Key Points
Question
Is savolitinib monotherapy more effective than sunitinib monotherapy on progression-free survival (PFS) in patients with MET-driven, unresectable and locally advanced, or metastatic papillary renal cell carcinoma (PRCC)?
Findings
In this phase 3, open-label, randomized clinical multicenter study including 60 patients with MET-driven PRCC, the primary end point was PFS. Although study enrollment was closed early, PFS was not statistically different for patients who received savolitinib or sunitinib, and the safety profile was superior with savolitinib.
Meaning
Further investigation of savolitinib as a treatment option for MET-driven PRCC is warranted.
This randomized clinical trial examines the effect of savolitinib monotherapy compared with sunitinib monotherapy on progression-free survival in patients with MET-driven, unresectable and locally advanced, or metastatic papillary renal cell carcinoma.
Abstract
Importance
Papillary renal cell carcinoma (PRCC) is the most common type of non–clear cell RCC. Because some cases of PRCC are MET-driven, MET inhibition could be a targeted treatment approach. In previous studies, savolitinib (AZD6094, HMPL-504, volitinib), a highly selective MET-tyrosine kinase inhibitor, demonstrated antitumor activity in this patient group.
Objective
To determine whether savolitinib is a better treatment option for this patient population, vs standard of care, sunitinib.
Design, Setting, and Participants
The SAVOIR phase 3, open-label, randomized clinical trial was a multicenter study carried out in 32 centers in 7 countries between July 2017 and the data cutoff in August 2019. Overall, 360 to 450 patients were to be screened to randomize approximately 180 patients. Patients were adults with MET-driven (centrally confirmed), metastatic PRCC, with 1 or more measurable lesions. Exclusion criteria included prior receipt of sunitinib or MET inhibitor treatment. Overall, 254 patients were screened.
Interventions
Patients received 600 mg of savolitinib orally once daily (qd), or 50 mg of sunitinib orally qd for 4 weeks, followed by 2 weeks without treatment.
Main Outcomes and Measures
The primary end point was progression-free survival (PFS, assessed by investigator and confirmed by blinded independent central review). Secondary end points included overall survival (OS), objective response rate (ORR), duration of response, and safety/tolerability.
Results
At data cutoff, 60 patients were randomized (savolitinib n = 33; sunitinib n = 27); most patients had chromosome 7 gain (savolitinib, 30 [91%]; sunitinib, 26 [96%]) and no prior therapy (savolitinib, 28 [85%]; sunitinib, 25 [93%]). For savolitinib and sunitinib, 4 (12%) and 10 (37%) patients were women, and the median (range) age was 60 (23-78) and 65 (31-77) years, respectively. Following availability of external data on PFS with sunitinib in patients with MET-driven disease, study enrollment was closed. Progression-free survival, OS, and ORR were numerically greater with savolitinib vs sunitinib. Median PFS was not statistically different between the 2 groups: 7.0 months (95% CI, 2.8-not calculated) for savolitinib and 5.6 months (95% CI, 4.1-6.9) for sunitinib (hazard ratio [HR], 0.71; 95% CI, 0.37-1.36; P = .31). For savolitinib and sunitinib respectively, grade 3 or higher adverse events (AEs) were reported in 14 (42%) and 22 (81%) of patients and AE-related dose modifications in 10 (30%) and 20 (74%). After discontinuation, 12 (36%) and 5 (19%) of patients on savolitinib and sunitinib respectively, received subsequent anticancer therapy.
Conclusions and Relevance
Although patient numbers and follow-up were limited, savolitinib demonstrated encouraging efficacy vs sunitinib, with fewer grade 3 or higher AEs and dose modifications. Further investigation of savolitinib as a treatment option for MET-driven PRCC is warranted.
Trial Registration
ClinicalTrials.gov Identifier: NCT03091192
Introduction
Kidney cancer is among the 10 most common cancers worldwide and is expected to account for 73 750 new cases in the US alone in 2020.1 Over 90% of kidney tumors are renal cell carcinoma (RCC), which consists of several heterogeneous subtypes with highly variable clinical courses and outcomes.2,3 Clear cell RCC (ccRCC) accounts for approximately 75% of all RCC and more than 80% of metastatic RCC; therefore, clear cell is the dominant histologic steering development of systemic therapies for all RCC subtypes.3,4,5 Over the past 2 decades, critical genomic findings such as the early loss of the VHL tumor suppressor gene in ccRCC paved the way for development of targeted therapies, which have improved outcomes in patients with ccRCC.6,7 Conversely, non-ccRCC (nccRCC) subtypes vary widely in their cytologic and molecular abnormalities.5 When treated with approved therapies for ccRCC, outcomes in nccRCC are demonstrably worse.3,4,8 This is presently the case for the dominant nccRCC subtype papillary RCC (PRCC), which accounts for approximately 15% of all RCC.9,10,11
Papillary RCC is histologically subclassified into the more indolent type-1 PRCC and aggressive type-2 PRCC; however, these classifications have poor consensus among pathologists, and molecular/genetic analyses appear to have greater utility.9,10,12 Type-1 PRCC has been associated with amplification of the MET gene on chromosome 7q31, which is thought to drive disease.9,10 Hereditary MET variations are rare but have been characterized and found to manifest as multifocal, bilateral type-1 PRCC tumors.13 The MET gene encodes a receptor tyrosine kinase that, in the tumor setting, drives proliferation, angiogenesis, and metastatic seeding.14 Aberrant MET activation may occur through genetic alterations, including: gain of chromosome 7; focal amplification of either MET or its ligand hepatocyte growth factor (HGF); or hyperactivating MET kinase domain variations.9,15 The MET gene has been found to be a major chromosome-level alteration in 81% of type-1 PRCC but also 46% of type-2 PRCC, whereas less common somatic variations in the kinase domain occur in 13% of all PRCC.9,16,17
