This phase 2 nonrandomized clinical trial evaluates the clinical activity and safety of the FOLFIRINOX regimen (leucovorin, fluorouracil, irinotecan, and oxaliplatin) as first-line treatment for patients with advanced gastroesophageal adenocarcinoma.
Key Points
Question
What is the clinical activity of FOLFIRINOX (leucovorin, fluorouracil, irinotecan, and oxaliplatin) as first-line treatment for advanced gastroesophageal adenocarcinoma?
Findings
In this single-arm phase 2 study, 41 patients with ERBB2-negative gastroesophageal cancer were treated with FOLFIRINOX alone, and 26 patients with ERBB2-positive disease were treated with FOLFIRINOX and trastuzumab. An objective response occurred in 61% of the ERBB2-negative group and 85% of the ERBB2-positive group.
Meaning
In patients with advanced gastroesophageal cancer, the FOLFIRINOX regimen appears to be an effective first-line treatment.
Abstract
Importance
Standard first-line regimens for patients with metastatic gastroesophageal adenocarcinomas have an approximate 40% objective response rate (ORR). The combination of leucovorin, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) has been efficacious as first-line therapy for other gastrointestinal cancers, such as pancreatic and colon cancers.
Objective
To evaluate the clinical activity and safety of FOLFIRINOX as first-line treatment for patients with advanced gastroesophageal adenocarcinoma.
Design, Setting, and Participants
This is an open-label, single-arm phase 2 study of first-line FOLFIRINOX in patients with advanced gastroesophageal adenocarcinoma. Estimated sample size included 41 patients with ERBB2-negative disease with 90% power to detect an ORR of 60% or greater with α of .10. No enrollment goal was planned for ERBB2-positive patients, but they were allowed to receive trastuzumab in combination with FOLFIRINOX.
Interventions
Starting doses were fluorouracil, 400 mg/m2 bolus, followed by 2400 mg/m2 over 46 hours; leucovorin, 400 mg/m2; irinotecan, 180 mg/m2; and oxaliplatin, 85 mg/m2. Trastuzumab was administered as a 6 mg/kg loading dose, followed by 4 mg/kg every 14 days in patients with ERBB2-positive disease.
Main Outcomes and Measures
The primary end point was ORR by the Response Evaluation Criteria in Solid Tumors, version 1.1. Secondary end points included safety profile, progression-free survival (PFS), overall survival (OS), and duration of response.
Results
From November 2013 to May 2018, 67 patients were enrolled (median [range] age, 59.0 [34-78] years; including 56 [84%] men), and 26 of 67 (39%) had ERBB2-positive disease. Median follow-up was 17.4 months. The ORR was 61%(95% CI, 44.5%-75.8%) (25 of 41) in the ERBB2-negative group and 85% (95% CI, 65.1%-95.6%) (22 of 26) in the ERBB2-positive group, including 1 patient with complete response. For ERBB2-negative patients, median PFS was 8.4 months and median OS was 15.5 months; for ERBB2-positive patients, median PFS was 13.8 months and median OS was 19.6 months. Fifty-six patients (84%) had dose modifications or treatment delays. The most common toxic effects were neutropenia (91%, n = 61), diarrhea (63%, n = 42), peripheral sensory neuropathy (61%, n = 41), and nausea (48%, n = 32), with no unexpected toxic effects.
Conclusions and Relevance
The FOLFIRINOX regimen with or without trastuzumab was associated with improved ORR and PFS in patients with advanced gastroesophageal adenocarcinoma in the first-line setting. This regimen may be a reasonable therapeutic option for patients with preserved performance status.
Trial Registration
ClinicalTrials.gov Identifier: NCT01928290
Introduction
Advanced gastric and esophageal malignant neoplasms are leading causes of cancer morbidity and mortality.1,2 In the US, approximately 45 000 new diagnoses and 27 000 deaths are reported annually.3 Standard first-line chemotherapy for advanced gastroesophageal cancers usually consists of a multidrug regimen, including a fluoropyrimidine and a platinum agent.4,5 While doublet chemotherapy regimens are often preferred because of lower toxicity, triplet regimens are used for patients with good performance status (PS).
Fluoropyrimidine and oxaliplatin doublet regimens, such as FOLFOX (leucovorin, fluorouracil, and oxaliplatin) or CAPOX (capecitabine and oxaliplatin), are commonly used as first-line treatment, with reported response rates of 35% to 45% and overall survival (OS) of 9 to 10 months.6,7,8,9 Irinotecan with fluorouracil has also been evaluated as a first-line regimen in phase 3 randomized clinical trials, showing noninferiority to cisplatin and fluorouracil combination with reported OS of about 9 months.10,11 Taken together, the efficacy of oxaliplatin or irinotecan in combination with fluorouracil for first-line treatment of advanced gastroesophageal cancer has been demonstrated and is reflected in the treatment guidelines.4
Triplet regimens using docetaxel combined with a platinum and fluoropyrimidine, including docetaxel, cisplatin, and fluorouracil (DCF) or docetaxel, oxaliplatin, and fluorouracil (FLOT), have been evaluated as first-line treatment options in the metastatic setting.12,13,14,15,16,17 Epirubicin, platinum, and fluoropyrimidine combinations have also been used as triplet therapy in the past8,18; however, this combination is no longer commonly used because of toxic effects and unclear efficacy improvement.19 The superiority of triplet over doublet regimens has been demonstrated, with the DCF regimen showing improved survival outcomes and response rates compared with cisplatin/fluorouracil.13 However, the DCF regimen also led to significantly higher incidence of adverse effects, including increased myelosuppression, worsened gastrointestinal toxic effects, and additional neuropathy, with up to 90% experiencing grade 3 or greater treatment-related toxic effects.13,14 Given the notable toxicity of this regimen, a randomized phase 2 study of a modified DCF (mDCF) regimen was conducted in 85 patients, which showed improved tolerability and survival compared with the conventional DCF regimen.20 The most prevalent grade 3/4 toxic effects included neutropenia, febrile neutropenia, fatigue, thromboembolism, and neuropathy. Therefore, the mDCF regimen has been considered a suitable first-line therapy option for patients with preserved PS.
