Significance
Maternal exposure to high levels of the herbicide glyphosate may increase the risk for autism spectrum disorder (ASD) in offspring; however, the underlying mechanisms remain largely unknown. Maternal glyphosate exposure during pregnancy and lactation caused ASD-like behavioral abnormalities and abnormal composition of gut microbiota in murine male offspring. Soluble epoxide hydrolase (sEH) in the brain of offspring after maternal glyphosate exposure was higher than controls. Treatment with an sEH inhibitor from pregnancy to weaning prevented the onset of ASD-like behavioral abnormalities in offspring after maternal glyphosate exposure. The glyphosate exposures used here exceed any reasonable dietary, environmental, or occupational exposure, but they indicate that increased sEH plays a role in ASD-like behaviors in offspring.
Keywords: glyphosate, gut microbiota, soluble epoxide hydrolase
Abstract
Epidemiological studies suggest that exposure to herbicides during pregnancy might increase risk for autism spectrum disorder (ASD) in offspring. However, the precise mechanisms underlying the risk of ASD by herbicides such as glyphosate remain unclear. Soluble epoxide hydrolase (sEH) in the metabolism of polyunsaturated fatty acids is shown to play a key role in the development of ASD in offspring after maternal immune activation. Here, we found ASD-like behavioral abnormalities in juvenile offspring after maternal exposure to high levels of formulated glyphosate. Furthermore, we found higher levels of sEH in the prefrontal cortex (PFC), hippocampus, and striatum of juvenile offspring, and oxylipin analysis showed decreased levels of epoxy-fatty acids such as 8 (9)-EpETrE in the blood, PFC, hippocampus, and striatum of juvenile offspring after maternal glyphosate exposure, supporting increased activity of sEH in the offspring. Moreover, we found abnormal composition of gut microbiota and short-chain fatty acids in fecal samples of juvenile offspring after maternal glyphosate exposure. Interestingly, oral administration of TPPU (an sEH inhibitor) to pregnant mothers from E5 to P21 prevented ASD-like behaviors such as social interaction deficits and increased grooming time in the juvenile offspring after maternal glyphosate exposure. These findings suggest that maternal exposure to high levels of glyphosate causes ASD-like behavioral abnormalities and abnormal composition of gut microbiota in juvenile offspring, and that increased activity of sEH might play a role in ASD-like behaviors in offspring after maternal glyphosate exposure. Therefore, sEH may represent a target for ASD in offspring after maternal stress from occupational exposure to contaminants.
Autism spectrum disorder (ASD) is a developmental disorder characterized by social and communication impairments, combined with limited or focused interests and repetitive behaviors (1, 2). Although the prevalence of ASD has been rising since the 1980s, the detailed reasons underlying this rise remain unknown (3, 4). In addition to genetic factors, accumulating evidence supports a significant contribution of environmental factors in ASD etiology (1, 2, 5, 6). Environmental factors, including exposures to synthetic chemicals during pregnancy and lactation, are suggested to play a role in the development of ASD. These chemicals include selective serotonin reuptake inhibitors (SSRIs), pesticides, phthalates, polychlorinated biphenyls, solvents, air pollutants, fragrances, and heavy metals (6–8).
Glyphosate [N-(phosphonomethyl)glycine] is the active ingredient in Roundup and other herbicides, and, because of its efficacy, excellent environmental profile, and low toxicity, it is the most widely used herbicide in the world (9, 10). Interestingly, a positive correlation was reported between the rise of glyphosate usage on corn and soy crops in the United States over the years 1995 to 2010 and the increase in ASD rates over the same period as reported in the US public school system (11–13). A recent population-based case-control study in California showed that the risk of ASD was associated with the use of glyphosate (odds ratio = 1.16) (14). For ASD with intellectual disability, estimated odds ratio were higher (by about 30%) with prenatal exposure to glyphosate (odds ratio = 1.33) (14). These reports suggest that possible relationships between glyphosate and ASD should be explored in animal models.