Savolitinib (AZD6094, HMPL-504, volitinib) is a potent and selective MET inhibitor under investigation in several malignant diseases. Savolitinib was advanced for clinical development based on promising single-agent activity in 2 patient-derived xenograft murine models of PRCC.18 In the first-in-human phase 1 study of savolitinib in 48 patients with advanced solid tumors, 3 patients experienced a partial response (PR).19 All 3 had PRCC and were retrospectively determined to have MET-driven disease. In a phase 2 study of savolitinib in 109 patients with PRCC, 8 of 44 patients (18%) who were determined to have MET-driven disease showed an objective response (all PR).20 No patients with MET-independent PRCC responded. Results from this study justified the investigation of savolitinib in a randomized clinical trial of MET-driven, locally advanced, or metastatic PRCC.20
Sunitinib, is an oral multikinase inhibitor approved for the treatment of advanced RCC. Sunitinib is considered the standard-of-care treatment option in PRCC.21,22,23 The activity of sunitinib in PRCC was previously reported in a single-arm study in the first-line setting: median progression-free survival (PFS), was 6.6 months (95% CI, 2.8-14.8) in type-1 PRCC and 5.5 (95% CI, 3.8-7.1) in type 2. Median overall survival (OS) was 17.8 (95% CI, 5.7-26.1) months and 12.4 (95% CI, 8.2-14.3) months in type 1 and type 2, respectively.24 Despite limited responses to vascular endothelial growth factor tyrosine kinase inhibitors (VEGF-TKIs) like sunitinib observed in these phase 2 studies, there are no proven treatments approved specifically for PRCC.
Here, we report the results of SAVOIR (NCT03091192), a phase 3, open-label, randomized clinical multicenter study to assess the efficacy and safety of savolitinib vs sunitinib in patients with MET-driven, unresectable, and locally advanced or metastatic PRCC.
Methods
Patients
The study included adult patients (≥18 years) who had MET-driven, unresectable, and locally advanced/metastatic histologically confirmed PRCC.25 An MET-driven tumor was defined as presence of any of the following molecular alterations, in the absence of co-occurring FH or VHL variations: chromosome 7 gain, MET amplification, MET kinase domain variations, or HGF amplification.26
The trial protocol is available in Supplement 1. For inclusion and exclusion criteria, see the eMethods in Supplement 2.
Study Design and Treatment
In this sponsor-blinded study, patients were randomized in a 1:1 ratio to receive treatment with 600 mg of oral savolitinib (or 400 mg if <50 kg) once daily, given continuously, or 50 mg of oral sunitinib once daily in 6-week cycles of 4 weeks of treatment followed by 2 weeks without treatment. Patients were stratified based on the International mRCC Database Consortium risk-group criteria27 using the number of predefined risk factors to assign patients into favorable, intermediate, or poor prognostic groups, as well as whether they were treatment-naïve or previously treated with or without a VEGF-TKI. The investigational agent savolitinib was provided by the trial sponsor, AstraZeneca. The comparator sunitinib was purchased from Pfizer, Inc.
Efficacy was assessed by imaging every 6 weeks (computed tomographic [CT] or magnetic resonance imaging [MRI]), corresponding to the start of each treatment cycle, and then every 12 weeks after the first year, until disease progression as defined by Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). All scan results were read by blinded independent central review (BICR) after notification of progressive disease (PD) by the investigator.
The study was approved by the independent institutional review board associated with each study center. The study was performed in accordance with the Declaration of Helsinki and was consistent with International Conference on Harmonisation/Good Clinical Practice guidelines, applicable regulatory requirements, and the AstraZeneca policy on bioethics and human biologic samples. Written informed consent was obtained from all participants.
End Points and Analysis
The primary end point was duration of PFS, defined as the interval between dates of randomization and first documentation of disease progression (assessed by investigator using RECIST 1.1 criteria and confirmed by BICR) or death, regardless of whether the patient withdrew from randomized therapy or received another anticancer therapy prior to progression. Secondary end points included OS, disease control rate (BICR), objective response rate (ORR), duration of response (DOR), and best percentage change in tumor size (all assessed by BICR using RECIST 1.1 criteria). For health-related quality of life methods and results, see eMethods and eResults in Supplement 2.
Statistical Analysis
Approximately 360 to 450 patients were planned to be screened, to randomize approximately 180 patients; however, recruitment to the study was closed prematurely on November 22, 2018. Further statistical methods are in eMethods in Supplement 2. Statistical calculations were performed with SAS statistical software (version 9.4. SAS Institute, Inc). Analysis of the data occurred between September 13, 2019 and October 29, 2019. The statistical analysis plan is available in Supplement 3.