The addition of trastuzumab to fluoropyrimidine and cisplatin doublet achieved superior survival outcomes and has been established as standard therapy for ERBB2 (formerly HER2)-positive metastatic gastroesophageal cancer by the ToGA trial.21 While adding ERBB2-directed therapy upon progression beyond first-line treatment has not been effective,22,23,24 several trials have since confirmed the benefit of adding trastuzumab to doublet chemotherapy for ERBB2-positive disease in the first-line setting.25,26,27,28,29 Addition of trastuzumab to epirubicin-containing triplet regimens has not been evaluated because of expected overlapping cardiotoxicity. Lapatinib, a dual tyrosine kinase inhibitor of epidermal growth factor receptor and ERBB2 with potentially lower cardiotoxicity risk, was combined with an epirubicin triplet, and this regimen did not show therapeutic benefit.30 Other trials have established the safety and clinical benefit with the addition of trastuzumab to non-epirubicin triplets in the first-line metastatic setting.31,32,33
Combinations of leucovorin, fluorouracil, irinotecan, and oxaliplatin, including the FOLFIRINOX regimen, are now increasingly used in gastrointestinal malignant neoplasms, including pancreatic and colorectal cancers.34,35,36,37,38,39,40 In pancreatic cancer, patients who received FOLFIRINOX had superior OS compared with standard gemcitabine in both metastatic and adjuvant settings.34,35 Common grade 3/4 treatment-related toxic effects included neutropenia, diarrhea, fatigue, and neuropathy.34,35 In metastatic colorectal cancer, the same combination as first-line treatment showed improved survival compared with the fluorouracil/irinotecan (FOLFIRI) regimen.36 The toxicity profile of the triplet compared with FOLFIRI showed increased grade 2/3 peripheral neurotoxicity and grade 3/4 neutropenia, making this a manageable regimen. However, the FOLFIRINOX regimen has not been prospectively evaluated in advanced gastroesophageal cancer. In this trial, we hypothesized that FOLFIRINOX would be a reasonable alternative triplet regimen, resulting in a similar response rate and survival with improved safety and tolerability for patients with advanced gastroesophageal cancer.
Methods
Study Design and Participants
This was a single-arm, phase 2 nonrandomized clinical trial (NCT01928290) performed at Siteman Cancer Center at Washington University in St Louis, Missouri. The trial protocol is provided in the Supplement. Patients with inoperable locally advanced, recurrent, or metastatic adenocarcinoma of the esophagus, stomach, or gastroesophageal junction were enrolled. Patients were required to have measurable disease as defined by the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.41 Other inclusion criteria included Eastern Cooperative Oncology Group (ECOG) PS of 2 or better and appropriate bone marrow and organ functions. Patients with unknown ERBB2 status at the time of enrollment were allowed to participate; however, patients with ERBB2-positive disease were required to have a left ventricular ejection fraction (LVEF) of 50% or greater prior to starting trastuzumab treatment. Exclusion criteria included receiving chemotherapy within 6 months prior to registration or other significant medical problems as defined by the protocol. Self-reported race/ethnicity in medical records was collected to describe patient characteristics. The protocol was approved by the institutional review board at Washington University. The trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All participants provided written informed consent prior to entering the study.
Procedures
The FOLFIRINOX regimen consisted of fluorouracil, 400 mg/m2 bolus, followed by 2400 mg/m2 infusion over 46 hours; leucovorin, 400 mg/m2; irinotecan, 180 mg/m2; and oxaliplatin, 85 mg/m2, given intravenously on days 1 and 15 of 28-day cycles. For the ERBB2-positive group, trastuzumab was given with a loading dose of 6 mg/kg for the first dose, followed by 4 mg/kg intravenously with subsequent treatments. Dose modifications for individual drugs were allowed based on toxic effect attributions. Computed tomography (CT) imaging studies were performed every 8 weeks, and the disease response was assessed by board-certified radiologists according to RECIST 1.1 prior to treatment continuation decisions. Supportive care, including administration of growth factors, was allowed. Palliative radiotherapy was not allowed because this was considered evidence of progressive disease. Patients were continued on a maintenance regimen of the treating physician’s choice once deemed to have achieved maximal response. Patients requiring dose modifications below 270 mg/m2 bolus and 1600 mg/m2 infusion fluorouracil, 120 mg/m2 irinotecan, and 50 mg/m2 oxaliplatin, or discontinuation of one of the agents without disease progression, were considered to be in maintenance and were monitored for response and survival. Patients were followed for toxic effects for up to 30 days after discontinuing the FOLFIRINOX regimen.
Outcomes
The primary objective was to determine the objective response rate (ORR) of patients treated with FOLFIRINOX for advanced gastroesophageal cancer. The ORR was defined as the proportion of patients achieving complete response and partial response as best response assessed by RECIST 1.1. Secondary end points included progression-free survival (PFS), OS, duration of response, and toxic effects and tolerability of FOLFIRINOX with or without trastuzumab. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Statistical Analyses
Based on Simon 2-stage minimax accrual design, a sample size of 41 patients with ERBB2-negative disease would provide 90% power at a 1-sided α of .10 to detect the ORR with FOLFIRINOX of 60% over historical ORR of 40%. If there were 12 or more responses in the first 28 patients, the trial was to be continued until 41 patients had been treated. There was a 55% chance to terminate the trial early if the true response rate was 40% or less. Formal sample size calculation and analysis plans were not specified for the ERBB2-positive group. All analyses were performed in the 2 cohorts separately.
Statistical analyses were performed using Stata, version 14.2 (StataCorp), and Prism, version 8 (GraphPad Software). Demographic information and baseline patient characteristics were summarized descriptively. The ORR was calculated in patients who underwent response assessment by clinical examination or at least 1 restaging CT scan after receiving FOLFIRINOX. Survival end points were evaluated in all patients who received at least 1 dose of FOLFIRINOX and described using the Kaplan-Meier product-limit method. Toxic effects and tolerability were assessed in all patients.
