Abstract
Background
The bacterial genus Exiguobacterium includes several species that inhabit environments with a wide range of temperature, salinity, and pH. This is why the microorganisms from this genus are known generically as polyextremophiles. Several environmental isolates have been explored and characterized for enzyme production as well as for bioremediation purposes. In this line, toxic metal(loid) reduction by these microorganisms represents an approach to decontaminate soluble metal ions via their transformation into less toxic, insoluble derivatives. Microbial-mediated metal(loid) reduction frequently results in the synthesis of nanoscale structures—nanostructures (NS) —. Thus, microorganisms could be used as an ecofriendly way to get NS.
Results
We analyzed the tolerance of Exiguobacterium acetylicum MF03, E. aurantiacum MF06, and E. profundum MF08 to Silver (I), gold (III), and tellurium (IV) compounds. Specifically, we explored the ability of cell-free extracts from these bacteria to reduce these toxicants and synthesize NS in vitro, both in the presence or absence of oxygen.
All isolates exhibited higher tolerance to these toxicants in anaerobiosis. While in the absence of oxygen they showed high tellurite- and silver-reducing activity at pH 9.0, whereas AuCl4− which was reduced at pH 7.0 in both conditions. Given these results, cell-free extracts were used to synthesize NS containing silver, gold or tellurium, characterizing their size, morphology and chemical composition. Silver and tellurium NS exhibited smaller size under anaerobiosis and their morphology was circular (silver NS), starred (tellurium NS) or amorphous (gold NS).
Conclusions
This nanostructure-synthesizing ability makes these isolates interesting candidates to get NS with biotechnological potential.
Keywords: Exiguobacterium, Metal(loid), Reduction, Aerobiosis, Anaerobiosis, Nanostructure
Background
The genus Exiguobacterium is quite diverse composed by Gram-positive, facultative anaerobic, non-sporulating, and motile rods. Bacteria from this genus have been isolated from a variety of environments including permafrost, salt lakes, deserts and even industrial wastes, suggesting a high plasticity, adaptation capacity, and tolerance to extreme environmental factors [1]. The ability to grow in such harsh conditions makes them interesting candidates to develop products with applications in biotechnology and bioremediation.
In recent years, research on Exiguobacterium has been of great interest mainly because it is considered as a source of enzymes that exhibit a broad range of thermal stability [1–3]. Numerous enzymes from these microorganisms exhibiting interesting activities have been reported including: i) an esterase from E. acetylicum, stable between pH 6.0–11.0 [4]; ii) a protease from Exiguobacterium sp. SKPB5, thermally stable between 4 and 60 °C at pH 4.0–9.0 [5]; iii) a dehydrogenase from Exiguobacterium spp. F42, stable between 4 and 45 °C [6]; iv) a β-glucosidase from Exiguobacterium sp GXG2 with activity ranging between 5 to 35 °C [7], among others. Regarding bioremediation applications, it has been observed that Exiguobacterium sp. 2Sz shows great potential to remove pesticides [8], Exiguobacterium sp. GS1 eliminates hexavalent chromium from water over a wide range of temperature and pH [9], while Exiguobacterium sp. WK6 reduces arsenate to arsenite at arsenic-polluted sites [10]. Other examples include Exiguobacterium sp. ZM-2, which reduces chromium (VI) to chromium (III) [11] and E. mexicanum that produces silver-containing nanostructures [12].
Here, our aim is to characterize Exiguobacterium strains able to form nanostructures (NS) when they are expose to silver, gold, or tellurium salts. These metal(loid)s have not known biological function and are considered as non-essential; on the contrary, they trigger high toxicity even at very low concentrations [13, 14]. This toxicity is exerted, at least in part, through the generation of reactive oxygen species (ROS), which is well-known to induce oxidative stress in the cell that damages important macromolecules such as proteins, DNA, and membranes [14]. Cells mutants lacking enzymes involved in ROS elimination exhibit enhanced sensitivity to tellurium and iron [15, 16]. In addition, the presence of Ag or Te salts produce, directly or indirectly, [4Fe-4S] center dismantling of certain enzymes, releasing concomitantly Fe2+ to cytoplasm that results in increased hydroxyl radical formation through the Fenton reaction [17, 18]. Nevertheless, some microorganisms can handle the presence of these toxics using a several resistance mechanisms or cell responses, including: i) decreased production of metal(loid)- transporters [19, 20]; ii) repair of oxidation sensitive molecules through enzymes or antioxidant production [21]; and iii) chemical modification of the metal(loid)s and their reduction to elemental state (frequently less toxic) [14, 22, 23] which usually leads to NS formation.
Microbial synthesis of NS is preferred compared to other strategies, because it is considered a safe method and friendly with the environment. However, microbial-mediated metal(loid) reduction is not fully understood [24, 25]. In fact, it has been observed that metal(loid) reduction can be a result of secondary activities of certain enzymes, as the case of the glutathione reductase in Pseudomonas sp. BNF22 (reduces Au3+ and Te4+) [26], catalase in Staphylococcus epidermidis and E. coli (reduces Te4+) [27], or the nitrate reductase in Fusarium oxysporum (reduces Ag+) [28]. Since the metal(loid) ion reduction under anaerobic conditions should not render in the formation of ROS, contrary to observed in the presence of oxygen [17, 29, 30]. In this work we examined the metal(loid) resistance, reduction and the ability to synthesize NS by specific environmental Exiguobacterium strains, both in the presence or absence of oxygen. We evaluated if different respirations induce bacterial responses for those toxicants which could lead to differential generation of NS.
Results
Exiguobacterium metal(loid) resistance
E. acetylicum MF03, E. aurantiacum MF06 and E. profundum MF08 were exposed to K2TeO3, AgNO3 or HAuCl4 under both aerobic and anaerobic conditions, to determine the minimal inhibitory concentrations (MIC) for each treatment (Table 1). Under anaerobic conditions, E. profundum MF08 was the most resistant strain to silver (MIC 0.25 mM). In turn, E. acetylicum MF03 was equally resistant to silver in both conditions, aerobic and anaerobic.
Table 1.
MICs of Ag (I), Au (III) and Te (IV) for the indicated Exiguobacterium strains
|
E. acetylicum MF03 |
E. aurantiacum MF06 |
E. profundum MF08 |
||||
|---|---|---|---|---|---|---|
| Metal(loid) | + O2 | - O2 | + O2 | - O2 | + O2 | - O2 |
| Ag (I) | 0.125 ± 0.06 | 0.125 ± 0.06 | 0.0625 ± 0.034 | 0.125 ± 0.06 | 0.125 ± 0.06 | 0.25 ± 0.13 |
| Au (III) | 0.25 ± 0.13 | 0.5 ± 0.27 | 0.125 ± 0.06 | 0.5 ± 0.27 | 0.25 ± 0.13 | 0.5 ± 0.27 |
| Te (IV) | 0.25 ± 0.13 | 2 ± 0.3 | 0.25 ± 0.13 | 1 ± 0.5 | 0.5 ± 0.27 | 4 ± 0.4 |
Concentrations are expressed in mM and the data represent the average of 6 independent trials
For gold, under anaerobic conditions either E. acetylicum MF03 and E. profundum MF08 showed resistance values that were two-fold higher than those observed in aerobiosis; whereas, gold resistance of E. aurantiacum MF06 was 4-fold higher in the absence of oxygen.