Results
Study Population
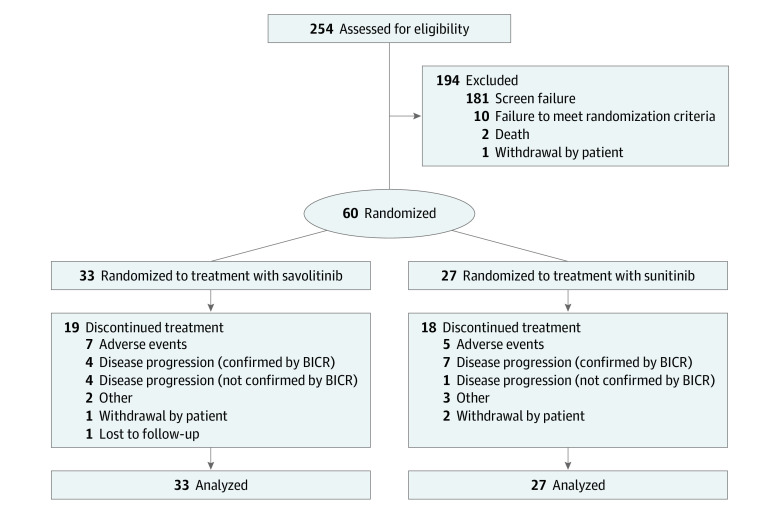
From July 2017 to November 2018, 254 patients were enrolled for screening; of these, a total of 60 (female n = 14, 23%) were randomized to either savolitinib (33 patients) or sunitinib (27 patients) treatment (Figure 1). For savolitinib and sunitinib, 4 (12%) and 10 (37%) patients were women, and the median (range) age was 60 (23-78) and 65 (31-77) years, respectively. The treatment groups were generally well-balanced (Table 1) (eTable 1 in Supplement 2). All patients randomized to savolitinib received 600 mg of savolitinib once daily. As of data cutoff, 23 (38%) patients were in ongoing treatment: 14 (42%) in the savolitinib group and 9 (33%) in the sunitinib group. Race and ethnicity were recorded by the investigator during screening to monitor the distribution of race and ethnicity across treatment groups, and to allow identification of race- or ethnicity-related treatment effects.
Figure 1. Patient Disposition.
BICR indicates blinded independent central review. Two patients were included based on investigator’s assessment of papillary renal cell carcinoma subtype, which did not receive positive confirmation on ad hoc revision of histopathologic analysis, as per protocol. These patients remained in the analysis set (1 patient in each group).
Table 1. Demographic Characteristics.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Savolitinib, 600 mg (n = 33) | Sunitinib, 50 mg (n = 27) | Total (n = 60) | |
| Age, median (range), y | 60 (23-78) | 65 (31-77) | 62 (23-78) |
| Sex | |||
| Male | 29 (88) | 17 (63) | 46 (77) |
| Female | 4 (12) | 10 (37) | 14 (23) |
| Race | |||
| White | 29 (88) | 23 (85) | 52 (87) |
| Black | 1 (3) | 1 (4) | 2 (3) |
| Asian | 2 (6) | 3 (11) | 5 (8) |
| Other | 1 (3) | 0 | 1 (2) |
| Countrya | |||
| France | 1 (3) | 0 | 1 (2) |
| Italy | 2 (6) | 3 (11) | 5 (8) |
| Russia | 7 (21) | 9 (33) | 16 (27) |
| Ukraine | 12 (36) | 5 (19) | 17 (28) |
| South Korea | 2 (6) | 3 (11) | 5 (8) |
| United States | 3 (9) | 0 | 3 (5) |
| Brazil | 6 (18) | 7 (26) | 13 (22) |
| IMDC risk group | |||
| Poor | 4 (12) | 3 (11) | 7 (12) |
| Intermediate | 22 (67) | 17 (63) | 39 (65) |
| Favorable | 7 (21) | 7 (26) | 14 (23) |
| Line of therapy | |||
| 1st line | 28 (85) | 25 (93) | 53 (88) |
| ≥2nd line with prior VEGF-TKI | 3 (9) | 0 | 3 (5) |
| ≥2nd line without prior VEGF-TKI | 2 (6) | 2 (7) | 4 (7) |
| Histology subtype | |||
| Type 1 | 10 (30) | 7 (26) | 17 (28) |
| Type 2 | 11 (33) | 10 (37) | 21 (35) |
| Unspecified | 10 (30) | 10 (37) | 20 (33) |
| Missing | 2 (6) | 0 | 2 (3) |
| Karnofsky performance status | |||
| 100% | 11 (33) | 4 (15) | 15 (25) |
| 90% | 15 (45) | 16 (59) | 31 (52) |
| 80% | 7 (21) | 7 (26) | 14 (23) |
| SAVOIR CTA-specific MET-driven (BICR)b | |||
| MET amplification | 1 (3) | 1 (4) | 2 (3) |
| HGF amplification | 1 (3) | 0 | 1 (2) |
| MET variation | 2 (6) | 3 (11) | 5 (8.3) |
| Chromosome 7 gain | 30 (91) | 26 (96) | 56 (93) |
Abbreviations: BICR, blinded independent central review; CTA, clinical trial assay; HGF, hepatocyte growth factor; IMDC, Independent Data Monitoring Committee; VEGF-TKI, vascular endothelial growth factor receptor tyrosine kinase inhibitor.
Patients were enrolled in 57 study centers, and of these, 32 study centers had patients randomized.
Patients can be counted in more than 1 subtype group for MET driven by SAVOIR CTA.
The low number of randomized patients was owing to this trial being halted prematurely because a concurrent retrospective molecular epidemiology study on the outcomes of patients with MET-driven PRCC on sunitinib suggested that MET-driven status did not appear to be a negative predictive factor for treatment outcomes.28 It was therefore concluded that the trial would be unlikely to detect a difference in efficacy between the treatment groups, and a decision was made to terminate recruitment.
Efficacy
For the primary end point of PFS, there were 17 of 33 patients (52%) with progression events in the savolitinib group vs 20 of 27 (74%) in the sunitinib group (hazard ratio [HR], 0.71; 95% CI, 0.4-1.4) (Figure 2A), which was not statistically significant (P = .31). The median PFS was 7.0 months (95% CI, 2.8 to not calculated [NC]) in the savolitinib group and 5.6 months (95% CI, 4.1-6.9) in the sunitinib group.