Results
Patients
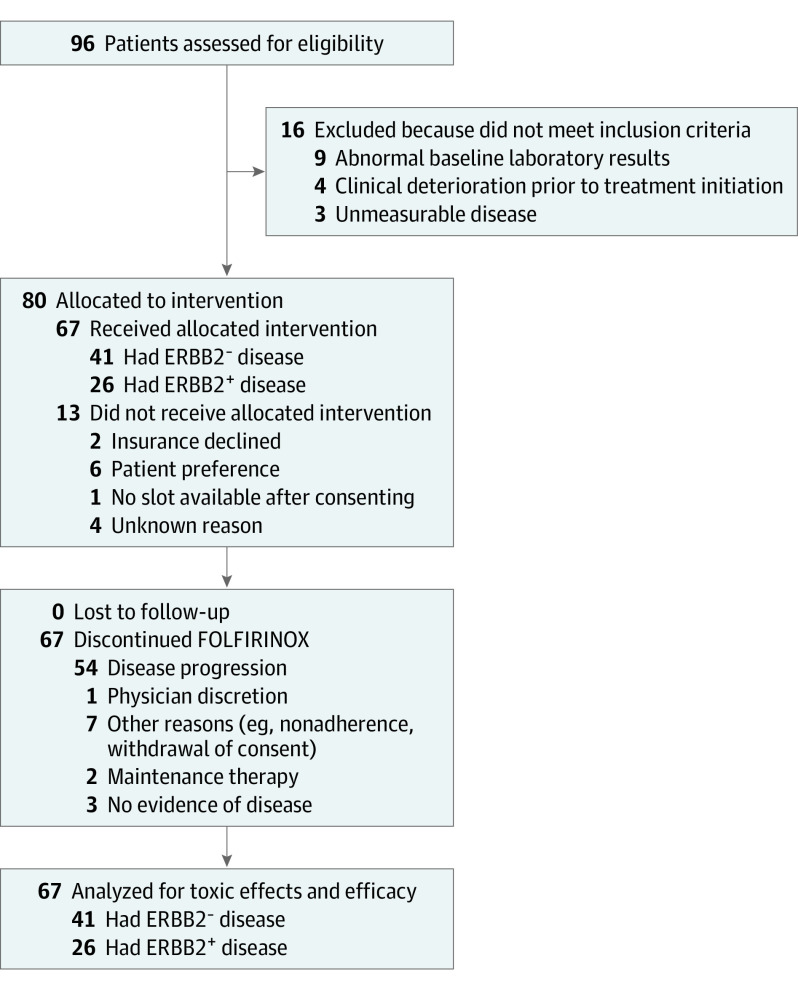
From November 2013 to May 2018, a total of 96 patients with advanced gastroesophageal adenocarcinoma provided informed consent, of which 67 were enrolled in the trial and received at least 1 dose of FOLFIRINOX. Details of patient recruitment and follow-up are reported in Figure 1 with patient characteristics summarized in Table 1. Last follow-up was in April 2020. Median follow-up time was 17.4 months (95% CI, 4.7-46.6) for all patients. Of the 67 patients enrolled, 41 patients had ERBB2-negative disease and received FOLFIRINOX; 26 had ERBB2-positive disease and received FOLFIRINOX with trastuzumab. Among the 41 patients in the ERBB2-negative group, 10 patients had esophageal cancer (24%), 10 had gastroesophageal junction cancer (24%), and 21 had gastric adenocarcinoma (51%). In the ERBB2-positive group, all 26 patients were men, and more patients had esophageal (17 [65%]) compared with gastric adenocarcinoma (6 [23%]). Most of the patients had a PS of 0 or 1 (100% of the ERBB2-negative group; 25 [96%] of the ERBB2-positive group, with 1 patient having a PS of 2). Forty-six patients remained on fluorouracil-based maintenance therapy following FOLFIRINOX treatment. Forty-three patients received at least 1 subsequent therapy after disease progression, and 15 received additional lines of therapy.
Figure 1. Study Flowchart.
Table 1. Patient Characteristics.
| Patients | No. (%) | ||
|---|---|---|---|
| All (N = 67) | ERBB2 negative (n = 41) | ERBB2 positive (n = 26) | |
| Age, median (range), y | 59.0 (34-78) | 59.0 (43-78) | 59.5 (34-73) |
| Male | 56 (84) | 30 (73) | 26 (100) |
| Female | 11 (16) | 11 (27) | 0 |
| Site of disease | |||
| Esophagus | 27 (40) | 10 (24) | 17 (65) |
| GEJ | 13 (19) | 10 (24) | 3 (12) |
| Gastric | 27 (40) | 21 (51) | 6 (23) |
| Histologic subtype | |||
| Diffuse | 18 (27) | 13 (32) | 5 (19) |
| Intestinal | 16 (24) | 9 (22) | 7 (27) |
| Not reported | 33 (49) | 19 (46) | 14 (54) |
| Presence of peritoneal disease | |||
| Yes | 14 (21) | 12 (29) | 2 (8) |
| No | 53 (79) | 29 (71) | 24 (92) |
| ECOG PS | |||
| 0 | 23 (34) | 11 (27) | 12 (46) |
| 1 | 43 (64) | 30 (73) | 13 (50) |
| 2 | 1 (2) | 0 | 1 (4) |
| Prior therapy | |||
| None (metastatic disease at diagnosis) | 59 (88) | 35 (85) | 24 (92) |
| Preoperative chemoradiation | 5 (7) | 5 (12) | 0 |
| Surgery only | 3 (4) | 1 (2) | 2 (8) |
| Subsequent maintenance therapy | |||
| None/second-line systemic therapy | 21 (31) | 16 (39) | 5 (19) |
| FOLFIRI (with or without trastuzumab) | 27 (40) | 14 (34) | 13 (50) |
| FOLFOX (with or without trastuzumab) | 11 (16) | 9 (22) | 2 (8) |
| Dose-reduced FOLFIRINOX | 2 (3) | 1 (2) | 1 (4) |
| Fluorouracil alone (with or without trastuzumab) | 3 (4) | 1 (2) | 2 (8) |
| Trastuzumab alone | 3 (4) | 0 | 3 (12) |
| FOLFIRINOX doses received, median (range) | 8.0 (1-22) | 8.0 (1-20) | 9.5 (1-22) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FOLFIRI, leucovorin, fluorouracil, and irinotecan; FOLFOX, leucovorin, fluorouracil, and oxaliplatin; FOLFIRINOX, leucovorin, fluorouracil, irinotecan, and oxaliplatin; GEJ, gastroesophageal junction; PS, performance status.