Epidemiological studies implicate prenatal environmental factors, including maternal immune activation (MIA), playing a key role in the etiology of developmental disorders such as ASD (15–19). There are a number of positive associations between maternal infections or inflammatory biomarkers and ASD (15, 16, 20). Collectively, MIA during pregnancy can increase the risk of developmental disorders such as ASD in offspring.
Epoxy fatty acids (EpFAs) are produced from the corresponding polyunsaturated fatty acids by cytochrome P450 enzymes. Epoxyeicosatrienoic acids (EpETrEs) and epoxydocosapentaenoic acids (EpDPEs) are produced from arachidonic acid and docosahexaenoic acid (DHA), respectively. EpETrEs, EpDPEs, and some other EpFAs have potent antiinflammatory properties. However, these lipid mediators are metabolized rapidly into their corresponding diols by soluble epoxide hydrolase (sEH), and inhibition of sEH enhances the beneficial effects of EpFAs (21–24). Accumulating evidence demonstrate a key role of sEH in multiple animal models, including depression, ASD, schizophrenia, and Parkinson’s disease (25–32). Recently, we reported that sEH in the prefrontal cortex (PFC) plays a key role in the development of ASD-like behavioral abnormalities in juvenile offspring after MIA (30). However, there is no previous report showing the role of sEH in the pathogenesis of ASD in offspring after maternal exposure to formulated glyphosate.
The purpose of this study was to examine the role of sEH in the pathogenesis of ASD in offspring after maternal glyphosate exposure. First, we examined whether maternal glyphosate exposure causes ASD-like behavioral abnormalities in juvenile offspring. Second, we examined whether expression of sEH is altered in the brain regions of juvenile offspring after maternal glyphosate exposure. Furthermore, we performed oxylipin analysis of blood and brain regions from juvenile offspring. Moreover, we measured levels of N-methyl-D-aspartate receptor (NMDAR)-related amino acids in the blood and brain from juvenile offspring since NMDAR-related amino acids were altered in patients with ASD (33–36). Third, we performed 16S rRNA analysis and measurement of short-chain fatty acids of fecal samples in juvenile off\spring after maternal glyphosate exposure since abnormal composition of gut microbiota is shown in patients with ASD (36–40). Finally, we examined whether treatment with TPPU [1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)urea] (40–42), a potent sEH inhibitor, in pregnant mice from pregnancy to weaning could prevent behavioral abnormalities in juvenile offspring after maternal glyphosate exposure.
Results
General and Behavioral Data of Mother and Juvenile Offspring after Maternal Glyphosate Exposure.
First, we examined whether maternal glyphosate exposure could affect the general and behavioral outcomes in male offspring (SI Appendix, Fig. S1A). Previous studies used drinking water containing 1% Roundup [0.38% (wt/vol) glyphosate] during pregnancy and lactation (43, 44). For this study, water or formulated glyphosate [0.039% (wt/vol) glyphosate] were given to pregnant mice from E5 to P21 (weaning). The mortality of pregnant mice at the highest concentration (0.39%) in our hands was 100%, although the mortality of lower concentrations (0.039% and 0.098%) was 0% (SI Appendix, Table S1), even though the 0.39% concentration has been previously used in other studies (43, 44). This discrepancy may have been due to different formulations of glyphosate or mouse strain differences (ddy vs. ICR) between the studies. Body weight of pregnant mice was increased gradually after maternal glyphosate (0.039 to 0.293%) exposure, whereas body weight of pregnant mice treated with the high concentration (0.39%) did not increase (SI Appendix, Fig. S1B). The mortality of offspring in the 0.039% glyphosate group was 0%, and juvenile offspring after maternal 0.039% glyphosate exposure did not show any behavioral abnormality such as locomotion, social interaction deficits in a three-chamber test, and depression-like phenotype in the forced swimming test (SI Appendix, Fig. S1 C–E and Table S1). In contrast, we found social interaction deficits in juvenile offspring after maternal 0.098% glyphosate exposure. Therefore, we used 0.098% glyphosate in the subsequent experiments. This concentration corresponded with 1/80th of the glyphosate no-observed-adverse-effect level, as reported previously (45).
