Abstract
Persistent senescence seems to exert detrimental effects fostering ageing and age-related disorders, such as cancer. Chemotherapy is one of the most valuable treatments for cancer, but its clinical application is limited due to adverse side effects. Melatonin is a potent antioxidant and antiageing molecule, is nontoxic, and enhances the efficacy and reduces the side effects of chemotherapy. In this review, we first summarize the mitochondrial protective role of melatonin in the context of chemotherapeutic drug-induced toxicity. Thereafter, we tabulate the protective actions of melatonin against ageing and the harmful roles induced by chemotherapy and chemotherapeutic agents, including anthracyclines, alkylating agents, platinum, antimetabolites, mitotic inhibitors, and molecular-targeted agents. Finally, we discuss several novel directions for future research in this area. The information compiled in this review will provide a comprehensive reference for the protective activities of melatonin in the context of chemotherapy drug-induced toxicity and will contribute to the design of future studies and increase the potential of melatonin as a therapeutic agent.
1. Introduction
All organismal functions are affected by senescence, from the disorders of cellular protein production and alterations in the macroscopic characteristics of cells to the decline of organ or system functional efficiency, which may increase the development of age-related diseases such as cancer [1–4]. Chemotherapy is one of the main treatments for cancer patients [5, 6]. Chemotherapeutic agents are divided into several categories according to the factors of their effects, their chemical structures, and their relationships to other drugs [7]. The major categories of chemotherapeutic agents include anthracyclines (e.g., daunorubicin (DNR), doxorubicin (DOX), and epirubicin), alkylating agents (e.g., cyclophosphamide (CP), ifosfamide, melphalan, and busulfan), platinum (e.g., cisplatin and oxaliplatin), antimetabolites (e.g., 5-fluorouracil (5-FU), capecitabine, methotrexate (MTX), and gemcitabine), topoisomerase inhibitors (e.g., topotecan, irinotecan, etoposide, and teniposide), mitotic inhibitors (e.g., paclitaxel, docetaxel, vinblastine, and vincristine), and molecular-targeted agents (e.g., trastuzumab) [8, 9]. Despite advances in the development of effective chemotherapeutic drugs, their toxicity or adverse side effects to multiple organ systems and drug resistance have remained main barriers to their successful clinical application [7, 10]. For instance, alkylating agents and topoisomerase II inhibitors could increase the risk of secondary cancer (acute leukemia); anthracyclines, such as doxorubicin, can cause cardiotoxicity; and mitotic inhibitors may cause peripheral nerve damage [10].
Melatonin, a widely distributed and functionally diverse molecule, is also known as N-acetyl-5-methoxytryptamine [11–13]. In addition to influencing circadian rhythms, it modulates several molecular pathways related to antitumor effects, antiageing, anti-inflammation, sleep promotion, antivenom, body weight regulation, antidiabetic activity, and vasorelaxant and antifibrotic properties [14–18]. The roles of melatonin in alleviating chemotherapy drug-induced toxicity among the elderly have been widely considered, and a variety of new mechanisms have been confirmed [19–21]. Accumulated evidence suggests that melatonin enhances the efficacy and reduces the side effects of chemotherapy [22–24]. Pineal indoleamine has the double function of inhibiting cancer and protecting normal tissues, having low toxicity, being a highly effective free radical scavenger, and influencing mitochondrial homeostasis and functioning [25–27]. Furthermore, studies have demonstrated that melatonin was superior in preventing free radical destruction compared to other antioxidants, vitamin E, β-carotene, vitamin C, and garlic oil [28, 29]. Accordingly, the results generally showed that melatonin had a favorable therapeutic use in reducing chemotherapy drug-induced toxicity. However, the precise mechanisms of melatonin protection and the key cellular parameters of its influences still need to be clarified [30].
To study the protective actions of melatonin against chemotherapy drug-induced toxicity, herein we evaluated the available published documents regarding recent progress in this field. First, we present the evidence documenting the mitochondrial protective effect of melatonin on the toxicity of chemotherapy drugs. Second, we illustrate and discuss what is known about how melatonin protects against the detrimental roles of chemotherapy drug-induced toxicity while enhancing the efficacy of chemotherapeutic agents against tumor in various organs. Finally, we discuss several novel potential directions for future research in this field. Collectively, the information compiled here will provide a comprehensive reference for the actions of melatonin in protecting against chemotherapy drug-induced toxicity.
2. Correlation between Melatonin and Ageing
Accumulating studies indicate that melatonin is an antiageing agent that may retard the consequences of senescence [31]. Ageing results in circadian rhythm disorders, thus deregulating the melatonin synthesis [1]. The decrease of the melatonin peak in the elderly at night is consistent with the hypothesis that melatonin is related to senescence [32]. Melatonin mediated the important signaling pathways such as SIRT1 that is an ageing inhibitor whose upregulation may be associated with ageing [33]. Furthermore, senescence is an important pathogenic factor, which decreases the efficiency of the respiratory chain, increases electron leakage, generates free radicals, reduces the production of ATP, and finally leads to mitochondrial dysfunction [34], which can be ameliorated by melatonin. Additionally, melatonin may prolong life-span [35]. Chronic nighttime administration of melatonin significantly extended the life-span of mice and increased their immunocompetence [36, 37]. A recent report indicated that the synthesis of melatonin in mitochondria may be a key aspect of indole's role in ageing [14].
3. Senior Patients Are More Susceptible to the Side Effects of Chemotherapy
Senescence gradually restricts the functional reserve of multiple organ systems and affects the pharmacokinetics and pharmacodynamics of chemotherapy drugs, which may affect both the efficacy and the toxicity of chemotherapy [38]. The rate of functional complications of chemotherapy increases with age, including chronic cardiomyopathy, myelosuppression, nephrotoxicity, neutropenic infections, and chronic peripheral neuropathy [38]. Interestingly, there is a considerable overlap in the common biological changes that occur in normal ageing and chemotherapy treatment [39]. As the cause or consequence of mitochondrial dysfunction, oxidative stress is one of the main driving factors. Increasing reactive oxygen species (ROS) and reactive nitrogen species (RNS) levels occur in ageing and age-related diseases, which were also found in chemotherapy treatment [40]. Besides, ageing is related to cellular senescence, DNA damage, inflammation, mitochondrial dysfunction, and telomere length shortening, and chemotherapy is also similarly associated with all of these processes [39]. Pinder et al. demonstrated that there was a statistically significant increase in the risk of congestive heart failure in senior women who received anthracyclines [41]. The accumulation of cisplatin in the kidney of elderly mice was higher than that of young mice, which was highly correlated with the age-dependent sensitivity of cisplatin-induced nephrotoxicity. In addition, the changes in the inflammatory response and antioxidant signals of the elderly kidney led to the age-dependent susceptibility to kidney injury [42]. It was also indicated that senior patients are more susceptible to experience cognitive decline associated with chemotherapy for breast cancer than younger patients [43, 44]. Senescence appears to interact with cognitive reserve and increases the risk of cognitive decline after chemotherapy [43]. The above studies have shown that the biological processes of the ageing body's response to chemotherapy and degenerative changes overlap with each other, thus raising the hypothesis that ageing may increase the side effects of chemotherapy.
4. The Mitochondrial Protective Role of Melatonin in the Context of Chemotherapy Drug-Induced Toxicity in Ageing
Mitochondrial dysfunction has been identified as an important event in chemotherapy-related toxicity in ageing [45, 46]. The mitochondrion is an organelle for ATP production and determines cell fate [30]. An important feature of mitochondria is that they are closely related to the senescence process, including generation of free radicals, production of ROS and RNS, amplification of damage caused by free radicals, and regulation of the apoptotic pathway due to interference with mitochondrial membrane potential and susceptibility to oxidative/nitrosative stress [1]. Furthermore, chemotherapy drugs often produce free radicals, which are a key cause of cell death [47]. ROS damage mitochondrial DNA (mtDNA), leading to the activation of the extrinsic apoptotic pathway (Figure 1) [48]. In addition, ROS interfere with calcium homeostasis and induce lipid peroxidation, reducing mitochondrial redox potential and opening the mitochondrial permeability transition pore (mPTP), thus resulting in membrane potential loss and cytochrome c release [48]. Excessive free radicals directly cause oxidative damage to the mitochondrial respiratory chain and metabolic enzymes, which further contribute to more electron leakage and free radical production (Figure 1) [49, 50]. Moreover, DOX reduces or inhibits the activity of the cellular antioxidant defense system that further leads to the oxidative stress [46, 51]. This leads to additional molecular damage thereby generating a vicious cycle that eventually leads to cell death [52, 53].
Figure 1.
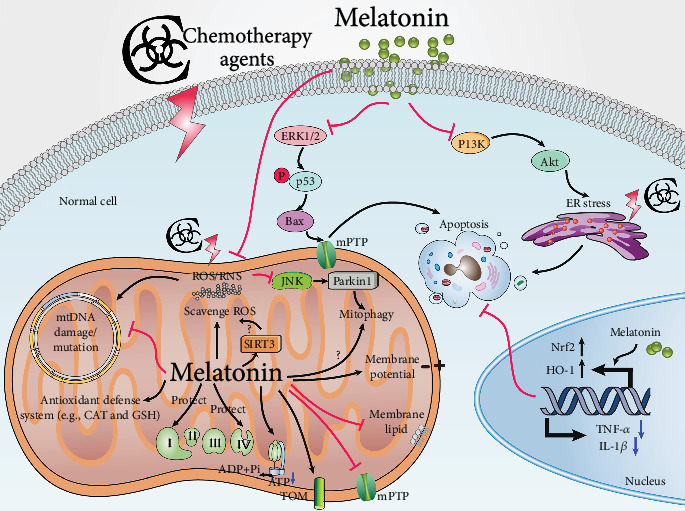
The mechanisms underlying cytoplasmic organellar dysfunction after chemotherapy and melatonin's protective effects under these conditions. Melatonin reverses chemotherapy-induced ER stress, as well as nucleus and mitochondrial dysfunction. In the mitochondrion, chemotherapy drugs lead to electron leakage and excessive free radical production. The ROS directly causes oxidative damage to the mitochondrial respiratory chain, further resulting in elevated electron leakage, free radical production, and ATP depletion. Moreover, ROS injures mitophagy, mtDNA, and the mitochondrial membrane structure (TOM complex reduction and mitochondrial membrane lipid peroxidation increases and elevates mPTP opening), leading to membrane potential loss and proapoptosis factor release. Apart from directly scavenging free radicals, melatonin protects against mtDNA damage/mutation, activates the antioxidant defense system, activates SIRT3 to scavenge ROS, and upregulates the TOM complex, the entry gate for the majority of precursor proteins that are imported into the mitochondria. However, the role of melatonin in mitophagy is less clear. Melatonin inhibits chemotherapy-induced stimulation of ERK1/2, followed via enhanced phosphorylation of p53 by the upregulation of genes such as Bax, thus resulting in mPTP opening. In the nucleus, melatonin upregulates Nrf2 and HO-1 expression and decreases TNF-α and IL-1β levels, thus contributing to cell protection. In the ER, melatonin reverses chemotherapy-induced ER stress via the inhibition of the PI3K/AKT pathway. As a consequence, melatonin protects diverse organs after chemotherapy. Abbreviations: Akt, protein kinase B; ATP, adenosine triphosphate; IL-1β, interleukin-1β; mPTP, mitochondrial permeability transition pore; ER, endoplasmic reticulum; ERK, extracellular regulated protein kinases; HO-1, heme oxygenase-1; JNK, c-Jun-N-terminal kinases; mtDNA, mitochondrial DNA; PI3K, phosphoinositide 3 kinase; ROS, reactive oxygen species; SIRT3, silent information regulator 3; SP, substance P; TOM, translocases in the outer membrane.
To reduce cell death, it is essential to break the vicious circle between free radical production and mitochondrial injury [14]. Melatonin, an effective free radical scavenger, is highly concentrated in mitochondria, which enhances its ability to resist mitochondrial oxidative damage [54, 55]. However, senescence can lead to the deterioration of circadian rhythmicity; thus, it causes disorders in melatonin synthesis [1, 56]. A certain amount of evidence has been accumulated showing that melatonin supplementation counteracts the exacerbating effects of senescence by inhibiting oxidative stress, mitochondrial dysfunction, and inflammation [1, 57]. Various studies have demonstrated that melatonin protected against mitochondrial dysfunction because of its direct free radical-scavenging activity and its indirect antioxidant properties [30, 58]. Melatonin effectively combats chemotherapy-mediated mitochondrial dysfunction by increasing the expression and activity of the mitochondrial respiration chain complexes (complexes I and IV), thereby increasing ATP production (Figure 1) [59]. When melatonin inhibits oxidative stress, lipid peroxidation is repressed and the mitochondrial membrane structure is protected [60]. It is also documented that melatonin regulates mitochondrial membrane permeability by modulating the translocases in the outer membrane (TOM) complex and mPTP activity (Figure 1) [61, 62]. In consequence, melatonin protects the mitochondrial membrane potential and inhibits the release of proapoptotic proteins [63, 64].
Compared with other antioxidants, the most attractive property of melatonin is that its metabolites also regulate the mitochondrial redox status by scavenging ROS and RNS and maintaining bioenergetic homeostasis and their antiapoptotic effects [30, 65]. Melatonin and its metabolites form a free radical-scavenging cascade, which makes melatonin highly effective even at low concentrations and can protect the organs against radical-induced damage continuously [66]. Furthermore, cyclic 3-hydroxymelatonin, a major metabolite of melatonin, protects mitochondrial cytochrome c against free radical-induced damage, and therefore it may inhibit apoptosis induced by oxidative cytochrome c release from mitochondria [67].
Additionally, compared with other antioxidants, melatonin has a protective effect on the heart without affecting DOX's antitumor activity, which is a unique characteristic of melatonin [68, 69]. Interestingly, some beneficial effects of melatonin on mitochondrial respiration are independent of its antioxidant activity but are related to its high redox potential [70]. This unique property allows melatonin to interact with the complexes of the electron transport chain, where it donates or accepts electrons thereby increasing electron flow in a way that other antioxidants do not [71]. Melatonin also exerts indirect protective effects through other pathways that do not involve radical scavenging. Melatonin enhances antioxidant defense systems by stimulating gene expression and the activity of antioxidants [59, 72] and improving the de novo synthesis of glutathione (GSH) by promoting the activity of gamma-glutamylcysteine synthetase [73].
Recent studies have revealed that melatonin inhibits the production of nitric oxide synthase (NOS) at the level of NOS gene transcription [74, 75]. Additionally, it was also shown that melatonin could selectively reduce mitochondria-induced NOS levels in the heart, thereby improving mitochondrial function in patients with sepsis [76]. In addition to the above effects, melatonin also maintains a healthy mitochondrial network by regulating mitochondrial biogenesis, dynamics, autophagy and mitophagy, mitochondrial fission and fusion, and its action on mitochondrial sirtuin activity [30, 77].
5. The Role of Melatonin in Anthracycline-Induced Organ Failure
The anthracyclines are the most widely used anticancer drugs in the treatment of human cancers, including their use in acute leukemia, Hodgkin's and non-Hodgkin's lymphoma, and breast cancer [78]. Like all other anticancer agents, anthracyclines are double-edged swords because they may result in the development of tumor cell resistance, and they are toxic to healthy tissues, especially the heart [78]. DOX and DNR are the original anthracyclines isolated from the pigment-producing bacterium Streptomyces peucetius in the 1960s. DOX differs from DNR by a single hydroxyl group, which has spurred researchers worldwide to identify five DOX/DNR analogs, one (idarubicin) of which is available in the United States [78]. A number of studies have indicated that DOX-induced cardiotoxicity is based on elevated oxidative stress via increasing ROS and lipid peroxidation, together with reducing the antioxidants and sulfhydryl groups [79, 80]. Compared with other organs, the heart has abundant mitochondria which are sources and targets of ROS, so that it is vulnerable to DOX-induced oxidative damage [45]. Moreover, the heart consumes more oxygen and has limited antioxidant defense systems compared with other tissues [81]. Thus, cardiomyocytes expressed low levels of catalase (CAT) and that antioxidant selenium-dependent glutathione- (GSH-) peroxidase-1 is inactivated when exposed to DOX, thereby reducing cytosolic antioxidant Cu-Zn superoxide dismutase [46, 51].
Although many approaches are designed to prevent or mitigate DOX toxicity, there are limits to the ability of these therapies to protect organs from injury, especially the heart. In contrast, the antioxidant melatonin has been effectively used to reduce cardiomyocyte damage [82, 83]. Melatonin plays a cardioprotective role against DOX-induced damage, including by elevating the ST segment and reducing the R-amplitude, decreasing the serum levels of cardiac injury markers, protecting antioxidant enzyme activity, reducing lipid peroxidation, and altering lipid profiles in the serum in rats (Table 1) [84]. Melatonin ameliorated oxidative stress by controlling iron and binding protein levels in DOX-treated rats [85]. Moreover, melatonin promotes the activity of protective antioxidative enzymes in myocardial cells subjected to the action of DOX. The protective effect is due to increased GSH levels and stimulation of CAT activity by melatonin in cardiomyocytes during DOX exposure (Table 1) [86]. Myocardial zinc accumulation may protect against DOX-induced oxidative stress, and melatonin inhibits the DOX-induced drop in plasma zinc levels, indicating that melatonin may have an action in maintaining plasma zinc levels [87]. Additionally, cardiac function was improved and lipid peroxidation was reduced after melatonin treatment, indicating that melatonin has a protective effect on DOX toxicity by attenuating lipid peroxidation (Figure 2) [88–90]. DOX binds to cardiolipin to form an irreversible complex; thus, it inhibits oxidative phosphorylation and prevents cardiolipin from acting as a cofactor of mitochondrial respiratory enzymes [91, 92]. Melatonin protects the mitochondria via inhibiting cardiolipin oxidation that would facilitate the mPTP, resulting in cell death [93]. Melatonin combined with DOX successfully inhibited DOX-induced apoptosis through AMPK-dependent mechanisms, indicating its potential as a cell death protectant in DOX chemotherapy [94, 95]. Another study reported that the protective effect of melatonin was due in part to inhibiting DOX-induced cardiomyocyte apoptosis by preventing DNA fragmentation (Figure 2) [30, 96, 97]. Furthermore, melatonin not only resists cardiotoxicity induced by DOX therapy, but it also enhances its antitumor activity more than vitamin E [98]. DOX causes serious injury when extravasated. Kesik et al. found that melatonin ameliorated DOX-induced skin necrosis in rats. Moreover, it could enhance the sensitivity of tumor to DOX in vivo [22, 99]. Melatonin administered in parallel with DNR reduced the proportion of apoptotic cardiomyocytes [100]. Epirubicin increased nitrosative stress only in heart tissue, and the cardioprotective effect of melatonin partially resulted from its suppression of epirubicin-induced nitrosative stress [101]. These results reveal that melatonin has a protective effect against epirubicin-induced cardiotoxicity [101].
Table 1.
Protective effects and mechanisms of melatonin action against the side effect induced by chemotherapy agents.
| Chemotherapy agents | Experimental studies | Drugs and doses | Administration route | Outcomes | Underlying mechanisms | References |
|---|---|---|---|---|---|---|
| Anthracyclines | NIH3T3 cells | DOX (2.6 μM for 24 h) + melatonin (1 μM for 24 h) | Countered apoptosis generated by DOX alone | AMPK-Ppar gamma-dependent mechanisms | [94] | |
| Male Wistar-Albino rats | DOX (18 mg/kg) + melatonin (10 mg/kg/day, 7 days) | Intraperitoneal | Protected the heart against DOX-induced cardiotoxicity | Melatonin treatment prevented the elevation of the ST segment and R amplitude, as well as the elevation of cardiac injury markers and lipid peroxidation, and it prevented the decrease of antioxidant enzyme activity | [84] | |
| Male Sprague-Dawley rats | DOX (10 mg/kg) + melatonin (15 mg/kg) | Intraperitoneal | Melatonin controlled oxidative stress and modulated iron, ferritin, and transferrin levels | Ameliorated oxidative stress by controlling iron and binding protein levels | [85] | |
| Buffalo strain rats | DOX (2.5 mg/kg) + melatonin (20 mg/kg) | Intraperitoneal | Melatonin stimulated the activity of protective antioxidative enzymes in myocardial cells of rats | Melatonin increased GSH levels and stimulated CAT activity | [86] | |
| Sprague-Dawley rats | DOX (15 mg/kg) + melatonin (84 mg/kg) | Intraperitoneal | Melatonin maintained the plasma zinc levels | Zinc accumulation protects against oxidative stress and melatonin inhibited the DOX-induced decrease in plasma zinc levels | [87] | |
| Male Wistar rats | DOX (7.5 mg/kg) + melatonin (6.0 mg/kg) | Intraperitoneal | Melatonin prevented DOX-induced lipid peroxidation in rat liver | Melatonin-induced gene expression changes | [87] | |
| Male Wistar rats | Melatonin (90 mg/kg) | Intraperitoneal | Cardiac function was improved and lipid peroxidation was decreased | Melatonin provides protection against DOX toxicity via an attenuation of lipid peroxidation | [88] | |
| Ehrlich ascite carcinoma-bearing mice | DOX (4 mg/kg/week, 2 weeks) + melatonin (5 mg/kg/day, 15 days) | Intraperitoneal | Melatonin protected against cardiotoxicity and enhanced its antitumor activity to a more significant extent than did vitamin E | The cardiac contents of total protein, GSH, and SOD were increased, while the cardiac content of MDA was decreased | [98] | |
| Male Wistar rats | Epirubicin (10 mg/kg) + melatonin (200 mg/kg) | Intraperitoneal | Melatonin protected against cardiotoxicity induced by epirubicin | Melatonin was partially attributed to the suppression of epirubicin-induced nitrozative stress | [101] | |
|
| ||||||
| Alkylating agents | HBE cells | Yperite or with MEC + melatonin (100 μM) | Melatonin prevented mustard-induced anoikis | Inhibition of caspase-dependent mitochondrial permeability transition | [120] | |
| NMRI mice | CP (200 mg/kg) + melatonin at different concentrations (2.5, 5, 10, and 20 mg/kg) | Intraperitoneal | Melatonin prevented CP-induced oxidative toxicity in mouse lung tissues | The activities of the antioxidant defense system, ROS scavenging, and free radical quenching were increased | [106] | |
| Female Wistar rats | CP (75 mg/kg) + melatonin (5 mg/kg) | Intraperitoneal | Melatonin significantly improved bladder symptoms and histological damage due to CP-induced cystitis | Diminishing bladder oxidative stress, blocking iNOS and peroxynitrite production, upregulating HO-1, and downregulating the expression of SP | [107] | |
| Male Sprague-Dawley rats | CP (150 mg/kg) + melatonin (10 mg/kg) | Intraperitoneal | Melatonin treatment reduced bladder damage and apoptosis | Upregulating Nrf2 and nuclear transcription factor NF-κB expression | [109] | |
| Male ICR mice | CP (150 mg/kg) + melatonin (10 mg/kg) | Intraperitoneal | Melatonin cotreatment prevented the development of hyperplastic urothelium | Decreasing proliferation and apoptotic indices and causing the higher differentiation state of superficial urothelial cells | [110] | |
| Male Wistar albino rats | CP (100 mg/kg) + melatonin (10 mg/kg) | Intraperitoneal | Melatonin may reduce CP-induced testicular damage | The antioxidative properties of indoleamine existed in the chemical structure | [111] | |
| NMRI mice | CP (60 mg/kg) + melatonin (2.5, 5, 10, and 20 mg/kg) | Intraperitoneal | Melatonin has potent antigenotoxic effects and suppression of chromosome aberrations | Scavenging of free radicals and increased antioxidant status | [112] | |
| Albino Wistar rats | CP (75 mg/kg) + melatonin (40 or 100 mg/kg) | Intraperitoneal | Melatonin resulted in global ANS activity elevation, with a marked sympathetic tone predominance | Melatonin modulates autonomic activity via nonreceptor mechanisms | [114] | |
| Male Wistar rats | HN2 (0.5 mg/kg) + melatonin (20 mg/kg or 40 mg/kg) | Intraperitoneal | Melatonin reduced mustard-induced toxicity in the lungs | Melatonin restored oxidative and nitrosative stress markers in a dose-dependent manner | [117] | |
| Male Sprague-Dawley rats | MEC (3.5 mg/kg) + melatonin (100 mg/kg) | MEC via transdermal route and melatonin via intraperitoneal route | Melatonin has anti-inflammatory properties, as well as antioxidant properties | These increases and elevated NOx levels were ameliorated | [196] | |
|
| ||||||
| Platinum | Hepatocellular carcinoma HepG2 cells | Melatonin (1 mM) + cisplatin (20 μM) | Melatonin attenuated cisplatin-induced HepG2 cell death | Regulation of mTOR and ERCC1 expressions | [130] | |
| SK-LU-1 cell line | Cisplatin (11 and 4 μM) + melatonin (1 or 2 mM) | Melatonin enhanced cisplatin-induced cytotoxicity and apoptosis | Elevating mitochondrial membrane depolarization, activating caspases-3/7, and inducing cell cycle arrest in the S phase | [132] | ||
| SH-SY5Y cells | Melatonin (10 μM, 50 μM) + Oxa (100 μM) | Melatonin protects against the oxaliplatin-induced pain and neuropathic deficits | Preventing the loss of mitochondrial membrane potential (Ψm), inhibiting Bcl-2/Bax ratio and releasing sequestered cytochrome c, and promoting neuritogenesis | [136] | ||
| HT-29 cells | Oxa (0-50 μM) + melatonin (15 and 30 μM) | Melatonin improved mitochondrial electron transport chain function and maintained cellular bioenergetics by improving the ATP levels | Ameliorating nitrooxidative stress and preventing nitrosylation of proteins and loss of antioxidant enzymes | [135] | ||
| SH-SY5Y cells | Oxa (10 μM, 50 μM, and 100 μM) + melatonin (10 μM) | Melatonin attenuated oxaliplatin-induced apoptosis | Inhibition of GSH depletion and Mcl-1 downregulation | [136] | ||
| Male Sprague Dawley rats | Cisplatin (7 mg/kg) + melatonin (5 mg/kg) | Intraperitoneal | Melatonin markedly reduced renal cytotoxicity and DNA fragmentation | Scavenge hydroxyl radical (•OH) directly | [123] | |
| Male Wistar rats | Melatonin (4 mg/kg, 10 days) + cisplatin (7 mg/kg) | Intraperitoneal | Melatonin suppressed cisplatin-induced nephrotoxicity | Increasing Nrf2 accumulation in the nuclear fraction and increasing the expression of HO-1 | [122] | |
| Female Swiss mice | Melatonin (5, 10, or 20 mg/kg) + cisplatin (5 mg/kg) | Intraperitoneal | Melatonin effectively protected the ovaries against cisplatin-induced damage | The MT1 receptor and melatonin antioxidant effects | [125] | |
| Female CD-1 mice | Cisplatin (2 mg/kg) + melatonin (15 or 30 mg/kg) | Intraperitoneal | Melatonin attenuated cisplatin-induced follicle loss | Preventing the phosphorylation of PTEN/AKT/FOXO3a pathway | [126] | |
| Wistar rats | Melatonin (10 mg/kg) + Oxa (4 mg/kg) | Intraperitoneal | Melatonin ameliorated the mitochondrial lipid peroxidation levels and protein carbonyl content | Modulating altered nonenzymatic and enzymatic antioxidants and complex enzymes of mitochondria | [54] | |
| Male Sprague-Dawley rats | Melatonin (20 mg/kg + Oxa 5 mg/kg) | Intraperitoneal | Melatonin had anti-inflammatory and antiallodynia effects | Melatonin inhibited synthesis of inflammatory mediators | [138] | |
|
| ||||||
| Antimetabolite | Male Wistar rats | Melatonin (20 and 40 mg/kg) + MTX (7 mg/kg) | Intraperitoneal | Melatonin reduced small intestinal damage and ameliorates MTX-induced enteritis | Attenuating oxidative stress and restoring the activities of the antioxidant enzymes | [141] |
| Male Wistar rats | Melatonin (20 and 40 mg/kg) + MTX (7 mg/kg) | Intraperitoneal | Melatonin protected against MTX-induced small intestinal damage | Attenuation of nitrosative stress, protein tyrosine nitration, and PARP activation | [142] | |
| Male Wistar rats | MTX (7 mg/kg) + melatonin (40 mg/kg) | Intraperitoneal | Melatonin reduced renal damage via antioxidant and anti-inflammatory activities | Reduction of oxidative stress and alteration in the activity of antioxidant enzymes, as well as elevation in myeloperoxidase activity | [143] | |
| Male Sprague Dawley rats | MTX (13.4 mg/kg) + melatonin (10 mg/kg) | Intraperitoneal | Melatonin prevented MTX-induced hepatotoxicity | Through their antioxidant- and radical-scavenging activities | [145] | |
|
| ||||||
| Mitotic inhibitors | Sprague Dawley rats, the 50B11 immortalized DRG neuronal stem cell line | Paclitaxel (100 μM) + melatonin (1 μM) | Intraperitoneal | Melatonin protected against neuropathic pain and limits paclitaxel-induced mitochondrial dysfunction in vitro | Limiting the development of mechanical hypersensitivity | [153] |
|
| ||||||
| Molecular-targeted agents | Male Sprague-Dawley rats | Trastuzumab (10 mg/kg) + melatonin (10 mg/kg, 2 days) | Intraperitoneal | Melatonin was effective in preventing trastuzumab-induced cardiotoxicity | Reversing oxidative stress markers | [156] |
Abbreviations: CAT, catalase; CP, cyclophosphamide; DOX, doxorubicin; GSH, glutathione; MEC, mechlorethamin; MTX, methotrexate; Oxa, oxaliplatin; PARP, poly(ADP-ribose)-polymerase.
Figure 2.
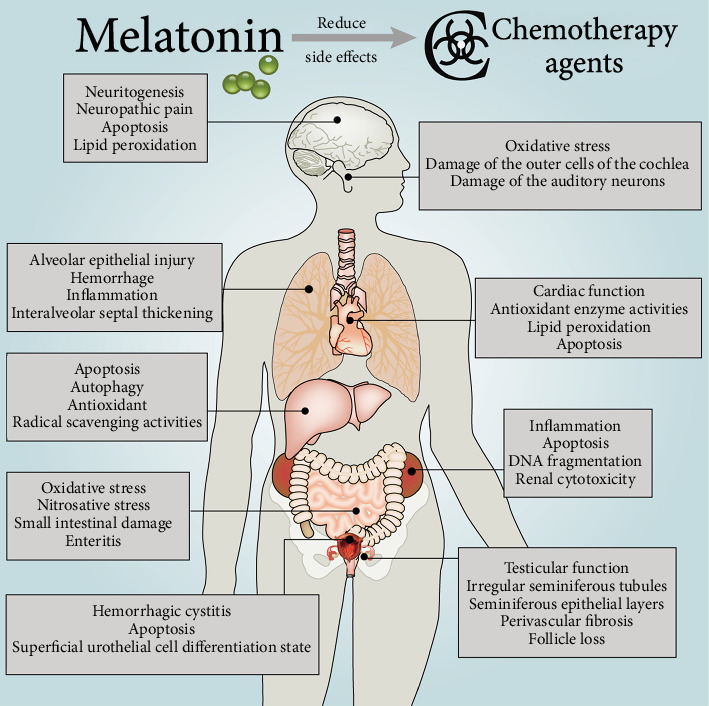
Protection of melatonin against chemotherapy drug-induced damage in various organs.
6. The Role of Melatonin in Alkylating Agent-Induced Organ Failure
6.1. Cyclophosphamide
CP is most widely used an alkylating agent, and its antineoplastic and immunomodulating activities have been approved for early and advanced breast cancer [102]. CP alkylates DNA, forming DNA-DNA cross-links; thus, it inhibits DNA synthesis and causes cell death [103]. It was also shown that CP exerts its toxic effect via enhancing free radicals and other reactive oxygen species that cause lipid peroxidation and cell damage while melatonin provided an antioxidant defense with a highly chemoprophylactic effect on CP-induced cytotoxicity [104, 105]. Oxidative stress markers and the corresponding adaptability of the antioxidant defense system were increased after CP administration, indicating that oxidative stress plays a central role in CP-induced injury to the lung. Melatonin prevents CP-induced oxidative toxicity in pulmonary tissue [106]. Furthermore, by reducing bladder oxidative stress and blocking the production of nitric oxide synthase and peroxynitrite, melatonin upregulates heme oxygenase-1 (HO-1) and downregulates substance P (SP) expression, significantly improving bladder symptoms and lowering histological damage in CP-induced cystitis in rats (Table 1) [107, 108]. Another work reported that melatonin administration decreased bladder injury and apoptosis due to the upregulation of Nrf2 and nuclear transcription factor NF-κB expression [109]. Moreover, melatonin cotreatment inhibited the development of hyperplastic urothelium, statistically significantly reduced cell proliferation and apoptosis index, and promoted the differentiation of superficial urothelial cells after CP treatment (Figure 2) [110]. CP caused spermatic tubule malformation, reduced the epithelium of spermatic tubules, and caused significant maturation stagnation and perivascular fibrosis [58]. Melatonin significantly improved the histopathologic appearance of a CP-damaged testis, indicating that the protective effect of melatonin on CP-induced testicular injury may be due to the antioxidant properties of indoleamine [58, 111]. Additionally, melatonin had potent antigenotoxic effects and suppressed chromosome aberrations against CP-induced toxicity in mice, which may relate to the scavenging of free radicals and elevated antioxidant status [112, 113]. Interestingly, the treatment of CP-induced hemorrhagic cystitis in rats with melatonin is characterized by the increased activity of the global autonomic nervous system (ANS) and a significant predominance of sympathetic tone, suggesting that melatonin may regulate autonomic activity through nonreceptor mechanisms [114].
6.2. Nitrogen Mustard
Nitrogen mustards, also known as DNA alkylating agents, are an important class of drugs for cancer treatment [115]. These anticancer drugs are used effectively in myelogenic leukemia; Hodgkin disease; lung, testicle, ovarian, and breast cancers; and several lymphomas [116]. Nonetheless, nitrogen mustards are highly reactive and lack selectivity; thus, they produce several adverse side effects [115]. Accumulation of inflammatory cells and increased proinflammatory cytokines, reactive oxygen species, nitric oxide, and peroxynitrite contribute to the pathogenesis of mustard-induced toxicity. Mechlorethamin (MEC), a nitrogen mustard, can result in alveolar epithelial injury, hemorrhage, inflammation, edema, and interalveolar septal thickening in lung tissues. Melatonin has anti-inflammatory properties, has the documented ability to alleviate mustard-induced toxicity, and acts as an iNOS inhibitor and a peroxynitrite scavenger in the lungs [117, 118]. Moreover, both inflammation and oxidative stress may be mechanisms in MEC-induced nephrotoxicity. TNF-α and IL-1β levels enhanced markedly with MEC application; melatonin ameliorated these increases and elevated NOx levels in kidney tissue. This supports the opinion that melatonin has anti-inflammatory and antioxidant properties [119]. Suppression of the mitochondrial pathway by melatonin significantly inhibited mustard-induced anoikis, indicating that suppression of caspase-dependent mitochondrial permeability transition preserves airway epithelial cells from mustard-induced apoptosis [120].
7. The Role of Melatonin in Platinum-Induced Organ Failure
7.1. Cisplatin
Cisplatin is widely used as a chemotherapeutic agent for the treatment of various malignant tumors in pediatric and adult patients, including non-small-cell lung cancer (NSCLC) and breast, testicular, and ovarian carcinomas [121]. However, the use of cisplatin is limited by its serious side effects such as nephrotoxicity and ototoxicity [122]. The decrease of antioxidant status induced by cisplatin results in the disorders of antioxidant defense against free radical damage [122]. Melatonin and its metabolites protect against cisplatin toxicity [123]. Melatonin directly scavenges the highly toxic hydroxyl radicals (•OH) and significantly attenuates renal cytotoxicity and DNA fragmentation induced by cisplatin [123, 124]. Melatonin treatment promoted the accumulation of Nrf2 and increased the expression of HO-1 in the cytosolic fraction, indicating that melatonin inhibits cisplatin-induced nephrotoxicity by activating the Nrf2/HO-1 pathway (Table 1) [122]. Cisplatin exhausts the dormant follicle pool in mouse ovaries via excessive activation of the primordial follicles without inducing follicular apoptosis. Pretreatment with melatonin effectively preserved the ovaries from cisplatin-induced injury, an effect mediated by the MT1 membrane melatonin receptor [125]. Furthermore, melatonin reduces cisplatin-induced follicle loss via blocking the phosphorylation of the PTEN/AKT/FOXO3a pathway (Figure 2) [126]. Cisplatin-treatment markedly impaired testicular function, but combined treatment with melatonin prevented the testicular toxicity in rats [111, 127]. Thus, melatonin is a potential therapeutic agent for protecting the reproductive system during chemotherapy.
The mechanism of the ototoxicity caused by cisplatin molecular damage is based on the generation of ROS, which interferes with the physiology of the organ of Corti. As an antioxidant and immune modulator, melatonin has been used to treat cisplatin ototoxicity using transtympanic local application in low doses [128, 129]. Melatonin attenuates cisplatin-induced cell death and reduced phosphorylated p53 apoptotic protein, cleaved caspase 3, and Bax levels, while enhancing the antiapoptotic Bcl-2 gene and protein expression [130]. It also reversed the effects of cisplatin through inhibiting the overexpression of mTOR and ERCC 1 and increasing the expression levels of Beclin-1 and microtubule-associated protein-light chain3-II, bringing about the development of intracellular autophagosomes [130]. These findings suggest that melatonin alleviated cisplatin-induced cell death in HepG2 cells by balancing the roles of apoptotic- and autophagy-related proteins [130].
Chemotherapy with cisplatin also has various vascular side effects. A recent report indicates that melatonin treatment protects the aorta during cisplatin-based chemotherapy [131]. Melatonin increased cisplatin-induced cytotoxicity and apoptosis in human lung adenocarcinoma cells [132]. Melatonin combined with chemotherapy had no effect on survival and adverse events in patients with advanced NSCLC, but showed a trend of improving health-related quality of life [133], which suggests that melatonin has the potential to treat NSCLC in combination with cisplatin.
7.2. Oxaliplatin
Oxaliplatin is a third-generation platinum compound which is active against colorectal growth, but its clinical application is limited attributed to peripheral neuropathy progression [134]. Mitochondrial dysfunction has been considered to be the main pathological mechanism of oxaliplatin-induced neurotoxicity, and the suppression of autophagy may also aggravate the death of neurons [135]. Melatonin has neuroprotective roles in oxaliplatin-induced peripheral neuropathy [135]. Moreover, melatonin protects against the oxaliplatin-induced pain and neuropathic deficits in rats [54, 135]. Melatonin suppressed the loss of mitochondrial membrane potential and Bcl-2/Bax ratio, as well as the release of sequestered cytochrome c, while promoting neuritogenesis in oxaliplatin-stimulated neuro-2a cells [135, 136]. Melatonin lowered oxaliplatin-induced mitochondrial lipid peroxidation levels and protein carbonyl content and regulated the changes of mitochondrial nonenzymatic and enzymatic antioxidants and complex respiratory enzymes [54]. It also improved oxaliplatin-mediated nitrooxidative stress to prevent nitrosylation of proteins and loss of antioxidant enzymes; thus, it ameliorates the function of the mitochondrial electron transport chain and maintains the biological energy of cells by increasing ATP levels [135]. The protective effects of melatonin are also partially due to the prevention of oxaliplatin-induced neuronal apoptosis via promoting the autophagy pathway of the peripheral and dorsal root ganglia (DRG) [135]. Melatonin was also found to inhibit proteolytic activation of caspase 3, inactivation of poly(ADP-ribose) polymerase, and DNA damage, thus allowing SH-SY5Y cells resistant to apoptotic cell death [136]. A recent study shows that melatonin reduces oxaliplatin-induced apoptosis via preventing GSH depletion and Mcl-1 downregulation in renal carcinoma Caki cells [137]. It has been documented that the neuroinflammatory response in the dorsal horn of the spinal cord is a key factor in oxaliplatin-induced pain. Melatonin has been reported to have anti-inflammatory and antiallodynia effects in preclinical and clinical pain studies [138].