Figure 2. Kaplan-Meier Curves.
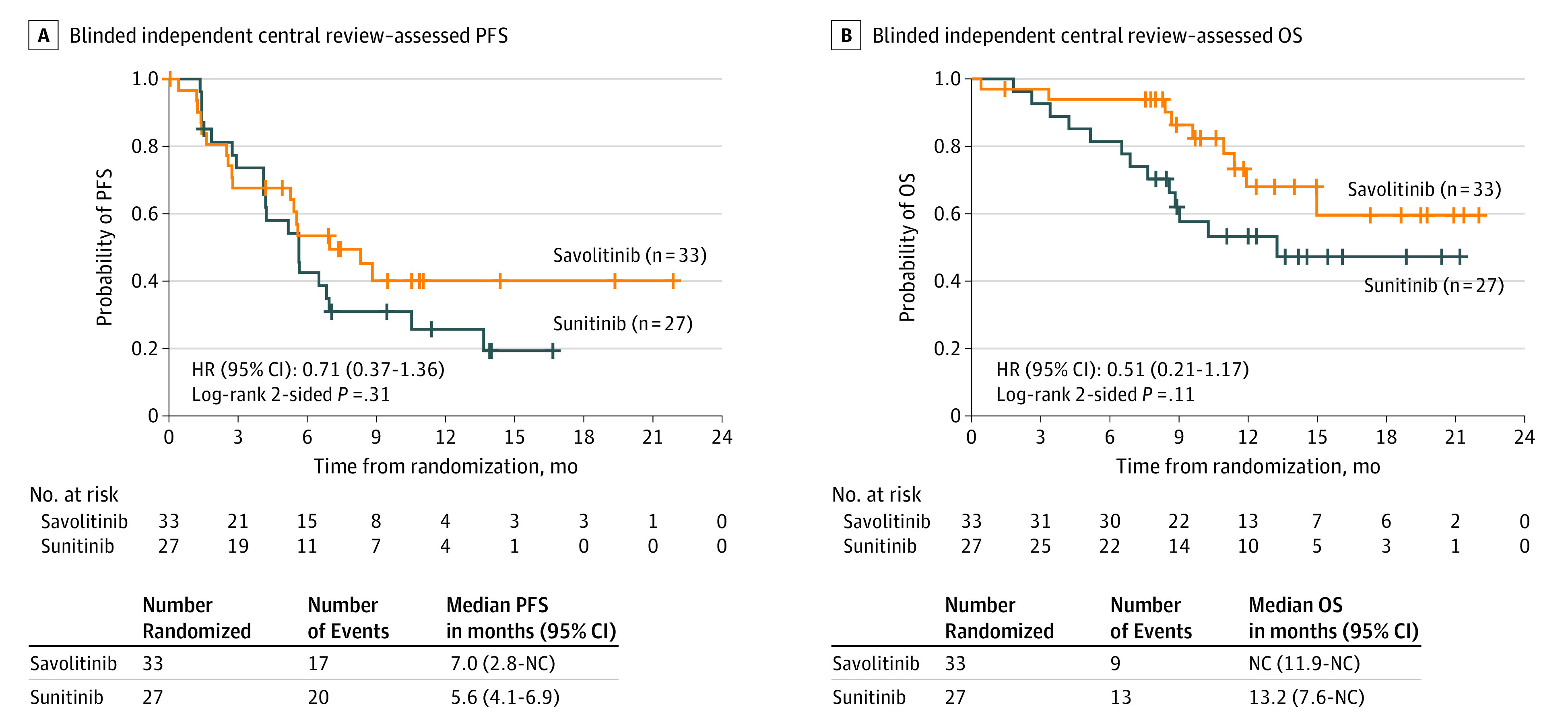
BICR indicates blinded independent central review; HR, hazard ratio; NC, not calculated; OS, overall survival; PFS, progression-free survival.
Nine patients (27%) from the savolitinib group died, vs 13 (48%) with sunitinib. The median OS was not reached for the savolitinib group (95% CI, 11.9-NC) and 13.2 months (95% CI, 7.6-NC) for the sunitinib group. The observed OS HR was 0.51 (95% CI, 0.2-1.2; P = .11) (Figure 2B). The ORR showed 9 of 33 (27%) patients (95% CI, 13.3-45.5) in the savolitinib group had a response, compared with 2 of 27 (7%) patients (95% CI, 0.9-24.3) in the sunitinib group; all responses were partial. Efficacy outcomes stratified by prognostic groups can be found in eTable 2 in Supplement 2.