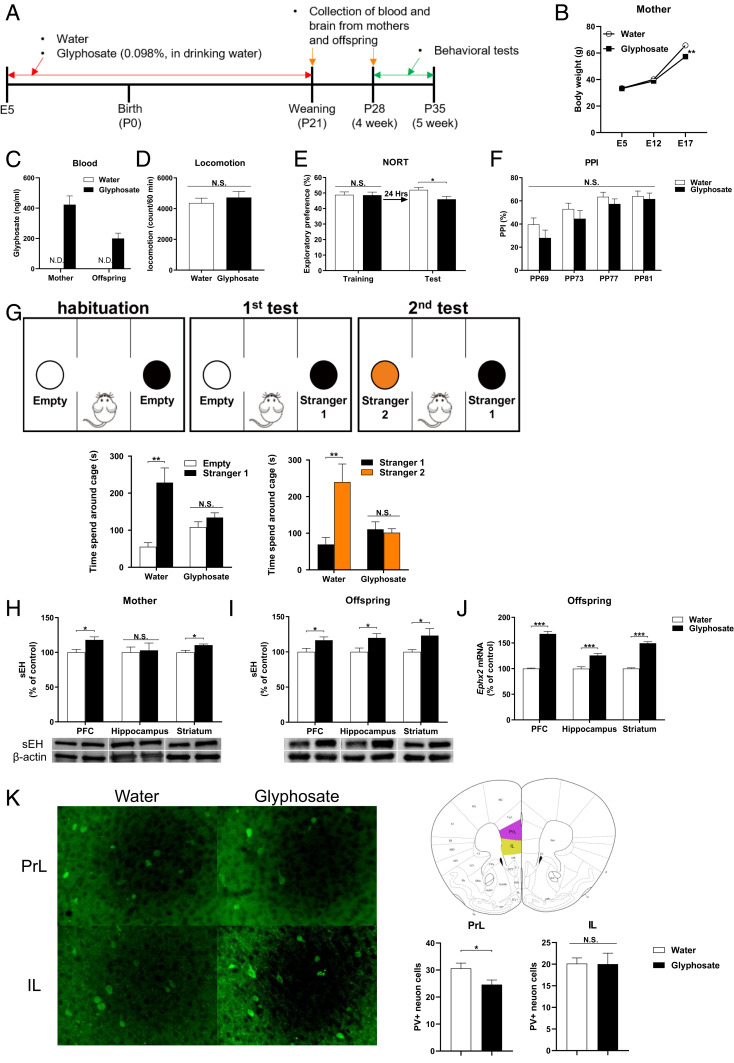
Body weight of glyphosate-exposed mothers was significantly lower than that of water-exposed mothers at E17 (Fig. 1 A and B). On P21 (weaning), we could detect blood levels of glyphosate in the mothers treated with 0.098% glyphosate and their offspring, although glyphosate was not detected in the dams and offspring of the water-treated group (Fig. 1 A and C). Locomotion and prepulse inhibition (PPI; for psychosis) were not different between the two groups (Fig. 1 D and F). In the novel object recognition test (NORT), offspring after maternal glyphosate exposure showed cognitive deficits (Fig. 1E). In the three-chamber test, juvenile offspring after maternal glyphosate exposure showed social interaction deficits compared to the water-treated group (Fig. 1G). The data suggest that maternal exposure to glyphosate in a commercial formation (SI Appendix, Supplementary Material for composition) causes ASD-like cognitive deficits and social interaction deficits in juvenile offspring.
Fig. 1.