Efficacy
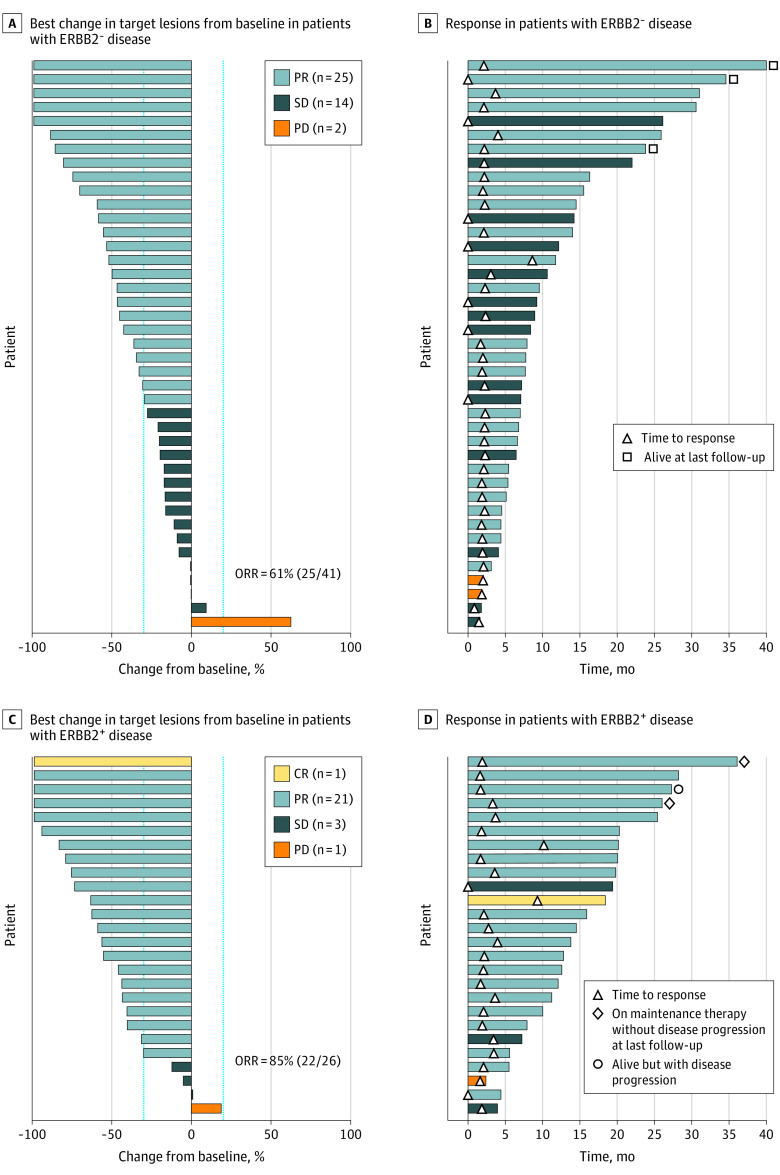
For the ERBB2-negative group, all 41 patients had at least 1 CT scan after study treatment to assess objective response. Thirty-eight patients had disease progression, and 3 were alive off treatment and did not have any evidence of disease at last follow-up. Twenty-five patients (61%) had partial response as their best radiographic response, resulting in an ORR of 61% (95% CI, 44.5%-75.8%) (Figure 2). Five patients had complete resolution of measurable target lesions as assessed by RECIST 1.1 but had persistent esophageal or gastric wall thickening noted on the imaging studies; therefore, they were considered to have had partial responses. Among the 14 patients who had stable disease as their best response, 11 had stable disease for 6 months or longer. Median PFS was 8.4 months (95% CI, 6.8-12.2), and the 12-month PFS rate was 34.2% (95% CI, 20.3-48.5). Median OS was 15.5 months (95% CI, 10.6-19.6), and the 12-month OS rate was 58.5% (95% CI, 42.1-71.8) (Figure 3). For patients who had objective responses, median duration of response was 5.8 months (95% CI, 3.5-13.5), and median time to best response was 2.1 months (95% CI, 1.7-4.0).
Figure 2. Treatment Response Among 67 Patients With ERBB2-Negative and ERBB2-Positive Advanced Gastroesophageal Cancer Treated With FOLFIRINOX.
A, Best percentage change in target lesions from baseline in patients with ERBB2-negative disease. Each bar represents a patient. Dotted lines indicate changes in target lesion size of −30% and +20%. B, Time to and duration of response in patients with ERBB2-negative disease. C, Best percentage change in target lesions from baseline in patients with ERBB2-positive disease. D, Time to and duration of response in patients with ERBB2-positive disease.
CR indicates complete response; FOLFIRINOX, leucovorin, fluorouracil, irinotecan, and oxaliplatin; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 3. Progression-Free Survival (PFS) and Overall Survival (OS) of Patients With Advanced Gastroesophageal Cancer Treated With First-line FOLFIRINOX .
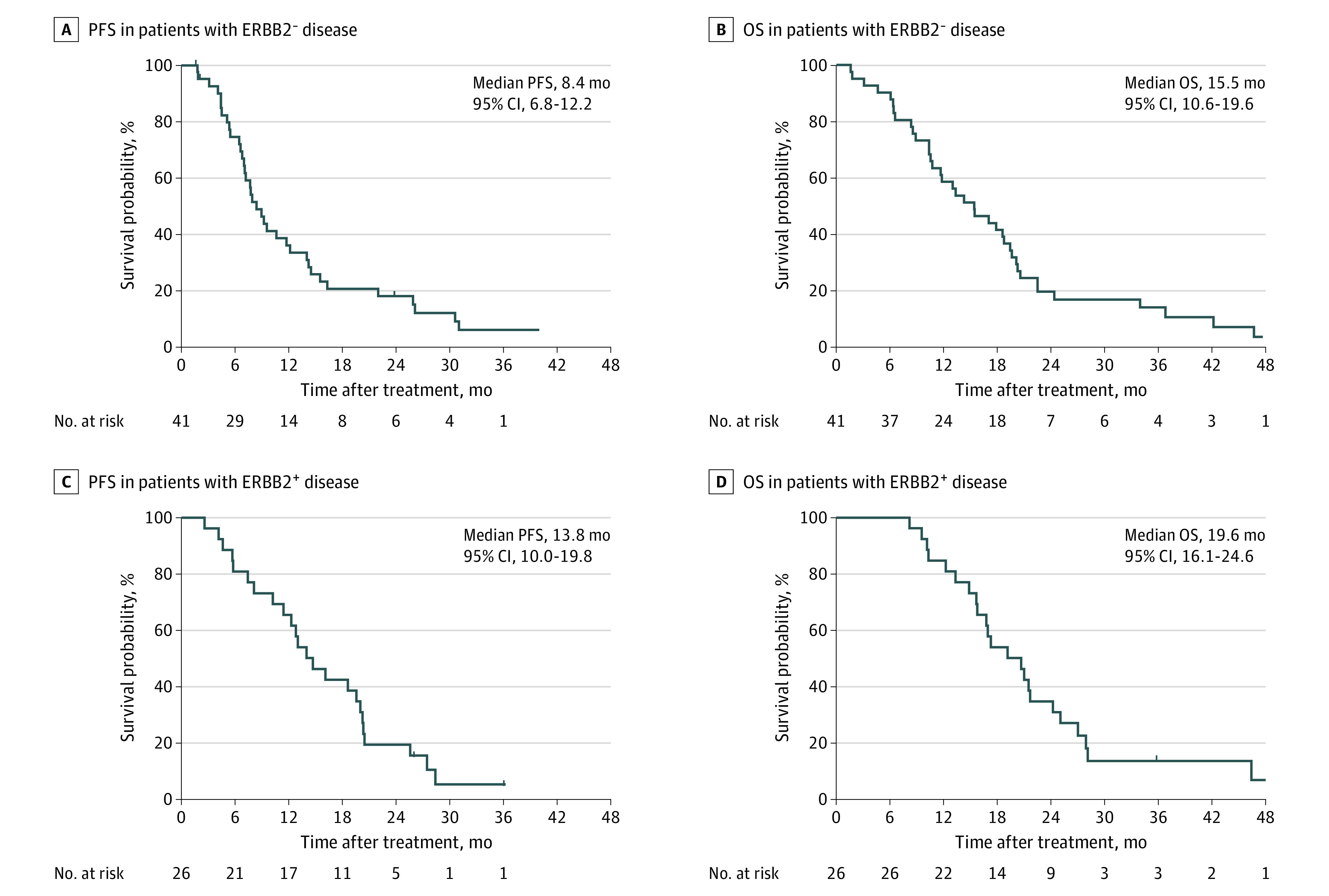
A, PFS in patients with ERBB2-negative disease. B, OS in patients with ERBB2-negative disease. C, PFS in patients with ERBB2-positive disease. D, OS in patients with ERBB2-positive disease.
FOLFIRINOX indicates a combination of leucovorin, fluorouracil, irinotecan, and oxaliplatin.
In the ERBB2-positive group, 3 patients remained alive and 25 patients had disease progression at last follow-up. One patient did not have a CT scan after study treatment; however, the patient had clinical disease progression and was considered evaluable for response. All other patients had at least 1 CT scan after study treatment. Of 26 patients, 1 had complete response and 21 had partial response; therefore, ORR was 85% (95% CI, 65.1%-95.6%) in this group. Three patients had stable disease as best response. Median PFS in the ERBB2-positive group was 13.8 months (95% CI 10.0-19.8), and the 12-month PFS rate was 65.4% (95% CI, 44.0-80.3). Median OS was 19.6 months (95% CI, 16.1-24.6), and the 12-month OS rate was 84.6% (95% CI, 64.0-93.9). For patients who had objective responses, median duration of response was 10.5 months (95% CI, 7.9-18.4), and median time to best response was 2.1 months (95% CI, 1.6-9.3).