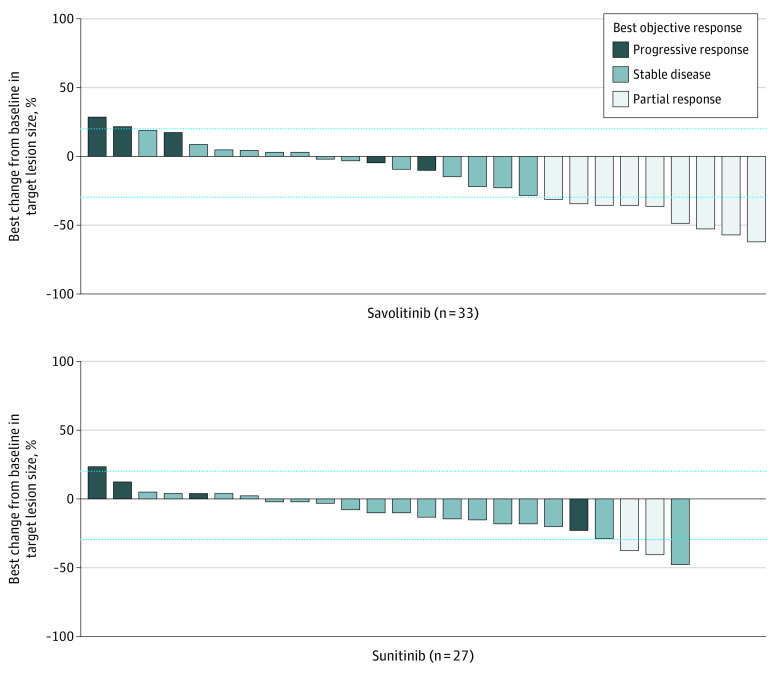
As of the data cutoff of August 19, 2019, no responding patients in the savolitinib group had disease progression, compared with 1 of 2 responding patients in the sunitinib group. It was not possible to calculate median DOR from the data because there were too few events; 3 responders treated with savolitinib were followed for more than 6 months after onset of response. For disease control rate, at the 6-month time point, there were 16 of 33 (48%) and 10 of 27 (37%) patients in the savolitinib and sunitinib groups, respectively, and at the 12-month time point there were 10 of 33 (30%) and 6 of 27 (22%) patients in the savolitinib and sunitinib groups, respectively. More patients in the savolitinib group showed a decrease in target lesion size, particularly those with PR to therapy (Figure 3).
Figure 3. Target Lesion Size, Best Percentage Change Waterfall Plot by BICR In 27 Patients Treated With Savolitinib and 24 Treated With Sunitiniba.
BICR indicates blinded independent central review.
aNine patients (savolitinib n = 6; sunitinib n = 3) were not included in the target lesion size plot: no target lesions present at baseline that were selected as target lesions for the purpose of BICR assessment (n = 7: savolitinib n = 5, sunitinib n = 2); no postbaseline target lesion assessment captured (savolitinib n = 1; sunitinib n = 1).
All time-to-events analyses should be interpreted with caution because of the limited number of patients enrolled and the limited follow-up due to the study’s premature termination.
Safety
Adverse events (AEs) of any cause occurred in 30 of 33 (91%) of the savolitinib group and 100% of the sunitinib group (eTable 3 in Supplement 2). Twenty-two patients died during the study; with 3 deaths attributed to AEs, all in the sunitinib group. In the savolitinib group, AEs led to discontinuation in 6 (18%) patients, vs 5 (19%) in the sunitinib group. Exposure was similar between groups: median total treatment duration of 7.6 (lower-upper quartile, 1.8-9.3) months in the savolitinib group vs 5.7 (lower-upper quartile, 3.7-12.0) months in the sunitinib group.
The most common AEs with savolitinib were peripheral edema (11 [33%]), increased creatinine levels (9 [27%]), aspartate aminotransferase increased (8 [24%]), and alanine aminotransferase increased (8 [24%]); with sunitinib, the most common AEs were anemia (12 [44%]), nausea (9 [33%]), decreased appetite (8 [30%]), thrombocytopenia (7 [26%]), and palmar-plantar erythrodysesthesia syndrome (7 [26%]) (Table 2) (eTable 4 in Supplement 2). Of note, thrombocytopenia and/or neutropenia was seen in 10 (37%) patients who received sunitinib vs no patients who received savolitinib. There was a similar proportion of serious AEs (SAEs) reported in both treatment groups: 8 [24%] vs 8 [30%] for savolitinib and sunitinib, respectively, with none reported by more than 1 patient and no notable differences between the groups.
Table 2. Most Common Adverse Events (AEs) Independent of Causality, Reported in 20% or More of Patients in Either Treatment.
| Variable | No. (%) | |||
|---|---|---|---|---|
| Savolitinib, 600 mg (n = 33) | Sunitinib, 50 mg (n = 27) | |||
| All | Grade ≥3 | All | Grade ≥3 | |
| Any AE | 30 (91) | 14 (42) | 27 (100) | 22 (81) |
| Alanine aminotransferase increased | 8 (24) | 5 (15) | 3 (11) | 2 (7) |
| Anemia | 2 (6) | 0 | 12 (44) | 4 (15) |
| Aspartate aminotransferase increased | 8 (24) | 4 (12) | 5 (19) | 2 (7) |
| Blood creatinine increased | 9 (27) | 0 | 2 (7) | 0 |
| Cough | 4 (12) | 0 | 6 (22) | 0 |
| Decreased appetite | 1 (3) | 0 | 8 (30) | 1 (4) |
| Diarrhea | 0 | 0 | 6 (22) | 1 (4) |
| Dyspnea | 7 (21) | 1 (3) | 4 (15) | 0 |
| Hypertension | 1 (3) | 0 | 6 (22) | 4 (15) |
| Nausea | 2 (6) | 0 | 9 (33) | 0 |
| Edema peripheral | 11 (33) | 0 | 3 (11) | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 0 | 0 | 7 (26) | 0 |
| Thrombocytopenia | 0 | 0 | 7 (26) | 2 (7) |
| Yellow skin | 0 | 0 | 4 (15) | 0 |
Of patients who discontinued treatment (19 [58%] vs 18 [67%] for savolitinib and sunitinib, respectively), the most common reason in both groups was disease progression, whether or not this was confirmed by BICR. There were fewer patients with a dose interruption in the savolitinib arm (9 [27%]) than in the sunitinib arm (15 [56%]). Most cases of dose interruption across both treatments were due to AEs: 8 (24%) and 15 (52%) in the savolitinib and sunitinib arms, respectively. After discontinuation, 12 patients (36%) taking savolitinib and 5 patients (19%) taking sunitinib received subsequent anticancer therapy (eTable 5 in Supplement 2).