Social interaction deficits, increased expression of sEH, and decreased PV immunoreactivity in the brain from juvenile offspring after maternal glyphosate exposure. (A) Schedule of treatment, behavioral tests, and sample collection. (B) Change of body weight of pregnant mothers (n = 6). (C) Blood levels of glyphosate in the mothers and offspring at P21. Data are shown as mean ± SEM (mother n = 7, offspring n = 10). (D) Locomotion. Data are shown as mean ± SEM (n = 7 or 8). (E) Novel object recognition test (NORT). Data are shown as mean ± SEM (n = 8). (F) Prepulse inhibition (PPI) test. Data are shown as mean ± SEM (n = 8). (G) Three-chamber social interaction test. (Left) Two-way ANOVA (glyphosate, F1,22 = 4.747, P = 0.040; stranger, F1,22 = 141.2, P < 0.001; interaction, F1,22 = 76.77, P < 0.001). (Right) Two-way ANOVA (glyphosate, F1,22 = 9.760, P = 0.005; stranger, F1,22 = 26.75, P < 0.001; interaction, F1,22 = 33.38, P < 0.001). Data are shown as mean ± SEM (n = 6 or 7). (H) Protein expression of sEH in the PFC, hippocampus, and striatum of mothers. Data are shown as mean ± SEM (n = 4 or 5). (I) Protein expression of sEH in the PFC, hippocampus, and striatum from juvenile offspring (P28). Data are shown as mean ± SEM (n = 10). (J) Gene expression of Ephx2 mRNA in the mouse brain regions from juvenile offspring (P28). Data are shown as mean ± SEM (n = 8). (K) PV immunoreactivity in the prelimbic area (PrL) and infralimbic (IL) of mPFC. The values represent the mean ± SEM (n = 8; *P < 0.05, **P < 0.01, ***P < 0.001 compared to control group by Student t test). N.S., not significant.
Increased Expression of sEH in the Brain of Juvenile Offspring after Maternal Glyphosate Exposure.
We measured the expression of sEH in the brain since increased expression of sEH in the PFC plays a role in the ASD-like behaviors after MIA (30). Protein levels of sEH in the PFC and striatum, but not hippocampus, from mothers treated with glyphosate were significantly higher than those of water-treated mice (Fig. 1H). Protein levels of sEH in the PFC, hippocampus, and striatum from juvenile offspring (P28) after maternal glyphosate exposure were significantly higher than those of water-treated mice (Fig. 1I). Furthermore, gene expression of sEH (or Ephx2) mRNA in the PFC, hippocampus, and striatum from juvenile offspring (P28) after maternal glyphosate exposure was significantly higher than those of water-treated mice (Fig. 1J).
Next, we performed parvalbumin (PV) immunohistochemistry in the brain from juvenile mice (Fig. 1K). PV immunoreactivity in the prelimbic (PrL), but not IL (infralimbic), of medial PFC in the offspring of maternal glyphosate exposure was significantly lower than that of the water-treated group (Fig. 1K).
Oxylipin Analysis of Blood and Brain Regions.
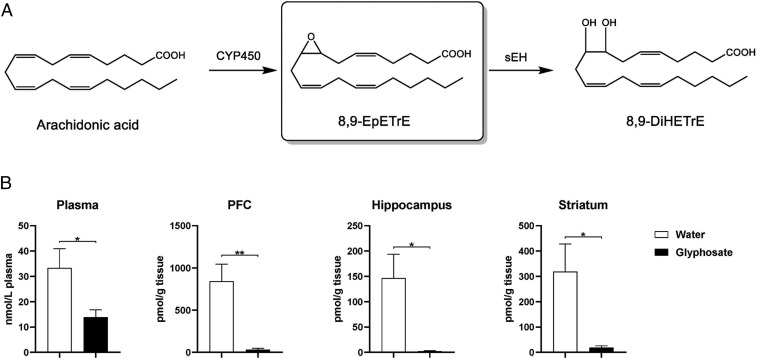
Using oxylipin analysis, we measured the levels of eicosanoid metabolites in the blood, PFC, hippocampus, and striatum from juvenile offspring (P28) after maternal glyphosate exposure (SI Appendix, Fig. S2 and Tables S2–S5). Blood levels of many epoxides were significantly lower in juvenile offspring after maternal glyphosate exposure (SI Appendix, Table S2). We found higher levels of 8 (9)-EpETrE [8,9-epoxy-5Z,11Z,14Z-eicosatrienoic acid] compared to other EpFAs in the mouse brain. Levels of 8 (9)-EpETrE in the PFC, hippocampus, and striatum were significantly lower in juvenile offspring (P28) after maternal glyphosate exposure (Fig. 2 and SI Appendix, Tables S3–S5). Lower levels of 8 (9)-EpETrE in the brain regions from juvenile offspring after maternal glyphosate exposure support the increased expression of sEH in these regions. In contrast, tissue levels of other EpFAs in the PFC, hippocampus, and striatum from juvenile offspring after maternal glyphosate exposure were significantly higher than those of control mice (SI Appendix, Tables S3–S5).