Safety
All patients who received at least 1 treatment with FOLFIRINOX were included in the safety evaluation (n = 67) as shown in Table 2. Neutropenia was the most common hematologic toxic effect in all patients receiving FOLFIRINOX with or without trastuzumab, with 79% of all patients experiencing grade 3/4 neutropenia. Two patients in the ERBB2-negative group had febrile neutropenia; this was not seen in the ERBB2-positive group. Of those who had grade 3/4 neutropenia, 18 patients (34%) received granulocyte–colony-stimulating factor to avoid further dose reduction.
Table 2. Adverse Events Reported in 10% or More Patients During Treatment or Within 30 Days of Treatment With FOLFIRINOX Regimen.
| Adverse event | No. (%) | |||||
|---|---|---|---|---|---|---|
| All (N = 67) | ERBB2 negative (n = 41) | ERBB2 positive (n = 26) | ||||
| All | ≥Grade 3 | All | ≥Grade 3 | All | ≥Grade 3 | |
| Hematologic events | ||||||
| Neutrophil count decreased | 61 (91) | 53 (79) | 38 (93) | 36 (88) | 23 (89) | 17 (65) |
| Febrile neutropenia | 2 (3) | 2 (3) | 2 (5) | 2 (5) | 0 | 0 |
| Platelet count decreased | 5 (8) | 3 (5) | 5 (12) | 3 (7) | 0 | 0 |
| Anemia | 4 (6) | 1 (2) | 3 (7) | 1 (2) | 1 (4) | 0 |
| Nonhematologic events | ||||||
| Diarrhea | 42 (63) | 9 (13) | 27 (66) | 4 (10) | 15 (58) | 5 (19) |
| Peripheral sensory neuropathy | 41 (61) | 2 (3) | 29 (71) | 2 (5) | 12 (46) | 0 |
| Nausea | 32 (48) | 4 (6) | 23 (56) | 2 (5) | 9 (35) | 2 (8) |
| Fatigue | 30 (45) | 4 (6) | 21 (51) | 4 (10) | 9 (35) | 0 |
| Anorexia | 27 (40) | 3 (5) | 21 (51) | 3 (7) | 6 (23) | 0 |
| Alopecia | 26 (39) | 0 | 20 (49) | 0 | 6 (23) | 0 |
| Constipation | 20 (30) | 0 | 16 (39) | 0 | 4 (15) | 0 |
| Oral mucositis | 20 (30) | 0 | 13 (32) | 0 | 7 (27) | 0 |
| Vomiting | 20 (30) | 4 (6) | 16 (39) | 3 (7) | 4 (15) | 1 (4) |
| Abdominal pain | 17 (25) | 1 (2) | 11 (27) | 0 | 6 (23) | 1 (4) |
| Dizziness | 16 (24) | 0 | 13 (32) | 0 | 3 (12) | 0 |
| Rasha | 13 (19) | 0 | 12 (29) | 0 | 1 (4) | 0 |
| Flatulence | 13 (19) | 0 | 11 (27) | 0 | 2 (8) | 0 |
| Edema of the limbs | 12 (18) | 0 | 9 (22) | 0 | 3 (12) | 0 |
| Headache | 12 (18) | 0 | 10 (24) | 0 | 2 (8) | 0 |
| Cough | 11 (16) | 0 | 7 (17) | 0 | 4 (15) | 0 |
| Dyspnea | 11 (16) | 0 | 8 (20) | 0 | 3 (12) | 0 |
| Dysgeusia | 10 (15) | 0 | 5 (12) | 0 | 5 (19) | 0 |
| Infectionsb | 9 (13) | 5 (8) | 5 (12) | 3 (7) | 4 (15) | 2 (8) |
| Depression | 9 (13) | 0 | 7 (17) | 0 | 2 (8) | 0 |
| Insomnia | 9 (13) | 0 | 4 (10) | 0 | 5 (19) | 0 |
| Epistaxis | 9 (13) | 0 | 7 (17) | 0 | 2 (8) | 0 |
| Thromboembolic events | 9 (13) | 6 (9) | 7 (17) | 5 (12) | 2 (8) | 1 (4) |
| Chills | 8 (12) | 0 | 2 (5) | 0 | 6 (23) | 0 |
| Dysphagia | 7 (10) | 0 | 4 (10) | 0 | 3 (12) | 0 |
| Anxiety | 7 (10) | 0 | 5 (12) | 0 | 2 (8) | 0 |
| Hiccups | 7 (10) | 0 | 5 (12) | 0 | 2 (8) | 0 |
Abbreviation: FOLFIRINOX, leucovorin, fluorouracil, irinotecan, and oxaliplatin.