Discussion
There remains an urgent unmet need for effective therapies in PRCC. Herein we have shown that, in a limited number of patients, savolitinib was associated with a superior safety and tolerability profile and numerically improved efficacy compared with sunitinib. However, premature termination of the study and the limited number of patients randomized is a key limitation and makes definitive conclusions on safety and efficacy difficult to draw.
In this study, the primary end point was PFS. There was no statistically significant difference between the PFS times for the 2 treatments, though PFS rates were numerically higher at months 6, 9, and 12 in the savolitinib group than in the sunitinib group, hence some separation seen in the Kaplan-Meier curves, beyond approximately 6 months. However, it should be noted that the number of patients at risk at these points was low. In addition, savolitinib was associated with a numerically better survival rate (with early separation of the Kaplan-Meier curves), a higher ORR, and a higher proportion of disease control at both the 6- and 12-month time points. Sunitinib performed in line with previous prospective studies of patients with PRCC unselected for MET status.21,22,24,28
Though the proportion of patients reporting an AE was similar in both treatment regimens, more patients in the sunitinib group reported AEs that were possibly treatment related, as well as AEs that were grade 3 or higher. Similarly, around a quarter of patients receiving savolitinib, vs over half of patients on sunitinib, had a treatment interruption. In both cohorts, most interruptions were due to AEs; however, the proportion was lower in the savolitinib group than the sunitinib group. Lastly, it is noteworthy that more patients discontinued sunitinib than savolitinib and more patients from the savolitinib arm received a subsequent therapy, vs the sunitinib arm. This is possibly owing to savolitinib having a better tolerability profile than sunitinib, so patients were more likely to be able to tolerate further treatment. Treatment exposure was similar between the 2 groups. However, as with the efficacy outcomes, the low patient numbers must be taken into consideration when comparing these findings. In addition, the treatment regimen should be considered: here, sunitinib was received once daily for 4 weeks, followed by 2 weeks without treatment. Elsewhere, a schedule of 2 weeks of treatment, followed by 1 week without, has been associated with improved safety outcomes.29
When SAVOIR was conceived, there was a dearth of data on the natural disease history and/or epidemiology of MET-driven PRCC. A crucial assumption for this study was that MET-driven status in PRCC is a negative predictor for treatment outcomes on sunitinib.30,31 The molecular epidemiology study was initiated for this reason. The analysis for that study indicated that the PFS of patients with PRCC treated with sunitinib was longer than anticipated in the SAVOIR power calculation. The SAVOIR protocol specified that a new power calculation should be performed when the results of molecular epidemiology study became available. With the longer PFS, a much larger study would be needed, so it was decided to stop recruitment for SAVOIR. In addition, because the molecular epidemiology study suggested a trend toward more favorable sunitinib treatment outcomes in MET-driven vs MET-independent metastatic PRCC, it was decided that the SAVOIR study would not benefit from a reassessment of study size given the expected similarity in efficacy between the 2 treatment groups.20,40 Due to the study’s early termination, the percentage of screened patients who were randomized was lower than the planned target of 40%, which was roughly the incidence of MET-driven disease from the phase 2 study.20 In addition, another key difference between SAVOIR and the phase 2 study was in the definition of MET-driven disease, as here, patients with VHL and FH variations were excluded.
Previous studies have tested MET-targeted therapy in patients with PRCC: the first prospective trial to do so was the phase 2 open-label biomarker study of the oral multikinase inhibitor foretinib in 74 patients.32 Though the trial failed to reach its response rate end point of 25%, when patients were stratified by MET status, 5 of 10 patients (50%) with a germline MET variation experienced a response compared with 5 of 57 (9%) without a germline MET variation (all PR). Somatic MET variations (1/5; 20%), MET amplification (0/2; 0%), and chromosome 7 gain (1/18; 5%) did not correlate with activity. Crizotinib, another oral multikinase inhibitor, was employed in a phase 2, open-label, biomarker study testing for efficacy in patients with MET-driven type-1 PRCC.33 The investigators defined MET driven as a variation in exons 16 to 19 of the MET gene, and because only patients with centrally confirmed diagnoses of type-1 PRCC were eligible, only 4 MET-driven patients were treated, of whom 2 experienced PR (50%). Importantly, the study also raised the question of the role of MET amplification in a post hoc analysis. From a safety standpoint, crizotinib’s most frequent AE was edema (47.8%). Importantly, both foretinib and crizotinib have significant polypharmacology with other kinases in addition to MET.34,35
Results of earlier studies in MET-driven PRCC raise the question of optimal study population for targeting MET. Response rates to MET-targeted therapy in patients with MET variations were higher than those with chromosome 7 copy number alterations or MET amplifications.20 However, these patients are rare in an already rare subgroup of MET-driven PRCC: only 5 (8%) patients in this study had an MET variation, whereas most (56, 93%) had gain of chromosome 7. It is therefore plausible that a narrower definition of MET-driven status would identify patients who experience significant benefit with savolitinib therapy; however, this raises new difficulties in trial design and recruitment and would only benefit a minority of patients.
In the SWOG S1500, multiarm, phase 2 trial originally comparing cabozantinib, crizotinib, savolitinib, and sunitinib in patients with metastatic PRCC (not selected for MET status), the savolitinib arm was closed early and is yet to report.36 A phase 1/2 study of savolitinib in combination with the anti–PD-L1 antibody, durvalumab, in patients with metastatic PRCC showed 27% ORR (n = 41)37; importantly, MET-driven status (defined by immunohistochemical analysis) was not associated with a significantly higher ORR (40%).38 However, given the involvement of dysregulated pathways beyond the MET pathway and the growing importance of combination therapies in metastatic RCC,39 perhaps savolitinib could be investigated as part of an effective treatment strategy in this patient population: using an increased number of patients, MET confirmation with next-generation sequencing, and a longer follow-up period to better assess the combination.