As for tellurium, all strains were more resistant to tellurite under anaerobic conditions: 8- fold for E. acetylicum MF03 and E. profundum MF08, and and 4-fold E. aurantiacum MF06. In parallel to MICs determinations, all Exiguobacterium strains were analyzed for their metal(loid) resistance in solid medium by determining growth inhibition zones (Figure S1). Consistent with MIC determinations, all strains -excepting E. acetylicum exposed to silver (Figure S1 A)- showed higher resistance to these toxicants under anaerobic conditions.
Metal(loid)-reducing activity
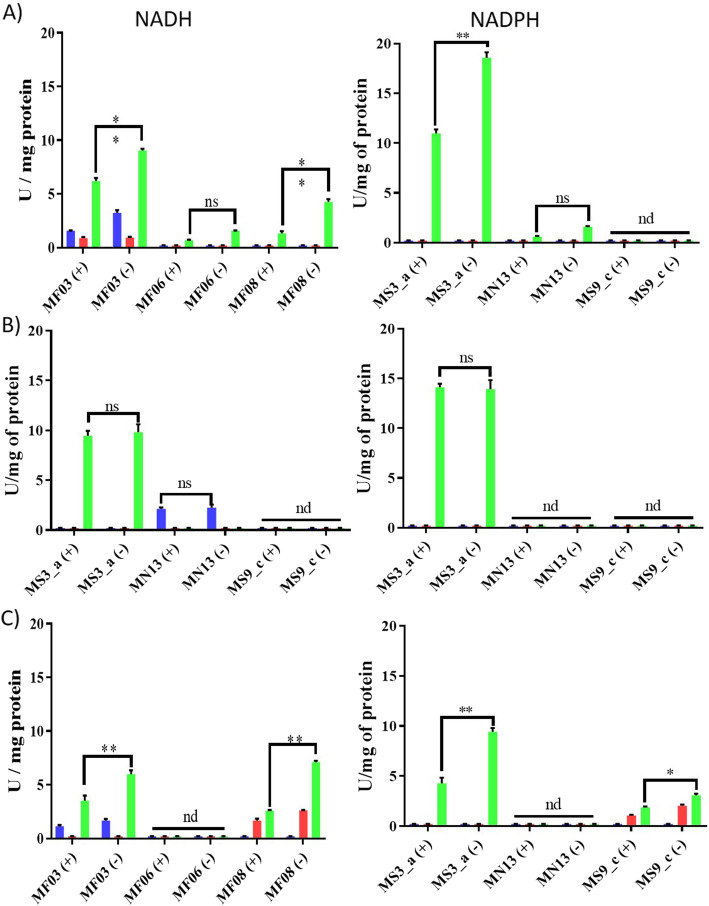
Cell-free extracts were tested metal(loid) for reduction of Ag (I) (Fig. 1a), Au (III) (Fig. 1b), and Te (IV) (Fig. 1c). Extracts prepared from cultures in different growth phases were evaluated at 37 °C both in aerobic and anaerobic conditions at pH 7.0, 8.0, and 9.0, and in the presence of NADH or NADPH as electron donor. Hypothesizing that bioreduction is an enzymatic process in nature, denatured negative controls were conducted in which crude extracts were treated with 1% SDS or heated at 90 °C for 10 min prior to the assay (not shown). Metal(loid)-reducing activity was found to be higher in extracts from cells grown to mid exponential phase (OD600 0.6) (not shown). While E. acetylicum MF03 silver-reducing activity was higher at pH 9.0, in anaerobiosis and NADPH as cofactor, E. aurantiacum MF06 showed higher Ag+-reducing activity at pH 9.0, irrespective of the electron donor or the presence of oxygen. In turn, E. profundum MF08 extracts showed higher activity at pH 9.0, with NADH under anaerobic conditions (Fig. 1a).
Fig. 1.
Metal(loid) reduction by Exiguobacterium strains under aerobic and anaerobic conditions. Reduction assays of Ag (a), Au (b) and Te (c) were carried out as described in Methods. Blue, red and green bars represent pH 7.0, 8.0 or 9.0, respectively. (+), aerobic test, (−) anaerobic test. Bars represent the average of 3 independent trials. **, p < 0.01; *, p < 0.05; nd, not determined; ns, not significant
Regarding Au (III), there were no significant differences in reducing activity regarding the presence or absence of oxygen. Particularly, E. acetylicum MF03 displayed maximal gold-reducing activity at pH 9.0 and NADPH, whereas E. aurantiacum MF06 shower higher activity at pH 7.0 using NADH as the pyridine cofactor. E. profundum MF08 did not show Au (III)-reducing activity under the tested conditions (Fig. 1b).
Te (IV) was efficiently reduced by crude extracts of E. acetylicum MF03 (pH 9.0, NADPH, no oxygen) and E. profundum MF08 (pH 9.0, NADH, no oxygen, Fig. 1c). E. aurantiacum MF06 did not show tellurite reduction activity, irrespective of the tested condition.
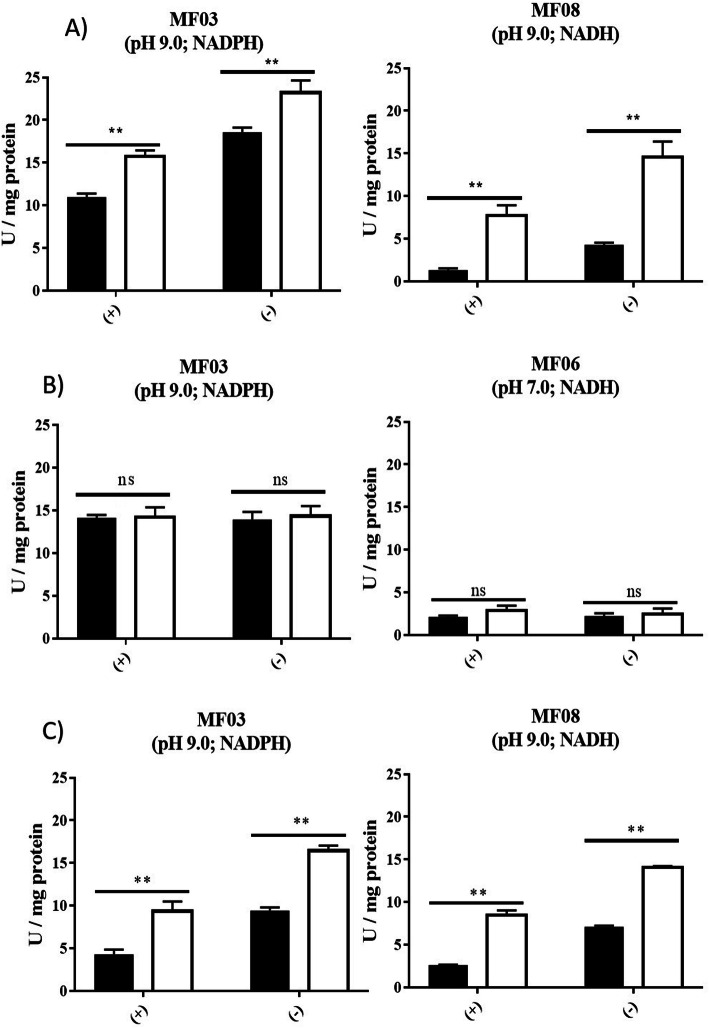
Thus, E. acetylicum MF03 and E. profundum MF08 were used for silver reduction (Fig. 2a), E. acetylicum MF03 and E. aurantiacum MF06 for Au (III) reduction (Fig. 2b) and tellurite reduction was assessed using E. acetylicum MF03 and E. profundum MF08 (Fig. 2c).