Rash includes acneiform rash, maculopapular rash, and palmar-plantar erythrodysesthesia syndrome.
Infection includes sepsis, pneumonia, upper respiratory tract infection, catheter-related infection, neutropenic enterocolitis, skin infection, and urinary tract infection.
Gastrointestinal toxic effects, including diarrhea, nausea, vomiting, and anorexia, were common. Nine patients experienced grade 3/4 diarrhea; however, the majority of these gastrointestinal toxic effects were grade 1/2. Three patients in the ERBB2-negative group and 1 patient in the ERBB2-positive group had grade 3 thromboembolic events, and 2 patients in the ERBB2-negative group experienced grade 4 thromboembolic events. Peripheral sensory neuropathy was reported in 41 patients (61%), with 27 (66%) in the ERBB2-negative group and 12 (46%) in the ERBB2-positive group. Two patients in the ERBB2-negative group and none in the ERBB2-positive group reported grade 3 neuropathy while on FOLFIRINOX treatment.
Three patients in the ERBB2-positive group reported asymptomatic reduction in LVEF by greater than 10%, including 1 patient who had greater than 20% decrease, resulting in trastuzumab being held during the course of treatment. All 3 patients had recovery in LVEF when trastuzumab was held, and 2 patients resumed trastuzumab treatment without further decrease in LVEF or dose interruption. One patient discontinued trastuzumab treatment per patient preference.
A majority of patients (56 [84%]) required dose modification during their treatment with FOLFIRINOX because of treatment-related toxic effects. Thirty-five (85%) patients in the ERBB2-negative group and 21 patients (81%) in the ERBB2-positive group had at least 1 dose modification during FOLFIRINOX treatment. The most common reasons for first dose modifications included grade 3/4 neutropenia, exhibited by 36 patients (88%) in the ERBB2-negtive group and 17 (65%) in the ERBB2-positive group; grade 3 diarrhea (4 patients); grade 3 fatigue (3 patients); and sensory neuropathy (2 patients). Reasons for second dose modifications included grade 3 (11 patients) or grade 4 (2 patients) neutropenia and grade 3 diarrhea (3 patients). Among those requiring dose modification, 31 of 56 (55%) required modification after the first dose, and an additional 12 (20%) required modification by the third dose. Fifty-five patients had their first dose modification within the first 3 months.
Discussion
There is an unmet need for efficacious, well-tolerated treatments for patients with advanced gastroesophageal cancer. We present here the final results of a phase 2 clinical trial evaluating the efficacy of the FOLFIRINOX regimen in the treatment of advanced gastroesophageal cancer and show expected tolerance with outstanding ORRs in both ERBB2-positive (85%) and ERBB2-negative (61%) groups. Moreover, among the ERBB2-negative group, 11 had prolonged stable disease after starting treatment with FOLFIRINOX. These response rates are significantly higher than historical response rates of other regimens. Survival outcomes are at least comparable to, if not better than, previously published first-line regimens15,20; median PFS was 8.4 months in the ERBB2-negative group and 13.8 months in the ERBB2-positive group, and respective median OS was 15.5 and 19.6 months. In summary, our study indicates that FOLFIRINOX is a reasonable first-line option in patients with advanced gastroesophageal cancer with good PS.
Our results cannot be directly compared with the results of previous trials, as many evaluating first-line chemotherapy included both ERBB2-positive and ERBB2-negative populations without adding trastuzumab. Superior survival outcomes in the ERBB2-positive group reported in our study compared with the ERBB2-negative group are consistent with current knowledge. To our knowledge, our study is the first clinical trial to explore a combination of trastuzumab with FOLFIRINOX in advanced gastroesophageal cancer. While we have not statistically compared ERBB2-negative and ERBB2-positive groups, toxic effects distribution appeared similar in both groups. Specifically, no additional cardiac toxic effects were reported other than those with reversible LVEF reduction, which was expected and consistent with previously reported incidences.42,43
A recent phase 2 study of perioperative FOLFIRINOX for resectable gastroesophageal cancer reported that 32 of 34 patients achieved R0 resection.44 The remarkable response rates seen in both ERBB2-negative and ERBB2-positive groups, short time to response, and overall tolerability also support the use of the FOLFIRINOX regimen in the perioperative setting. Reported ORR with the modified DCF regimen in the metastatic setting was 49%.20 The current standard of the FLOT regimen for perioperative treatment results in 25% of patients having ypT1 tumors and 49% achieving ypN0 stage.45 We anticipate that FOLFIRINOX would provide comparable results to the results of the FLOT4 trial.45 Notably, previous trials for perioperative regimens,45,46,47 including FLOT4, did not evaluate the effect of ERBB2 status or the addition of ERBB2-directed therapy. The ongoing INNOVATION trial (NCT02205047) will elucidate the role of ERBB2-directed therapy in perioperative setting.48 The ORR of 85% seen in our trial in the ERBB2-positive group supports the addition of ERBB2-directed therapy to FOLFIRINOX to enhance response rates in patients with advanced ERBB2-positive gastroesophageal cancer.
