Abstract
Drought events are a major challenge for many horticultural crops, including grapes, which are often cultivated in dry and warm climates. It is not understood how the cuticle contributes to the grape berry response to water deficit (WD); furthermore, the cuticular waxes and the related biosynthetic pathways are poorly characterized in this fruit. In this study, we identified candidate wax-related genes from the grapevine genome by phylogenetic and transcriptomic analyses. Developmental and stress response expression patterns of these candidates were characterized across pre-existing RNA sequencing data sets and confirmed a high responsiveness of the pathway to environmental stresses. We then characterized the developmental and WD-induced changes in berry cuticular wax composition, and quantified differences in berry transpiration. Cuticular aliphatic wax content was modulated during development and an increase was observed under WD, with wax esters being strongly up-regulated. These compositional changes were related to up-regulated candidate genes of the aliphatic wax biosynthetic pathway, including CER10, CER2, CER3, CER1, CER4, and WSD1. The effect of WD on berry transpiration was not significant. This study indicates that changes in cuticular wax amount and composition are part of the metabolic response of the grape berry to WD, but these changes do not reduce berry transpiration.
Keywords: Cuticle, fruit, transpiration, triterpenoids, Vitis vinifera (grapevine), water deficit, wax esters
Water deficit caused an increase in grape berry aliphatic cuticular wax load and changes in wax composition, yet these modifications did not reduce the berry cuticle transpiration rate.
Introduction
The plant cuticle covers all primary aerial organs forming the outermost layer of a plant’s ‘skin’, and is the interface between the plant and environment, protecting it from biotic and abiotic stresses (reviewed in Yeats and Rose, 2013). The cuticle is a specialized lipidic modification of plant cell walls, which is largely composed of a cutin polymer that acts as a macromolecular scaffold for cuticular waxes. These waxes are intercalated within the cutin polymer and deposited on the cuticle surface as epicuticular wax crystals or as a film (Yeats and Rose, 2013).
Cuticular waxes are primarily composed of very long chain (VLC) aliphatic compounds, and can also contain triterpenoids and other metabolites such as sterols and flavonoids (Bernard and Joubès, 2013). The cuticular aliphatic wax biosynthetic pathway synthesizes a range of VLC compounds, including fatty acids, primary alcohols, acyl esters (wax esters), alkanes, aldehydes, secondary alcohols, and ketones.
The first stage of cuticular aliphatic wax biosynthesis (Supplementary Fig. S1 at JXB online) involves the elongation of the fatty acyl-CoA-thioesters by the multienzyme fatty acid elongase (FAE) complex producing VLC fatty acids (VLCFAs) ranging from C18 to C34 in length. The FAE complex is composed of multiple enzymes which include a ketoacyl-CoA synthase (KCS), a β-ketoacyl-CoA reductase (KCR), a β-hydroxyacyl-CoA dehydratase, and an enoyl-CoA reductase (Haslam and Kunst, 2013a). In Arabidopsis, these four components of the FAE are encoded by the genes CER6, KCR1, PAS2, and CER10, respectively (Fiebig et al., 2000; Zheng et al., 2005; Bach et al., 2008; Beaudoin et al., 2009). Extension of VLCFAs past C28 in length requires the involvement of a subfamily of BAHD named CER2-LIKEs that function together with CER6 to produce longer acyl-CoA thioesters (Haslam et al., 2012, 2015; Haslam and Kunst, 2013a).
VLCFA-CoAs are then modified to different wax components via two parallel metabolic pathways, which form primary alcohols and alkanes as their major products. In the alcohol-forming pathway, VLCFA-CoAs are reduced to alcohols by a fatty acyl-CoA reductase (FAR), CER4 (Rowland et al., 2006). The alcohol products can then be esterified to fatty acids to generate wax esters (Li et al., 2008). In the alkane-forming pathway, two related proteins, CER1 and CER3, function together to reduce VLCFA-CoAs to aldehydes, and then convert the aldehyde intermediates to alkanes by a catalytic process that remains very poorly understood (Bourdenx et al., 2011; Bernard et al., 2012).
The cuticle protects the plant from biotic and abiotic stresses, and water deficit (WD) has been of particular interest since the cuticle restricts water loss from plant surfaces. Seo et al. (2011) showed that the up-regulation of cuticular wax biosynthetic genes (including KCS1, KCS2, KCS6, CER1, and WSD1) accompanies a major increase in wax content in Arabidopsis in response to WD. Increases in wax content in response to WD have been observed in tobacco (Cameron et al., 2006), Arabidopsis (Kosma et al., 2009), sesame (Kim et al., 2007), and poplar (Xu et al., 2016). In all four cases, alkanes were the dominant aliphatic wax in the cuticle and showed the greatest accumulation upon WD.
There are substantial differences between the cuticles of vegetative tissues and those of fleshy fruits. Fruit cuticles are usually astomatous and considerably thicker than leaf cuticles (reviewed in Martin and Rose, 2014) and affect the post-harvest quality of fruits through their role as a barrier to dehydration and pathogens (reviewed in Petit et al., 2017; Lara et al., 2019). Large compositional diversity is found between cuticles of different fruit species (reviewed in Lara et al., 2015) where compounds such as triterpenoids and flavonoids are also present, and the proportion of aliphatic waxes in the total cuticle can vary greatly, ranging from 5% in tomatoes (Domínguez et al., 2009) to 30–50% in olives (Huang et al., 2017).
The grape berry cuticle is rich in both aliphatic waxes and triterpenoids, like most fleshy fruits (Lara et al., 2015). Oleanolic acid (OA) and its precursors (erythrodiol, β-amyrin) are the major triterpenoids on grapes (Radler, 1965). The cuticular triterpenoid and aliphatic wax content varies greatly between grapevine varieties; for example, OA was 42% of the total wax content in the Muscat d’Alsace berries and 80% in Sylvaner berries (Pensec et al., 2014). Changes in cuticular waxes are possibly among the first signs of ripening in green berries as reported in a viticultural book of the 19th century (Lacoste, 1865). Interestingly, the total amount of chloroform-extracted cuticular waxes increases during early berry development and peaks before or at veraison (onset of ripening) (Rogiers et al., 2004; Pensec et al., 2014). The grape berry cuticle becomes thinner during ripening as the berry expands (Rogiers et al., 2004), resulting in a decrease in wax content per unit area (Pensec et al., 2014). The composition of berry cuticular waxes also changes with development. The relative OA content decreases with ripening as OA biosynthesis is outpaced by aliphatic wax synthesis (Pensec et al., 2014).
Despite a negative relationship between cuticle thickness and post-harvest water loss, as was found in tomato mutants (Girard et al., 2012), cuticle permeability also depends on its composition and its arrangements in various cuticle layers (Vogg et al., 2004; Leide et al., 2007; Jetter and Riederer, 2016). In the case of grape berries, both cuticular wax thickness and the water transpiration rate through the cuticle decrease over the course of berry development (Scharwies and Tyerman, 2017), which is not congruent with a simple correspondence between decreased cuticular wax thickness and increased transpiration (Rogiers et al., 2004).
Grapes are often cultivated in dry and warm Mediterranean climates and subjected to prolonged droughts that limit berry growth (reviewed in Lovisolo et al., 2016). Interestingly, genes annotated as wax ester synthases have been found to be up-regulated under WD in the grape berry (Savoi et al., 2017). While the general pattern of development of the grape berry cuticle is known, there are unanswered questions. Does the aliphatic wax composition change over development, and, if so, how? How does the cuticular wax of the grape berry change and affect water loss from the berry in response to WD stress?
In the current study we hypothesized that WD would increase the biosynthesis of cuticular aliphatic wax in the grape berry cuticle and potentially decrease berry transpiration. To determine if this is indeed the case, we first conducted in silico analyses to identify candidate grapevine cuticular wax-related genes. Then we characterized the expression profiles of cuticular wax-related genes and assessed changes in cuticular wax content during berry development and under prolonged WD. Finally, these changes were related to rates of transpiration in control and WD berries.
Materials and methods
Identification of candidate cuticular wax-related genes in grapevine and in silico RNA sequencing meta-analysis
BLASTp searches were performed using BLAST+v2.3 (Camacho et al., 2009). We used genes described in the published literature as query sequences (Supplementary Table S1) to search for (e-value cut-off: 1e−10) and identify all potential biosynthetic and transcription factor (TF) gene family members in the organism (Arabidopsis thaliana, Medicago truncatula, or Vitis vinifera) from which the query sequence came. Next, the potential family members were used as a query sequences in a second round of BLASTp searches to identify (e-value cut-off 1e−10) all potentially related homologs in grapevine and Arabidopsis. Protein sequences for Arabidopsis were retrieved from TAIR (www.arabidopsis.org) and those from grapevine were retrieved from the 12X V1 version of the genome (Jaillon et al., 2007).
