Key Points
Question
Does administration of adjuvant imatinib for 3 years after macroscopically complete surgery prolong overall survival (OS) compared with 1-year administration in patients with localized gastrointestinal stromal tumor (GIST) with high-risk features?
Findings
This secondary analysis of a randomized clinical trial of 397 patients with high-risk GIST found that, after a median follow-up time of 10 years after the study entry, patients randomly allocated to receive 3 years of imatinib had longer recurrence-free survival and OS than patients allocated to 1 year of imatinib: Approximately one-half of deaths were avoided with the longer treatment. No new adverse safety signals were detected.
Meaning
Three years of treatment with adjuvant imatinib was associated with improved OS compared with 1 year of imatinib in patients with high-risk GIST.
This secondary analysis evaluates the effect of adjuvant imatinib on overall survival in patients who have a high estimated risk for gastrointestinal stromal tumor recurrence after macroscopically complete surgery.
Abstract
Importance
Adjuvant imatinib is associated with improved recurrence-free survival (RFS) when administered after surgery to patients with operable gastrointestinal stromal tumor (GIST), but its influence on overall survival (OS) has remained uncertain.
Objective
To evaluate the effect of adjuvant imatinib on OS of patients who have a high estimated risk for GIST recurrence after macroscopically complete surgery.
Design, Setting, and Participants
In this open-label, randomized (1:1), multicenter phase 3 clinical trial conducted in Finland, Germany, Norway, and Sweden, 400 patients who had undergone macroscopically complete surgery for GIST with a high estimated risk for recurrence according to the modified National Institutes of Health Consensus Criteria were enrolled between February 2004 and September 2008. Data for this follow-up analysis were analyzed from September to November, 2019.
Interventions
Imatinib 400 mg/d administered orally for either 12 months or 36 months after surgery.
Main Outcomes And Measures
The primary end point was RFS; the secondary objectives included OS and treatment safety.
Results
The intention-to-treat cohort consisted of 397 patients (12-month group, 199; 36-month group, 198; 201 men and 196 women; median [IQR] age, 62 (51-69) years and 60 (51-67) years, during a median follow-up time of 119 months after the date of randomization, 194 RFS events and 96 OS events were recorded in the intention-to-treat population. Five-year and 10-year RFS was 71.4% and 52.5%, respectively, in the 36-month group and 53.0% and 41.8% in the 12-month group (hazard ratio [HR], 0.66; 95% CI, 0.49-0.87; P = .003). In the 36-month group, 5-year OS and 10-year OS rates were 92.0% and 79.0%, respectively, and in the 12-month group 85.5% and 65.3% (HR, 0.55; 95% CI, 0.37-0.83; P = .004). The results were similar in the efficacy population, from which 15 patients who did not have GIST in central pathology review and 24 patients who had intra-abdominal metastases removed at surgery were excluded (36-month group, 10-year OS 81.6%; 12-month group, 66.8%; HR, 0.50; 95% CI, 0.32-0.80; P = .003). No new safety signals were detected.
Conclusions and Relevance
Three years of adjuvant imatinib is superior in efficacy compared with 1 year of imatinib. Approximately 50% of deaths may be avoided during the first 10 years of follow-up after surgery with longer adjuvant imatinib treatment.
Trial Registration
ClinicalTrials.gov Identifier: NCT00116935
Introduction
The survival outcomes of patients with gastrointestinal stromal tumor (GIST) with overt metastases improved greatly after the approval of imatinib.1 Yet, the median survival time of patients with advanced GIST is still only about 2 to 3 years. Administration of imatinib in the adjuvant setting to patients who have a high risk of recurrence despite macroscopically complete surgery is an attractive strategy for improving survival. Imatinib remains the only approved tyrosine kinase inhibitor (TKI) for adjuvant treatment of GIST.