The safety and toxicity seen in our trial is comparable to previous trials of FOLFIRINOX in other cancers.34 Neutropenia and gastrointestinal toxic effects were the most common adverse events noted, as expected. Relatively low incidences of grade 3/4 sensory neuropathy was noted in our study (3% of all patients), which may be due to our practice patterns of reducing oxaliplatin dose earlier when neuropathy emerges during treatment. While neutropenia was common, febrile neutropenia was observed in only 2 patients (3%). Grade 3/4 infection was reported in 5 patients (8%). These toxicity profiles favor FOLFIRINOX over other triple-drug regimens, such as DCF or FLOT.15,20,45 The mDCF regimen was associated with 20% of patients experiencing grade 3/4 thromboembolism and 9% experiencing febrile neutropenia; FLOT was associated with 18% of patients experiencing grade 3/4 infections and 7% experiencing grade 3/4 neuropathy. It is also encouraging that toxicity profiles were similar in both patient groups with and without trastuzumab.
Limitations
We acknowledge that this was a nonrandomized single-arm trial conducted at a single academic institution. The majority of patients required FOLFIRINOX dose modifications, as is common practice currently,35,40,49,50 with either irinotecan or oxaliplatin started at a lower dose than the full dose level used in our trial. We suspect that modified doses may have been associated with lower incidences of neutropenia during the course of treatment. While toxic effects from the combination regimen would not have reduced the efficacy of the treatment, they may be negatively associated with survival time. Approximately half of the patients did not have histologic subtype reported; thus, we are not able to demonstrate whether the requirement of measurable disease at enrollment enriched for patients with nondiffuse subtypes. Even though this trial was not designed with a sample size to formally detect improved outcomes in the ERBB2-positive group, our results showed a numerically superior ORR. The addition was based on known benefit of added trastuzumab for ERBB2-positive disease21; thus, omitting trastuzumab would have potentially compromised optimal clinical care in this population.
Conclusions
We conclude that the triple-drug FOLFIRINOX regimen is associated with an impressive ORR and no unexpected toxic effects in first-line treatment of advanced gastroesophageal cancer. Moreover, it appears that trastuzumab can be safely administered in combination with FOLFIRINOX in patients with ERBB2-positive disease. Larger trials are necessary to confirm these findings. The compelling response rates seen with the FOLFIRINOX regimen may provide more rapid disease responses in locally advanced settings and a less toxic regimen compared with FLOT. A prospective study to evaluate dose-modified FOLFIRINOX with or without trastuzumab in the neoadjuvant setting is planned.
Trial Protocol
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700-713. doi: 10.1158/1055-9965.EPI-13-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(7):855-883. doi: 10.6004/jnccn.2019.0033 [DOI] [PubMed] [Google Scholar]
- 5.Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Batran SE, Hartmann JT, Probst S, et al. ; Arbeitsgemeinschaft Internistische Onkologie . Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26(9):1435-1442. doi: 10.1200/JCO.2007.13.9378 [DOI] [PubMed] [Google Scholar]
- 7.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20(4):666-673. doi: 10.1093/annonc/mdn717 [DOI] [PubMed] [Google Scholar]
- 8.Cunningham D, Starling N, Rao S, et al. ; Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom . Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36-46. doi: 10.1056/NEJMoa073149 [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26(1):141-148. doi: 10.1093/annonc/mdu472 [DOI] [PubMed] [Google Scholar]
- 10.Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19(8):1450-1457. doi: 10.1093/annonc/mdn166 [DOI] [PubMed] [Google Scholar]
- 11.Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Fédération Francophone de Cancérologie Digestive, Fédération Nationale des Centres de Lutte Contre le Cancer, and Groupe Coopérateur Multidisciplinaire en Oncologie) study. J Clin Oncol. 2014;32(31):3520-3526. doi: 10.1200/JCO.2013.54.1011 [DOI] [PubMed] [Google Scholar]
- 12.Ajani JA, Fodor MB, Tjulandin SA, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol. 2005;23(24):5660-5667. doi: 10.1200/JCO.2005.17.376 [DOI] [PubMed] [Google Scholar]
- 13.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. ; V325 Study Group . Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24(31):4991-4997. doi: 10.1200/JCO.2006.06.8429 [DOI] [PubMed] [Google Scholar]
- 14.Van Cutsem E, Boni C, Tabernero J, et al. Docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer: a randomized phase II study. Ann Oncol. 2015;26(1):149-156. doi: 10.1093/annonc/mdu496 [DOI] [PubMed] [Google Scholar]
- 15.Al-Batran SE, Hartmann JT, Hofheinz R, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2008;19(11):1882-1887. doi: 10.1093/annonc/mdn403 [DOI] [PubMed] [Google Scholar]
- 16.Al-Batran SE, Pauligk C, Homann N, et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: a randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). Eur J Cancer. 2013;49(4):835-842. doi: 10.1016/j.ejca.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 17.Ochenduszko S, Puskulluoglu M, Konopka K, et al. Comparison of efficacy and safety of first-line palliative chemotherapy with EOX and mDCF regimens in patients with locally advanced inoperable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma: a randomized phase 3 trial. Med Oncol. 2015;32(10):242. doi: 10.1007/s12032-015-0687-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Findlay M, Cunningham D, Norman A, et al. A phase II study in advanced gastro-esophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF). Ann Oncol. 1994;5(7):609-616. doi: 10.1093/oxfordjournals.annonc.a058932 [DOI] [PubMed] [Google Scholar]
- 19.Elimova E, Janjigian YY, Mulcahy M, et al. It is time to stop using epirubicin to treat any patient with gastroesophageal adenocarcinoma. J Clin Oncol. 2017;35(4):475-477. doi: 10.1200/JCO.2016.69.7276 [DOI] [PubMed] [Google Scholar]
- 20.Shah MA, Janjigian YY, Stoller R, et al. Randomized multicenter phase II study of modified docetaxel, cisplatin, and fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: a study of the US Gastric Cancer Consortium. J Clin Oncol. 2015;33(33):3874-3879. doi: 10.1200/JCO.2015.60.7465 [DOI] [PubMed] [Google Scholar]