For further selection of biosynthetic gene candidates, multiple sequence alignment and dendrogram construction (Fig. 1) were carried out with Phylogeny.fr (Dereeper et al., 2008). Sequences were aligned with MUSCLE (v3.8.31) using default settings. After alignment, gaps and/or poorly aligned regions were removed employing Gblocks (v0.91b) (Talavera and Castresana, 2007) using default settings, except for a minimum block length after gap cleaning of 5, and minimum number of sequences for a flank position=55%. Dendrograms were reconstructed using the maximum likelihood method implemented in the PhyML program (v3.1/3.0 aLRT) (Guindon et al., 2010) with default settings. Reliability for the internal branch was assessed using 100 bootstrap replicates. Dendrograms were drawn with TreeDyn (v198.3) (Chevenet et al., 2006).
Fig. 1.
Protein sequence phylogenetic relationships of putative grapevine (Vitis vinifera L.) homologs of Arabidopsis biosynthetic genes involved in cuticular aliphatic wax biosynthesis. PAS2 (A), KCR1 (B), CER10 (C), KCS6 (D), and CER2 (F) are involved in fatty acid elongation. CER3 and CER1 (E) are part of the alkane-forming branch, and CER4 (G) and WSD1 (H) are part of the alcohol-forming branch. BAS (I) and CYP716A (J) are part of the oleanolic acid biosynthetic pathway. Numbers represent bootstrap values (100 bootstrap replicates), with branches of values <50 being collapsed together. Bold brackets denote clades that contain the characterized Arabidopsis sequences (in bold) involved in cuticular wax synthesis, and genes that are most closely related and are the likeliest functionally related homologs. Asterisks denote genes selected for study in the water deficit experiment. Scale bars are shown in each panel.
Transcriptomic meta-analyses were conducted using publicly available data sets that examined different grapevine tissues, developmental stages, and genotypes, under several biotic and abiotic environmental stresses (Supplementary Table S2). Briefly, single-end and paired-end Illumina data sets were first processed by trimming for read quality and removal of adaptor sequences using Trimmomaticv0.36 (Bolger et al., 2014) with the following settings: LEADING, 3; TRAILING, 3; SLIDINGWINDOW, 4:15; MINLEN, 40; AVGQUAL, 20. Trimmed reads were then aligned to the PN40024 12X grapevine genome (Jaillon et al., 2007) using HISAT2v2.04 (Kim et al., 2015) with default settings. For ABI SOLiD data sets, PASSv2.30 aligner (Campagna et al., 2009) was used for quality trimming and alignment to the PN40024 12X grapevine genome with the following settings: -p 1111111001111111, -check_block 5000, -csfastq, -flc 1, -seeds_steps 3, -fid 90, -b, -l, -fle 40. Read count summarization was then performed on all the aligned reads with the grapevine V1 annotation (Jaillon et al., 2007) using featureCounts (Liao et al., 2014) on default settings.
EdgeR (Lun et al., 2016) was used to calculate fragments/reads per kilobase of transcript per million mapped reads (FPKM/RPKM) transcript abundance (for differential expression (DE) analysis comparing the developmental stages or treatments (e.g. biotic and abiotic stresses) and controls. Significant DE was determined if the false discovery rate (FDR) was <0.05.
Plant material and water deficit experiment
To assess the expression of candidate genes across grapevine tissues, we generated cDNA libraries from roots, stems, buds, apexes, leaves, tendrils, flowers, berry skins, berry flesh, and berry seeds of 2-year old Gewürztraminer (V. vinifera L.) grapevines at various developmental stages (Supplementary Table S3).
In order to investigate the impact of water deficit on cuticular waxes, a greenhouse experiment was carried out from 10 May to 5 August 2016 using 2-year-old own-rooted Merlot grapevines grown in 7.5 liter pots. Environmental light was supplemented by Phillips Green Power LED top lighting (spectra: deep red, medium blue, white lights) during the early phases of the experiment until 2 June 2016 in order to guarantee a minimum of 140 µmol m−2 s−1 of photosynthetically active radiation (PAR) at the table surface for 16 h per day. Two parallel irrigation treatments were applied, starting on 13 May at 30 days after anthesis (DAA) until the last sampling on 5 August at 113 DAA. Treatments consisted of control (CT) plants that were watered (nutrient-free water with dripper irrigation system) on a daily basis to maintain a leaf water potential above –0.8 MPa (Castellarin et al., 2007a), and WD plants that were watered as needed to maintain an average leaf water potential between –1.6 MPa and –1.8 MPa that relates to severe water deficit for grapevines (Castellarin et al., 2007a; Charrier et al., 2018). Leaf water potential was measured at 14.00 h with a Scholander pressure chamber (PMS Instrument Company) according to Castellarin et al. (2007b). A range of 2–6 fully expanded leaves per treatment were measured weekly.
Five biological replicates were considered for each irrigation treatment, and each biological replicate consisted of a group of three vines, each with 1–3 developing clusters, for a total of 30 vines. In the greenhouse, the vines were spaced ~50 cm apart, and treatments and biological replicates were spatially arranged in a randomized manner. Vines were trimmed at the 18–20th node and secondary shoots were also removed from the plants throughout the experiment to maintain constant total leaf areas. Lastly, all other growing conditions were kept consistent regardless of the treatment and were based on the standard growing practices at the UBC Horticulture Greenhouse.
Six berries were collected for cuticular wax analysis from each biological replicate at 27 DAA (pre-treatment), 41 DAA (green berries), 68 DAA (mid-veraison), 82 and 96 DAA (ripening), and 111 DAA (late ripening). Another six berries were collected from each biological replicate at 10.30 h at the same developmental stages for performing gene expression analysis. An additional two berries were sampled from each biological replicate for SEM analyses at 28 DAA (pre-treatment), 42 DAA (green berries), 72 DAA (late veraison), 83 and 96 DAA (ripening), and 113 DAA (late ripening). Finally, 20 randomly selected berries from each treatment were sampled for measuring the rate of water transpiration at 28 DAA (pre-treatment), 48 DAA (green berries), 75 DAA (late veraison), 97 DAA (ripening), and 111 DAA (late ripening). At pre-treatment (27 and 28 DAA), berry samples were collected only from CT vines. During veraison (68, 72, and 75 DAA), one set of green berries (representing berries that have not started the ripening process) and another of red berries (representing berries that have started the ripening process) were collected for each of the analyses reported above.
In order to create little to no disturbance of the cuticular wax layer and avoid wiping of waxes from the berry surface during the sampling, berries were held with tweezers through the pedicel and carefully trimmed off the cluster with a pair of scissors. The berries for wax extraction and transpiration rates were then placed in 40 ml wide mouth glass test tubes, while those for gene expression analysis were placed in zip-lock bags. Berry development was tracked by measuring berry weight of the collected samples. Berry total soluble solids (TSS) were measured from the juice of berries collected for wax and RNA analyses with a digital refractometer (Sper Scientific).
Cuticular wax extraction and quantification
Berry samples collected from the WD greenhouse experiment were brought to the laboratory just after sampling for wax extraction, which used an adapted version of the protocol described in Haslam et al. (2012) and in Haslam and Kunst (2013b). Pictures of the grape berries were taken before extraction, and their dimensions were measured using the GIMPv2.8.4 graphics editor. The two conjugate diameters of the berry were measured, and an average radius was calculated for each berry, from which the surface area was calculated with A=4πr2. The total surface area of a replicate was equal to the added surface areas of all the six berries in the replicate.
Grape berries were each submerged for 30 s with gentle swirling in 10 ml of chloroform with 10 µg of tetracosane as an internal standard. One chloroform bath for each replicate was performed using 40 ml wide mouth glass test tubes. Samples were then transferred to 11 ml glass screw-cap tubes, dried using an N2 gas stream and heat block (45 °C), and finally stored at –30 °C until ready for further processing.
Dried wax samples were resuspended in 930 µl of chloroform, after which 100 µl of the sample was aliquoted into an Agilent 250 µl vial insert and placed into an Agilent 2 ml wide opening screw-top vial. Samples where then dried under an N2 gas stream and heat block. Afterwards, 10 µl of pyridine and 10 µl of BSTFA+TMCS (99:1) were added to silylate hydroxyl and carboxylic acid groups, at 80 °C for 1 h. Samples were finally ready for GC-MS analysis after drying under an N2 gas stream and heat block, and then resuspended in 50 µl of chloroform.
For GC-MS analysis, a sample volume of 1 µl was injected into an Agilent Technologies 6890N (G1530N) GC using an Agilent Technologies J&W DB-1ms column (122-0132) with 30 m length, 250 µm diameter, and 0.25 µm film thickness. The GC used a pulsed splitless mode with a constant gas flow of helium and the following program: 45 °C for 2 min; ramp 45 °C min–1 to 210 °C; hold 1 min; ramp 5 °C min–1 to 340 °C; hold 24 min. The separated peaks were detected using an Agilent Technologies 5975 inert XL Mass Selective detector.
