Abstract
Objectives
Our study shows that glucagon-like peptide-1 (GLP-1) is secreted within human islets and may play an unexpectedly important paracrine role in islet physiology and pathophysiology. It is known that α cells within rodent and human pancreatic islets are capable of secreting GLP-1, but little is known about the functional role that islet-derived GLP-1 plays in human islets.
Methods
We used flow cytometry, immunohistochemistry, perifusions, and calcium imaging techniques to analyse GLP-1 expression and function in islets isolated from cadaveric human donors with or without type 2 diabetes. We also used immunohistochemistry to analyse GLP-1 expression within islets from pancreatic biopsies obtained from living donors.
Results
We have demonstrated that human islets secrete ∼50-fold more GLP-1 than murine islets and that ∼40% of the total human α cells contain GLP-1. Our results also confirm that dipeptidyl peptidase-4 (DPP4) is expressed in α cells. Sitagliptin increased GLP-1 secretion from cultured human islets but did not enhance glucose-stimulated insulin secretion (GSIS) in islets from non-diabetic (ND) or type 2 diabetic (T2D) donors, suggesting that β cell GLP-1 receptors (GLP-1R) may already be maximally activated. Therefore, we tested the effects of exendin-9, a GLP-1R antagonist. Exendin-9 was shown to reduce GSIS by 39% and 61% in ND islets and T2D islets, respectively. We also observed significantly more GLP-1+ α cells in T2D islets compared with ND islets obtained from cadaveric donors. Furthermore, GLP-1+ α cells were also identified in pancreatic islet sections obtained from living donors undergoing surgery.
Conclusions
In summary, we demonstrated that human islets secrete robust amounts of GLP-1 from an α cell subpopulation and that GLP-1R signalling may support GSIS to a greater extent in T2D islets.
Keywords: Human islets, Glucagon-like peptide-1, GLP-1, Glucagon, Alpha-cell, Beta-cell
Highlights
-
•
Here we show that glucagon-like peptide-1 (GLP-1) is secreted from a subpopulation of α cells within human islets.
-
•
Human islets secrete ∼50-fold more GLP-1 than murine islets and that ∼40% of the total human α cells contain GLP-1.
-
•
We observed significantly more GLP-1+ α cells in islets from donors with type 2 diabetes than in islets from donors with no diabetes.
-
•
GLP-1+ α cells can also be detected in pancreatic islet sections obtained from living donors undergoing surgery.
-
•
GLP-1 receptor signaling may support insulin secretion to a greater extent in type 2 diabetes.
1. Introduction
It is known that α cells within rodent and human pancreatic islets are capable of secreting glucagon-like peptide-1 (GLP-1) [[1], [2], [3]] under certain conditions and the enzyme responsible for GLP-1 cleavage and subsequent inactivation, dipeptidyl peptidase-4 (DPP4), is expressed in α cells within human islets [4,5]. Despite the evidence for the production and inactivation of GLP-1 in islets, the functional role that islet-derived GLP-1 might play in human islets remains unclear.
GLP-1 therapies such as DPP4 inhibitors and GLP-1R peptide agonists have been developed and are now in wide clinical use using the classical incretin effect as a physiological framework [6]. The incretin effect describes stimulated GLP-1 release from the entero-endocrine L cells of the gut to act on pancreatic β cells, thus potentiating insulin secretion in response to nutrient ingestion [7]. Early reports have shown proglucagon cleavage to be tissue-specific with GLP-1 being produced in the L cells and negligible amounts found in islets. In contrast, glucagon secretion is limited to pancreatic islets with little if any being produced from the L cells [[8], [9], [10]]. However, there have also been reports of GLP-1 secretion from islets [11,12] and it has been proposed that GLP-1 may also act as a paracrine factor in islets [2,13,14], whereby α-cell GLP-1 secretion, dependent on prohormone convertase 1/3 (PC1/3) proglucagon cleavage, acts on adjacent β cells to augment function and insulin secretion. Despite the controversy regarding a functional role for intra-islet GLP-1, this paracrine concept has received advanced support from genetic mouse studies showing intra-islet GLP-1 is required for whole-body glucose homeostasis [15,16]. Although the physiological relevance of intra-islet GLP-1 in these genetic models has been demonstrated, it is currently unknown whether intra-islet GLP-1 is relevant for human physiology and pathophysiology such as T2D, and little information is available as to whether this proposed GLP-1 paracrine axis functionally exists in human islets.
Human islet architecture argues for perhaps a greater functional paracrine role for intra-islet GLP-1 than in mouse islets because the α cells within human islets are greater in number and interspersed throughout the islet compared with the mouse islet where α cells form a mantle around the periphery [17]. Consequently, human α cells are organized to facilitate paracrine interactions with other islet cells [18]. Moreover, α cell glucagon secretion has been reported to determine the glycemic set-point in mice and humans, suggesting an important integration of α and β cell communication in glucose homeostasis [19]. As a result of the paucity of functional information on intra-islet GLP-1 from human tissue, we therefore studied isolated human islets from ND and T2D cadaveric donors as well as pancreatic biopsy sections obtained from living donors during surgery.
2. Methods
2.1. Mouse pancreatic islets
All animal studies followed the guidelines issued by the University of Alberta Animal Care and Use Committee (protocol # AU00286). Pancreatic islets from male C57BL/6 mice (ages 12–24 weeks) were isolated by collagenase Type V (Sigma–Aldrich) digestion of the pancreas and purified by Histopaque density gradient. Mouse islets were cultured in RPMI 1640 media (11.1 mM glucose) supplemented with 10% FBS and 1% penicillin/streptomycin.
