Significance
Microbiota-derived short-chain fatty acids influence processes important for the intestinal health of hosts and the activities of resident bacteria. Herein, we identified the Campylobacter jejuni BumSR TCS as an indirect sensor of butyrate. Because the BumSR TCS is important for infection of humans for diarrheal disease and optimal commensal colonization of avian hosts, a butyrate response by BumSR likely promotes molecular eavesdropping for C. jejuni recognition of lower intestinal niches across hosts. Further analysis suggests a noncanonical mechanism for signal transduction in which BumS functions mainly as a phosphatase, rather than as a kinase, to control activity of the cognate BumR response regulator. Our findings indicate a complex and multifactorial mechanism for enacting butyrate-dependent responses in a bacterium.
Keywords: Campylobacter jejuni, butyrate, short-chain fatty acids, BumS, two-component signal transduction system
Abstract
Campylobacter jejuni monitors intestinal metabolites produced by the host and microbiota to initiate intestinal colonization of avian and animal hosts for commensalism and infection of humans for diarrheal disease. We previously discovered that C. jejuni has the capacity to spatially discern different intestinal regions by sensing lactate and the short-chain fatty acids acetate and butyrate and then alter transcription of colonization factors appropriately for in vivo growth. In this study, we identified the C. jejuni butyrate-modulated regulon and discovered that the BumSR two-component signal transduction system (TCS) directs a response to butyrate by identifying mutants in a genetic screen defective for butyrate-modulated transcription. The BumSR TCS, which is important for infection of humans and optimal colonization of avian hosts, senses butyrate likely by indirect means to alter transcription of genes encoding important colonization determinants. Unlike many canonical TCSs, the predicted cytoplasmic sensor kinase BumS lacked in vitro autokinase activity, which would normally lead to phosphorylation of the cognate BumR response regulator. Instead, BumS has likely evolved mutations to naturally function as a phosphatase whose activity is influenced by exogenous butyrate to control the level of endogenous phosphorylation of BumR and its ability to alter transcription of target genes. To our knowledge, the BumSR TCS is the only bacterial signal transduction system identified so far that mediates responses to the microbiota-generated intestinal metabolite butyrate, an important factor for host intestinal health and homeostasis. Our findings suggest that butyrate sensing by this system is vital for C. jejuni colonization of multiple hosts.
A complex ecosystem develops in the intestines of humans and animals that is influenced by the physiology and diet of the hosts and the metabolic capacities of diverse members of the microbiota (1, 2). As a result, specific biogeographies form in each intestinal tract region that are composed of metabolic byproducts and the microbiota that feed upon and contribute to these metabolites (1, 3). Short-chain fatty acids (SCFAs) such as butyrate, propionate, and acetate are important metabolites produced by an established intestinal microbiota that also contribute to the health of the host (4, 5). SCFAs are generated predominantly by clostridial species that ferment dietary fibers and sugars and can reach ∼130 mM in the lumen of the lower intestines (4–6). While all SCFAs have roles in host physiology, butyrate is particularly important for lower intestinal homeostasis as a primary energy source for colonocytes and its functions in inflammatory processes (7, 8). In contrast, the organic acid lactate generated by Lactobacillus species in large quantities is more constrained to the upper intestinal tract regions (9–11). Lactate is an energy source for commensal bacteria and pathogens while also promoting immune modulatory and intestinal barrier maintenance properties for the host (12–14).
Due to the abundance and intestinal distribution of SCFAs and lactate, bacterial pathogens can use these metabolites as biogeographical cues to discriminate among different regions of the host intestines (15–19). Furthermore, some pathogenic bacteria sense and exploit these metabolites for expansion and consequently induce inflammation during dysbiosis (12, 20–22). This phenomenon is particularly apparent among foodborne intestinal pathogens, including Salmonella enterica serovar Typhimurium and enterohemorrhagic Escherichia coli that sense SCFAs through a two-component signal transduction system (TCS) composed of the sensor kinase BarA and its cognate response regulator (annotated as UvrY in E. coli and SirA in Salmonella) to regulate virulence (23–25). In E. coli, BarA may directly sense formate to influence virulence gene expression and metabolism (25). However, acetate influences signal transduction independently of BarA by its conversion to acetyl-phosphate (AcP) through acetogenesis, thereby serving as a phosphodonor for UvrY to alter gene expression (25). Similarly, acetate activates Salmonella SirA through AcP synthesis to promote expression of invasion genes (23). Butyrate and propionate inhibit Salmonella invasion gene expression independent of BarA and SirA (23, 26). Propionate, via conversion to propionyl-CoA, leads to posttranslational regulation of the HilD response regulator (23, 24). However, identification of a butyrate sensor in these bacteria and others has remained elusive.
Another prominent cause of diarrheal disease in humans is Campylobacter jejuni, but little is known regarding how C. jejuni senses the intestinal biogeography (19, 27, 28). Upon oral ingestion, C. jejuni establishes infection mainly in the colon and rectum, causing a mild, watery to inflammatory, bloody diarrhea (29). In contrast, C. jejuni is a commensal organism in the intestinal tract of many avian species and other important animals in agriculture. In the natural avian host, infection can occur within days after hatch with C. jejuni establishing a prolonged, asymptomatic commensal colonization primarily in the ceca and large intestines of the lower intestinal tract with little resulting inflammation (30–33). Consequently, zoonotic cases of campylobacteriosis are often attributed to handling or consumption of contaminated retail chicken meats (34, 35).
To establish commensalism in avian hosts and cause disease in humans, we hypothesized that C. jejuni recognizes intestinal niches that support growth, which include the mucus layer lining the crypts of the lower intestinal epithelium, by sensing molecular cues produced by the microbiota. We previously acquired evidence that C. jejuni spatially discriminates among different regions of the avian intestinal tract by modulating gene expression in response to microbiota-derived metabolites (19). We discovered that C. jejuni induces transcription of genes encoding nutrient acquisition systems, energy generation pathways, and other colonization determinants in the presence of physiologic concentrations of the SCFAs butyrate and acetate. As such, regions of the avian lower intestinal tract where C. jejuni preferentially colonizes and grows abundantly are enriched in these SCFAs (19, 36, 37). In contrast, transcription of beneficial colonization factors are repressed in the presence of the organic acid lactate, which is enriched in the upper intestinal tract where C. jejuni does not robustly colonize (19, 38, 39). Since the spatial gradients of microbiota-generated SCFAs and lactate are oriented similarly in the avian and human host and at similar concentrations (3, 6, 40–42), we propose that C. jejuni senses these same metabolites to impact gene expression in the intestinal tract of humans to promote infection and diarrheal disease.
In this work, we discovered that C. jejuni senses the microbiota-derived SCFA butyrate via the noncanonical BumSR TCS. This system, encoded by Cjj81176_1484 and Cjj81176_1483 in the C. jejuni strain 81-176 genome (here annotated as BumS and BumR, respectively), was previously analyzed, although the signals detected by this TCS remained unknown (43). Additionally, BumR has been shown to be important for promoting infection of humans, suggesting that directing a response to butyrate is important for colonization of hosts (44). Our data suggest that the predicted BumS sensor kinase of this TCS appears to lack natural kinase activity and instead functions as a phosphatase. We propose that BumS likely senses butyrate indirectly to influence BumS phosphatase activity to ultimately control the function of the cognate BumR response regulator and modulate transcription of colonization factors necessary for infection of humans and avian species.
Results
The C. jejuni BumSR TCS Mediates Butyrate-Dependent Responses.
We previously identified a collection of genes from C. jejuni strain 81-176 whose expression was altered in the presence of SCFAs (19). We investigated this gene set to identify a promoter that we could link to a promoterless astA gene (encoding arylsulfatase) to function as a quantitative and qualitative transcriptional reporter for analyses (45–49). We identified the promoter of peb3, encoding a glycoprotein that may function as a transporter for phosphate-containing metabolites, as a robust transcriptional reporter when fused to astA (50, 51). When wild-type (WT) C. jejuni was grown in Campylobacter-defined media (CDM), which contains amino acids and keto acids as the primary carbon sources for C. jejuni growth (52), supplemented with 12.5 mM butyrate, and buffered to pH 7.0, expression of the peb3::astA transcriptional reporter was reduced 10.2-fold compared to growth in CDM without butyrate (Fig. 1A). This butyrate concentration is within the physiological range present in the ceca and lower intestinal regions of chickens and humans (10 to 34 mM) (6, 36, 37, 40, 42).
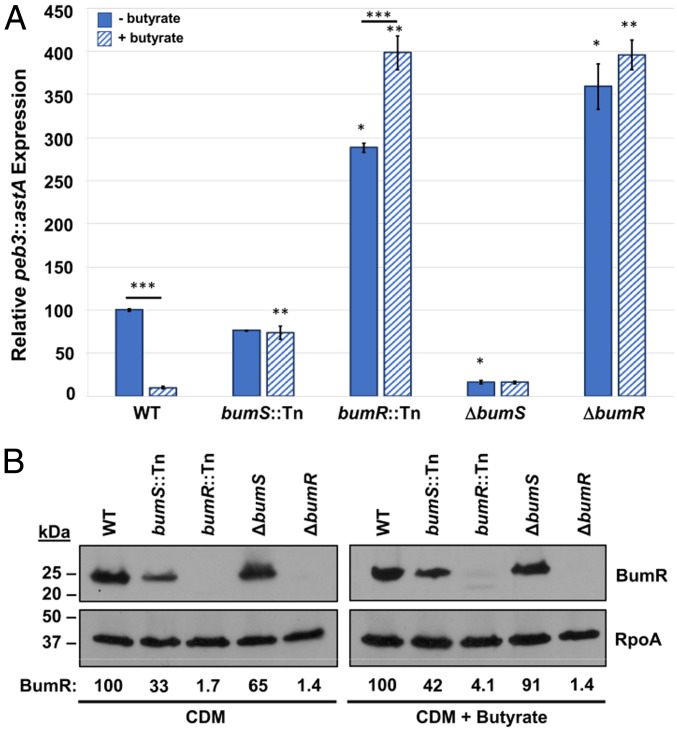
Fig. 1.