Fig. 2.
Assessing metal(loid)-reducing activity of crude extracts from E. acetylicum MF03, E. aurantiacum MF06 and E. profundum MF08. Reduction of Ag (a), Au (b) and Te (c) was carried out as described in Methods. Black and white bars represent no treatment or toxicant exposure, respectively. +, aerobic tests; −, anaerobic tests. Bars represent the average of 3 independent trials. **, p < 0.01; ns, not significant
To induce a cellular response by bacteria, a pretreatment with 1/8 of the MIC was performed for each toxicant; the aim was to favor the expression of genes encoding proteins involved in resistance to these elements, particularly reducing enzymes. At this concentration, a slight decrease of Exiguobacterium growth was observed regarding untreated control (data not shown). Extracts from E. acetylicum MF03 and E. profundum MF08 exposed to sublethal doses of silver salts showed increased Ag+-reducing activity regarding those grown in LB medium alone (Fig. 2a). On the other hand, no significant differences were observed in Au (III)-reducing activity by E. acetylicum MF03 or E. aurantiacum extracts under all conditions tested (Fig. 2b). Finally, and like Ag (I), Te (IV) reduction by extracts of E. acetylicum MF03 and E. profundum MF08 was more efficient than that observed in extracts prepared from cells not exposed to tellurite (Fig. 2c). With these results we were able to determine the optimal pH conditions and cofactor preference to be used later for NS synthesis.
Generation and characterization of nanostructures
As above mentioned, conditions were set in reduction trials. Then, the ability of crude extracts from Exiguobacterium strains to generate NS -in the presence or absence of oxygen- was assessed. AgNS synthesis was conducted using crude extracts from E. acetylicum MF03 (pH 9.0, NADPH) or E. profundum MF08 (pH 9.0, NADH). In turn, while AuNS were generated using crude extracts of E. acetylicum MF03 (pH 9.0, NADPH) and E. aurantiacum MF06 (pH 7.0, NADH), TeNS formation was assessed using crude extracts of E. acetylicum MF03 (pH 9.0, NADPH) and E. profundum MF08 (pH 9.0, NADH). AgNS (Fig. 3), AuNS (Fig. 4) and TeNS (Fig. 5) relative size and morphology were characterized by transmission electron microscopy (TEM) and their chemical composition was assessed by energy dispersion spectroscopy (EDS).
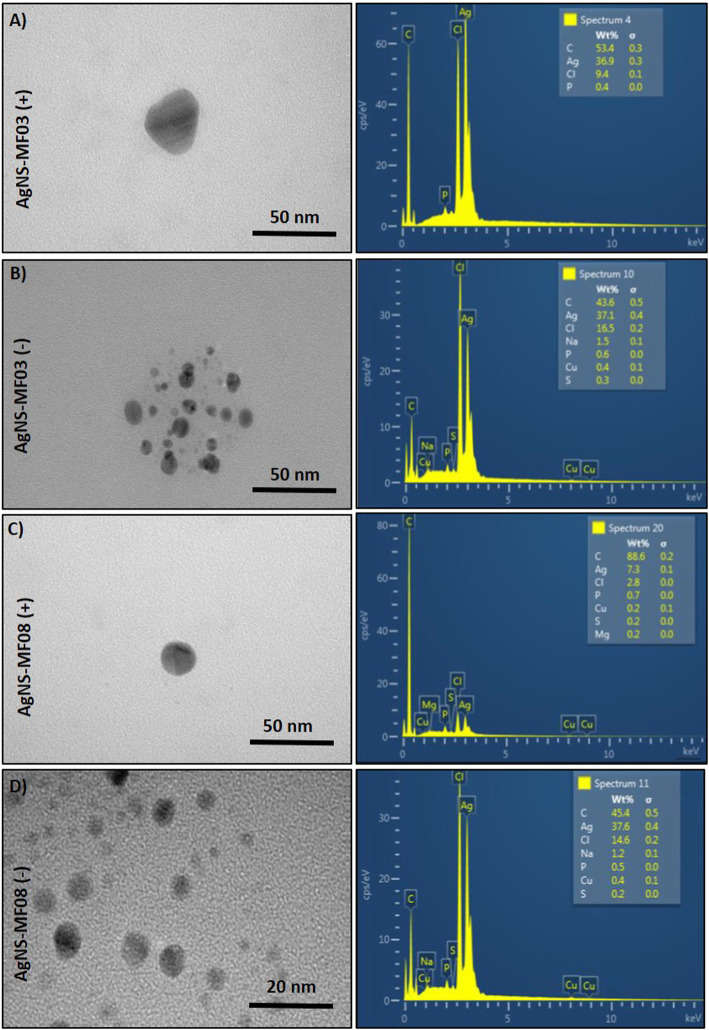
Fig. 3.
Characterization of silver nanostructures. Electronic micrographs (left) and EDS analysis (right) of in vitro generated AgNS, under anaerobic (a) and anaerobic conditions (b) by crude extracts of E. acetylicum MF03. c and d, AgNS generated aerobically and anaerobically, respectively, by E. profundum MF08
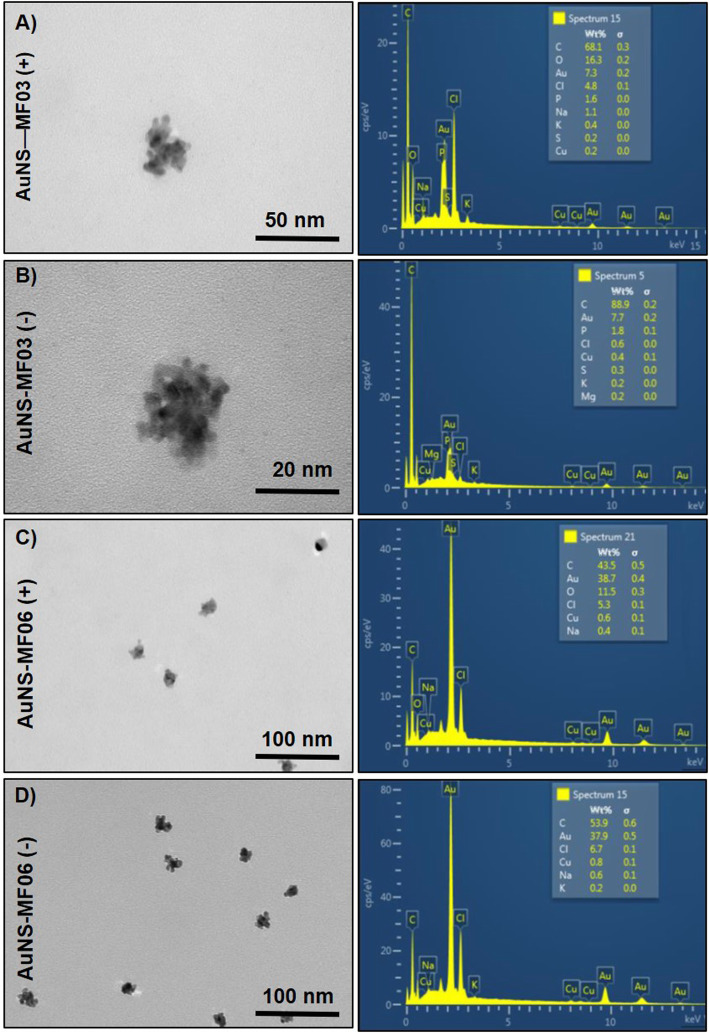
Fig. 4.