The National Comprehensive Cancer Network of the US guidelines recommend postoperative imatinib for at least 36 months for high-risk GISTs based on the randomized clinical trials conducted,2,3,4,5,6,7 and the European Society for Medical Oncology guidelines for 3 years as the standard treatment for patients with a significant risk for relapse.8 Both recommendations were influenced by the results of the Scandinavian Sarcoma Group XVIII/German (SSGXVIII/AIO) clinical trial that compared 3 years of adjuvant imatinib with 1 year of imatinib. The SSGXVIII/AIO trial has been analyzed twice, after median patient follow-up durations of 54 months4 and 90 months.6 In both analyses overall survival (OS) was longer in the 3-year group than in the 1-year group, but whether imatinib improves OS after extended follow-up remains uncertain.
We amended the SSGXVIII/AIO study protocol to allow a third analysis of the trial. In this analysis we investigated survival when the last patient entered reached 10 years of follow-up after the date of randomization. This is the maximum follow-up time available because the patients were scheduled for 10 years of follow-up. We also collected data about cardiac events and second cancers.
Methods
Design
The SSGXVIII/AIO trial (NCT00116935) is an open-label, multicenter, randomized, phase 3 study. The study participants were assigned in a 1:1 ratio to adjuvant imatinib, 400 mg, once daily orally for either 12 or 36 months after surgery. The primary end point was RFS, secondary objectives included OS.4
National or regional ethics boards or the institutional review committees approved the study. The participants provided written informed consent prior to randomization. The study was conducted according to the Good Clinical Practice guidelines.
Patients
Eligible patients underwent macroscopically complete surgery for a tumor that was histologically verified as KIT-positive GIST.4 For inclusion, GIST was required to have a high estimated risk of recurrence according to the modified National Institutes of Health (NIH) consensus criteria.9 The exclusion criteria included inoperable, metastatic, or recurrent GIST, and administration of imatinib preoperatively for GIST.4
Study Procedures
The trial protocol and statistical analysis plan are available in Supplement 1. The patients were allocated to groups using computer-generated random numbers.4 Staging was performed within 4 weeks prior to initiating imatinib using computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen and the pelvis, and CT or radiography of the chest. Imaging of the abdomen and the pelvis with CT or MRI, blood tests, and physical examination were carried out periodically during treatment and follow-up.4,6 Adverse events were captured and graded. The histologic diagnosis of GIST and risk stratification were done by local pathologists and reviewed centrally. KIT (NCBI Entrez gene 3815) exons 9, 11, 13, and 17, and PDGFRA (NCBI Entrez gene 5156) exons 12, 14, and 18 were sequenced centrally.4
Statistical Analysis
The study protocol was amended to allow a third analysis of the trial data in April 2017.
The analysis was scheduled when the last patient entered to the study and still in follow-up had completed the full study follow-up period (10 years after surgery). The data collection cutoff date was December 31, 2018.
Participant RFS was defined as the time interval between the date of randomization and the date of first documentation of GIST recurrence or death, whichever occurred first, censoring patients alive without recurrence on the date of last follow-up. Participant OS was defined as the time period from the date of randomization to death, censoring patients alive on the date of last follow-up. Patients lost to follow-up were censored on the date of the last follow-up visit.
The estimated study sample size was 400 patients.4 Patients who signed informed consent and were randomized formed the intention-to-treat population (ITT). The efficacy population consisted of patients who signed consent, had GIST confirmed at the central pathology assessment, and did not have metastases removed at laparotomy.
Survival between groups was compared using the Kaplan-Meier method and an unstratified log-rank test (P values), and an unstratified Cox proportional hazards model (HRs). The subgroup analyses were performed similarly; each subgroup variable category was entered at a time. The subgroup analyses were predefined in the Statistical Analysis Plan (written in March 2010) (Supplement 1), and the factors included were the high-risk GIST features defined in the modified NIH consensus criteria,9 age at diagnosis, and the site of KIT mutation. The P values are 2-tailed and not adjusted for multiple testing. Statistical analyses were done with SAS statistical software for Windows (version 9.4, SAS Institute Inc). Data were analyzed between September and November, 2019.