The BumSR TCS is required for butyrate-modulated expression of peb3. (A) Expression of the peb3::astA transcriptional reporter in WT C. jejuni ΔastA and isogenic bumS and bumR mutants. The bumS and bumR mutants examined include a representative bumS::Tn and bumR::Tn mutant isolated in our genetic screen to identify Tn mutants defective for butyrate-dependent repression of peb3::astA expression and previously constructed C. jejuni ΔbumS and ΔbumR mutants with in-frame deletion of the coding sequence of each gene. C. jejuni strains were grown for 16 h in CDM alone (solid blue bars) or CDM supplemented with 12.5 mM butyrate (hatched blue bars) as indicated. The level of peb3::astA expression in each mutant is relative to the level of expression in WT C. jejuni grown without butyrate, which was set to 100 units. Results from a representative assay with each sample analyzed in triplicate are shown. Error bars indicate SDs of the average level of expression from three samples. Statistical significance was calculated in GraphPad Prism by ANOVA with Tukey’s test: * indicates the mutant grown in CDM alone had significantly increased or decreased expression relative to the WT strain grown in CDM alone; ** indicates the mutant grown with butyrate had a significantly increased or decreased expression relative to the WT strain grown with butyrate; *** indicates a strain showed a significantly different level of expression when grown in the presence of butyrate compared to growth in CDM alone (P < 0.05). (B) Production of BumR in WT and isogenic bumS and bumR mutants. Immunoblot analysis of the level of BumR in whole-cell lysates of WT and mutant strains after growth in CDM alone or CDM supplemented with 12.5 mM butyrate. RpoA served as a control for protein loading. Immunoblots were performed in triplicate, and values underneath the immunoblots represent quantified BumR mean signals relative to RpoA and normalized to WT, which was set to 100.
WT C. jejuni peb3::astA appears as a light blue colony when grown on CDM with 12.5 mM butyrate containing X-S (the chromogenic substrate for arylsulfatase). We conducted a genetic screen to identify genes required for butyrate sensing by performing transposon (Tn) mutagenesis with WT C. jejuni peb3::astA and examining darkhelmet Tn mutants on CDM with butyrate and X-S. We identified 12 out of over 10,000 Tn mutants with a shift to a darker blue colony phenotype relative to WT on CDM with butyrate. Six transposons were located within Cjj81176_1484 and the remaining six were in the gene immediately downstream, Cjj81176_1483, in the 81-176 genome. Due to our findings presented below, we annotated Cjj81176_1484 as bumS and Cjj81176_1483 as bumR for their requirement in butyrate-modulated gene expression. Repression of peb3::astA expression in the presence of butyrate was abolished in bumS::Tn and bumR::Tn mutants (representative Tn mutants are shown in Fig. 1A). C. jejuni bumS::Tn and bumR::Tn exhibited a 7.6-fold and 40.9-fold increase in peb3::astA expression, respectively, in the presence of butyrate compared to WT with butyrate (Fig. 1A).
We verified that the BumSR TCS was responsible for butyrate-modulated responses by examining C. jejuni ΔbumS and ΔbumR from our strain collection that contained in-frame deletions of large portions of the coding sequences of each gene (43). In comparison to the bumS::Tn mutant, ΔbumS demonstrated a 4.6-fold reduction in peb3-astA expression regardless of butyrate supplementation. C. jejuni ΔbumR behaved similarly as the bumR::Tn mutant with increased peb3::astA expression compared to WT regardless of butyrate supplementation (Fig. 1A). Immunoblot analysis revealed that BumR levels were mostly similar in WT C. jejuni and ΔbumS, but were further reduced in bumS::Tn likely because of polar effects on bumR expression due to insertion of the transposon in bumS (Fig. 1B). These different BumR levels in bumS::Tn and ΔbumS likely contributed to the differences in peb3::astA expression between the mutants (Fig. 1B). Butyrate did not cause alterations of BumR levels in WT C. jejuni, suggesting that bumSR expression is independent of butyrate (Fig. 1B). Our findings that mutation of bumS and bumR result in contrasting effects suggest that signal transduction through the BumSR TCS resulted in BumS influencing the activity of BumR as a transcriptional repressor, at least for peb3 expression.
In cis complementation of ΔbumS and ΔbumR with native promoters and the respected genes inserted into rdxA on the chromosome reversed the phenotypes of each mutant (SI Appendix, Fig. S1) (53, 54). Butyrate supplementation reduced peb3 transcription in each complemented strain, similar to the butyrate-dependent repression of peb3 transcription in WT C. jejuni. These results indicate that the BumSR TCS is responsible for sensing butyrate either directly or indirectly and mediating butyrate-modulated transcription.
BumR Receiver Domain Mutants Are Impaired for Butyrate-Modulated Gene Expression.
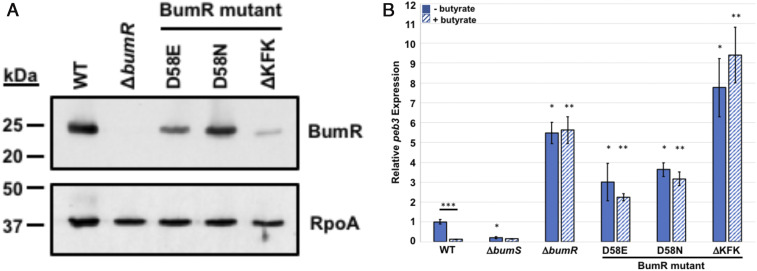
Previous analyses revealed that D58 of the BumR receiver domain is the site of phosphorylation with AcP as a phosphodonor (43). Whereas the D58E or D58N mutation of BumR prevented autophosphorylation, a D58A mutation destabilized the protein (43). In a study of C. jejuni infection of human volunteers, BumR was discovered to be important for infection (44). In this previous study, the inoculum for infection of humans unknowingly contained a mixed population of WT C. jejuni and a mutant with a 9-base pair in-frame deletion in bumR that altered a KFKKFK sequence at position 46 to 51 in the BumR receiver domain to a single KFK unit (BumRΔKFK), which is near the site of BumR phosphorylation at D58 (43). This mixed inoculum given to human volunteers was discovered by SNP analysis after the infection study was completed. C. jejuni bumRΔKFK was outcompeted by WT C. jejuni and not recovered from any human volunteers, demonstrating that WT BumR was important for infection of humans (44). However, a mechanistic reason for why and how the bumRΔKFK mutation affected processes in C. jejuni was not determined.
We recreated bumRΔKFK in WT C. jejuni strain 81-176 and examined this mutant along with the bumRD58E and bumRD58N mutants for butyrate-modulated peb3 transcription. In whole-cell lysates, BumRΔKFK levels were greatly reduced likely due to instability of the protein, with BumRD58E and BumRD58N only slightly or modestly reduced compared to WT BumR (Fig. 2A), suggesting that bumRΔKFK might have the same phenotype as ΔbumR. Analysis of peb3 transcription in WT and mutant strains grown with or without 12.5 mM butyrate revealed that mutation of D58 affects BumSR activity as peb3 transcription was not repressed by butyrate in bumRD58E and bumRD58N (Fig. 2B). Thus, D58, as the phosphorylation site of BumR, is important for BumSR TCS signal transduction in response to butyrate. As expected, bumRΔKFK like ΔbumR showed augmented levels of peb3 transcription relative to WT C. jejuni that was not repressed by butyrate. These results suggest that butyrate modulation of gene expression is defective with the bumRΔKFK allele. Since this mutation also abolished the ability of C. jejuni to infect humans, our findings suggest that butyrate sensing and alteration of transcription via BumSR TCS signal transduction is highly important for in vivo growth in humans and progression to diarrheal disease.
Fig. 2.
Effect of BumR receiver domain mutations on butyrate-modulated transcription. (A) Production of BumR in WT C. jejuni and bumR mutants. Immunoblot analysis of the level of BumR in whole-cell lysates of WT and mutant strains after growth in Mueller–Hinton media. RpoA served as a control for protein loading. (B) qRT-PCR analysis of peb3 transcription in WT C. jejuni and bumR mutants grown in CDM alone or CDM supplemented with 12.5 mM butyrate. The expression of peb3 in CDM alone (solid blue bars) and CDM with butyrate (hatched blue bars) in WT C. jejuni and mutant strains are shown. The level of peb3 transcription in WT grown in CDM alone as measured by qRT-PCR was set to 1. Expression of peb3 in WT grown in CDM with butyrate or mutants grown with or without butyrate is shown relative to the WT strain grown in CDM alone. Results from a representative assay with each sample analyzed in triplicate are shown. Error bars indicate SDs of the average level of expression from three samples. Statistical significance of ΔCT values relative to secD reference gene was calculated in GraphPad Prism by ANOVA with Tukey’s test: * indicates the mutant grown in CDM alone had significantly increased or decreased expression relative to the WT strain grown in CDM alone; ** indicates the mutant grown with butyrate had a significantly increased or decreased expression relative to the WT strain grown with butyrate; *** indicates a strain showed a significantly different level of expression when grown in the presence of butyrate compared to growth in CDM alone (P < 0.05).