The GC-MS data were analyzed with Agilent Technologies MSD ChemStation E.01.01.335. Separated peaks were identified by comparing their fragmentation patterns with the Wiley Chemical Compound Library W9N08.L. Quantification was obtained by the construction of calibration curves of one representative standard for each class of compounds and using the internal standard (tetracosane) method. Hexacosane, hexacosanoic acid, hexacosanol, hexacosanal, behenyl palmitate, erythrodiol, and OA were used as representative standards for alkanes, fatty acids, alcohols, aldehydes, esters, and triterpenoids, respectively. The cuticular wax composition of each sample was then expressed in terms of wax (µg) per tissue surface area (cm2) extracted.
RNA extraction and reverse tanscription–quantitative PCR (RT–qPCR) gene expression analysis
All tissues collected for the RNA extractions were snap-frozen and stored at –80 °C. Berry skins for the WD greenhouse experiment were peeled off after snap-freezing and dropped again in liquid nitrogen. Tissues were then ground into a fine powder in liquid nitrogen using a mortar and pestle and used for the RNA extraction (Wong et al., 2016).
Total RNA was extracted from ~50 mg of powdered tissue using a Sigma Aldrich Spectrum™ Plant Total RNA Kit with the standard extraction protocol. RNA was then quantified using a Nanodrop (Thermo Scientific™ ND-1000 Spectrophotometer). Aliquots of 1 μg of RNA were digested with Thermo Scientific™ DNase I to remove genomic DNA, and cDNA was synthesized with a Thermo Scientific™ RevertAid First Strand cDNA Synthesis Kit.
Primers (Supplementary Table S4) for candidate genes were designed using the IDT PrimerQuest Tool (www.idtdna.com/primerquest) and NCBI Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast). Expression of candidate genes relative to that of VviUbiquitin and VviAP47, for the cDNA tissue library and WD experiment, respectively, was measured by qPCR (Applied Biosystems 7500 Real Time PCR System; Life Technologies 7500 Software v2.0.6) using the 2−ΔCT method (Savoi et al., 2017).
SEM
Berry sections were mounted onto SEM stubs, dried, and sputter coated using a Cressington 208HR High Resolution Sputter Coater with 12 nm Au/Pd. The images were taken using a Hitachi S-4700 Field Emission scanning electron microscope with 5 kV acceleration voltage at 10 µA and a working distance of 15 mm at a tilt of 34°. For the developmental time course, only CT berries (one berry per time point) were analyzed. For the comparison between CT and WD berries, only samples collected at 113 DAA (late ripening) were analyzed. Three biological replicates (one berry per biological replicate) per treatment were imaged. For each sample analyzed, images were taken from at least three different areas of the berry surface.
Quantification of berry transpiration
The surface area dimensions and weight of the grapes (20 berries per treatment) were measured as described above. Berries were handled with care in order to minimally disturb the cuticular wax surface, and were placed in a sealed desiccation chamber with their cut pedicels sealed with Vaseline. Sealing with Vaseline was compared with paraffin wax—previously used for similar purposes by Rogiers et al. (2004)—and was determined to be equally effective (data not shown). In the desiccation chamber, the berries were left to dehydrate in the dark at constant temperature (23 °C) and relative humidity (32%) over the course of 7–9 d, with their weights measured daily (Rogiers et al., 2004; Zhang and Keller, 2015). Relative humidity within the chamber was maintained constant with a saturated solution of MgCl2. The rate of water loss was then expressed in terms of water weight (g) per berry skin surface area (cm2) per hour as in Rogiers et al. (2004).
Statistical analysis
Two-sample Student’s t-test in Microsoft Excel v15.40 was used to determine statistical significance (*P-value <0.05) between treatments (CT versus WD) at each time point for cuticular wax concentration, gene expression values, and transpiration rates of individual berries. A univariate repeated measures ANOVA test was performed with JMP v9 (© SAS Institute Inc.) to analyze the effect of the irrigation treatments (CT and WD) on water transpiration across the cuticle.
A Pearson correlation analysis and subsequent hierarchical clustering was performed using R (R Core Team, 2019) on gene expression and cuticular wax values from the water deficit experiment. All values were log(x+1) transformed for analysis.
Results
Identification and expression profiling of grapevine cuticular wax-related genes
Forty-six putative homologs in families related to cuticular aliphatic wax biosynthesis, and 17 involved in OA biosynthesis, were chosen based on their homology to previously characterized Arabidopsis genes and used for expression analyses (Fig. 1).
We examined the expression levels and DE patterns of the putative grapevine homologs in order to narrow down the list of candidates to the likeliest functionally relevant homologs. In silico analyses of the expression patterns of the identified candidate genes were performed examining their expression across different grapevine tissues: root, leaf, inflorescence, flower, berry (whole), and berry skin (Supplementary Fig. S2). We then validated the in silico expression results of the candidate genes we selected for study by in vivo RT–qPCR analysis in different grapevine tissues, which included root, stem, bud, shoot, leaf, tendril, berry skin and flesh, and seeds (Supplementary Fig. S3). The results confirmed the in silico analysis, showing that the candidate genes were mostly expressed in berry skin and leaves, and that seeds also strongly expressed aliphatic wax-related genes.
Additional in silico analyses examined the candidate response to abiotic and biotic stresses (Supplemenrtary Fig. S4). The effects of WD on the whole-berry transcriptome was investigated for Tocai Friulano and Merlot. The general trend of WD stress was an up-regulation of genes predicted to function in cuticular wax biosynthesis, and down-regulation of those predicted to be involved in OA biosynthesis. The two cultivars responded differently to WD stress; Tocai Friulano berries showed little change in expression, while Merlot berries experienced a much stronger response. In contrast to WD stress, both high temperature stress and Botrytis cinerea infection caused down-regulation of the majority of differentially expressed homologs that we associated with cuticular wax biosynthesis (Supplementary Fig. S4).
We picked (Supplementary Fig. S5) the likeliest fruit-specific functional homologs by selecting candidate genes that were the most closely related to the characterized biosynthetic genes (Fig. 1), and showed expression in grape tissue (Supplementary Fig. S2) and/or DE response to WD in Merlot grapes (Supplementary Fig. S4). This left 20 homologs (Supplementary Table S4) involved in cuticular wax synthesis and three homologs involved in OA synthesis for study in grape berry skin under WD stress. Additionally, the top BLASTp homologs for five transcription factors and an E3 ubiquitin ligase that regulate cuticular wax synthesis were also selected for study (Supplementary Table S4).
Changes in berry cuticular wax composition during development and under water deficit
The grapevines exposed to WD consistently experienced lower leaf water potential, and WD berries were lower in weight and higher in concentration of soluble solids (Supplementary Fig. S6).
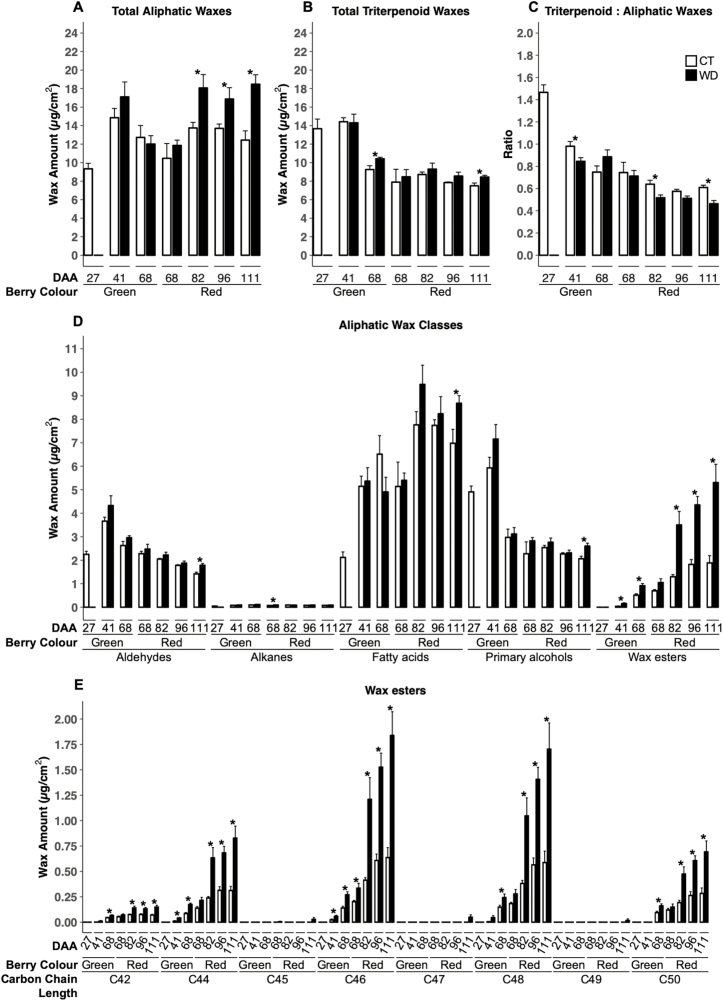
In CT berries, the aliphatic wax load per unit area (μg cm–2) (Fig. 2A) was highest at 41 DAA and then slightly decreased by the end of development (111 DAA) as the berry expanded. WD induced an increase of total aliphatic waxes at 82, 96, and 111 DAA. The triterpenoid load (Fig. 2B) followed a similar trend to that of aliphatic waxes. When the ratio of total triterpenoids to total aliphatic waxes was calculated (Fig. 2C), a clear trend was observed where the ratio was highest at 27 DAA and steadily decreased with berry development to stabilize during ripening. The ratio was lower in WD in both green and red berries.
