Key Points
Question
What are the safety and efficacy profiles of rechallenge with an immune checkpoint inhibitor (ICI) in patients with metastatic renal cell carcinoma?
Findings
In this cohort study of 69 patients treated between 2012 and 2019, the overall response rate of ICI rechallenge was 23%. In addition, ICI rechallenge was reasonably safe, with 11 patients (16%) experiencing a grade 3 or higher immune-related adverse event.
Meaning
The findings of this cohort study suggest that ICI rechallenge may be safe and reasonably effective for the treatment of patients with metastatic renal cell carcinoma.
This cohort study examines the safety and efficacy of immune checkpoint inhibitor rechallenge in patients with metastatic renal cell carcinoma.
Abstract
Importance
Several immune checkpoint inhibitors (ICIs) are approved for use in patients with metastatic renal cell carcinoma (mRCC), but the efficacy and safety of ICI rechallenge in mRCC is unknown.
Objective
To evaluate the safety and efficacy of ICI rechallenge in patients with mRCC.
Design, Setting, and Participants
This multicenter, retrospective cohort study included consecutive patients with mRCC from 9 institutions in the US who received at least 2 separate lines of ICI (ICI-1, ICI-2) between January 2012 and December 2019.
Exposure
Receipt of an ICI (anticytotoxic T-lymphocyte-associated protein 4, anti–programmed cell death protein 1, or anti–programmed cell death ligand 1), alone or in combination with other therapies, in at least 2 separate lines of therapy for mRCC.
Main Outcomes and Measures
Investigator-assessed best overall response and immune-related adverse events.
Results
A total of 69 patients were included. Median (range) age at diagnosis of mRCC was 61 (36-86) years. Of these, 50 were men and 19 were women. The most common therapies received at ICI-1 were single-agent ICI (n = 27 [39%]) or ICI in combination with targeted therapy (n = 29 [42%]), while at ICI-2, the most common therapies were single-agent ICI (n = 26 [38%]) or dual ICI (n = 22 [32%]). Most patients discontinued ICI-1 owing to disease progression (n = 50 [72%]) or toxic effects (n = 16 [23%]). The overall response rates at ICI-1 and ICI-2 were 37% and 23%, respectively. The likelihood of a response at ICI-2 was greatest among patients who had previously responded to ICI-1 (7 of 24 [29%]), although responses at ICI-2 were seen in those who had progressive disease as their best response following ICI-1 (3 of 14 [21%]) as well as in those who received single-agent ICI at ICI-2 (7 of 23 [30%]). Grade 3 or higher immune-related adverse events were seen in 18 patients (26%) and 11 patients (16%) at ICI-1 and ICI-2, respectively. There were no treatment-related deaths.
Conclusions and Relevance
The findings of this multicenter cohort study suggest that ICI rechallenge in patients with mRCC may be safe and reasonably efficacious, with an overall response rate of 23%. Data from prospective studies are needed to validate these findings and determine the role of sequential ICI regimens in treatment of mRCC.
Introduction
Over the past 5 years, several immune checkpoint inhibitors (ICIs) have been approved for the treatment of metastatic renal cell carcinoma (mRCC). These include nivolumab, either alone or in combination with ipilimumab, as well as pembrolizumab and avelumab, both in combination with axitinib, a vascular endothelial growth factor-directed tyrosine kinase inhibitor. These therapies produce response rates of 40% to 60%, and median overall survival now approaches 4 years in patients with intermediate or poor risk treated with ipilimumab and nivolumab.1,2
Nonetheless, most patients with mRCC receiving ICIs ultimately experience disease progression and require subsequent therapies. Oncologists may attempt ICI rechallenge given that some activity with rechallenge has been seen in patients with melanoma3 and non–small cell lung cancer.4 However, to our knowledge there are no data to support this approach in patients with mRCC. We therefore conducted a multicenter cohort study to assess the safety and efficacy of ICI rechallenge (ICI-2) in patients with mRCC who had previously received ICI in an earlier line of therapy (ICI-1). We hypothesized that response to ICI-2 would be lower than to ICI-1 and that toxic effects may be increased.