BumR Is Required for Commensal Colonization of Avian Hosts at Early Time Points of Infection.
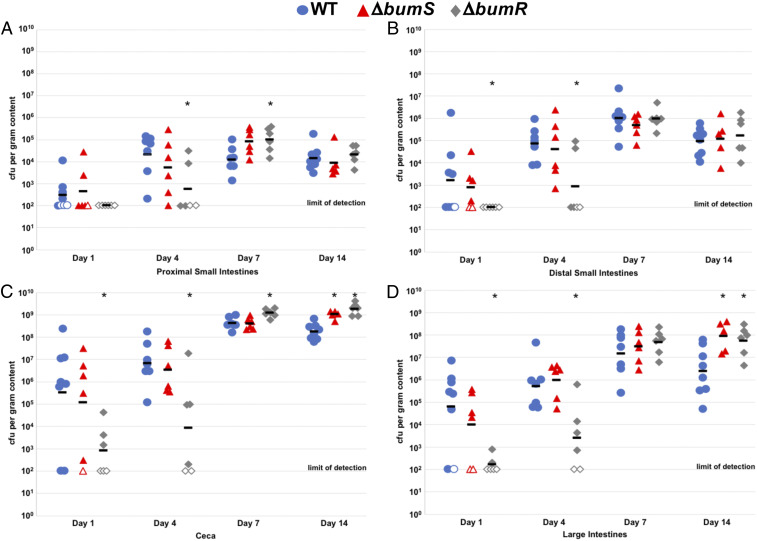
Although BumR is important for infection of human volunteers (44), we previously analyzed the commensal colonization capacity of C. jejuni ΔbumS and ΔbumR for the ceca of day-of-hatch chicks at day 7 postinfection and found that the mutants colonized as well as WT C. jejuni (43). For more thorough investigation of the BumSR TCS in commensal colonization of chicks, we compared the ability of WT C. jejuni and isogenic ΔbumS and ΔbumR mutants to colonize different regions of the avian intestinal tract over time. Day-of-hatch chicks were orally gavaged with C. jejuni strains and the levels of C. jejuni in the proximal and distal small intestines, ceca, and large intestines from day 1 to 14 postinfection were determined. BumR was required for optimal colonization throughout the intestinal tract at days 1 and 4 postinfection, as ΔbumR showed 3- to 782-fold reductions in colonization compared to WT C. jejuni (Fig. 3 A–D). In contrast, ΔbumS only showed a 6-fold lower colonization capacity in the large intestines at day 1 postinfection (Fig. 3 A–D). Similar to our previous analysis, we did not observe reductions of colonization by the mutants at day 7 and 14 postinfection of day-of-hatch chicks (43). We did observe enhanced colonization by ΔbumS and ΔbumR relative to WT in some regions of the intestinal tract, most noticeably in the large intestines at day 14 postinfection (Fig. 3 A–D). Our results, combined with the previous human volunteer study, indicate that BumR is required for infection of the human host and optimal colonization of the avian host, at least in young chicks. Our findings also suggest that BumS and BumR possibly have modulatory effects during prolonged commensal colonization.
Fig. 3.
Colonization dynamics of WT C. jejuni and isogenic ΔbumS and ΔbumR mutants over time in the avian intestinal tract. Day of hatch chicks were orally infected with ∼100 colony-forming units (CFU) of WT C. jejuni (blue circles), isogenic ΔbumS (red triangles), and ΔbumR (gray diamonds) mutants. Chicks were killed at days 1, 4, 7, or 14 postinfection and the levels of each C. jejuni strain in A the proximal small intestine, (B) distal small intestines, (C) ceca, and (D) large intestines was determined (reported as CFU per gram of content). Each closed symbol represents the level of C. jejuni in a single chick. Open symbols represent chicks with C. jejuni levels below the limit of detection (<100 CFU per gram of content). Horizontal bars represent geometric mean for each group. Statistical analysis was performed using the Mann–Whitney U test (*P < 0.05).
The C. jejuni BumSR TCS Impacts Expression of Colonization Determinants.
To understand the impact of butyrate on C. jejuni gene expression, we performed a transcriptome analysis of WT C. jejuni grown with or without a physiological concentration of butyrate to identify the butyrate-modulated regulon. Because we observed differences in peb3 expression and requirements for commensal colonization between ΔbumS and ΔbumR, we hypothesized that the transcriptomes of the two mutants likely differ. Therefore, we also performed RNA sequencing (RNAseq) analysis to compare the transcriptomes of WT C. jejuni, ΔbumS, and ΔbumR to fully reveal the BumSR regulon.
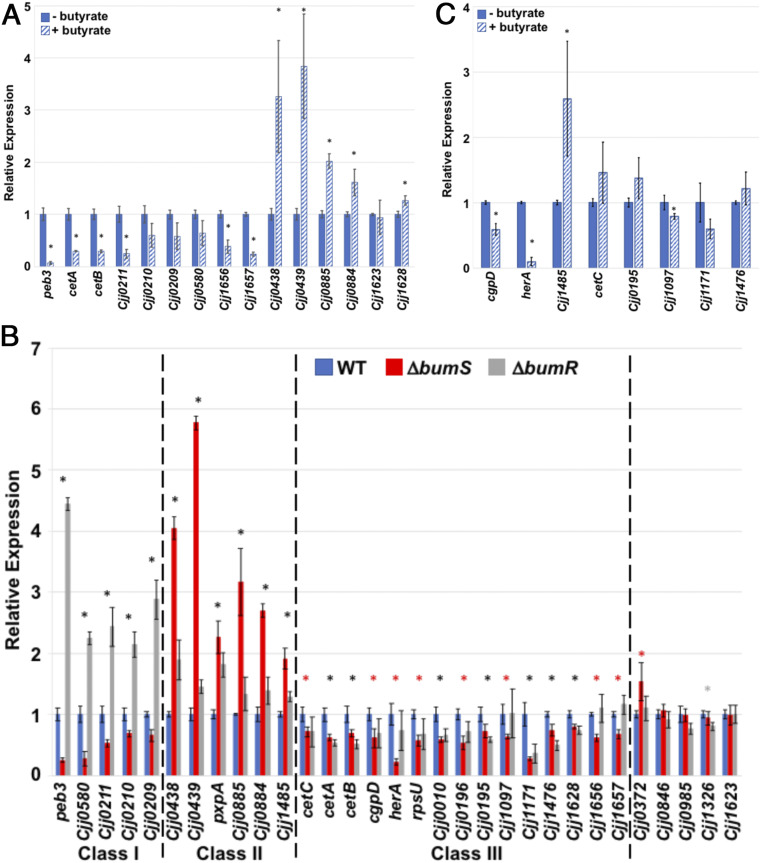
RNAseq analysis of the transcriptomes of WT C. jejuni with or without exposure to 12.5 mM butyrate revealed at least 11 potential genes with significantly altered transcription (SI Appendix, Table S1 and Dataset S1). Validation of RNAseq analysis by qRT-PCR revealed significant repression of some genes (and some cotranscribed operonic genes) in the presence of butyrate (Fig. 4A). Supplementation with butyrate led to 14.5-fold decreased expression of peb3 (the reporter gene in our genetic screen to identify the BumSR TCS), 3.4-fold reduction of cetAB (encoding an energy taxis system), and 4.1-fold reduction of Cjj0211 (the first gene of an operon encoding an iron acquisition system) (Fig. 4A). Note, for simplicity in describing genes from the 81-176 genome, we simplified the locus tags so that “Cjj0211” represents “Cjj81176_0211,” for example. We also found Cjj1656 and Cjj1657 showed 2.6- and 4.2-fold reductions in expression in the presence of butyrate, respectively. We confirmed that other genes were induced upon butyrate exposure (Fig. 4A). Transcription of Cjj0438 and Cjj0439, which compose a gluconate dehydrogenase complex required for colonization of chicks that we previously found to be regulated by the BumSR TCS (43, 55), was induced 3.5-fold upon butyrate supplementation (Fig. 4A).
Fig. 4.
Analysis of C. jejuni butyrate-modulated genes and the BumSR regulon. (A) qRT-PCR analysis of transcription of genes initially identified by RNAseq analysis of WT C. jejuni to be modulated in expression by 12.5 mM butyrate during growth in CDM. The expression of each gene in WT C. jejuni grown in CDM alone (solid blue bars) as measured by qRT-PCR was set to 1. Expression of each gene in WT C. jejuni grown in CDM supplemented with 12.5 mM butyrate (hatched blue bars) is shown relative to WT grown in CDM alone. (B) qRT-PCR analysis of transcription of genes initially identified by RNAseq analysis to be altered in C. jejuni ΔbumS and/or ΔbumR relative to WT C. jejuni. The expression of each gene in WT C. jejuni grown in CDM alone (solid blue bars) as measured by qRT-PCR was set to 1. Expression of each gene in ΔbumS (red bars) and ΔbumR (gray bars) is shown relative to WT. Dotted lines indicates divisions between gene sets (classes I to III) show similar effects in expression due to deletion of bumS and/or bumR. (C) Analysis of the ability of BumSR TCS regulon members to be modulated by butyrate for transcription. Select genes that were altered in expression in C. jejuni ΔbumS or ΔbumR were analyzed for butyrate-modulated expression in WT C. jejuni grown in CDM alone (solid blue bars) and in CDM supplemented with 12.5 mM butyrate (hatched blue bars) by qRT-PCR. The expression of each gene in WT C. jejuni grown in CDM alone was set to 1. Expression of each gene in WT C. jejuni grown in CDM supplemented with 12.5 mM butyrate is shown relative to WT grown in CDM alone. For (A–C), results from a representative assay with each sample analyzed in triplicate are shown. Error bars indicate SDs of the average level of expression from three samples. Statistically significant differences in gene expression between WT C. jejuni grown with or without butyrate is indicated by * in A and C (P < 0.05). For (B), different colored asterisks indicate statistically significant differences (P < 0.05) in gene expression between: WT C. jejuni and both ΔbumS and ΔbumR, black *; WT C. jejuni and ΔbumS only, red *; and WT C. jejuni and ΔbumR only, gray * (P < 0.05). Statistical analysis of ΔCT values relative to secD reference gene was calculated by the Student’s t test.
Comparison of the transcriptomes of WT C. jejuni and isogenic ΔbumS and ΔbumR mutants grown in CDM without butyrate revealed 46 potential genes with significantly altered transcription (SI Appendix, Table S2 and Datasets S2–S5). Four potential genes were differentially expressed between WT C. jejuni and ΔbumS and 33 potential genes were differentially expressed between WT C. jejuni and ΔbumR. Moreover, nine potential genes were significantly altered in expression in both ΔbumS and ΔbumR compared to WT C. jejuni (SI Appendix, Table S2 and Datasets S2 and S3). We confirmed the results of the RNAseq analysis via qRT-PCR, and by combining the data from both experiments, genes could be grouped into specific categories based on their expression profiles. Transcription of some genes (class I) including peb3 and Cjj0580 were repressed by BumR as transcription was increased in ΔbumR (Fig. 4B). However, ΔbumS exhibited an opposing phenotype with decreased expression of these genes compared to WT. Another set of genes (class II) that includes the Cjj0438 and Cjj0885 operons were increased in expression in both ΔbumS and ΔbumR compared to WT, suggesting that BumR represses transcription of these genes (Fig. 4B). A third set of genes (class III), which includes cetAB, herA, and rpsU, were repressed in ΔbumS with either slight repression or no change in expression in ΔbumR (Fig. 4B). The BumSR regulon did contain genes modulated by butyrate supplementation in WT C. jejuni, but not originally identified to be differentially expressed in our RNAseq analysis of WT grown with or without butyrate (Fig. 4C and Datasets S1–S5). We were able to expand the butyrate-modulated regulon as cgpD and herA were reduced in expression in WT C. jejuni in the presence of butyrate, and Cjj1485 expression was induced with butyrate supplementation (Fig. 4C). Other BumSR regulon members showed only modest-to-no significant alterations in expression with butyrate supplementation (Fig. 4C). Our findings for how ΔbumS and ΔbumR differently impact expression of genes such as peb3, the Cjj0438 operon, and cetAB suggest complexity in molecular mechanisms of signal transduction through the BumSR TCS and for different modes of regulating transcription of genes within its regulon.
Given that we found BumR to be important for the initial colonization of chicks, we analyzed whether some butyrate-modulated genes within the BumSR regulon were involved in colonization in a natural chick model of commensalism. For this experiment, day-of-hatch chicks were orally gavaged with WT C. jejuni or an isogenic mutant and the levels of C. jejuni in the proximal and distal small intestines, ceca, and large intestines were determined at days 4 and 7 postinfection (SI Appendix, Fig. S2). Mutants lacking a specific BumSR regulon member demonstrated a range of colonization capacities for the avian intestinal tract. Mutants in peb3 and Cjj0580, which have similar expression profiles in WT C. jejuni supplemented with butyrate and in the ΔbumS and ΔbumR mutants, exhibited similar colonization defects with 68- to 688-fold reductions in the ceca and large intestines at an early time point postinfection (SI Appendix, Fig. S2 C and D). The peb3 mutant colonization defect continued for the large intestines and became more prominent in some other intestinal areas at day 7. Mutants lacking Cet energy taxis system components (CetA, CetB, or CetC; refs. 56–58), which generally depend on both BumS and BumR for WT level of expression, were not defective for cecal colonization, but were lower in other areas of the intestinal tract especially at day 7 postinfection (SI Appendix, Fig. S2 A–D). In contrast, the mutant lacking herA encoding a hemerythrin (59), which is mainly dependent on BumS for expression, was not defective for colonization in any intestinal areas. Considering that deletion of bumS or bumR results in lack of expression for some genes and derepression and overexpression of others, linking any one BumSR regulon member to general colonization defects or enhancements observed with ΔbumS and ΔbumR is hindered. Rather, the BumSR TCS controls expression of butyrate-modulated determinants, of which many can influence commensal colonization in different areas of the intestinal tract over time.
The BumSR TCS Specifically Responds to Butyrate but Not Acetate or Lactate.
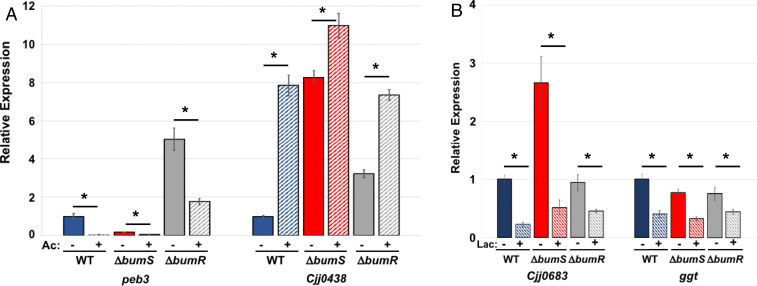
We previously discovered that C. jejuni senses and responds to physiological levels of intestinal acetate and l-lactate in addition to butyrate (19). In this prior study, we also analyzed whether C. jejuni senses and responds to propionate, but only detected a transcriptional change to propionate at a concentration ∼10-fold greater than physiological levels present in the lower intestinal tract of humans and avian species. We analyzed whether BumSR sensing is specific for butyrate or if it has an expanded repertoire for acetate and lactate. Expression of two butyrate-modulated genes of the BumSR regulon, peb3 and Cjj0438, was also modulated when WT C. jejuni was grown in CDM with a physiological concentration of 100 mM acetate (Fig. 5A). The reported levels of acetate in the lower intestinal regions of chickens ranges for 47 to 124 mM (36, 37). In the presence of acetate, peb3 transcription was reduced 28.5-fold and Cjj0438 was increased 7.9-fold in WT C. jejuni. In ΔbumS and ΔbumR, we continued to observe significant repression of peb3 transcription and induction of Cjj0438 expression in the presence of acetate (Fig. 5A), suggesting that a system other than the BumSR TCS is responsible for sensing and responding to acetate.
Fig. 5.
The BumSR TCS is not required for acetate- or lactate-modulated gene expression. (A) qRT-PCR analysis of transcription of peb3 and Cjj0438 in WT C. jejuni and ΔbumS or ΔbumR mutants grown in CDM alone or CDM supplemented with 100 mM acetate. The expression of peb3 and Cjj0438 in WT C. jejuni grown in CDM alone (sold blue bars) as measured by qRT-PCR was set to 1. Expression of genes in WT grown in CDM supplemented with acetate (hatched blue bars), ΔbumS without (solid red bars), or with acetate (hatched red bars), or ΔbumR without (solid gray bars) or with acetate (hatched gray bars) is shown relative to WT grown in CDM alone. (B) qRT-PCR analysis of transcription of Cjj0683 and ggt in WT C. jejuni and ΔbumS or ΔbumR mutants grown in CDM alone or CDM supplemented with 25 mM l-lactate. The expression of Cjj0683 and ggt in WT C. jejuni grown in CDM alone (sold blue bars) as measured by qRT-PCR was set to 1. Expression of genes in WT grown in CDM supplemented with l-lactate (hatched blue bars), ΔbumS without (solid red bars) or with l-lactate (hatched red bars), or ΔbumR without (solid gray bars) or with l-lactate (hatched gray bars) is shown relative to the WT strain grown in CDM alone. Results from a representative assay with each sample analyzed in triplicate are shown. Error bars indicate SDs of the average level of expression from three samples. Statistical significance of ΔCT values relative to recA reference gene (for acetate analysis in A) or secD reference gene (for lactate analysis in B) was calculated in GraphPad Prism by ANOVA with Sidak’s test: * indicates statistically significant differences in gene expression between a strain grown in CDM alone and in CDM supplemented with acetate or lactate (P < 0.05).
Given that transcription of several genes within the BumSR regulon were not affected by l-lactate, we analyzed expression of Cjj0683 and ggt, which we previously found were modulated by l-lactate (19). Reported levels of lactate in the upper intestinal regions of chickens range from 21 to 72 mM (38, 39). In WT C. jejuni grown in CDM with 25 mM l-lactate, significant transcriptional repression of both genes was observed (Fig. 5B). Transcriptional repression was also evident in ΔbumS and ΔbumR when grown in l-lactate (Fig. 5B). Therefore, we conclude that the BumSR TCS specifically directs a response to butyrate and not other SCFAs or organic acids such as acetate or l-lactate.
Evidence for BumS Controlling BumR Activity Primarily through Phosphatase Activity.
Canonical bacterial TCSs respond to environmental stimuli and mediate outputs by first sensing a specific signal via the sensory domain of the kinase, which leads to autophosphorylation on a conserved histidine residue (60, 61). The phosphohistidine is a substrate for autophosphorylation of a cognate response regulator, which causes a conformational change in the effector domain to alter the activity of the protein (60). For response regulators that are transcription factors, phosphorylation affects the ability of these proteins to bind DNA and function as activators or repressors of gene expression. Many sensor kinases function as phosphatases to control the level of phosphorylation and activity of a cognate response regulator when not functioning as an autokinase (62). This phosphatase activity contributes to signaling fidelity and elimination of crosstalk between signal transduction systems in bacteria (63).
Most bacterial sensor kinases contain transmembrane domains to localize to the cytoplasmic membrane. However, BumS is predicted to be a cytoplasmic sensor histidine kinase as no transmembrane domains are predicted in its protein sequence. Based on the canonical TCS signal transduction model, H195 in the H box of BumS is the best candidate as the site of autophosphorylation. We previously confirmed D58 in the receiver domain of BumR as the site of phosphorylation (43). Further sequence analysis of BumS reveals potential alterations in two regions that could affect autokinase activity. One region includes the H box, where the histidine that is the site of autophosphorylation is usually followed by an aspartate or glutamate and a hydrophobic residue (most often a leucine or isoleucine) (64, 65). In BumS, H195 is followed by a glutamine and tryptophan. The second potentially altered domain is the DxGxG motif in the D box of the histidine kinase and GHL ATPase superfamilies that coordinates a magnesium ion to bind ATP (65). The sequence of the D box in BumS is DxAxG; this glycine-to-alanine alteration eliminated autokinase activity in CheA sensor kinases (66, 67).
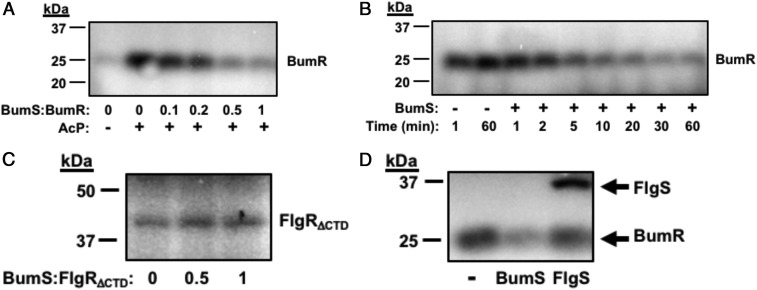
Repeated attempts to observe in vitro autophosphorylation of recombinant BumS purified from E. coli were unsuccessful, even in the presence of physiological concentrations of butyrate or with recombinant BumR (SI Appendix, Fig. S3 A and B). As a control, we observed both in vitro autophosphorylation of another C. jejuni sensor histidine kinase, FlgS, and phosphorylation of a mutant form of its cognate response regulator, FlgRΔCTD, which is more promiscuous for autophosphorylation by AcP, as previously described (SI Appendix, Fig. S3 A and B; refs. 49, 68, and 69). These findings suggest that WT BumS does not possess observable in vitro autokinase activity at least by these conditions employed and may not be responsible for the phosphorylation of BumR in C. jejuni.
We then investigated whether BumS functions as a phosphatase with phospho-BumR as a substrate. For these analyses, we added Ac[32P] to promote autophosphorylation of BumR and then observed whether levels of phospho-BumR were reduced upon addition of BumS. We observed that BumS caused robust dephosphorylation of BumR that was dependent on the concentration of BumS and time (Fig. 6 A and B). BumS phosphatase activity was specific for BumR as BumS was unable to dephosphorylate the noncognate phosphorylated FlgRΔCTD response regulator (Fig. 6C). Furthermore, FlgS was unable to dephosphorylate BumR (Fig. 6D), also indicating that dephosphorylation of BumR is an activity specific for BumS. In the course of this experimentation, we observed that BumR is naturally more readily autophosphorylated with Ac[32P] than is FlgRΔCTD (∼65-fold more; SI Appendix, Fig. S3C).
Fig. 6.
BumS phosphatase activity is specific for phospho-BumR. (A) Dephosphorylation of phospho-BumR by BumS. After autophosphorylation of BumR by Ac[32P], increasing concentrations of BumS were added as indicated by BumS:BumR molar ratios and then incubated for 10 min. (B) Dephosphorylation of phospho-BumR by BumS over time. After autophosphorylation of BumR by Ac[32P], mock treated or BumS at a 1:2 BumS:BumR molar ratio was added and incubated for up to 60 min. Reactions were stopped at various times indicated. (C) Dephosphorylation of phospho-FlgRΔCTD by BumS. After autophosphorylation of FlgRΔCTD by Ac[32P], BumS was added at 1:2 or 1:1 BumS:FlgRΔCTD molar ratio for 20 min. (D) Comparison of dephosphorylation of phospho-BumR by BumS and FlgS. After autophosphorylation of BumR by Ac[32P], BumS or FlgS were added at 1:2 molar ratio to BumR and the reactions were incubated for 10 min.
We next tested whether butyrate might be sensed by BumS and impact BumS phosphatase activity for BumR. Titration of butyrate up to 12.5 mM, which we observed to impact butyrate-modulated gene expression, did not influence the phosphatase activity of BumS (SI Appendix, Fig. S3D). These findings suggest that butyrate-modulated gene expression likely involves indirect sensing of butyrate by BumS to alter BumS phosphatase activity to control the level of phosphorylation of BumR and its activity as a response regulator. Due to the lack of detectable in vitro BumS kinase activity, BumR phosphorylation in C. jejuni is hypothesized to occur by other noncognate kinases or metabolite phosphodonors.
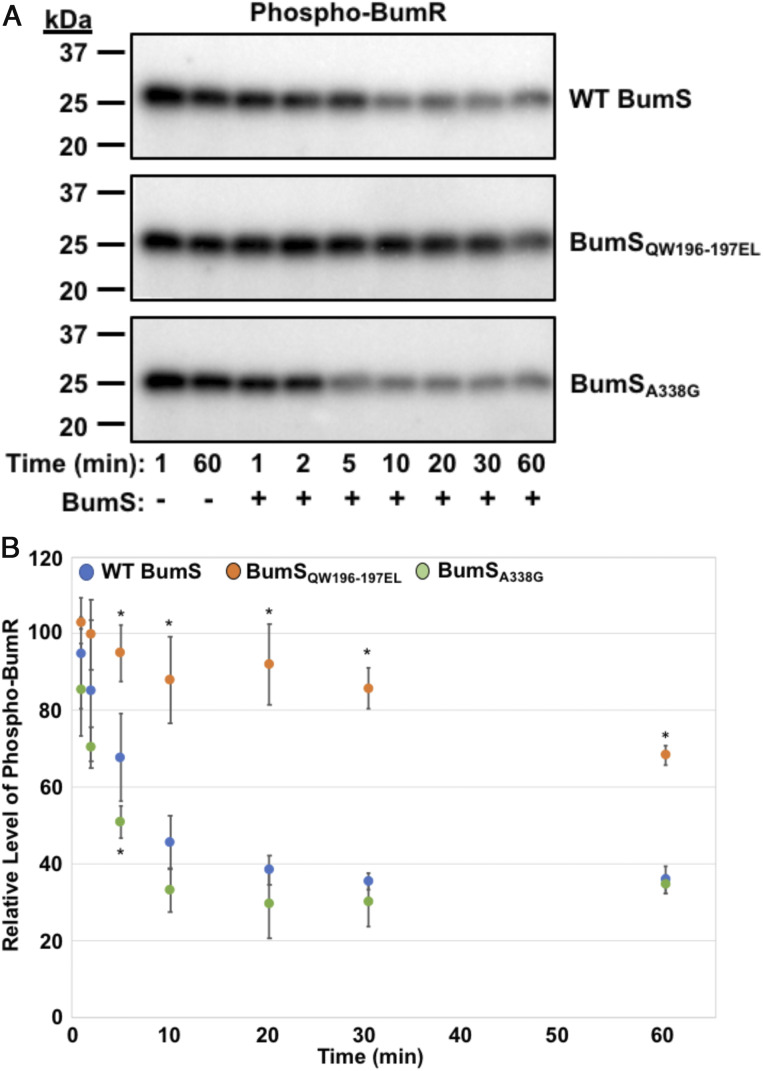
As discussed above, WT BumS contains alterations in the H and D boxes that could impair its ability to function naturally as an autokinase to ultimately serve as a direct phosphodonor for BumR. BumS sequences across many C. jejuni strains also conserve these alterations in the H and D boxes found in WT BumS in C. jejuni strain 81-176, which was used throughout this study. We tested whether kinase activity could be restored to BumS by altering residues in the H and D boxes to consensus residues present in a majority of active kinases. As such, we purified two recombinant BumS proteins, BumSQW196-7EL and BumSA338G (“restored” H and D boxes, respectively) and a third protein that combined the three mutations (BumSHD box). None of the mutations alone or combined restored autokinase activity to BumS (SI Appendix, Fig. S4). We then assessed whether BumS phosphatase activity was affected by these mutations. Addition of WT BumS to phospho-BumR at a 1:2 ratio resulted in 50% dephosphorylation of BumR between 5 and 10 min of incubation (Fig. 7 A and B). A slightly enhanced level of BumR dephosphorylation was noted for BumSA338G, indicating that A338 was not required for phosphatase activity. In contrast, BumSQW196-7EL exhibited significantly decreased phosphatase activity toward BumR with no more than a 20% reduction in phospho-BumR over time (Fig. 7 A and B). These findings suggest that BumS may have evolved these mutations to primarily serve as a phosphatase rather than a kinase to mediate control of BumR activity.
Fig. 7.
BumS phosphatase activity is dependent on specific H box residues. (A and B) In vitro phosphatase activities WT BumS, BumSQW196-7EL (an H box mutant), and BumSA338G (a D box mutant) over time. After autophosphorylation of BumR by Ac[32P], BumS proteins were added at 1:2 BumS:BumR molar ratios for up to 60 min. Reactions were stopped at various times indicated. (A) Representative phosphatase assay of WT BumS or BumS mutants for phospho-BumR. (B) Quantification of three independent phosphatase assays for WT BumS, BumSQW196-7EL, and BumSA338G. The level of phospho-BumR in each assay was normalized to phospho-BumR at 1 min of mock sample, which was set to 100%. No appreciable decrease in phospho-BumR in the absence of BumS was observed for the duration of the experiment (up to 60 min) as observed in A. Error bars indicate SDs of the relative level of phospho-BumR at indicated time after addition of BumS from three independent experiments. Statistical significance to compare WT to mutant proteins was calculated in GraphPad Prism by ANOVA with Dunnett’s test (*P < 0.05).
BumR Binding to Target Promoters Is Modulated by Phosphorylation.
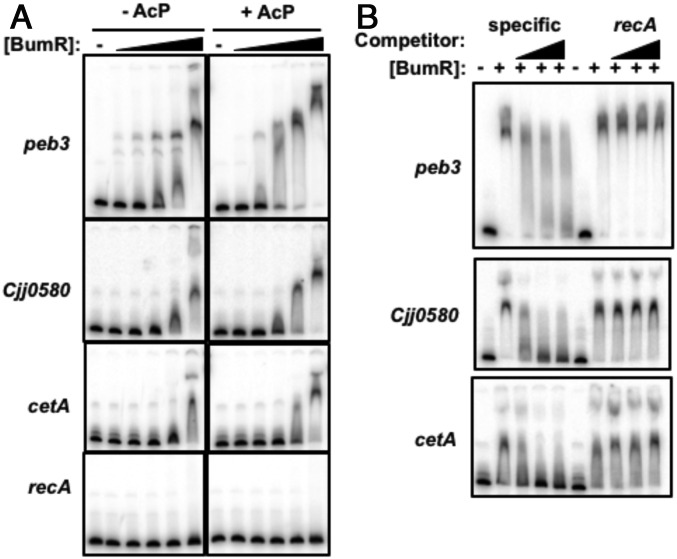
Since BumR activates and represses expression of different genes in its regulon, we examined the effect of BumR phosphorylation on promoter binding via electrophoretic mobility shift assays (EMSAs). We tested peb3, Cjj0580, and cetA promoter DNA for binding by BumR; a recA promoter probe served as a negative control to ensure specificity of BumR binding. Unphosphorylated BumR bound all promoters except recA at the highest BumR concentration tested (1.5 µM; Fig. 8A). Pretreatment of BumR with AcP increased binding at lower concentrations of BumR to all promoters except the recA negative control probe, suggesting that phosphorylated BumR augments DNA-binding activity to activate or repress transcription at different promoters. Competition assays with excess unlabeled promoter DNA or recA promoter DNA indicated that BumR specifically interacted with all target promoters (Fig. 8B). These findings are consistent with our previous results that control of the level of phosphorylation of BumR influences direct interactions with target promoter and the level of transcription of genes in the BumSR regulon (43).
Fig. 8.
Electrophoretic mobility shift assays for analysis of DNA-binding activity of BumR. (A) Comparison of BumR binding to target promoter with or without phosphorylation. Recombinant BumR alone or after autophosphorylation with Li-AcP was added to peb3, Cjj0580, cetA, or recA promoter DNAs at concentrations ranging from 0 to 1.5 μM. (B) Binding of 1.5 μm BumR after autophosphorylation with Li-AcP to radiolabeled promoter DNAs for peb3, Cjj0580, or cetA in the presence of increasing concentrations of the same unlabeled specific competitor DNA or unlabeled noncompetitor DNA (recA promoter).
Discussion
In this report, we identified a system and potential mechanism for a bacterium to sense and respond to the microbiota-generated SCFA butyrate, a metabolite with important activities in intestinal health and homeostasis. The C. jejuni BumSR TCS altered gene expression that impacted host colonization in the presence of butyrate. This work, combined with our previous analyses (19), demonstrate that C. jejuni senses and responds to multiple microbiota-generated metabolites, including the SCFAs butyrate and acetate and the organic acid lactate. As the BumSR TCS specifically responds to butyrate but not acetate or lactate, we propose C. jejuni employs different sensing systems in vivo to collectively monitor spatial gradients of specific metabolites and process this information to identify lower intestinal regions ideal for supporting growth and then altering gene expression appropriately. Continued exploration will reveal how butyrate sensing by the BumSR TCS likely converges into a complex network of signaling systems each possibly specific for different metabolites to result in optimal responses to ensure colonization of human and avian hosts.
A previous study with human volunteers found that a BumSR TCS mutant, due to an in-frame deletion within bumR that we found to make the respective protein unstable, is important for C. jejuni to infect humans (44). However, the activity of the TCS and how it contributed to in vivo growth and pathogenesis of disease in humans was not determined. Our findings suggest that directing a response to butyrate by the BumSR TCS and the resultant signal transduction that culminates in modulation of transcription of colonization and virulence factors is likely important for C. jejuni to infect humans. We also found that WT BumR was required for optimal levels of colonization of chicks at early time points after infection, but not at later time points. As our colonization assay ended at day 14 postinfection of chickens, we have yet determined whether the BumSR TCS may be important for a persistent infection lasting many weeks or for transmission from one host to another. Assuming that the BumSR TCS responds to butyrate in humans, butyrate sensing seems to be more important for C. jejuni to promote infection of humans than colonization of avian hosts. If so, we speculate that evolution of the BumSR TCS along with other virulence determinants potentially encoded within the C. jejuni BumSR regulon may have allowed the bacterium to extend its host range from a commensal in the avian host to a pathogen of humans. Thus, a number of members of the BumSR regulon that we identified whose expression is modulated upon sensing butyrate may be important for promoting infection of humans to result in diarrheal disease.
To our knowledge, the C. jejuni BumSR TCS is the only bacterial TCS identified thus far to be responsible for sensing and responding to butyrate. A search for bacterial species that contain a cognate pair of BumS and BumR orthologs only revealed hits in other C. jejuni strains and the closely related diarrheal pathogen Campylobacter coli. Butyrate has been shown to repress transcription of Salmonella virulence genes to cause reduced invasion of intestinal epithelial cells (23, 26). However, the only mechanism linked to how butyrate affects Salmonella so far has been the reduced transcription of hilD, encoding a transcription factor that derepresses expression of HilA, which is a positive activator of SPI1 gene expression (26). It is not known how butyrate is sensed directly by Salmonella to alter expression of the HilD-HilA pathway. Butyrate can also influence expression of the E. coli locus of enterocyte effacement through the Lrp master regulator, but it is mechanistically unclear how butyrate is sensed (17).
A question that remains from our analyses presented is whether BumS directly or indirectly senses butyrate. Data supporting that BumS directly senses butyrate are that simply supplementing C. jejuni with exogenous butyrate in vitro resulted in transcriptional changes that were abolished by mutation of bumS and bumR. However, butyrate did not influence the in vitro kinase or phosphatase activity of BumS in our assays. BumS lacks predicted transmembrane domains and is presumed to be a cytoplasmic sensor kinase with a predicted PAS domain as a major feature of its sensor region. This composition implies that BumS may detect a cytoplasmic signal. If butyrate is the direct cue sensed by BumS, then we would expect butyrate to be transported into the cytoplasm to function as a signal. We have not yet analyzed whether butyrate is transported by an active or passive process into the C. jejuni cytoplasm. Alternatively, microbiota-generated intestinal butyrate might be sensed indirectly by the BumSR TCS by being converted into another factor that is a cytoplasmic cue directly sensed by BumS. Bioinformatic analysis of the C. jejuni genome, however, does not identify any encoded canonical metabolic pathways for utilization of butyrate or its conversion to another metabolite. In a preliminary experiment, we attempted to detect changes in concentration of potential butyrate-derived metabolites after growth of C. jejuni in the presence of butyrate, but detection of these metabolites was difficult, requiring further experimentation and analysis development; therefore, our analysis was inconclusive. Alternatively, exogenous butyrate may influence the activity of another metabolic pathway in C. jejuni that creates a factor directly sensed by BumS. Our genetic screen to identify C. jejuni mutants with defects in butyrate-modulated responses only identified mutants with transposon insertions in bumS or bumR, and not other such auxiliary components. It is possible that our transposon mutagenesis screen may not have been saturating to identify every factor required for the indirect sensing of butyrate. Alternatively, if butyrate is converted by C. jejuni into a cytoplasmic cue or influences a metabolic system to generate such a cue that is sensed directly by BumS, this system could be essential for viability, providing a reason for why it was not identified in our genetic screen. Because butyrate is not required for normal C. jejuni growth, we assume that such an auxiliary system has a butyrate-independent primary activity essential for viability and a secondary butyrate-dependent activity to create a cue directly sensed by BumS.
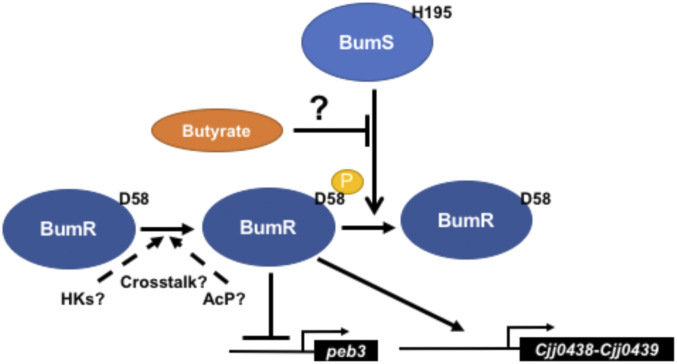
Based on our findings, we propose a model for BumSR signal transduction influenced by exogenous butyrate (Fig. 9). A salient feature of our model is BumS functioning prominently as a phosphatase rather than as a kinase to control the activity of BumR as a transcriptional regulator, with butyrate influencing a cytoplasmic signal that inhibits the BumS phosphatase activity to impact the level of phosphorylation of BumR. Using peb3 as an example, we can highlight aspects of our model. Deletion of bumR increases peb3 transcription, indicating that BumR serves as a transcriptional repressor in this context. Phosphorylation of BumR increased binding of BumR to the peb3 promoter, indicating that high levels of phospho-BumR are required for full repression of peb3. Since ΔbumS demonstrated high repression of peb3 transcription, BumR likely exists in a mostly phosphorylated form in ΔbumS to cause full repression. Addition of butyrate to WT C. jejuni decreased peb3 expression, suggesting that butyrate sensed by BumS inhibits its phosphatase activity to increase phospho-BumR levels and repress peb3 transcription. In contrast, Cjj0438 transcription was influenced in the opposite manner; both butyrate exposure and deletion of bumS stimulated transcription. BumR also showed increased binding to the Cjj0438 promoter upon phosphorylation (43). For this scenario, inhibiting the phosphatase activity of BumS (either with butyrate supplementation or deletion of the gene) leads to increased levels of phospho-BumR to activate transcription of Cjj0438.
Fig. 9.
Model for butyrate-modulated transcription of C. jejuni genes via the BumSR TCS. BumR is hypothesized to be endogenously phosphorylated by other kinases or high-energy phosphodonors in the cytoplasm of C. jejuni. BumS is proposed to primarily function as a phosphatase to control the level of phosphorylation of BumR and its activity as a transcriptional regulator. BumR shows increased affinity for target promoter DNA in its phosphorylated form and can function as a repressor or activator depending on the promoter. Exogenous butyrate produced by the intestinal microbiota is either sensed directly by BumS after transport to the cytoplasm, sensed indirectly by its conversion to another metabolite that is sensed by BumS, or sensed indirectly by its influence on another pathway that alters the level of a factor directly sensed by BumS. In any case, increased levels of exogenous butyrate are expected to have a negative influence on BumS phosphatase activity, which results in increased phospho-BumR levels to influence transcriptional outcomes.
Our proposed model for signal transduction through the BumSR TCS and how BumS functions is unusual as most sensor histidine kinases of bacterial TCSs studied to date sense a signal that influences its autokinase activity for phosphotransfer to its cognate response regulator as the primary mechanism to impact the activity of the regulator. Instead, we propose that butyrate sensing regulates the phosphatase activity of BumS for BumR, with BumS possibly lacking innate kinase activity. It is known that many bacterial sensor histidine kinases serve as phosphatases for their cognate response regulators when not functioning as kinases in the absence of their specific signals. Our model suggests that crosstalk with other C. jejuni sensor histidine kinases or high-energy phosphodonors such as AcP contributes to endogenous BumR phosphorylation on D58. In our cursory analysis, BumR did seem to be more readily phosphorylated by AcP than another C. jejuni response regulator (FlgRΔCTD) in vitro, suggesting that BumR may be fairly susceptible to phosphorylation by noncognate phosphodonors in C. jejuni. Thus, the BumSR TCS is apparently unique on two fronts—in sensing and responding to butyrate and in employing signal-dependent sensor phosphatase activity to exert control of its response regulator to modulate gene expression.
Examination of the amino acid sequence of BumS and motifs conserved in the protein histidine kinase family support our biochemical assays for BumS functioning as a phosphatase rather than a kinase. Although we did not perform ATP binding assays with recombinant BumS, the natural alteration in its D box from the canonical DxGxG motif to DxAxG motif present in BumS may prevent BumS from binding ATP and functioning as a kinase. Furthermore, many histidine kinases possess an H box in which the conserved autophosphorylated histidine residue is followed by an aspartate or glutamate and then a hydrophobic residue (65). In BumS, the predicted histidine that would normally be autophosphorylated, H195, is followed by a glutamine and the aromatic amino acid tryptophan. Of note, five of the six C. jejuni sensor histidine kinases of other TCSs all conserve the normal residues following the histidine that is the site of phosphorylation in the H box; the exception is CheA, whose orthologs in other bacterial species also do not have an H box (65). This H box alteration in BumS could have also caused BumS to no longer possess autokinase activity. We attempted to potentially restore BumS as a kinase by converting A338 to glycine to repair the DxGxG motif in the D box, along with converting Q196 and W197 to glutamate and leucine in the H box, respectively, which are the more standard amino acid residues at these positions in other kinases (65). However, combinations of single, double, and triple point mutants did not restore BumS kinase activity, which could suggest that BumS may have evolved multiple alterations besides those examined to more efficiently function as a phosphatase than a kinase. We actually demonstrated that mutation of Q196 and W197 to the canonical amino acids found in most histidine kinase greatly diminished BumS phosphatase activity without restoring kinase activity. Thus, these two noncanonical residues may represent a few evolutionary adaptations that have occurred to enhance the phosphatase activity of BumS that now drives the major mechanism by which BumS controls BumR activity, which is influenced by sensing exogenous butyrate via direct or indirect means. Overall, our work indicates a noncanonical, complex, and multifactorial mechanism for a bacterium to sense microbiota-derived butyrate in the intestines of hosts to promote infection of humans for diarrheal disease and colonization of avian hosts for commensalism.
Materials and Methods
Materials and methods describing the growth conditions, plasmid construction, C. jejuni mutant construction, transposon mutagenesis and genetic screening procedures, arylsulfatase assays, immunoblotting, chick colonization assays, RNAseq and qRT-PCR analysis, protein expression and purification, in vitro kinase and phosphatase assays, and EMSAs are described in detail in SI Appendix. All bacterial strains and plasmids constructed and used in this work are included in SI Appendix, Tables S3 and S4, respectively. All use of animals in experimentation has been approved by the institutional animal care and use committee at the University of Texas Southwestern Medical Center.
Data Availability.
All protocols and data discussed in the paper are available in the main text and SI Appendix. All strains and plasmids generated in this report will be made promptly available to readers.
Supplementary Material
Acknowledgments
This work was supported by Public Health NIH grants R01AI065539 (D.R.H.), R01HD095830 (D.R.H.), R01AI129940 (M.S.T.), R01AI138576 (M.S.T.), and R01AI150098 (M.S.T.). K.N.G. was supported by NIH training grant T32 AI007520. M.J.P. was supported by NSF Graduate Research Fellowship 049347-05. We thank Dr. Deborah Ribardo for assistance with chick colonization and qRT-PCR analyses.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: RNASeq data are publicly available for download at the National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO) at GEO accession GSE142852.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1922719117/-/DCSupplemental.