It should be noted that using MET as a biomarker remains challenging because different testing methods detect different subsets of patients with MET-based disease, and it is therefore unclear which biomarker is the best predictor for sensitivity to MET-targeted therapies.25
Limitations
Premature termination of the study and the limited number of patients randomized are key limitations of this study and make definitive conclusions on safety and efficacy difficult to draw.
Conclusions
Overall, in SAVOIR, early termination of recruitment precludes definitive conclusions from being drawn owing to the small data set. Though none of the study end points reached significance, the limited efficacy data favored savolitinib over sunitinib in this study, and savolitinib showed a superior safety and tolerability profile. Though the retrospective molecular epidemiology study suggested that MET-driven status did not appear to be a negative predictive factor for treatment outcomes, our clinical findings suggest differently and thus, given the potential to improve treatment for MET-driven PRCC with savolitinib, a new study of the same population is being considered at this time.
Trial Protocol
eMethods
eResults
eTable 1. Extent of disease at baseline
eTable 2. Progression-Free Survival And Overall Survival Outcomes Stratified By Prognostic Groups
eTable 3. Safety Outcomes
eTable 4. Most Common Adverse Events Independent of Causality, Reported in Over 10% of Patients in Either Treatment
eTable 5. Post-Discontinuation Disease-Related Anticancer Therapy
eReferences
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60(4):615-621. doi: 10.1016/j.eururo.2011.06.049 [DOI] [PubMed] [Google Scholar]
- 3.Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. doi: 10.1038/nrdp.2017.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vera-Badillo FE, Templeton AJ, Duran I, et al. Systemic therapy for non-clear cell renal cell carcinomas: a systematic review and meta-analysis. Eur Urol. 2015;67(4):740-749. doi: 10.1016/j.eururo.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 5.Albiges L, Flippot R, Rioux-Leclercq N, Choueiri TK. Non-clear cell renal cell carcinomas: from shadow to light. J Clin Oncol. 2018;36:3624-3631. doi: 10.1200/JCO.2018.79.2531 [DOI] [PubMed] [Google Scholar]
- 6.Linehan WM. Genetic basis of kidney cancer: role of genomics for the development of disease-based therapeutics. Genome Res. 2012;22(11):2089-2100. doi: 10.1101/gr.131110.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376(4):354-366. doi: 10.1056/NEJMra1601333 [DOI] [PubMed] [Google Scholar]
- 8.Bellmunt J, Dutcher J. Targeted therapies and the treatment of non-clear cell renal cell carcinoma. Ann Oncol. 2013;24(7):1730-1740. doi: 10.1093/annonc/mdt152 [DOI] [PubMed] [Google Scholar]
- 9.Linehan WM, Spellman PT, Ricketts CJ, et al. ; Cancer Genome Atlas Research Network . Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374(2):135-145. doi: 10.1056/NEJMoa1505917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akhtar M, Al-Bozom IA, Al Hussain T. Papillary renal cell carcinoma (PRCC): an update. Adv Anat Pathol. 2019;26(2):124-132. doi: 10.1097/PAP.0000000000000220 [DOI] [PubMed] [Google Scholar]
- 11.Graham J, Wells JC, Donskov F, et al. Cytoreductive nephrectomy in metastatic papillary renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol Oncol. 2019;2(6):643-648. doi: 10.1016/j.euo.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong ECL, Di Lena R, Breau RH, et al. Morphologic subtyping as a prognostic predictor for survival in papillary renal cell carcinoma: Type 1 vs. type 2. Urol Oncol. 2019;37(10):721-726. doi: 10.1016/j.urolonc.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 13.Haas NB, Nathanson KL. Hereditary kidney cancer syndromes. Adv Chronic Kidney Dis. 2014;21(1):81-90. doi: 10.1053/j.ackd.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Xia M, Jin K, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17(1):45. doi: 10.1186/s12943-018-0796-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon HM, Lee J. MET: roles in epithelial-mesenchymal transition and cancer stemness. Ann Transl Med. 2017;5(1):5. doi: 10.21037/atm.2016.12.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albiges L, Guegan J, Le Formal A, et al. MET is a potential target across all papillary renal cell carcinomas: result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res. 2014;20(13):3411-3421. doi: 10.1158/1078-0432.CCR-13-2173 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt L, Junker K, Nakaigawa N, et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene. 1999;18(14):2343-2350. doi: 10.1038/sj.onc.1202547 [DOI] [PubMed] [Google Scholar]
- 18.Schuller AG, Barry ER, Jones RD, et al. The MET inhibitor AZD6094 (savolitinib, HMPL-504) induces regression in papillary renal cell carcinoma patient-derived xenograft models. Clin Cancer Res. 2015;21(12):2811-2819. doi: 10.1158/1078-0432.CCR-14-2685 [DOI] [PubMed] [Google Scholar]
- 19.Gan HK, Millward M, Hua Y, et al. First-in-human phase I study of the selective MET inhibitor, savolitinib, in patients with advanced solid tumors: safety, pharmacokinetics, and antitumor activity. Clin Cancer Res. 2019;25(16):4924-4932. doi: 10.1158/1078-0432.CCR-18-1189 [DOI] [PubMed] [Google Scholar]