Methods
After approval was obtained from each participating institution’s review board, data from 9 centers in the US were obtained from consecutive patients who received at least 2 separate lines of ICI (alone or in combination with other therapies) for mRCC between January 2012 and December 2019. Patient written informed consent was waived based on the deidentified nature of the data in this study. In most cases, therapy at ICI-2 was different from that at ICI-1; however, patients who received nivolumab after ipilimumab and nivolumab (or vice versa) were included if they had received at least 1 intervening, non–ICI-based therapy. Maintenance nivolumab administered immediately after ipilimumab, and nivolumab, or addition of ipilimumab to nivolumab, was not considered a separate line of ICI.
The primary outcomes were best overall radiographic response (per Response Evaluation Criteria in Solid Tumors version 1.1) and immune-related adverse events (irAEs, graded using Common Terminology Criteria for Adverse Events version 5.0). Both were assessed by investigators via review of medical and treatment records. Patients without a follow-up scan after the start of therapy were considered nonevaluable for response. Time to progression was defined as duration between start of therapy and radiographic and/or clinical progression or death, or censored at the date of last follow-up. The Kaplan-Meier method was used for survival analyses and 2-sided P < .05 was considered significant. Statistical analysis was performed using Stata version 16.1 (StataCorp).
Results
Patient Characteristics
A total of 69 patients were included in this study (Table 1), of whom 50 were men and 19 were women. Median (range) age at diagnosis of metastatic disease was 61 (36-86) years and most (60 [87%]) had clear cell histology; median follow-up was 3.2 years (95% CI, 2.7-4.1). Most patients received single-agent ICI (n = 27 [39%]) or ICI in combination with a targeted therapy (TT; n = 29 [42%]) at ICI-1, while the most frequent therapies at ICI-2 were single-agent ICI (n = 26 [38%]) or dual ICI (n = 22 [32%]).
Table 1. Baseline Characteristics of the Cohort.
| Characteristic | No. (%) |
|---|---|
| Total No. | 69 |
| Age at diagnosis, median (range), y | 61 (36-86) |
| Clear cell histology | 60 (87) |
| IMDC risk at time of ICI-1 therapy | |
| Good | 13 (19) |
| Intermediate | 45 (65) |
| Poor | 8 (12) |
| Not available | 3 (4) |
| Line of ICI-1 therapy, median (range) | 1 (1-6) |
| ICI alone | 27 (39) |
| ICI + ICI | 9 (13) |
| ICI + TT | 29 (42) |
| ICI + chemotherapy | 2 (3) |
| ICI + investigational agent | 2 (3) |
| Reasons for discontinuation of ICI-1 | |
| Disease progression | 50 (72) |
| Toxic effects | 16 (23) |
| Other | 3 (4) |
| Line of ICI-2 therapy, median (range) | 3 (2-8) |
| ICI alone | 26 (38) |
| ICI + ICI | 22 (32) |
| ICI + TT | 13 (19) |
| ICI + chemotherapy | 1 (1) |
| ICI + investigational agent | 7 (10) |
Abbreviations: ICI, immune checkpoint inhibitor; ICI-1, first line of ICI therapy; ICI-2, ICI rechallenge in patients who have already received an earlier line of ICI therapy; IMDC, International Metastatic RCC Database Consortium; TT, targeted therapy.
Efficacy of ICI Rechallenge
Details of ICI therapies received, including response, are shown in the eFigure in the Supplement. A total of 68 patients were evaluable for response at ICI-1; the overall response rate (ORR) was 37% (n = 25), while 43% (n = 29) and 21% (n = 14) of patients had stable disease (SD) and progressive disease (PD), respectively.
At ICI-2, the ORR was 23% (n = 15), while 41% (n = 26) and 36% (n = 23) of patients had SD and PD, respectively; 5 patients were nonevaluable. There were no complete responses with either ICI-1 or ICI-2. Median time to progression was significantly longer with ICI-1 compared with ICI-2 (8.2 months [95% CI, 5.7-10.6] vs 5.7 months [95% CI, 3.2-7.6], Wilcoxon P = .045).
Characteristics of Responders to ICI-2
There were 15 patients who responded to ICI-2. Of these, 7 (47%) received single-agent ICI, 5 (33%) received ICI + ICI, and 3 (20%) received ICI + TT. A total of 7 (47%) responded to ICI-1, while 4 (27%) had SD to ICI-1 and 3 (20%) had PD to ICI-1. A total of 6 (40%) discontinued ICI-1 due to toxic effects.