References
- 1.Conlon M. A., Bird A. R., The impact of diet and lifestyle on gut microbiota and human health. Nutrients 7, 17–44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillman E. T., Lu H., Yao T., Nakatsu C. H., Microbial ecology along the gastrointestinal tract. Microbes Environ. 32, 300–313 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donaldson G. P., Lee S. M., Mazmanian S. K., Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14, 20–32 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong J. M., de Souza R., Kendall C. W., Emam A., Jenkins D. J., Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 40, 235–243 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Tan J., et al. , The role of short-chain fatty acids in health and disease. Adv. Immunol. 121, 91–119 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T., Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach Knudsen K. E., et al. , Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 10, E1499 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roediger W. E., The starved colon–Diminished mucosal nutrition, diminished absorption, and colitis. Dis. Colon Rectum 33, 858–862 (1990). [DOI] [PubMed] [Google Scholar]
- 9.Reuter G., The Lactobacillus and Bifidobacterium microflora of the human intestine: Composition and succession. Curr. Issues Intest. Microbiol. 2, 43–53 (2001). [PubMed] [Google Scholar]
- 10.Stanley D., Hughes R. J., Moore R. J., Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 98, 4301–4310 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Pessione E., Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2, 86 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillis C. C., et al. , Dysbiosis-associated change in host metabolism generates lactate to support Salmonella growth. Cell Host Microbe 23, 54–64.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrote G. L., Abraham A. G., Rumbo M., Is lactate an undervalued functional component of fermented food products? Front. Microbiol. 6, 629 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan S. H., Louis P., Flint H. J., Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 70, 5810–5817 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lustri B. C., Sperandio V., Moreira C. G., Bacterial chat: Intestinal metabolites and signals in host-microbiota-pathogen interactions. Infect. Immun. 85, e00476-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez-Doria J. D., Sperandio V., Nutrient and chemical sensing by intestinal pathogens. Microbes Infect. 15, 759–764 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakanishi N., et al. , Regulation of virulence by butyrate sensing in enterohaemorrhagic Escherichia coli. Microbiology 155, 521–530 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Tobe T., Nakanishi N., Sugimoto N., Activation of motility by sensing short-chain fatty acids via two steps in a flagellar gene regulatory cascade in enterohemorrhagic Escherichia coli. Infect. Immun. 79, 1016–1024 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luethy P. M., et al. , Microbiota-derived short-chain fatty acids modulate expression of Campylobacter jejuni determinants required for commensalism and virulence. mBio 8, e00417-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faber F., et al. , Host-mediated sugar oxidation promotes post-antibiotic pathogen expansion. Nature 534, 697–699 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes E. R., et al. , Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe 21, 208–219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiga L., et al. , An oxidative central metabolism enables Salmonella to utilize microbiota-derived succinate. Cell Host Microbe 22, 291–301.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawhon S. D., Maurer R., Suyemoto M., Altier C., Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 46, 1451–1464 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Hung C. C., et al. , The intestinal fatty acid propionate inhibits Salmonella invasion through the post-translational control of HilD. Mol. Microbiol. 87, 1045–1060 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavez R. G., Alvarez A. F., Romeo T., Georgellis D., The physiological stimulus for the BarA sensor kinase. J. Bacteriol. 192, 2009–2012 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gantois I., et al. , Butyrate specifically down-regulates Salmonella pathogenicity island 1 gene expression. Appl. Environ. Microbiol. 72, 946–949 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun X., et al. , Microbiota-derived metabolic factors reduce campylobacteriosis in mice. Gastroenterology 154, 1751–1763.e2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tack D. M., et al. , Preliminary incidence and trends of infections with pathogens transmitted commonly through food–Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2015-2018. MMWR Morb. Mortal. Wkly. Rep. 68, 369–373 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skirrow M. B., Blaser M. J., “Clinical aspects of Campylobacter infection” in Campylobacter, Nachamkin I., Blaser M. J., Eds. (ASM Press, Washington, DC, ed. 2, 2000), pp. 69–88. [Google Scholar]
- 30.Lindblom G.-B., Sjörgren E., Kaijser B., Natural campylobacter colonization in chickens raised under different environmental conditions. J. Hyg. (Lond.) 96, 385–391 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pokamunski S., Kass N., Borochovich E., Marantz B., Rogol M., Incidence of Campylobacter spp. in broiler flocks monitored from hatching to slaughter. Avian Pathol. 15, 83–92 (1986). [DOI] [PubMed] [Google Scholar]
- 32.Beery J. T., Hugdahl M. B., Doyle M. P., Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 54, 2365–2370 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendrixson D. R., DiRita V. J., Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol. Microbiol. 52, 471–484 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Friedman C. R., et al. ; Emerging Infections Program FoodNet Working Group , Risk factors for sporadic Campylobacter infection in the United States: A case-control study in FoodNet sites. Clin. Infect. Dis. 38 (suppl. 3), S285–S296 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Hale C. R., et al. , Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clin. Infect. Dis. 54 (suppl. 5), S472–S479 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Molnár A., et al. , Composition of diet modifies colonization dynamics of Campylobacter jejuni in broiler chickens. J. Appl. Microbiol. 118, 245–254 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Rehman H. U., Vahjen W., Awad W. A., Zentek J., Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch. Anim. Nutr. 61, 319–335 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Meyer B., Bessei W., Vahjen W., Zentek J., Harlander-Matauschek A., Dietary inclusion of feathers affects intestinal microbiota and microbial metabolites in growing Leghorn-type chickens. Poult. Sci. 91, 1506–1513 (2012) Erratum in: Poult. Sci.91, 2079 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Rehman H., Böhm J., Zentek J., Effects of differentially fermentable carbohydrates on the microbial fermentation profile of the gastrointestinal tract of broilers. J. Anim. Physiol. Anim. Nutr. (Berl.) 92, 471–480 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Cummings J. H., Short chain fatty acids in the human colon. Gut 22, 763–779 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cummings J. H., Macfarlane G. T., The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 70, 443–459 (1991). [DOI] [PubMed] [Google Scholar]
- 42.Macfarlane G. T., Gibson G. R., Cummings J. H., Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol. 72, 57–64 (1992). [DOI] [PubMed] [Google Scholar]
- 43.Luethy P. M., Huynh S., Parker C. T., Hendrixson D. R., Analysis of the activity and regulon of the two-component regulatory system composed by Cjj81176_1484 and Cjj81176_1483 of Campylobacter jejuni. J. Bacteriol. 197, 1592–1605 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crofts A. A., et al. , Campylobacter jejuni transcriptional and genetic adaptation during human infection. Nat. Microbiol. 3, 494–502 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hendrixson D. R., DiRita V. J., Transcription of σ54-dependent but not σ28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol. Microbiol. 50, 687–702 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Hendrixson D. R., A phase-variable mechanism controlling the Campylobacter jejuni FlgR response regulator influences commensalism. Mol. Microbiol. 61, 1646–1659 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Hendrixson D. R., Restoration of flagellar biosynthesis by varied mutational events in Campylobacter jejuni. Mol. Microbiol. 70, 519–536 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boll J. M., Hendrixson D. R., A regulatory checkpoint during flagellar biogenesis in Campylobacter jejuni initiates signal transduction to activate transcription of flagellar genes. mBio 4, e00432-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boll J. M., Hendrixson D. R., A specificity determinant for phosphorylation in a response regulator prevents in vivo cross-talk and modification by acetyl phosphate. Proc. Natl. Acad. Sci. U.S.A. 108, 20160–20165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linton D., Allan E., Karlyshev A. V., Cronshaw A. D., Wren B. W., Identification of N-acetylgalactosamine-containing glycoproteins PEB3 and CgpA in Campylobacter jejuni. Mol. Microbiol. 43, 497–508 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Min T., et al. , Specificity of Campylobacter jejuni adhesin PEB3 for phosphates and structural differences among its ligand complexes. Biochemistry 48, 3057–3067 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Leach S., Harvey P., Wali R., Changes with growth rate in the membrane lipid composition of and amino acid utilization by continuous cultures of Campylobacter jejuni. J. Appl. Microbiol. 82, 631–640 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Barrero-Tobon A. M., Hendrixson D. R., Flagellar biosynthesis exerts temporal regulation of secretion of specific Campylobacter jejuni colonization and virulence determinants. Mol. Microbiol. 93, 957–974 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ribardo D. A., Bingham-Ramos L. K., Hendrixson D. R., Functional analysis of the RdxA and RdxB nitroreductases of Campylobacter jejuni reveals that mutations in rdxA confer metronidazole resistance. J. Bacteriol. 192, 1890–1901 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pajaniappan M., et al. , A temperature-regulated Campylobacter jejuni gluconate dehydrogenase is involved in respiration-dependent energy conservation and chicken colonization. Mol. Microbiol. 68, 474–491 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hendrixson D. R., Akerley B. J., DiRita V. J., Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol. Microbiol. 40, 214–224 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Elliott K. T., Dirita V. J., Characterization of CetA and CetB, a bipartite energy taxis system in Campylobacter jejuni. Mol. Microbiol. 69, 1091–1103 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reuter M., van Vliet A. H., Signal balancing by the CetABC and CetZ chemoreceptors controls energy taxis in Campylobacter jejuni. PLoS One 8, e54390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kendall J. J., Barrero-Tobon A. M., Hendrixson D. R., Kelly D. J., Hemerythrins in the microaerophilic bacterium Campylobacter jejuni help protect key iron-sulphur cluster enzymes from oxidative damage. Environ. Microbiol. 16, 1105–1121 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao R., Stock A. M., Biological insights from structures of two-component proteins. Annu. Rev. Microbiol. 63, 133–154 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhate M. P., Molnar K. S., Goulian M., DeGrado W. F., Signal transduction in histidine kinases: Insights from new structures. Structure 23, 981–994 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huynh T. N., Stewart V., Negative control in two-component signal transduction by transmitter phosphatase activity. Mol. Microbiol. 82, 275–286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laub M. T., Goulian M., Specificity in two-component signal transduction pathways. Annu. Rev. Genet. 41, 121–145 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Mascher T., Helmann J. D., Unden G., Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70, 910–938 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolanin P. M., Thomason P. A., Stock J. B., Histidine protein kinases: Key signal transducers outside the animal kingdom. Genome Biol. 3, REVIEWS3013 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ellefson D. D., Weber U., Wolfe A. J., Genetic analysis of the catalytic domain of the chemotaxis-associated histidine kinase CheA. J. Bacteriol. 179, 825–830 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirschman A., Boukhvalova M., VanBruggen R., Wolfe A. J., Stewart R. C., Active site mutations in CheA, the signal-transducing protein kinase of the chemotaxis system in Escherichia coli. Biochemistry 40, 13876–13887 (2001). [DOI] [PubMed] [Google Scholar]
- 68.Joslin S. N., Hendrixson D. R., Analysis of the Campylobacter jejuni FlgR response regulator suggests integration of diverse mechanisms to activate an NtrC-like protein. J. Bacteriol. 190, 2422–2433 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joslin S. N., Hendrixson D. R., Activation of the Campylobacter jejuni FlgSR two-component system is linked to the flagellar export apparatus. J. Bacteriol. 191, 2656–2667 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All protocols and data discussed in the paper are available in the main text and SI Appendix. All strains and plasmids generated in this report will be made promptly available to readers.