Characterization of gold nanostructures. Electron micrographs (left) and EDS analysis (right) of in vitro generated Au-NS, under aerobic (a) and anaerobic (b) conditions by crude extracts of E. acetylicum MF03; AuNS generated under aerobic (c) and anaerobic (d) conditions by E. aurantiacum MF06
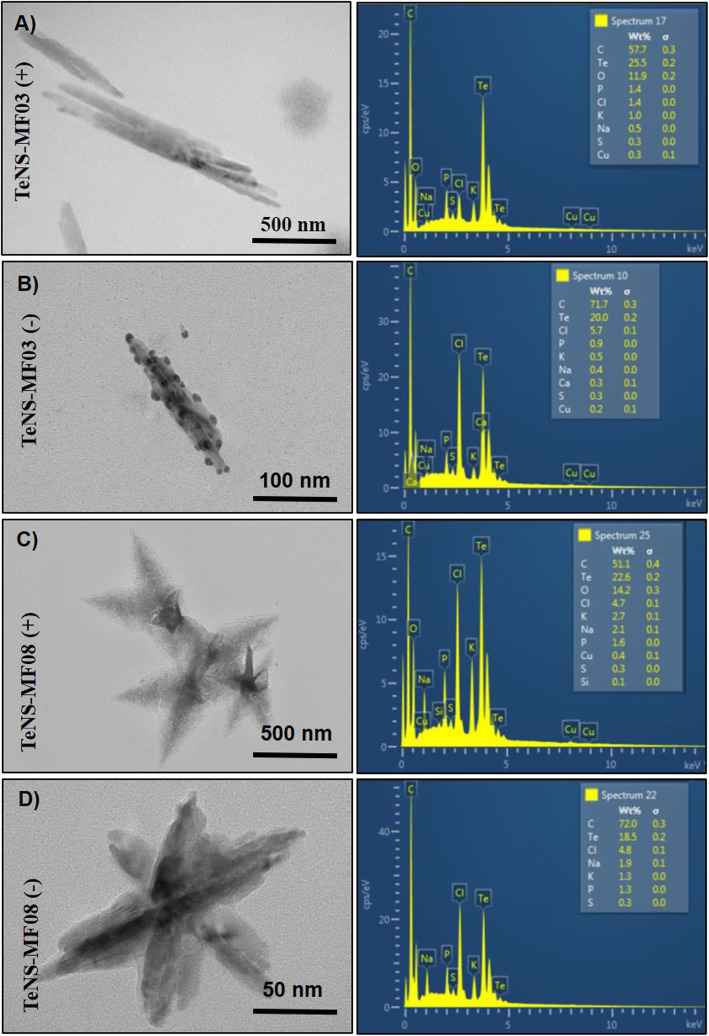
Fig. 5.
Characterization of tellurium nanostructures. Electronic micrographs (left) and EDS analysis (right) of NS from Te generated in vitro. TeNS generated aerobically (a) and anaerobically (b) by crude extracts of E. acetylicum MF03 and TeNS generated aerobically (c) and anaerobically (d) by E. profundum MF08
Aerobically synthesized AgNS of ~ 40 nm by E. acetylicum MF03 showed triangular morphology (Fig. 3a), whereas those synthesized anaerobically were ~ 20 nm and were rather circular (Fig. 3b). These NS were composed mainly of carbon and silver both in aerobic (36.9% Ag) and anaerobic (37.1% Ag) conditions. On the other hand, AgNS generated by E. profundum MF08 in aerobic or anaerobic conditions exhibited circular morphology. While their size in aerobiosis was ~ 25 nm and were composed mainly of carbon (88%) and 7% silver (Fig. 3c), in anaerobic conditions they were ~ 15 nm with 45% carbon and 37.6% silver (Fig. 3d).
AuNS generated by E. acetylicum MF03 under aerobic (Fig. 4a) and anaerobic conditions (Fig. 4b) were ~ 25 nm and showed irregular morphology. They were composed mainly of carbon and gold (7.3 and 7.7%, respectively). On the other hand, AuNS generated by E. aurantiacum MF06, both in aerobiosis and anaerobiosis (Fig. 4), also exhibited irregular morphology of similar size (~ 25 nm), whose composition was mainly carbon with a gold content of 38.7% (aerobic, Fig. 4c) and 37.9% (anaerobic, Fig. 4d).
Finally, TeNS generated by E. acetylicum MF03 under aerobic and anaerobic conditions showed an elongated morphology of similar size, particularly in anaerobic conditions where they exhibited further electrodense zones. Their chemical composition was mainly carbon and tellurium (25.5%) under aerobic conditions. TeNS generated by E. profundum MF08 both in aerobiosis (Fig. 5c) and anaerobiosis (Fig. 5d) resulted in the formation of starred structures. However, their size in aerobiosis exceeded the nanoscale, being 10-fold bigger than their anaerobic counterparts (~ 100 nm).
Discussion
Based on 16S rRNA phylogenetic studies of Exiguobacterium genus, it has shown that both E. acetylicum and E. profundum display three branch points from the common ancestor with E. aurantiacum. Additionally, E. profundum and E. aurantiacum show privileged homology since they are clustered in a joint node, despite showing higher speciation [3]. At the genome level, the draft sequence of E. aurantiacum, E. profundum [31] and the sequence/assembly of E. acetylicum are available in the databases.
The distinctive feature belonging to this genus is their ability to grow under extreme environmental conditions, including a wide temperature range (− 12–55 °C) besides nutrient limitation situations [32, 33]. The robustness and tolerance against harsh conditions showed by Exiguobacterium members turn them in suitable candidates for industrial applications, useful in bioremediation and bioabsorption processes of metals and metalloids [34–37].
Exiguobacterium strains used in this research were previously isolated from different regions in Chile that display a combination of extreme environments such as high salinity, desiccation, high and low temperatures, and volcanic interventions [38]. Most of these factors are responsible for generating oxidative stress to microorganisms, a situation that also occurs often upon bacterial exposure to metal(loid)s. This is corroborated, because in Chile it has been identified and characterized strains of Exiguobacterium obtained from the Salar del Huasco which have arsenic resistance [1, 2].
In this work, resistance for the three elements was higher in the absence of oxygen, a result that was expected because of the absence of ROS, which otherwise would generate oxidative stress [14, 29].