- 20.Choueiri TK, Plimack E, Arkenau HT, et al. Biomarker-based phase II trial of savolitinib in patients with advanced papillary renal cell cancer. J Clin Oncol. 2017;35(26):2993-3001. doi: 10.1200/JCO.2017.72.2967 [DOI] [PubMed] [Google Scholar]
- 21.Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016;17(3):378-388. doi: 10.1016/S1470-2045(15)00515-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tannir NM, Jonasch E, Albiges L, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): a randomized multicenter phase 2 trial. Eur Urol. 2016;69(5):866-874. doi: 10.1016/j.eururo.2015.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escudier B, Porta C, Schmidinger M, et al. ; ESMO Guidelines Committee . Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):706-720. doi: 10.1093/annonc/mdz056 [DOI] [PubMed] [Google Scholar]
- 24.Ravaud A, Oudard S, De Fromont M, et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: a phase II study (SUPAP) by the French Genitourinary Group (GETUG). Ann Oncol. 2015;26(6):1123-1128. doi: 10.1093/annonc/mdv149 [DOI] [PubMed] [Google Scholar]
- 25.Hartmaier RJ, Han J-Y, Cho BC, et al. Abstract 4897: Detection of MET-mediated EGFR tyrosine kinase inhibitor (TKI) resistance in advanced non-small cell lung cancer (NSCLC): biomarker analysis of the TATTON study. Cancer Res. 2019;79(suppl 13):4897. doi: 10.1158/1538-7445.AM2019-4897 [DOI] [Google Scholar]
- 26.Frigault MM, Signoretti S, Markovets A, et al. Abstract 4541: MET gene copy number gains evaluated by NGS is more predictive than other methods to enrich for papillary RCC patients sensitive to savolitinib, a selective MET inhibitor. Cancer Res. 2018;78(suppl 13):4541. doi: 10.1158/1538-7445.AM2018-4541 [DOI] [Google Scholar]
- 27.Kroeger N, Xie W, Lee JL, et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer. 2013;119(16):2999-3006. doi: 10.1002/cncr.28151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molina AM, Feldman DR, Ginsberg MS, et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Invest New Drugs. 2012;30(1):335-340. doi: 10.1007/s10637-010-9491-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonasch E, Slack RS, Geynisman DM, et al. Phase II study of two weeks on, one week off sunitinib scheduling in patients with metastatic renal cell carcinoma. J Clin Oncol. 2018;36(16):1588-1593. doi: 10.1200/JCO.2017.77.1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moosavi F, Giovannetti E, Saso L, Firuzi O. HGF/MET pathway aberrations as diagnostic, prognostic, and predictive biomarkers in human cancers. Crit Rev Clin Lab Sci. 2019;56(8):533-566. doi: 10.1080/10408363.2019.1653821 [DOI] [PubMed] [Google Scholar]
- 31.Macher-Goeppinger S, Keith M, Endris V, et al. MET expression and copy number status in clear-cell renal cell carcinoma: prognostic value and potential predictive marker. Oncotarget. 2017;8(1):1046-1057. doi: 10.18632/oncotarget.13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choueiri TK, Vaishampayan U, Rosenberg JE, et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol. 2013;31(2):181-186. doi: 10.1200/JCO.2012.43.3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schöffski P, Wozniak A, Escudier B, et al. Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type 1 patients with MET mutations or amplification. EORTC 90101 CREATE trial. Eur J Cancer. 2017;87:147-163. doi: 10.1016/j.ejca.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 34.Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16(13):3507-3516. doi: 10.1158/1078-0432.CCR-10-0574 [DOI] [PubMed] [Google Scholar]
- 35.Ou SH. Crizotinib: a novel and first-in-class multitargeted tyrosine kinase inhibitor for the treatment of anaplastic lymphoma kinase rearranged non-small cell lung cancer and beyond. Drug Des Devel Ther. 2011;5:471-485. doi: 10.2147/DDDT.S19045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pal SK, Tangen CM, Thompson IM, et al. A randomized, phase II efficacy assessment of multiple MET kinase inhibitors in metastatic papillary renal carcinoma (PRCC): SWOG S1500. J Clin Oncol. 2017;35(suppl 15):TPS4599. doi: 10.1200/JCO.2017.35.15_suppl.TPS4599 [DOI] [Google Scholar]
- 37.Powles T, Larkin JMG, Patel P, et al. A phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO). J Clin Oncol. 2019;37(suppl 7):545. doi: 10.1200/JCO.2019.37.7_suppl.545 [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez CS, Larkin JMG, Patel P, et al. Overall survival results for durvalumab and savolitinib in metastatic papillary renal cancer. J Clin Oncol. 2020;38(suppl 6):619. doi: 10.1200/JCO.2020.38.6_suppl.619 [DOI] [Google Scholar]
- 39.Escudier B. Combination therapy as first-line treatment in metastatic renal-cell carcinoma. N Engl J Med. 2019;380(12):1176-1178. doi: 10.1056/NEJMe1900887 [DOI] [PubMed] [Google Scholar]
- 40.Albiges L, Heng DYC, Lee J-L, et al. MET status and treatment outcomes in papillary renal cell carcinoma (PRCC): pooled analysis of historical data. J Clin Oncol. 2020;38(suppl 15):e19321. doi: 10.1200/JCO.2020.38.15_suppl.e19321 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods
eResults
eTable 1. Extent of disease at baseline
eTable 2. Progression-Free Survival And Overall Survival Outcomes Stratified By Prognostic Groups
eTable 3. Safety Outcomes
eTable 4. Most Common Adverse Events Independent of Causality, Reported in Over 10% of Patients in Either Treatment
eTable 5. Post-Discontinuation Disease-Related Anticancer Therapy
eReferences
Statistical Analysis Plan
Data Sharing Statement