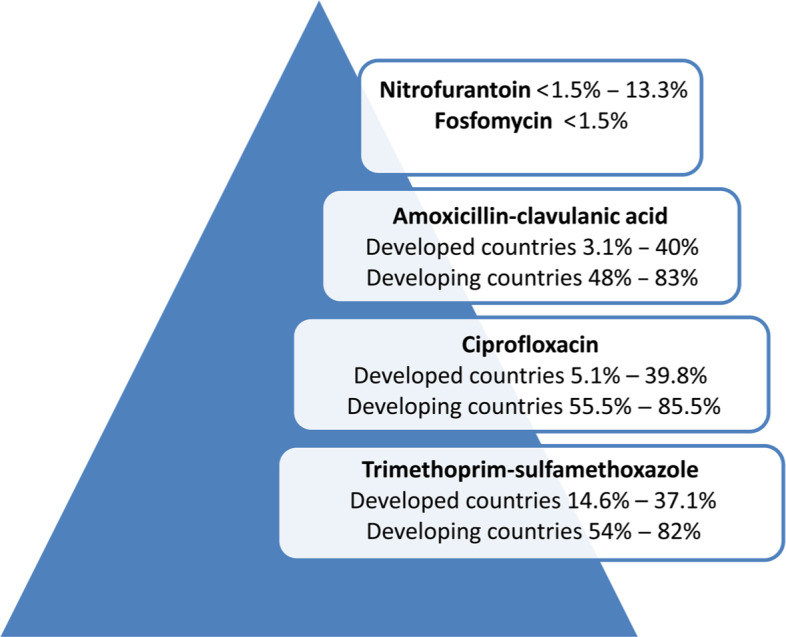
The resistance of UPEC to antimicrobials using in the treatment of UTIs.
Amoxicillin-clavulanic acid; the developed countries (USA, 3.1–40%; Germany, 5.3%; Poland, 13.9%; England, 30%; France, 37.6%), developing countries (Nepal, 48%; Pakistan, 71%; Jordan, 83%). Ciprofloxacin; developed countries (USA, 5.1–12.1%; Belgium, 12.9%; Germany, 10.5–17.3%; Switzerland.17.4%; England, 20.4%; France, 24.8%; Spain, 39.8%), developing countries (Jordan, 55.5%, Mongolia, 58.1%; Pakistan, 60.8%; Nepal, 64.6%; Ethiopia, 85.5%). Trimethoprim-sulfamethoxazole; developed countries (Belgium, 14.6%; USA, 17.4%; Germany, 18.45%; Poland, 21.4%; Switzerland, 24.5%; Spain, 30.9%; France, 37.1%), developing countries (Iran, 54%; Mexico, 66%; Ethiopia, 68.5%; Mongolia, 70.9%; Jordan, 73,1%; Pakistan, 82%).
Resistance to nitrofurantoin
According to the European Association of Urology guidelines (Bonkat et al. 2017), nitrofurantoin is recommended for the treatment of uncomplicated cystitis as first-line empiric therapy. At present, the resistance of UPEC to nitrofurantoin is very low, favoring its use as a first-line antibacterial agent. A retrospective analysis performed by Sanchez et al. (2016) showed that in the United States nitrofurantoin retains a high level of antibiotic activity against urinary E. coli. A comparison of the reports from the period of 2003 to 2012 revealed that resistance of E. coli isolates from adults to nitrofurantoin only slightly increased (from 0.7% to 0.9%). The rates of UPEC resistance in Germany, Belgium, and Spain in 2013–2014 were below 1.5% (Kresken et al. 2016). A slightly higher percentage of UPEC resistant to nitrofurantoin was observed among isolates from elderly hospitalized patients in Argentina (2.3%) (Delpech et al. 2018). While in Brazil the rate of UPEC isolates resistant to nitrofurantoin was 6.6% (Cunha et al. 2016). In European countries (Romania, Poland, France) the percentage of nitrofurantoin resistant UPEC isolated from outpatients and hospitalized patients ranged from 3% to 3.8% (Ciontea et al. 2018; Kot et al. 2016; Lavigne et al. 2016). The study concerning E. coli resistance rates at urology clinics in the Netherlands revealed that nitrofurantoin was active against 95% of strains (van der Donk et al. 2012). Among UPEC from inpatients and outpatients in Bosnia and Herzegovina, 8.23% isolates were resistant to nitrofurantoin (Abduzaimovic et al. 2016). Lack of susceptibility to nitrofurantoin among E. coli isolates from the urine of patients hospitalized in different hospitals in England was low (4.6–6.9%) (Abernethy et al. 2017). E. coli strains isolated from the hospitalized patients with UTIs in Mongolia (Munkhdelger et al. 2017), Pakistan (Ali et al. 2016), and Iran (Raeispour and Ranjbar 2018) showed that 5.4%, 6%, and 10% of strains were resistant to this antimicrobial agent, respectively. The study conducted by Prasada et al. (2019) in India showed that the resistance of UPEC isolated from hospitalized patients to nitrofurantoin was 13.3% and did not significantly change over 5 years (2013–2017). A similar rate of UPEC resistance to nitrofurantoin (12.7%) in Mexico was reported by Ramírez-Castillo et al. (2018). These results show that nitrofurantoin remains the drug of choice for the treatment of uncomplicated cystitis, although it should not be used for the treatment of pyelonephritis since its concentration in the renal parenchyma is too low. Additional characteristics, as high efficiency, cost-effectiveness, and weak adverse environmental impact suggest that nitrofurantoin should be the first-choice treatment in uncomplicated UTI in women.
Resistance to fosfomycin
Fosfomycin is an “old” antibiotic used for the treatment of drug-resistant bacterial infections. Fosfomycin shows activity against several Enterobacteriaceae species, including those expressing extended-spectrum β-lactamases (ESBL) and metallo-β-lactamases (MBL). Fosfomycin is currently approved for use in some European countries as a single 3 g dose for treating uncomplicated UTIs caused by E. coli in women (Dijkmans et al. 2017). The results of Hirsch et al. (2015) showed that all E. coli strains investigated were susceptible to fosfomycin. The comparative study on UPEC susceptibility to fosfomycin conducted in Germany, Belgium, and Spain, showed that only < 1.5% of isolates were resistant to this antibiotic (Kresken et al. 2016). In UTI caused by non-resistant uropathogens, clinical cure rates ranged from 87% to 93%, while microbiological cure rates ranged from 80% to 83%. The treatments of UTIs caused by MDR uropathogens with fosfomycin demonstrated an overall microbiological cure rate of 59% (Neuner et al. 2012). Antibacterial efficacy of fosfomycin is weaker compared to other first-line agents used in the treatment of uncomplicated UTIs, and thus this drug should be avoided if there is a suspicion of early pyelonephritis.
Resistance to trimethoprim-sulfamethoxazole
TMP-SMZ is another important and widely used first-line antimicrobial in the treatment of uncomplicated cystitis. The comparative study performed in the USA by Yamaji et al. (2018) showed that frequencies of TMP-SMZ resistance in UPEC isolates obtained from outpatients with UTI symptoms in 1999–2000 and in 2016–2017 did not significantly change (resistance slightly increased from 16.9% to 17.1%). However, increasing resistance to this drug has recently been was observed in many countries. A majority of studies show resistance at or above the accepted level of 20%, and thus TMP-SMZ should not be used in empiric treatment (Bartoletti et al. 2016). High resistance rate to TMP-SMZ (24.5%) among E. coli isolated from urine in 2012–2015 was reported in Switzerland by Erb et al. (2018). The considerable geographic and age-related differences in resistance of UPEC to TMP-SMZ were observed, and the highest resistance was noted in certain regions of Europe in young women. Studies of TMP-SMZ activity against UPEC revealed resistance in European countries (Belgium, Germany, Poland, Switzerland, France, Spain, Bosnia and Herzegovina, and Romania) between 14.6% and 60% (Abduzaimovic et al. 2016; Kot et al. 2016; Kresken et al. 2016; Lavigne et al. 2016; Ciontea et al. 2018; Erb et al. 2018; Hitzenbichler et al. 2018). The TMP-SXT resistance rate in India in 2013 was 52%, and in 2017 it increased up to 59.6% (Prasada et al. 2019). A similar rate of resistance of UPEC isolates against TMP-SXT (50.6%) was observed in Brazil (Cunha et al. 2016). The activity of TMP-SMZ against UPEC in other regions of the world was significantly lower as the frequencies of TMP-SMZ resistant UPEC isolates ranged from 72.7% in Mexico to 82% in Pakistan (Ali et al. 2016; Ramírez-Castillo et al. 2018). High resistance to TMP-SMZ was also observed in Iran (54%), Ethiopia (68.5%), Mongolia (70.9%), and Jordan (73,1%) (Raeispour and Ranjbar 2016; Munkhdelger et al. 2017; Regasa Dadi et al. 2018; Shakhatreh et al. 2018). These results indicate that in many countries TMP-SMZ should not be used in empiric UTI therapy due to the high frequency of UPEC resistant to this antimicrobial.
Resistance to fluoroquinolones
Fluoroquinolones (FQs) are recommended for empirical oral antimicrobial therapy in uncomplicated pyelonephritis (Bonkat et al. 2017) and they are frequently used for the treatment of UTIs (Drekonja et al. 2013; Yamasaki et al. 2015; Walker et al. 2016). The increasing emergence of E. coli resistant to FQs was reported worldwide, and it has emerged probably due to the excessive use of these antibiotics. The resistance of UPEC to FQs was reported from different countries and the level of resistance is significant. The results of Prasada et al. (2019) revealed a -high rate of fluoroquinolone resistance in UPEC in India (> 60%). The results of the meta-analysis on resistance to ciprofloxacin in the community- and hospital-acquired UTIs showed that UPEC resistance to ciprofloxacin was higher in the hospital when compared to the community setting (Fasugba et al. 2015). In Europe, resistance to FQs was reported in 22% of strains, and the prevalence of fluoroquinolone-resistant UPEC strains was about 31% among hospitalized patients in the United States (Asadi Karam et al. 2019). In many parts of the world, > 20% of E. coli isolated from patients with community-acquired uncomplicated UTI and > 50% of E. coli isolated from complicated UTI showed resistance to FQs (Talan et al. 2016). In Poland, resistance to FQs was observed in about 30% of UPEC (Michno et al. 2018). MDR UPEC demonstrated much higher rates of resistance to FQs, ranging from 49% to 72% (Walker et al. 2016). Among FQs, ciprofloxacin is the most commonly prescribed for UTIs because it is available in oral and intravenous preparations. In the group of UPEC strains isolated in 2013–2014 from outpatients in Brasilia, 18.8% were resistant to ciprofloxacin, and resistance to ciprofloxacin was associated with multidrug resistance (Moreira da Silva et al. 2017). In the United States in the period from 2013 to 2014, 12.1% of E. coli isolates from patients with acute uncomplicated and complicated pyelonephritis were resistant to this antibiotic (Talan et al. 2016). A similar percentage of UPEC resistant to ciprofloxacin was noted in other research conducted in the United States in 2016–2017 for isolates from the urine samples from outpatients with symptoms of UTI (Yamaji et al. 2018). While a higher rate of resistance to ciprofloxacin was detected in UPEC from elderly hospitalized patients in Argentina (42.8%) (Delpech et al. 2018), and in UPEC isolates from the community and hospital-acquired infections in Mexico (47.3%) (Ramírez-Castillo et al. 2018). Blaettler et al. (2009) found that in Switzerland, over 10 years (1997–2007), resistance to ciprofloxacin increased significantly from 1.8% to 15.9%, which coincided with an increase in ciprofloxacin use in this country. In 2000 in Switzerland FQs were prescribed for treatment of uncomplicated UTIs in 64% of cases. In the recent study conducted in Switzerland in 2012–2015, an increase of resistance to ciprofloxacin from 15.9% (Blaettler et al. 2009) to 17.4% (Erb et al. 2018) was reported. Although total antibiotic usage in Switzerland is lower than in other European countries, the fluoroquinolone consumption in this country for treatments of urological outpatients comprises 20.1% of all antibiotics (Helsana 2014) and it is rather high when compared to an average of 7.3% in other European countries (Filippini et al. 2004), which may explain the increasing fluoroquinolone resistance in UPEC isolates. In 2013–2014 in Belgium, Germany, and Spain the percentage of ciprofloxacin-resistant UPEC strains was 12.9, 17.3, and 39.8%, respectively (Kresken et al. 2016). The research conducted in England showed that among E. coli isolates from the urine samples from patients, who later developed bacteremia, 20.4% and 15.5% of isolates were resistant to ciprofloxacin in the year and four weeks before the bacteremia onsets. (Abernethy et al. 2017). Resistance to ciprofloxacin was significantly higher in developing countries (Ethiopia – 85.5%, Nepal – 64.6%, Pakistan – 60.8%, Mongolia – 58.1%, Jordan – 55.5%) (Ali et al. 2016, Khatri et al. 2017; Munkhdelger et al. 2017; Regasa Dadi et al. 2018; Shakhatreh et al. 2018) than in developed countries (USA – 5.1%, Germany – 10.5%, Switzerland – 17.4%, France – 24.8%) (Lavigne et al. 2016; Erb et al. 2018; Hitzenbichler et al. 2018; Yamaji et al. 2018). The study conducted in Switzerland showed also that patient age over 65 years was associated with higher E. coli resistance to ciprofloxacin (Erb et al. 2018). According to many authors, the widespread use of fluoroquinolones in the outpatients is the cause of the continuous increase in resistance to this drug. Therefore, ciprofloxacin should be avoided in first-line treatment of UTIs and be used only in more severe infections or as an alternative when the recommended agents cannot be used. Restrictions of ciprofloxacin usage should be enhanced especially in developing countries, where no regulations concerning the use of this antibiotic currently apply (Asadi Karam et al. 2019).
Resistance to amoxicillin-clavulanic acid
Amoxicillin-clavulanic acid was recommended as first-line therapy for mild and moderate pyelonephritis or complicated UTI or as an alternative empiric therapy for uncomplicated cystitis (Cheung et al. 2017). Resistance rates for amoxicillin-clavulanic acid vary regionally. In the United States in 2012, the prevalence of UPEC isolates resistant to this antibiotic in the age groups: below 17, 18–64, and over 65 years were 3.1%, 3.9%, and 5.5%, respectively (Sanchez et al. 2016). Morrill et al. (2017) studied E. coli urinary isolates collected in the period from 2009 to 2013 from inpatients and outpatients in the Veterans Affairs Care System in the United States and showed that rates of resistance to amoxicillin or ampicillin/beta-lactamase inhibitors were approximately 40%. Ramírez-Castillo et al. (2018) have recently found that 23.6% of UPEC isolates were resistant to amoxicillin-clavulanic acid in Mexico. The resistance rate to amoxicillin-clavulanic acid among E. coli isolates from the urine samples taken from patients of tertiary care hospital in Germany in 2015–2017 was 5.3% (Hitzenbichler et al. 2018). Between 2015 and 2017 in Romania, (Ciontea et al. 2018), and in Bosnia and Herzegovina in 2016, 29.0% and 19.6% of UPEC isolates collected from outpatients were resistant to amoxicillin-clavulanic acid (Abduzaimovic et al. (2016). In Poland in women with uncomplicated UTIs 3.3% of UPEC were resistant to amoxicillin-clavulanic acid in 2003–2006 (Naber et al. 2008). In 2007–2008 the percentage of amoxicillin-clavulanic acid-resistant UPEC isolated from hospital-acquired UTIs in Poland was 13.9% (Kot et al. 2016). In the group of UPEC isolates obtained in 2009–2010 in the urology units in France, 37.6% were resistant to this antimicrobial (Lavigne et al. 2016) Among UPEC isolated in Argentina from patients over 70 years old with UTIs, without urinary catheters and antimicrobial therapy, 28.6% were resistant to this antibacterial agent (Delpech et al. 2018). In England, 30% of E. coli isolates from the urine samples from hospitalized patients showed resistance to amoxicillin-clavulanic acid (Abernethy et al. 2017). High level of resistance to this antimicrobial was reported for UPEC isolates from children hospitalized in 2015–2016 in Nepal (48%) (Parajuli et al. 2017), and in Pakistan in the group of UPEC isolated from outpatients (71%) (Ali et al. 2016). While very high resistance to amoxicillin-clavulanic acid (83%) was showed for UPEC isolates from hospitalized patients in Jordan (Shakhatreh et al. 2018). These results demonstrate that the levels of UPEC resistance to amoxicillin-clavulanic acid varied between geographical regions or patient populations. For this reason, the empiric regimens for uncomplicated and complicated UTIs should be guided by the local susceptibility of E. coli. However, definitive regimens should be developed according to the susceptibility results of UPEC, when available.
Resistance to other antibiotics
Some UPEC isolates can be resistant to ampicillin and first-generation oral cephalosporins (Moya-Dionisio et al. 2016). The resistance to cefuroxime (second-generation cephalosporin) in Belgium, Germany, and Spain was 5.5%, 12.8%, and 16.6%, respectively (Kresken et al. 2016). E. coli isolates from the urine samples from hospitalized patients in England were found to be resistant (13.8–21.3%) to third-generation cephalosporins (cefotaxime/ceftazidime) (Abernethy et al. 2017). The percentage of UPEC susceptible to third generation cephalosporins in Romania was 87% (Ciontea et al. 2018). While, the other research conducted in this country demonstrated that the resistance rate was 47.52% for ampicillin, and 41.16% for tetracycline (Cristea et al. 2019). The resistance of E. coli isolates from the urinary tract isolated in the urology department in France to amoxicillin, ticarcillin, nalidixic acid was high and reached 61.4%, 59%, and 31.9%, respectively (Lavigne et al. 2016). In Iran, the resistance to ampicillin, ceftazidime and nalidixic acid was higher than 50%, while amikacin and gentamicin showed high activity against UPEC (89.1% and 82.4% of sensitive isolates, respectively) (Faghri et al. 2016). The resistance against gentamicin and amikacin of UPEC isolated from outpatients in Pakistan was 29%, and 4%, respectively (Ali et al. 2016). In Mexico, the resistance rates to antibiotics belonging to aminoglycosides were 28.2%, 19.1%, 10%, and 5.5% for gentamycin, tobramycin, amikacin, and netilmicin, respectively (Ramírez-Castillo et al. 2018). The carbapenems, piperacillin-tazobactam, and amikacin were highly active (> 95% susceptibility) against E. coli isolates from UTIs collected from 2010 to 2014 in Canada and the United States (Lob et al. 2016). Carbapenems (ertapenem, imipenem, meropenem, doripenem) are recommended for the treatment of acute uncomplicated pyelonephritis, complicated UTI, and urosepsis (Bonkat et al. 2017). Shahbazi et al. (2018) showed that all UPEC isolates from patients with UTI in Teheran were negative for carbapenemases. Another study on UPEC isolates from Iran confirmed a lack of resistance to meropenem in isolates from outpatients and inpatients (Faghri et al. 2016). Ali et al. (2016) described a high activity of meropenem in Pakistan since only 1.3% of UPEC were resistant to this antibiotic. However, a recent study from Saudi Arabia about the presence of carbapenem-resistant uropathogenic E. coli clones in community-acquired UTIs reported the occurrence of carbapenemases NDM-1 and 5 (the New Delhi metallo-lactamase), and carbapenemases of the OXA-181 type in these strains (Abd El Ghany et al. 2018). The emergence of carbapenem-resistant uropathogenic E. coli isolates makes treatment of these infections increasingly challenging.
Mechanisms of UPEC resistance to antibiotics
Resistance to β-lactams is related to the production of different types of β-lactamase enzymes. Among the genes often located on plasmids are those coding multiple types of β-lactamases (bla genes) (Adamus-Białek et al. 2018). β-lactamases hydrolyze the amide bond of the four- membered β-lactam ring of β-lactam antibiotics (penicillin, cephalosporin, monobactams, and carbapenems) (Noyal et al. 2009). ESBL are enzymes that confer resistance to β-lactam antibiotics (all penicillins, cephalosporins, and monobactams), except for carbapenems, cephamycins, and β-lactamase inhibitors (Baudry et al. 2009). ESBL are the predominant source of Enterocacteriaceae resistance to 3rd- and 4th-generation cephalosporins and they developed as a result of mutations in the genes coding for ancestral enzymes blaTEM-1, blaTEM-2, and blaSHV-1 (Dashti et al. 2006). Three classes of β-lactamases including TEM and SHV, and since 2000 a new group of ESBL, CTX-M (cefotaximases), were observed among ESBL produced by UPEC (Ojdana et al. 2014; Shahbazi et al. 2018). Genetic analyses of UPEC from hospitalized patients in different hospital wards in Lodz (Poland) revealed that TEM-1 was present in almost all investigated strains (Adamus-Białek et al. 2018). Among UPEC isolated from 2013 to 2015 from patients hospitalized in a Department of Internal Medicine and Nephrology in southern Poland, 8% of the strains produced ESBL (Michno et al. 2018). In France, 7.6% of UPEC produce ESBL with the predominance of CTX-M (Lavigne et al. 2016). The CTX-M enzymes are active against cefotaxime and ceftriaxone and less active against ceftazidime (Bhat et al. 2012). UPEC producing ESBL are particularly often detected in developing countries (Iran – 37.1%, Nepal – 38.9%, Pakistan – 40%, and Jordan – about 50%) (Ali et al. 2016; Parajuli et al. 2017; Shakhatreh et al. 2018). Prasada et al. (2019) revealed that in India the percentage of ESBL-producing UPEC increased from 45.2 to 59.6% over 5 years (2013–2017). The frequency of ESBL-producing E. coli isolates is different in various parts of the world and sometimes even in various hospitals within the country. In addition to resistance to β-lactam antibiotics, ESBL-producing E. coli isolates are also resistant to other antimicrobial agents, such as aminoglycosides, tetracycline, and trimethoprim/sulfamethoxazole (Rezai et al. 2015). Shahbazi et al. (2018) has found that higher number of ESBL-producing UPEC isolates were resistant to aminoglycosides and quinolones when compared to the UPEC strains that not produce ESBL. Carbapenems (imipenem and meropenem) represent the best option for the treatment of UTIs caused by ESBL-producing strains (Idil et al. 2016). Cephalosporins, penicillins, and monobactams should be used with β-lactamase inhibitors (Bartoletti et al. 2016).
Quinolones and fluoroquinolones are extensively used worldwide in the treatment of UTIs and their common use led to increased resistance in UPEC. The mechanism of fluoroquinolone action is based on binding to and impeding the activity of topoisomerase II (DNA gyrase) and topoisomerase IV (parC and parE) (Komp Lindgren et al. 2003). DNA gyrase is encoded by the gyrA and gyrB genes (Pourahmad Jaktaji and Mohiti 2010). The resistance of E. coli to quinolones frequently results from a mutation in the gyrA and gyrB genes that catalyze DNA supercoiling. The point mutations in gyrA protein N-terminal sequence (amino acids 67 (Ala-67) to 106 (Gln-106)) strongly correlate with phenotypic resistance to quinolones and fluoroquinolones, and this sequence is named a quinolone resistance-determining region (QRDR) (Friedman et al. 2001). Investigation of mutations in codons 83 and 106 of the gyrA gene in UPEC isolates in Iran presented the significant relationship between mutations in the gyrA gene and quinolone and fluoroquinolone resistance pattern of UPEC isolates (Shenagari et al. 2018). Other genes responsible for the resistance to quinolones and fluoroquinolones are the qnr genes (qnrA, qnrB, and qnrC), being the most important PMQR (plasmid-mediated quinolone resistance) genes that induce antibiotic resistance by inhibition of binding of quinolones to DNA gyrase and topoisomerases (Shahbazi et al. 2018).
Other mechanisms of E. coli resistance to quinolones and fluoroquinolones are related to the presence of efflux pumps and decreased uptake of the antibiotics due to changes in the outer membrane porin proteins (Asadi Karam et al. 2019). Abdelhamid and Abozahra (2017) showed that the increased expression of the efflux pump-coding genes acrA and mdfA was related to the growing resistance to levofloxacin, which confirms that efflux pump systems contribute to fluoroquinolone resistance in urinary E. coli isolates.
The mechanism of fosfomycin action is unique because it irreversibly inhibits an early stage of bacterial cell wall biosynthesis, leading to bacterial cell lysis and death (Dijkmans et al. 2017). The active transport proteins used to transport both glucose-6-P and glycerol-3-P are also used by fosfomycin to reach bacterial cytoplasm (Popovic et al. 2010). Fosfomycin in the cytoplasm is an analog of phosphoenolpyruvate (PEP) and binds UDP-GlcNAc enolpyruvyl transferase, inactivating this enzyme, which is essential for peptidoglycan biosynthesis. The resistance to fosfomycin is due to three mechanisms that have already been described. One of them is based on decreased uptake of fosfomycin by the bacterial cells due to mutations in the genes that encode the glycerol-3-phosphate transporter or the glucose-6-phosphate transporter (Kadner and Winkler 1973; Tsuruoka and Yamada 1975). The second mechanism is based on point mutations in the binding site of UDP-GlcNAc enolpyruvyl transferase (Kim et al. 1996). The third mechanism of resistance is related to the inactivation of fosfomycin by enzymatic cleavage of the oxirane ring of the antibiotic or by phosphorylation of the phosphonate group. The opening of the oxirane ring may be catalyzed by glutathione transferase (FosA), L-cysteine thiol transferase (Fos B) or fosfomycin-specific epoxide hydrolase (FosX) (Rigsby et al. 2005).
Nitrofurantoin is recommended for the treatment of uncomplicated cystitis and, currently, the resistance of UPEC to nitrofurantoin is very low. Resistance to nitrofurantoin did not evolve as fast as to other drugs because of this antimicrobial acts at multiple targets in the bacterial cell (Veeraraghavan and Shakti 2015). Sandegren et al. (2008) identified mutations conferring resistance to nitrofurantoin and found that the mutation frequency is approximately 10−7/cell in E. coli. The mutations in the nsfA and nfsB genes that encode oxygen-insensitive nitroreductases were responsible for nitrofurantoin resistance. It was also found that the growth of bacterial cells in the presence of nitrofurantoin at therapeutic concentrations was greatly reduced in nitrofurantoin-resistant mutants. It may indicate that resistant mutants in the presence of nitrofurantoin were probably unable to establish an infection (Sandegren et al. 2008).
The resistance of UPEC lineages
The multilocus sequence-typing (MLST) technique is widely used to study ExPEC lineages. Sequence types (STs) 10, 69, 73, 95, 127, and 131 defined by MLST were isolated as pandemic clones of ExPEC from human infections, including UTIs and bloodstream infections (Tartof et al. 2005; Adams-Sapper et al. 2013; Riley 2014). Globally, these genotypes account for more than 50% of ExPEC infections (Gibreel et al. 2012; Adams-Sapper et al. 2013; Kallonen et al. 2017). The isolates belonging to the lineage ST131 are multidrug-resistant (Banerjee and Johnson 2014; Petty et al. 2014). Adams-Sapper et al. (2013) who investigated E. coli isolates from bloodstream infections in the United States, found that ST131 was the most common genotype, including 92% of multidrug-resistant isolates. UPEC isolates from patients in the Northwest region of England that belonged to lineage ST131 exhibited higher levels of antibiotic resistance when compared to ST127 isolates that were the most widely susceptible to antibiotics (Gibreel et al. 2012). The UPEC of ST131 line from a tertiary care hospital in Saudi Arabia was also significantly associated with high levels of antibiotic resistance and 60% of ST131 carried CTX-M-14 and CTX-M-15 (Alghoribi et al. 2015). ST73 was the lineage most frequently identified by Kallonen et al. (2017) among isolates associated with bacteremia in England, and isolates belonging to this lineage were susceptible to most antibiotics. The authors suggested that drug resistance was not a primary determinant for the prevalence of E. coli lineages responsible for invasive diseases, and the frequency of E. coli lineages is associated with the presence of new lineages outside the hospital. Yamaji et al. (2018) compared the clonal distribution of UPEC in the same community during two periods. The UPEC strains belonging to ST 95, 127, 73, 69, 131, and 10 were responsible for 56% of UTI cases in 1999–2000. In the period 2016–2017, the same STs caused 64% of UTI cases. The study of Yamaji et al. (2018) showed that 46.4% of the isolates resistant to ampicillin in 2016–2017 belonged to four genotypes (ST 95, 127, 73, and 131), while in 1999–2000 they comprised only 21.8%. The increase of resistance to ampicillin was observed only in these genotypes. Yamaji et al. (2018) reported that ST69 included the highest percentage of TMP-SMZ-resistant isolates during two study periods. In 2016–2017, 58% of ciprofloxacin-resistant isolates represented the ST131 lineage. ST69, 127, and 131 comprised 70% of isolates with CTX-M. Among E. coli extraintestinal isolates from Iran, the highest rates of the multidrug-resistance phenotype were detected in ST131 (85.7%), and ST69 (41.7%) lineages (Hojabri et al. 2019). Similarly to the results by Yamaji et al. (2018), the resistance to TMP-SMZ was detected mainly in the ST69 lineage. The widespread occurrence of new multidrug-resistant E. coli clonal group ST1193 has recently been demonstrated in the United States (Tchesnokova et al. 2019). This clonal group of strains was isolated from younger adults. ST1193 isolates were resistant to fluoroquinolones and often co-resistant to TMP-SXT and tetracycline, but currently, remain susceptible to most β-lactam antibiotics.
Conclusion
The antibiotic therapy is important in the UTI treatment but in recent years it is becoming more challenging due to increasing resistance of UTIs to routinely applied antibiotics. High resistance of UPEC to FQs used as third-line empiric treatment for therapy of uncomplicated cystitis in pyelonephritis or second-line empiric treatment in complicated UTI requires a rational policy of prescription of these drugs. The awareness of the resistance rates of E. coli in a given area and the established guidelines for appropriate first-line antibiotic treatment should be critical in the empirical treatment of UTIs, especially for the controlled use of fluoroquinolones.
Acknowledgments
This work was supported by the Siedlce University of Natural Science and Humanities (Scientific Research Project No. 316/12/S).
Footnotes
Conflict of interest
The author does not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
ORCID
Barbara Kot 0000-0002-6191-8275
Literature
- Abd El Ghany M, Sharaf H, Al-agamy MH, Shibl A, Hill-Cawthorne GA, Hong PY.. Genomic characterization of NDM-1 and 5, and OXA-181 carbapenemases in uropathogenic Escherichia coli isolates from Riyadh, Saudi Arabia. PLoS One. 2018. Aug 15; 13(8):e0201613 10.1371/journal.pone.0201613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhamid SM, Abozahra RR.. Expression of the fluoroquinolones efflux pump genes acrA and mdfA in urinary Escherichia coli isolates. Pol J Microbiol. 66(1):25–30. [DOI] [PubMed] [Google Scholar]
- Abduzaimovic A, Aljicevic M, Rebic V, Vranic S, Abduzaimovic K, Sestic S.. Antibiotic resistance in urinary isolates of Escherichia coli. Mater Sociomed. 2016;28(6):416–419. 10.5455/msm.2016.28.416-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abernethy J, Guy R, Sheridan EA, Hopkins S, Kiernan M, Wilcox MH, Johnson AP, Hope R. E. coli bacteraemia sentinel surveillance group. . Epidemiology of Escherichia coli bacteraemia in England: results of an enhanced sentinel surveillance programme. J Hosp Infect. 2017;95(4):365–375. 10.1016/j.jhin.2016.12.008 [DOI] [PubMed] [Google Scholar]
- Adams-Sapper S, Diep BA, Perdreau-Remington F, Riley LW.. Clonal composition and community clustering of drug-susceptible and -resistant Escherichia coli isolates from bloodstream infections. Antimicrob Agents Chemother. 2013. Jan;57(1):490–497. 10.1128/AAC.01025-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamus-Białek W, Baraniak A, Wawszczak M, Głuszek S, Gad B, Wróbel K, Bator P, Majchrzak M, Parniewski P.. The genetic background of antibiotic resistance among clinical uropathogenic Escherichia coli strains. Mol Biol Rep. 2018. Oct;45(5):1055–1065. 10.1007/s11033-018-4254-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghoribi MF, Gibreel TM, Farnham G, Al Johani SM, Balkhy HH, Upton M.. Antibiotic-resistant ST38, ST131 and ST405 strains are the leading uropathogenic Escherichia coli clones in Riyadh, Saudi Arabia. J Antimicrob Chemother. 2015. Oct;70(10):2757–2762. 10.1093/jac/dkv188 [DOI] [PubMed] [Google Scholar]
- Ali I, Rafaque Z, Ahmed S, Malik S, Dasti JI.. Prevalence of multidrug resistant uropathogenic Escherichia coli in Potohar region of Pakistan. Asian Pac J Trop Biomed. 2016. Jan;6(1):60–66. 10.1016/j.apjtb.2015.09.022 [DOI] [Google Scholar]
- Asadi Karam MR, Habibi M, Bouzari S.. Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol Immunol. 2019. Apr;108:56–67. 10.1016/j.molimm.2019.02.007 [DOI] [PubMed] [Google Scholar]
- Bahagon Y, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM.. Prevalence and predictive features of bacteremic urinary tract infection in emergency department patients. Eur J Clin Microbiol Infect Dis. 2007. May 4;26(5):349–352. 10.1007/s10096-007-0287-3 [DOI] [PubMed] [Google Scholar]
- Banerjee R, Johnson JR.. A new clone sweeps clean: the enigmatic emergence of Escherichia coli sequence type 131. Antimicrob Agents Chemother. 2014. Sep;58(9):4997–5004. 10.1128/AAC.02824-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoletti R, Cai T, Wagenlehner FM, Naber K, Bjerklund Johansen TE.. Treatment of urinary tract infections and antibiotic stewardship. Eur Urol Suppl. 2016. Jul;15(4):81–87. 10.1016/j.eursup.2016.04.003 [DOI] [Google Scholar]
- Baudry PJ, Nichol K, DeCorby M, Lagacé-Wiens P, Olivier E, Boyd D, Mulvey MR, Hoban DJ, Zhanel GG.. Mechanisms of resistance and mobility among multidrug-resistant CTX-M-producing Escherichia coli from Canadian intensive care units: the 1st report of QepA in North America. Diagn Microbiol Infect Dis. 2009. Mar;63(3):319–326. 10.1016/j.diagmicrobio.2008.12.001 [DOI] [PubMed] [Google Scholar]
- Bhat MA, Sageerabanoo S, Kowsalya R, Sarkar G.. The occurrence of CTX-M3 type extended spectrum beta lactamases among Escherichia coli causing urinary tract infections in a tertiary care hospital in puducherry. J Clin Diagn Res. 2012;6(7):1203–1206. [Google Scholar]
- Bischoff S, Walter T, Gerigk M, Ebert M, Vogelmann R.. Empiric antibiotic therapy in urinary tract infection in patients with risk factors for antibiotic resistance in a German emergency department. BMC Infect Dis. 2018. Dec;18(1):56 10.1186/s12879-018-2960-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaettler L, Mertz D, Frei R, Elzi L, Widmer AF, Battegay M, Flückiger U.. Secular trend and risk factors for antimicrobial resistance in Escherichia coli isolates in Switzerland 1997–2007. Infection. 2009. Dec;37(6):534–539. 10.1007/s15010-009-8457-0 [DOI] [PubMed] [Google Scholar]
- Bonkat G, Pickard R, Bartoletti R, Bruyère F, Geerlings SE, Wagenlehner F, Wullt B.. Guidelines on urological infections [Internet]. Arnhem (The Netherlands): European Association of Urology; 2017. [cited 2019 May 31]. Available from https://uroweb.org/wp-content/uploads/Urological-Infections-2017-pocket.pdf [Google Scholar]
- Brumbaugh AR, Smith SN, Mobley HLT.. Immunization with the yersiniabactin receptor, FyuA, protects against pyelonephritis in a murine model of urinary tract infection. Infect Immun. 2013. Sep; 81(9):3309–3316. 10.1128/IAI.00470-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cek M, Tandoğdu Z, Wagenlehner F, Tenke P, Naber K, Bjerklund-Johansen TE.. Healthcare-associated urinary tract infections in hospitalized urological patients – a global perspective: results from the GPIU studies 2003–2010. World J Urol. 2014. Dec;32(6):1587–1594. 10.1007/s00345-013-1218-9 [DOI] [PubMed] [Google Scholar]
- Cheung A, Karmali G, Noble S, Song H.. Antimicrobial stewardship initiative in treatment of urinary tract infections at a rehabilitation and complex continuing care hospital. Can J Hosp Pharm. 2017. Apr 28;70(2):144–149. 10.4212/cjhp.v70i2.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciontea AS, Cristea D, Andrei MM, Popa A, Usein CR.. In vitro antimicrobial resistance of urinary Escherichia coli isolates from outpatients collected in a laboratory during two years, 2015–2017. Roum Arch Microbiol Immunol. 2018;77(1):28–32. [Google Scholar]
- Cristea VC, Gheorghe I, Barbu IC, Popa LI, Ispas B, Grigore GA, Bucatariu I, Popa GL, Angelescu M-C, Velican A, et al. Snapshot of phylogenetic groups, virulence, and esistance markers in Escherichia coli uropathogenic strains isolated from outpatients with urinary tract infections in Bucharest, Romania. BioMed Res Int. 2019;Article ID 5712371, 8 pages. 10.1155/2019/5712371 [DOI] [PMC free article] [PubMed]
- Cunha MA, Assunção GLM, Medeiros IM, Freitas MR.. Antibiotic resistance patterns of urinary tract infections in a northeastern Brazilian capital. Rev Inst Med Trop São Paulo. 2016;58(0):2 10.1590/S1678-9946201658002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti AA, West P, Paton R, Amyes SG.. Characterization of extended-spectrum -lactamase (ESBL)-producing Kuwait and UK strains identified by the Vitek system, and subsequent comparison of the Vitek system with other commercial ESBL-testing systems using these strains. J Med Microbiol. 2006. Apr 01;55(4):417–421. 10.1099/jmm.0.46177-0 [DOI] [PubMed] [Google Scholar]
- Delpech G, Allende NG, Lissarrague S, Sparo M.. Antimicrobial resistance of uropathogenic Escherichia coli from elderly patients at a general hospital, Argentina. Open Infect Dis J. 2018. Jul 19;10(1):79–87. 10.2174/1874279301810010079 [DOI] [Google Scholar]
- Dijkmans AC, Zacarías NVO, Burggraaf J, Mouton JW, Wilms E, van Nieuwkoop C, Touw DJ, Stevens J, Kamerling IMC.. Fosfomycin: pharmacological, clinical and future perspectives. Antibiotics (Basel). 2017. Oct 31;6(4):24 10.3390/antibiotics6040024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drekonja DM, Rector TS, Cutting A, Johnson JR.. Urinary tract infection in male veterans: treatment patterns and outcomes. JAMA Intern Med. 2013. Jan 14;173(1):62–68. 10.1001/2013.jamainternmed.829 [DOI] [PubMed] [Google Scholar]
- Erb S, Frei R, Tschudin Sutter S, Egli A, Dangel M, Bonkat G, Widmer AF.. Basic patient characteristics predict antimicrobial resistance in E. coli from urinary tract specimens: a retrospective cohort analysis of 5246 urine samples. Swiss Med Wkly. 2018. Nov 15;148:w14660 10.4414/smw.2018.14660 [DOI] [PubMed] [Google Scholar]
- ECDCP. Point prevalence survey of healthcare associated infections and antimicrobial use in European Acute Care Hospitals, 2011–2012. Stockholm (Sweden): European Center for Disease Control and Prevention; 2013. [Google Scholar]
- Faghri J, Dehbanipour R, Rastaghi S, Sedighi M, Maleki N.. High prevalence of multidrug-resistance uropathogenic Escherichia coli strains, Isfahan, Iran. J Nat Sci Biol Med. 2016;7(1):22–26. 10.4103/0976-9668.175020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasugba O, Gardner A, Mitchell BG, Mnatzaganian G.. Ciprofloxacin resistance in community- and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2015. Dec;15(1):545 10.1186/s12879-015-1282-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini M, Masiero G, Moschetti K.. Socioeconomic determinants of regional differences in outpatient antibiotic consumption: evidence from Switzerland. Health Policy. 2006. Aug;78(1):77–92. 10.1016/j.healthpol.2005.09.009 [DOI] [PubMed] [Google Scholar]
- Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ.. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015. May;13(5):269–284. 10.1038/nrmicro3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SM, Lu T, Drlica K.. Mutation in the DNA gyrase A Gene of Escherichia coli that expands the quinolone resistance-determining region. Antimicrob Agents Chemother. 2001. Aug 01; 45(8):2378–2380. 10.1128/AAC.45.8.2378-2380.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibreel TM, Dodgson AR, Cheesbrough J, Fox AJ, Bolton FJ, Upton M.. Population structure, virulence potential and antibiotic susceptibility of uropathogenic Escherichia coli from Northwest England. J Antimicrob Chemother. 2012. Feb 01;67(2):346–356. 10.1093/jac/dkr451 [DOI] [PubMed] [Google Scholar]
- Habibi A, Khameneie MK.. Antibiotic resistance properties of uropathogenic Escherichia coli isolated from pregnant women with history of recurrent urinary tract infections. Trop J Pharm Res. 2016. Sep 05;15(8):1745–1750. 10.4314/tjpr.v15i8.21 [DOI] [Google Scholar]
- Helsana. Helsana-arzneimittelreport. Zürich (Switzerland): Helsana-Gruppe; 2014. [Google Scholar]
- Hirsch EB, Raux BR, Zucchi PC, Kim Y, McCoy C, Kirby JE, Wright SB, Eliopoulos GM.. Activity of fosfomycin and comparison of several susceptibility testing methods against contemporary urine isolates. Int J Antimicrob Agents. 2015. Dec;46(6):642–647. 10.1016/j.ijantimicag.2015.08.012 [DOI] [PubMed] [Google Scholar]
- Hitzenbichler F, Simon M, Holzmann T, Iberer M, Zimmermann M, Salzberger B, Hanses F.. Antibiotic resistance in E. coli isolates from patients with urinary tract infections presenting to the emergency department. Infection. 2018. Jun;46(3):325–331. 10.1007/s15010-018-1117-5 [DOI] [PubMed] [Google Scholar]
- Hof H. [Candiduria! What now? Therapy of urinary tract infections with Candida]. Urologe. 2017. Feb;56(2):172–179. 10.1007/s00120-016-0219-x [DOI] [PubMed] [Google Scholar]
- Hojabri Z, Mirmohammadkhani M, Darabi N, Arab M, Pajand O.. Characterization of antibiotic-susceptibility patterns and virulence genes of five major sequence types of Escherichia coli isolates cultured from extraintestinal specimens: a 1-year surveillance study from Iran. Infect Drug Resist. 2019. Apr;12:893–903. 10.2147/IDR.S199759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idil N, Candan ED, Rad AY, Aksoz N.. High trimethoprim-sulfamethoxazole resistance in ciprofloxacin-resistant Escherichia coli strains isolated from urinary tract infection. Minerva Biotecnol. 2016;28(3):159–163. [Google Scholar]
- Jakobsen L, Spangholm DJ, Pedersen K, Jensen LB, Emborg HD, Agersø Y, Aarestrup FM, Hammerum AM, Frimodt-Møller N.. Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. Int J Food Microbiol. 2010. Aug 15;142(1-2):264–272. 10.1016/j.ijfoodmicro.2010.06.025 [DOI] [PubMed] [Google Scholar]
- Kadner RJ, Winkler HH.. Isolation and characterization of mutations affecting the transport of hexose phosphates in Escherichia coli. J Bacteriol. 1973. Feb;113(2):895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallonen T, Brodrick HJ, Harris SR, Corander J, Brown NM, Martin V, Peacock SJ, Parkhill J.. Systematic longitudinal survey of invasive Escherichia coli in England demonstrates a stable population structure only transiently disturbed by the emergence of ST131. Genome Res. 2017. Aug;27(8):1437–1449. 10.1101/gr.216606.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri S, Pant ND, Neupane S, Bhandari S, Banjara MR.. Biofilm production in relation to extended spectrum beta-lactamase production and antibiotic resistance among uropathogenic Escherichia coli. Janaki Medical College J Med Sci. 2017. Aug 09;5(1):61–63. 10.3126/jmcjms.v5i1.17989 [DOI] [Google Scholar]
- Kim DH, Lees WJ, Kempsell KE, Lane WS, Duncan K, Walsh CT.. Characterization of a Cys115 to Asp substitution in the Escherichia coli cell wall biosynthetic enzyme UDP-GlcNAc enolpyruvyl transferase (MurA) that confers resistance to inactivation by the antibiotic fosfomycin. Biochemistry. 1996. Jan;35(15):4923–4928. 10.1021/bi952937w [DOI] [PubMed] [Google Scholar]
- Komp Lindgren P, Karlsson A, Hughes D.. Mutation rate and evolution of fluoroquinolone resistance in Escherichia coli isolates from patients with urinary tract infections. Antimicrob Agents Chemother. 2003. Oct 01;47(10):3222–3232. 10.1128/AAC.47.10.3222-3232.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kot B, Wicha J, Grużewska A, Piechota M, Wolska K, Obrębska M.. Virulence factors, biofilm-forming ability, and antimicrobial resistance of urinary Escherichia coli strains isolated from hospitalized patients. Turk J Med Sci. 2016;46(6):1908–1914. 10.3906/sag-1508-105 [DOI] [PubMed] [Google Scholar]
- Kresken M, Körber-Irrgang B, Biedenbach DJ, Batista N, Besard V, Cantón R, García-Castillo M, Kalka-Moll W, Pascual A, Schwarz R, et al. Comparative in vitro activity of oral antimicrobial agents against Enterobacteriaceae from patients with community-acquired urinary tract infections in three European countries. Clin Microbiol Infect. 2016. Jan;22(1):63.e1–63.e5. 10.1016/j.cmi.2015.08.019 [DOI] [PubMed] [Google Scholar]
- Lavigne JP, Thibault M, Costa P, Combescure C, Sotto A, Cariou G, Ronco E, Lanotte P, Bruyère F, Coloby P, et al. Resistance and virulence potential of uropathogenic Escherichia coli strains isolated from patients hospitalized in urology departments: a French prospective multicentre study. J Med Microbiol. 2016. Jun 01;65(6):530–537. 10.1099/jmm.0.000247 [DOI] [PubMed] [Google Scholar]
- Lob SH, Nicolle LE, Hoban DJ, Kazmierczak KM, Badal RE, Sahm DF.. Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010–2014. Diagn Microbiol Infect Dis. 2016. Aug;85(4):459–465. 10.1016/j.diagmicrobio.2016.04.022 [DOI] [PubMed] [Google Scholar]
- Mann R, Mediati DG, Duggin IG, Harry EJ, Bottomley AL.. Metabolic adaptations of uropathogenic E. coli in the urinary tract. Front Cell Infect Microbiol. 2017. Jun 08;7:241 10.3389/fcimb.2017.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan LK, Hunstad DA.. Urinary tract infection: pathogenesis and outlook. Trends Mol Med. 2016. Nov;22(11):946–957. 10.1016/j.molmed.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellata M, Johnson JR, Curtiss R 3rd.. Escherichia coli isolates from commercial chicken meat and eggs cause sepsis, meningitis and urinary tract infection in rodent models of human infections. Zoonoses Public Health. 2018. Feb;65(1):103–113. 10.1111/zph.12376 [DOI] [PubMed] [Google Scholar]
- Micali S, Isgro G, Bianchi G, Miceli N, Calapai G, Navarra M.. Cranberry and recurrent cystitis: more than marketing? Crit Rev Food Sci Nutr. 2014. Jan;54(8):1063–1075. 10.1080/10408398.2011.625574 [DOI] [PubMed] [Google Scholar]
- Michno M, Sydor A, Wałaszek M, Sułowicz W.. Microbiology and drug resistance of pathogens in patients hospitalized at the Nephrology Department in the South of Poland. Pol J Microbiol. 2018;67(4):517–524. 10.21307/pjm-2018-061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira da Silva RCR, de Oliveira Martins Júnior P, Gonçalves LF, de Paulo Martins V, de Melo ABF, Pitondo-Silva A, de Campos TA.. Ciprofloxacin resistance in uropathogenic Escherichia coli isolates causing community-acquired urinary infections in Brasília, Brazil. J Glob Antimicrob Resist. 2017. Jun;9:61–67. 10.1016/j.jgar.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Morrill HJ, Morton JB, Caffrey AR, Jiang L, Dosa D, Mermel LA, LaPlante KL.. Antimicrobial Resistance of Escherichia coli Urinary Isolates in the Veterans Affairs Health Care System. Antimicrob Agents Chemother. 2017. May;61(5):e02236-16. 10.1128/AAC.02236-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya-Dionisio V, Díaz-Zabala M, Ibáñez-Fernández A, Suárez-Leiva P, Martínez-Suárez V, Ordóñez-Álvarez FA, Santos-Rodríguez F.. [Uropathogen pattern and antimicrobial susceptibility in positive urinary cultures isolates from paediatric patients]. Rev Esp Quimioter. 2016. Jun;29(3):146–150. [PubMed] [Google Scholar]
- Munkhdelger Y, Gunregjav N, Dorjpurev A, Juniichiro N, Sarantuya J.. Detection of virulence genes, phylogenetic group and antibiotic resistance of uropathogenic Escherichia coli in Mongolia. J Infect Dev Ctries. 2017. Jan 30;11(01):51–57. 10.3855/jidc.7903 [DOI] [PubMed] [Google Scholar]
- Naber KG, Schito G, Botto H, Palou J, Mazzei T.. Surveillance study in Europe and Brazil on clinical aspects and Antimicrobial Resistance Epidemiology in Females with Cystitis (ARESC): implications for empiric therapy. Eur Urol. 2008. Nov;54(5):1164–1178. 10.1016/j.eururo.2008.05.010 [DOI] [PubMed] [Google Scholar]
- Neuner EA, Sekeres J, Hall GS, van Duin D.. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012. Nov;56(11):5744–5748. 10.1128/AAC.00402-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom L, Liu CM, Price LB.. Foodborne urinary tract infections: a new paradigm for antimicrobial-resistant foodborne illness. Front Microbiol. 2013;4:29 10.3389/fmicb.2013.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyal MJC, Menezes GA, Harish BN, Sujatha S, Parija SC.. Simple screening tests for detection of carbapenemases in clinical isolates of nonfermentative Gram-negative bacteria. Indian J Med Res. 2009. Jun;129(6):707–712. [PubMed] [Google Scholar]
- Ojdana D, Sacha P, Wieczorek P, Czaban S, Michalska A, Jaworowska J, Jurczak A, Poniatowski B, Tryniszewska E.. The occurrence of blaCTX-M, blaSHV, and blaTEM genes in extended-spectrum β-lactamase-positive strains of Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis in Poland. Int J Antibiot. 2014; Art. ID 935842: 7 pages. 10.1155/2014/935842 [DOI] [Google Scholar]
- Paniagua-Contreras GL, Monroy-Pérez E, Rodríguez-Moctezuma JR, Domínguez-Trejo P, Vaca-Paniagua F, Vaca S.. Virulence factors, antibiotic resistance phenotypes and O-serogroups of Escherichia coli strains isolated from community-acquired urinary tract infection patients in Mexico. J Microbiol Immunol Infect. 2017. Aug;50(4):478–485. 10.1016/j.jmii.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Parajuli NP, Maharjan P, Parajuli H, Joshi G, Paudel D, Sayami S, Khanal PR.. High rates of multidrug resistance among uropathogenic Escherichia coli in children and analyses of ESBL producers from Nepal. Antimicrob Resist Infect Control. 2017. Dec;6(1):9 10.1186/s13756-016-0168-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty NK, Ben Zakour NL, Stanton-Cook M, Skippington E, Totsika M, Forde BM, Phan MD, Gomes Moriel D, Peters KM, Davies M, et al. Global dissemination of a multidrug resistant Escherichia coli clone. Proc Natl Acad Sci USA. 2014. Apr 15; 111(15):5694–5699. 10.1073/pnas.1322678111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M, Steinort D, Pillai S, Joukhadar C.. Fosfomycin: an old, new friend? Eur J Clin Microbiol Infect Dis. 2010. Feb;29(2):127–142. 10.1007/s10096-009-0833-2 [DOI] [PubMed] [Google Scholar]
- Pourahmad Jaktaji R, Mohiti E.. Study of Mutations in the DNA gyrase gyrA Gene of Escherichia coli. Iran J Pharm Res. 2010. Winter; 9(1):43–48. [PMC free article] [PubMed] [Google Scholar]
- Prasada S, Bhat A, Bhat S, Shenoy Mulki S, Tulasidas S.. Changing antibiotic susceptibility pattern in uropathogenic Escherichia coli over a period of 5 years in a tertiary care center. Infect Drug Resist. 2019. May;12:1439–1443. 10.2147/IDR.S201849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeispour M, Ranjbar R.. Antibiotic resistance, virulence factors and genotyping of Uropathogenic Escherichia coli strains. Antimicrob Resist Infect Control. 2018. Dec;7(1):118 10.1186/s13756-018-0411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Castillo FY, Moreno-Flores AC, Avelar-González FJ, Márquez-Díaz F, Harel J, Guerrero-Barrera AL.. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: cross-sectional study. Ann Clin Microbiol Antimicrob. 2018. Dec;17(1):34 10.1186/s12941-018-0286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regasa Dadi B, Abebe T, Zhang L, Mihret A, Abebe W, Amogne W.. Drug resistance and plasmid profile of uropathogenic Escherichia coli among urinary tract infection patients in Addis Abeba. J Infect Dev Ctries. 2018. Aug 31;12(08):608–615. 10.3855/jidc.9916 [DOI] [PubMed] [Google Scholar]