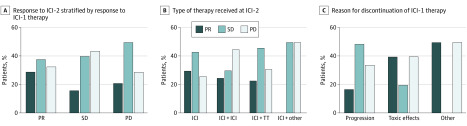
Responses to ICI-2 stratified by response at ICI-1, reason for discontinuation of ICI-1, and the type of therapy received at ICI-2 are shown in the Figure. The ORR at ICI-2 was higher in patients who responded to ICI-1 (7 of 24 [29%]) compared with those who had SD (4 of 25 [16%]) or PD (3 of 14 [21%]), while it was similar in patients receiving single-agent ICI (n = 7 [30%]), dual ICI (n = 5 [25%]), or ICI in combination with TT (n = 3 [23%]) at ICI-2. Of note, among 6 of 7 evaluable patients who received dual anti–programmed cell death protein 1 (anti–PD-1) and anti–cytotoxic T-lymphocyte-associated protein (anti–CTLA-4) inhibition at ICI-2 after anti–programmed cell death ligand 1 (anti–PD-L1) therapy at ICI-1, 1 had a partial response (ORR, 17%).
Figure. Responses to Immune Checkpoint Inhibitor Rechallenge (ICI-2) in Selected Patient Populations.
There were a total of 68 evaluable patients at first course of immune checkpoint inhibitor therapy (ICI-1) and 64 evaluable patients at ICI-2. PD indicates progressive disease; PR, partial response; SD, stable disease; TT, targeted therapy.
Safety of ICI Rechallenge
A total of 49 patients (71%) and 31 patients (45%) experienced an irAE of any grade with ICI-1 and ICI-2, respectively. Grade 3 or higher irAEs were seen in 18 patients (26%) and 11 patients (16%) with ICI-1 and ICI-2, respectively (Table 2). A total of 3 patients (27%) who had a grade 3 or higher irAE at ICI-2 had previously experienced a grade 3 or higher irAE with ICI-1. There were no treatment-related deaths. The risk of experiencing an irAE with ICI-2 was higher in patients who had an irAE with ICI-1 (n = 20 [41%]) compared with those who did not (n = 4 [20%]).
Table 2. Details of Grade 3 or Higher Immune-Related Adverse Events Seen With Initial Immune Checkpoint Inhibitor Therapy (ICI-1) and ICI Rechallenge (ICI-2).
| Immune-related adverse event | No. (%) |
|---|---|
| ICI-1 | |
| Any adverse event ≥grade 3 | 18 (26) |
| Elevated liver enzymes | 6 (9) |
| Elevated amylase/lipase | 4 (6) |
| Pneumonitis | 3 (4) |
| Thyroiditis | 1 (1) |
| Hypophysitis | 1 (1) |
| Adrenalitis | 1 (1) |
| Hypercalcemia | 1 (1) |
| Hypertension | 1 (1) |
| Hypertriglyceridemia | 1 (1) |
| ICI-2 | |
| Any adverse event ≥grade 3 | 11 (16) |
| Elevated liver enzymes | 1 (1) |
| Elevated amylase/lipase | 3 (4) |
| Pneumonitis | 1 (1) |
| Arthritis | 2 (3) |
| Colitis | 1 (1) |
| Hyperglycemia | 1 (1) |
| Nausea/vomiting | 1 (1) |
| Fatigue | 1 (1) |
Discussion
In this multicenter cohort study, we found that ICI rechallenge in patients with mRCC was not associated with an increase in immunotherapy-related toxic effects and had an ORR of 23%. This response rate is similar to that seen with single-agent nivolumab in the second-line setting.5
There has been growing interest in ICI rechallenge in solid tumors after discontinuation or disease progression following a prior ICI. Outcomes of ICI rechallenge have been reported in patients with melanoma, with large trials of nivolumab and pembrolizumab in ipilimumab-refractory patients showing an ORR of 20% to 30%,3,6 although response rates are lower with anti–PD-1 rechallenge after prior anti–PD-1.7 In non–small cell lung cancer, an ORR of 43% was seen in 14 patients who had completed 2 years of therapy in the KEYNOTE-010 trial and were then retreated with pembrolizumab,4 although retrospective series have shown no responses when patients switch to a different anti–PD-1/anti–PD-L1 agent.8,9
In patients with mRCC, a case report of 2 patients rechallenged with a different anti–PD-1/anti–PD-L1 inhibitor showed no responses.10 However, the concept of salvage ipilimumab for patients who are either stable or progress while taking nivolumab is gaining traction and being tested in several clinical trials, although preliminary data from one such trial (Tailored Immunotherapy Approach With Nivolumab in Advanced Renal Cell Carcinoma [TITAN-RCC])11 suggested that this approach has limited efficacy (approximately 10% response). A retrospective study assessing the efficacy of ipilimumab and nivolumab after prior ICI in 30 patients was presented in 2019,12 and showed an ORR of 17% and no increase in toxic effects. Additionally, a response was seen in 23% of patients with mRCC who resumed ICI after experiencing toxic effects and who had not responded prior to temporary discontinuation of ICI.13
In this regard, to our knowledge, our study is the largest reporting on outcomes of ICI rechallenge in patients with mRCC and confirms that it is relatively safe and reasonably efficacious. Responses were seen in patients receiving a single-agent ICI at rechallenge as well as in those for whom PD was the best response to prior ICI. This suggests that ICI refractoriness may be overcome by using a different ICI, or, perhaps, that intervening therapy may alter the tumor microenvironment, thereby sensitizing patients to subsequent ICI.14
Limitations
The key limitation of our study is the retrospective nature of response determination and toxic effects by investigators. Nevertheless, our results provide some support for ICI rechallenge while we await data from prospective studies assessing sequential or add-on ICI in patients with mRCC.
Conclusions
In this cohort study, ICI rechallenge was safe and demonstrated moderate efficacy in patients with mRCC, including as a single agent and after prior nonresponse to ICI. Data from prospective trials are needed to validate these findings and determine the role for sequential ICI in mRCC.
eFigure 1.
References
- 1.Tannir NM, Frontera OA, Hammers HJ, et al. Thirty-month follow-up of the phase III CheckMate 214 trial of first-line nivolumab + ipilimumab (N+I) or sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2020;37(7):2547. [Google Scholar]
- 2.Rini BI, Plimack ER, Stus V, et al. ; KEYNOTE-426 Investigators . Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127. doi: 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- 3.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908-918. doi: 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst RS, Garon EB, Kim DW, et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1–positive, advanced non–small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol. 2020;38(14):1580-1590. doi: 10.1200/JCO.19.02446 [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, McDermott DF, et al. ; CheckMate 025 Investigators . Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803-1813. doi: 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384. doi: 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 7.Betof Warner A, Palmer JS, Shoushtari AN, et al. Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J Clin Oncol. 2020:JCO1901464. doi: 10.1200/JCO.19.01464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita K, Yamamoto Y, Kanai O, et al. Retreatment with anti-PD-1 antibody in non-small cell lung cancer patients previously treated with anti-PD-L1 antibody. Thorac Cancer. 2020;11(1):15-18. doi: 10.1111/1759-7714.13241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita K, Uchida N, Yamamoto Y, et al. Retreatment with anti-PD-L1 antibody in advanced non-small cell lung cancer previously treated with anti-PD-1 antibodies. Anticancer Res. 2019;39(7):3917-3921. doi: 10.21873/anticanres.13543 [DOI] [PubMed] [Google Scholar]
- 10.Martini DJ, Lalani AA, Bossé D, et al. Response to single agent PD-1 inhibitor after progression on previous PD-1/PD-L1 inhibitors: a case series. J Immunother Cancer. 2017;5(1):66. doi: 10.1186/s40425-017-0273-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm MO, Schmidinger M, Duran Martinez I, et al. Tailored immunotherapy approach with nivolumab in advanced renal cell carcinoma (TITAN-RCC). Ann Oncol. 2019;30(suppl 5):v851-v934. doi: 10.1093/annonc/mdz394.051 [DOI] [Google Scholar]
- 12.Gul A, Shah NJ, Mantia C, et al. Ipilimumab plus nivolumab (Ipi/Nivo) as salvage therapy in patients with immunotherapy (IO)-refractory metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2019;37(7):669. doi: 10.1200/JCO.2019.37.7_suppl.66931877086 [DOI] [Google Scholar]
- 13.Abou Alaiwi S, Xie W, Nassar AH, et al. Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune-related adverse events in metastatic renal cell carcinoma. J Immunother Cancer. 2020;8(1):e000144. doi: 10.1136/jitc-2019-000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30(2):219-235. doi: 10.1093/annonc/mdy551 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1.