Results
Patients
Between February 4, 2004 and September 29, 2008, 400 patients were accrued in Finland, Germany, Norway, and Sweden. Three patients who were randomized without the patient signing informed consent were excluded from the analyses. Therefore, the ITT cohort consisted of 397 patients (12-month group, 199; 36-month group, 198; 201 men and 196 women; median [IQR] age, 62 (51-69) years and 60 (51-67) years, respectively). The characteristics of the patients and their tumors were similar in the groups (eTable 1 in Supplement 2).
To form the efficacy population, we excluded from the ITT population 15 (3.8%) patients who did not have GIST at tumor central pathology review and 24 (6.0%) patients who had metastases removed in addition to the primary tumor at laparotomy. The efficacy population consisted of 358 patients (12-month group, 181; 36-month group, 177; eFigure 1 in Supplement 2).
Survival
The median follow-up time after the date of randomization was 119 months (12-month group, 118 months; 36-month group, 119 months). Seventeen (4.3%) patients were lost to follow-up (12-month group, 7; 36-month group, 10). In the ITT population 106 and 88 RFS events were recorded in the 12-month and 36-month groups, respectively, and in the efficacy population 92 and 79 events.
The patients assigned to 36 months of imatinib had longer RFS compared with those assigned to 12 months of imatinib (Figure 1). In the ITT population, the Kaplan-Meier 5-year and 10-year RFS estimates were 71.4% and 52.5%, respectively, in the 36-month group, and 53.0% and 41.8% in the 12-month group (HR, 0.66; 2-sided 95% CI, 0.49-0.87; P = .003). In the efficacy population, the 10-year RFS estimates were 52.4% and 44.2% in the 36-month and 12-month groups, respectively (HR, 0.70; 95% CI, 0.52-0.94; P = .02).
Figure 1. Kaplan-Meier Estimates of Survival Outcomes.
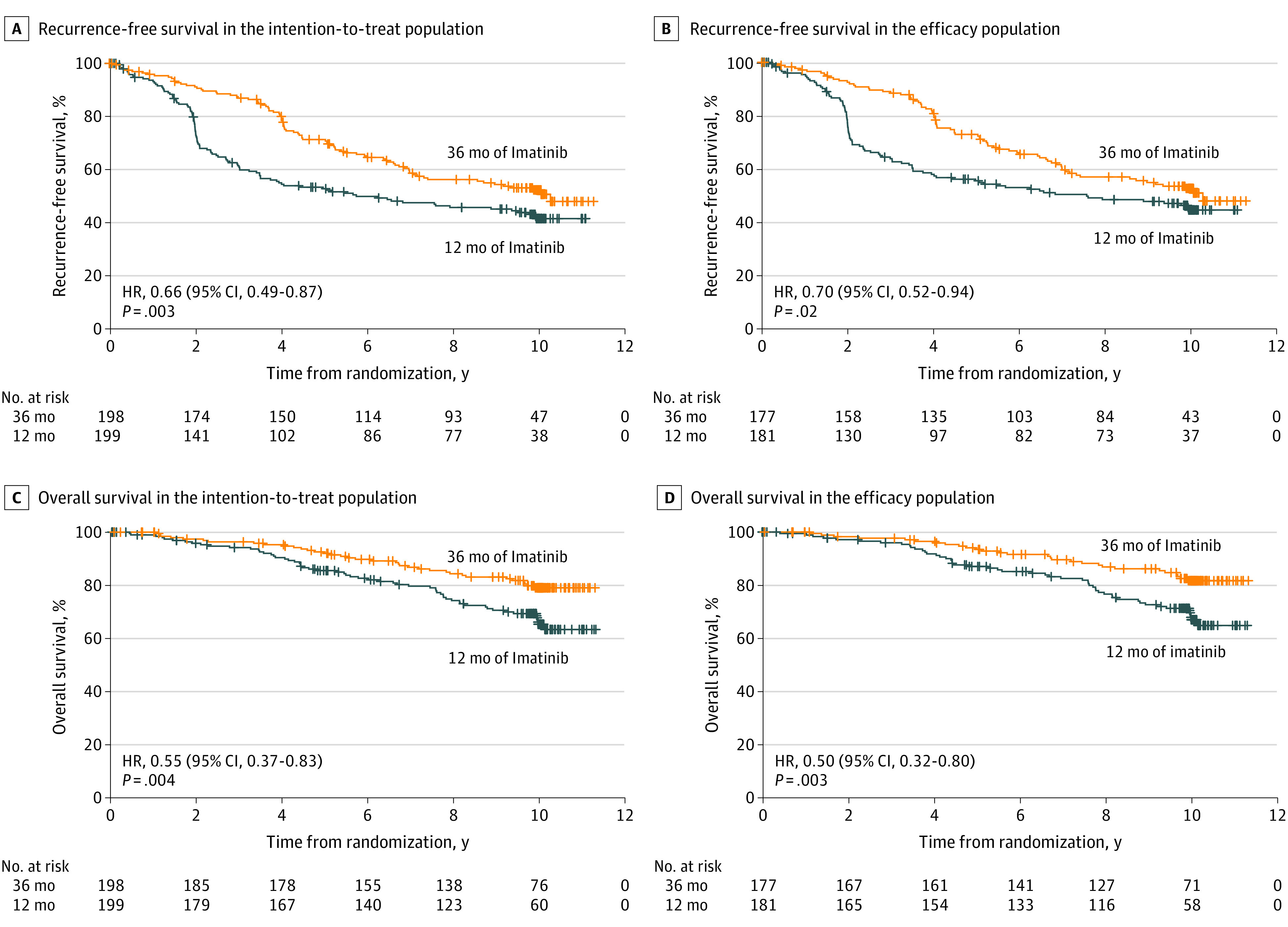
A, Recurrence-free survival in the intention-to-treat population. B, recurrence-free survival in the efficacy population. C, Overall survival in the intention-to-treat population. D, Overall survival in the efficacy population.
Ninety-six patients died during the follow-up (12-month group, 60; 36-month group, 36). Most patients (79 [82%]) died with overtly metastatic GIST (12-month group, 49 [82%]; 36-month group, 30 [83%]). Five-year and 10-year OS was 92.0% and 79.0%, respectively, in the 36-month group in the ITT population, and 85.5% and 65.3% in the 12-month group (HR, 0.55; 95% CI, 0.37-0.83; P = .004). In the efficacy population 81.6% and 66.8% of the patients survived for at least 10 years after the date of randomization in the 36-month group and the 12-month group, respectively (HR, 0.50; 95% CI, 0.32-0.80; P = .003).
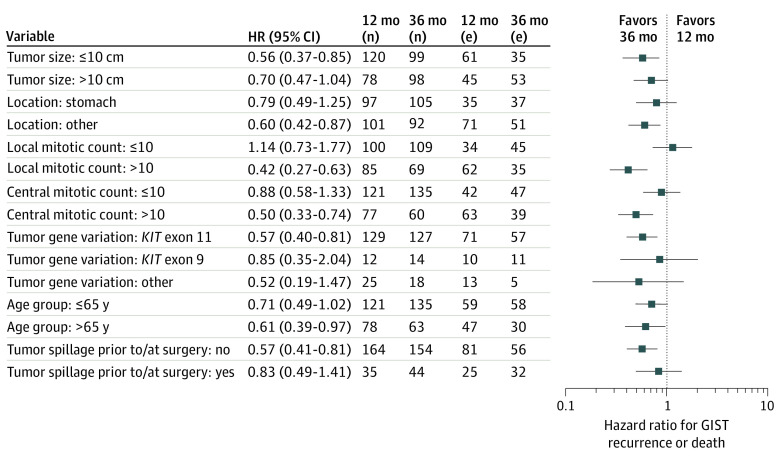
Subgroup analyses of RFS generally favored 36 months of treatment in the ITT population (Figure 2). Patients with high tumor mitotic counts benefited more from the longer treatment than patients with smaller counts. The findings were generally similar when OS was analyzed instead of RFS (eFigure 2 in Supplement 2).