Fig. 2.
Oxylipin analysis of blood and brain regions. (A) Arachidonic acid is metabolized to 8,9-EpETrE by several cytochrome P450 enzymes. Subsequently, 8,9-EpETrE is metabolized to 8,9-DiHETrE by sEH. (B) Levels of 8,9-EpETrE in the plasma, PFC, hippocampus, and striatum from juvenile offspring (P28). The values represent the mean ± SEM (n = 8 to 10; *P < 0.05, **P < 0.01 compared to control group by Student t test).
Measurement of Amino Acids in the Blood and Brain.
Next, we measured levels of NMDAR-related amino acids (i.e., glutamate, glutamine, glycine, D-serine, L-serine, GABA) in the plasma and brains of juvenile offspring (P28) after maternal glyphosate exposure. Maternal glyphosate exposure caused significant reductions of glutamate in the plasma and brain regions. In addition, maternal glyphosate exposure caused significant reductions of other amino acids (i.e., glycine, L-serine, GABA) in the PFC (SI Appendix, Table S6). The data suggest abnormalities in NMDAR-related neurotransmission in the PFC of juvenile offspring after maternal glyphosate exposure.
16S rRNA Analysis and Measurement of Short-Chain Fatty Acids of Fecal Samples of Juvenile Offspring after Maternal Glyphosate Exposure.
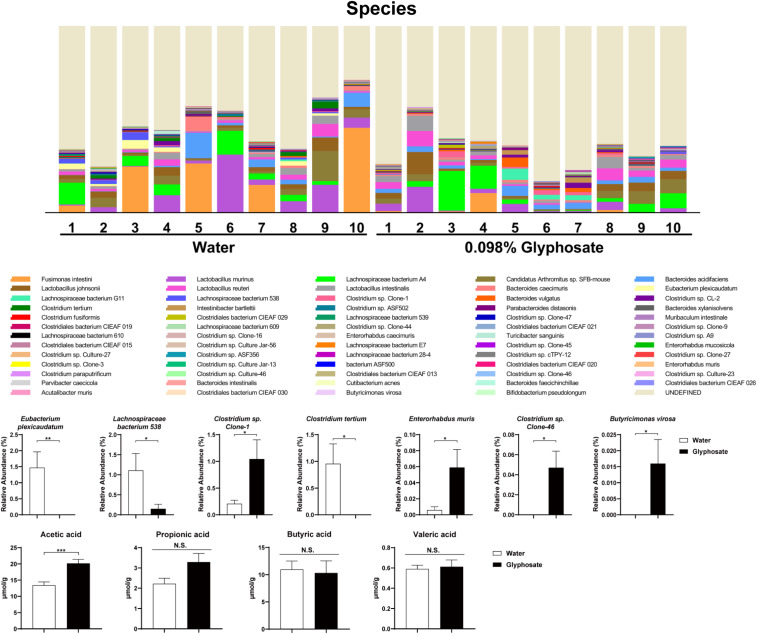
We performed 16S rRNA analysis of fecal samples of offspring (P28). Maternal glyphosate exposure caused abnormal composition of gut microbiota in juvenile offspring (Fig. 3). At the species level, the relative abundance of Eubacterium plexicaudatum, Lachnospiraceae bacterium 538, and Clostridium tertium was significantly lower in the juvenile offspring after maternal glyphosate exposure compared to the water-treated group (Fig. 3). In contrast, the relative abundance of Clostridium sp. Clone-1, Enterorhabdus muris, Clostridium sp. Clone-46, and Butyricimonas virosa was significantly higher in juvenile offspring after maternal glyphosate exposure compared to the water-treated group (Fig. 3). Furthermore, levels of acetic acid in the fecal samples of the offspring were significantly increased after maternal glyphosate exposure (Fig. 3). Other short-chain fatty acids including propionic acid, butyric acid, and valeric acid were not different. The data suggest that maternal exposure to formulated glyphosate causes abnormal composition of gut microbiota in juvenile offspring.
Fig. 3.
Composition of gut microbiota in fecal samples of juvenile offspring. (A) Histogram of microbiota at species level of offspring (P28). (B) Several bacteria were significantly altered in the offspring after maternal glyphosate exposure. Data are shown as mean ± SEM (n = 10; *P < 0.05, **P < 0.01 compared to control group by Student t test).
Effects of TPPU on ASD-Like Behaviors in Juvenile Offspring of Maternal Glyphosate Exposure.
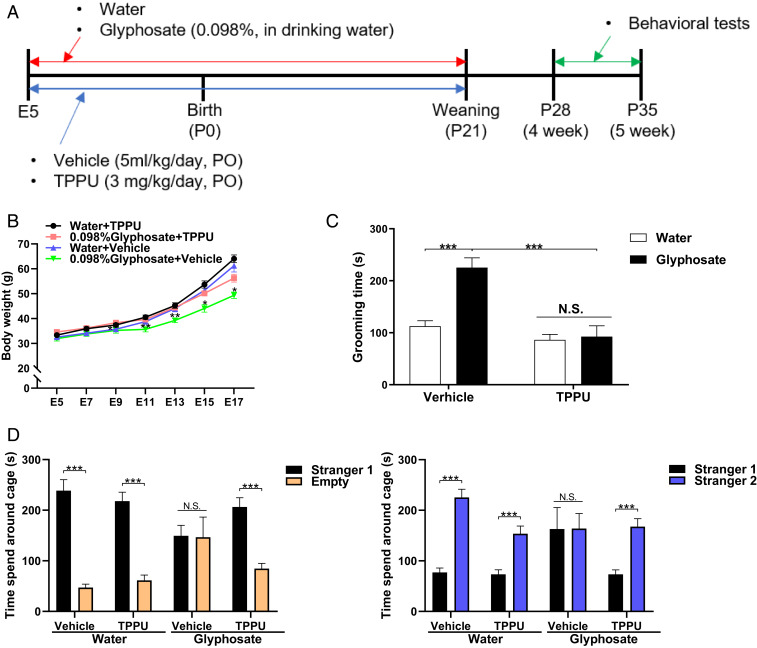
Water or glyphosate was given to pregnant mice from E5 to P21. In addition, the pregnant mice were orally administered vehicle (5 mL/kg/d) or vehicle and TPPU (3 mg/kg/d) from E5 to P21. Behavioral tests such as grooming test and three-chamber social interaction test were performed from P28 to P35 (Fig. 4A). Body weight was significantly increased in TPPU-treated glyphosate-exposed mothers compared to vehicle-treated glyphosate exposure mothers (Fig. 4B). Treatment with TPPU significantly ameliorated the increased grooming time of juvenile offspring after maternal glyphosate exposure (Fig. 4C). In the three-chamber social interaction test, treatment with TPPU significantly improved social interaction deficits in juvenile offspring after maternal glyphosate exposure (Fig. 4D).
Fig. 4.