8. The Role of Melatonin in Antimetabolite-Induced Organ Failure
MTX, a structural analogue of folic acid, is one of the most effective and potent anticancer drugs used for leukemia and other malignancies [139]. It is an important component in the treatment regime of acute lymphoblastic leukemia, lymphoma, osteosarcoma, and breast cancer, as well as in head and neck cancer [139]. However, its high toxicity, including gastrointestinal, renal, nervous, hepatic, and bone marrow toxicity, limits its use. The main toxic effects of MTX are intestinal injury and enterocolitis resulting in malabsorption and diarrhea [140], which are the major causes of morbidity in children and adults [141]. It is demonstrated that melatonin reduces MTX-induced oxidative stress and small intestinal damage in rats, indicating that supplementation with exogenous melatonin can significantly attenuate MTX-induced intestinal injury, and may be beneficial to the improvement of human enteritis induced by MTX (Figure 2) [141]. Moreover, a preclinical study reported that melatonin protected against MTX-induced small intestinal damage through reducing nitrosative stress, protein tyrosine nitration, and poly(ADP-ribose)-polymerase (PARP) activation (Table 1) [142]. Melatonin pretreatment attenuated MTX-induced oxidative stress, changed antioxidant enzyme activity, and improved myeloperoxidase activity, suggesting that melatonin may decrease renal damage via antioxidant and anti-inflammatory actions [143, 144]. Melatonin prevents MTX-induced hepatotoxicity through antioxidant- and radical-scavenging activities in male rats [145]. MTX treatment brings about enhanced malondialdehyde levels and myeloperoxidase activity and reduces GSH levels in the blood, liver, and kidney. These effects were reversed by melatonin, suggesting that melatonin may have a high therapeutic benefit when used with MTX [146].
9. The Role of Melatonin in Mitotic Inhibitor-Induced Organ Failure
Taxanes, including paclitaxel and docetaxel, are commonly used chemotherapeutic agents for a variety of malignancies [147, 148]. Paclitaxel has a wide range of anticancer effects. In the process of cell division, paclitaxel inhibits cell cycle and induces cell death by stabilizing microtubules and interfering with microtubule disassembly [149]. In addition to ameliorating disease-specific outcomes, taxanes also can cause considerable morbidity. The most common and particularly troublesome toxicity is taxane-induced peripheral neuropathy [150]. Melatonin plays a beneficial role in taxane-related neuropathy [151, 152]. Patients treated with melatonin during taxane chemotherapy had a lower incidence of neuropathy, suggesting that melatonin may be useful in preventing or reducing taxane-induced neuropathy and in maintaining quality of life [151]. Moreover, melatonin protected rats from paclitaxel-induced neuropathic pain and mitochondrial dysfunction in vitro (Figure 2) [153]. Mitochondrial dysfunction associated with oxidative stress in peripheral nerves has been considered as a potential mechanism [153]. The potential of melatonin to decrease mitochondrial injury and neuropathic pain due to paclitaxel has been documented [153].
10. The Role of Melatonin in Molecular-Targeted Agent-Induced Organ Failure
Trastuzumab, a humanized monoclonal antibody that can be used against the extracellular domain of human epidermal growth factor receptor 2 (HER2), is an important component of the adjuvant therapy and metastasis therapy for HER2-positive breast cancers. The herceptin adjuvant study reported that adjuvant trastuzumab treatment for 1 year improves disease-free survival and overall survival in patients with HER2-positive early breast cancer [154]. However, its side effects limit the use of adjuvant trastuzumab treatment, including cardiotoxicity, fever and chills, shortness of breath, muscle weakness, cutaneous rash, diarrhea, and headache [155]. Trastuzumab is an effective agent for the treatment of various neoplastic diseases. Oxidative stress markers and serum CK-MB levels were highly enhanced after treatment with trastuzumab; these changes were also reversed by melatonin treatment which resulted in near normal levels, which suggested that melatonin is effective in preventing trastuzumab-induced cardiotoxicity (Table 1) [156].
11. Melatonin Enhances the Efficacy of Chemotherapy Agents against Tumors
Mounting evidence indicates that melatonin exerts a variety of anticancer properties at different stages of tumor progression and metastasis [157–160]. Moreover, the combination of melatonin and chemotherapies has been reported to improve the effectiveness of anticancer drugs [23, 161]. Melatonin significantly enhanced the cytotoxicity of the chemotherapy drugs against cancer cells. Consistently, each of the chemotherapy drugs with melatonin increased the ratio of cells entering mitochondrial apoptosis due to ROS overproduction, mitochondrial membrane depolarization, and highly expanded DNA fragmentation [162, 163]. It was also reported that melatonin does not interfere with the action of DOX on cancer cells but actually enhances the action of the anticancer drug possibly by inhibiting the outflow of P-glycoprotein-mediated DOX from cancer cells [23]. Melatonin potentiates cisplatin-induced apoptosis and cell cycle arrest in human lung adenocarcinoma cells [132]. Costimulation of HeLa cells with cisplatin in the presence of melatonin further increased cellular apoptosis, improved the mitochondrial structure and function, and significantly increased caspase-9-dependent mitochondrial apoptosis [161]. Melatonin inactivated mitophagy via blocking c-Jun-N-terminal kinases (JNK)/Parkin, resulting in the suppression of antiapoptotic mitophagy, indicating that melatonin enhances human cervical cancer HeLa cell apoptosis induced by cisplatin through inhibiting the JNK/Parkin/mitophagy axis [161, 164]. Furthermore, melatonin substantially augmented the 5-FU-mediated inhibition of cell proliferation, colony formation, cell migration, and invasion of colon cancer cells [165]. It was shown that melatonin and 5-FU synergistically induced cell cycle arrest by activating the caspase/PARP-dependent apoptosis pathway [165]. Furthermore, melatonin exaggerated the antitumor role of 5-FU by inhibiting the phosphorylation of the phosphatidylinositol 3-kinase (PI3K)/AKT/iNOS signaling pathways or promoting the translocation of NF-κB p50/p65 from the nuclei to the cytoplasm, abrogating their binding to the iNOS promoter; thus, it inhibits iNOS signaling [165, 166]. Additionally, melatonin intensified the antitumor actions of paclitaxel in the endoplasmic reticulum endometrial cancer cell line, which express MT1 melatonin receptors [167].
12. Potential Future Directions and Conclusions
Senescence is a process of gradual functional deterioration of physiological mechanisms as time goes on. About half of human deaths are linked to ageing-related chronic diseases, including neurological disorders, diabetes, cardiovascular diseases, and cancer [168, 169]. Mitochondrial dysfunction is the main driver of these processes, which occur in ageing and age-related disorders; they were also found in chemotherapy treatment [40]. Mitochondria are major production sites of free radicals and related toxic species [170]. Abnormal mitochondrial function, increased ROS production, damaged mitochondrial DNA, decreased respiratory complex activities, and augmented electron leakage and mPTP opening played key roles in the pathophysiology of chemotherapy agent-induced toxicity in ageing [30]. Consistent with the amphiphilic nature of melatonin, it easily crosses all biological barriers and gains access to all compartments of the cell, and it is highly concentrated in mitochondria, indicating its ability to resist mitochondrial oxidative damage [171, 172]. Melatonin was first implicated in modulating nuclear SIRT1 during the biological process of cancer [173, 174]. SIRT3 indirectly reduces cellular ROS to prevent the cardiac hypertrophic response [175]. Moreover, both MnSOD and catalase levels of SIRT3 transgenic mice were increased, implying that SIRT3 was partly responsible for the enhancement of the antioxidant defense mechanisms of the heart [175]. These evidences potentially indicate that melatonin may have the ability to regulate mitochondrial sirtuins during DOX-induced cardiotoxicity [176]. It is demonstrated that mtDNA lesions caused by ROS or ERK1/2 activation directly induced by DOX, followed by elevated phosphorylation of p53, upregulated genes such as Bax [177]. After melatonin pretreatment, ERK2, phosphorylated p38, HSP-70, phosphorylated p53, c-Jun, and other crucial stress protein levels returned to normal [178]. Additionally, excessive DNA damage and enhanced ROS production induced by DOX, resulting in PARP hyperactivation and energy depletion, promotes necroptosis [179, 180].
Melatonin also activates mitochondrial STAT3 via the SAFE pathway of decreasing myocardial IR damage [181–183]. In cultured neonatal rat cardiomyocytes and isolated rat hearts, melatonin precondition alleviates IR-induced mitochondrial oxidative damage by activating the JAK2/STAT3 signaling pathway, as well as enhances mitochondrial mitophagy via activating the AMPK-OPA1 signaling pathways [184–187]. Thus, melatonin may protect against chemotherapy agent-induced mitochondrial oxidative damage through similar pathways. Apart from melatonin as an effective free radical scavenger, it also maintains a healthy mitochondrial network by regulating mitochondrial biogenesis, dynamics, and mitophagy [188]. Melatonin has multiple mitochondrial benefits in Alzheimer's disease, by significantly reducing ROS-mediated mitochondrial fission, mitochondrial membrane potential depolarization, and mitochondrial tardiness, thereby stabilizing cardiolipin; collectively, these actions reduce enhanced mitochondria-mediated cell death [189].
It is well documented that DOX causes endoplasmic reticulum (ER) dilation, indicating that DOX may also affect ER function apart from actions in the mitochondrion [190]. ER is involved in protein folding, calcium homeostasis, and lipid biosynthesis [191]. ER stress refers to the accumulation of unfolded proteins induced by oxidative stress, ischemic injury, calcium homeostasis disorders, and/or enhanced expression of folded defective proteins [191]. DOX stimulates the ER transmembrane stress sensor, activating transcription factor 6, while suppressing X-box binding protein 1 expression, a gene downstream of activating transcription factor 6 [190]. The reduced expression of X-box binding protein 1 lowered the ER chaperone glucose-regulated protein 78 level that is crucial in adaptive responses to ER stress [190]. The results of this study revealed that DOX promoted the apoptosis response induced by ER stress without inducing the ER chaperone glucose-regulated protein 78, further elevating ER stress in the hearts (Figure 1). Moreover, doxorubicin activated caspase-12, an ER membrane-resident apoptotic molecule, which leads to cardiomyocyte apoptosis and cardiac dysfunction [190]. Melatonin reverses tunicamycin-induced ER stress by preventing the PI3K/AKT pathway, and it promotes cytotoxic response to DOX via enhancing C/EBP-homologous protein (CHOP) as well as decreasing surviving in human hepatocellular carcinoma cells [192, 193]. Therefore, in addition to protecting mitochondrial homeostasis, maintaining ER homeostasis may also be an important mechanism for melatonin to participate in antichemotherapeutic drug injury. This is an area where more intensive investigation is warranted.
A number of clinical studies have shown that melatonin treatment improves the efficacy and decreases the side roles of chemotherapy, prolongs survival time, and promotes quality of life for patients [82, 90, 194, 195]. The beneficial effects of melatonin administration are partially results from its direct free radical-scavenging activity and its indirect antioxidant properties [30]. Increasing reports on the role of melatonin in animal experiments and clinical trials will undoubtedly deepen our understanding of the protective and beneficial mechanisms of melatonin during chemotherapy. One clinical trial included 70 cancer patients (advanced NSCLC) who were treated with a combination of cisplatin plus etoposide or the chemotherapy drugs plus melatonin. On the basis of complete and partial tumor response rate, melatonin enhanced the effect of cisplatin plus etoposide, and improved the 1-year survival rate. Furthermore, the incidences of myelosuppression, neuropathy, and cachexia were significantly reduced, indicating that patients treated with melatonin had better tolerance to chemotherapy [23]. Another clinical trial treated a total of 250 patients with metastatic solid tumors who were given a variety of different chemotherapies alone or in combination with melatonin [23]. The objective tumor regression rate and the 1-year survival rate were again improved by melatonin cotreatment. Moreover, melatonin significantly alleviated the incidence of thrombocytopenia, neurotoxicity, cardiotoxicity, stomatitis, and asthenia [23]. The patients included in these studies were in the advanced stages of disease where any one treatment is likely to have little effect. Therefore, any benefit of melatonin therapy seems exceptional and applying melatonin therapy in the early stages of cancer seems reasonable, with the promise of greater benefits [23]. Although other clinical trials (NCT01557478, Phase 3; NCT02454855, Phase 3) related to melatonin alleviating the toxicity and improving the efficacy of chemotherapy have yet to be published, it seems necessary to use it more widely given the molecule's apparently beneficial properties and low toxicity. It is suggested that the therapeutic value of melatonin in chemotherapy-induced toxicity and its relationship with mitochondrial dysfunction in further double-blind placebo-controlled studies be evaluated; we anticipate a bounty of additional beneficial findings on the actions of melatonin cotreatment with chemotherapy agents in the next decade.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81572252, 81871866), Natural Science Foundation of Shaanxi Province (2016SF-308; 2019SF-033), the Project of Tangdu Hospital, The Fourth Military Medical University (2015 Key Talents; 2018 Key Talents), and the Excellent Doctoral Support Project of the Fourth Military Medical University (2018D09).
Contributor Information
Jing Han, Email: hanjing_cns@163.com.
Xiaofei Li, Email: lxfchest@fmmu.edu.cn.
Xiaolong Yan, Email: yanxiaolong@fmmu.edu.cn.
Conflicts of Interest
The authors declare no competing interests regarding the publication of this manuscript.
Authors' Contributions
YXL, HJ, and LXF designed the study. MZQ, XLQ, and LD searched the literature and wrote the manuscript. ZXY, DSY, LWM, and ZJ searched the literature and made the table, and MZQ drew the figures. RJR helped to revise the grammar and sentences. All authors read and approved the final manuscript. Zhiqiang Ma, Liqun Xu, and Dong Liu contributed equally to this work.
References
- 1.Majidinia M., Reiter R. J., Shakouri S. K., Yousefi B. The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Ageing Research Reviews. 2018;47:198–213. doi: 10.1016/j.arr.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Kubben N., Misteli T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nature Reviews Molecular Cell Biology. 2017;18(10):595–609. doi: 10.1038/nrm.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Z., Xin Z., Hu W., et al. Forkhead box O proteins: crucial regulators of cancer EMT. Seminars in Cancer Biology. 2018;50:21–31. doi: 10.1016/j.semcancer.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Ma Z., Zhang X., Xu L., et al. Pterostilbene: mechanisms of its action as oncostatic agent in cell models and in vivo studies. Pharmacological Research. 2019;145, article 104265 doi: 10.1016/j.phrs.2019.104265. [DOI] [PubMed] [Google Scholar]
- 5.Liu F. S. Mechanisms of chemotherapeutic drug resistance in cancer therapy—a quick review. Taiwanese Journal of Obstetrics and Gynecology. 2009;48(3):239–244. doi: 10.1016/S1028-4559(09)60296-5. [DOI] [PubMed] [Google Scholar]
- 6.da Silva-Diz V., Lorenzo-Sanz L., Bernat-Peguera A., Lopez-Cerda M., Muñoz P. Cancer cell plasticity: impact on tumor progression and therapy response. Seminars in Cancer Biology. 2018;53:48–58. doi: 10.1016/j.semcancer.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Sak K. Chemotherapy and dietary phytochemical agents. Chemotherapy Research and Practice. 2012;2012:11. doi: 10.1155/2012/282570.282570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X. Z. A new classification system of anticancer drugs – based on cell biological mechanisms. Medical Hypotheses. 2006;66(5):883–887. doi: 10.1016/j.mehy.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Lamson D. W., Brignall M. S. Antioxidants in cancer therapy; their actions and interactions with oncologic therapies. Alternative Medicine Review. 1999;4(5):304–329. [PubMed] [Google Scholar]
- 10.Ma H., Das T., Pereira S., et al. Efficacy of dietary antioxidants combined with a chemotherapeutic agent on human colon cancer progression in a fluorescent orthotopic mouse model. Anticancer Research. 2009;29(7):2421–2426. [PubMed] [Google Scholar]
- 11.Kanwar M. K., Yu J., Zhou J. Phytomelatonin: recent advances and future prospects. Journal of Pineal Research. 2018;65(4, article e12526) doi: 10.1111/jpi.12526. [DOI] [PubMed] [Google Scholar]
- 12.Xia Y., Chen S., Zeng S., et al. Melatonin in macrophage biology: current understanding and future perspectives. Journal of Pineal Research. 2019;66(2, article e12547) doi: 10.1111/jpi.12547. [DOI] [PubMed] [Google Scholar]
- 13.Kennaway D. J. A critical review of melatonin assays: past and present. Journal of Pineal Research. 2019;(article e12572) doi: 10.1111/jpi.12572. [DOI] [PubMed] [Google Scholar]
- 14.Reiter R. J., Rosales-Corral S., Tan D. X., Jou M. J., Galano A., Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cellular and Molecular Life Sciences. 2017;74(21):3863–3881. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y. T., Yang C. C., Shao P. L., Huang C. R., Yip H. K. Melatonin-mediated downregulation of ZNF746 suppresses bladder tumorigenesis mainly through inhibiting the AKT-MMP-9 signaling pathway. Journal of Pineal Research. 2019;66(1, article e12536) doi: 10.1111/jpi.12536. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez D. I., González-Fernández B., Crespo I., et al. Melatonin modulates dysregulated circadian clocks in mice with diethylnitrosamine-induced hepatocellular carcinoma. Journal of Pineal Research. 2018;65(3, article e12506) doi: 10.1111/jpi.12506. [DOI] [PubMed] [Google Scholar]
- 17.Xiang S., Dauchy R. T., Hoffman A. E., et al. Epigenetic inhibition of the tumor suppressor ARHI by light at night-induced circadian melatonin disruption mediates STAT3-driven paclitaxel resistance in breast cancer. Journal of Pineal Research. 2019;(article e12586) doi: 10.1111/jpi.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma R., Sahota P., Thakkar M. M. Melatonin promotes sleep in mice by inhibiting orexin neurons in the perifornical lateral hypothalamus. Journal of Pineal Research. 2018;65(2, article e12498) doi: 10.1111/jpi.12498. [DOI] [PubMed] [Google Scholar]
- 19.Schaper J., Meiser E., Stammler G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circulation Research. 1985;56(3):377–391. doi: 10.1161/01.RES.56.3.377. [DOI] [PubMed] [Google Scholar]
- 20.Ventura-Clapier R., Garnier A., Veksler V. Energy metabolism in heart failure. The Journal of Physiology. 2004;555(1):1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mi P., Zhang Q. P., Li S. B., et al. Melatonin protects embryonic development and maintains sleep/wake behaviors from the deleterious effects of fluorene-9-bisphenol in zebrafish (Danio rerio) Journal of Pineal Research. 2019;66(1, article e12530) doi: 10.1111/jpi.12530. [DOI] [PubMed] [Google Scholar]
- 22.Ma C., Li L. X., Zhang Y., et al. Protective and sensitive effects of melatonin combined with adriamycin on ER+ (estrogen receptor) breast cancer. European Journal of Gynaecological Oncology. 2015;36(2):197–202. [PubMed] [Google Scholar]
- 23.Reiter R. J., Tan D. X., Sainz R. M., Mayo J. C., Lopez-Burillo S. Melatonin: reducing the toxicity and increasing the efficacy of drugs. Journal of Pharmacy and Pharmacology. 2002;54(10):1299–1321. doi: 10.1211/002235702760345374. [DOI] [PubMed] [Google Scholar]
- 24.Lee J. H., Yun C. W., Han Y. S., et al. Melatonin and 5-fluorouracil co-suppress colon cancer stem cells by regulating cellular prion protein-Oct4 axis. Journal of Pineal Research. 2018;65(4, article e12519) doi: 10.1111/jpi.12519. [DOI] [PubMed] [Google Scholar]
- 25.Tengattini S., Reiter R. J., Tan D. X., Terron M. P., Rodella L. F., Rezzani R. Cardiovascular diseases: protective effects of melatonin. Journal of Pineal Research. 2008;44:16–25. doi: 10.1111/j.1600-079X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 26.García J. J., López-Pingarrón L., Almeida-Souza P., et al. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. Journal of Pineal Research. 2014;56(3):225–237. doi: 10.1111/jpi.12128. [DOI] [PubMed] [Google Scholar]
- 27.Lee F. Y., Sun C. K., Sung P. H., et al. Daily melatonin protects the endothelial lineage and functional integrity against the aging process, oxidative stress, and toxic environment and restores blood flow in critical limb ischemia area in mice. Journal of Pineal Research. 2018;65(2, article e12489) doi: 10.1111/jpi.12489. [DOI] [PubMed] [Google Scholar]
- 28.Anwar M. M., Meki A. R. M. A. Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2003;135(4):539–547. doi: 10.1016/S1095-6433(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 29.Gultekin F., Delibas N., Yasar S., Kilinc I. In vivo changes in antioxidant systems and protective role of melatonin and a combination of vitamin C and vitamin E on oxidative damage in erythrocytes induced by chlorpyrifos-ethyl in rats. Archives of Toxicology. 2001;75(2):88–96. doi: 10.1007/s002040100219. [DOI] [PubMed] [Google Scholar]
- 30.Govender J., Loos B., Marais E., Engelbrecht A. M. Mitochondrial catastrophe during doxorubicin-induced cardiotoxicity: a review of the protective role of melatonin. Journal of Pineal Research. 2014;57(4):367–380. doi: 10.1111/jpi.12176. [DOI] [PubMed] [Google Scholar]
- 31.Reiter R. J., Tan D., Rosales-Corral S., Galano A., Zhou X., Xu B. Mitochondria: central organelles for melatonin’s antioxidant and anti-aging actions. Molecules. 2018;23(2):p. 509. doi: 10.3390/molecules23020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong S. M., Redman J. R. Melatonin: a chronobiotic with anti-aging properties? Medical Hypotheses. 1991;34(4):300–309. doi: 10.1016/0306-9877(91)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Ramis M. R., Esteban S., Miralles A., Tan D. X., Reiter R. J. Caloric restriction, resveratrol and melatonin: role of SIRT1 and implications for aging and related-diseases. Mechanisms of Ageing and Development. 2015;146-148:28–41. doi: 10.1016/j.mad.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Green D. R., Galluzzi L., Kroemer G. Mitochondria and the autophagy–inflammation–cell death axis in organismal aging. Science. 2011;333(6046):1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardeland R. Melatonin and the theories of aging: a critical appraisal of melatonin’s role in antiaging mechanisms. Journal of Pineal Research. 2013;55(4):325–356. doi: 10.1111/jpi.12090. [DOI] [PubMed] [Google Scholar]
- 36.Pierpaoli W., Regelson W. Pineal control of aging: effect of melatonin and pineal grafting on aging mice. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(2):787–791. doi: 10.1073/pnas.91.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierpaoli W., Bulian D. The pineal aging and death program: life prolongation in pre-aging pinealectomized mice. Annals of the New York Academy of Sciences. 2005;1057(1):133–144. doi: 10.1196/annals.1356.008. [DOI] [PubMed] [Google Scholar]
- 38.Carreca I., Balducci L. Cancer chemotherapy in the older cancer patient. Urologic Oncology: Seminars and Original Investigations. 2009;27(6):633–642. doi: 10.1016/j.urolonc.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Vega J. N., Dumas J., Newhouse P. A. Cognitive effects of chemotherapy and cancer-related treatments in older adults. The American Journal of Geriatric Psychiatry. 2017;25(12):1415–1426. doi: 10.1016/j.jagp.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kudryavtseva A. V., Krasnov G. S., Dmitriev A. A., et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016;7(29):44879–44905. doi: 10.18632/oncotarget.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinder M. C., Duan Z., Goodwin J. S., Hortobagyi G. N., Giordano S. H. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. Journal of Clinical Oncology. 2007;25(25):3808–3815. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 42.Wen J., Zeng M., Shu Y., et al. Aging increases the susceptibility of cisplatin-induced nephrotoxicity. Age. 2015;37(6):p. 112. doi: 10.1007/s11357-015-9844-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahles T. A., Saykin A. J., McDonald B. C., et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. Journal of Clinical Oncology. 2010;28(29):4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schilder C. M., Seynaeve C., Beex L. V., et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. Journal of Clinical Oncology. 2010;28(8):1294–1300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 45.Doroshow J. H. Anthracycline antibiotic-stimulated superoxide, hydrogen peroxide, and hydroxyl radical production by NADH dehydrogenase. Cancer Research. 1983;43(10):4543–4551. [PubMed] [Google Scholar]
- 46.Li T., Danelisen I., Singal P. K. Early changes in myocardial antioxidant enzymes in rats treated with adriamycin. Molecular and Cellular Biochemistry. 2002;232(1-2):19–26. doi: 10.1023/A:1014862912783. [DOI] [PubMed] [Google Scholar]
- 47.Takemura G., Fujiwara H. Doxorubicin-induced cardiomyopathy: from the cardiotoxic mechanisms to management. Progress in Cardiovascular Diseases. 2007;49(5):330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y. W., Shi J., Li Y. J., Wei L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Archivum Immunologiae et Therapiae Experimentalis. 2009;57(6):435–445. doi: 10.1007/s00005-009-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zorov D. B., Juhaszova M., Sollott S. J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological Reviews. 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musatov A., Robinson N. C. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radical Research. 2012;46(11):1313–1326. doi: 10.3109/10715762.2012.717273. [DOI] [PubMed] [Google Scholar]
- 51.Doroshow J. H., Locker G. Y., Myers C. E. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. The Journal of Clinical Investigation. 1980;65(1):128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singal P. K., Iliskovic N., Li T., Kumar D. Adriamycin cardiomyopathy: pathophysiology and prevention. The FASEB Journal. 1997;11(12):931–936. doi: 10.1096/fasebj.11.12.9337145. [DOI] [PubMed] [Google Scholar]
- 53.Tang J. Y., Farooqi A. A., Ou-Yang F., et al. Oxidative stress-modulating drugs have preferential anticancer effects - involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Seminars in Cancer Biology. 2018 doi: 10.1016/j.semcancer.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Waseem M., Tabassum H., Parvez S. Neuroprotective effects of melatonin as evidenced by abrogation of oxaliplatin induced behavioral alterations, mitochondrial dysfunction and neurotoxicity in rat brain. Mitochondrion. 2016;30:168–176. doi: 10.1016/j.mito.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Galano A., Reiter R. J. Melatonin and its metabolites vs oxidative stress: from individual actions to collective protection. Journal of Pineal Research. 2018;65(1, article e12514) doi: 10.1111/jpi.12514. [DOI] [PubMed] [Google Scholar]
- 56.Gao T., Wang Z., Dong Y., et al. Role of melatonin in sleep deprivation‐induced intestinal barrier dysfunction in mice. Journal of Pineal Research. 2019;(article e12574) doi: 10.1111/jpi.12574. [DOI] [PubMed] [Google Scholar]
- 57.Sagrillo-Fagundes L., Assunção Salustiano E. M., Ruano R., Markus R. P., Vaillancourt C. Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. Journal of Pineal Research. 2018;65(4, article e12520) doi: 10.1111/jpi.12520. [DOI] [PubMed] [Google Scholar]
- 58.Chabra A., Shokrzadeh M., Naghshvar F., Salehi F., Ahmadi A. Melatonin ameliorates oxidative stress and reproductive toxicity induced by cyclophosphamide in male mice. Human & Experimental Toxicology. 2014;33(2):185–195. doi: 10.1177/0960327113489052. [DOI] [PubMed] [Google Scholar]
- 59.Reiter R. J., Tan D. X., Osuna C., Gitto E. Actions of melatonin in the reduction of oxidative stress. Journal of Biomedical Science. 2000;7(6):444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 60.Mukherjee D., Ghosh A. K., Dutta M., et al. Mechanisms of isoproterenol‐induced cardiac mitochondrial damage: protective actions of melatonin. Journal of Pineal Research. 2015;58(3):275–290. doi: 10.1111/jpi.12213. [DOI] [PubMed] [Google Scholar]
- 61.Nair S. M., Rahman R. M. A., Clarkson A. N., et al. Melatonin treatment following stroke induction modulates L-arginine metabolism. Journal of Pineal Research. 2011;51(3):313–323. doi: 10.1111/j.1600-079X.2011.00891.x. [DOI] [PubMed] [Google Scholar]
- 62.Jou M. J., Peng T. I., Reiter R. J. Protective stabilization of mitochondrial permeability transition and mitochondrial oxidation during mitochondrial Ca2+ stress by melatonin’s cascade metabolites C3-OHM and AFMK in RBA1 astrocytes. Journal of Pineal Research. 2019;66(1, article e12538) doi: 10.1111/jpi.12538. [DOI] [PubMed] [Google Scholar]
- 63.Chen H. H., Chen Y. T., Yang C. C., et al. Melatonin pretreatment enhances the therapeutic effects of exogenous mitochondria against hepatic ischemia–reperfusion injury in rats through suppression of mitochondrial permeability transition. Journal of Pineal Research. 2016;61(1):52–68. doi: 10.1111/jpi.12326. [DOI] [PubMed] [Google Scholar]
- 64.Beer M., Seyfarth T., Sandstede J., et al. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. Journal of the American College of Cardiology. 2002;40(7):1267–1274. doi: 10.1016/S0735-1097(02)02160-5. [DOI] [PubMed] [Google Scholar]
- 65.Hu W., Ma Z., Jiang S., et al. Melatonin: the dawning of a treatment for fibrosis? Journal of Pineal Research. 2016;60(2):121–131. doi: 10.1111/jpi.12302. [DOI] [PubMed] [Google Scholar]
- 66.Galano A., Tan D. X., Reiter R. J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. Journal of Pineal Research. 2013;54(3):245–257. doi: 10.1111/jpi.12010. [DOI] [PubMed] [Google Scholar]
- 67.Tan D. X., Hardeland R., Manchester L. C., Galano A., Reiter R. J. Cyclic-3-hydroxymelatonin (C3HOM), a potent antioxidant, scavenges free radicals and suppresses oxidative reactions. Current Medicinal Chemistry. 2014;21(13):1557–1565. doi: 10.2174/0929867321666131129113146. [DOI] [PubMed] [Google Scholar]
- 68.Liu X., Chen Z., Chua C. C., et al. Melatonin as an effective protector against doxorubicin-induced cardiotoxicity. American Journal of Physiology-Heart and Circulatory Physiology. 2002;283(1):H254–H263. doi: 10.1152/ajpheart.01023.2001. [DOI] [PubMed] [Google Scholar]
- 69.Siveski-Iliskovic N., Hill M., Chow D. A., Singal P. K. Probucol protects against adriamycin cardiomyopathy without interfering with its antitumor effect. Circulation. 1995;91(1):10–15. doi: 10.1161/01.CIR.91.1.10. [DOI] [PubMed] [Google Scholar]
- 70.Tan D.-X., Manchester L. C., Reiter R. J., et al. Melatonin directly scavenges hydrogen peroxide: a potentially new metabolic pathway of melatonin biotransformation. Free Radical Biology & Medicine. 2000;29(11):1177–1185. doi: 10.1016/S0891-5849(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 71.Martin M., Macias M., Escames G., et al. Melatonin‐induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. Journal of Pineal Research. 2000;28(4):242–248. doi: 10.1034/j.1600-079X.2000.280407.x. [DOI] [PubMed] [Google Scholar]
- 72.Fischer T. W., Kleszczynski K., Hardkop L. H., Kruse N., Zillikens D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. Journal of Pineal Research. 2013;54(3):303–312. doi: 10.1111/jpi.12018. [DOI] [PubMed] [Google Scholar]
- 73.Urata Y., Honma S., Goto S., et al. Melatonin induces γ-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radical Biology & Medicine. 1999;27(7-8):838–847. doi: 10.1016/S0891-5849(99)00131-8. [DOI] [PubMed] [Google Scholar]
- 74.Jiménez-Ortega V., Cano P., Cardinali D. P., Esquifino A. I. 24-Hour variation in gene expression of redox pathway enzymes in rat hypothalamus: effect of melatonin treatment. Redox Report. 2009;14(3):132–138. doi: 10.1179/135100009X392548. [DOI] [PubMed] [Google Scholar]
- 75.Pozo D., Reiter R. J., Calvo J. R., Guerrero J. M. Inhibition of cerebellar nitric oxide synthase and cyclic GMP production by melatonin via complex formation with calmodulin. Journal of Cellular Biochemistry. 1997;65(3):430–442. doi: 10.1002/(SICI)1097-4644(19970601)65:3<430::AID-JCB12>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 76.Ortiz F., García J. A., Acuña-Castroviejo D., et al. The beneficial effects of melatonin against heart mitochondrial impairment during sepsis: inhibition of iNOS and preservation of nNOS. Journal of Pineal Research. 2014;56(1):71–81. doi: 10.1111/jpi.12099. [DOI] [PubMed] [Google Scholar]
- 77.Boga J. A., Caballero B., Potes Y., et al. Therapeutic potential of melatonin related to its role as an autophagy regulator: a review. Journal of Pineal Research. 2019;66(1, article e12534) doi: 10.1111/jpi.12534. [DOI] [PubMed] [Google Scholar]
- 78.Weiss R. B. The anthracyclines: will we ever find a better doxorubicin? Seminars in Oncology. 1992;19(6):670–686. [PubMed] [Google Scholar]
- 79.Lee K. M., Lee I. C., Kim S. H., et al. Melatonin attenuates doxorubicin‐induced testicular toxicity in rats. Andrologia. 2012;44(Supplement 1):796–803. doi: 10.1111/j.1439-0272.2011.01269.x. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y., Li L., Xiang C., Ma Z., Ma T., Zhu S. Protective effect of melatonin against adriamycin-induced cardiotoxicity. Experimental and Therapeutic Medicine. 2013;5(5):1496–1500. doi: 10.3892/etm.2013.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quiles J. L., Huertas J. R., Battino M., Mataix J., Ramírez-Tortosa M. C. Antioxidant nutrients and adriamycin toxicity. Toxicology. 2002;180(1):79–95. doi: 10.1016/S0300-483X(02)00383-9. [DOI] [PubMed] [Google Scholar]
- 82.Sahna E., Parlakpinar H., Ozer M. K., Ozturk F., Ozugurlu F., Acet A. Melatonin protects against myocardial doxorubicin toxicity in rats: role of physiological concentrations. Journal of Pineal Research. 2003;35(4):257–261. doi: 10.1034/j.1600-079X.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 83.LIU D., Ma Z., Di S., et al. AMPK/PGC1α activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis. Free Radical Biology & Medicine. 2018;129:59–72. doi: 10.1016/j.freeradbiomed.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 84.Bilginoğlu A., Aydin D., Ozsoy Ş., Aygün H. Protective effect of melatonin on adriamycin-induced cardiotoxicity in rats. Turk Kardiyoloji Dernegi Arsivi-Archives of the Turkish Society of Cardiology. 2014;42(3):265–273. doi: 10.5543/tkda.2014.36089. [DOI] [PubMed] [Google Scholar]
- 85.Othman A. I., El-Missiry M. A., Amer M. A., Arafa M. Melatonin controls oxidative stress and modulates iron, ferritin, and transferrin levels in adriamycin treated rats. Life Sciences. 2008;83(15-16):563–568. doi: 10.1016/j.lfs.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 86.Dziegiel P., Murawska-Cialowicz E., Jethon Z., et al. Melatonin stimulates the activity of protective antioxidative enzymes in myocardial cells of rats in the course of doxorubicin intoxication. Journal of Pineal Research. 2003;35(3):183–187. doi: 10.1034/j.1600-079X.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 87.Morishima I., Okumura K., Matsui H., et al. Zinc accumulation in adriamycin‐induced cardiomyopathy in rats: Effects of melatonin, a cardioprotective antioxidant. Journal of Pineal Research. 1999;26(4):204–210. doi: 10.1111/j.1600-079X.1999.tb00585.x. [DOI] [PubMed] [Google Scholar]
- 88.Xu M. F., Ho S., Qian Z. M., Tang P. L. Melatonin protects against cardiac toxicity of doxorubicin in rat. Journal of Pineal Research. 2001;31(4):301–307. doi: 10.1034/j.1600-079X.2001.310403.x. [DOI] [PubMed] [Google Scholar]
- 89.Kocak G., Erbil K. M., Ozdemir I., et al. The protective effect of melatonin on adriamycin-induced acute cardiac injury. The Canadian Journal of Cardiology. 2003;19(5):535–541. [PubMed] [Google Scholar]
- 90.Öz E., Erbaş D., Sürücü H. S., Düzgün E. Prevention of doxorubicin-induced cardiotoxicity by melatonin. Molecular and Cellular Biochemistry. 2006;282(1-2):31–37. doi: 10.1007/s11010-006-1153-9. [DOI] [PubMed] [Google Scholar]
- 91.Goormaghtigh E., Chatelain P., Caspers J., Ruysschaert J. M. Evidence of a complex between adriamycin derivatives and cardiolipin: possible role in cardiotoxicity. Biochemical Pharmacology. 1980;29(21):3003–3010. doi: 10.1016/0006-2952(80)90050-7. [DOI] [PubMed] [Google Scholar]
- 92.Goormaghtigh E., Huart P., Brasseur R., Ruysschaert J. M. Mechanism of inhibition of mitochondrial enzymatic complex I–III by adriamycin derivatives. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1986;861(1):83–94. doi: 10.1016/0005-2736(86)90406-2. [DOI] [PubMed] [Google Scholar]
- 93.Paradies G., Petrosillo G., Paradies V., Reiter R. J., Ruggiero F. M. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. Journal of Pineal Research. 2010;48(4):297–310. doi: 10.1111/j.1600-079X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 94.GUVEN C., TASKIN E., AKCAKAYA H. Melatonin prevents mitochondrial damage induced by doxorubicin in mouse fibroblasts through AMPK-PPAR gamma-dependent mechanisms. Medical science monitor : international medical journal of experimental and clinical research. 2016;22:438–446. doi: 10.12659/MSM.897114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tan W., Zhong Z., Carney R. P., et al. Deciphering the metabolic role of AMPK in cancer multi-drug resistance. Seminars in Cancer Biology. 2018;56:56–71. doi: 10.1016/j.semcancer.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 96.Pérez‐González A., Castañeda‐Arriaga R., Álvarez‐Idaboy J. R., Reiter R. J., Galano A. Melatonin and its metabolites as chemical agents capable of directly repairing oxidized DNA. Journal of Pineal Research. 2019;66(2, article e12539) doi: 10.1111/jpi.12539. [DOI] [PubMed] [Google Scholar]
- 97.Anderson G. Linking the biological underpinnings of depression: Role of mitochondria interactions with melatonin, inflammation, sirtuins, tryptophan catabolites, DNA repair and oxidative and nitrosative stress, with consequences for classification and cognition. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2018;80:255–266. doi: 10.1016/j.pnpbp.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 98.Wahab M. H., Akoul E. S., Abdel-Aziz A. A. Modulatory effects of melatonin and vitamin E on doxorubicin-induced cardiotoxicity in Ehrlich ascites carcinoma-bearing mice. Tumori Journal. 2000;86(2):157–162. doi: 10.1177/030089160008600210. [DOI] [PubMed] [Google Scholar]
- 99.Kesik V., Kurt B., Tunc T., et al. Melatonin ameliorates doxorubicin-induced skin necrosis in rats. Annals of Plastic Surgery. 2010;65(2):250–253. doi: 10.1097/SAP.0b013e3181bb4b4e. [DOI] [PubMed] [Google Scholar]
- 100.Dziegiel P., Surowiak P., Rabczyński J., Zabel M. Effect of melatonin on cytotoxic effects of daunorubicin on myocardium and on transplantable Morris hepatoma in rats. Polish Journal of Pathology. 2002;53(4):201–204. [PubMed] [Google Scholar]
- 101.Guven A., Yavuz O., Cam M., Ercan F., Bukan N., Comunoglu C. Melatonin protects against epirubicin-induced cardiotoxicity. Acta Histochemica. 2007;109(1):52–60. doi: 10.1016/j.acthis.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 102.Joslin C. A., Kunkler P. B., Evans I. H., Jones V., Wong K. Cyclophosphamide in the management of advanced breast cancer. Proceedings of the Royal Society of Medicine. 1970;63(1):81–84. doi: 10.1177/003591577006300135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fleming R. A. An overview of cyclophosphamide and ifosfamide pharmacology. Pharmacotherapy. 1997;17(5, Part 2):146s–154s. [PubMed] [Google Scholar]
- 104.Al-Malki A. L. Synergestic effect of lycopene and melatonin against the genesis of oxidative stress induced by cyclophosphamide in rats. Toxicology and Industrial Health. 2014;30(6):570–575. doi: 10.1177/0748233712459916. [DOI] [PubMed] [Google Scholar]
- 105.Kaya H., Oral B., Ozgüner F., Tahan V., Babar Y., Delibaş N. The effect of melatonin application on lipid peroxidation during cyclophosphamide therapy in female rats. Zentralblatt fur Gynakologie. 1999;121(10):499–502. [PubMed] [Google Scholar]
- 106.Shokrzadeh M., Chabra A., Naghshvar F., Ahmadi A., Jafarinejhad M., Hasani-Nourian Y. Protective effects of melatonin against cyclophosphamide-induced oxidative lung toxicity in mice. Drug Research. 2015;65(6):281–286. doi: 10.1055/s-0034-1371801. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Q. H., Zhou Z. S., Lu G. S., Song B., Guo J. X. Melatonin improves bladder symptoms and may ameliorate bladder damage via increasing HO-1 in rats. Inflammation. 2013;36(3):651–657. doi: 10.1007/s10753-012-9588-5. [DOI] [PubMed] [Google Scholar]
- 108.Topal T., Oztas Y., Korkmaz A., et al. Melatonin ameliorates bladder damage induced by cyclophosphamide in rats. Journal of Pineal Research. 2005;38(4):272–277. doi: 10.1111/j.1600-079X.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 109.Tripathi D. N., Jena G. B. Effect of melatonin on the expression of Nrf2 and NF-κB during cyclophosphamide-induced urinary bladder injury in rat. Journal of Pineal Research. 2010;48(4):324–331. doi: 10.1111/j.1600-079X.2010.00756.x. [DOI] [PubMed] [Google Scholar]
- 110.ZUPANCIC D., VIDMAR G., JEZERNIK K. Melatonin prevents the development of hyperplastic urothelium induced by repeated doses of cyclophosphamide. Virchows Archiv : an international journal of pathology. 2009;454(6):657–666. doi: 10.1007/s00428-009-0765-3. [DOI] [PubMed] [Google Scholar]
- 111.Ilbey Y. O., Ozbek E., Simsek A., Otunctemur A., Cekmen M., Somay A. Potential chemoprotective effect of melatonin in cyclophosphamide- and cisplatin-induced testicular damage in rats. Fertility and Sterility. 2009;92(3):1124–1132. doi: 10.1016/j.fertnstert.2008.07.1758. [DOI] [PubMed] [Google Scholar]
- 112.Shokrzadeh M., Naghshvar F., Ahmadi A., Chabr A., Jeivad F. The potential ameliorative effects of melatonin against cyclophosphamide-induced DNA damage in murine bone marrow cells. European Review for Medical and Pharmacological Sciences. 2014;18(5):605–611. [PubMed] [Google Scholar]
- 113.Ferreira S. G., Peliciari-Garcia R. A., Takahashi-Hyodo S. A., et al. Effects of melatonin on DNA damage induced by cyclophosphamide in rats. Brazilian Journal of Medical and Biological Research. 2013;46(3):278–286. doi: 10.1590/1414-431x20122230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dobrek L., Baranowska A., Thor P. J. Indirect autonomic nervous system activity assessment with heart rate variability in rats with cyclophosphamide-induced hemorrhagic cystitis treated with melatonin or agomelatine. Contemporary Oncology. 2015;5(5):368–373. doi: 10.5114/wo.2015.52739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saha P., Debnath C., Berube G. Steroid-linked nitrogen mustards as potential anticancer therapeutics: a review. The Journal of Steroid Biochemistry and Molecular Biology. 2013;137:271–300. doi: 10.1016/j.jsbmb.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 116.Lawley P. D. Alkylation of DNA and its aftermath. BioEssays. 1995;17(6):561–568. doi: 10.1002/bies.950170615. [DOI] [PubMed] [Google Scholar]
- 117.Ucar M., Korkmaz A., Reiter R. J., et al. Melatonin alleviates lung damage induced by the chemical warfare agent nitrogen mustard. Toxicology Letters. 2007;173(2):124–131. doi: 10.1016/j.toxlet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 118.Macit E., Yaren H., Aydin I., et al. The protective effect of melatonin and S-methylisothiourea treatments in nitrogen mustard induced lung toxicity in rats. Environmental Toxicology and Pharmacology. 2013;36(3):1283–1290. doi: 10.1016/j.etap.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 119.Hardeland R. elatonin and inflammation—Story of a double‐edged blade. Journal of Pineal Research. 2018;65(4, article e12525) doi: 10.1111/jpi.12525. [DOI] [PubMed] [Google Scholar]
- 120.Sourdeval M., Lemaire C., Deniaud A., et al. Inhibition of caspase-dependent mitochondrial permeability transition protects airway epithelial cells against mustard-induced apoptosis. Apoptosis. 2006;11(9):1545–1559. doi: 10.1007/s10495-006-8764-1. [DOI] [PubMed] [Google Scholar]
- 121.Ferroni P., Della-Morte D., Palmirotta R., et al. Platinum-based compounds and risk for cardiovascular toxicity in the elderly: role of the antioxidants in chemoprevention. Rejuvenation Research. 2011;14(3):293–308. doi: 10.1089/rej.2010.1141. [DOI] [PubMed] [Google Scholar]
- 122.Kilic U., Kilic E., Tuzcu Z., et al. Melatonin suppresses cisplatin-induced nephrotoxicity via activation of Nrf-2/HO-1 pathway. Nutrition & Metabolism. 2013;10(1):p. 7. doi: 10.1186/1743-7075-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hara M., Yoshida M., Nishijima H., et al. Melatonin, a pineal secretory product with antioxidant properties, protects against cisplatin‐induced nephrotoxicity in rats. Journal of Pineal Research. 2001;30(3):129–138. doi: 10.1034/j.1600-079X.2001.300301.x. [DOI] [PubMed] [Google Scholar]
- 124.Majidinia M., Sadeghpour A., Mehrzadi S., Reiter R. J., Khatami N., Yousefi B. Melatonin: a pleiotropic molecule that modulates DNA damage response and repair pathways. 2017;63(1, article e12416) doi: 10.1111/jpi.12416. [DOI] [PubMed] [Google Scholar]
- 125.Barberino R. S., Menezes V. G., Ribeiro A. E. A. S., et al. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biology of Reproduction. 2017;96(6):1244–1255. doi: 10.1093/biolre/iox053. [DOI] [PubMed] [Google Scholar]
- 126.Jang H., Lee O. H., Lee Y., et al. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. 2016;60:336–347. doi: 10.1111/jpi.12316. [DOI] [PubMed] [Google Scholar]
- 127.Madhu P., Reddy K. P., Reddy P. S. Role of melatonin in mitigating chemotherapy-induced testicular dysfunction in Wistar rats. Drug and Chemical Toxicology. 2016;39(2):137–146. doi: 10.3109/01480545.2015.1055359. [DOI] [PubMed] [Google Scholar]
- 128.Lopez-Gonzalez M. A., Guerrero J. M., Rojas F., Delgado F. Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. Journal of Pineal Research. 2000;28(2):73–80. doi: 10.1034/j.1600-079X.2001.280202.x. [DOI] [PubMed] [Google Scholar]
- 129.Demir M. G., Altintoprak N., Aydin S., Kosemihal E., Basak K. Effect of transtympanic injection of melatonin on cisplatin-induced ototoxicity. The Journal of International Advanced Otology. 2015;11(3):202–206. doi: 10.5152/iao.2015.1094. [DOI] [PubMed] [Google Scholar]
- 130.Bennukul K., Numkliang S., Leardkamolkarn V. Melatonin attenuates cisplatin-induced HepG2 cell death via the regulation of mTOR and ERCC1 expressions. World Journal of Hepatology. 2014;6(4):230–242. doi: 10.4254/wjh.v6.i4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Atalik K. E., Keles B., Uyar Y., Dündar M. A., Öz M., Esen H. H. Vasoprotection by melatonin and quercetin in rats treated with cisplatin. Indian Journal of Experimental Biology. 2010;48:1188–1193. [PubMed] [Google Scholar]
- 132.Plaimee P., Weerapreeyakul N., Barusrux S., Johns N. P. Melatonin potentiates cisplatin-induced apoptosis and cell cycle arrest in human lung adenocarcinoma cells. Cell Proliferation. 2015;48(1):67–77. doi: 10.1111/cpr.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sookprasert A., Johns N. P., Phunmanee A., et al. Melatonin in patients with cancer receiving chemotherapy: a randomized, double-blind, placebo-controlled trial. Anticancer Research. 2014;34(12):7327–7337. [PubMed] [Google Scholar]
- 134.Argyriou A. A., Polychronopoulos P., Iconomou G., Chroni E., Kalofonos H. P. A review on oxaliplatin-induced peripheral nerve damage. Cancer Treatment Reviews. 2008;34(4):368–377. doi: 10.1016/j.ctrv.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 135.Areti A., Komirishetty P., Akuthota M., Malik R. A., Kumar A. Melatonin prevents mitochondrial dysfunction and promotes neuroprotection by inducing autophagy during oxaliplatin-evoked peripheral neuropathy. Journal of Pineal Research. 2017;62(3) doi: 10.1111/jpi.12393. [DOI] [PubMed] [Google Scholar]
- 136.Waseem M., Sahu U., Salman M., et al. Melatonin pre-treatment mitigates SHSY-5Y cells against oxaliplatin induced mitochondrial stress and apoptotic cell death. PLoS One. 2017;12(7, article e0180953) doi: 10.1371/journal.pone.0180953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Um H. J., Kwon T. K. Protective effect of melatonin on oxaliplatin‐induced apoptosis through sustained Mcl‐1 expression and anti‐oxidant action in renal carcinoma Caki cells. Journal of Pineal Research. 2010;49(3):283–290. doi: 10.1111/j.1600-079X.2010.00793.x. [DOI] [PubMed] [Google Scholar]
- 138.Wang Y. S., Li Y. Y., Cui W., et al. Melatonin attenuates pain hypersensitivity and decreases astrocyte-mediated spinal neuroinflammation in a rat model of oxaliplatin-induced pain. Inflammation. 2017;40(6):2052–2061. doi: 10.1007/s10753-017-0645-y. [DOI] [PubMed] [Google Scholar]
- 139.Kolli V. K., Abraham P., Rabi S. Methotrexate-induced nitrosative stress may play a critical role in small intestinal damage in the rat. Archives of toxicology. 2008;82(10):763–770. doi: 10.1007/s00204-008-0287-9. [DOI] [PubMed] [Google Scholar]
- 140.Fazzi P. Pharmacotherapeutic management of pulmonary sarcoidosis. American Journal of Respiratory Medicine. 2003;2(4):311–320. doi: 10.1007/bf03256659. [DOI] [PubMed] [Google Scholar]
- 141.Kolli V. K., Abraham P., Isaac B., Kasthuri N. Preclinical efficacy of melatonin to reduce methotrexate-induced oxidative stress and small intestinal damage in rats. Digestive Diseases and Sciences. 2013;58(4):959–969. doi: 10.1007/s10620-012-2437-4. [DOI] [PubMed] [Google Scholar]
- 142.Kolli V. K., Kanakasabapathy I., Faith M., et al. A preclinical study on the protective effect of melatonin against methotrexate-induced small intestinal damage: effect mediated by attenuation of nitrosative stress, protein tyrosine nitration, and PARP activation. Cancer Chemotherapy and Pharmacology. 2013;71(5):1209–1218. doi: 10.1007/s00280-013-2115-z. [DOI] [PubMed] [Google Scholar]
- 143.Abraham P., Kolli V. K., Rabi S. Melatonin attenuates methotrexate‐induced oxidative stress and renal damage in rats. Cell Biochemistry and Function. 2010;28(5):426–433. doi: 10.1002/cbf.1676. [DOI] [PubMed] [Google Scholar]
- 144.Oguz E., Kocarslan S., Tabur S., Sezen H., Yilmaz Z., Aksoy N. Effects of lycopene alone or combined with melatonin on methotrexate-induced nephrotoxicity in rats. Asian Pacific Journal of Cancer Prevention. 2015;16(14):6061–6066. doi: 10.7314/APJCP.2015.16.14.6061. [DOI] [PubMed] [Google Scholar]
- 145.Montasser A. O. S., Saleh H., Ahmed-Farid O. A., Saad A., Marie M. A. S. Protective effects of Balanites aegyptiaca extract, melatonin and ursodeoxycholic acid against hepatotoxicity induced by methotrexate in male rats. Asian Pacific Journal of Tropical Medicine. 2017;10(6):557–565. doi: 10.1016/j.apjtm.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 146.Çevik H., Şehirli A. Ö., Yeğen B. Ç., Şener G. Melatonin prevents methotrexate‐induced hepatorenal oxidative injury in rats. Journal of Pineal Research. 2003;34(4):282–287. doi: 10.1034/j.1600-079X.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 147.Ghersi D., Wilcken N., Simes R. J. A systematic review of taxane-containing regimens for metastatic breast cancer. British Journal of Cancer. 2005;93(3):293–301. doi: 10.1038/sj.bjc.6602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Frederiks C. N., Lam S. W., Guchelaar H. J., Boven E. Genetic polymorphisms and paclitaxel- or docetaxel-induced toxicities: a systematic review. Cancer Treatment Reviews. 2015;41(10):935–950. doi: 10.1016/j.ctrv.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 149.Zhang D., Yang R., Wang S., Dong Z. Paclitaxel: new uses for an old drug. Drug Design, Development and Therapy. 2014;8:279–284. doi: 10.2147/dddt.s56801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schneider B. P., Hershman D. L., Loprinzi C. Symptoms: chemotherapy-induced peripheral neuropathy. In: Ganz P., editor. Improving Outcomes for Breast Cancer Survivors. Vol. 862. Cham: Springer; 2015. pp. 77–87. (Advances in experimental medicine and biology). [DOI] [PubMed] [Google Scholar]
- 151.Nahleh Z., Pruemer J., Lafollette J., Sweany S. Melatonin, a promising role in taxane-related neuropathy. Clinical Medicine Insights: Oncology. 2010;4 doi: 10.4137/cmo.s4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee H., Lee H. J., Jung J. H., Shin E. A., Kim S. H. Melatonin disturbs SUMOylation‐mediated crosstalk between c‐Myc and nestin via MT1 activation and promotes the sensitivity of paclitaxel in brain cancer stem cells. Journal of Pineal Research. 2018;65(2, article e12496) doi: 10.1111/jpi.12496. [DOI] [PubMed] [Google Scholar]
- 153.Galley H. F., Mccormick B., Wilson K. L., Lowes D. A., Colvin L., Torsney C. Melatonin limits paclitaxel-induced mitochondrial dysfunction in vitro and protects against paclitaxel-induced neuropathic pain in the rat. Journal of Pineal Research. 2017;63(4, article e12444) doi: 10.1111/jpi.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gianni L., Dafni U., Gelber R. D., et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. The Lancet Oncology. 2011;12(3):236–244. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 155.Ghani E. A., Kerr I., Dada R. Grade 3 trastuzumab-induced neutropenia in breast cancer patient. Journal of Oncology Pharmacy Practice. 2014;20(2):154–157. doi: 10.1177/1078155213487394. [DOI] [PubMed] [Google Scholar]
- 156.Ozturk M., Ozler M., Kurt Y. G., et al. Efficacy of melatonin, mercaptoethylguanidine and 1400W in doxorubicin‐ and trastuzumab‐induced cardiotoxicity. Journal of Pineal Research. 2011;50(1):89–96. doi: 10.1111/j.1600-079X.2010.00818.x. [DOI] [PubMed] [Google Scholar]
- 157.Reiter R., Rosales-Corral S., Tan D. X., et al. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. International Journal of Molecular Sciences. 2017;18(4):p. 843. doi: 10.3390/ijms18040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mayo J. C., Hevia D., Quiros-Gonzalez I., et al. IGFBP3 and MAPK/ERK signaling mediates melatonin-induced antitumor activity in prostate cancer. Journal of Pineal Research. 2017;62(1, article e12373) doi: 10.1111/jpi.12373. [DOI] [PubMed] [Google Scholar]
- 159.Lu K. H., Su S. C., Lin C. W., et al. Melatonin attenuates osteosarcoma cell invasion by suppression of C-C motif chemokine ligand 24 through inhibition of the c-Jun N-terminal kinase pathway. Journal of Pineal Research. 2018;65(3, article e12507) doi: 10.1111/jpi.12507. [DOI] [PubMed] [Google Scholar]
- 160.Ma Z., Liu D., Di S., et al. Histone deacetylase 9 downregulation decreases tumor growth and promotes apoptosis in non‐small cell lung cancer after melatonin treatment. Journal of Pineal Research. 2019;(article e12587) doi: 10.1111/jpi.12587. [DOI] [PubMed] [Google Scholar]
- 161.Chen L., Liu L., Li Y., Gao J. Melatonin increases human cervical cancer HeLa cells apoptosis induced by cisplatin via inhibition of JNK/Parkin/mitophagy axis. In vitro Cellular & Developmental Biology Animal. 2018;54(1):1–10. doi: 10.1007/s11626-017-0200-z. [DOI] [PubMed] [Google Scholar]
- 162.Uguz A. C., Cig B., Espino J., et al. Melatonin potentiates chemotherapy‐induced cytotoxicity and apoptosis in rat pancreatic tumor cells. Journal of Pineal Research. 2012;53(1):91–98. doi: 10.1111/j.1600-079X.2012.00974.x. [DOI] [PubMed] [Google Scholar]
- 163.Pariente R., Pariente J. A., Rodríguez A. B., Espino J. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin‐induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. Journal of Pineal Research. 2016;60(1):55–64. doi: 10.1111/jpi.12288. [DOI] [PubMed] [Google Scholar]
- 164.Zhou H., Du W., Li Y., et al. Effects of melatonin on fatty liver disease: the role of NR4A1/DNA‐PKcs/p53 pathway, mitochondrial fission, and mitophagy. Journal of Pineal Research. 2018;64(1) doi: 10.1111/jpi.12450. [DOI] [PubMed] [Google Scholar]
- 165.Gao Y., Xiao X., Zhang C., et al. Melatonin synergizes the chemotherapeutic effect of 5‐fluorouracil in colon cancer by suppressing PI3K/AKT and NF‐κB/iNOS signaling pathways. Journal of Pineal Research. 2016;62(2) doi: 10.1111/jpi.12380. [DOI] [PubMed] [Google Scholar]
- 166.Yang Q., Jiang W., Hou P. Emerging role of PI3K/AKT in tumor-related epigenetic regulation. Seminars in Cancer biology. 2019 doi: 10.1016/j.semcancer.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 167.Sanchez-Barcelo E. J., Mediavilla M. D., Alonso-Gonzalez C., Reiter R. J. Melatonin uses in oncology: breast cancer prevention and reduction of the side effects of chemotherapy and radiation. Expert Opinion on Investigational Drugs. 2012;21(6):819–831. doi: 10.1517/13543784.2012.681045. [DOI] [PubMed] [Google Scholar]
- 168.Liu D., Xu L., Zhang X., et al. Snapshot: implications for mTOR in aging-related ischemia/reperfusion injury. Aging and Disease. 2019;10(1):116–133. doi: 10.14336/AD.2018.0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ma Z., Liu D., Li W., et al. STYK1 promotes tumor growth and metastasis by reducing SPINT2/HAI-2 expression in non-small cell lung cancer. Cell Death & Disease. 2019;10(6) doi: 10.1038/s41419-019-1659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Su S. C., Hsieh M. J., Yang W. E., Chung W. H., Reiter R. J., Yang S. F. Cancer metastasis: mechanisms of inhibition by melatonin. Journal of Pineal Research. 2017;62(1, article e12370) doi: 10.1111/jpi.12370. [DOI] [PubMed] [Google Scholar]
- 171.Manchester L. C., Coto-Montes A., Boga J. A., et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. Journal of Pineal Research. 2015;59(4):403–419. doi: 10.1111/jpi.12267. [DOI] [PubMed] [Google Scholar]
- 172.Venegas C., García J. A., Escames G., et al. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. Journal of Pineal Research. 2012;52(2):217–227. doi: 10.1111/j.1600-079X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 173.Jung-Hynes B., Reiter R. J., Ahmad N. Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. Journal of Pineal Research. 2010;48(1):9–19. doi: 10.1111/j.1600-079X.2009.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jung-Hynes B., Schmit T. L., Reagan-Shaw S. R., Siddiqui I. A., Mukhtar H., Ahmad N. Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. Journal of Pineal Research. 2011;50(2):140–149. doi: 10.1111/j.1600-079X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Scher M. B., Vaquero A., Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes & Development. 2007;21(8):920–928. doi: 10.1101/gad.1527307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhu S., Dong Z., Ke X., et al. The roles of sirtuins family in cell metabolism during tumor development. Seminars in Cancer Biology. 2018 doi: 10.1016/j.semcancer.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 177.KIM J. S., He L., Lemasters J. J. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochemical and Biophysical Research Communications. 2003;304(3):463–470. doi: 10.1016/S0006-291X(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 178.Mukherjee D., Ghosh A. K., Bandyopadhyay A., et al. Melatonin protects against isoproterenol‐induced alterations in cardiac mitochondrial energy‐metabolizing enzymes, apoptotic proteins, and assists in complete recovery from myocardial injury in rats. Journal of Pineal Research. 2012;53(2):166–179. doi: 10.1111/j.1600-079X.2012.00984.x. [DOI] [PubMed] [Google Scholar]
- 179.Xu X., Chua C. C., Zhang M., et al. The role of PARP activation in glutamate-induced necroptosis in HT-22 cells. Brain Research. 2010;1343:206–212. doi: 10.1016/j.brainres.2010.04.080. [DOI] [PubMed] [Google Scholar]
- 180.Yu S. W., Wang H., Poitras M. F., et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297(5579):259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 181.Nduhirabandi F., Lamont K., Albertyn Z., Opie L. H., Lecour S. Role of toll-like receptor 4 in melatonin-induced cardioprotection. Journal of Pineal Research. 2016;60(1):39–47. doi: 10.1111/jpi.12286. [DOI] [PubMed] [Google Scholar]
- 182.Zhou H., Ma Q. Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. Journal of Pineal Research. 2018;64(3) doi: 10.1111/jpi.12471. [DOI] [PubMed] [Google Scholar]
- 183.Han D., Wang Y., Chen J., et al. Activation of melatonin receptor 2 but not melatonin receptor 1 mediates melatonin‐conferred cardioprotection against myocardial ischemia/reperfusion injury. Journal of Pineal Research. 2019;(article e12571) doi: 10.1111/jpi.12571. [DOI] [PubMed] [Google Scholar]
- 184.Yang Y., Duan W., Jin Z., et al. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. Journal of Pineal Research. 2013;55(3):275–286. doi: 10.1111/jpi.12070. [DOI] [PubMed] [Google Scholar]
- 185.Zhang Y., Wang Y., Xu J., et al. Melatonin attenuates myocardial ischemia‐reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK‐OPA1 signaling pathways. Journal of Pineal Research. 2019;66(2, article e12542) doi: 10.1111/jpi.12542. [DOI] [PubMed] [Google Scholar]
- 186.Lochner A., Marais E., Huisamen B. Melatonin and cardioprotection against ischaemia/reperfusion injury: what’s new? A review. Journal of Pineal Research. 2018;65(1, article e12490) doi: 10.1111/jpi.12490. [DOI] [PubMed] [Google Scholar]
- 187.Zhao Z., Lu C., Li T., et al. The protective effect of melatonin on brain ischemia and reperfusion in rats and humans: in vivo assessment and a randomized controlled trial. Journal of Pineal Research. 2018;65(4, article e12521) doi: 10.1111/jpi.12521. [DOI] [PubMed] [Google Scholar]
- 188.Jayaraman B., Smith A. M., Fernandes J. D., Frankel A. D. Oligomeric viral proteins: small in size, large in presence. Critical Reviews in Biochemistry and Molecular Biology. 2016;51(5):379–394. doi: 10.1080/10409238.2016.1215406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hsiao C. W., Peng T. I., Peng A. C., et al. Long‐term Aβ exposure augments mCa2+‐independent mROS‐mediated depletion of cardiolipin for the shift of a lethal transient mitochondrial permeability transition to its permanent mode in NARP cybrids: a protective targeting of melatonin. Journal of Pineal Research. 2013;54(1):107–125. doi: 10.1111/jpi.12004. [DOI] [PubMed] [Google Scholar]
- 190.Fu H. Y., Sanada S., Matsuzaki T., et al. Chemical endoplasmic reticulum chaperone alleviates doxorubicin-induced cardiac dysfunction. Circulation Research. 2016;118(5):798–809. doi: 10.1161/CIRCRESAHA.115.307604. [DOI] [PubMed] [Google Scholar]
- 191.Minamino T., Komuro I., Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circulation Research. 2010;107(9):1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 192.Fan L., Sun G., Ma T., et al. Melatonin reverses tunicamycin‐induced endoplasmic reticulum stress in human hepatocellular carcinoma cells and improves cytotoxic response to doxorubicin by increasing CHOP and decreasing Survivin. Journal of Pineal Research. 2013;55(2):184–194. doi: 10.1111/jpi.12061. [DOI] [PubMed] [Google Scholar]
- 193.Park H. J., Park J. Y., Kim J. W., et al. Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation. Journal of Pineal Research. 2018;64(2, article e12458) doi: 10.1111/jpi.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim C., Kim N., Joo H., et al. Modulation by melatonin of the cardiotoxic and antitumor activities of adriamycin. Journal of Cardiovascular Pharmacology. 2005;46(2):200–210. doi: 10.1097/01.fjc.0000171750.97822.a2. [DOI] [PubMed] [Google Scholar]
- 195.Ahmed H. H., Mannaa F., Elmegeed G. A., Doss S. H. Cardioprotective activity of melatonin and its novel synthesized derivatives on doxorubicin-induced cardiotoxicity. Bioorganic & Medicinal Chemistry. 2005;13(5):1847–1857. doi: 10.1016/j.bmc.2004.10.066. [DOI] [PubMed] [Google Scholar]
- 196.Kunak Z. I., Macit E., Yaren H., et al. Protective effects of melatonin and S-methylisothiourea on mechlorethamine induced nephrotoxicity. The Journal of Surgical Research. 2012;175(1):e17–e23. doi: 10.1016/j.jss.2011.11.002. [DOI] [PubMed] [Google Scholar]