2.2. Human islets and pancreatic sections
De-identified human primary islets isolated from deceased donors were obtained from the Alberta Diabetes Institute IsletCore and the Clinical Islet Laboratory, University of Alberta, in accordance with institutional human ethics guidelines. Details on the islet and donor parameters are provided in the supplementary material. If the islet preparation was <90% pure, islets were handpicked to obtain >90% purity. Islets were cultured in DMEM (5.5 mM glucose) supplemented with 10% FBS and 1% penicillin/streptomycin for static incubations and perifusions. For flow cytometry and live imaging, islets were cultured in CMRL medium (5.5 mM glucose) supplemented with 0.5% BSA, 1% Insulin-Transferrin-Selenium (ITS), 1% Glutamax, and 1% penicillin/streptomycin. Sections of isolated human islets and pancreatic sections were formalin fixed (Z-fix), paraffin embedded, and sectioned at 5-μm thick. Diagnosis of type 2 diabetes was determined from medical records and an HbA1c > 6.5%. Whole pancreatic sections from cadaveric donors were obtained from the Alberta Diabetes Institute IsletCore. Human pancreatic biopsies were generously provided by Dr. Marko Barovic and Dr. Michele Solimena from the Paul Langerhans Institute, Dresden, Germany for islet sections from living donors. Live-donor biopsies were collected from type-2 diabetic and non-diabetic patients undergoing treatment for either chronic pancreatitis or malignant pancreatic tumors. Biopsies were acquired from the bodies and tails of the intact pancreases during laparoscopic surgery, or from the bodies of excised whole pancreases after a pancreatectomy. Immediately after surgery, the biopsies were fixed in 4% PFA at room temperature for 2–3 h. After fixation, the tissue samples were thoroughly washed and stored in PBS with added 0.02% sodium azide at 4 °C until paraffin embedding. Samples were paraffin-embedded and sectioned at the Paul Langerhans Institute before shipment to the University of Alberta.
2.3. Antibodies for immunofluorescent microscopy
Anti-glucagon Rab [EP370] (ab92517) and Anti-GLP-1 (amidated) Mab [8G9] (ab26278) were purchased from Abcam. Anti-insulin guinea pig polyclonal DAKO (A056401-2) was purchased from Agilent Technologies. Alexa Fluor goat anti-rabbit 488, Alexa Fluor goat anti-mouse 568, and Alexa Fluor goat anti-guinea pig 647 were used as secondary antibodies and purchased from ThermoFisher Scientific.
2.4. Antibodies for flow cytometry
PE mouse anti-glucagon [U16-850] and Alexa Flour 647 mouse anti-insulin [T56-706] were purchased from BD Biosciences. Anti-GLP-1 (amidated) Mab [8G9] (ab26278) and anti-PC1/3 rabbit polyclonal (a154246) were purchased from Abcam. Anti-GLP-1 (amidated) was detected using anti-mouse IgG-biotin (13–4013) and streptavidin-eFluor 450 (48–4317) purchased from eBioscience. Anti-PC1/3 was probed using anti-rabbit IgG-FITC (11–4839) purchased from eBioscience. Mouse anti-human CD26/DPP4-PEcy7 (BA5b) was purchased from Biolegend.
2.5. Compounds
Sitagliptin phosphate monohydrate was purchased from Biovision, reconstituted in water, and used at 200 nM concentration for islet culture and perifusion experiments. Exendin-9 (9–39) salt was purchased from Bachem, reconstituted in PBS, and used at 100 nM concentration for perifusion experiments. Interleukin-1β (IL-1β) (Genscript, Z02922-10) was reconstituted in PBS and used at 50 ng/ml for islet culture experiments.
2.6. Flow cytometry
Human islets were washed in citric saline and dispersed mechanically with a 21G needle and tuberculin syringe. For cell surface antigen detection, dispersed cells were stained with antibody cocktails in FACS buffer (PBS, 1% FCS, 0.02% sodium azide, 1 mM EDTA) for 30 min on ice. Cells were washed twice with FACS buffer after staining. For intracellular antigen staining, the dispersed cells were treated with BD Cytofix/Cytoperm (BD Bioscience) for 30 min on ice and washed twice with BD Perm/Wash buffer (BD Bioscience). Intracellular antigens were stained with antibody cocktails in BD Perm/Wash buffer for 30 min on ice, and the cells were washed twice with BD Perm/Wash buffer after staining. Cell events were collected on a BD Fortessa X-20 analyser, and data were analysed with FlowJo software (Tree Star).
2.7. Islet cultures and perifusions
For islet culture incubations, 40 islets (human or mouse) were kept in a humidified incubator at 37 °C and 5% CO2 and cultured in 200uL of Ham's F10 (6.1 mM or 5.5 mM glucose) supplemented with 10% FBS and 1% penicillin/streptomycin. 10% FBS was removed for the serum-free culture. The islets were treated with compounds (sitagliptin 200 nM or IL-1β 50 ng/ml) for 24 or 48 h. The supernatant was taken and stored at −20 °C for active GLP-1 analysis.
Islet perifusions were performed using a Brandel SF-06 system (Gaithersburg, MD) and a KRBH buffer containing (mM) 115 NaCl, 5 KCl, 24 NaHCO3, 2.5 CaCl2, 1 MgCl2, 10 HEPES, 0.1% BSA, (pH 7.4) supplemented with the appropriate glucose concentration. The KRBH buffer and islet perifusion chamber were maintained at 37 °C. Following a 3-hour static preincubation with treatment compounds in 2.5 mM glucose buffer, 25 islets were perfused at 250 μL/min with 2.5 mM glucose buffer for 20 min. Next, islets were perfused in 11.1 mM glucose buffer for 50 min. Perfusate was collected at timed intervals and stored at −20 °C for insulin analysis.
2.8. Calcium imaging
Human pancreatic islets were incubated with 2 μM Fluo-4 AM for 1 h in a humidified incubator at 37 °C and 5% CO2. They were placed in the recording chamber of an Olympus IX83 inverted fluorescence microscope equipped with an UPLANApo 10X objective. We used a multichannel gravity-fed Warner Instruments TC-324B temperature-controlled perfusion system, maintained at 37 °C. Excitation was performed with a X-Cite 120LEDBoost (Excelitas) light source and image acquisition was performed with an Andor iXon ultra camera using Olympus cellSens software. The fluorescence of whole human pancreatic islets was measured at a sampling frequency of 0.33 Hz. The islets were perfused with a solution containing (mM) 120 NaCl, 4.8 KCl, 1.2 MgCl2, 2.5 CaCl2 and 10 HEPES (pH 7.4) supplemented with the indicated amount of glucose and Exendin-9. Depolarization of the islets with 30 mM KCl was used as a positive control at the end of the measurement. Ca2+ events and signal dynamics of active subregions within the islets, before or during the application of Exendin-9, were analysed.
2.9. Hormone secretion assays
Active GLP-1 levels from islet culture media samples (human and mouse) were quantified using the electrochemiluminescent assay active GLP-1 (v2) kit (K150JWC-1), MesoScale Discovery (MSD). The MSD active GLP-1 (7–36) antibody was validated in-house against high purity synthetic peptides and was found to be highly selective for the GLP-1 (7–36) isoform when compared with GLP-1 (9–36), GLP-1 (1–36), and glucagon (Figure 2F). Total GLP levels in human islet culture media samples were quantified with the electrochemiluminescent assay MSD total GLP-1 (v2) kit (K150JVC-1). Insulin levels in supernatants from static incubations and perfusate samples were quantified with Stellux Chemiluminescent Human Insulin ELISA, ALPCO.