- 21.Bang YJ, Van Cutsem E, Feyereislova A, et al. ; ToGA Trial Investigators . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697. doi: 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 22.Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640-653. doi: 10.1016/S1470-2045(17)30111-0 [DOI] [PubMed] [Google Scholar]
- 23.Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19(10):1372-1384. doi: 10.1016/S1470-2045(18)30481-9 [DOI] [PubMed] [Google Scholar]
- 24.Kagawa S, Muraoka A, Kambara T, et al. A multi-institution phase II study of docetaxel and S-1 in combination with trastuzumab for HER2-positive advanced gastric cancer (DASH study). Cancer Chemother Pharmacol. 2018;81(2):387-392. doi: 10.1007/s00280-017-3505-4 [DOI] [PubMed] [Google Scholar]
- 25.Gong J, Liu T, Fan Q, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer. 2016;16:68. doi: 10.1186/s12885-016-2092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kataoka H, Mori Y, Shimura T, et al. A phase II prospective study of the trastuzumab combined with 5-weekly S-1 and CDDP therapy for HER2-positive advanced gastric cancer. Cancer Chemother Pharmacol. 2016;77(5):957-962. doi: 10.1007/s00280-016-3013-y [DOI] [PubMed] [Google Scholar]
- 27.Kim YS, Sym SJ, Baek MY, et al. Low-dose capecitabine plus trastuzumab as first-line treatment in patients 75 years of age or older with HER2-positive advanced gastric cancer: a pilot study. Cancer Chemother Pharmacol. 2015;76(6):1267-1272. doi: 10.1007/s00280-015-2881-x [DOI] [PubMed] [Google Scholar]
- 28.Ryu MH, Yoo C, Kim JG, et al. Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer. 2015;51(4):482-488. doi: 10.1016/j.ejca.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 29.Kurokawa Y, Sugimoto N, Miwa H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer. 2014;110(5):1163-1168. doi: 10.1038/bjc.2014.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moehler M, Schad A, Maderer A, et al. ; EORTC Gastrointestinal Tract Cancer Group . Lapatinib with ECF/X in the first-line treatment of metastatic gastric cancer according to HER2neu and EGFR status: a randomized placebo-controlled phase II study (EORTC 40071). Cancer Chemother Pharmacol. 2018;82(4):733-739. doi: 10.1007/s00280-018-3667-8 [DOI] [PubMed] [Google Scholar]
- 31.Mondaca S, Margolis M, Sanchez-Vega F, et al. Phase II study of trastuzumab with modified docetaxel, cisplatin, and 5 fluorouracil in metastatic HER2-positive gastric cancer. Gastric Cancer. 2019;22(2):355-362. doi: 10.1007/s10120-018-0861-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsui Y, Sato Y, Miyamoto H, et al. Trastuzumab in combination with docetaxel/cisplatin/S-1 (DCS) for patients with HER2-positive metastatic gastric cancer: feasibility and preliminary efficacy. Cancer Chemother Pharmacol. 2015;76(2):375-382. doi: 10.1007/s00280-015-2807-7 [DOI] [PubMed] [Google Scholar]
- 33.Roviello G, Petrioli R, Nardone V, et al. Docetaxel, oxaliplatin, 5FU, and trastuzumab as first-line therapy in patients with human epidermal receptor 2-positive advanced gastric or gastroesophageal junction cancer: preliminary results of a phase II study. Medicine (Baltimore). 2018;97(20):e10745. doi: 10.1097/MD.0000000000010745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conroy T, Desseigne F, Ychou M, et al. ; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup . FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 35.Conroy T, Hammel P, Hebbar M, et al. ; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group . FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395-2406. doi: 10.1056/NEJMoa1809775 [DOI] [PubMed] [Google Scholar]
- 36.Falcone A, Ricci S, Brunetti I, et al. ; Gruppo Oncologico Nord Ovest . Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670-1676. doi: 10.1200/JCO.2006.09.0928 [DOI] [PubMed] [Google Scholar]
- 37.Cremolini C, Antoniotti C, Lonardi S, et al. Activity and safety of cetuximab plus modified FOLFOXIRI followed by maintenance with cetuximab or bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: a randomized phase 2 clinical trial. JAMA Oncol. 2018;4(4):529-536. doi: 10.1001/jamaoncol.2017.5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609-1618. doi: 10.1056/NEJMoa1403108 [DOI] [PubMed] [Google Scholar]
- 39.Oki E, Kato T, Bando H, et al. A multicenter clinical phase II study of FOLFOXIRI plus bevacizumab as first-line therapy in patients with metastatic colorectal cancer: QUATTRO study. Clin Colorectal Cancer. 2018;17(2):147-155. doi: 10.1016/j.clcc.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 40.Mizrahi JD, Rogers JE, Hess KR, et al. Modified FOLFIRINOX in pancreatic cancer patients age 75 or older. Pancreatology. 2020;20(3):501-504. doi: 10.1016/j.pan.2020.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 42.Dang C, Guo H, Najita J, et al. Cardiac outcomes of patients receiving adjuvant weekly paclitaxel and trastuzumab for node-negative, ERBB2-positive breast cancer. JAMA Oncol. 2016;2(1):29-36. doi: 10.1001/jamaoncol.2015.3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Azambuja E, Ponde N, Procter M, et al. A pooled analysis of the cardiac events in the trastuzumab adjuvant trials. Breast Cancer Res Treat. 2020;179(1):161-171. doi: 10.1007/s10549-019-05453-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catenacci DVT, Chase L, Lomnicki S, et al. Evaluation of the association of perioperative UGT1A1 genotype-dosed gFOLFIRINOX with margin-negative resection rates and pathologic response grades among patients with locally advanced gastroesophageal adenocarcinoma: a phase 2 clinical trial. JAMA Netw Open. 2020;3(2):e1921290. doi: 10.1001/jamanetworkopen.2019.21290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Batran SE, Homann N, Pauligk C, et al. ; FLOT4-AIO Investigators . Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948-1957. doi: 10.1016/S0140-6736(18)32557-1 [DOI] [PubMed] [Google Scholar]
- 46.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715-1721. doi: 10.1200/JCO.2010.33.0597 [DOI] [PubMed] [Google Scholar]
- 47.Cunningham D, Allum WH, Stenning SP, et al. ; MAGIC Trial Participants . Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11-20. doi: 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 48.Wagner AD, Grabsch HI, Mauer M, et al. EORTC-1203-GITCG—the “INNOVATION”-trial: effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer. 2019;19(1):494. doi: 10.1186/s12885-019-5675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramanathan RK, McDonough SL, Philip PA, et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J Clin Oncol. 2019;37(13):1062-1069. doi: 10.1200/JCO.18.01295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alistar A, Morris BB, Desnoyer R, et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017;18(6):770-778. doi: 10.1016/S1470-2045(17)30314-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol