Abstract
Norcantharidin (NCTD) is a demethylated derivative of cantharidin, which is an anticancer active ingredient of traditional Chinese medicine, and is currently used clinically as a routine anti-cancer drug in China. Clarifying the anticancer effect and molecular mechanism of NCTD is critical for its clinical application. Here, we summarized the physiological, chemical, pharmacokinetic characteristics and clinical applications of NCTD. Besides, we mainly focus on its potential multi-target anticancer activities and underlying mechanisms, and discuss the problems existing in clinical application and scientific research of NCTD, so as to provide a potential anticancer therapeutic agent for human malignant tumors.
Keywords: NCTD, Antitumor agent, Anticancer activities, Mechanism
Background
Since Tu Youyou was awarded the 2015 Nobel Prize in physiology or medicine for the discovery of artemisinin used for malaria treatment, traditional Chinese medicines (TCMs) and natural medicine are getting more attention. A growing body of evidences indicate that TCMs contain anticancer ingredient. Norcantharidin (NCTD), a demethylated derivative of cantharidin which is an active ingredient of TCM—Mylabris [1–3], is currently used clinically as an optional anticancer drug in China, because of its relatively synthesized facility, potential anticancer activity, and less side-effects such as myelosuppression, gastrointestinal and urinary tract toxicity [1–5]. Increasing evidences show that NCTD not only effectively inhibited the proliferation of many tumor cells in vitro and in vivo, including hepatoma HepG2 [6–8], SMMC-7721 [8, 9] and BEL-7402 [10, 11], gallbladder cancer GBC-SD cells [12, 13], colon cancer CT26 and HT29 cells [14, 15], breast cancer cells [16, 17], leukemia K562 [18] and HL-60 cells [4, 5, 19], melanoma A375 cells [20], and oral cancer KB cells [21], but also decreased tumor growth and prolonged survival in animal models in vivo [17, 22]. As an efficacious anticancer drug, it has been used to treat hepatic cancer, gastric cancer and leucopenia patients in China for many years. To deepen the understanding of the characteristics and clinical application of NCTD is of great significance for NCTD to work as an anticancer drug in clinic. Here, we review the physiological, chemical, pharmacokinetic characteristics and clinical uses, especially, potential multi-target anticancer activities such as inducing apoptosis, inhibiting proliferation, blocking invasion/metastasis, antiangiogenesis, anti-vasculogenic mimicry, anti-lymphangiogenesis and underlying mechanisms of NCTD, so as to provide a potential anticancer therapeutic agent for human malignant tumors.
Physiological, chemical and pharmacokinetic characteristics
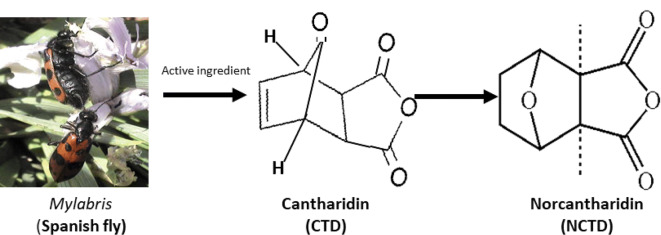
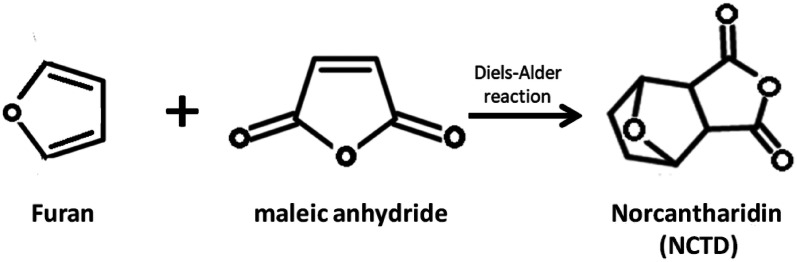
Norcantharidin (NCTD, 7-oxabicyclo[2.2.1] heptane-2,3-dicarboxylic anhydride) is a demethylated analogue of cantharidin (CTD). The molecular formula is C8H8O4 and the molecular formula is 168.15 g/mol. NCTD can not only be extracted from TCM Mylabris (Spanish fly) [1–4] (Fig. 1), but also can be synthesized from furan and maleic anhydride via the Diels–Alder reaction [23] (Fig. 2). It is a colorless, odorless, slightly irritating crystalline powder, being slightly soluble in water and ethanol, and soluble in hot water and acetone. This small-molecule synthetic compound has low-cytotoxic features and few side effects such as less marrow suppression (myelosuppression), low toxicity of gastrointestinal and urinary tract, because of removing 1,2 methyl groups on the chemical structure of CTD [1–5].
Fig. 1.
The origin, evolvement and molecular formula of norcantharidin (NCTD). Mylabris, also known as Spanish fly, is a traditional Chinese medicine. Cantharidin (CTD), a 7-oxabicyclo [2.2.1] heptane-2, 3-dicarboxylic acid derivative, a natural toxin and the active ingredient with antitumor properties extracted from a traditional Chinese medicine Mylabris. NCTD (7-oxabicyclo [2.2.1] heptane-2, 3-dicarboxylic anhydride), with a molecular formula of C8H8O4 and formula weight of 168.15 g/mol, is the demethylated analog and the low-cytotoxic derivative of CTD with antitumor properties
Fig. 2.
Synthesis of NCTD by furan and maleic anhydride through Diels–Alder reaction. NCTD can be synthesized by furan and maleic anhydride through Diels–Alder reaction under appropriate conditions
In pharmacokinetics, radionuclide and whole-body autoradiography showed that NCTD was rapidly absorbed by intragastric administration in mice with 3H-norcantharidin, reached a higher concentration within 15 min and 2 h after dosing in the kidney, liver, tumor, stomach, intestines, heart and lung. NCTD was highly distributed in the bile duct, liver, kidney, heart and lung by intravenous administration, reached the peak concentration in liver and cancer tissues within 15 min after dosing. After 6 h, the concentration decreased significantly by being excreted from the urethra. Most of drugs were excreted from the kidneys within 24 h, and were rarely accumulated in the various organs of the body [24]. Thus, NCTD is less likely to cause drug accumulation poisoning.
Clinical uses
As an efficacious anticancer drug, NCTD has been used to treat cancer patients clinically in China for many years. Two thousand years ago, Mylabris (Spanish fly), a traditional Chinese medicine, was used to treat “abdominal mass” in China [1–4]. Later, an active ingredient of Mylabris—CTD was artificially extracted and be used to treat many human tumors as a natural toxin [1–4]. Afterwards, in order to alleviate side effects of CTD such as gastrointestinal and urinary tract toxicity, NCTD was extracted from CTD, or was synthesized from furan and maleic anhydride [1–4, 23]. Now, NCTD is clinically used as a routine anticancer drug in China.
Clinical indications of NCTD include: (1) It is used to treat patients with digestive tumors, such as hepatocellular cancer, esophageal cancer, gastric cancer, and colorectal cancer and it shows better curative effect; (2) It is used to treat other cancer patients, such as lung, breast and ovarian cancers and has certain curative effect; (3) Also, it is used as premedication or in combination with other antineoplastic drugs. In addition, NCTD can also be used for hepatitis, liver cirrhosis and leukopenia.
Usage of NCTD includes oral, intravenous administration and local injection. For oral, 5–15 mg (most dose can be added to 30 mg) NCTD is used for one time, 3 times a day, 1 months for 1 courses, generally 3 courses. For intravenous infusion or intravenous drip, 10–20 mg a day, added to the 5% glucose injection 250–500 ml, in a slow drop by intravenous drip; or added to the 5% glucose injection 10–20 ml, by slow intravenous injection; 1 month for 1 treatment course. And for local injection, 20–40 mg/times, once a week, 2–4 times for 1 courses.
Growing clinical evidences demonstrated that NCTD was an efficacious anticancer drug for cancer patients. Table 1 illustrates the clinical uses of NCTD and the related results [25–48]. No matter NCTD is used alone via oral, intravenous administration, intro-tumor injection, or in combination with chemotherapy, radiotherapy and other therapies such as interventional therapy (IVT), transcatheter arterial chemoembolization (TACE) and TCMs can reduce tumors, improve symptoms and life quality, alleviate side effects, and prolong survival time in most patients with mid-advanced stage tumors such as hepatocellular cancer, esophageal cancer, gastric cancer, lung cancer, ovarian cancer, non-Hodgkin lymphoma and so on [25–48]. Thus, NCTD is believed as a useful adjunct anticancer drug in clinical treatment of mid-advanced stage tumors and in the prevention of post-operational recurrent tumors.
Table 1.
Clinical uses of NCTD in treatment of cancer patients and the related results and outcomes
| Cancers | n | Therapies and usages of NCTD | Efficient (CR + PR) | Symptoms or LQ improving | Tumor marker decreasing | Tumor size reducing | Survival time prolonging | Side effects alleviating | References | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | Dose | Course | Group | |||||||||
| PHC (I–III stage) | 244 | po or iv | 10 mg, tid; or 5–20 mg, qd, iv | 1–18 month | 58.6% | (AFP) 39% | Yes, 40.7% |
MST 7 month 1 year SR 30% |
Yes, WBC↑72% | [25] | ||
| PHC (I–III stage) | 86 | iti |
iti, 20 mg, qw po, 10 mg, tid |
4 weeks 3–6 months |
iti vs. po | Yes, P < 0.05 | Yes, P < 0.05 | Yes, P < 0.05 | 1 year SR, P < 0.05 | Yes, P < 0.05 | [26] | |
| PHC (I–III stage) | 41 | iv + po |
10 mg, qd, iv 5 mg, qd, po |
1 month 1–3 months |
Yes, P < 0.05 | Yes, 31.7% |
MST 6.8 month 1 year SR 17.7% |
Yes, WBC↑59% | [27] | |||
| PHC (II–III stage) | 76 | po + Chem |
po, 10 mg, tid Chem., FAM regimen |
3–12weeks |
po + Chem vs. Chem |
– | (AFP) 39% |
66% vs. 35% P < 0.05 |
NS | NS | [28] | |
| PHC (II–III stage) | 75 | po + TCM |
10 mg, tid, po GFL, 10 tab, tid, po |
3 months |
NCTD + GFL vs. NCTD or GFL |
Yes |
CR + PR, P < 0.05 84% vs. 7% or 53% |
Yes |
1 year SR, P < 0.05 41% vs. 27% or 12% |
Yes | [29] | |
| PHC (Ad) | 54 | iti | NCTD-P407, 2–4 ml, qw | 2–3 weeks | NCTD-P407 vs. TACE | Yes, P < 0.05 | NS | NS | NS | Yes, P < 0.05 | [30] | |
| PHC (Ad) | 56 | iti |
NCTD-P407, 2–4 ml, qw Ethanol 4-8 ml, qw |
2–3 weeks 6–8 weeks |
NS | NS | 1 year SR, P < 0.05 | NS | [31] | |||
| PHC (Ad) | 80 | po + IVT |
IVT, 1/m × 4 po, 5–10 mg, tid |
4 months 3 months |
po + IVT vs. placebo + IVT placebo + IVT |
Yes, P < 0.05 | Yes, P < 0.05 | Yes, P < 0.05 | [32] | |||
| PHC (Ad) | 43 | iv + Chem |
30 mg, iv, qd × 10 5-FU + CF regimen |
20 days |
iv + Chem vs. Chem |
Yes, P < 0.05 | Yes, P < 0.05 | Yes, P < 0.05 | [33] | |||
| PHC (Ad) | 47 | iv + TACE | 10–20 mg, iv qd | 1–2 months |
iv + TACE vs. TACE |
Yes, P < 0.05 | Yes, P < 0.05 | [34] | ||||
| PHC (Ad) | 60 | po + TCM | 10–15 mg, po, tid | 3 months |
po + TCM vs. TCM |
Yes, P < 0.05 | Yes, P < 0.05 | [35] | ||||
| PHC (Ad) | 79 | po + TCM | 15 mg, po, tid | 2 month | po + TCM vs. Chem/IVT | Yes, P < 0.05 | Yes, P < 0.05 |
MST, 16 month vs. 11 month P < 0.01 |
Yes, P < 0.05 | [36] | ||
| SHC | 60 | po + Chem | 15 mg, po, tid | 3 months |
po + Chem vs. Chem |
Yes, P < 0.05 | Yes, P < 0.05 | Yes, P < 0.05 | [37] | |||
| GC (Ad) | 50 | iv + Chem | 30 mg, iv qd × 7–10 | 6 weeks |
iv + Chem vs. Chem |
NS | Yes, P = 0.02 | NS | Yes, P < 0.05 | [38] | ||
| GC II-III (post-op.) | 82 | po + Chem |
15 mg, po, tid PLF regimen |
6 months 4 weeks × 6 |
po + Chem vs. Chem |
3 year SR, P < 0.05 3 year RR, P < 0.05 |
Yes, P < 0.05 | [39] | ||||
| EC | 58 | iv + RT |
30 mg, iv, qd × 10 RT,200GY, qd × 5 |
4 weeks 2 weeks |
iv + RT vs. RT |
Yes, P < 0.05 | Yes, P < 0.05 | Yes, P < 0.05 | Yes, P < 0.05 | [40] | ||
| CC (III stage) | 264 | iv + RT |
20–30 mg, iv, qd RT,20GY, qd × 5 |
6–8 weeks |
iv + RT vs. RT |
NS | Yes, P < 0.05 | Yes, P < 0.05 | [41] | |||
| NHL | 86 | iv + Chem |
15–25 mg, iv, qd CHOP regimen |
2 weeks |
iv + Chem vs. Chem |
NS | Yes, P < 0.05 | NS | NS | Yes, P < 0.05 | [42] | |
| NHL | 57 | iv + Chem |
30–40 mg, iv, qd CTOP regimen |
2 weeks |
iv + Chem vs. Chem |
NS | Yes, P < 0.05 | NS | Yes, P < 0.05 | [43] | ||
| LC (Ad) | 60 | iv + Chem |
20 mg, iv, qd × 7 CVI regimen |
9 weeks |
iv + Chem vs. Chem |
Yes, P < 0.05 | NS | [44] | ||||
| NSCLC (Ad) | 50 | iv + Chem |
20 mg, iv, qd × 7 DP regimen |
iv + Chem vs. Chem |
Yes, P < 0.05 | Yes, P < 0.05 | [45] | |||||
| NSCLC (III-IVstage) | 85 | iv + Chem |
60–100 ml, iv, qd × 14 PTC protocol |
8 weeks |
iv + Chem vs. Chem |
Yes, P < 0.05 | Yes, P < 0.05 | Yes, P < 0.05 | Yes, P < 0.01 | [46] | ||
| NSCLC (III-IVstage) | 180 | iv + Chem |
30 mg, iv, qd × 21 GC protocol |
9 weeks |
iv + Chem vs. Chem |
Yes, P = 0.007 | Yes, P < 0.05 | Yes, P < 0.05 | [47] | |||
| NSCLC (Ad) | 80 | iv + Chem |
40 ml, iv, qd × 14 DDP protocol |
8 weeks |
iv + Chem vs. Chem |
Yes, P < 0.05 | Yes, P < 0.01 | Yes, P < 0.05 | Yes, P < 0.01 | [48] | ||
NCTD, norcantharidin; PHC, primary hepatic cancer; SHC, secondary hepatic cancer; GC, gastric cancer; EC, esophageal cancer; CC, cervical cancer; NHL, non-Hodgkin lymphoma; LC, lung cancer; NSCLC, non-small cell lung cancer; Ad, advanced; Chem., chemotherapy; RT, radiotherapy; IVT, interventional therapy; TCM, traditional Chinese medicine; P407, Poloxamer 407; po, per os; iv, intravenous drip; iti, intro-tumor injection; TACE, transcatheter arterial chemoembolization; qd, one a day, quaque die; tid, three times a day, ter in die; qw, one a week; LQ, life quality, Karuafsky score; MST, median survival time; SR, survival rate; CR, complete response; PR, partial response; P < 0.05, statistically significant difference; NS, no significant difference
Multi-target anticancer activities and underlying mechanisms
The multi-target anticancer activities and underlying mechanisms of NCTD in treatment of different cancer models and cell lines have been reported. Here, we systematically review the potential anticancer activities and underlying molecular mechanisms of NCTD in vitro and in vivo.
Inhibiting proliferation and inducing apoptosis
In recent years, a large number of researches have been carried out to study the effects of NCTD on inhibiting proliferation and inducing apoptosis in different cancer models (Table 2). NCTD has a cytotoxic effect on a variety of tumor cells. Significant anti-proliferative and apoptotic effects are observed in NCTD-treated tumor cells [7, 49, 50]. At the same time, relevant studies have confirmed that NCTD has no myelosuppression and can induce hematopoiesis via bone marrow stimulation while exerting its anticancer activity [4, 5]. NCTD has no effect on the viability of normal peripheral blood mononuclear cells (MNC) [51, 52]. These are incomparable advantages over many traditional anticancer drugs. In addition, NCTD has a synergistic effect with a variety of anticancer drugs, such as cisplatin and gefitinib [53, 54].
Table 2.
Relevant researches of NCTD on inhibiting proliferation and inducing apoptosis
| Cancers | Cell lines | Basic mechanisms | Pathways | Accompanying roles | Experiment | References |
|---|---|---|---|---|---|---|
| Leukemia | K562 | DNA synthesis inhibition; G2/M phase cell-cycle arrest | In vitro | [18] | ||
| HL-60 | G2/M cell-cycle arrest and apoptosis | Inducing apoptosis via a caspases- dependent pathway, regulated by JNK activation signaling | [19] | |||
| Jurkat | S phase cell-cycle arrest; activation of cytochrome c, caspase-9, -3; PARP cleavage | Regulation of ATM | With no effect on the viability of normal MNCs | [51] | ||
| Jurkat T | G2/M phase cell-cycle arrest, down-regulating the expression of calcineurin, reducing calcineurin phosphatase activity | Activation of P38 and ERK1/2 | With no myelosuppression | [52] | ||
| HL-60 | S and G2/M-phase arrest;DNA synthesis inhibition | [55] | ||||
| Jurkat, Ramos | Inducing the degradation of Cdc6 | [65] | ||||
| Jurkat | Decreasing β-catenin protei | Inhibiting Wnt/β-catenin signaling | [70] | |||
| HL-60 | Inhibiting DNA replication, and induce apoptosis and caspase-3-dependent cleavage of Cdc6 | [133] | ||||
| MV4-11 | Modulating the expression of several molecules, including HLF, SLUG, NFIL3 and c-myc | With no myelosuppression, inducing haemopoiesis |
In vivo In vitro |
[4] | ||
| K562, HL-60 | DNA synthesis inhibition; G2/M phase cell-cycle arrest; producing interleukin (IL)-1β, colony stimulating activity (CSA) and tumor necrosis factor (TNF)-alpha | Inhibition of PP2A | Transient leukocytosis, less nephrotoxic and phlogogenic side-effects; stimulating hematopoiesis | [5] | ||
| L1210 | Inhibiting the serine/threonine protein PP2A | Without myelosuppression, inducing haemopoiesis | [62] | |||
| Z138, Mino | G2/M, G1 cell-cycle arrest, upregulating caspase-3, -8, and -9, suppressing NF-κB-regulated gene products, such as cyclin D1, BAX, survivin, Bcl-2, XIAP, and cIAP | Inhibiting PI3K–Akt–NF-κB signaling pathway | [72] | |||
| Hepatocellular cancer | HepG2 | Xenograft growth inhibition | Prolonging host survival | In vivo | [50] | |
| HepG2 | Activation of ERK and JNK; modulation of NF-kappa B and AP-1 | In vitro | [6] | |||
|
HepG2 Hep3B Huh-7 |
M-phase cell-cycle arrest; phosphorylation of p21, Cdc25C; regulation of cyclin B1-associated kinase activity; phosphorylation of Bcl-2 and Bcl-X(L), activation of caspase-3, -9 | [7] | ||||
| SMMC-7721 BEL-7402 | Inducing the activation of caspase-9, -3 and the cleavage of PARP, and downregulating the expression of Bcl-2, Bcl-X(L) and Mcl-1. | [11] | ||||
| HepG2 | Cytotoxic effect | [49] | ||||
| Hep3B | Downregulating TGF-β1 and Smad7, up-regulated Smad4 | Altering TGF-β1/Smads signaling | With cisplatin synergistic effect | [53] | ||
| HepG2 | G2/M phase cell-cycle arrest, upregulating Bax, and downregulating Bcl-2 | With EVO synergistic effect | [56] | |||
| BEL-7402 | M phase cell-cycle arrest; decreasing Bcl-2 expression | [58] | ||||
| HepG2 | Inducing the degradation of Cdc6 | [65] | ||||
| HepG2 | Inhibiting pre-RCs assembly, inducing degradation of Cdc6 and Mcm2, inhibiting the nuclear translocation of Mcm6, G1/S phase cell-cycle arrest, inhibiting DNA replication | Inhibiting pre-RCs assembly via degrading initiation protein Cdc6, Mcm2, and Mcm6 | With Cdc6 depletion synergistic effect | [66] | ||
| SMMC-7721 | Upregulating caspase-3, cytochrome c, AIF, and Bax, downregulating Bcl-2 | Activation of JNK and mitochondrial pathways | [134, 135] | |||
| HepG2 | Downregulating Bcl-2, upregulating Bax, reduction of Bcl-2/Bax ratio | Caspase-3, and -9 activities | [136] | |||
| HepG2 | An increase in ROS production, loss of mitochondrial membrane potential and release of cytochrome c (cyto-c) from the mitochondria to the cytosol and downregulating Bcl-2, upregulating Bax levels. Increasing caspase-9, -3 and PARP | Through ROS generation and mitochondrial pathway | [3] | |||
| Hep3B with deficiency of p53. | G(2)M or G(0)G(1) phase cell-cycle arrest, activation of caspase-3, -10 | Activation of a p53-independent pathway (caspase-3 and -10) via TRAIL/DR5 signal transduction | [137] | |||
| HepG2 | Downregulating LC3-II, an autophagosome marker; upregulating Bax, cytochrome c, caspase-3, -9, PARP, ROS production; disrupting MMP | Inhibiting autophagy via ROS generation and mitochondrial apoptosis pathway activation | Atg5 siRNA enhances the anticancer action | [138] | ||
| HepG2 SMMC-7721 | Inhibiting of Mcl-1, thus enhancing the release of cytochrome C, ABT-737, inducing apoptosis | Solving the ABT-737 drug resistance problem | [139] | |||
| SMCC-7721 SK-Hep-1 | G2/M phase cell-cycle arrest; upregulating FAM46C, mitigating DEN-initiated HCC in mice; inhibiting Ras, p-MEK1/2, p-ERK1/2 | Up-regulating FAM46C and inhibiting ERK1/2 signaling |
In vivo In vitro |
[57] | ||
| Hep3B | Inhibiting PP5 via activating AMPK signaling | [140] | ||||
| HepG2 HepG2/ADM hepatoma Hepal-1 | Inhibiting cell viability, decreasing CD4+ CD25+ T cells, downregulating FoxP3 in vitro; suppressing tumor formation, downregulating Tregs, FoxP3, CTLA-4, TGF-β, IL-10 in vivo | Downregulating regulatory T cells accumulation | With CLSO synergistic effect | [141] | ||
| Gallbladder cancer | GBC-SD | Inhibiting PCNA and Ki-67 expression | In vitro | [12, 67, 142] | ||
| GBC-SD | Inhibiting PCNA, Ki-67, cyclin D1, Bcl-2, Survivin; upregulation of p27, Bax |
In vivo In vitro |
[143, 144] | |||
| GBC-SD | Inhibiting cyclin D1, Bcl-2, Survivin; upregulating p27, Bax; S phase cell- cycle arrest | [145] | ||||
| Colorectal cancer |
Colo205 HT-29 SW480 |
G2/M phase cell-cycle arrest, activation of CD95 receptor/ligand and caspase 8 | In vitro | [59] | ||
| CT26 | Cell cycle arrest in the S and G2/M phases, inducing anoikis-mediated apoptosis | JNK activation | [60] | |||
| Six cell lines | Caspase-3, -8, -9 and MAPK activity | [68] | ||||
| HT-29 | Inhibiting integrin αvβ6-ERK | [146] | ||||
| HCT116, HT29 | G2/M phase cell-cycle arrest; downregulating EGFR, p-EGFR, c-Met, p-c-Met, and cyclinD1, Rb, CDK-4; increasing cleaved PARP and caspase-3 | Affecting cell cycle- and apoptosis-related signaling | Substituting for gefitinib | [147] | ||
| Breast cancer | MCF-7 | Repressing cell adhesion to platelets via downregulating α2 integrin | Activating protein kinase C pathway via PP2A inhibition | Inhibiting adhesion and migration | In vitro | [63] |
| MCF-7 | Inhibiting MAPK and the dephosphorylation of erk1, 2 | [148] | ||||
| ER-HS-578T ER + MCF-7 | Activation of MAPK and STAT pathways | [149] | ||||
| Bcap-37 | Increased ROS, decreased MMP, induced DNA damage and reduced G1, G2/M peak | [150] | ||||
|
MDA-MB-231 MDA-MB-468 BT-549 SKBR-3 MCF-7 BT474 |
Dual inhibition of pAkt and pERK1/2 signaling |
In vitro In vivo |
[16] | |||
| Highly-metastatic MDA-MB-231 | G2/M phase cell-cycle arrest; up-regulating Bax, down-regulating Bcl-2, Bcl-2/Bax ratio, p-Akt, NF-kappaB | Inhibiting the Akt and NF-kappaB signaling | Suppressing tumor growth in vivo | [73] | ||
| Gastric cancer | AGS | G0/G1 phase cell-cycle arrest; increasing ROS production, cytochrome c, AIF and Endo G release; upregulating BAX, BID, caspase-3, -8, -9; downregulating MMP, caspase-4, -12 | Through mitochondria- and caspase-dependent pathways | In vitro | [151] | |
| Melanoma | A375-S2 | Caspase-3, -9 activation and Bax upregulaton and Bcl-2 downregulation | In vitro | [152] | ||
| A375-S2 | Activation of JNK and p38 MAPK | [153] | ||||
| U266 | Potentializing the chemosensitivity to ADR | Regulating NF-κB/IκBα signaling pathway and NF-κB-regulated gene products including survivin, Bcl-2, Bax and VEGF | With ADR synergistic effect | [154] | ||
|
WM115A, 1205Lu Sbcl2, WM35 |
Increased cytochome c, Bax and caspase-3, decreased Bcl-2 and NF-κB2 | Activation of a TR3 dependent pathway | Improving survival |
In vitro In vivo |
[20] | |
| Downregulating IKKα and p-IκBα, inducing the accumulation of IκBα and inhibiting activation of NF-κB, potentializing the chemosensitivity to BTZ | Inhibiting NF-κB signaling pathway | With BTZ synergistic effect | [155] | |||
| NSCLC |
EGFR mutation − A549 EGFR mutation + PC9 |
G2/M phase cell- cycle arrest, enhancing the anticancer effects of gefitinib and cisplatin | With gefitinib and cisplatin synergistic effect | In vitro | [54] | |
|
A549 H1299 Calu6 |
Repressing YAP and its downstream targets CYR61 and CTGF; arresting cell cycle, inducing senescence | Repressing YAP signal pathway | Inhibiting EMT, motile, invasion via enhancing E-cadherin and decreasing fibronectin/vimentin | [80] | ||
| A549 | Downregulating Bcl-2, upregulating Bax, reducing Bcl-2/Bax ratio and viability | With trichostatin A, celecoxib, lovastatin, synergistic effect |
In vitro In vivo |
[157] | ||
| Oral cancer | KB cell | Induced significant cytotoxicity | In vitro | [21] | ||
| SAS, Ca9-22 | Activation of caspase-9, enhancing Bax, downregulating Bcl-2, Bcl-XL | [108] | ||||
| Medulloblastoma | DAOY, UW228 | Loss of β-catenin activation; reduce of β-catenin expression | Inhibition of Wnt/β-catenin signaling | Ability to cross the blood–brain barrier |
In vitro In vivo |
[71] |
| Glioma | U87, C6 | Inhibiting phospho-MEK, phospho-ERK, Bcl-2 and Mcl-1 | Blocking Raf/MEK/ERK pathway | In vitro | [157] | |
| Neuroblastoma | SH-SY5Y | Inhibiting MAPK and the dephosphorylation of erk1,2 | In vitro | [148] | ||
| SK-N-SH | Uppressing proliferation and cloning ability G2/M phase cell-cycle arrest; inducing mitophagy, autophagy; reducing MMP; downregulating cyclin B1, Cdc2, TOM20, SQSTM1/p62, p-AKT, mTOR; upregulating p21, beclin1, LC3-II, caspase-3, -9, p-AMPK; regulating Bax/Bcl-2, Bax/Mcl-1 | The AMPK, AKT/mTOR, and JNK/c-Jun signaling pathways are widely involved in these processes via activation of JNK/c-Jun pathway | [158] | |||
| Cervical cancer | HeLa | Inducing the degradation of Cdc6. | In vitro | [65] | ||
| HeLa | Up-regulation of caspase-3, -8, -9, and Bax; down-regulation of Bcl-xL. | Activation of ERK and JNK. | [159] | |||
| HeLa | G2/M cell-cycle arrest; downregulating ΔΨ(m), Bcl-2, cyclin B and cdc2; upregulating Bax, cytochrome c, p21 and p-cdc25c | Activating p38-NF-κB signaling pathway; p38-NF-κB-promoted mitochondria- associated apoptosis and G2/M cell cycle arrest | [160] | |||
| Bladder cancer | TSGH 8301 | S, G1phase cell-cycle arrest; upregulating caspase-3, -8, -9 and Fas, FasL, Bax, Bid, cytochrome c, and ROS production; downregulating ΔΨ(m), ERK, JNK, p38 | Activation of ROS-modulated Fas receptor, caspse-3, -8, -9 mitochondrial -dependent and -independent pathways | In vitro | [161] | |
| Prostate cancer | DU145 | Inhibiting DNA replication and pre-RCs, inducing mitotic catastrophe | Blocking ATR-dependent checkpoint pathway; degrading initiation protein Cdc6 | With paclitaxel synergistic effec | In vitro | [162] |
| DU145 | Downregulating PCNA, MnSOD; destructing MMP, ROS-mediated DNA damage; depleting ATP; activating AMPK | ROS-mediated mitochondrial dysfunction and energy depletion | [163] | |||
| Increasing autophagy; inducing autophagic cell death, cell proliferation arrest; upregulating Beclin-1; suppressing miR-129-5p | Inducing autophagy-related cell death through Beclin-1, upregulation by miR-129-5p suppression | [164] | ||||
| 22Rv1, Du145 | Increased oligonucleosomal formation, PARP cleavage; upregulating cytochrome c, caspase-3, -8, -9, Fas, DR5, RIP, TRADD; increased ratios of pro-/anti-apoptotic proteins and decreased expression of IAP family member proteins, including cIAP1 and survivin | Inducing both intrinsic and extrinsic apoptotic pathways | [165] | |||
| Mitochondria dysfunction, modulating Akt signaling via increasing nuclear translocation and interaction with Mcl-1 | Suppressing Mcl-1 via epigenetic upregulation of miR-320d |
In vitro In vivo |
[166] | |||
| Osteosarcoma | 143B, SJSA | Inducing G2/M cell cycle arrest | Blocking the Akt/mTOR signaling pathway | In vitro | [167] | |
|
MG63 HOS |
The induction of autophagy, the triggering of ER stress and the inactivation of the c-Met/Akt/mTOR pathway | The inhibition of the c-Met/Akt/mTOR signaling pathway |
In vitro In vivo |
[22] | ||
| Glioblastoma |
RT-2 U251 |
G(2)/M phase arrest and post-G(2)/M apoptosis in RT-2 cell line | Adenoviral p53 gene therapy enhances chemosensitivity of tumor cells to NCTD. | In vitro | [168] | |
| Giant cell tumor of bone (GCTB) | Suppressing the PI3K/AKT signaling pathway through upregulating the expression of miR-30a | Modulating the miR-30a/MTDH/AKT cell signaling pathway | In vitro | [169] |
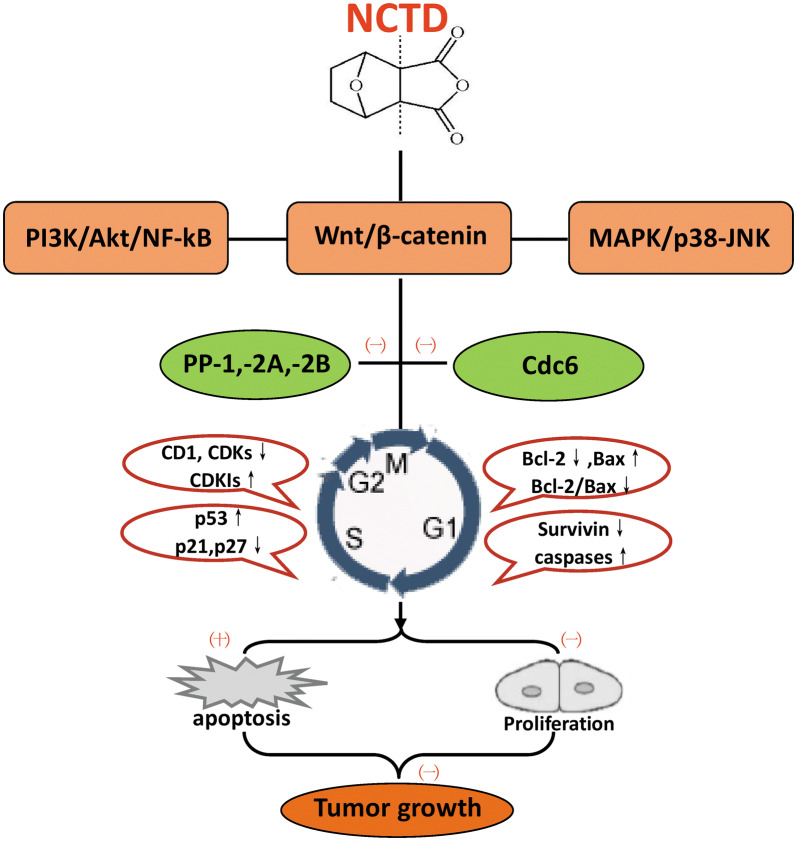
The anti-proliferation and pro-apoptotic effects of NCTD depend on the complex interactions between different molecules (Fig. 3). On the one hand, the inhibitory effect of NCTD on proliferation is mainly achieved through cell cycle arrest and inhibition of DNA synthesis by inhibiting the expression of cyclins, cyclin-dependent kinases (CDKs) and increasing the expression of cyclin-dependent kinase inhibitors (CDKIs, such as p21Cip/Waf1, p27kip1); On the other hand, NCTD can also induce apoptosis by increasing the expression of pro-apoptotic protein such as P53, Bax, Caspases, and reducing the expression of anti-apoptotic proteins such as Bcl-2 (B-cell lymphoma-2) and survivin. These mechanisms have been confirmed in a variety of tumor cell lines such as leukemia K562 and HL-60 [18, 55], hepatoma HepG2, SMMC-7721 and BEL-7402 [56–58], colorectal cancer CT26 and HCT-15 cells [59, 60], etc. It is generally believed that serine/threonine protein phosphatases, such as protein phosphatase type 1 (PP1), protein phosphatase-2A (PP2A) and protein phosphatase-2B (PP2B), play important roles in intracellular signal transduction, whose inhibition is an excellent target for the development of novel anti-cancer agents [5, 61, 62]. Some studies have confirmed that NCTD, as a PP2A inhibitor, can inhibit cancer cell proliferation and induce apoptosis by inhibiting the activity of PP2A [5, 62, 63]. In addition, DNA replication-initiation protein Cdc6 (cell division cycle protein 6) is an effective target to disturb DNA replication [64]. Other studies have found that NCTD can inhibit cell proliferation by inducing Cdc6 degradation [65, 66]. In gallbladder cancer, it was reported that NCTD inhibited the expression of GBC-SD cell proliferation-related gene proteins PCNA (proliferating cell nuclear antigen) and Ki-67, this may be one of the mechanisms by which NCTD inhibit the proliferation and growth of tumor cells [12, 67].
Fig. 3.
The “multi-points priming” mechanisms of NCTD on inhibiting proliferation and inducing apoptosis. NCTD: norcantharidin; PI3K: phosphoinositide 3 kinase; NF-κB: nuclear factor-kappa B; MAPK: mitogen-activated protein kinase; JNK: Jun N-terminal kinase; PP1: protein phosphatase type 1; PP2A: protein phosphatase 2A; PP2B: protein phosphatase 2B; Cdc6: cell division cycle protein 6; CD1: cyclin D1; CDKs: cyclin-dependent kinases; CDKIs: cyclin-dependent kinase inhibitors; Bcl-2: B-cell lymphoma-2; (−): Inhibition; (+): Promotion or inducing
NCTD inhibited proliferation and induced apoptosis in cancer cells is dose- and time-dependent [51, 55], and is regulated by both extrinsic and intrinsic signaling pathways [34]. MAPK (mitogen-activated protein kinase) can be divided into four subfamilies: ERK (extracellular regulated protein kinases), p38, JNK (Jun N-terminal kinase) and ERK5. MAPK-related signaling pathways are widely involved in NCTD-induced apoptosis [68]. For instance, NCTD-induced apoptosis in leukemia HL-60 cells is regulated by activating JNK signaling [19], and apoptosis in hepatocellular cancer HepG2 cells induced by NCTD is dependent on ERK and JNK activity [6]. The Wnt/β-catenin signaling pathway is considered to be another target for antitumor drugs [69]. Some studies have shown that NCTD can reduce the proliferation of leukemia Jurkat cells by inhibiting Wnt/β-catenin signaling [70]. Due to the ability to cross the blood–brain barrier, NCTD can also significantly inhibit the growth of medulloblastoma through Wnt/β-catenin signaling pathway [71]. In addition, NCTD can inhibit the expression of the proliferation-related protein cyclin D1, downregulate the expression of anti-apoptotic protein, and upregulate the expression of pro-apoptotic protein by blocking PI3K (phosphoinositide 3 kinase)/Akt/NF-κB (nuclear factor-kappa B) pathway [72, 73]. So, the PI3K/Akt/NF-κB pathway has been shown to be another signal pathway for the regulation of NCTD-mediated anti-proliferation and pro-apoptosis.
Inhibiting tumor invasion/metastasis
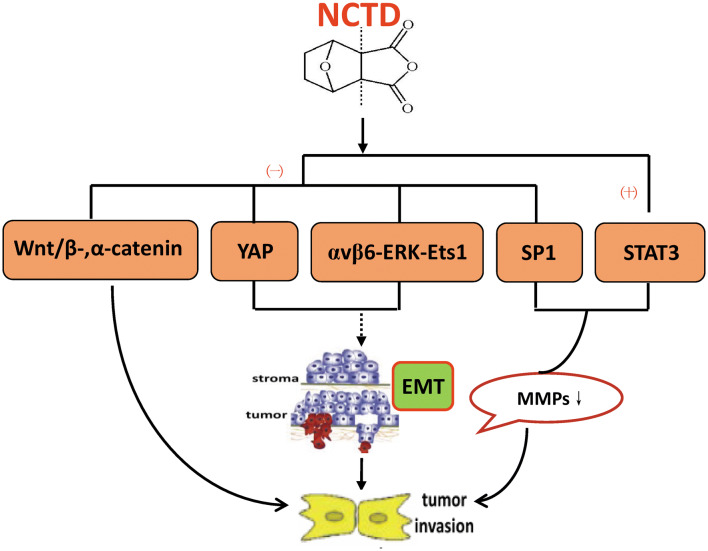
Two major protein families are involved in NCTD against tumor invasion and metastasis, including matrix metalloproteinases (MMPs) and adhesion molecules [74]. The MMP family, particularly MMP-2 and MMP-9, has gelatinase activity and is capable of proteolytic cleavage of plasminogen in extracellular matrix [75]. Cell adhesion molecules such as α-catenin and b-catenin have the function of adhering tumor cells to other cellular and matrix components [76], both of them play an important role in local invasion and distant metastasis.
It has been confirmed that NCTD has anti-invasion and anti-metastasis effects in many kinds of tumor cells (Table 3). Some experiments indicated that NCTD reduces the activity of MMP-2 and MMP-9 by upregulating the transcription factor STAT1 (signal transducers and activators of transcription 1) and inhibiting the transactivation of Sp1 (specificity protein 1), thereby inhibiting the invasion and metastasis of tumor cells [77, 78]. Another study showed that NCTD has the ability to reduce the expression of α-catenin and β-catenin in colorectal cancer CT26 cells, suggesting that the anti-invasive and anti-metastatic activity of NCTD may be related to the regulation of these adhesion molecules [75]. Furthermore, epithelial–mesenchymal transition (EMT) is widely involved in the invasion and metastasis of malignant epithelial tumors [79]. NCTD inhibits the EMT process in non-small cell lung cancer, colorectal cancer and hepatocellular cancer cells via the αvβ6-ERK-Ets1 (E-Twenty-Six-1) signaling pathway blocking and NCTD-mediated Yes-associated protein (YAP) inhibition [78, 80, 81]. These regulatory mechanism of NCTD against tumor invasion and metastasis is detailed in Fig. 4.
Table 3.
Relevant researches of NCTD against invasion and metastasis for multiple cell lines in different cancer models
| Cancers | Cell lines | Basic mechanisms | Pathways | Accompanying roles | Experiment | References |
|---|---|---|---|---|---|---|
| Gallbladder cancer | GBC-SD | Upregulating TIMP-2 and MMP-2/TIMP-2 ratio, downregulating MMP-2 | In vitro | [142] | ||
| Colorectal cancer | CT26 | Downregulating MMP-9 and gelatinase; inhibiting the DNA-binding activity of Sp1 | Inhibiting Sp1 transcriptional activity | In vitro | [77] | |
|
HT-29 WiDr |
Downregulating αvβ6, MMP-3, MMP-9, N-cadherin, vimentin, p-ERK, p-Ets1; up-regulating E-cadherin | Inhibiting EMT by blocking αvβ6-ERK-Ets1 signaling pathway | [78] | |||
| CT26 | Down-expressing MMP-2, -9 and Desmoglein, N-cadherin, α- and β-catenin; reducing pulmonary metastasis. | Prolonging mice survival |
In vitro In vivo |
[74] | ||
| NSCLC |
A549 PC9 |
Inhibiting migration; enhancing the anticancer effects of gefitinib and cisplatin | Not altering p-EGFR | With gefitinib and cisplatin synergistic effect | In vitro | [54] |
|
A549 H1299 Calu6 |
Interfering the YAP-mediated cell progression and metastasis; inhibiting EMT, motile, invasion via enhancing E-cadherin and decreasing fibronectin/vimentin; repressing YAP and its downstream CYR61, CTGF | Repressing YAP signal pathway | [80] | |||
| A549 | Suppressing migration | Inhibiting p-Akt, NF-κB | With trichostatin A, celecoxib, lovastatin, synergistic effect |
In vitro Ex vivo |
[156] | |
| Breast cancer | MCF-7 | Inhibiting adhesion and migration, repressing cell adhesion to platelets via downregulating α2 integrin | Activating protein kinase C pathway via PP2A inhibition. via protein kinase C pathway-dependent, downregulation of α2 integrin | In vitro | [63] | |
| Hepatocellular cancer |
Huh7 SK-Hep1 |
Downregulating MMP-9, u-PA, p-ERK1/2, NF-kB, FAK; upregulating PAI-1 and TIMP-1 | Inhibiting the phosphorylation of ERK1/2 and NF-kB signaling pathway | In vitro | [170] | |
| SMMC-7721, MHCC-97H | Suppressing cell motility and invasiveness; up-regulating FAM46C; suppressing TGF-β/Smad signaling, EMT | Up-regulating FAM46C via brocking EMT process and TGF-β/Smad signaling | [9] | |||
|
HCCLM3 SMMC-7721 |
Inhibiting IL-6-induced EMT and cell invasiveness, and JAK/STAT3/TWIST signaling | Inhibiting IL-6-induced EMT via JAK2/STAT3/TWIST signaling | [81] | |||
| Osteosarcoma |
MG63 HOS |
Inhibiting the expression of MMP-2 and MMP-9 |
In vitro In vivo |
[22] | ||
| Giant cell tumor of bone (GCTB) | Inhibiting the EMT process | Modulating the miR-30a/MTDH/AKT cell signaling pathway | In vitro | [169] |
Fig. 4.
Underlying regulatory targets of NCTD against invasion and metastasis. NCTD: norcantharidin; YAP: Yes-associated protein; ERK: extracellular regulated protein kinases; Ets1: E-Twenty-Six-1; Sp1: specificity protein 1; STAT1: signal transducers and activators of transcription 1; MMPs: matrix metalloproteinases; EMT: epithelial–mesenchymal transition. (−): Inhibition; (+): Promotion or inducing
Anti-angiogenesis and anti-vasculogenic mimicry
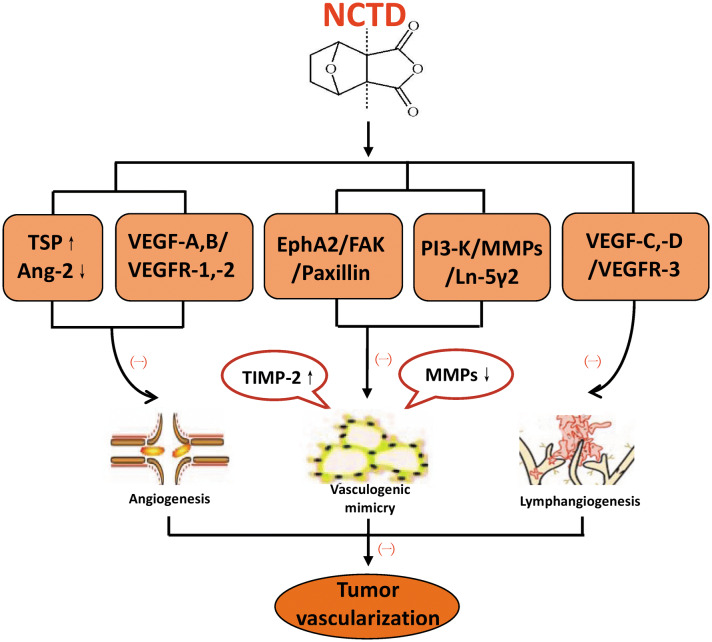
Angiogenesis and effective blood supply are basic conditions for tumor growth and metastasis [82]. Multiple angiogenic growth factors and cytokines play important roles in regulating tumor angiogenesis, such as vascular endothelial growth factor (VEGF) and its corresponding receptor, thrombospondin (TSP), angiogenin (Ang), and tissue metalloproteinase inhibitor (TIMP) family. In gallbladder and colorectal cancer, it has been confirmed that NCTD can inhibit angiogenesis, induce apoptosis of vascular endothelial cells, downregulate the expression of angiogenic factors such as VEGF, VEGFR-2 (vascular endothelial growth factor receptor-2), Ang-2, and upregulate the expression of anti-angiogenic factors such as TSP and TIMP-2 [83–86]. So, NCTD may be a potential anti-angiogenic drug for cancer treatment.
Tumor vasculogenic mimicry (VM) refers to a novel tumor blood supply pattern that occurs in certain highly aggressive malignancies and is associated with poor clinical outcomes and poor prognosis [87]. TIMP-2 has anti-VM activity in some highly aggressive malignancies [88]. Furthermore, the PI3-K (phosphatidylinositol 3-kinase)/MMPs (matrix metalloproteinases)/Ln-5γ2 (laminin 5γ2) and EphA2 (ephrin type a receptor 2)/FAK (focal adhesion kinase)/Paxillin signaling pathways are two critical pathways for the control of VM formation [89], while MMP-2 and MT1-MMP (membrane type 1-matrix metalloproteinase) are key molecules and important mediators of these two pathways, regulating VM formation in invasive malignant cells [90]. NCTD is believed as a potential anti-VM active drug, its anti-VM mechanisms mainly involves two aspects: NCTD downregulates the expression of MMP-2 and MT1-MMP via inhibiting EphA2/FAK/Paxillin signaling pathway, thereby enhancing the anti-VM activity of TIMP-2; in turn, a decrease in MMP-2 and MT1-MMP activity inhibits PI3-K/MMPs/Ln-5γ2 signaling and exerts an anti-VM effect on malignant cells [13, 91–93].
Anti-lymphangiogenesis
Lymphatic metastasis is one of the important metastatic pathways of tumors, and tumor lymphatic tube formation (lymphangiogenesis) plays an important role in tumor growth, metastasis and prognosis [94]. Lymphatic endothelial growth factors, including two members of the VEGF family, VEGF-C and VEGF-D, as well as their cognate receptor VEGFR-3, are the main regulators of tumor lymphangiogenesis and is of great significance in tumor lymph node metastasis [95–97]. In recent years, some researchers have reported that NCTD is an effective lymphangiogenesis inhibitor. The basic mechanism of NCTD anti-lymphangiogenesis refers to directly or indirectly downregulate the expression of VEGF-C, VEGF-D and VEGFR-3 at protein and mRNA levels, which has been proved in human lymphatic endothelial cells (HLECs) and human colonic adenocarcinomas (HCACs) [98–100]. In addition, NCTD in combine with sorafenib or mF4-31C1 enhanced the ability of anti-lymphangiogenesis in human colonic adenocarcinomas [100].
The relevant researches and mechanisms of NCTD inhibiting tumor vascularization (Angiogenesis, VM and lymphangiogenesis) are summarized in Table 4 and Fig. 5.
Table 4.
Relevant studies of NCTD anti-angiogenesis, anti-VM, and anti-lymphangiogenesis
| Anticancer activities | Cancers | Cell lines | Basic mechanisms | Pathways | Accompanying roles | Experiment | References |
|---|---|---|---|---|---|---|---|
| Anti-angiogenesis | Gallbladder cancer | GBC-SD | Inhibiting capillary-like tube formation of HUVECs in vitro; destroying angiogenesis and CAM capillaries; decreasing xenograft MVD and vascular perfusion in vivo; downregulating VEGF, Ang-2; upregulating TSP, TIMP-2 | Prolonging xenograft-mice survival | In vitro | [84] | |
| GBC-SD | Lower MVD and PCNA/apoptosis ratio, smaller tumor volume; down-regulating VEGF and Ang-2, and up-regulating TSP and TIMP2; MVD positively correlating with VEGF, Ang-2n and negatively correlating with TSP and TIMP2 |
In vitro In vivo |
[83] | ||||
| Colorectal cancer | HCT116 | Inhibiting xenograft growth and tumor angiogenesis in vivo; reducing migration, adhesion and vascular network tube formation of HUVECs in vitro; downregulating VEGF and VEGFR-2 | Downregulating VEGF and VEGFR-2 | In vivo | [85] | ||
| CT26 | Inhibiting viability, adhesion, migration, capillary-like tube formation of HUVECs, and the release of pro-angiogenic factors from HUVECs; inducing anoikis; down-regulating VEGF, integrin β1, vimentin, p-JNK and p-ERK | Down-regulating VEGF and inhibiting MAPK (JNK/ERK) signaling | Without renal or hepatic toxicity |
In vitro In vivo |
[14] | ||
| LOVO | Inhibiting VEGF-induced proliferation, migration, invasion, capillary tube formation of HUVECs and LOVO proliferation; inhibiting tumor angiogenesis and tumor growth in vivo; inhibiting VEGFR2/MEK/ERK pathway | Blocking VEGFR2/MEK/ERK | [86] | ||||
| Anti-VM | Gallbladder cancer | GBC-SD | Inhibiting proliferation, invasion, migration, VM formation in vitro and in vivo; downregulating EphA2, FAK and Paxillin | Blocking the EphA2/FAK/Paxillin signaling pathway | Prolonging xenograft mice survival |
In vitro In vivo |
[13] |
| GBC-SD | Inhibiting proliferation, growth, invasion, migration and VM formation in vitro and in vivo; downregulating MMP-2, MT1-MMP, PI3-K, Ln-5γ2 | Suppression of the PI3-K/MMPs/Ln-5γ2 signaling pathway | [91] | ||||
| GBC-SD | MMP‑2, MT1‑MMP relating tumor VM In vitro; a poor survival in VM+ patients with high MMP‑2, MT1‑MMP expression; inhibiting tumor growth, VM formation, VM hemodynamic in vivo; inhibiting proliferation, invasion, migration and VM‑like networks in vitro; downregulating MMP‑2 and MT1‑MMP in vivo and in vitro; thus, enhancing TIMP‑2 antitumor and anti‑VM activities | Enhancing TIMP-2 anti-VM via downregulating MMP-2 and MT1-MMP | With TIMP-2 synergistic effect; prolonging xenograft mice survival | [92] | |||
| Melanoma | A375 | Suppressing MMP-2 expression |
In vitro In vivo |
[83] | |||
| Anti-lymphangiogenesis | HLEC | HDLECs | Inhibiting proliferation, migration, invasion, lymphatic tube formation (lymphangiogenesis), inducing apoptosis; downregulating VEGF-C, VEGF-D and VEGFR-3 expression | Blocking VEGF-C,-D, VEGFR-3 | In vitro | [98] | |
| HDLECs | Inhibiting growth, lymphatic tube formation; inducing apoptosis; downregulating VEGF-C and VEGF-D expression | Downregulating the expression of VEGF-C and VEGF-D | [99] | ||||
| Colorectal cancer | HT-29 | S-phase cell-cycle arrest; Inhibiting proliferation, migration, invasion, lymphatic tube formation in vitro and tumor growth and lymphangiogenesis in vivo; downregulating Ki-67, Bcl-2, LYVE-1, D2-40, CK20 and their LMVD, and VEGF-A, VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 in vitro and in vivo | Blocking the VEGF-A,-C,-D, VEGFR-2, -3 “multi-points priming” mechanisms | With mF4-31C1 or Sorafenib synergistic effect |
In vitro In vivo |
[100] | |
| AML | TSC-null cell 21-101 | Inhibiting proliferation of TSC2−, TSC2+ cells with rapamycin | An additive effect between rapamycin and NCTD in inhibiting lymphangiogenesis | In vitro | [171] |
Fig. 5.
The “more targets” mechanisms of NCTD against tumor vascularization (angiogeneses, VM and lymphangiogenesis). NCTD: norcantharidin; TSP: thrombospondin; Ang-2: angiogenin-2; VEGF: vascular endothelial growth factor; VEGFR: vascular endothelial growth factor receptor; EphA2: ephrin type a receptor 2; FAK: focal adhesion kinase; PI3-K: phosphatidylinositol 3-kinase; MMPs: matrix metalloproteinases; Ln-5γ2: laminin 5γ2; TIMP: tissue metalloproteinase inhibitor. (−): Inhibition; (+): Promotion or inducing
Overcoming multi-drug resistance
Multi-drug resistance (MDR) refers to tumor cells develop resistance to anti-tumor drugs, as well as producing cross-resistance to other antineoplastics with different structures and mechanisms [101]. As one of the main problems in clinical tumor chemotherapy, MDR directly affects the efficacy of chemotherapy drugs and even lead to treatment failure [102].
In human breast cancer cells, NCTD may overcome MDR through inhibiting sonic hedgehog (Shh) signaling and its downstream MDR-1/P-gp expression [103], which has been shown to increase resistance to a variety of structurally unrelated antitumor drugs [104]. Bcl-2 family proteins Bcl-2 and Bcl-xL are resistant to multiple chemotherapeutic agents in a variety of cell lines [105–107], and it was reported that NCTD downregulated the expression of Bcl-2 and Bcl-xL in oral cancer cells [108]. In addition, Bcl-2 family inhibitors ABT-737 and ABT-263 are two promising anticancer agents with anticancer activity against a variety of cancer cells [109, 110]. NCTD significantly enhances ABT-263 and ABT-737-mediated anticancer activity, and overcomes the increased ABT-737 resistance caused by elevated Mcl-1 levels in cancer cells [111–113]. Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are widely used in anti-tumor therapy for non-small cell lung cancer (NSCLC) [114]. HGF (hepatocyte growth factor) overexpression is a major factor contributing to acquired resistance caused by EGFR-TKI [115]. NCTD can overcome HGF-induced EGFR-TKI resistance in EGFR-mutant lung cancer cells by inhibition of the Met/PI3K/Akt pathway [116]. Therefore, NCTD may be a potential agent to reverse MDR (Table 5).
Table 5.
Summary of related research on NCTD overcoming multidrug resistance
| Cancers | Cell lines | Basic mechanisms | Pathways | Accompanying roles | Experiment | References |
|---|---|---|---|---|---|---|
| Oral cancer | SAS, Ca9-22 | Activation of caspase-9, enhancing Bax, downregulating Bcl-2, Bcl-XL | In vitro | [108] | ||
| Breast cancer | MCF-7S, MCF-7R, MDA-MB-231, BT-474 | Inhibiting Shh signaling and expression of its downstream mdr-1/P-gp expression | In vitro | [103] | ||
| MDA-MB-231, MDA-MB-468, MDA-MB-415, AU565 | Inhibiting SMAC mimetic Birinapant-mediated cell viability and promoting apoptosis and cell death; reducing c-FLIP; enhancing Birinapant-triggered caspase-8/caspase-3, Inhibiting caspase-8 | Downregulation of c-FLIP | With SMAC mimetics promoting Birinapant-mediated anticancer activity | [172] | ||
| Hepatocellular cancer | Multiple HCC cell lines | Inducing transcriptional repression of Mcl-1 and enhancing ABT-737-mediated cell viability inhibition and apoptosis; activation of mitochondrial apoptosis pathway, involving cytosolic release of cytochrome c, cleavage of caspase-9, -3 | Enhancing ABT-737-induced apoptosis by transcriptional repression of Mcl-1 | Enhancing ABT-737 therapeutic efficacy | In vitro | [111] |
| HepG2, SMMC-7721 | ABT-737 plus NCTD have stronger proliferation inhibition, greater apoptosis induce and stronger Mcl-1 inhibiting, thus enhancing the release of cytochrome C and ABT-737 inducing apoptosis | With ABT-737 solving resistance of ABT-737 to liver cancer | [112] | |||
| Neuroblastoma | SH-SY5Y CHLA-119 | Enhancing ABT-263-mediated apoptosis, inhibiting cell viability and clonal formation; upregulating Noxa with cytosolic release of cytochrome c, activation of caspase-9, -3, and cleavage of PARP | Enhancing ABT-263-mediated anticancer activity by upregulation of Noxa | In vitro | [113] | |
| Hepatocellular cancer; Cervical cancer |
HepG2 Hela |
Inhibiting PTX-induced Cdc6 up-regulation, maintaining Cdk1 activity, and repressing Cohesin/Rad21 cleavage, thus reducing mitotic slippage and overcoming PTX resistance | Reducing mitotic slippage and overcoming PTX resistance via inhibiting Cdc6 | In vitro | [155] | |
| Pancreatic cancer | PANC-1, CFPAC-1 | Repressing cell growth and stemness marker CD44, CD24, EPCAM, CD44(+)/CD24(+)/EPCAM(+) proportion, and β-catenin pathway-dependent manner; strengthening the cytotoxicity of gemcitabine and erlotinib | Repressing the stemness of pancreatic cancer cells through repressing β-catenin pathway, strengthening the cytotoxicity of gemcitabine, erlotinib | Strengthening the cytotoxicity of gemcitabine, erlotinib | In vitro | [173] |
| NSCLC |
PC-9 HCC827 |
Reversing resistance to EGFR-TKIs induced by exogenous and endogenous HGF in EGFR mutant lung cancer cells via inhibiting the Met/PI3K/Akt pathway; NCTD plus gefitinib regressing tumor growth and Akt phosphory in vivo | Inhibition of Met/PI3k/Akt pathway | With EGFR-TKIs in vitro, with gefitinib in vivo |
In vitro In vivo |
[116] |
| Lymphoma | Multiple myeloma cells | Induction of G2/M arrest; down-regulating IKKα and p-IκBα | Inactivation of NF-kB signaling pathway | Enhancing bortezomib- antimyeloma activity |
In vitro In vivo |
[174] |
Promoting tumor cell demethylation
Tumorigenesis is a process of interaction between genetic and epigenetic mechanisms. DNA methylation is an important epigenetic regulator closely related to the occurrence and development of tumors [117]. Abnormal DNA methylation is involved in the pathogenesis of tumors. DNA hypomethylation promotes gene expression, while DNA hypermethylation inhibits gene expression [118, 119]. Hypermethylation of RASSF1A (a tumor suppressor gene) results in loss of function in human tumor cells [120]. It was reported that NCTD can inhibit RASSF1A methylation and inducing its re-expression in hepatocellular cancers [121]. Moreover, the Wnt/β-catenin signaling pathway is closely related to a variety of neoplastic diseases and is activated in tumor formation [122, 123]. Wnt inhibitory factor-1 (WIF-1), as a Wnt antagonist, has the function of inhibiting Wnt signal transduction. And due to hypermethylation of the promoter, WIF-1 silencing occurs in some tumor cells [124]. Studies have demonstrated that NCTD can activate WIF-1 to inhibit Wnt signaling pathway through promoter demethylation in NSCLC and glioma cells [125, 126] (Table 6).
Table 6.
Studies of NCTD on promoting demethylation, modulating immune response and some other anticancer activities
| Anticancer activities | Cancers | Cell lines | Basic mechanisms | Pathways | Accompanying roles | Experiment | Referencess |
|---|---|---|---|---|---|---|---|
| Promoting demethylation | NSCLC | Inhibiting proliferation, invasion, migration; inducing apoptosis and cell-cycle arrest; blocking β-beta-catenin; altering Bax, caspase-3, Bcl-2; activating WIF-1 and SFRP1; promoting WIF-1 demethylation, thus inhibits Wnt signal pathway | Promoting demethylation of WIF-1 | Activating WIF-1 and SFRP1 | In vitro | [125] | |
| Glioma |
LN229 U251 |
Inhibiting proliferation, migration, invasion; inducing apoptosis and G2 phase cell-cycle arrest; downregulating Bcl-2, activating caspase-3; promoting WIF-1 and its demethylation; suppressing Wnt/β-catenin signaling, cyclin B1, and β-catenin/TCF-4; Bcl-2 and cleaved caspase-3 | Inhibiting Wnt/β-catenin pathway via promoting WIF-1 demethylation | Activating WIF-1 and SFRP1 | In vitro | [126] | |
| Hepatocellular cancer | HepG2 | Inhibiting proliferation and RASSF1A methylation in a dose-dependent manner | Inhibiting RASSF1A methylation | In vitro | [121] | ||
| Modulating immune responses | Macrophages | Promoting the phosphorylation of AKT/p65 and transcriptional activity of NF-κB | Upregulation of AKT/NF-κB signaling pathway |
In vitro In vivo |
[127] | ||
| Peripheral blood mononuclear cell (PBMC) | Blocking PHA-induced cyclins D3, E, A and B and IL-2 mRNAs expression; improving production of cyclin D3, E, A and B and IL-2; Cell cycle G0/G1 arrest; blocking cell proliferation | In vitro | [128] | ||||
| Suppressing tumor glucose oxidative metabolism | Morris Hepatoma 7777 | Suppressing tumour 14C-labelled glucose oxidative metabolism in rat Morris hepatoma |
In vitro In vivo |
[130] | |||
| Inhibiting NAT activity | Hepatocellular cancer | HepG2 | NAT activity on acetylation of 2-aminofluorene (AF) and p-aminobenzoic acid (PABA) were examined, inhibiting NAT activity | In vitro | [131] | ||
| The effect on leukemic stem cells | Acute myeloid leukemia | MV4-11 | Decreasing HLF, inducing apoptosis by modulating HLF, SLUG, NFIL3 and c-myc, thereby inducing p53 and the mitochondrial caspase cascade, producing no myelosuppression |
In vitro In vivo |
[4] | ||
| Modulating macrophage polarization | Hepatocellular cancer | HepG2, mouse hepatoma H22, BMDM Raw 264.7 | Inhibiting tumor growth, survival and invasion, decreasing a shift from M2 to M1 polarization and CD4+/CD25+ Foxp3 T cells in HCC microenvironment; inhibiting STAT3; enhancing M1 polarization through increasing miR-214 expression; inhibited β-catenin | Through miR-214 modulating macrophage polarization |
In vitro In vivo |
[23] |
Modulating immune responses
The immune system plays a very important role in the development of tumors. The inflammatory response is a common and serious complication due to the continued damage to the immune system by the cancer itself and anti-cancer drugs. NCTD positively regulates macrophage-mediated immune responses via the AKT/NF-κB signaling pathway, helping to clear invading pathogens [127]; NCTD also reduces tissue inflammation by suppressing PBMC (human peripheral blood mononuclear cells) proliferation and cytokine gene expression and production [128]. In addition, the increased production of IL-10 will block the effect of specific T lymphocytes on tumor cells [129], and NCTD inhibits the production of IL-10 in PBMC induced by PHA (phytohemagglutinin) [128] (Table 6).
Others
NCTD has also been reported to have some other anticancer activities, including inhibition of tumor glucose oxidative metabolism [130]; inhibition of NAT (N-acetyltransferase) activity [131]; regulation of macrophage polarization [175]; regulation of leukemia stem cell activity [4] (Table 6). Due to the lack of relevant researches, it is necessary to further verify the relevant mechanisms and applications in the clinic.
Discussion
In recent years, the anti-tumor effect of TCMs has aroused extensive attention. However, due to the complexity of components, difficulty in extraction and high toxicity, the clinical application of many anti-tumor TCMs is limited. NCTD, as a demethylation product of CTD, can be extracted from CTD or synthesized artificially at a low cost. In addition, its physical and chemical properties are clear, so it is convenient for basic and clinical research. These prerequisites are helpful for the promotion of NCTD in clinical practice.
On the basis of summarizing the relevant literature, we found that there are two main ways of clinical application of NCTD. First of all, NCTD can be used as an anti-tumor drug alone in the treatment of liver cancer, gastric cancer and other tumors, especially for advanced malignant tumors that have lost the opportunity of operation. Secondly, it is used as an adjuvant of other anti-tumor drugs, which is currently the most important way for NCTD applied in clinic. Some studies have shown that the combination of NCTD with other anticancer drugs, or as an adjuvant to chemotherapy or interventional therapy, can help to improve the efficacy, increase the tolerance of patients, reduce side effects, and improve the prognosis [28, 30, 33].
Adverse reactions and serious complications of NCTD are rare. Gastrointestinal symptoms such as nausea and vomiting may occur when the oral dose or injection is excessive. A study has shown that patients with advanced liver cancer who take NCTD more than 45 mg/day will have significant gastrointestinal response [25]. It has also been reported that when the dosage of NCTD reaches 600 mg, the patients may have slight gastrointestinal symptoms, but it will be relieved soon after the drug is stopped or the alkaline agent is taken [27]. A large number of clinical studies have proven that patients treated with NCTD have no obvious symptoms of urinary irritation, no adverse effects on liver and renal function, and no myelosuppression [27, 28, 32].
Among the three routes of administration, oral administration and intravenous administration are simple and safe. The disadvantage is that the drug is eliminated quickly in the body, resulting in poor anti-tumor effect. It is reported that the half-life of NCTD in blood is short, only about 0.26 h [17]. Local injection is mainly used for some solid tumors, especially for advanced liver cancer which can not be treated by surgery. Compared with the former two, this method has better curative effect. However, due to the invasive operation, there are some risks such as bleeding, cancer rupture and so on.
NCTD has the disadvantages of poor water solubility, short half-life and low tumor targeting efficiency, which limits its clinical application [132, 176]. Therefore, a variety of NCTD analogues have been developed to improve the clinical applicability and efficacy. These NCTD analogues can be divided into two categories: new NCTD reagents and drug delivery systems. For example, it has been reported a new type of NCTD conjugate recently, called CNC conjugates (NCTD-conjugated carboxymethyl chitosan). Compared with the same dose of free NCTD, CNC conjugates have higher therapeutic concentration and longer half-life. It can not only enhance the inhibitory effect on cancer cells, but also reduce side effects [177, 178]. In addition, some other NCTD derivatives and liposomes, such as NOC15 (N-farnesyloxy-norcantharimide) [179] and SG-NCTD-LIP (NCTD-loaded liposomes modified with stearyl glycyrrhetinate) [176], also can effectively improve the anticancer activity and reduce the toxicity of NCTD. However, although these studies have shown that NCTD analogues have a very broad application prospect, most of the existing NCTD analogues have no obvious selectivity for tumors and targets. And it should be noted that most of the relevant researches are in the stage of basic research at present, whether these NCTD analogues can be applied to clinical needs to be confirmed by a large number of clinical experiments.
Conclusions
Collectively, NCTD, as a demethylation derivative of traditional Chinese medicine, has been clinically used to treat cancer patients, and is gradually believed as a useful adjunct anticancer drug, especially for the patients with mid-advanced and postoperational recurrent cancers. The underlying molecular mechanisms of NCTD anticancer activities maybe “multi-factor”, “more targets” and “multi-points priming” mechanisms, include inhibiting proliferation, inducing apoptosis, inhibiting tumor invasion and metastasis, anti-neoangiogenesis (including anti-angiogenesis and anti-VM), anti-lymphangiogenesis, overcoming multiple drug resistance, promoting tumor cell demethylation, modulating immune responses and so on. Numerous clinical applications and drug experiments have also demonstrated that NCTD has effective and “multi-factor” anticancer activities, especially in apoptotic inducement in human cancer cells by “more targets” and “multi-points priming” mechanisms. But other mechanisms of NCTD’s anticancer effects such as anti-angiogenesis, anti-VM, anti-lymphangiogenesis as well as overcoming multiple drug resistance are seldom reported. It is necessary to improve the relevant research, which is of great significance for the development of NCTD as a potential chemotherapeutic agent.
Acknowledgements
Not applicable.
Abbreviations
- TCM
Traditional Chinese medicine
- NCTD
Norcantharidin
- CTD
Cantharidin
- IVT
Interventional therapy
- TACE
Transcatheter arterial chemoembolization
- MNC
Mononuclear cells
- Bcl-2
B-cell lymphoma-2
- PP2A
Protein phosphatase 2A
- Cdc6
Cell division cycle protein 6
- PCNA
Proliferating cell nuclear antigen
- MAPK
Mitogen-activated protein kinase
- ERK
Extracellular regulated protein kinases
- JNK
Jun N-terminal kinase
- PI3K
Phosphoinositide 3 kinase
- NF-κB
Nuclear factor-kappa B
- MMPs
Matrix metalloproteinases
- STAT1
Signal transducers and activators of transcription 1
- Sp1
Specificity protein 1
- EMT
Epithelial–mesenchymal transition
- YAP
Yes-associated protein
- VM
Vasculogenic mimicry
- VEGF
Vascular endothelial growth factor
- TSP
Thrombospondin
- Ang
Angiogenin
- TIMP
Tissue metalloproteinase inhibitor
- VEGFR
Vascular endothelial growth factor receptor
- Ln-5γ2
Laminin 5γ2
- EphA2
Ephrin type a receptor 2
- FAK
Focal adhesion kinase
- MT1-MMP
Membrane type 1-matrix metalloproteinase
- HLECs
Human lymphatic endothelial cells
- HCACs
Human colonic adenocarcinomas
- MDR
Multi-drug resistance
- Shh
Sonic hedgehog
- EGFR-TKIs
Epidermal growth factor receptor- tyrosine kinase inhibitors
- NSCLC
Non-small cell lung cancer
- HGF
Hepatocyte growth factor
- WIF-1
Wnt inhibitory factor-1
- PBMC
Peripheral blood mononuclear cells
- PHA
Phytohemagglutinin
- NAT
N-acetyltransferase
- CNC conjugates
NCTD-conjugated carboxymethyl chitosan
- NOC15
N-farnesyloxy-norcantharimide
- SG-NCTD-LIP
NCTD-loaded liposomes modified with stearyl glycyrrhetinate
Authors’ contributions
All authors contributed in the preparation of this manuscript. MSP and JC made contributions to acquisition, compiling and analysis of the data, writing this manuscript. YZF was responsible for design and revising of this manuscript. YZF is the corresponding author and the guarantor. All authors read and approved the final manuscript.
Funding
This work was supported by funds from the National Nature Science Foundation of China (Nos. 30672073, 81072004 and 81372614), the Natural Science Foundation Project in Shanghai (No. 13ZR1432300) and the Science and Technology Commission Foundation in Shanghai (Nos. 19411966300 and 19140902302).
Availability of data and materials
All available data and material can be accessed.
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors consent for the publication of this review.
Competing interests
The authors declare that they have no potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mu-Su Pan, Email: panmusu@hotmail.com.
Jin Cao, Email: susancj508@hotmail.com.
Yue-Zu Fan, Email: fanyuezu@hotmail.com.
References
- 1.Wang GS. Medical uses of Mylabris in ancient China and recent studies. J Ethnopharmacol. 1989;26:147–162. doi: 10.1016/0378-8741(89)90062-7. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Z, Chi J, Han B, Liu W. Preparation and pharmacological evaluation of norcantharidin-conjugated carboxymethyl chitosan in mice bearing hepatocellular carcinoma. Carbohydr Polym. 2017;174:282–290. doi: 10.1016/j.carbpol.2017.06.072. [DOI] [PubMed] [Google Scholar]
- 3.Chang C, Zhu YQ, Mei JJ, Liu SQ, Luo J. Involvement of mitochondrial pathway in NCTD-induced cytotoxicity in human hepG2 cells. J Exp Clin Cancer Res. 2010;29(1):145. doi: 10.1186/1756-9966-29-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorn DC, Kou CA, Png KJ, Moore MA. The effect of cantharidins on leukemic stem cells. Int J Cancer. 2009;124(9):2186–2199. doi: 10.1002/ijc.24157. [DOI] [PubMed] [Google Scholar]
- 5.Liu Xu-Hui, Blazsek I., Comisso M., Legras S., Marion S., Quittet P., Anjo A., Wang Guang-Sheng, Misset J.L. Effects of norcantharidin, a protein phosphatase type-2A inhibitor, on the growth of normal and malignant haemopoietic cells. European Journal of Cancer. 1995;31(6):953–963. doi: 10.1016/0959-8049(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 6.Chen YN, Cheng CC, Chen JC, Tsauer W, Hsu SL. Norcantharidin-induced apoptosis is via the extracellular signal-regulated kinase and c-Jun-NH2-terminal kinase signaling pathways in human hepatoma HepG2 cells. Br J Pharmacol. 2003;140(3):461–470. doi: 10.1038/sj.bjp.0705461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YN, Chen JC, Yin SC, Wang GS, Tsauer W, Hsu SF, et al. Effector mechanisms of norcantharidin-induced mitotic arrest and apoptosis in human hepatoma cells. Int J Cancer. 2002;100(2):158–165. doi: 10.1002/ijc.10479. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Zhang L, Hu W, Hu ZH, Bei YY, Xu JY, et al. Norcantharidin- associated galactosylated chitosan nanoparticles for hepatocyte-targeted delivery. Nanomedicine. 2010;6(2):371–381. doi: 10.1016/j.nano.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Wan XY, Zhai XF, Jiang YP, Han T, Zhang QY, Xin HL. Antimetastatic effects of norcantharidin on hepatocellular carcinoma cells by up-regulating FAM46C expression. Am J Transl Res. 2017;9(1):155. [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H, Guo W, Xu B, Li M, Cui J. Anticancer activity and mechanisms of norcantharidin-Nd3II on hepatoma. Anticancer Drugs. 2007;18(10):1133–1137. doi: 10.1097/CAD.0b013e3282eeb1c5. [DOI] [PubMed] [Google Scholar]
- 11.Li G, Zhang S, Lü JF, Wu Y, Li J, Zhang G, et al. Molecular mechanism of norcantharidin inducing apoptosis in liver cancer cells. Zhonghua Yi Xue Za Zhi. 2010;90(30):2145. [PubMed] [Google Scholar]
- 12.Fan YZ, Fu JY, Zhao ZM, Chen CQ. Effect of norcantharidin on proliferation and invasion of human gallbladder carcinoma GBC-SD cells. World J Gastroenterol. 2005;11(16):2431–2437. doi: 10.3748/wjg.v11.i16.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Sun W, Zhang WZ, Ge CY, Zhang JT, Liu ZY, et al. Inhibition of tumor vasculogenic mimicry and prolongation of host survival in highly aggressive gallbladder cancers by norcantharidin via blocking the ephrin type a receptor 2/focal adhesion kinase/paxillin signaling pathway. PLoS ONE. 2014;9(1):64. doi: 10.1371/journal.pone.0096982. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Chen YJ, Tsai YM, Kuo CD, Ku KL, Shie HS, Liao HF. Norcantharidin is a small-molecule synthetic compound with anti-angiogenesis effect. Life Sci. 2009;85(17–18):642–651. doi: 10.1016/j.lfs.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Yeh CB, Su CJ, Hwang JM, Chou MC. Therapeutic effects of cantharidin analogues without bridging ether oxygen on human hepatocellular carcinoma cells. Eur J Med Chem. 2010;45(9):3981–3985. doi: 10.1016/j.ejmech.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 16.He Q, Xue S, Tan Y, Zhang L, Shao Q, Xing L, et al. Dual inhibition of Akt and ERK signaling induces cell senescence in triple-negative breast cancer. Cancer Lett. 2019;448:94–104. doi: 10.1016/j.canlet.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Xiao Y, Lin HP, Reichel D, Bae Y, Lee EY, et al. In vivo β-catenin attenuation by the integrin α5-targeting nano-delivery strategy suppresses triple negative breast cancer stemness and metastasis. Biomaterials. 2019;188:160–172. doi: 10.1016/j.biomaterials.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Yi S, Wass J, Vincent P, Iland H. Inhibitory effect of norcantharidin on K562 human myeloid leukemia cells in vitro. Leuk Res. 1991;15(10):883. doi: 10.1016/0145-2126(91)90163-n. [DOI] [PubMed] [Google Scholar]
- 19.Wang SC, Chow JM, Chien MH, Lin CW, Chen HY, Hsiao PC, et al. Cantharidic acid induces apoptosis of human leukemic HL-60 cells via c-Jun N-terminal kinase-regulated caspase-8/-9/-3 activation pathway. Environ Toxicol. 2018;33:514–522. doi: 10.1002/tox.22537. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Yu H, Kumar SM, Martin JS, Bing Z, Sheng W, et al. Norcantharidin induces melanoma cell apoptosis through activation of TR3 dependent pathway. Cancer Biol Ther. 2011;12(11):1005–1014. doi: 10.4161/cbt.12.11.18380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kok SH, Hong CY, Kuo MY, Lee CH, Lee JJ, Lou IU, et al. Comparisons of norcantharidin cytotoxic effects on oral cancer cells and normal buccal keratinocytes. Oral Oncol. 2003;39(1):19–26. doi: 10.1016/s1368-8375(01)00129-4. [DOI] [PubMed] [Google Scholar]
- 22.Mei L, Sang W, Cui K, Zhang Y, Chen F, Li X, et al. Norcantharidin inhibits proliferation and promotes apoptosis via c-Met/Akt/mTOR pathway in human osteosarcoma cells. Cancer Sci. 2019;110:582–595. doi: 10.1111/cas.13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tu GG, Zhan JF, Lv QL, Wang JQ, Kuang BH, Li SH. Synthesis and antiproliferative assay of norcantharidin derivatives in cancer cells. Med Chem. 2014;10:376–381. doi: 10.2174/15734064113099990037. [DOI] [PubMed] [Google Scholar]
- 24.Wei CM, Wang BJ, Ma Y, Sun ZP, Li XL, Guo RC. Pharmacokinetics and biodistribution of ~ 3H-norcantharidin in mice. Acta Pharm Sin. 2007;42(5):516. [PubMed] [Google Scholar]
- 25.Wang GS, Zhong HY, Huang JK, Lu FX, Yang KZ, Liu ZC, et al. Treatment of 244 cases of primary hepatocellular carcinoma with norcantharidin. Chin Pharm J. 1986;02:90–93. [Google Scholar]
- 26.Yang MY, Wu Z, Liang BY, Yu QP, Jian GF, Lin JM, et al. Clinical observation of oral and tumor center injection of norcantharidin in the treatment of primary liver cancer. J Pharm Res. 1992;01:45–47. [Google Scholar]
- 27.Huang DT. Treatment for 41 cases of primary liver cancer with norcantharidin. Acta Med Sin. 1996;01:39–40. [Google Scholar]
- 28.Zhou YY, Yang HY, Liu GX, Deng WJ. Norcantharidin combined with chemotherapy for advanced hepatocellular carcinoma. Chin J Clin Oncol Rehabil. 1997;03:75. [Google Scholar]
- 29.Liu A, Shan DQ. Combination of Ganfule and norcantharidin in the treatment of advanced hepatocellular carcinoma. Neimongol J Tradit Chin Med. 1998;04:6. [Google Scholar]
- 30.Ling CQ, Chen J, Chen Z, Huang XQ, Gao XF, Zheng XM, et al. Clinical study of intratumoral injecting sustained-release norcantharidin-poloxamer 407 preparation in treatment of primary liver cancer. Acad J Second Mil Med Univ. 2000;11:1074–1076. [Google Scholar]
- 31.Chen Z, Zhai XF, Jiang D, Ling CQ. Comparing therapeutic effects of intratumoral injection of norcantharidin poloxamer 407 slow released preparation and absolute ethanol on primary liver cancer. Acad J Second Mil Med Univ. 2001;07:606–608. [Google Scholar]
- 32.Zheng YL, Shi CL, He YX. Clinical study of norcantharidin in the treatment of liver cancer. Hebei Med. 2005;09:820–821. [Google Scholar]
- 33.Luan ZP, Li XD, Ma M. Clinical study of norcantharidin injection combined with fluorouracil in the treatment of advanced liver cancer. Hebei Med J. 2005;07:542–543. [Google Scholar]
- 34.Fan CX. The observation of the effects of disodium norcantharidate in the treatment of advanced primary liver cancer. J Basic Clin Oncol. 2010;23(01):50–51. [Google Scholar]
- 35.Jiang F, Cai RC, Xin Y. Clinical study of high-dose epimedium combined with norcantharidin in the treatment of primary liver cancer. Pract Clin J Integ Tradit Chin West Med. 2011;11(6):49. [Google Scholar]
- 36.Chu YP, Shen L, Bai Y. Efficacy observation of liver cancer at the later stage for the elderly treated with integration of norcantharidin and Chinese medicine. World J Integ Tradit West Med. 2012;258(1):224–229. [Google Scholar]
- 37.Zhou WL, Kao J, Fan QL. Clinical observation of norcantharidin tablets in the treatment of 30 cases of liver metastasis from gastrointestinal cancer. Shandong Med J. 2005;20:32–33. [Google Scholar]
- 38.Chen SH, Wang JH, Tan QH, Tian SY. Sodium norcantharidin combined with DF regimen in the treatment of advanced gastric carcinoma. J Basic Clin Oncol. 2013;26(04):311–313. [Google Scholar]
- 39.Zhang LT, Xiang H. Clinical efficacy of norcantharidin combined with conventional chemotherapy treating postoperative gastric cancer. Med Recapit. 2013;19(11):2087–2088. [Google Scholar]
- 40.Zhao PZ. Go to a sodium cantharidate clinical observation of treatment of esophageal cancer with radiotherapy. Med J Chin People’s Health. 2010;22(13):1648–1650. [Google Scholar]
- 41.Feng BH. Therapeutic effect of radiotherapy combined with sodium norcantharidin for stage III cervical cancer. Chin Foreign Med Treat. 2010;29(03):40–41. [Google Scholar]
- 42.Wu GX, Yang ZH, Chen EB. Clinical observation of chemotherapy combined with compound cantharidin injection in the treatment of malignant lymphoma. J Chin Physician. 2005;10:1425–1426. [Google Scholar]
- 43.Chen WM, Zeng GY. Clinical research on norcantharidin injection combined with CTOP scheme for treating non-Hodgkin’s lymphoma. Fujian Med J. 2012;34(01):85–87. [Google Scholar]
- 44.Zhi XJ, Li GL. Clinical observation on 30 cases of advanced lung cancer treated with norcantharidin sodium. J Hebei North Univ. 2008;03:60. [Google Scholar]
- 45.Guan ZF. Clinical observation on 50 cases of advanced non-small cell lung cancer treated with sodium norcantharidin. J Qiqihar Med Univ. 2010;31(17):2727. [Google Scholar]
- 46.Gong D, Wang MH, Zhang S, Wang XY, Chen MY, Fang F, et al. Aidi injection combined with paclitaxel-containing chemotherapy protocol in treatment of 42 patients with non-small-cell lung carcinoma in stage III–IV. Med Pharm J Chin PLA. 2014;26(08):81–84. [Google Scholar]
- 47.Li ZY, Ma QT, Zhang Y, Wang XC, Liu YL. Gemcitabine and ciplatin combined with norcantharidin sodium for the treatment of advanced NSCLC. Chin J Cancer Prev Treat. 2014;21(04):293–295. [Google Scholar]
- 48.Li YG, Wu JZ, Liu HM, Zhang LX. Effect of sodium cantharidate injection on immune function in patients with non-small cell lung cancer. Chin J Gerontol. 2015;35(06):1538–1540. [Google Scholar]
- 49.Ma Q, Feng Y, Deng K, Shao H, Sui T, Zhang X, et al. Unique responses of hepatocellular carcinoma and cholangiocarcinoma cell lines toward cantharidin and norcantharidin. J Cancer. 2018;9:2183–2190. doi: 10.7150/jca.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang EB, Tang WY, Zhang K, Cheng LY, Mack PO. Norcantharidin inhibits growth of human HepG2 cell-transplanted tumor in nude mice and prolongs host survival. Cancer Lett. 1997;117:93–98. doi: 10.1016/s0304-3835(97)00206-1. [DOI] [PubMed] [Google Scholar]
- 51.Liao HF, Su SL, Chen YJ, Chou CH, Kuo CD. Norcantharidin preferentially induces apoptosis in human leukemic Jurkat cells without affecting viability of normal blood mononuclear cells. Food Chem Toxicol. 2007;45:1678–1687. doi: 10.1016/j.fct.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Liao HF, Chen YJ, Chou CH, Wang FW, Kuo CD. Norcantharidin induces cell cycle arrest and inhibits progression of human leukemic Jurkat T cells through mitogen-activated protein kinase-mediated regulation of interleukin-2 production. Toxicol In Vitro. 2011;25(1):206–212. doi: 10.1016/j.tiv.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Li KY, Shi CX, Huang JZ, Tang KL. Cisplatin plus norcantharidin alter the expression of TGF-β1/Smads signaling pathway in hepatocellular carcinoma. Bratisl Lek Listy. 2017;118:85–88. doi: 10.4149/BLL_2017_018. [DOI] [PubMed] [Google Scholar]
- 54.Lee YC, Lee LM, Yang CH, Lin AM, Huang YC, Hsu CC, et al. Norcantharidin suppresses cell growth and migration with enhanced anticancer activity of gefitinib and cisplatin in human non-small cell lung cancer cells. Oncol Rep. 2013;29:237–243. doi: 10.3892/or.2012.2118. [DOI] [PubMed] [Google Scholar]
- 55.Jiang YM, Meng ZZ, Yue GX, Chen JX. Norcantharidin induces HL-60 cells apoptosis in vitro. Evid Based Complement Altern Med. 2012;2012:154271. doi: 10.1155/2012/154271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Zahng J, You Z, Liao H. Inhibitory effect of norcantharidin combined with evodiamine on the growth of human hepatic carcinoma cell line HepG2 in vitro. Chin J Cell Mol Immunol. 2014;30(8):824–828. [PubMed] [Google Scholar]
- 57.Zhang QY, Yue XQ, Jiang YP, Han T, Xin HL. Author correction: FAM46C is critical for the anti-proliferation and pro-apoptotic effects of norcantharidin in hepatocellular carcinoma cells. Sci Rep. 2017;7(1):17576. doi: 10.1038/s41598-017-17244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun ZX, Ma QW, Zhao TD, Wei YL, Wang GS, Li JS. Apoptosis induced by norcantharidin in human tumor cells. World J Gastroenterol. 2000;6(2):263–265. doi: 10.3748/wjg.v6.i2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng F, Wei YQ, Tian L, Yang L, Zhao X, Lu Y, et al. Induction of apoptosis by norcantharidin in human colorectal carcinoma cell lines: involvement of the CD95 receptor/ligand. J Cancer Res Clin Oncol. 2002;128(4):223–230. doi: 10.1007/s00432-002-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen YJ, Kuo CD, Tsai YM, Yu CC, Wang GS, Liao HF. Norcantharidin induces anoikis through Jun-N-terminal kinase activation in CT26 colorectal cancer cells. Anticancer Drugs. 2008;19(1):55–64. doi: 10.1097/CAD.0b013e3282f18826. [DOI] [PubMed] [Google Scholar]
- 61.Mumby M. PP2A: unveiling a reluctant tumor suppressor. Cell. 2007;130(1):21–24. doi: 10.1016/j.cell.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 62.Hart ME, Chamberlin AR, Walkom C, Sakoff JA, McCluskey A. Modified norcantharidins; synthesis, protein phosphatases 1 and 2A inhibition, and anticancer activity. Bioorg Med Chem Lett. 2004;14:1969–1973. doi: 10.1016/j.bmcl.2004.01.093. [DOI] [PubMed] [Google Scholar]
- 63.Shou LM, Zhang QY, Li W, Xie X, Chen K, Lian L, et al. Cantharidin and norcantharidin inhibit the ability of MCF-7 cells to adhere to platelets via protein kinase C pathway-dependent downregulation of α2 integrin. Oncol Rep. 2013;30:1059–1066. doi: 10.3892/or.2013.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ayad Nagi G. CDKs give Cdc6 a license to drive into S phase. Cell. 2005;122(6):825–827. doi: 10.1016/j.cell.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Li JL, Cai YC, Hu ZM, Gao JM. Norcantharidin inhibits DNA replication initiation protein Cdc6 in cancer cells. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:1851–1853. [PubMed] [Google Scholar]
- 66.Chen S, Qu X, Wan P, Li QW, Wang Z, Guo F, et al. Norcantharidin inhibits pre-replicative complexes assembly of HepG2 cells. Am J Chin Med. 2013;41:665–682. doi: 10.1142/S0192415X13500468. [DOI] [PubMed] [Google Scholar]
- 67.Fan YZ, Fu JY, Zhao ZM, Chen CQ. Influence of norcantharidin on proliferation, proliferation-related gene proteins proliferating cell nuclear antigen and Ki-67 of human gallbladder carcinoma GBC-SD cells. HBPD INT. 2004;3:603–607. [PubMed] [Google Scholar]
- 68.Yang PY, Chen MF, Tsai CH, Hu DN, Chang FR, Wu YC. Involvement of caspase and MAPK activities in norcantharidin-induced colorectal cancer cell apoptosis. Toxicol In Vitro. 2010;24(3):766–775. doi: 10.1016/j.tiv.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 69.Janssens N, Janicot M, Perera T. The Wnt-dependent signaling pathways as target in oncology drug discovery. Invest New Drugs. 2006;24:263–280. doi: 10.1007/s10637-005-5199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chuang KA, Lieu CH, Tsai WJ, Wu MH, Chen YC, Liao JF, et al. Evaluation of anti-Wnt/β-catenin signaling agents by pGL4-TOP transfected stable cells with a luciferase reporter system. Braz J Med Biol Res. 2010;43:931–941. doi: 10.1590/s0100-879x2010007500091. [DOI] [PubMed] [Google Scholar]
- 71.Cimmino F, Scoppettuolo MN, Carotenuto M, De Antonellis P, Dato VD, De Vita G, et al. Norcantharidin impairs medulloblastoma growth by inhibition of Wnt/β-catenin signaling. J Neurooncol. 2012;106(1):59–70. doi: 10.1007/s11060-011-0645-y. [DOI] [PubMed] [Google Scholar]
- 72.Lv H, Li Y, Du H, Fang J, Song X, Zhang J. The synthetic compound norcantharidin induced apoptosis in mantle cell lymphoma in vivo and in vitro through the PI3K-Akt-NF-κB signaling pathway. Evid Based Complement Altern Med. 2013;2013:461487. doi: 10.1155/2013/461487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang Y, Liu Q, Liu K, Yagasaki K, Zhang G. Suppression of growth of highly-metastatic human breast cancer cells by norcantharidin and its mechanisms of action. Cytotechnology. 2009;59(3):201–208. doi: 10.1007/s10616-009-9210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen YJ, Shieh CJ, Tsai TH, Kuo CD, Ho LT, Liu TY, et al. Inhibitory effect of norcantharidin, a derivative compound from blister beetles, on tumor invasion and metastasis in CT26 colorectal adenocarcinoma cells. Anticancer Drugs. 2005;16(3):293–299. doi: 10.1097/00001813-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 75.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 76.Makrilia N, Kollias A, Manolopoulos L, Syrigos K. Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest. 2009;27:1023–1037. doi: 10.3109/07357900902769749. [DOI] [PubMed] [Google Scholar]
- 77.Chen YJ, Chang WM, Liu YW, Lee CY, Jang YH, Kuo CD, et al. A small-molecule metastasis inhibitor, norcantharidin, downregulates matrix metalloproteinase-9 expression by inhibiting Sp1 transcriptional activity in colorectal cancer cells. Chem Biol Interact. 2009;181(3):440–446. doi: 10.1016/j.cbi.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Peng C, Li Z, Niu Z, Niu W, Xu Z, Gao H, et al. Norcantharidin suppresses colon cancer cell epithelial–mesenchymal transition by inhibiting the αvβ6-ERK-Ets1 signaling pathway. Sci Rep. 2016;6(1):20500. doi: 10.1038/srep20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loboda A, Nebozhyn MV, Watters JW, Buser CA, Shaw PM, Huang PS, et al. EMT is the dominant program in human colon cancer. BMC Med Genomics. 2011;4:9. doi: 10.1186/1755-8794-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo J, Wu Y, Yang L, Du J, Gong K, Chen W, et al. Repression of YAP by NCTD disrupts NSCLC progression. Oncotarget. 2017;8:2307–2319. doi: 10.18632/oncotarget.13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao Y, Li W, Liu R, Guo Q, Li J, Bao Y, et al. Norcantharidin inhibits IL-6-induced epithelial–mesenchymal transition via the JAK2/STAT3/TWIST signaling pathway in hepatocellular carcinoma cells. Oncol Rep. 2017;38:1224–1232. doi: 10.3892/or.2017.5775. [DOI] [PubMed] [Google Scholar]
- 82.Folkman Judah. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–30. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 83.Fan YZ, Chen CQ, Zhao ZM, Sun W. Effects of norcantharidin on angiogenesis of human gallbladder carcinoma and its anti-angiogenic mechanisms. Nat Med J Chin. 2006;86:693–699. [PubMed] [Google Scholar]
- 84.Zhang JT, Fan YZ, Chen CQ, Zhao ZM, Sun W. Norcantharidin: a potential antiangiogenic agent for gallbladder cancers in vitro and in vivo. Int J Oncol. 2012;40:1501–1514. doi: 10.3892/ijo.2011.1314. [DOI] [PubMed] [Google Scholar]
- 85.Yu T, Hou F, Liu M, Zhou L, Li D, Liu J, et al. Norcantharidin anti-angiogenesis activity possibly through an endothelial cell pathway in human colorectal cancer. Asian Pac J Cancer Prev. 2012;13(2):499–503. doi: 10.7314/apjcp.2012.13.2.499. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, Ji Q, Liu X, Chen X, Chen Z, Qiu Y, et al. Norcantharidin inhibits tumor angiogenesis via blocking VEGFR2/MEK/ERK signaling pathways. Cancer Sci. 2013;104(5):604–610. doi: 10.1111/cas.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Albini A, Melchiori A, Santi L, Liotta LA, Brown PD, Stetler-Stevenson WG. Tumor cell invasion inhibited by TIMP-2. J Natl Cancer Inst. 1991;83(11):775–779. doi: 10.1093/jnci/83.11.775. [DOI] [PubMed] [Google Scholar]
- 89.Fan YZ, Sun W. Molecular regulation of vasculogenic mimicry in tumors and potential tumor-target therapy. World J Gastrointest Surg. 2010;2:117–127. doi: 10.4240/wjgs.v2.i4.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, et al. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61:6322–6327. [PubMed] [Google Scholar]
- 91.Zhang JT, Sun W, Zhang WZ, Ge CY, Liu ZY, Zhao ZM, et al. Norcantharidin inhibits tumor growth and vasculogenic mimicry of human gallbladder carcinomas by suppression of the PI3-K/MMPs/Ln-5γ2 signaling pathway. BMC Cancer. 2014;14:193. doi: 10.1186/1471-2407-14-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu W, Sun W, Zhang JT, Liu ZY, Li XP, Fan YZ. Norcantharidin enhances TIMP-2 anti-vasculogenic mimicry activity for human gallbladder cancers through downregulating MMP-2 and MT1-MMP. Int J Oncol. 2015;46:627–640. doi: 10.3892/ijo.2014.2753. [DOI] [PubMed] [Google Scholar]
- 93.Wang Z, You D, Lu M, He Y, Yan S. Inhibitory effect of norcantharidin on melanoma tumor growth and vasculogenic mimicry by suppressing MMP-2 expression. Oncol Lett. 2017;13:1660–1664. doi: 10.3892/ol.2017.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Achen MG, Stacker SA. Tumor lymphangiogenesis and metastatic spread-new players begin to emerge. Int J Cancer. 2006;119:1755–1760. doi: 10.1002/ijc.21899. [DOI] [PubMed] [Google Scholar]
- 95.Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Siegfried G, Basak A, Cromlish JA, Benjannet S, Marcinkiewicz J, Chrétien M, et al. The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J Clin Invest. 2003;111:1723–1732. doi: 10.1172/JCI17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu ZY, Qiu HO, Yuan XJ, Ni YY, Sun JJ, Jing W, et al. Suppression of lymphangiogenesis in human lymphatic endothelial cells by simultaneously blocking VEGF-C and VEGF-D/VEGFR-3 with norcantharidin. Int J Oncol. 2012;41:1762–1772. doi: 10.3892/ijo.2012.1603. [DOI] [PubMed] [Google Scholar]
- 99.Yuan X, Chen Y, Li X, Zhang G, Jin D, Zhao H, et al. Norcantharidin inhibits lymphangiogenesis by downregulating the expression of VEGF-C and VEGF-D in human dermal lymphatic endothelial cells in vitro. Pharmacology. 2015;95:1–9. doi: 10.1159/000362418. [DOI] [PubMed] [Google Scholar]
- 100.Li XP, Jing W, Sun JJ, Liu ZY, Zhang JT, Sun W, et al. A potential small-molecule synthetic antilymphangiogenic agent norcantharidin inhibits tumor growth and lymphangiogenesis of human colonic adenocarcinomas through blocking VEGF-A,-C,-D/VEGFR-2,-3 “multi-points priming” mechanisms in vitro and in vivo. BMC Cancer. 2015;15(1):527. doi: 10.1186/s12885-015-1521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 102.Li Y, Gao X, Yu Z, Liu B, Pan W, Li N, et al. Reversing multidrug resistance by multiplexed gene silencing for enhanced breast cancer chemotherapy. ACS Appl Mater Interfaces. 2018;10:15461–15466. doi: 10.1021/acsami.8b02800. [DOI] [PubMed] [Google Scholar]
- 103.Chen YJ, Kuo CD, Chen SH, Chen WJ, Huang WC, Chao KS, et al. Small-molecule synthetic compound norcantharidin reverses multi-drug resistance by regulating Sonic hedgehog signaling in human breast cancer cells. PLoS ONE. 2012;7:e37006. doi: 10.1371/journal.pone.0037006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sims-Mourtada J, Izzo JG, Ajani J, Chao KS. Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene. 2007;26:5674–5679. doi: 10.1038/sj.onc.1210356. [DOI] [PubMed] [Google Scholar]
- 105.Miyashita T, Reed JC. Bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res. 1992;52:5407–5411. [PubMed] [Google Scholar]
- 106.Simonian PL, Grillot DA, Nuñez G. Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood. 1997;90:1208–1216. [PubMed] [Google Scholar]
- 107.Noutomi T, Chiba H, Itoh M, Toyota H, Mizuguchi J. Bcl-xL confers multi-drug resistance in several squamous cell carcinoma cell lines. Oral Oncol. 2002;38(1):41–48. doi: 10.1016/s1368-8375(00)00098-1. [DOI] [PubMed] [Google Scholar]
- 108.Kok SH, Cheng SJ, Hong CY, Lee JJ, Lin SK, Kuo YS, et al. Norcantharidin-induced apoptosis in oral cancer cells is associated with an increase of proapoptotic to antiapoptotic protein ratio. Cancer Lett. 2005;217(1):1–52. doi: 10.1016/j.canlet.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 109.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 110.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 111.Zhang S, Li G, Ma X, Wang Y, Liu G, Feng L, et al. Norcantharidin enhances ABT-737-induced apoptosis in hepatocellular carcinoma cells by transcriptional repression of Mcl-1. Cell Signal. 2012;24:1803–1809. doi: 10.1016/j.cellsig.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 112.Ren J, Li G, Zhao W, Lin L, Ye T. Norcantharidin combined with ABT-737 for hepatocellular carcinoma: therapeutic effects and molecular mechanisms. World J Gastroenterol. 2016;22:3962–3968. doi: 10.3748/wjg.v22.i15.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang X, Gu Z, Li G, Zhang S, Cao Z, Yang Z, et al. Norcantharidin enhances ABT-263-mediated anticancer activity in neuroblastoma cells by upregulation of Noxa. Oncol Rep. 2014;32:716–722. doi: 10.3892/or.2014.3228. [DOI] [PubMed] [Google Scholar]
- 114.Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Jänne PA, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28:357–360. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yano S, Yamada T, Takeuchi S, Tachibana K, Minami Y, Yatabe Y, et al. Hepatocyte growth factor expression in EGFR mutant lung cancer with intrinsic and acquired resistance to tyrosine kinase inhibitors in a Japanese cohort. J Thorac Oncol. 2011;6(12):2011–2017. doi: 10.1097/JTO.0b013e31823ab0dd. [DOI] [PubMed] [Google Scholar]
- 116.Wu H, Fan F, Liu Z, Shen C, Wang A, Lu Y. Norcantharidin combined with EGFR-TKIs overcomes HGF-induced resistance to EGFR-TKIs in EGFR mutant lung cancer cells via inhibition of Met/PI3k/Akt pathway. Cancer Chemother Pharmacol. 2015;76:307–315. doi: 10.1007/s00280-015-2792-x. [DOI] [PubMed] [Google Scholar]
- 117.Varley KE, Gertz J, Bowling KM, Parker SL, Reddy TE, Pauli-Behn F, et al. Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res. 2013;23:555–567. doi: 10.1101/gr.147942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lund AH, Lohuizen MV. Epigenetics and cancer. Gene Dev. 2004;18(19):2315–2335. doi: 10.1101/gad.1232504. [DOI] [PubMed] [Google Scholar]
- 119.Yang X, Gao L, Zhang S. Comparative pan-cancer DNA methylation analysis reveals cancer common and specific patterns. Brief Bioinform. 2017;18:761–773. doi: 10.1093/bib/bbw063. [DOI] [PubMed] [Google Scholar]
- 120.Hesson LB, Cooper WN, Latif F. The role of RASSF1A methylation in cancer. Dis Markers. 2013;23(1–2):73. doi: 10.1155/2007/291538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y, Xu M, Di ZH, Zhang J, Mao XQ, Sun HB. Regulation of demethylation and re-expression of RASSF1A gene in hepatocellular carcinoma cell lines treated with NCTD in vitro. J Cancer Res Ther. 2015;11:818–822. doi: 10.4103/0973-1482.146126. [DOI] [PubMed] [Google Scholar]
- 122.Mazieres J, He B, You L, Xu Z, Jablons DM. Wnt signaling in lung cancer. Cancer Lett. 2005;222(1):1–10. doi: 10.1016/j.canlet.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 123.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4(5):1–10. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim SA, Kwak J, Nam HY, Chun SM, Lee BW, Lee HJ, et al. Promoter methylation of WNT inhibitory factor-1 and expression pattern of WNT/β-catenin pathway in human astrocytoma: pathologic and prognostic correlations. Mod Pathol. 2013;26:626–639. doi: 10.1038/modpathol.2012.215. [DOI] [PubMed] [Google Scholar]
- 125.Xie J, Zhang Y, Hu X, Lv R, Xiao D, Jiang L, et al. Norcantharidin inhibits Wnt signal pathway via promoter demethylation of WIF-1 in human non-small cell lung cancer. Med Oncol. 2015;32:145. doi: 10.1007/s12032-015-0592-0. [DOI] [PubMed] [Google Scholar]
- 126.Xie D, Xie J, Wan Y, Ma L, Qi X, Wang K, et al. Norcantharidin blocks Wnt/β-catenin signaling via promoter demethylation of WIF-1 in glioma. Oncol Rep. 2016;35:2191–2197. doi: 10.3892/or.2016.4559. [DOI] [PubMed] [Google Scholar]
- 127.Zhao Q, Qian Y, Li R, Tan B, Han H, Liu M, et al. Norcantharidin facilitates LPS-mediated immune responses by up-regulation of AKT/NF-κB signaling in macrophages. PLoS ONE. 2012;7:e44956. doi: 10.1371/journal.pone.0044956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen YC, Chang SC, Wu MH, Chuang KA, Wu JY, Tsai WJ, et al. Norcantharidin reduced cyclins and cytokines production in human peripheral blood mononuclear cells. Life Sci. 2009;84:218–226. doi: 10.1016/j.lfs.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 129.Bergmann C, Strauss L, Wang Y, Szczepanski MJ, Lang S, Johnson JT, et al. T regulatory type 1 cells in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease. Clin Cancer Res. 2008;14(12):3706–3715. doi: 10.1158/1078-0432.CCR-07-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mack P, Ha XF, Cheng LY. Efficacy of intra-arterial norcantharidin in suppressing tumour 14C-labelled glucose oxidative metabolism in rat Morris hepatoma. HPB Surg. 1996;10:65–72. doi: 10.1155/1996/63403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu LT, Chung JG, Chen JC, Tsauer W. Effect of norcantharidin on N-acetyltransferase activity in HepG2 cells. Am J Chin Med. 2001;29:161–172. doi: 10.1142/S0192415X01000186. [DOI] [PubMed] [Google Scholar]
- 132.Zhang H, Jiang Y, Ni X, Chen L, Wu M, Liu J, et al. Glycyrrhetinic acid- modified norcantharidin nanoparticles for active targeted therapy of hepatocellular carcinoma. J Biomed Nanotechnol. 2018;14:114–126. doi: 10.1166/jbn.2018.2467. [DOI] [PubMed] [Google Scholar]
- 133.Li JL, Cai YC, Liu XH, Xian LJ. Norcantharidin inhibits DNA replication and induces apoptosis with the cleavage of initiation protein Cdc6 in HL-60 cells. Anticancer Drugs. 2006;17(3):307. doi: 10.1097/00001813-200603000-00009. [DOI] [PubMed] [Google Scholar]
- 134.Li XQ, Shao SH, Fu GL, Han SH, Gao H. Study on norcantharidin-induced apoptosis in SMMC-7721 cells through mitochondrial pathways. Chin J Integr Med. 2010;16(5):448–452. doi: 10.1007/s11655-010-0538-5. [DOI] [PubMed] [Google Scholar]
- 135.Li XQ, Shao SH, Han XH, Fan ZZ, Sun J, Yin PH, et al. Norcantharidin induces apoptosis in SMMC-7721 cells via the c-Jun-NH2-terminal kinase signaling pathways. Chin J Hepatol. 2010;18(2):146–147. doi: 10.3760/cma.j.issn.1007-3418.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 136.Chang C, Zhu Y, Tang X, Tao W. The anti-proliferative effects of norcantharidin on human HepG2 cells in cell culture. Mol Biol Rep. 2011;38:163–169. doi: 10.1007/s11033-010-0090-6. [DOI] [PubMed] [Google Scholar]
- 137.Yeh CH, Yang YY, Huang YF, Chow KC, Chen MF. Induction of apoptosis in human Hep3B hepatoma cells by norcantharidin through a p53 independent pathway via TRAIL/DR5 signal transduction. Chin J Integr Med. 2012;18:676–682. doi: 10.1007/s11655-012-1206-8. [DOI] [PubMed] [Google Scholar]
- 138.Xiong X, Wu M, Zhang H, Li J, Lu B, Guo Y, et al. Atg5 siRNA inhibits autophagy and enhances norcantharidin-induced apoptosis in hepatocellular carcinoma. Int J Oncol. 2015;47:1321–1328. doi: 10.3892/ijo.2015.3103. [DOI] [PubMed] [Google Scholar]
- 139.Ren J, Li G, Zhao W, Lin L, Ye T. Norcantharidin combined with ABT-737 for hepatocellular carcinoma: therapeutic effects and molecular mechanisms. World J Gastroenterol. 2016;22(15):3962–3968. doi: 10.3748/wjg.v22.i15.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen YL, Hung MH, Chu PY, Chao TI, Tsai MH, Chen LJ, et al. Protein phosphatase 5 promotes hepatocarcinogenesis through interaction with AMP-activated protein kinase. Biochem Pharmacol. 2017;138:49–60. doi: 10.1016/j.bcp.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 141.Wang D, Yang C, Wang Z, Yang Y, Li D, Ding X, et al. Norcantharidin combined with Coix seed oil synergistically induces apoptosis and inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation. Sci Rep. 2017;7:9373. doi: 10.1038/s41598-017-09668-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 142.Fan YZ, Fu JY, Zhao ZM, Chen CQ. The in vitro effect of norcantharidin on proliferation and invasion of human gallbladder carcinoma GBC-SD cells and its mechanism. Zhonghua Zhong Liu Za Zhi. 2004;26:271–274. [PubMed] [Google Scholar]
- 143.Fan YZ, Fu JY, Zhao ZM, Chen CQ. Inhibitory effect of norcantharidin on the growth of human gallbladder carcinoma GBC-SD cells in vitro. HBPD INT. 2007;6(1):72–80. [PubMed] [Google Scholar]
- 144.Fan YZ, Zhao ZM, Fu JY, Chen CQ. Anti-tumor mechanism of norcantharidin for the implanted tumors of human gallbladder carcinoma in nude mice in vivo. Chin J Surg. 2006;44(9):618–622. [PubMed] [Google Scholar]
- 145.Fan YZ, Zhao ZM, Fu JY, Chen CQ, Sun W. Norcantharidin inhibits growth of human gallbladder carcinoma xenografted tumors in nude mice by inducing apoptosis and blocking the cell cycle in vivo. HBPD INT. 2010;9(4):414–422. [PubMed] [Google Scholar]
- 146.Peng C, Liu X, Liu E, Xu K, Niu W, Chen R, et al. Norcantharidin induces HT-29 colon cancer cell apoptosis through the alphavbeta6-extracellular signal-related kinase signaling pathway. Cancer Sci. 2010;100(12):2302–2308. doi: 10.1111/j.1349-7006.2009.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Qiu P, Wang S, Liu M, Ma H, Zeng X, Zhang M, et al. Norcantharidin inhibits cell growth by suppressing the expression and phosphorylation of both EGFR and c-Met in human colon cancer cells. BMC Cancer. 2017;17(1):55. doi: 10.1186/s12885-016-3039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Williams LA, Möller W, Merisor E, Kraus W, Rösner H. In vitro anti- proliferation/cytotoxic activity of cantharidin (Spanish Fly) and related derivatives. West Indian Med J. 2003;52(1):10–13. [PubMed] [Google Scholar]
- 149.Yang PY, Chen MF, Kao YH, Hu DN, Chang FR, Wu YC. Norcantharidin induces apoptosis of breast cancer cells: involvement of activities of mitogen activated protein kinases and signal transducers and activators of transcription. Toxicol In Vitro. 2011;25(3):699–707. doi: 10.1016/j.tiv.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 150.Liu D, Shi P, Yin X, Chen Z, Zhang X. Effect of norcantharidin on the human breast cancer Bcap-37 cells. Connect Tissue Res. 2012;53(6):5. doi: 10.3109/03008207.2012.694928. [DOI] [PubMed] [Google Scholar]
- 151.Zheng LC, Yang MD, Kuo CL, Lin CH, Fan MJ, Chou YC, et al. Norcantharidin-induced apoptosis of AGS human gastric cancer cells through reactive oxygen species production, and caspase- and mitochondria-dependent signaling pathways. Anticancer Res. 2016;36(11):6031–6042. doi: 10.21873/anticanres.11192. [DOI] [PubMed] [Google Scholar]
- 152.An WW, Wang MW, Tashiro S, Onodera S, Ikejima T. Norcantharidin induces human melanoma A375-S2 cell apoptosis through mitochondrial and caspase pathways. J Korean Med Sci. 2004;19(4):560–566. doi: 10.3346/jkms.2004.19.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.An WW, Wang MW, Tashiro S, Onodera S, Ikejima T. Mitogen-activated protein kinase-dependent apoptosis in norcan-tharidin-treated A375-S2 cells is proceeded by the activation of protein kinase C. Chin Med J. 2005;118:198–203. [PubMed] [Google Scholar]
- 154.Song XN, Du HF, Yu LJ, Meng YF, Lü HY, Sun LX, et al. Norcantharidin potentialize the chemosensitivity of adriamycin through the NF-κB/IκBα signaling pathway. Zhonghua Xue Ye Xue Za Zhi. 2011;32(12):809–813. [PubMed] [Google Scholar]
- 155.He Y, Yan D, Zheng D, Hu Z, Li H, Li J. Cell division cycle 6 promotes mitotic slippage and contributes to drug resistance in paclitaxel-treated cancer cells. PLoS ONE. 2016;11:e0162633. doi: 10.1371/journal.pone.0162633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Luan J, Duan H, Liu Q, Yagasaki K, Zhang G. Inhibitory effects of norcantharidin against human lung cancer cell growth and migration. Cytotechnology. 2010;62(4):349–355. doi: 10.1007/s10616-009-9250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zheng J, Du W, Song LJ, Zhang R, Sun LG, Chen FG, et al. Norcantharidin induces growth inhibition and apoptosis of glioma cells by blocking the Raf/MEK/ERK pathway. World J Surg Oncol. 2014;12(1):207. doi: 10.1186/1477-7819-12-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Han Z, Li B, Wang J, Zhang X, Li Z, Dai L, et al. Norcantharidin inhibits SK-N-SH neuroblastoma cell growth by induction of autophagy and apoptosis. Technol Cancer Res Treat. 2017;16:33–44. doi: 10.1177/1533034615624583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.An WW, Gong XF, Wang MW, Tashiro S, Onodera S, Ikejima T. Norcantharidin induces apoptosis in HeLa cells through caspase, MAPK, and mitochondrial pathways. Acta Pharmacol Sin. 2004;25:1502–1508. [PubMed] [Google Scholar]
- 160.Dong X, Li JC, Jiang YY, Xia MY, Tashiro S, Onodera S, et al. P38-NF-κB-promoted mitochondria-associated apoptosis and G2/M cell cycle arrest in norcantharidin-treated HeLa cells. J Asian Nat Prod Res. 2012;14:1008–1019. doi: 10.1080/10286020.2012.693481. [DOI] [PubMed] [Google Scholar]
- 161.Yu CC, Ko FY, Yu CS, Lin CC, Huang YP, Yang JS, et al. Norcantharidin triggers cell death and DNA damage through S-phase arrest and ROS-modulated apoptotic pathways in TSGH 8301 human urinary bladder carcinoma cells. Int J Oncol. 2012;41:1050–1060. doi: 10.3892/ijo.2012.1511. [DOI] [PubMed] [Google Scholar]
- 162.Chen S, Wan P, Ding W, Li F, He C, Chen P, et al. Norcantharidin inhibits DNA replication and induces mitotic catastrophe by degrading initiation protein Cdc6. Int J Mol Med. 2013;32:43–50. doi: 10.3892/ijmm.2013.1359. [DOI] [PubMed] [Google Scholar]
- 163.Shen B, He PJ, Shao CL. Norcantharidin induced DU145 cell apoptosis through ROS-mediated mitochondrial dysfunction and energy depletion. PLoS ONE. 2013;8:e84610. doi: 10.1371/journal.pone.0084610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xiao W, Dai B, Zhu Y, Ye D. Norcantharidin induces autophagy-related prostate cancer cell death through Beclin-1 upregulation by miR-129-5p suppression. Tumor Biol. 2016;37(12):15643–15648. doi: 10.1007/s13277-015-4488-6. [DOI] [PubMed] [Google Scholar]
- 165.Yang PY, Hu DN, Kao YH, Lin IC, Chou CY, Wu YC. Norcantharidin induces apoptosis in human prostate cancer cells through both intrinsic and extrinsic pathways. Pharmacol Rep. 2016;68:874–880. doi: 10.1016/j.pharep.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 166.Lin CL, Chen CM, Lin CL, Cheng CW, Lee CH, Hsieh YH. Norcantharidin induces mitochondrial-dependent apoptosis through Mcl-1 inhibition in human prostate cancer cells. Biochim Biophys Acta Mol Cell Res. 2017;1864(10):1867. doi: 10.1016/j.bbamcr.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 167.Zhu Y, Mi Y, Wang Z, Jia X, Jin Z. Norcantharidin inhibits viability and induces cell cycle arrest and apoptosis in osteosarcoma. Oncol Lett. 2019;17:456–461. doi: 10.3892/ol.2018.9615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hong CY, Huang SC, Lin SK, Lee JJ, Chueh LL, Lee CH, et al. Norcantharidin-induced post-G2/M apoptosis is dependent on wild-type p53 gene. Biochem Biophys Res Commun. 2000;276:278–285. doi: 10.1006/bbrc.2000.3341. [DOI] [PubMed] [Google Scholar]
- 169.Chen F, Wang S, Wei Y, Wu J, Huang G, Chen J, et al. Norcantharidin modulates the miR-30a/Metadherin/AKT signaling axis to suppress proliferation and metastasis of stromal tumor cells in giant cell tumor of bone. Biomed Pharmacother. 2018;103:1092–1100. doi: 10.1016/j.biopha.2018.04.100. [DOI] [PubMed] [Google Scholar]
- 170.Yeh CB, Hsieh MJ, Hsieh YH, Chien MH, Chiou HL, Yang SF. Antimetastatic effects of norcantharidin on hepatocellular carcinoma by transcriptional inhibition of MMP-9 through modulation of NF-kB activity. PLoS ONE. 2012;7:e31055. doi: 10.1371/journal.pone.0031055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yue M, Pacheco G, Cheng T, Li J, Wang Y, Henske EP, et al. Evidence supporting a lymphatic endothelium origin for angiomyolipoma, a TSC2(−) tumor related to lymphangioleiomyomatosis. Am J Pathol. 2016;186:1825–1836. doi: 10.1016/j.ajpath.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhao L, Yang G, Bai H, Zhang M, Mou D. NCTD promotes Birinapant-mediated anticancer activity in breast cancer cells by downregulation of c-FLIP. Oncotarget. 2017;8:26886–26895. doi: 10.18632/oncotarget.15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang WJ, Wu MY, Shen M, Zhi Q, Liu ZY, Gong FR, et al. Cantharidin and norcantharidin impair stemness of pancreatic cancer cells by repressing the β-catenin pathway and strengthen the cytotoxicity of gemcitabine and erlotinib. Int J Oncol. 2015;47:1912–1922. doi: 10.3892/ijo.2015.3156. [DOI] [PubMed] [Google Scholar]
- 174.Du HF, Yu LJ, Meng YF, Lv HY, Meng J, Song XN, et al. Norcantharidin enhances bortezomib-antimyeloma activity in multiple myeloma cells in vitro and in nude mouse xenografts. Leuk Lymphoma. 2013;54(3):607–618. doi: 10.3109/10428194.2012.720371. [DOI] [PubMed] [Google Scholar]
- 175.Lu S, Gao Y, Huang X, Wang X. Cantharidin exerts anti-hepatocellular carcinoma by miR-214 modulating macrophage polarization. Int J Biol Sci. 2014;10(4):415–425. doi: 10.7150/ijbs.8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhu J, Zhang W, Wang DD, Li SZ, Wu W. Preparation and characterization of norcantharidin liposomes modified with stearyl glycyrrhetinate. Exp Ther Med. 2018;16:1639–1646. doi: 10.3892/etm.2018.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chi JH, Jiang ZW, Chen XT, Peng YF, Liu WH, Han BS, et al. Studies on anti-hepatocarcinoma effect, pharmacokinetics and tissue distribution of carboxymethyl chitosan based norcantharidin conjugates. Carbohydr Polym. 2019;226:115297. doi: 10.1016/j.carbpol.2019.115297. [DOI] [PubMed] [Google Scholar]
- 178.Chi JH, Jiang ZW, Qiao J, Peng YF, Liu WS, Han BQ. Synthesis and anti-metastasis activities of norcantharidin-conjugated carboxymethyl chitosan as a novel drug delivery system. Carbohydr Polym. 2019;214:80–89. doi: 10.1016/j.carbpol.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 179.Chang MC, Wu JY, Liao HF, Chen YJ, Guo CD. N-Farnesyloxy-norcantharimide inhibits progression of human leukemic Jurkat T cells through regulation of mitogen-activated protein kinase and interleukin-2 production. Anticancer Drugs. 2015;26:1034–1042. doi: 10.1097/CAD.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All available data and material can be accessed.