Figure 2.
Sitagliptin increases active GLP-1 levels in cultured islets, but does not increase GSIS from ND and T2D islets. A. Active GLP-1 secretion from human islets in culture showing the inhibitory effects of sitagliptin on DPP4 over 24 h. N = 4 donors. B. Active GLP-1 secretion from human islets treated with sitagliptin in serum-free culture (5.5 mM glucose) for 48 h to exclude the effect of serum DPP4. N = 3 donors. C. Active GLP-1 secretion increases with sitagliptin treatment in control media or IL-1 beta (50 ng/ml) co-incubation. N = 3 donors. D. Sitagliptin is unable to increase insulin secretion with glucose-stimulated perifusions of ND islets. 25 islets per lane, N = 6 donors. E. Sitagliptin is unable to increase insulin secretion with glucose-stimulated perifusions of T2D islets. 25 islets per lane, N = 3 donors. For all experiments the sitagliptin concentration used (200 nM) was in the therapeutic range. F. GLP-1 antibody validation plot illustrating that the GLP-1 assay used in this study is highly selective for active GLP-1 compared with the inactive GLP-1 peptides ((9–36) and (1–36) and glucagon. Statistical significance for the data was determined using a paired Student's t-test or two-way ANOVA. ∗, P < 0.05, ∗∗, P < 0.01, ∗∗∗, P < 0.001. Error bars indicate SEM.
2.10. Immunofluorescence microscopy
Paraffin-embedded human islet sections were rehydrated and subjected to antigen retrieval by microwaving the slides for 15 min in a citrate buffer (pH 6.0). After cooling, sections were blocked with 20% normal goat serum for 1 h, incubated with primary antibodies for 1 h, and then incubated with secondary antibodies and DAPI (1:1000) for 1 h. ProLong Gold antifade mountant was applied and the slide sections were mounted on coverslips. Primary antibodies were as follows: 1:1000, anti-glucagon Rab [EP370] (ab92517), anti-insulin guinea pig polyclonal DAKO (A056401-2); 1:2000 anti-GLP-1 amidated Mab ([8G9] (ab26278)). This amidated GLP-1 antibody was validated in house and detected the C-terminal amidated 1–36, 7–36, and 9–36 GLP-1 isoforms but not glucagon (Figure 1L). Secondary antibodies were incubated at 1:200 dilution. For epifluorescence microscopy, islet sections were imaged with an Olympus IX83 inverted fluorescence microscope and a UPLanSApo 20X objective. Excitation was performed with an X-Cite 120LEDBoost (Excelitas) light source with the appropriate filter set for DAPI, AF488, AF568, and AF647. Images were captured with a Hamamatsu Orca Flash4.0 camera operated with Olympus cellSens software. Image analysis was performed using FIJI and a customized macro. DAPI staining was used to identify ROIs (cells). Where appropriate, thresholds were set to identify positive staining in the green (AF488), red (AF568), or far-red (AF647) channels.
Figure 1.
Human islets contain a GLP-1 secreting α cell subpopulation A. Human islets secrete ∼50-fold more active GLP-1 than mouse islets. B. Representative images of human islets stained for glucagon, GLP-1, and double-positive staining (merge, arrows denote examples of double-positive cells) indicating a subpopulation of α cells. Scale bar: 20 μm. C. Quantification of glucagon + cells shown as % total islet cells. N = 6 donors. D. Quantification of amidated GLP-1+ cells shown as % total glucagon + cells. N = 6 donors. E. Representative merged image of amidated GLP-1/glucagon staining from a section of whole human pancreas (arrows denote examples of double-positive cells). Scale bar: 50 μm. F. Representative flow cytometry contour plots of dispersed islet cells. Left, insulin and glucagon expression were analysed in total dispersed islets, allowing the identification of insulin-/glucagon-, insulin+, and glucagon + cell populations. Right, the insulin-/glucagon-, insulin+, and glucagon + cell populations were further analysed for forward scatter (FSC) and expression of amidated GLP-1. Amidated GLP-1+ cells were gated within each cell population. G. Percentages of amidated GLP-1+ cells in the insulin-/glucagon-, insulin+, and glucagon + cell populations. N = 6 donors. H. Representative flow cytometry histograms showing PC1/3 expression levels within GLP-1-/glucagon+, GLP-1+/glucagon+, and insulin + cells. I. Relative expression of PC1/3 expression in GLP-1-/glucagon + cells, GLP-1+/glucagon + cells, and insulin + cells. Median fluorescent intensities (MFIs) of PC1/3 staining were calculated within each cell fraction, normalized relative to the insulin + cell fraction, and compiled as percent expression to insulin + cells. N = 4 donors. J. Representative flow cytometry histograms showing DPP4 expression in insulin-/glucagon-, insulin+, and glucagon + cells. K. Relative expression of DPP4 expression in insulin-/glucagon-, insulin+, and glucagon + cells. MFIs of DPP4 staining were calculated within each cell population, were normalized relative to the insulin-/glucagon-cell fraction and compiled as fold expression relative to insulin-/glucagon-cells. N = 3 donors. L. Islet section antibody validation immunoblot showing that the antibody used in this study to detect amidated forms of GLP-1 does not detect glucagon. Statistical significance for the data was determined using a paired Student's t-test. ∗, P < 0.05, ∗∗∗, P < 0.001. Error bars indicate SEM.