Figure 2. Recurrence-Free Survival in Prespecified Subgroups.
GIST indicates gastrointestinal stromal tumor; (n) indicates the number of patients; (e) indicates the number of events (either GIST recurrence or death).
Survival After GIST Recurrence
We performed an exploratory analysis not defined in the study protocol or in the statistical analysis plan to investigate the duration of survival from the date of GIST recurrence to the date of death or the last follow-up visit in the subset of 175 patients whose GIST recurred during the follow-up (12-month group, 94; 36-month group, 81). Participant OS was similar in the 2 groups after the date of GIST recurrence (12-month group, median survival 6.7 years; 36-month group, 6.4 years; HR, 1.00; 95% CI, 0.62-1.61; P > .99). Most patients in each group survived at least for 5 years after the date of GIST recurrence (12-month group, 60.8%; 36-month group, 66.9%).
Cardiac Safety and Second Cancers
Similar numbers of cardiac events were recorded in the groups. Twelve (6.2%) of the 194 evaluable patients in the 12-month group and 10 (5.1%) of the 198 patients in the 36-month group had 1 or more cardiac events (eTable 2 in Supplement 2). Only 1 patient died from cardiac failure.
Twenty-four (12.1%) and 34 (17.2%) patients in the 12-month group and in the 36-month group, respectively, were diagnosed with cancer other than GIST during the follow-up. The most commonly detected cancer was prostate cancer (7 patients in each group), followed by basal cell carcinoma of the skin (12-month group, 3; 36-month group, 1), and melanoma (12-month group, 3; 36-month group, 1). Other types of cancers were detected in 3 or fewer individuals. Seven (3.5%) and 8 (4.0%) patients in the 12-month and the 36-month groups, respectively, died from cancer other than GIST.
Discussion
Adjuvant imatinib administered for 3 years to patients with a high risk of GIST recurrence after macroscopically complete surgery was associated with prolonged RFS and OS. The HRs for OS observed suggest that the 3-year treatment may prevent approximately one half of deaths during the first decade of follow-up compared with 1-year of imatinib. In the 2 earlier analyses of the SSGXVIII/AIO clinical trial the point estimates of the HR for OS were roughly similar as in the current analysis,4,6 but the statistical significance was only borderline, possibly owing to a shorter follow-up time and fewer events in the analyses.
Neither a clinical trial that compared 1 year of adjuvant imatinib to 1 year of placebo, nor a trial that compared 2 years of adjuvant imatinib to observation found an association between imatinib treatment with prolonged OS.2,3,5 In an analysis consisting of pooled individual patient data from 10 population-based GIST series where each patient was treated with surgery alone without adjuvant therapy, the 10-year RFS was 40% in the subset of patients with localized high-risk GIST defined with the modified NIH risk criteria,10 which is a similar 10-year RFS rate as we found in this study when the patients were treated with surgery plus 1 year of adjuvant imatinib. Taken together, these data suggest that to obtain a survival benefit the duration of adjuvant imatinib needs to be relatively long.
Despite the unfavorable GIST prognostic features, OS was high particularly in the 36-month group. Participant OS after GIST recurrence was similar in the 2 groups, and the median survival time with overtly metastatic GIST exceeded 6 years. This duration of survival does not compare unfavorably to patient series treated with first-line imatinib and without adjuvant imatinib,11 suggesting that adjuvant imatinib may not markedly impair the efficacy of subsequent TKI treatments. This hypothesis needs, however, to be viewed with caution owing to confounding factors, such as availability of novel effective treatments for advanced GIST.12,13 The longitudinal imaging of the study patients may have resulted in early detection of recurrences, potentially leading to a lead time bias and slow emergence of gene mutations that confer drug resistance.
No new safety signals were detected. Some findings suggest that imatinib is cardiotoxic, sometimes associated with congestive heart failure,14 but only 1 patient was diagnosed with cardiac failure. The most commonly diagnosed second cancer was prostate cancer, but only 1 patient died from prostate cancer.