Fig. 2.
Cuticular wax composition in berries at 27, 41, 68, 82, 96, and 111 days after anthesis (DAA) of grapevines (Vitis vinifera L.) exposed to two irrigation treatments: well-irrigated (control, CT) and deficit irrigated (water deficit, WD). Total aliphatic waxes (A), total triterpenoid waxes (B), triterpenoids:aliphatic waxes ratio (C), individual functional group classes of aliphatic waxes (D), and wax ester composition (E) are reported. Please refer to Supplementary Figs S7–S11 for detailed wax composition of other functional group classes. At 68 DAA, green and red berries were analyzed independently. Error bars represent ±SE, and significant differences between treatments were determined by two-sample t-test (*P<0.05).
When aliphatic waxes are separated into their functional classes (Fig. 2D), VLC primary alcohols and aldehydes were high during early berry development and decreased as berries ripened. In contrast, VLCFAs and wax esters increased as berries developed. Alkanes were the lowest of the aliphatic waxes and remained relatively stable throughout berry development and ripening.
Under WD treatment, alkanes were higher than in CT in red veraison berries (68 DAA), while VLCFAs, primary alcohols, and aldehydes were higher in late-ripening (111 DAA) berries. The amount of total wax esters was increased in response to WD at the majority of stages (41, 68 green, 82, 96, and 111 DAA) and involved mostly C42, C44, C46, C48, and C50 wax esters (Fig. 2E). The most abundant chain lengths of VLCFAs, aldehydes, and primary alcohols were greater in WD berries at late ripening (111 DAA) (Supplementary Figs S7–9), whereas C21, C23, and C25 alkanes were found in greater amounts only in WD berries during veraison (68 DAA) (Supplementary Fig. S10). Higher OA content was also found in WD berries during ripening (68 green, 96, and 111 DAA) (Supplementary Fig. S11).
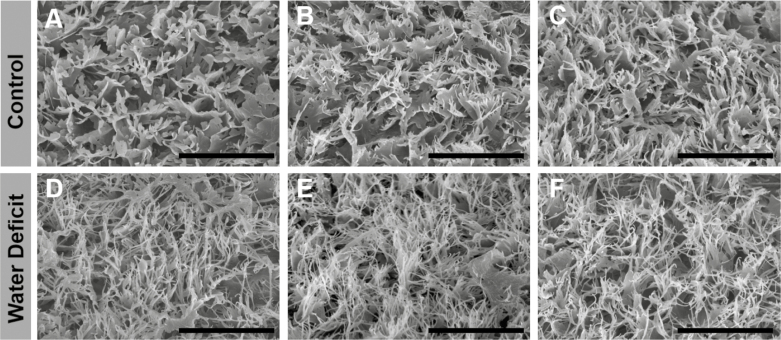
Reflecting these compositional changes, SEM images of epicuticular wax crystals showed that their structures changed with berry development (Supplementary Fig. S12). Crystal structures were also qualitatively different between treatments in late-ripening (113 DAA) berries, with CT crystals having a more ‘broad-leaf’-like shape and WD crystals having a more ‘spindly’-like shape (Fig. 3).
Fig. 3.
Ultrastructural morphology of cuticular waxes on grape berries at 113 days after anthesis (DAA). SEM images of the cuticular wax ultrastructure on grape berries of grapevines (Vitis vinifera L.) exposed to two irrigation treatments: (A–C) well-irrigated (control, CT); (D and E) deficit irrigated (water deficit, WD). Each image represents one biological replicate. The scale bar represents 5 µm in all images.
Expression of candidate genes during development and under water deficit
Almost all of the selected genes putatively involved in aliphatic wax and OA biosynthesis and regulation exhibited the same expression pattern over the course of development in CT conditions (Figs 4–6) and had high correlation [Pearson correlation coefficent (PCC) ≥0.5] to each other along with aldehyde, primary alcohol, alkane, and triterpenoid levels (Supplementary Fig. S13). The homologs experienced the highest expression levels early in berry development (27 and 41 DAA), intermediate levels at veraison (green and red berries at 68 DAA), and lowest expression levels in later ripening stages (96 and 111 DAA). The exceptions to this expression pattern included a CER1-like (VIT_15s0021g00050), a WSD1-like (VIT_15s0046g00490), and VviERF045 (VIT_04s0008g06000) that correlated into a separate group with VLCFAs and wax esters. These genes showed lower expression in early development and higher expression during ripening.
Fig. 4.
Expression of candidate genes involved in the fatty acid elongation of the biosynthetic cuticular wax pathway in berry skins of grapevines (Vitis vinifera L.) exposed to two irrigation treatments: well-irrigated (control, CT) and deficit irrigated (water deficit, WD). Error bars represent ±SE, and significant differences between treatments were determined by two-sample t-test (*P<0.05).
WD had an effect on the expression of many of the candidate genes tested. Among those involved in fatty acid elongation (Fig. 4), WD decreased the expression of three of the four KCS-like (VIT_05s0020g04540 was both down-regulated and up-regulated at different times, and VIT_15s0048g02720 was unaffected by WD), and of KCR1-like (VIT_01s0137g00180) and PAS2-like (VIT_00s0313g00040) homologs, but only in green berries. In contrast, WD increased the expression of the CER10-like (VIT_13s0019g01260) homolog and one CER2-like (VIT_18s0001g07640) homolog in green and red, and green berries, respectively.
Candidate genes associated with the alkane- and alcohol-forming pathways were affected by WD stress (Fig. 5). In green berries, the lowest (VIT_09s0018g01360) and highest (VIT_11s0037g01210) expressed CER3-like homologs were down-regulated and up-regulated by WD, respectively. CER1-like homologs experienced very low expression levels, though VIT_15s0021g00050 was up-regulated by WD in red berries. Similarly, both CER4-like homologs had very low expression, with VIT_06s0080g00120 being up-regulated by WD in green berries. All three WSD1-like homologs were affected by WD, VIT_15s0046g00480 was up-regulated in green berries, VIT_15s0046g00490 was up-regulated in both green and red berries, while VIT_15s0046g00710 was down-regulated in green berries and then up-regulated in red berries at 68 DAA.
Fig. 5.
Expression of candidate genes involved in the alcohol- and alkane-forming branches of the biosynthetic cuticular wax pathway in berry skins of grapevines (Vitis vinifera L.) exposed to two irrigation treatments: well-irrigated (control, CT) and deficit irrigated (water deficit, WD). Error bars represent ±SE, and significant differences between treatments were determined by two-sample t-test (*P<0.05).
Of the five TFs we tested that are predicted to regulate aliphatic wax biosynthesis, WD affected the expression of two (Fig. 6). DEWAX-like (VIT_16s0013g01000) was down-regulated at 96 DAA and MYB96-like (VIT_17s0000g06190) was up-regulated at both 41 and 96 DAA.
Fig. 6.
Expression of candidate genes involved in the oleanolic acid biosynthesis, and transcription factors (TFs) and an E3 ubiquitin ligase involved in regulating cuticular wax development of berry skins of grapevines (Vitis vinifera L.) exposed to two irrigation treatments: well-irrigated (control, CT) and deficit irrigated (water deficit, WD). Error bars represent ±SE, and significant differences between treatments were determined by two-sample t-test (*P<0.05).
One OA biosynthesis homolog (Fig. 6), BAS-like (VIT_09s0054g01220), was down-regulated by WD at 41 DAA and in red berries at 68 DAA.
Transpiration rate through the berry cuticle
The rate of water transpiration through the cuticle decreased as berries developed, then remained stable from 97 DAA to the end of the experiment (Fig. 7). There was no significant difference in the average rate of water transpiration (mg cm−2 h–1) through the berry cuticle between WD and CT treatments at any developmental stage tested. Moreover, when the cumulative amount of water lost per skin area (mg cm−2) at each measurement time (hours from T0), within each experiment (developmental stage: 48, 75, 97, and 111 DAA) was considered, no differences between treatments were seen.