3. Results
3.1. Human islets contain a GLP-1 secreting α-cell subpopulation
As human islets possess ∼40% α cells compared with ∼20% α cells in mouse islets [17], it is reasonable to expect that human islets would secrete ∼2-fold more active GLP-1 when compared with mouse islets. However, we observed a ∼50-fold increase in active GLP-1 in human islet cultures compared with mouse islets suggesting that GLP-1 secretion from α cells may be more important to human islet function (Figure 1A). Because the number of α cells is greater and GLP-1 secretion is higher in human islets, we hypothesized that a significant α-cell subpopulation would express the post-processed amidated form of GLP-1. Human islet sections were stained with antibodies for glucagon and amidated GLP-1. The amidated GLP-1 antibody was validated for selectivity using an in-house immunoblot assay (Figure 1L) that shows that the antibody can detect amidated forms of GLP-1, but not glucagon. We determined that 40% of the total cells in human islets are α cells and that ∼40% of the α cells are a subpopulation that is positive for GLP-1 (Figure 1B–D). Importantly, GLP-1 expression was also found in a subpopulation of α cells in pancreatic biopsies, indicating that the presence of intra-islet GLP-1 is not an artifact of islet isolation and culture (Figure 1E). To study this subpopulation further, flow cytometry was used to segregate three cell populations within human islets: insulin-/glucagon-, insulin+, and glucagon+ (Figure 1F). Our results show that GLP-1 expression is found only in a subpopulation of glucagon+ α cells that constitutes ∼50% of the total α cell number (Figure 1F,G), a value that is consistent with the value of 40% observed in human islet sections (Figure 1D). PC1/3 is required for the proteolytic cleavage of GLP-1 from proglucagon and we observed a significant increase in PC1/3 expression in glucagon+/GLP-1+ α cells compared with glucagon+/GLP-1- cells (Figure 1H,I). We further characterized the α cell population using DPP4 expression and confirmed that DPP4 expression is enriched in α cells [4,5] when compared with β cells (Figure 1J,K). However, we did not observe any differences in DPP4 expression between GLP-1+ and GLP-1- α cells (data not shown).
3.2. Sitagliptin increases active GLP-1 levels in culture, but does not enhance GSIS in ND and T2D islets
Because human α cells express DPP4, we tested the ability of sitagliptin (a DPP4 inhibitor) to increase active GLP-1 levels in human islet cultures. We observed a significant increase in active GLP-1 levels with sitagliptin treatment in long-term cultures (24–48 h, Figure 2A,B,C). We also tested the ability of sitagliptin to increase active GLP-1 in a serum-free culture and confirmed that intra-islet DPP4 is being inhibited, rather than any DPP4 that may be in the culture media (Figure 2B). As inflammatory cytokines may increase GLP-1 secretion, we tested the effects of IL-1β, however the sitagliptin-mediated increase in active GLP-1 was not affected by that cytokine challenge (Figure 2C). As sitagliptin increased active GLP-1, we hypothesized that sitagliptin would augment GSIS. However, under perifusion conditions, sitagliptin-treated ND islets did not show any increase in GSIS (Figure 2D). Because DPP4 inhibitors were successfully used to increase circulating active GLP-1 levels and restore insulin secretion in T2D patients [20], we tested whether sitagliptin would enhance GSIS in T2D islets. Again, no increase in insulin secretion was observed in perifused GSIS experiments, indicating that DPP4 inhibition in T2D islets does not directly contribute to increased GSIS (Figure 2E). To validate the selectivity of the active GLP-1 assay used in these studies, we tested the active GLP-1 (7–36) antibody against several other proglucagon cleavage peptides. We observed that the antibody was highly selective for the active GLP-1 (7–36) peptide, whereas no signal was detected with either the (1–36) or (9–36) GLP-1 peptides or glucagon (Figure 2F).
3.3. Islet GLP-1Rs contribute to insulin secretion in ND and T2D islets
Because sitagliptin treatment did not enhance GSIS in ND and T2D islets, one plausible explanation is that the GLP-1Rs may be maximally stimulated by the robust amount of intra-islet proglucagon peptides that are present, such as GLP-1 (Figure 1A). To explore this concept, we used the GLP-1R antagonist, exendin-9, under the GSIS perifusion conditions and observed a 25% decrease in insulin secretion that occurs primarily at high glucose in ND islets (Figure 3A), suggesting that GLP-1R signalling contributes to insulin secretion in ND islets. In T2D islets, we observed an even larger decrease in insulin secretion of 62% that occurred at both low- and high-glucose conditions (Figure 3B), suggesting that insulin secretion is strongly supported by GLP-1R signalling in T2D. Indeed, when the change in insulin secretion over time is analysed for ND and T2D islets, the percent change from control is 61% greater in T2D islets than in ND islets (Figure 3C). As fluctuations in intracellular calcium correlate with β-cell insulin secretion, we investigated whether treating human islets with exendin-9 would alter calcium dynamics. ND islets were loaded with the calcium sensitive indicator Fluo-4 and stimulated with glucose (10 mM). Exendin-9 caused a reduction in calcium dynamics in whole islets as well as dynamic sub-regions of islets (Figure 3D,E). Calcium events and signal dynamics of sub-regions were significantly decreased in the presence of Exendin-9 when compared with the high (10 mM) glucose control (Figure 3F,G).
Figure 3.
Islet GLP-1Rs contribute to insulin secretion in ND islets and to a larger extent in T2D islets. A. Exendin-9 (100 nM) inhibits GLP-1Rs to reduce insulin secretion at high glucose with glucose-stimulated perifusions of ND islets. B. Exendin-9 reduces insulin secretion at high and low glucose in T2D islet perifusions. C. Area under the curve (AUC) for % change in insulin secretion from control is larger for T2D islets than ND islets (calculated from the perifusion data in panels A and B). D. Example trace of a human islet perfused with 3 mM glucose (0–5 min) and 10 mM glucose (5–60 min) supplemented with 100 nM Exendin-9 (30–55 min). There is less variation in the intracellular calcium dynamics upon perfusion with Exendin-9. E. Example traces of the fluorescence in discrete sub-regions of the islet represented in D, individual regions are more dynamic compared with the average signal of the whole islet. F. The number of calcium events counted in selected regions during perfusion with 10 mM glucose, either in the presence or absence of 100 nM Exendin-9. G. The dynamics of the Fluo-4 calcium signal represented as the standard deviation of the signal over the mean fluorescence in the same conditions as F. Data and analysis in D-G is based on four experiments with four islets from a single donor, focusing on five sub-regions per islet. The mean insulin content for non-diabetic islets is 25.93 ng/islet, while the mean insulin content for type 2 diabetic islets is 13.77 ng/islet. Statistical significance for the data was determined using a paired Student's t-test or two-way ANOVA. ∗, P < 0.05, ∗∗, P < 0.01, ∗∗∗, P < 0.001. Error bars indicate SEM.
3.4. The GLP-1 expressing α cell subpopulation is increased in T2D islets
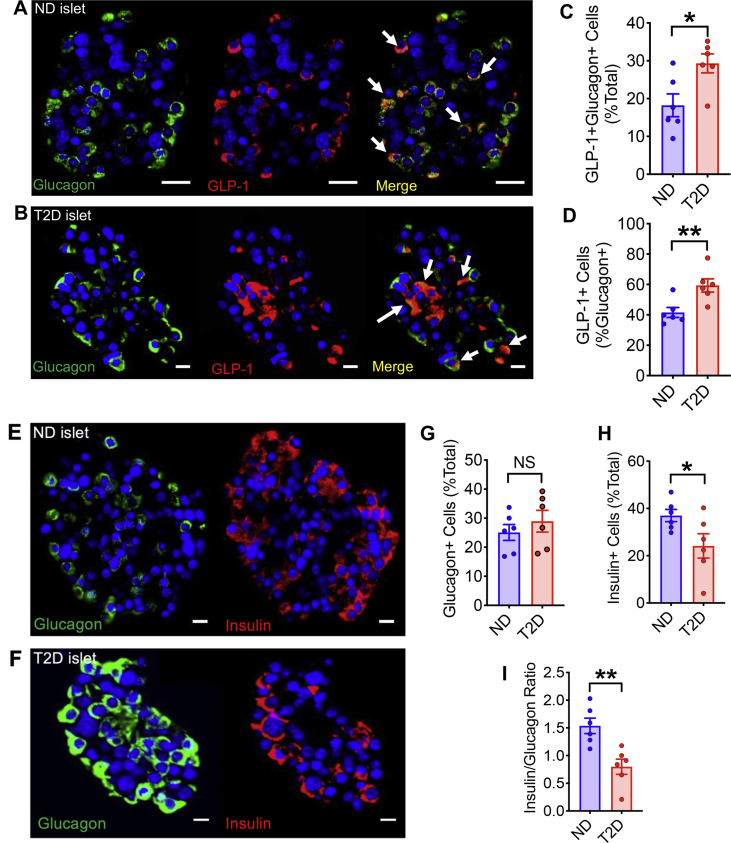
Because GLP-1 expression in pancreatic α cells has been associated with metabolic stress and β cell injury [21,22], we hypothesized that the GLP-1+ α cell subpopulation may be increased in T2D. To test this concept, we obtained islet sections from T2D and ND cadaveric donors and stained for glucagon and amidated GLP-1. Double positive staining for glucagon and GLP-1 was observed in both ND and T2D islet sections (Figure 4A,B). Image analysis revealed that the GLP-1+/glucagon + subpopulation was significantly increased from 18 to 29% of total islet cells in T2D islets when compared with ND islets (Figure 4C). Importantly, the GLP-1+ subpopulation increased from 40 to 60% within the α cells of T2D islets (Figure 4D). We then immuno-stained islet sections from the same donors for glucagon and insulin to identify the α and β cell populations, respectively (Figure 4E,F). Image analysis of T2D islets showed no change in αcell density, but a significant decrease in β cell density (Figure 4G,H) resulting in a significant decrease in the insulin+/glucagon + cell ratio, confirming that the islets are phenotypically diabetic (Figure 4I).
Figure 4.
The GLP-1 expressing α cell subpopulation is increased in T2D islets. Representative image of a ND islet (A) and a T2D islet (B) with staining for DAPI, glucagon and amidated GLP-1 in the glucagon + cells (merge, arrows denote examples of double positive cells). Scale bar: 20 μm. C. GLP-1+/glucagon + cells increased as % total in T2D islets compared to ND islets. D. GLP-1+ α cell subpopulation is increased in T2D islets. E,F. Representative images of a ND and T2D islet respectively showing positive staining for DAPI, glucagon and insulin. Scale bar: 10 μm. G. Glucagon+ (α) cells as a % of total islets cells does not change in T2D islets. H. Insulin+ (β) cells decrease in T2D islets indicating the islets have experienced β cell loss. I. The insulin/glucagon ratio decreases in T2D islets indicating they are phenotypically diabetic. N = 6 ND donors and N = 6 T2D donors matched for age, BMI, and sex. Statistical significance for the data was determined using a paired Student's t-test. ∗, P < 0.05, ∗∗, P < 0.01. Error bars indicate SEM.
3.5. The GLP-1 expressing α-cell subpopulation is present in islets obtained from living donors
Although intra-islet GLP-1 has previously been detected in human and rodent islets, it has been suggested that this is because of stress-induced alterations in proglucagon peptide processing resulting from ischemia, isolation, and culturing. Therefore, it is important to determine whether intra-islet GLP-1 is present in human islets that have not undergone these stressors. Accordingly, we analysed pancreatic sections obtained from living donors undergoing surgery for pancreatitis or malignancy because the presence of intra-islet GLP-1 in those sections would provide further evidence for a physiological role in humans. Analysis of pancreatic sections from eleven living donors revealed that the islets had a similar percentage of insulin+ β cells and insulin+/glucagon + cell (β/α) ratio (Figure 5A–C) to islets obtained from cadaveric donors (Figure 4H,I). Furthermore, immuno-staining for glucagon and amidated GLP-1 (Figure 5D–F) revealed that ∼70% of the glucagon + cells were also positive for GLP-1 (Figure 5G). Four of the eleven living donors had type 2 diabetes, so we compared insulin+, glucagon+ and GLP-1+ cell populations between non-diabetic donors and those with type 2 diabetes. As was previously seen in islet sections from cadaveric donors (Figure 4H), we also observed a significant reduction in insulin + cells in type 2 diabetic islets from living donors (Figure 5H). However, in contrast to our cadaveric donor data (Figure 4D), we did not observe any increase in the percentage of GLP-1+/glucagon + cells in islets from donors with type 2 diabetes (Figure 5I).
Figure 5.
A subpopulation of GLP-1+/Glucagon+ (α) cells can be detected in islets within pancreatic sections obtained from living donors undergoing pancreatic surgery. A. Representative images of glucagon+ and insulin + staining from a single human islet. B,C. Pancreatic section grouped data showing (B) the number of insulin + cells as a % of the total number of cells (DAPI staining in blue) and (C) the ratio of insulin + to glucagon + cells. D. Representative images from a single human islet showing glucagon+, GLP-1 + staining and their co-localization (merge, arrows denote examples of double positive cells). E-G. Pancreatic section grouped data showing the number of: (E) Glucagon + cells and (F) GLP-1+/glucagon + double positive cells as a % of the total number of cells (DAPI staining in blue), (G) GLP-1+ cells as a % of the total number of glucagon + cells. H. Grouped data from pancreatic sections obtained from non-diabetic (ND) and type 2 diabetic (T2D) living donors. Islets were stained for insulin, glucagon, and GLP-1. I. Grouped data expressed as a % of the number of GLP-1+ cells of the total glucagon + cell population. Pancreatic sections were obtained from eleven patients undergoing surgery for either a malignancy or pancreatitis (B,C,E-G, circle or square data points, respectively). Seven patients were non-diabetic (ND) and four had diagnosed type 2 diabetes (T2D). Statistical significance for the data was determined using a paired Student's t-test. ∗, P < 0.05. Error bars indicate SEM.
4. Discussion
The existence of intra-islet GLP-1 within rodent and human islets has been previously documented [2,11,12], however the presence of GLP-1 within islets does not necessarily provide evidence for any functional role. Despite several recent studies using genetic mouse models providing support for a functional role of intra-islet GLP-1 [15,16], the concept of a paracrine role for GLP-1 within human islets remains controversial and requires more evidence obtained from live tissue. As the GLP-1 antibody specificity is central to our methods and data interpretation, we have performed extensive in-house validation of the antibodies to confirm the following: 1) the active GLP-1 assay used detects only the active GLP-1 (7–36) isoform and not the 1–37, 9–37 inactive GLP-1 isoforms or glucagon (Figure 1L); and 2) the GLP-1 amidated C-terminal antibody used for quantification of the GLP-1 expressing α cell subpopulation does not cross-react with glucagon (Figure 2F).
Our results corroborate previous findings [23] that isolated human islets secrete robust amounts of active GLP-1 and that GLP-1 is likely originating from a significant subpopulation of α cells. Furthermore, the key prohormone convertase PC1/3 required for the proglucagon processing to release GLP-1 [10] is significantly increased in the GLP-1+ α cell subpopulation. Importantly, we observed an increase in the GLP-1+ α cell population in T2D islets, suggesting that metabolic factors such as hyperglycemia and chemokine action may regulate intra-islet GLP-1 expression. Indeed, hyperglycemia is associated with α cell PC1/3 expression in diabetic rodent models [23,24]. β cell injury has been shown to increase GLP-1 expression in human islets that involves the expression and secretion of the chemokine, SDF-1α [21], whereby β cells secrete SDF-1α that acts upon α cells to increase PC1/3 expression and GLP-1 production. Furthermore, the cytokine IL-6, produced from skeletal muscle and adipose tissue, has been shown to function as a metabolic signal that triggers islet α cells to increase islet GLP-1 production [22].
The possibility remains that intra-islet GLP-1 expression is increased as a result of the use of genetically modified models and/or the peri- and post-isolation procedure of human islets that may result in the β cell injury described above. However, we observed the presence of a significant subpopulation of GLP-1+ α cells in islets from whole pancreas sections that did not undergo any isolation stress, as well as in pancreatic sections obtained from living donors undergoing surgery [25,26]. Furthermore, we observed a significant increase in the number of GLP-1+ α cells in islets from T2D cadaveric donors when compared with ND islets, despite the isolation procedure and culturing conditions being identical. It should be noted that we did not observe a similar finding in islet sections from living donors. One possible explanation for this discrepancy is that all the donors undergoing surgery had pre-existing pancreatic disease (malignancy or pancreatitis) that may have masked any differences between donors with or without type 2 diabetes. We also acknowledge that a potential limitation of our study is the lack of information on any anti-diabetic medications being taken by the donors that might affect the results of our study. In conjunction, our results provide additional evidence for a potential physiological role for GLP-1 in human islets rather than an epiphenomenon resulting from isolation and culturing. The recent observation also supports the concept that active GLP-1 can be detected in human pancreatic tissue extracts at levels that were higher than observed in mouse pancreases [27].
We observed a strong GLP-1 secretory phenotype coupled with a high percentage of GLP-1+ α cells in human islets, therefore the potential for a functional paracrine role for GLP-1R signalling in human islets is of importance. Using the GLP-1R antagonist exendin-9, we have demonstrated that GLP-1R signalling plays a significant role in modulating intracellular calcium and GSIS and that islets from T2D donors are dependent on GLP-1R activation for >60% of insulin secretion. This observation is further supported by a significant increase in GLP-1+ α cells in T2D islets. Moreover, the effect of exendin-9 on insulin secretion at low glucose levels in T2D islets is more pronounced in T2D islets and may partially explain the higher levels of insulin secretion at low glucose observed in T2D islets. Because exendin-9 is a specific antagonist of GLP-1Rs, a plausible explanation is that exendin-9 is competitively inhibiting the actions of intra-islet GLP-1 on β cell GLP-1Rs [28], although an alternative explanation may be that glucagon also directly activates GLP-1Rs to stimulate insulin secretion. Additionally, a recent study that used gcgr−/− and GLP-1R−/− genetic mouse models suggests that intra-islet GLP-1 levels are very low in wild-type mice and that glucagon can regulate insulin secretion via GLP-1R signalling, albeit at lower potency than GLP-1 [29]. However, previous reports indicate that glucagon acts via the glucagon receptor [30,31]. Moreover, GLP-1 is a ∼400-fold more potent agonist at the GLP-1R than glucagon [32]. In this study we determined that there are large inherent differences in both the levels of intra-islet GLP-1 secretion and α cell population density between human and mouse islets that further highlight the controversy surrounding a paracrine role for GLP-1 in human physiology if only mouse models are used. Furthermore, due to the inherent phenotypic variability of human islets when compared to mice, this variation constitutes a limitation of our study. However, we have recently analysed GLP-1 secretion from human islets in association with islet isolation and donor parameters that attempts to characterise this potential limitation [33]. In the absence of difficult to obtain in vivo human data to further support the concept of intra-islet GLP-1, our study provides additional evidence for a paracrine GLP-1R signalling axis in human islets, perhaps via the localized high levels of GLP-1 secretion observed in this study. Future studies that quantify GLP-1R protein expression in the cell membranes of β cells of ND and T2D islets will help to establish if the increased GLP-1 expression we observe in the α cells of T2D islets is associated with an increase in its canonical receptor on β cells. However, in light of recent findings from mouse and human islets, a direct role for α cell derived glucagon acting upon β cell GLP-1Rs should also be considered [34,35].
The role for DPP4 and the clinically used DPP4 inhibitors on this intra-islet GLP-1 axis is also of interest. We tested the effects of the DPP4 inhibitor sitagliptin to evaluate whether some of the clinical efficacy of this class of drugs can be attributed to a direct intra-islet effect. Our flow cytometry analysis showed that DPP4 expression is relatively restricted to α cells, arguing for a regulatory role for DPP4 of α cell substrates such as GLP-1. As previously shown [4,36,37], we were also able to increase active GLP-1 in long-term human islet cultures. However, short-term perifusion of human islets with sitagliptin did not significantly increase GSIS in either ND or T2D islets; a result that is in direct contrast to previous human islet studies [36,37]. This discrepancy may be a result of various isolation, culture, and experimental conditions among research groups. Furthermore, we cannot exclude the possibility that intra-islet glucagon levels might contribute significantly to, or perhaps even dominate, activation of the GLP-1Rs in our perifusion experiments [34,35,38], thus masking any enhancement in GSIS by increased levels of active GLP-1. Finally, DPP4 inhibitors may also improve islet function and survival and therefore indirectly enhance β cell function and insulin secretion [36,37].
In conclusion, our results provide evidence for the robust secretion of active GLP-1 from a subpopulation of α cells and an important paracrine role for GLP-1R signalling within human islets. The α-cell subpopulation is increased in T2D and is associated with a greater dependency on GLP-1R signalling for insulin secretion, suggesting that the α and β cells within human islets have adapted in T2D to amplify the paracrine pathway in an attempt to support insulin secretion.
Acknowledgments
We would like to thank Dr. Michele Solimena, Dr. Marko Barovic, and their teams at the Paul Langerhans Institute Dresden of the Helmholtz Center Munich at the University Hospital and Medical Faculty of the Technical University of Dresden for generously providing the pancreatic sections from living donors undergoing surgery [25,26]. This research program is supported by the BMBF funded German Centre for Diabetes Research (DZD e.V.); and the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement n° 115881 (RHAPSODY), which includes financial contributions from European Union's Framework Programme Horizon 2020, EFPIA, the Swiss State Secretariat for Education‘ Research and Innovation (SERI) under contract number 16.0097. We thank the Human Organ Procurement and Exchange (HOPE) program in Alberta and the Trillium Gift of Life Network (TGLN) in Ontario for their work obtaining human pancreas for research islet isolations by the ADI IsletCore. We also thank organ donors and their families for generously supporting diabetes research.
P.E.L. holds the Dr. Charles A. Allard Chair in Diabetes Research. This research was supported by grants from the Canadian Institutes of Health Research (CIHR, to P.E.L. and P.EM.) and the Dr. Rod Eidem Diabetes Research Fund (P.E.L.). Trainee funding was provided by the Alberta Diabetes Foundation (S.A.C., J.J.) and the Faculty of Medicine and Dentistry (S.A.C.). K.P. is an FWO [PEGASUS]2 Marie Skłodowska-Curie Fellow and received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement (665501) with the Research Foundation Flanders. Author contributions: S.A.C. designed and performed experiments, analysed data, designed figures, and co-wrote the manuscript. D.G. designed and performed the flow cytometry experiments, analysed the data, and designed figures. M.H. performed the perfusion experiments and hormone assays and analysed the data. J.J. and N.S. optimized and performed the immune-staining of islet sections and J.J. quantified cell sub-populations. A.B. designed experiments, coordinated the project, and contributed to data analysis and interpretation. P.E.M. and K.P. contributed to the study design, data interpretation, and writing the manuscript. P.E.L. conceived the concept, aided in study design, data interpretation, and the co-writing of the manuscript. Conflict of interest: none of the authors have any conflicts to declare. All data generated or analysed during this study are included in the published article (and its online supplementary files).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2020.101014.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Marchetti P., Lupi R., Bugliani M., Kirkpatrick C.L., Sebastiani G., Grieco F.A. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia. 2012;55(12):3262–3272. doi: 10.1007/s00125-012-2716-9. [DOI] [PubMed] [Google Scholar]
- 2.Fava G.E., Dong E.W., Wu H. Intra-islet glucagon-like peptide 1. Journal of Diabetes and its Complications. 2016;30(8):1651–1658. doi: 10.1016/j.jdiacomp.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller T.D., Finan B., Bloom S.R., D'Alessio D., Drucker D.J., Flatt P.R. Glucagon-like peptide 1 (GLP-1) Molecular Metabolism. 2019;30(September):72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omar B.A., Liehua L., Yamada Y., Seino Y., Marchetti P., Ahrén B. Dipeptidyl peptidase 4 (DPP-4) is expressed in mouse and human islets and its activity is decreased in human islets from individuals with type 2 diabetes. Diabetologia. 2014;57(9):1876–1883. doi: 10.1007/s00125-014-3299-4. [DOI] [PubMed] [Google Scholar]
- 5.Segerstolpe Å., Palasantza A., Eliasson P., Andersson E.M., Andréasson A.C., Sun X. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metabolism. 2016;24(4):593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metabolism. 2018;27(4):740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Campbell J.E., Drucker D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metabolism. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Holst J.J., Bersani M., Johnsen A.H., Kofod H., Hartmann B., Ørskov C. Proglucagon processing in porcine and human pancreas. Journal of Biological Chemistry. 1994;269(29):18827–18833. [PubMed] [Google Scholar]
- 9.Rouille Y., Martin S., Steiner D.F. Differential processing of proglucagon by the subtilisin-like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon-like peptide. Journal of Biological Chemistry. 1995;270(44):26488–26496. doi: 10.1074/jbc.270.44.26488. [DOI] [PubMed] [Google Scholar]
- 10.Rouillé Y., Kantengwa S., Irminger J.C., Halban P.A. Role of the prohormone convertase PC3 in the processing of proglucagon to glucagon-like peptide 1. Journal of Biological Chemistry. 1997;272(52):32810–32816. doi: 10.1074/jbc.272.52.32810. [DOI] [PubMed] [Google Scholar]
- 11.Heller S., Aponte G. Intra-islet regulation of hormone by glucagon-like peptide- l- ( 7-36 ) secretion amide. American Journal of Physiology. 1995;269:G852–G860. doi: 10.1152/ajpgi.1995.269.6.G852. [DOI] [PubMed] [Google Scholar]
- 12.Masur K., Tibaduiza E.C., Chen C., Ligon B., Beinborn M. Basal receptor activation by locally produced glucagon-like peptide-1 contributes to maintaining β-cell function. Molecular Endocrinology. 2005;19(5):1373–1382. doi: 10.1210/me.2004-0350. [DOI] [PubMed] [Google Scholar]
- 13.Donath M.Y., Burcelin R. GLP-1 effects on islets: hormonal, neuronal, or paracrine? Diabetes Care. 2013;36(SUPPL.2):2–5. doi: 10.2337/dcS13-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Alessio D. Is GLP-1 a hormone: whether and When? J Diabetes Invest. 2016;7(April):50–55. doi: 10.1111/jdi.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Traub S., Meier D.T., Schulze F., Dror E., Nordmann T.M., Goetz N. Pancreatic α cell-derived glucagon-related peptides are required for β cell adaptation and glucose homeostasis. Cell Reports. 2017;18(13):3192–3203. doi: 10.1016/j.celrep.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Chambers A.P., Sorrell J.E., Haller A., Roelofs K., Hutch C.R., Kim K.-S. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metabolism. 2017;25(4):927–934. doi: 10.1016/j.cmet.2017.02.008. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabrera O., Berman D.M., Kenyon N.S., Ricordi C., Berggren P.O., Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caicedo A. Paracrine and autocrine interactions in the human islet. Seminars in Cell & Developmental Biology. 2013;24(1):11–21. doi: 10.1016/j.pestbp.2011.02.012.Investigations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Diaz R., Molano R.D., Weitz J.R., Abdulreda M.H., Berman D.M., Leibiger B. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metabolism. 2018;27(3):549–558. doi: 10.1016/j.cmet.2018.01.015. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulvihill E.E., Drucker D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocrine Reviews. 2014;35(6):992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z., Stanojevic V., Avadhani S., Yano T., Habener J.F. Stromal cell-derived factor-1 (SDF-1)/chemokine (C-X-C motif) receptor 4 (CXCR4) axis activation induces intra-islet glucagon-like peptide-1 (GLP-1) production and enhances beta cell survival. Diabetologia. 2011;54(8):2067–2076. doi: 10.1007/s00125-011-2181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellingsgaard H., Hauselmann I., Schuler B., Habib A.M., Baggio L.L., Meier D.T. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nature Medicine. 2011;17(11):1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen A.M.K., Bödvarsdottir T.B., Nordestgaard D.N.E., Heller R.S., Gotfredsen C.F., Maedler K. Upregulation of alpha cell glucagon-like peptide 1 (GLP-1) in Psammomys obesus - an adaptive response to hyperglycaemia? Diabetologia. 2011;54(6):1379–1387. doi: 10.1007/s00125-011-2080-1. [DOI] [PubMed] [Google Scholar]
- 24.Nie Y., Nakashima M., Brubaker P.L., Li Q.L., Perfetti R., Jansen E. Regulation of pancreatic PC1 and PC2 associated with increased glucagon- like peptide 1 in diabetic rats. Journal of Clinical Investigation. 2000;105(7):955–965. doi: 10.1172/JCI7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solimena M., Schulte A.M., Marselli L., Ehehalt F., Richter D., Kleeberg M. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia. 2018;61(3):641–657. doi: 10.1007/s00125-017-4500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barovic M., Distler M., Schöniger E., Radisch N., Aust D., Weitz J. Metabolically phenotyped pancreatectomized patients as living donors for the study of islets in health and diabetes. Molecular Metabolism. 2019;27:S1–S6. doi: 10.1016/j.molmet.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Y., Koehler J.A., Baggio L.L., Powers A.C., Sandoval D.A., Drucker D.J. Gut-proglucagon-derived peptides are essential for regulating glucose homeostasis in mice. Cell Metabolism. 2019;30(5):976–986. doi: 10.1016/j.cmet.2019.08.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marie J.C., Boissard C., Skoglund G., Rosselin G., Breant B. Glucagon acts through its own receptors in the presence of functional glucagon-like peptide-1 receptors on hamster insulinoma. Endocrinology. 1996;137(10):4108–4114. doi: 10.1210/endo.137.10.8828464. [DOI] [PubMed] [Google Scholar]
- 29.Svendsen B., Larsen O., Gabe M.B.N., Christiansen C.B., Rosenkilde M.M., Drucker D.J. Insulin secretion depends on intra-islet glucagon signaling. Cell Reports. 2018;25(5):1127–1134. doi: 10.1016/j.celrep.2018.10.018. e2. [DOI] [PubMed] [Google Scholar]
- 30.Huypens P., Ling Z., Pipeleers D., Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43(8):1012–1019. doi: 10.1007/s001250051484. [DOI] [PubMed] [Google Scholar]
- 31.Kawai K., Yokota C., Ohashi S., Watanabe Y., Yamashita K. Evidence that glucagon stimulates insulin secretion through its own receptor in rats. Diabetologia. 1995;38(3):274–276. doi: 10.1007/BF00400630. [DOI] [PubMed] [Google Scholar]
- 32.Runge S., Wulff B.S., Madsen K., Bräuner-Osborne H., Knudsen L.B. Different domains of the glucagon and glucagon-like peptide-1 receptors provide the critical determinants of ligand selectivity. British Journal of Pharmacology. 2003;138(5):787–794. doi: 10.1038/sj.bjp.0705120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell S.A., Hubert M., Johnson J., Salamon N., Light P.E. The DPP4 inhibitor sitagliptin increases active GLP-1-1 levels from human islets and may increase islet cell survival prior to transplantation. OBM Transplantation. 2019;3(2) doi: 10.21926/obm.transplant.1902069. 1–1. [DOI] [Google Scholar]
- 34.Zhu L., Dattaroy D., Pham J., Wang L., Barella L.F., Cui Y. Intraislet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight. 2019;4(10):1–15. doi: 10.1172/jci.insight.127994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capozzi M.E., Svendsen B., Encisco S.E., Lewandowski S.L., Martin M.D., Lin H. β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight. 2019;4(5):1–15. doi: 10.1172/jci.insight.126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah P., Ardestani A., Dharmadhikari G., Laue S., Schumann D.M., Kerr-Conte J. The DPP-4 inhibitor linagliptin restores β-cell function and survival in human isolated islets through GLP-1 stabilization. Journal of Clinical Endocrinology & Metabolism. 2013;98(7):1163–1172. doi: 10.1210/jc.2013-1029. [DOI] [PubMed] [Google Scholar]
- 37.Bugliani M., Syed F., Paula F.M.M., Omar B.A., Suleiman M., Mossuto S. DPP-4 is expressed in human pancreatic beta cells and its direct inhibition improves beta cell function and survival in type 2 diabetes. Molecular and Cellular Endocrinology. 2018;473:186–193. doi: 10.1016/j.mce.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen R., Martini L., Schwartz T.W., Elling C.E. Characterization of glucagon-like peptide-1 receptor β-arrestin 2 interaction: a high-affinity receptor phenotype. Molecular Endocrinology. 2005;19(3):812–823. doi: 10.1210/me.2004-0312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.