Effects of TPPU on ASD-like behavioral abnormalities in juvenile offspring after maternal glyphosate exposure. (A) Schedule of treatment and behavioral tests. Water or glyphosate (0.098%) was given to pregnant mice. Vehicle (5 mL/kg/d) or TPPU (3 mg/kg/d) was administered orally to pregnant mice from E5 to P21. Subsequently, all mice received normal water. Grooming test and three-chamber social interaction test were performed from P28 to P35. (B) Change of body weight of mothers (n = 5 or 6). Two-way ANOVA (glyphosate, F1,17 = 7.66, P = 0.013; TPPU, F1,17 = 9.14, P = 0.008; interaction, F1,17 = 1.59, P = 0.225). (C) Grooming test. Treatment with TPPU significantly attenuated the increased grooming time in juvenile offspring after maternal glyphosate exposure. Two-way ANOVA (glyphosate, F1,36 = 14.19, P = 0.001; TPPU, F1,36 = 25.34, P < 0.001; interaction, F1,36 = 11.31, P = 0.002). Data are shown as mean ± SEM (n = 10; ***P < 0.01 compared to glyphosate + vehicle group). (D) Three-chamber social interaction test. (Left) Three-way ANOVA (glyphosate, F1,56 = 9.948, P = 0.003; TPPU, F1,56 = 0.301, P = 0.585; stranger, F1,56 = 135.27, P < 0.001; interaction [glyphosate × TPPU], F1,56 = 0.845, P = 0.362; interaction [glyphosate × stranger], F1,56 = 38.61, P < 0.001; interaction [TPPU × stranger], F1,56 = 3.593, P = 0.063; interaction [glyphosate × TPPU × stranger], F1,56 = 11.06, P = 0.002). (Right) Three-way ANOVA (glyphosate, F1,56 = 4.168, P = 0.046; TPPU, F1,56 = 21.96, P < 0.001; stranger, F1,56 = 104.28, P < 0.001; interaction [glyphosate × TPPU], F1,56 = 1.902, P = 0.173; interaction [TPPU × stranger], F1,56 = 3.870, P = 0.054; interaction [glyphosate × stranger], F1,56 = 13.10, P = 0.001; interaction [glyphosate × TPPU × stranger], F1,56 = 19.69, P < 0.001). Data are shown as mean ± SEM (n = 8; ***P < 0.01). N.S., not significant.
Discussion
The present results demonstrate a role of sEH in the onset of ASD-like behaviors in murine offspring after maternal glyphosate exposure. The major findings of the present study are as follows. First, exposure to high levels (0.098%) of glyphosate during pregnancy and lactation caused ASD-like behaviors in juvenile offspring. Second, expression of sEH protein in the PFC, hippocampus, and striatum from juvenile offspring after maternal glyphosate exposure was higher than that of the control group. Oxylipin analysis showed a marked reduction of 8 (9)-EpETrE in the plasma, PFC, hippocampus, and striatum from juvenile offspring after maternal glyphosate exposure, supporting higher levels of sEH in these regions. Third, maternal glyphosate exposure caused reduced PV immunoreactivity in the prelimbic of medial PFC in the offspring compared to the water-treated group. Furthermore, maternal glyphosate exposure caused significant alterations of NMDAR-related amino acids in the blood and brain of offspring. Fourth, maternal glyphosate exposure caused significant abnormal composition of gut microbiota and increased levels of acetic acid in the fecal samples from juvenile offspring. Finally, repeated treatment with TPPU in glyphosate-treated pregnant mice from pregnancy (E5) to weaning (P21) prevented the onset of ASD-like behaviors (i.e., increased grooming time and social interaction deficits) in juvenile offspring after maternal glyphosate exposure. Collectively, these findings suggest that the sEH enzyme plays a key role in the development of ASD-like behavioral abnormalities in offspring after maternal glyphosate exposure, and that sEH inhibitors may prove to be promising prophylactic or therapeutic drugs for ASD.
In this study, we found increased expression of sEH protein in the PFC of juvenile offspring after maternal glyphosate exposure, consistent with our report using MIA (30). Thus, it seems that increases in the sEH in the PFC and other regions (hippocampus and striatum) might play a role in the behavioral and biochemical abnormalities seen in juvenile offspring after maternal glyphosate exposure. Previously, we reported higher levels of EPHX2 mRNA in the postmortem brain samples from ASD patients (30). These findings suggest that increased metabolism of EpFAs to the corresponding diols by increased sEH may play a role in the pathogenesis of ASD, although further detailed studies on how maternal glyphosate exposure induces abnormalities in the eicosanoid metabolism by sEH and behavioral abnormalities in offspring are needed.