Fig. 7.
Rates of water transpiration of the whole berry at different stages of development in grapevines (Vitis vinifera L.) exposed to two irrigation treatments: well-irrigated (control, CT) and deficit irrigated (water deficit, WD). Stages of development that were measured were 27 (A), 48 (B), 75 (C), 97 (D), and 111 (E) days after anthesis (DAA). Error bars represent ±SE; significant differences in the cumulative amount of water lost for each developmental stage were determined by univariate repeated measures ANOVA (U.R.M. ANOVA). The effects of treatment, time, and their interaction are reported. In the table (F), the average transpiration rate and the significance value of the t-test at each developmental stage are reported.
Discussion
Evolution of the wax profile during berry development
The Merlot grape berry cuticular wax has a large triterpenoid content like other fleshy fruits (Lara et al., 2015), a similar aliphatic wax class composition to other grapevine varieties (Radler, 1965), and a chain length distribution of aliphatic waxes very similar to that of other grapes (Radler, 1965) and plant species (Cameron et al., 2006; Kim et al., 2007; Kosma et al., 2009). The decrease in total wax amount and triterpenoid content observed during berry maturation is typical of grape berries (Commenil et al., 1997; Pensec et al., 2014), and is common, though not universal, among other fruit species. In contrast, tomato cuticles progressively accumulate triterpenoids throughout fruit development (Lara et al., 2015).
The onset of ripening (veraison) is a pivotal phenological stage in the regulation of the cuticular aliphatic wax biosynthetic pathway. The shift in wax composition suggests major changes in the expression of the associated biosynthetic genes occurring at veraison, which was observed. Almost all aliphatic wax and OA biosynthetic genes decreased in expression, while at the same time WSD1-like homologs and VviERF045 increased in expression. These changes in wax metabolism at the onset of ripening are consistent with several major changes of berry metabolism and in gene expression previously reported (Wong et al., 2016).
The amounts of OA and the expression of predicted biosynthetic genes early in development quickly decreased from veraison onwards, which is consistent with previous work reporting that OA accumulation occurs before veraison (Pensec et al., 2014). In addition, Fukushima et al. (2011) had functionally characterized cytochrome P450 enzymes that produced OA in grapevine, but the loci IDs that they provided (GSVIVT01032218001 and GSVIVT01032223001 for CYP716A17 and CYP716A15, respectively) do not match their characterized proteins. Based on our analyses, we propose that instead, CYP716A17 is VIT_11s0065g00130 (100% alignment) and that CYP716A15 is probably VIT_11s0065g00040 since they are a very close match.
VviERF045 has been proposed to be a key regulator in berry ripening (Palumbo et al., 2014), and has been shown, using transgenic grapevine lines, to regulate expression of several grapevine genes putatively involved in cuticular wax biosynthesis (Leida et al., 2016). Specifically, the WSD1-like homolog VIT_15s0046g00490 was up-regulated when VviERF045 was overexpressed (Leida et al., 2016). In our experiment, there was a strong correlation (PCC ≥0.5) (Supplementary Fig. S13) between the expression of VviERF045, VIT_15s0046g00490, and total wax ester and VLCFA content during berry development. This correlation supports the idea that VviERF045 is a key regulator that controls the shift in the cuticular aliphatic wax pathway towards increased VLCFA and wax ester synthesis from veraison onwards during berry development.
Changes in the berry cuticular waxes under water deficit
Our in silico transcriptomic analysis indicated that cuticular wax-related genes were differentially expressed in grape berries exposed to water stress; particularly in Merlot grapes, which showed general up-regulation of the genes associated with the aliphatic cuticular wax biosynthetic pathway, and down-regulation of genes associated with OA biosynthesis. Not all grapevine cultivars behaved in the same way. For example, Tocai Friulano grape berries exposed to WD lacked expression of cuticular wax-related genes, suggesting differences, possibly related to their native eco-physiological niche, in the cuticle response between varieties analogous to differences observed for other metabolic and physiological responses to WD (Hochberg et al., 2013a, b; Levin et al., 2019).
Prolonged WD stress resulted in smaller berries with higher total soluble solids, as previously observed (Castellarin et al., 2007a). This decrease in size was also associated with an increase in cuticular wax content. It is unlikely that this increase resulted from the decreased surface area of WD berries without changes in wax biosynthesis. If it had, we would expect to see increases in all cuticular waxes, and an unchanged total triterpenoids/total aliphatic waxes ratio between treatments. This was not the case, as the ratio was lower and some cuticular waxes were not affected by WD, such as alkanes (Supplementary Fig. S10), β-amyrin, and erythrodiol (Supplementary Fig. S11).
We observed several instances where wax compositional changes in WD berries did not correspond to the expression of the associated biosynthetic genes. Aliphatic wax content increased, while genes of the FAE complex were down-regulated. Increases in alkane content were observed at veraison, but CER3-like or CER1-like genes were not up-regulated at that point.
The most striking inconsistency was that primary alcohol content increased in late-ripening WD berries, when CER4-like expression was not detected. This indicates that primary alcohol synthesis and accumulation continued while the tested CER4-like homologs were absent. More distantly related CER4 homologs were not expressed according to our in silico analysis (data not shown), suggesting that the gene responsible for primary alcohol synthesis was not identified. We searched for any additional homologs by a BLASTp search of the V2 grapevine genome annotation (Vitulo et al., 2014), a tBLASTn search directly in the 12X grapevine genome (Jaillon et al., 2007), and tBLASTn searches of Tannat (Da Silva et al., 2013) and Merlot (Wong et al., 2016) de novo assembled transcriptomes. None of these searches found any additional homologs.
Wax esters increased under WD by >2.5 times the CT levels by harvest time. This preference for wax esters contrasts with other species where alkanes are primarily increased under WD stress (Cameron et al., 2006; Kim et al., 2007; Kosma et al., 2009; Xu et al., 2016). The up-regulation of two WDS1-like homologs before and after veraison corresponded to the times when wax ester amounts were increased in WD berries. Additionally, the strong correlation (PCC ≥0.5) (Supplementary Fig. S13) seen between wax ester content and VIT_15s0046g000490 expression make this gene the likeliest WSD1-like functional homolog.
Based on the effect of WD on their expression, the DE of MYB96-like and DEWAX-like homologs indicates that they may modulate the cuticular wax pathway under WD in the grape berry, similar to their counterparts in Arabidopsis (Oshima et al., 2013; Go et al., 2014; Cui et al., 2016; Lee et al., 2016).
Water transpiration through the berry cuticle
Cuticular aliphatic waxes have previously been demonstrated to impede water transpiration through an artificial membrane and are responsible for forming the water-impermeable barrier on grape berries, whereas OA does not contribute to berry cuticular impermeability (Grncarevic and Radler, 1971; Casado and Heredia, 1999). Thus, one would hypothesize that WD berries should have experienced a lower transpiration rate once they start to accumulate greater amounts of waxes, but instead no decrease in transpiration was observed in WD berries during ripening or at harvest. Experiments with the response to WD stress in Arabidopsis (Kosma et al., 2009; Seo et al., 2011; Patwari et al., 2019), tobacco tree (Cameron et al., 2006), and wheat (Bi et al., 2017) leaves revealed a decrease in the rate of water loss accompanying an increase of cuticular aliphatic wax amount. In contrast, the minor changes in the transpiration rate in this study suggest that the changes in cuticular wax are not associated with decreased berry water loss under water deficit.
The majority of the variables affecting water transpiration through the cuticle were controlled for in these experiments in order to have a high certainty that any water loss was through the cuticle. Water loss through berry stomata should be negligible since they are found at a much lower density than on leaves, and are sealed with cuticular wax shortly after anthesis (Palliotti and Cartechini, 2001). Additionally, the berries were kept in the dark during the transpiration experiments to induce the closure of any potentially functional stomata. Water loss through the cut pedicel was eliminated by sealing the wound.
Water loss in fruits depends on waxes (reviewed in Petit et al., 2017; Lara et al., 2019), but it does not always correlate to the total amount of cuticular waxes (López-Castañeda et al., 2010; Parsons et al., 2013). The amount of specific aliphatic waxes, such as alkanes, and the ratio of alkanes to total non-aliphatic wax compounds can have a strong effect on cuticular water loss, as seen in peppers (Parsons et al., 2013). The importance of wax composition was confirmed using tomato mutants, where a decrease in the proportion of n-alkanes of chain lengths longer than C28 and a concomitant increase in cyclic triterpenoids increased water loss (Leide et al., 2007). In contrast, in our experiment, alkanes were mostly absent from the cuticle and, based on the changes in transpiration and wax composition during development, there appears to be a strong relationship between berry water loss and VLCFA and wax ester content.