It is unknown whether longer than 3 years of adjuvant imatinib administration confers benefits compared with 3 years of imatinib treatment. Two randomized clinical trials that compare either 5 years or 6 years of adjuvant imatinib to 3 years of imatinib treatment (NCT02413736 and NCT02260505, respectively) are accruing patients.
Limitations
The 10-year median follow-up time may still be too short for the full evaluation of adjuvant imatinib. Recurrence-free survival events continued to occur throughout the follow-up. The dose of imatinib administered may have been too low for patients with KIT exon 9 mutation,15 which is a potential study limitation. Seventeen patients were lost to follow-up.
Conclusions
These findings suggest that 3 years of adjuvant imatinib is superior in efficacy compared with 1 year of imatinib in the treatment of patients with GIST with a high risk of GIST recurrence after macroscopically complete surgery, when the risk is estimated using the modified NIH criteria.9,10 This analysis suggests that approximately 50% of deaths could be avoided during the first 10 years of follow-up after surgery with the longer treatment.
Trial Protocol and Statistical Analysis Plan
eTable 1. Key Baseline Patient And GIST Characteristics
eTable 2. Patients With a Cardiac Event
eFigure 1. CONSORT Diagram
eFigure 2. Overall Survival in Prespecified Subgroups
Data Sharing Statement
References
- 1.von Mehren M, Joensuu H. Gastrointestinal stromal tumors. J Clin Oncol. 2018;36(2):136-143. doi: 10.1200/JCO.2017.74.9705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dematteo RP, Ballman KV, Antonescu CR, et al. ; American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team . Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104. doi: 10.1016/S0140-6736(09)60500-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casali PG, Le Cesne A, Poveda Velasco A, et al. Time to definitive failure to the first tyrosine kinase inhibitor in localized GI stromal tumors treated with imatinib as an adjuvant: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup randomized trial in collaboration with the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol. 2015;33(36):4276-4283. doi: 10.1200/JCO.2015.62.4304 [DOI] [PubMed] [Google Scholar]
- 4.Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272. doi: 10.1001/jama.2012.347 [DOI] [PubMed] [Google Scholar]
- 5.Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32(15):1563-1570. doi: 10.1200/JCO.2013.51.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joensuu H, Eriksson M, Sundby Hall K, et al. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol. 2016;34(3):244-250. doi: 10.1200/JCO.2015.62.9170 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network guidelines Version 4.2019. https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf. Accessed April 20, 2020.
- 8.Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. ESMO/European Sarcoma Network Working Group. Ann Oncol. 2018;29(suppl 4):iv267. doi: 10.1093/annonc/mdy320 [DOI] [PubMed] [Google Scholar]
- 9.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39(10):1411-1419. doi: 10.1016/j.humpath.2008.06.025 [DOI] [PubMed] [Google Scholar]
- 10.Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265-274. doi: 10.1016/S1470-2045(11)70299-6 [DOI] [PubMed] [Google Scholar]
- 11.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26(4):620-625. doi: 10.1200/JCO.2007.13.4403 [DOI] [PubMed] [Google Scholar]
- 12.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329-1338. doi: 10.1016/S0140-6736(06)69446-4 [DOI] [PubMed] [Google Scholar]
- 13.Demetri GD, Reichardt P, Kang YK, et al. ; GRID study investigators . Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295-302. doi: 10.1016/S0140-6736(12)61857-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerkelä R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12(8):908-916. doi: 10.1038/nm1446 [DOI] [PubMed] [Google Scholar]
- 15.Debiec-Rychter M, Sciot R, Le Cesne A, et al. ; EORTC Soft Tissue and Bone Sarcoma Group; Italian Sarcoma Group; Australasian GastroIntestinal Trials Group . KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42(8):1093-1103. doi: 10.1016/j.ejca.2006.01.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. Key Baseline Patient And GIST Characteristics
eTable 2. Patients With a Cardiac Event
eFigure 1. CONSORT Diagram
eFigure 2. Overall Survival in Prespecified Subgroups
Data Sharing Statement