To determine optimal parameters, reduction assays of TeO32−, AuCl4− and Ag+ by crude extracts were carried out at pH values 7.0–9.0, in the presence of NADH or NADPH as the electron donor. Particularly, this study worked in the optimal temperature conditions (37 °C), however, it would be interesting to study the minimum and maximum ranges that the system supports, since Exiguobacterium is a polyextremophilic microorganism. Crude extract-mediated reducing activity was higher at pH 9.0 for most tested metal(loid)s, irrespective of the presence of oxygen (Fig. 1). This could reflect the fact that most proteins displaying metal(loid)-reducing activity contain catalytic sites including vicinal cysteine residues that play an important role in the reduction process [39]. Therefore, at basic pH, deprotonation of thiol groups from these cysteines would occur, giving rise to the highly reactive thiolate anion (−S−) [40]. Another possible explanation is that enzymes exhibiting metal(loid)-reducing activity are tolerant to alkali, as has been described for most enzymes of biotechnological interest isolated from Exiguobacterium strains [3]. In turn, E. aurantiacum MF06 crude extracts showed Au (III)-reducing activity at pH 7.0 (Fig. 1b), a situation that may occur because pH can influence metal(loid) speciation. This could result in the formation of complexes and/or deprotonation or protonation of functional groups in amino acids that participate in enzyme-substrate stabilization [41] (Panda and Deepa, 2011). The preference for NADH or NADPH as electron donor could reflect its stabilization at the enzyme’s active site [42].
For Ag+- and TeO32−-reducing activities, these were higher under anaerobic conditions, probably due to the limitation of ROS formation in this condition [14, 29]. To date, gold toxicity has not been associated to oxidative stress; the lack of significant differences in AuCl4− reduction by crude extracts from aerobically- or anaerobically grown cells supports this observation.
Assays that were carried out with crude extracts from cells that were previously exposed with sublethal doses of toxicants showed -in general- higher reducing activities than those coming from untreated cells both under aerobic and anaerobic conditions with the exception of Au (III) reduction. Since bacterial Ag and Te resistance is associated to enzymatic reduction [14], the observed results could reflect the expression of genes related to bacterial Ag (I) and/or Te (IV) resistance.
In addition, crude extracts of this genus have been previously used for nanoparticle synthesis. For instance, E. mexicanum extracts were able to synthesize silver nanoparticles of 5–40 nm, a process in which extracellular polymeric substances played a critical role both in silver reduction and nanoparticle stabilization [12]. Because of this, we used Exiguobacterium strains as an ecofriendly way to get NS.
NS synthesis by bacterial crude extracts or purified enzymes has not been widely reported. Indeed, most synthetic procedures are chemical in nature, in which mechanisms of NS formation involve two stages: nucleation and growth, processes that are affected by several factors including thermal energy, metal concentration and reaction rate, among others [43].
AgNS synthesized in aerobic conditions using crude extracts of E. acetylicum MF03 and E. profundum MF08 exhibited larger sizes than their anaerobic counterparts. However, the highest silver-reducing activity was observed precisely in the absence of oxygen. Given that, NS size could be affected by the activity of the enzyme, the following tests considered protein concentration as a critical parameter. Indeed, it was previously observed that during enzymatic synthesis of tellurium-containing NS, particle size was inversely related to enzyme concentration [26]. In addition, NS yield was higher under anaerobic conditions. These results could be explained by a higher metal(loid)-reducing as result of the absence of oxygen that could prevent electron leakage [44].
On the other hand, AuNS generated by E. acetylicum MF03 and E. aurantiacum MF06 did not show significant fluctuations in size or yield both in aerobiosis and anaerobiosis. However, what is relevant about these results is that when working with two different species of Exiguobacterium it is possible to obtain AuNS with different gold content, which from a biotechnological point of view is attractive, for example in the field of medicine.
Finally, aerobically- and anaerobically generated TeNS by E. acetylicum MF03 displayed similar sizes along with elongated morphologies; the exception was anaerobically-synthesized TeNS, which showed dense electron spots. This kind of elongated morphology of tellurium-containing NS was previously reported for Rhodobacter capsulatus [45]. In turn, TeNS generated by E. profundum MF08 (Fig. 5c-d) were much larger in aerobic conditions, which does not correlate with the reducing activity of crude extracts. Similarly, to what was proposed for AgNS, TeNs could be adopting different nucleation/growth processes that could explain this observation [43].
In general, the composition analysis of in vitro synthesized NS included the metal(loid) itself along with other, apparently unrelated elements. These include mainly carbon, oxygen and sulfur, probably indicating the organic origin of NS formation. Consistent with this, previous studies have shown that AuNS can be found in association with the enzyme glutathione reductase [46].
Biological processes for NS synthesis remain a challenge, not solely as a synthetic platform but in green purification techniques for subsequent characterizations. More efforts should be made to expand the characterization techniques applicable to these methods such as XRD, DLS with potential Z, FTIR, among others. When irregular NS with variable size and undefined organic layers are obtained, the results of these analyzes generate errors so they might be not reliable. In our case, XRD analyzes were not possible to perform because the surface of the nanostructures was not clean enough due to the biological processes that were used for the synthesis, this can be seen in the EDS analyzes in which many elements, of organic origin, they are identified, so the background is abundant. However, during TEM observation and navigation, SAED and FFT (Fourier transform) electron diffraction were explored, revealing the polycrystalline character of the samples without identifying preferential growth axes or phenomena of crystallographic and significant interest to report.
All these results allow us to demonstrate the great applicability of the Exiguobacterium genus in processes of resistance, reduction and generation of NS of metals and metalloids, which could be applied to help in developing effective co-cultures to improve the metal(loid) polluted sites like those described in Batool et al. [47]. Moreover, developing new bacterial-assisted techniques for reduced metal(loid) uptake of vegetables in the metal(loid)-contaminated soils [48].
Conclusion
The procedures of this study, related to NS synthesis of Ag, Au or Te obtained with Exiguobacterium strains under aerobic or anaerobic conditions opens the possibility of future controlled biological approaches which represent an interesting green methodology in the field of nanotechnology.
Methods
Bacterial strains and culture conditions
Bacterial strains Exiguobacterium acetylicum MF03, E. aurantiacum MF06 and E. profundum MF08 used in this study were previously characterized [38]. Cells were grown in the presence or absence of oxygen at 37 °C in Luria-Bertani culture medium (LB) with constant shaking (150 rpm) from 1% inoculum of a preculture grown overnight (approx 109 CFU/ml). Anaerobic assays were conducted inside a Coy chamber (Coy Laboratory Products, Inc.®), which provides a strict anaerobic environment (100% N2). Solutions, buffers and culture media (solid or liquid) were equilibrated before their use in the anaerobic atmosphere by introducing them to the chamber for at least 12 h for liquid medium and solutions and 3 h for solid media.
In liquid grown condition times required to achieve the optical density of 0.6 (mid-exponential phase), at 600 nm of wavelength (OD600), under aerobic conditions are 3, 6, and 4 h for E. acetylicum MF03, E. aurantiacum MF06, and E. profundum MF08, respectively. On the other hand, under anaerobic conditions all strains required an extra hour to reach the mid-exponential phase.
Determination of the minimal inhibitory concentration (MIC)
Using sterile stock solutions of 40 mM K2TeO3 [Te (IV)], 50 mM AgNO3 [Ag (I)] and 10 mM HAuCl4 [Au (III)], serial dilutions were made in 1 ml of LB medium using 48-well culture plates. Then, 10 μL of cells previously grown in LB to OD600 0.6 were added to each well and incubated with constant shaking (150 rpm) at 37 °C. Minimal inhibitory concentrations (MICs) were defined as the lowest concentration that inhibits the visible growth of a microorganism, and were determined as the average of six independent trials (n = 6) by monitoring turbidity at 600 nm after 24 h as described by Fuentes et al [49].
Crude extract preparation
Cultures were grown to OD600 0.6 at 37 °C in the absence or presence of a sublethal dose of the toxicant to be tested (1/8 MIC) and centrifuged at 9000 x g for 5 min at 4 °C. After discarding supernatants, sediments were suspended in 50 mM Tris-HCl buffer pH 7.0, 8.0 or 9.0, containing 0.1 mM PMSF. Cell suspensions were sonicated on ice with 4 pulses of 20 s each at 60% amplitude. The cell debris was discarded by centrifuging at 14,000 x g for 10 min at 4 °C and supernatants, containing soluble proteins, were collected and considered the crude extracts.
Determination of protein concentration
Protein concentration was quantified accordingly to Bradford protocol [50] using bovine serum albumin (BSA) as standard; the absorbance at 595 nm was determined using a Tecan INFINITE M200 Pro multiplate reader.
Determination of enzyme activity
Metal(loid)-reducing activity present in crude extracts was determined at 37 °C in a final volume of 200 μl which contained 20 μL of the corresponding extract in 50 mM Tris-HCl buffer pH 7.0, 8.0 or 9.0, 1 mM NAD(P) H, 1 mM β-mercaptoethanol and the toxicant to be evaluated (1 mM K2TeO3, 0.2 mM AgNO3 or 1 mM HAuCl4). Metal(loid) reduction was monitored at the highest absorption wavelength of the metal(loid) in the zero-state (500, 424 and 540 nm for Te, Ag and Au, respectively) using a Tecan multiplate reader INFINITE M200 Pro equipment. All experiments were performed in triplicate and with their respective controls. Controls excluding extracts were run in each case to rule out abiotic reduction.
An enzyme unit (U) was defined as the amount of enzyme required to increase the absorbance by 0.001 units/min at the respective wavelength. Similar reaction mixes -but containing 200 μg/ml protein- were used for NS biosynthesis. Optimal pH and electron donor concentration were determined, and reactions lasted 18 h.
In vitro synthesis and characterization of NS
Crude extracts from each strain (200 μg/mL protein) were used to produce nanostructures by incubation with 1 mM mM K2TeO3, 0.2 mM AgNO3 or 1 mM HAuCl4 for 16 h at the optimal conditions of temperature, pH, cofactor and growth conditions (as determined from reduction assays) in a final volume of 1 mL. Controls to rule out abiotic synthesis were run in each case.
The morphology of the synthesized nanostructures was analyzed by Transmission Electron Microscopy (TEM) using a Hitachi Transmission Electron Microscope HT7700 equipped with a thermionic Lanthanum Hexaboride (LaB6) filament under 120 kV of acceleration voltage. Each sample was prepared by placing a drop (20 μl) of nanostructure suspension on a 250-mesh copper grid previously covered with a carbon film. The NS chemical composition as determined by Energy dispersive X-ray spectroscopy (EDS) analysis; briefly, nanostructure suspensions were centrifuged at 13,000 x g for 60 min and after drying the sediment for 1 h at 40 °C, samples (approximately 20 μl), as well as their respective controls, were supported on glass slides and stained using the Gram-Hucker kit. Then they were fixed on conductive adhesive over pin stub mount and coated with a gold film to protect the surface from damage and calcinations and to minimize charge related artifacts. Analyses by Scanning Electron Microscopy (SEM) were carried out using a Zeiss EVO MA-10 microscope with a tungsten filament gun and by EDX spectra. Data were collected using an Oxford Instruments X-act system (connected to a microscope equipped with a Penta FET Precision detector). Samples were imaged at an accelerating voltage of 20 kV and 8 mm of working distance. All studies of electron microscopy were conducted at the Center for the Development of Nanoscience and Nanotechnology–CEDENNA, USACH.
Data analysis
Statistical analysis and graphs were carried out using GraphPad Prism 6.0 (GraphPad Software, Inc.). The confidence interval in the analysis of variance (ANOVA) was set at p < 0.05. The statistical significance was indicated as follows: ∗, p < 0.05, ∗∗, p < 0.01, ∗∗∗, p < 0.001 and ∗∗∗∗, p < 0.0001; ns, not significant.
Raw data from analyzes are in the supplementary information file named “Availability of Data”.
Supplementary information
Additional file 1.Figure 1S. Growth inhibition zones of the strains belonging to the Exiguobacterium genus exposed to Ag(I), Au(III) and Te(IV). Growth inhibition areas for E. acetylicum MF03 [A], E. aurantiacum MF06 [B] and E. profundum MF08 [C] were determined under aerobic (blue) and anaerobic (red) growth conditions. Bars indicate an average of 6 independent tests ± standard deviation. ****, Indicates significant statistical difference (p <0.0001) and ns, not significant.
Additional file 2. Availability of Data.
Acknowledgments
Authors thank Dr. Fabian Cornejo from Universidad de Santiago de Chile, Facultad de Química y Biología, for his constant support in carrying out the experiments.
Abbreviations
- AgNS
Silver nanostructure
- AuNS
Gold nanostructure
- EDX or EDS
X-ray energy dispersion spectroscopy
- MIC
Minimum inhibitory concentration
- NADH
Nicotinamide adenine dinucleotide
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NS
Nanostructure
- OD
Optical density
- PMSF
Phenylmethylsulfonyl fluoride
- SEM
Scanning electronic microscopy
- TEM
Transmission electronic microscopy
- TeNS
Tellurium nanostructure
Authors’ contributions
Conceived and designed the experiments: JO, MR, CV and FA. Performed the experiments: JO, MR, EV and CM. Analyzed the data: JO, MR, CM, EV, CV and FA. Contributed reagents/materials/analysis tools: CV and FA. Wrote the paper: CV and FA. All authors read and approved the final manuscript.
Funding
This work received financial support from FONDECYT (Fondo Nacional de Ciencia y Tecnología) Iniciación en la Investigación #11140334 (FA) and Regular #1160051 (CV) for reagents and equipments. Support from USA1799 Vridei 021943CV_GO (MR) and DICYT (Dirección de Investigación en Ciencia y Tecnología, Universidad de Santiago de Chile). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Javier Orizola, Email: javier.orizola@usach.cl.
Mirtha Ríos-Silva, Email: mirtha.r@gmail.com.
Claudia Muñoz-Villagrán, Email: c.munoz.villagran@gmail.com.
Esteban Vargas, Email: esteban.vargasr@usach.cl.
Claudio Vásquez, Email: claudio.vasquez@usach.cl.
Felipe Arenas, Email: felipe.arenass@usach.cl.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12896-020-00625-y.
References
- 1.Castro-Severyn J, Pardo-Esté C, Bracho S, Noe Y, Cabezas CE, Gariazzo V, Briones A, Morales N, Séveno M, Decourcelle M, Salvetat N, Remonsellez F, Castro-Nallar E, Molina F, Molina L, Saavedra C. Arsenic response of three altiplanic Exiguobacterium strains with different tolerance levels against the metalloid species: a proteomics study. Front Microbiol. 2019;10:2161. doi: 10.3389/fmicb.2019.02161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castro-Severyn J, Remonsellez F, Valenzuela SL, Salinas C, Fortt J, Aguilar P, Pardo-Esté C, Dorador C, Quatrini R, Molina F, Aguayo D, Castro-Nallar E, Saavedra C. Comparative genomics analysis of a new Exiguobacterium strain from Salar de Huasco reveals a repertoire of stress-related genes and arsenic resistance. Front Microbiol. 2017;8:456. doi: 10.3389/fmicb.2017.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasana RC, Pandey CB. Exiguobacterium: an overview of a versatile genus with potential in industry and agriculture. Crit Rev Biotechnol. 2018;38(1):141–156. doi: 10.1080/07388551.2017.1312273. [DOI] [PubMed] [Google Scholar]
- 4.Hwang BY, Kim JH, Kim J, Kim BG. Screening of Exiguobacterium acetylicum from soil samples showing enantioselective and alkalotolerant esterase activity. Biotechnol Bioproc E. 2005;10(4):367–371. [Google Scholar]
- 5.Kasana RC, Yadav SK. Isolation of a psychrotrophic Exiguobacterium sp. SKPB5 (MTCC 7803) and characterization of its alkaline protease. Curr. Microbiol. 2007;54(3):224–229. doi: 10.1007/s00284-006-0402-1. [DOI] [PubMed] [Google Scholar]
- 6.Wada M, Yoshizumi A, Furukawa Y, Kawabata H, Ueda M, Takagi H, Nakamori S. Cloning and overexpression of the Exiguobacterium sp. F42 gene encoding a new short chain dehydrogenase, which catalyzes the stereoselective reduction of ethyl 3-Oxo-3-(2-thienyl) propanoate to ethyl (S)-3-hydroxy-3-(2-thienyl)propanoate. Biosci Biotechnol Biochem. 2004;68(7):1481–1488. doi: 10.1271/bbb.68.1481. [DOI] [PubMed] [Google Scholar]
- 7.Yin B, Gu H, Mo X, Xu Y, Yan B, Li Q, Ou Q, Wu B, Guo C, Jiang C. Identification and molecular characterization of a psychrophilic GH1 β-glucosidase from the subtropical soil microorganism Exiguobacterium sp. GXG2. AMB Express. 2019;9(1):1–12. doi: 10.1186/s13568-019-0873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López L, Pozo C, Rodelas B, Calvo C, Juárez B, Martínez-Toledo MV, González-López J. Identification of bacteria isolated from an oligotrophic lake with pesticide removal capacities. Ecotoxicology. 2005;14(3):299–312. doi: 10.1007/s10646-003-6367-y. [DOI] [PubMed] [Google Scholar]
- 9.Okeke BC. Bioremoval of hexavalent chromium from water by a salt tolerant bacterium, Exiguobacterium sp. GS1. J Ind Microbiol Biotechnol. 2008;35(12):1571–1579. doi: 10.1007/s10295-008-0399-5. [DOI] [PubMed] [Google Scholar]
- 10.Anderson CR, Cook GM. Isolation and characterization of arsenate-reducing bacteria from arsenic-contaminated sites in New Zealand. Curr Microbiol. 2004;48(5):341–347. doi: 10.1007/s00284-003-4205-3. [DOI] [PubMed] [Google Scholar]
- 11.Alam MZ, Malik A. Chromate resistance, transport and bioreduction by Exiguobacterium sp. ZM-2 isolated from agricultural soil irrigated with tannery effluent. J Basic Microbiol. 2008;48(5):416–420. doi: 10.1002/jobm.200800046. [DOI] [PubMed] [Google Scholar]
- 12.Padman AJ, Henderson J, Hodgson S, Rahman PKSM. Biomediated synthesis of silver nanoparticles using Exiguobacterium mexicanum. Biotechnol Lett. 2014;36(10):2079–2084. doi: 10.1007/s10529-014-1579-1. [DOI] [PubMed] [Google Scholar]
- 13.Nies DH. Microbial heavy-metal resistance. Appl Microbiol Biot. 1999;51(6):730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- 14.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11(6):371. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 15.Touati D, Jacques M, Tardat B, Bouchard L, Despied S. Lethal oxidative damage and mutagenesis are generated by iron in delta fur mutants of Escherichia coli: protective role of superoxide dismutase. J Bacteriol. 1995;177(9):2305–2314. doi: 10.1128/jb.177.9.2305-2314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geslin C, Llanos J, Prieur D, Jeanthon C. The manganese and iron superoxide dismutases protect Escherichia coli from heavy metal toxicity. Res Microbiol. 2001;152(10):901–905. doi: 10.1016/s0923-2508(01)01273-6. [DOI] [PubMed] [Google Scholar]
- 17.Calderón IL, Elías AO, Fuentes EL, Pradenas GA, Castro ME, Arenas FA, Pérez JM, Vásquez CC. Tellurite-mediated disabling of [4Fe–4S] clusters of Escherichia coli dehydratases. Microbiology. 2009;155(6):1840–1846. doi: 10.1099/mic.0.026260-0. [DOI] [PubMed] [Google Scholar]
- 18.Xu FF, Imlay JA. Silver (I), mercury (II), cadmium (II), and zinc (II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl Environ Microbiol. 2012;78(10):3614–3621. doi: 10.1128/AEM.07368-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009;109(10):4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292(5526):2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 21.Harrison JJ, Tremaroli V, Stan MA, Chan CS, Vacchi-Suzzi C, Heyne BJ, Parsek MR, Ceri H, Turner RJ. Chromosomal antioxidant genes have metal ion-specific roles as determinants of bacterial metal tolerance. Environ Microbiol. 2009;11(10):2491–2509. doi: 10.1111/j.1462-2920.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 22.Silver S, Phung LT. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 1996;50(1):753–789. doi: 10.1146/annurev.micro.50.1.753. [DOI] [PubMed] [Google Scholar]
- 23.Silver S, Phung LT. A bacterial view of the periodic table: genes and proteins for toxic inorganic ions. J Ind Microbiol Biotechnol. 2005;32(11–12):587–605. doi: 10.1007/s10295-005-0019-6. [DOI] [PubMed] [Google Scholar]
- 24.Tsezos M. Biological removal of ions: principles and applications. Adv Mater Res. 2007;20:589–596. [Google Scholar]
- 25.Suresh AK. Metallic nanocrystallites and their interaction with microbial systems. Berlin: Springer Science and Business Media; 2012. [Google Scholar]
- 26.Pugin B, Cornejo FA, Muñoz-Díaz P, Muñoz-Villagrán CM, Vargas-Pérez JI, Arenas FA, Vásquez CC. Glutathione reductase-mediated synthesis of tellurium-containing nanostructures exhibiting antibacterial properties. Appl Environ Microbiol. 2014;80(22):7061–7070. doi: 10.1128/AEM.02207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calderón IL, Arenas FA, Pérez JM, Fuentes DE, Araya MA, Saavedra CP, Tantaleán JC, Pichuantes SE, Youderan PA, Vásquez CC. Catalases are NAD(P)H-dependent tellurite reductases. Plos One. 2006;1:e70. doi: 10.1371/journal.pone.0000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar SA, Abyaneh MK, Gosavi SW, Kulkarni SK, Pasricha R, Ahmad A, Khan MI. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol Lett. 2007;29(3):439–445. doi: 10.1007/s10529-006-9256-7. [DOI] [PubMed] [Google Scholar]
- 29.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57(1):395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 30.Pérez JM, Calderón IL, Arenas FA, Fuentes DE, Pradenas GA, Fuentes EL, Sandoval JM, Castro ME, Elías AO, Vásquez CC. Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS One. 2007;2(2):e211. doi: 10.1371/journal.pone.0000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vishnivetskaya, TA, Chauhan, A, Layton, AC, Pfiffner, SM, Huntemann, M, Copeland, A, Chen, A, Kyrpides, NC, Markowitz, VM, Palaniappan, K, Ivanova, N, Mikhailova, N, Ovchinnikova, G, Andersen, EW, Pati, A, Stamatis, D Reddy, TBK, Shapiro, N, Nordberg, HP, Cantor, MN, Hua, S, Woykec T. Draft genome sequences of 10 strains of the genus Exiguobacterium. Genome Announc 2014;2(5):e01058–e01014. [DOI] [PMC free article] [PubMed]
- 32.Vishnivetskaya TA, Siletzky R, Jefferies N, Tiedje JM, Kathariou S. Effect of low temperature and culture media on the growth and freeze-thawing tolerance of Exiguobacterium strains. Cryobiology. 2007;54(2):234–240. doi: 10.1016/j.cryobiol.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Vishnivetskaya TA, Kathariou S, Tiedje JM. The Exiguobacterium genus: biodiversity and biogeography. Extremophiles. 2009;13(3):541–555. doi: 10.1007/s00792-009-0243-5. [DOI] [PubMed] [Google Scholar]
- 34.Saba, Andreasen R., Li Y., Rehman Y., Ahmed M., Meyer R.L., Sabri A.N. Prospective role of indigenous Exiguobacterium profundum PT2 in arsenic biotransformation and biosorption by planktonic cultures and biofilms. Journal of Applied Microbiology. 2018;124(2):431–443. doi: 10.1111/jam.13636. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Luo W, Wang Q, He L, Sheng X. Metal(loid)-resistant bacteria reduce wheat cd and as uptake in metal(loid)-contaminated soil. Environ Pollut. 2018;241:529–539. doi: 10.1016/j.envpol.2018.05.088. [DOI] [PubMed] [Google Scholar]
- 36.Zannier F, Portero L, Ordoñez O, Martinez L, Farías M, Albarracin V. Polyextremophilic Bacteria from high altitude Andean lakes: arsenic resistance profiles and biofilm production. Biomed Res Int. 2019;9:1231975. doi: 10.1155/2019/1231975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jan Rahmatullah, Khan Muhammad Aaqil, Asaf Sajjad, Lubna, Lee In-Jung, Kim Kyung Min. Metal Resistant Endophytic Bacteria Reduces Cadmium, Nickel Toxicity, and Enhances Expression of Metal Stress Related Genes with Improved Growth of Oryza Sativa, via Regulating Its Antioxidant Machinery and Endogenous Hormones. Plants. 2019;8(10):363. doi: 10.3390/plants8100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figueroa M, Fernandez V, Arenas-Salinas M, Ahumada D, Muñoz-Villagrán C, Cornejo F, Vargas E, Latorre M, Morales E, Vásquez C, Arenas F. Synthesis and antibacterial activity of metal(loid) nanostructures by environmental multi-metal(loid) resistant bacteria and metal(loid)-reducing flavoproteins. Front Microbiol. 2018;9:959. doi: 10.3389/fmicb.2018.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arenas FA, Leal CA, Pinto CA, Arenas-Salinas MA, Morales WA, Cornejo FA, Díaz-Vásquez WA, Vásquez CC. On the mechanism underlying tellurite reduction by Aeromonas caviae ST dihydrolipoamide dehydrogenase. Biochimie. 2014;102(1):174–182. doi: 10.1016/j.biochi.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Vlamis-Gardikas A. The multiple functions of the thiol-based electron flow pathways of Escherichia coli: eternal concepts revisited. Biochim Biophys Acta. 2008;1780(11):1170–1200. doi: 10.1016/j.bbagen.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Panda T, Deepa K. Biosynthesis of gold nanoparticles. J Nanosci Nanotechnol. 2011;11(12):10279–10294. doi: 10.1166/jnn.2011.5021. [DOI] [PubMed] [Google Scholar]
- 42.Rigobello MP, Scutari G, Folda A, Bindoli A. Mitochondrial thioredoxin reductase inhibition by gold (I) compounds and concurrent stimulation of permeability transition and release of cytochrome c. Biochem Pharmacol. 2004;67(4):689–696. doi: 10.1016/j.bcp.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 43.Xiong Y, Lu X. Metallic nanoestructures: from controlled synthesis to applications. Cham: Springer International publishing; 2015. [Google Scholar]
- 44.Zare B, Faramarzi MA, Sepehrizadeh Z, Shakibaie M, Rezaie S, Shahverdi AR. Biosynthesis and recovery of rod-shaped tellurium nanoparticles and their bactericidal activities. Mater Res Bull. 2012;47(11):3719–3725. [Google Scholar]
- 45.Turner RJ, Borghese R, Zannoni D. Microbial reduction of tellurium metalloids as a tool in biotechnology. Biotechnol Adv. 2011;30:954–963. doi: 10.1016/j.biotechadv.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 46.Scott D, Toney M, Muzikár M. Harnessing the mechanism of glutathione reductase for synthesis of active site bound metallic nanoparticles and electrical connection to electrodes. J Am Chem Soc. 2008;130(3):865–874. doi: 10.1021/ja074660g. [DOI] [PubMed] [Google Scholar]
- 47.Batool S, Hussain A, Iqbal M, Javid A, Ali W, Bukhari S, Akmal M, Qazi J. Implication of highly metal-resistant microalgal-bacterial co-cultures for the treatment of simulated metal-loaded wastewaters. Int Microbiol. 2019;22(1):41–48. doi: 10.1007/s10123-018-0025-y. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Nie Z, He L, Wang Q, Sheng X. Isolation of as-tolerant bacteria and their potentials of reducing as and cd accumulation of edible tissues of vegetables in metal(loid)-contaminated soils. Sci Total Environ. 2017;579:179–189. doi: 10.1016/j.scitotenv.2016.10.239. [DOI] [PubMed] [Google Scholar]
- 49.Fuentes DE, Fuentes EL, Castro ME, Pérez JM, Araya MA, Chasteen TG, Pichuantes SE, Vásquez CC. Cysteine metabolism-related genes and bacterial resistance to potassium tellurite. J Bacteriol. 2007;189(24):8953–8960. doi: 10.1128/JB.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1.Figure 1S. Growth inhibition zones of the strains belonging to the Exiguobacterium genus exposed to Ag(I), Au(III) and Te(IV). Growth inhibition areas for E. acetylicum MF03 [A], E. aurantiacum MF06 [B] and E. profundum MF08 [C] were determined under aerobic (blue) and anaerobic (red) growth conditions. Bars indicate an average of 6 independent tests ± standard deviation. ****, Indicates significant statistical difference (p <0.0001) and ns, not significant.
Additional file 2. Availability of Data.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.