- Rezai MS, Salehifar E, Rafiei A, Langaee T, Rafati M, Shafahi K, Eslami G.. Characterization of multidrug resistant extended-spectrum beta-lactamase-producing Escherichia coli among uropathogens of pediatrics in North of Iran. BioMed Res Int. 2015;2015:1–7. 10.1155/2015/309478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigsby RE, Fillgrove KL, Beihoffer LA, Armstrong RN.. Fosfomycin resistance proteins: a nexus of glutathione transferases and epoxide hydrolases in a metalloenzyme superfamily. Methods Enzymol. 2005;401:367–379. 10.1016/S0076-6879(05)01023-2 [DOI] [PubMed] [Google Scholar]
- Riley LW. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin Microbiol Infect. 2014. May;20(5):380–390. 10.1111/1469-0691.12646 [DOI] [PubMed] [Google Scholar]
- Sanchez GV, Babiker A, Master RN, Luu T, Mathur A, Bordon J.. Antibiotic resistance among urinary isolates from female outpatients in the United States in 2003 and 2012. Antimicrob Agents Chemother. 2016. May;60(5):2680–2683. 10.1128/AAC.02897-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandegren L, Lindqvist A, Kahlmeter G, Andersson DI.. Nitrofurantoin resistance mechanism and fitness cost in Escherichia coli. J Antimicrob Chemother. 2008. Jun 10;62(3):495–503. 10.1093/jac/dkn222 [DOI] [PubMed] [Google Scholar]
- Sarowska J, Futoma-Koloch B, Jama-Kmiecik A, Frej-Madrzak M, Ksiazczyk M, Bugla-Ploskonska G, Choroszy-Krol I.. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathog. 2019. Dec;11(1):10 10.1186/s13099-019-0290-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger C, Kazemier BM, Geerlings SE.. Asymptomatic bacteriuria and urinary tract infections in special patient groups: women with diabetes mellitus and pregnant women. Curr Opin Infect Dis. 2014. Feb;27(1):108–114. 10.1097/QCO.0000000000000028 [DOI] [PubMed] [Google Scholar]
- Shahbazi S, Asadi Karam MR, Habibi M, Talebi A, Bouzari S.. Distribution of extended-spectrum β-lactam, quinolone and carbapenem resistance genes, and genetic diversity among uropathogenic Escherichia coli isolates in Tehran, Iran. J Glob Antimicrob Resist. 2018. Sep;14:118–125. 10.1016/j.jgar.2018.03.006 [DOI] [PubMed] [Google Scholar]
- Shakhatreh MAK, Swedan SF, Al-Odat MA. Khabour OF.. Uropathogenic Escherichia coli (UPEC) in Jordan: prevalence of urovirulence genes and antibiotic resistance. JKSUS. 2018. 10.1016/j.jksus.2018.03.009 [DOI]
- Shenagari M, Bakhtiari M, Mojtahedi A, Atrkar Roushan Z.. High frequency of mutations in gyrA gene associated with quinolones resistance in uropathogenic Escherichiacoli isolates from the north of Iran. Iran J Basic Med Sci. 2018. Dec;21(12):1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelov V, Naber K, Bjerklund Johansen TE.. Improved classification of urinary tract infection: future considerations. Eur Urol Suppl. 2016. Jul;15(4):71–80. 10.1016/j.eursup.2016.04.002 [DOI] [Google Scholar]
- Tabasi M, Karam MR, Habibi M, Mostafavi E, Bouzari S.. Genotypic characterization of virulence factors in Escherichia coli isolated from patients with acute cystitis, pyelonephritis and asymptomatic bacteriuria. J Clin Diagn Res. 2016;10(12):DC01-DC07. 10.7860/JCDR/2016/21379.9009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talan DA, Takhar SS, Krishnadasan A, Abrahamian FM, Mower WR, Moran GJ; EMERGEncy ID Net Study Group. . Fluoroquinolone-resistant and extended-spectrum β-lactamase-producing Escherichia coli infections in patients with pyelonephritis, United States. Emerg Infect Dis. 2016. Sep;22(9). 10.3201/eid2209.160148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof SY, Solberg OD, Manges AR, Riley LW.. Analysis of a uropathogenic Escherichia coli clonal group by multilocus sequence typing. J Clin Microbiol. 2005. Dec 01;43(12):5860–5864. 10.1128/JCM.43.12.5860-5864.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchesnokova VL, Rechkina E, Larson L, Ferrier K, Weaver JL, Schroeder DW, She R, Butler-Wu SM, Aguero-Rosenfeld ME, Zerr D, et al. Rapid and extensive expansion in the United States of a new multidrug-resistant Escherichia coli clonal group, sequence type 1193. Clin Infect Dis. 2019. Jan 07;68(2):334–337. 10.1093/cid/ciy525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney J, Hudson N, Alnifaidy H, Li JTC, Fung KH.. Risk factors for aquiring multidrug-resistant organisms in urinary tract infections: A systematic literature review. Saudi Pharm J. 2018. Jul;26(5):678–684. 10.1016/j.jsps.2018.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlizzi ME, Gribaudo G, Maffei ME.. Uropathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front Microbiol. 2017. Aug 15;8:1566 10.3389/fmicb.2017.01566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruoka T, Yamada Y.. Characterization of spontaneous fosfomycin (phosphonomycin)-resistant cells of Escherichia coli B in vitro. J Antibiot (Tokyo). 1975;28(11):906–911. 10.7164/antibiotics.28.906 [DOI] [PubMed] [Google Scholar]
- van der Donk CFM, van de Bovenkamp JHB, De Brauwer EIGB, De Mol P, Feldhoff KH, Kalka-Moll WM, Nys S, Thoelen I, Trienekens TAM, Stobberingh EE.. Antimicrobial resistance and spread of multi drug resistant Escherichia coli isolates collected from nine urology services in the Euregion Meuse-Rhine. PLoS One. 2012. Oct 17;7(10):e47707 10.1371/journal.pone.0047707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuwkoop C, Bonten TN, Wout JW, Becker MJ, Groeneveld GH, Jansen CL, van der Vorm ER, IJzerman EP, Rothbarth PH, TerMeer-Veringa EM, et al. Risk factors for bacteremia with uropathogen not cultured from urine in adults with febrile urinary tract infection. Clin Infect Dis. 2010. Jun;50(11):e69–e72. 10.1086/652657 [DOI] [PubMed] [Google Scholar]
- Veeraraghavan B, Shakti L.. Advantage and limitations of nitrofurantoin in multi-drug resistant Indian scenario. Indian J Med Microbiol. 2015;33(4):477–481. 10.4103/0255-0857.167350 [DOI] [PubMed] [Google Scholar]
- Velasco M, Martínez JA, Moreno-Martínez A, Horcajada JP, Ruiz J, Barranco M, Almela M, Vila J, Mensa J.. Blood cultures for women with uncomplicated acute pyelonephritis: are they necessary? Clin Infect Dis. 2003. Oct 15;37(8):1127–1130. 10.1086/378291 [DOI] [PubMed] [Google Scholar]
- Wagenlehner F, Tandogdu Z, Bartoletti R, Cai T, Cek M, Kulchavenya E, Köves B, Naber K, Perepanova T, Tenke P, et al. The global prevalence of infections in urology study: a long-term, worldwide surveillance study on urological infections. Pathogens. 2016. Jan 19;5(1):10 10.3390/pathogens5010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Lyman A, Gupta K, Mahoney MV, Snyder GM, Hirsch EB.. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016. Oct 01;63(7):960–965. 10.1093/cid/ciw396 [DOI] [PubMed] [Google Scholar]
- Yamaji R, Rubin J, Thys E, Friedman CR, Riley LW.. Persistent pandemic lineages of uropathogenic Escherichia coli in a college community from 1999 to 2017. J Clin Microbiol. 2018. Feb 07;56(4): e01834-17. 10.1128/JCM.01834-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki E, Yamada C, Jin X, Nair GB, Kurazono H, Yamamoto S.. Expression of marA is remarkably increased from the early stage of development of fluoroquinolone-resistance in uropathogenic Escherichia coli. J Infect Chemother. 2015. Feb;21(2):105–109. 10.1016/j.jiac.2014.10.007 [DOI] [PubMed] [Google Scholar]
- Zacchè MM, Giarenis I.. Therapies in early development for the treatment of urinary tract inflammation. Expert Opin Investig Drugs. 2016. May 03;25(5):531–540. 10.1517/13543784.2016.1161024 [DOI] [PubMed] [Google Scholar]