Furthermore, other components of the wax cuticle might also affect cuticular water permeability. In tomato, cutin content does not affect transpiration (Isaacson et al., 2009), but flavonoid accumulation in the cuticle appears to modulate wax deposition during ripening and subsequently cuticular water transpiration, as well as other biomechanical properties (Luque et al., 1995; España et al., 2014a). In our study, we focused on cuticular waxes and we did not consider changes in the cutin matrix. However, based to the normally observed overexpression of flavonoid genes (Castellarin et al., 2007b; Savoi et al., 2017), we might expect increased flavonoid content in response to WD; yet, the potential presence of flavonoids in the cuticle structure did not result in reduced water transpiration.
Since no significant change in berry transpiration in response to WD was observed, it brings into question the biological role of the increased wax content in WD berry cuticles. One explanation could be that the berry response is part of a systemic response (Kachroo and Robin, 2013) to WD stress, where cuticular wax load is globally increased to reduce transpiration rates, regardless of whether the change in wax load is effective on any particular organ. On a whole-plant level, this perspective is especially relevant where maximum berry transpiration levels have been shown to be ~0.07 mmol H2O m−2 s−1 (corresponding to ~0.45 mg H2O cm−2 h−1) (Zhang and Keller, 2015); such levels are much lower than those of the canopy, where rates are >50 times higher (Rogiers et al., 2009). When considering how much more canopy surface area there is relative to berries, the contribution of berry water loss to the whole plant is negligible.
Another explanation could be that the change in wax load has other important biological roles in berries, possibly protecting the berry cuticle against other unaccounted for environmental stresses.
Higher light conditions can occur on the more exposed WD clusters (Castellarin et al., 2007b), potentially increasing berry temperature. A greater wax amount could possibly result in higher light reflectance (Holmes and Keiller, 2002; Heredia-Guerrero et al., 2018) and/or heat capacity of the berry (Heredia-Guerrero et al., 2018), and thus could be reducing the higher light and temperature stress the berries would be experiencing. Alternatively, modulating wax content under WD could also help protect berries from pathogens since OA, which increased in concentration under WD, has antimicrobial properties (Pensec et al., 2014). Consistently, a correlation between resistance to B. cinerea infection and wax density/surface area on berries was found (Commenil et al., 1997), and grape response to B. cinerea involves the triggering of cutin and cuticular wax biosynthesis and affected the level of several cuticular compounds as well as of other secondary metabolites such as trans-resveratrol and gallic acid (Agudelo-Romero et al., 2015).
Effects of cuticular wax on fruit quality
Besides the role in water loss, the cuticle also affects the fruit susceptibility to pathogen infections, fruit firmness and texture, and fruit appearance (reviewed in Lara et al., 2019). The enrichment of longer chain aliphatic waxes, which we observed under WD stress, can result in larger wax crystals, and increased stiffness of the cuticle (reviewed in Heredia-Guerrero et al., 2018) and, on the contrary, wax removal leads to decreases in the elastic modulus and stiffness of fruits (Khanal et al., 2013; Tsubaki et al., 2013). A stiffer cuticle surface, which can also be due to an increase in the wax load (observed in our experiment), can make the fruit more resistant to fungal infections (reviewed in Domínguez et al., 2017). Additionally, increasing stiffness, and thus resistance to tissue expansion (reviewed in Heredia-Guerrero et al., 2018), could be a contributing factor to the berry size reduction under WD. In tomato, quantitative and qualitative changes in the cuticle, that included an increase in cuticular flavonoids and cutin depolymerization, during ripening were related to increases in stiffness and a decrease in extensibility (España et al., 2014a, b). Additionally, for tomato varieties that increase cuticle wax load and decrease transpiration rates when under drought stress, a direct relationship to increasing fruit firmness has been observed (Romero and Rose, 2019). Based on the relevance of the above parameters for wine and table grape quality, we call for more studies on the impact of cuticular changes on those parameters in grapevine.
Conclusion
Through phylogenetic and transcriptomic analyses, we identified putative grapevine homologs involved in the biosynthetic pathways for aliphatic cuticular waxes and OA. We demonstrate that normally developing berries experience a decrease in total cuticular wax (aliphatic waxes and triterpenoids combined) load per unit area with berry growth, corresponding to changes in the expression of related biosynthetic genes. During this development, aliphatic cuticular wax content changes substantially, which correlates with a major shift in gene expression centered around the onset of ripening, or veraison. Finally, we determined that genes tentatively associated with aliphatic cuticular wax biosynthesis are transcriptionally up-regulated in grape berries under WD, resulting in increased cuticular wax load with a particularly large increase in wax esters. Yet, this increase did not result in a decrease of the berry’s transpiration rate as expected, suggesting that the detected changes in cuticular wax may serve a different biological purpose for the berry.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Schematic of the aliphatic cuticular wax pathway.
Fig. S2. RPKM/FPKM heatmap of putative genes.
Fig. S3. Relative expression of the tissue atlas of the likeliest candidate genes.
Fig. S4. Differential expression heatmap of putative genes.
Fig. S5. Decision tree for selection of the likeliest candidate genes.
Fig. S6. Physiological parameters measured during the water deficit experiment.
Fig. S7. Cuticular VLCFA content of grape berries.
Fig. S8. Cuticular aldehyde content of grape berries.
Fig. S9. Cuticular primary alcohol content of grape berries.
Fig. S10. Cuticular alkane content of grape berries.
Fig. S11. Cuticular triterpenoid content of grape berries.
Fig. S12. SEM images of cuticular wax crystals during berry development.
Fig. S13. Pearson correlation heatmap of gene expression and wax content.
Table S1. Characterized genes involved in cuticular wax biosynthesis and regulation.
Table S2. RNA-seq data sets used for in silico analysis.
Table S3. Grapevine tissues used for cDNA library for in vivo validation.
Table S4. Primer sequences used for RT–qPCR.
Acknowledgements
This work was supported by the Natural Science and Engineering Research Council of Canada (10R23082) and the Canada Research Chair (950-230913) program. The authors are very grateful to the BC Wine and Grape Council for providing the Merlot cuttings, to Melina Biron for assistance in the greenhouse experiment, to Jedrzej Gozdzik and Reinhard Jetter for donating the hexacosanal standard, to Lina Madilao for helping with the GC-MS analyses, and to Derrick Horne at the UBC Bioimaging Facility for help with the scanning electron microscopy. The authors declare that they have no conflict of interests.
Glossary
Abbreviations
- ABA
abscisic acid
- BAS
β-amyrin synthase
- CER10
an ECR
- CER2
BAHD acyltransferase
- CER2-LIKE1
ECERIFERUM2-LIKE1
- CER2-LIKE2
ECERIFERUM2-LIKE2
- CER4
fatty acyl-CoA reductase
- CER6
a KCS
- CT
control
- DAA
days after anthesis
- DE
differential expression
- ECR
enoyl-CoA reductase
- ER
endoplasmic reticulum
- FAAR
fatty acyl-CoA reductase
- FAE
fatty acid elongase complex
- FPKM
fragments per kilobase of transcript per million mapped reads
- HCD
β-hydroxyacyl-CoA dehydratase
- KCR
β-ketoacyl-CoA reductase
- KCS
ketoacyl-CoA synthase
- OA
oleanolic acid
- PAS2
an HCD
- RPKM
reads per kilobase of transcript per million mapped reads
- TF
transcription factor
- VLC
very long chain
- VLCFA
very long chain fatty acid
- WD
water deficit
- WSD1
wax ester synthase/acyl-CoA:diacylglycerol acyltransferase
References
- Agudelo-Romero P, Erban A, Rego C, Carbonell-Bejerano P, Nascimento T, Sousa L, Martínez-Zapater JM, Kopka J, Fortes AM. 2015. Transcriptome and metabolome reprogramming in Vitis vinifera cv. Trincadeira berries upon infection with Botrytis cinerea. Journal of Experimental Botany 66, 1769–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach L, Michaelson LV, Haslam R, et al. 2008. The very-long-chain hydroxy fatty acyl-CoA dehydratase PASTICCINO2 is essential and limiting for plant development. Proceedings of the National Academy of Sciences, USA 105, 14727–14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin F, Wu X, Li F, Haslam RP, Markham JE, Zheng H, Napier JA, Kunst L. 2009. Functional characterization of the Arabidopsis beta-ketoacyl-coenzyme A reductase candidates of the fatty acid elongase. Plant Physiology 150, 1174–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A, Domergue F, Pascal S, Jetter R, Renne C, Faure JD, Haslam RP, Napier JA, Lessire R, Joubès J. 2012. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. The Plant Cell 24, 3106–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A, Joubès J. 2013. Arabidopsis cuticular waxes: advances in synthesis, export and regulation. Progress in Lipid Research 52, 110–129. [DOI] [PubMed] [Google Scholar]
- Bi H, Kovalchuk N, Langridge P, Tricker PJ, Lopato S, Borisjuk N. 2017. The impact of drought on wheat leaf cuticle properties. BMC Plant Biology 17, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdenx B, Bernard A, Domergue F, et al. 2011. Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiology 156, 29–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KD, Teece MA, Smart LB. 2006. Increased accumulation of cuticular wax and expression of lipid transfer protein in response to periodic drying events in leaves of tree tobacco. Plant Physiology 140, 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna D, Albiero A, Bilardi A, Caniato E, Forcato C, Manavski S, Vitulo N, Valle G. 2009. PASS: a program to align short sequences. Bioinformatics 25, 967–968. [DOI] [PubMed] [Google Scholar]
- Casado CG, Heredia A. 1999. Structure and dynamics of reconstituted cuticular waxes of grape berry cuticle (Vitis vinifera L.). Journal of Experimental Botany 50, 175–182. [Google Scholar]
- Castellarin SD, Matthews MA, Di Gaspero G, Gambetta GA. 2007a Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 227, 101–112. [DOI] [PubMed] [Google Scholar]
- Castellarin SD, Pfeiffer A, Sivilotti P, Degan M, Peterlunger E, Di Gaspero G. 2007b Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant, Cell & Environment 30, 1381–1399. [DOI] [PubMed] [Google Scholar]
- Charrier G, Delzon S, Domec JC, et al. 2018. Drought will not leave your glass empty: low risk of hydraulic failure revealed by long-term drought observations in world’s top wine regions. Science Advances 4, eaao6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevenet F, Brun C, Bañuls AL, Jacq B, Christen R. 2006. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics 7, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commenil P, Brunet L, Audran J. 1997. The development of the grape berry cuticle in relation to susceptibility to bunch rot disease. Journal of Experimental Botany 48, 1599–1607. [Google Scholar]
- Cui F, Brosché M, Lehtonen MT, Amiryousefi A, Xu E, Punkkinen M, Valkonen JP, Fujii H, Overmyer K. 2016. Dissecting abscisic acid signaling pathways involved in cuticle formation. Molecular Plant 9, 926–938. [DOI] [PubMed] [Google Scholar]
- Da Silva C, Zamperin G, Ferrarini A, et al. 2013. The high polyphenol content of grapevine cultivar tannat berries is conferred primarily by genes that are not shared with the reference genome. The Plant Cell 25, 4777–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, et al. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research 36, W465–W469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez E, España L, López-Casado G, Cuartero J, Heredia A. 2009. Biomechanics of isolated tomato (Solanum lycopersicum) fruit cuticles during ripening: the role of flavonoids. Functional Plant Biology 36, 613–620. [DOI] [PubMed] [Google Scholar]
- Domínguez E, Heredia-Guerrero JA, Heredia A. 2017. The plant cuticle: old challenges, new perspectives. Journal of Experimental Botany 68, 5251–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España L, Heredia-Guerrero JA, Reina-Pinto JJ, Fernández-Muñoz R, Heredia A, Domínguez E. 2014a Transient silencing of CHALCONE SYNTHASE during fruit ripening modifies tomato epidermal cells and cuticle properties. Plant Physiology 166, 1371–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España L, Heredia-Guerrero JA, Segado P, Benítez JJ, Heredia A, Domínguez E. 2014b Biomechanical properties of the tomato (Solanum lycopersicum) fruit cuticle during development are modulated by changes in the relative amounts of its components. New Phytologist 202, 790–802. [DOI] [PubMed] [Google Scholar]
- Fiebig A, Mayfield JA, Miley NL, Chau S, Fischer RL, Preuss D. 2000. Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems. The Plant Cell 12, 2001–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima EO, Seki H, Ohyama K, Ono E, Umemoto N, Mizutani M, Saito K, Muranaka T. 2011. CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant & Cell Physiology 52, 2050–2061. [DOI] [PubMed] [Google Scholar]
- Girard AL, Mounet F, Lemaire-Chamley M, et al. 2012. Tomato GDSL1 is required for cutin deposition in the fruit cuticle. The Plant Cell 24, 3119–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YS, Kim H, Kim HJ, Suh MC. 2014. Arabidopsis cuticular wax biosynthesis is negatively regulated by the DEWAX gene encoding an AP2/ERF-type transcription factor. The Plant Cell 26, 1666–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grncarevic M, Radler F. 1971. A review of the surface lipids of grapes and their importance in the drying process. American Journal of Enology and Viticulture 22, 80–86. [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59, 307–321. [DOI] [PubMed] [Google Scholar]
- Haslam TM, Haslam R, Thoraval D, et al. 2015. ECERIFERUM2-LIKE proteins have unique biochemical and physiological functions in very-long-chain fatty acid elongation. Plant Physiology 167, 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam TM, Kunst L. 2013a Extending the story of very-long-chain fatty acid elongation. Plant Science 210, 93–107. [DOI] [PubMed] [Google Scholar]
- Haslam TM, Kunst L. 2013b Wax analysis of stem and rosette leaves in Arabidopsis thaliana. Bioprotocols 3, e782. [Google Scholar]
- Haslam TM, Mañas-Fernández A, Zhao L, Kunst L. 2012. Arabidopsis ECERIFERUM2 is a component of the fatty acid elongation machinery required for fatty acid extension to exceptional lengths. Plant Physiology 160, 1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia-Guerrero JA, Guzman-Puyol S, Benítez JJ, Athanassiou A, Heredia A, Domínguez E. 2018. Plant cuticle under global change: biophysical implications. Global Change Biology 24, 2749–2751. [DOI] [PubMed] [Google Scholar]
- Hochberg U, Degu A, Fait A, Rachmilevitch S. 2013a Near isohydric grapevine cultivar displays higher photosynthetic efficiency and photorespiration rates under drought stress as compared with near anisohydric grapevine cultivar. Physiologia Plantarum 147, 443–452. [DOI] [PubMed] [Google Scholar]
- Hochberg U, Degu A, Toubiana D, Gendler T, Nikoloski Z, Rachmilevitch S, Fait A. 2013b Metabolite profiling and network analysis reveal coordinated changes in grapevine water stress response. BMC Plant Biology 13, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MG, Keiller DR. 2002. Effects of pubescence and waxes on the reflectance of leaves in the ultraviolet and photosynthetic wavebands: a comparison of a range of species. Plant, Cell & Environment 25, 85–93. [Google Scholar]
- Huang H, Burghardt M, Schuster AC, Leide J, Lara I, Riederer M. 2017. Chemical composition and water permeability of fruit and leaf cuticles of Olea europaea L. Journal of Agricultural and Food Chemistry 65, 8790–8797. [DOI] [PubMed] [Google Scholar]
- Isaacson T, Kosma DK, Matas AJ, et al. 2009. Cutin deficiency in the tomato fruit cuticle consistently affects resistance to microbial infection and biomechanical properties, but not transpirational water loss. The Plant Journal 60, 363–377. [DOI] [PubMed] [Google Scholar]
- Jaillon O, Aury JM, Noel B, et al. 2007. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449, 463–467. [DOI] [PubMed] [Google Scholar]
- Jetter R, Riederer M. 2016. Localization of the transpiration barrier in the epi- and intracuticular waxes of eight plant species: water transport resistances are associated with fatty acyl rather than alicyclic components. Plant Physiology 170, 921–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo A, Robin GP. 2013. Systemic signaling during plant defense. Current Opinion in Plant Biology 16, 527–533. [DOI] [PubMed] [Google Scholar]
- Khanal BP, Grimm E, Finger S, Blume A, Knoche M. 2013. Intracuticular wax fixes and restricts strain in leaf and fruit cuticles. New Phytologist 200, 134–143. [DOI] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL. 2015. HISAT: a fast spliced aligner with low memory requirements. Nature Methods 12, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Park SH, Jenks MA. 2007. Changes in leaf cuticular waxes of sesame (Sesamum indicum L.) plants exposed to water deficit. Journal of Plant Physiology 164, 1134–1143. [DOI] [PubMed] [Google Scholar]
- Kosma DK, Bourdenx B, Bernard A, Parsons EP, Lü S, Joubès J, Jenks MA. 2009. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiology 151, 1918–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste F. 1865. Guide pratique du vigneron: culture, vendange et vinification. Paris: Eugene Lacroix. [Google Scholar]
- Lara I, Belge B, Goulao LF. 2015. A focus on the biosynthesis and composition of cuticle in fruits. Journal of Agricultural and Food Chemistry 63, 4005–4019. [DOI] [PubMed] [Google Scholar]
- Lara I, Heredia A, Domínguez E. 2019. Shelf life potential and the fruit cuticle: the unexpected player. Frontiers in Plant Science 10, 770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Kim HU, Suh MC. 2016. MYB94 and MYB96 additively activate cuticular wax biosynthesis in Arabidopsis. Plant & Cell Physiology 57, 2300–2311. [DOI] [PubMed] [Google Scholar]
- Leida C, Dal Rì A, Dalla Costa L, Gómez MD, Pompili V, Sonego P, Engelen K, Masuero D, Ríos G, Moser C. 2016. Insights into the role of the berry-specific ethylene responsive factor VviERF045. Frontiers in Plant Science 7, 1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leide J, Hildebrandt U, Reussing K, Riederer M, Vogg G. 2007. The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: effects of a deficiency in a beta-ketoacyl-coenzyme A synthase (LeCER6). Plant Physiology 144, 1667–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AD, Williams LE, Matthews MA. 2019. A continuum of stomatal responses to water deficits among 17 wine grape cultivars (Vitis vinifera). Functional Plant Biology 11–25. [DOI] [PubMed] [Google Scholar]
- Li F, Wu X, Lam P, Bird D, Zheng H, Samuels L, Jetter R, Kunst L. 2008. Identification of the wax ester synthase/acyl-coenzyme A:diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis. Plant Physiology 148, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. [DOI] [PubMed] [Google Scholar]
- López-Castañeda J, Corrales-García J, Terrazas-Salgado T, Colinas-León T. 2010. Effect of vapor heat treatments on weight loss reduction and epicuticular changes in six varieties of cactus pear fruit (Opuntia spp.). Journal of the Professional Association for Cactus Development 12, 37–47. [Google Scholar]
- Lovisolo C, Lavoie-Lamoureux A, Tramontini S, Ferrandino A. 2016. Grapevine adaptations to water stress: new perspectives about soil/plant interactions. Theoretical and Experimental Plant Physiology 28, 53–66. [Google Scholar]
- Lun ATL, Chen Y, Smyth GK. 2016. It’s DE-licious: a recipe for differential expression analyses of RNA-seq experiments using quasi-likelihood methods in edgeR. In: Mathé E, Davis S, eds. Statistical genomics: methods and protocols. New York: Springer Science+Business Media, 391–416. [DOI] [PubMed] [Google Scholar]
- Luque P, Bruque S, Heredia A. 1995. Water permeability of isolated cuticular membranes: a structural analysis. Archives of Biochemistry and Biophysics 317, 417–422. [DOI] [PubMed] [Google Scholar]
- Martin LB, Rose JK. 2014. There’s more than one way to skin a fruit: formation and functions of fruit cuticles. Journal of Experimental Botany 65, 4639–4651. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Shikata M, Koyama T, Ohtsubo N, Mitsuda N, Ohme-Takagi M. 2013. MIXTA-like transcription factors and WAX INDUCER1/SHINE1 coordinately regulate cuticle development in Arabidopsis and Torenia fournieri. The Plant Cell 25, 1609–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palliotti A, Cartechini A. 2001. Developmental changes in gas exchange activity in flowers, berries, and tendrils of field-grown Cabernet Sauvignon. American Journal of Enology and Viticulture 52, 317–323. [Google Scholar]
- Palumbo MC, Zenoni S, Fasoli M, Massonnet M, Farina L, Castiglione F, Pezzotti M, Paci P. 2014. Integrated network analysis identifies fight-club nodes as a class of hubs encompassing key putative switch genes that induce major transcriptome reprogramming during grapevine development. The Plant Cell 26, 4617–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons EP, Popopvsky S, Lohrey GT, Alkalai-Tuvia S, Perzelan Y, Bosland P, Bebeli PJ, Paran I, Fallik E, Jenks MA. 2013. Fruit cuticle lipid composition and water loss in a diverse collection of pepper (Capsicum). Physiologia Plantarum 149, 160–174. [DOI] [PubMed] [Google Scholar]
- Patwari P, Salewski V, Gutbrod K, Kreszies T, Dresen-Scholz B, Peisker H, Steiner U, Meyer AJ, Schreiber L, Dörmann P. 2019. Surface wax esters contribute to drought tolerance in Arabidopsis. The Plant Journal 98, 727–744. [DOI] [PubMed] [Google Scholar]
- Pensec F, Pączkowski C, Grabarczyk M, Woźniak A, Bénard-Gellon M, Bertsch C, Chong J, Szakiel A. 2014. Changes in the triterpenoid content of cuticular waxes during fruit ripening of eight grape (Vitis vinifera) cultivars grown in the Upper Rhine Valley. Journal of Agricultural and Food Chemistry 62, 7998–8007. [DOI] [PubMed] [Google Scholar]
- Petit J, Bres C, Mauxion JP, Bakan B, Rothan C. 2017. Breeding for cuticle-associated traits in crop species: traits, targets, and strategies. Journal of Experimental Botany 68, 5369–5387. [DOI] [PubMed] [Google Scholar]
- R Core Team 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Radler F. 1965. The main constituents of the surface waxes of varieties and species of the genus Vitis. American Journal of Enology and Viticulture 16, 159–167. [Google Scholar]
- Rogiers SY, Greer DH, Hutton RJ, Landsberg JJ. 2009. Does night-time transpiration contribute to anisohydric behaviour in a Vitis vinifera cultivar? Journal of Experimental Botany 60, 3751–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogiers SY, Hatfield JM, Gunta Jaudzems V, White RG, Keller M. 2004. Grape berry cv. Shiraz epicuticular wax and transpiration during ripening and preharvest weight loss. American Journal of Enology and Viticulture 55, 121–127. [Google Scholar]
- Romero P, Rose JKC. 2019. A relationship between tomato fruit softening, cuticle properties and water availability. Food Chemistry 295, 300–310. [DOI] [PubMed] [Google Scholar]
- Rowland O, Zheng H, Hepworth SR, Lam P, Jetter R, Kunst L. 2006. CER4 encodes an alcohol-forming fatty acyl-coenzyme A reductase involved in cuticular wax production in Arabidopsis. Plant Physiology 142, 866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoi S, Wong DCJ, Degu A, Herrera JC, Bucchetti B, Peterlunger E, Fait A, Mattivi F, Castellarin SD. 2017. Multi-omics and integrated network analyses reveal new insights into the systems relationships between metabolites, structural genes, and transcriptional regulators in developing grape berries (Vitis vinifera L.) exposed to water deficit. Frontiers in Plant Science 8, 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharwies JD, Tyerman SD. 2017. Comparison of isohydric and anisohydric Vitis vinifera L. cultivars reveals a fine balance between hydraulic resistances, driving forces and transpiration in ripening berries. Functional Plant Biology 44, 324–338. [DOI] [PubMed] [Google Scholar]
- Seo PJ, Lee SB, Suh MC, Park MJ, Go YS, Park CM. 2011. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. The Plant Cell 23, 1138–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology 56, 564–577. [DOI] [PubMed] [Google Scholar]
- Tsubaki S, Sugimura K, Teramoto Y, Yonemori K, Azuma J. 2013. Cuticular membrane of Fuyu persimmon fruit is strengthened by triterpenoid nano-fillers. PLoS One 8, e75275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitulo N, Forcato C, Carpinelli EC, et al. 2014. A deep survey of alternative splicing in grape reveals changes in the splicing machinery related to tissue, stress condition and genotype. BMC Plant Biology 14, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogg G, Fischer S, Leide J, Emmanuel E, Jetter R, Levy AA, Riederer M. 2004. Tomato fruit cuticular waxes and their effects on transpiration barrier properties: functional characterization of a mutant deficient in a very-long-chain fatty acid beta-ketoacyl-CoA synthase. Journal of Experimental Botany 55, 1401–1410. [DOI] [PubMed] [Google Scholar]
- Wong DC, Lopez Gutierrez R, Dimopoulos N, Gambetta GA, Castellarin SD. 2016. Combined physiological, transcriptome, and cis-regulatory element analyses indicate that key aspects of ripening, metabolism, and transcriptional program in grapes (Vitis vinifera L.) are differentially modulated accordingly to fruit size. BMC Genomics 17, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Xiao L, Feng J, Chen N, Chen Y, Song B, Xue K, Shi S, Zhou Y, Jenks MA. 2016. Cuticle lipids on heteromorphic leaves of Populus euphratica Oliv. growing in riparian habitats differing in available soil moisture. Physiologia Plantarum 158, 318–330. [DOI] [PubMed] [Google Scholar]
- Yeats TH, Rose JK. 2013. The formation and function of plant cuticles. Plant Physiology 163, 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Keller M. 2015. Grape berry transpiration is determined by vapor pressure deficit, cuticular conductance, and berry size. American Journal of Enology and Viticulture 66, 454–462. [Google Scholar]
- Zheng H, Rowland O, Kunst L. 2005. Disruptions of the Arabidopsis enoyl-CoA reductase gene reveal an essential role for very-long-chain fatty acid synthesis in cell expansion during plant morphogenesis. The Plant Cell 17, 1467–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.