We found decreased levels of many EpFAs including 8 (9)-EpETrE in the blood of juvenile offspring after maternal glyphosate exposure compared to the water-treated group. Interestingly, tissue levels of 8 (9)-EpETrE, the abundant EpFA in the brain, were significantly lower in the PFC, hippocampus, and striatum from juvenile offspring after maternal glyphosate exposure than those of control mice, supporting an increased activity of sEH in these brain regions. The data on 8 (9)-EpETrE are consistent with our previous report using MIA model of ASD (30). Although the precise mechanisms underlying the relationship between 8 (9)-EpETrE and sEH in the brain from juvenile offspring after maternal glyphosate exposure are currently unclear, it seems that low levels of 8 (9)-EpETrE by increased levels of sEH in the brain may be involved in behavioral abnormalities of offspring after maternal glyphosate exposure. By contrast, other EpFAs were significantly higher in the brain regions of juvenile offspring after maternal glyphosate exposure than those of the water-treated group, although tissue levels of sEH in the brain regions were increased after maternal glyphosate exposure. Although the reasons underlying this discrepancy are currently unknown, it seems that multiple pathways may contribute to formation and degradation of EpFAs in the brain regions.
It is recognized that mechanism of action of glyphosate is to disrupt the shikimate pathway, which is absent from human cells. However, human gut microbiomes contain the shikimate pathway, which plays a key role in the synthesis of aromatic amino acids in both plants and microbiomes (11, 46–48). Therefore, it is suggested that exposure to glyphosate can affect gut microbiota in humans (6, 49). In this study, we found abnormal composition of gut microbiota such as Clostridium in juvenile offspring after maternal glyphosate exposure. A recent review pointed an interaction between Clostridium bacteria and ASD (50). In addition, we found higher levels of acetic acid in fecal samples of juvenile offspring after maternal glyphosate exposure. It is reported that fecal levels of acetic acid in children with ASD were higher than those in controls (51). It seems that increased intestinal permeability by acetic acid might play a role in fecal production of acetic acid since acetic acid plays a role in gut epithelial barrier function (51). Given the crucial role of gut microbiota in ASD pathogenesis (39, 52, 53), abnormal composition of gut microbiota may be, in part, involved in the ASD-like behaviors in offspring after maternal glyphosate exposure. At present, specific bacteria that can cause ASD were not yet identified. Therefore, further study on the role of gut microbiota on glyphosate-induced ASD is needed.
Maternal exposure to 0.098% glyphosate causes ASD-like behaviors and abnormal composition of gut microbiota in juvenile offspring. Although it is exceptionally unlikely that such exposure could be reached during human pregnancy, maternal exposure to high levels of technical glyphosate could have detrimental side effects in offspring. A cohort study on measurement of blood (or urine) levels of glyphosate in pregnant mothers who have offspring with or without ASD is of interest. Although the current animal data do not necessarily translate to humans, further study connecting animal data with the findings from epidemiological studies is needed to identify the detailed mechanisms of action of glyphosate exposure for ASD pathogenesis.
In conclusion, this study suggests that maternal exposure to high levels of formulated glyphosate might play a role in the etiology of ASD-like behaviors in murine offspring through increased activity of sEH in the brain, and sEH inhibitors could be a useful tool to dissect the mechanism. However, a recent comprehensive review on human exposure to glyphosate indicates that human exposure approaching these levels is exceptionally unlikely (54).
Materials and Methods
Details of the experimental protocols, including animals, maternal glyphosate exposure, measurement of glyphosate in the blood, oxylipin analysis, Western blot analysis, RT-PCR, behavioral tests, treatment of TPPU, immunohistochemistry, measurement of amino acids, gut microbiota analysis, and statistical analysis, are given in the SI Appendix.
Data Availability.
All data in the paper are included in the dataset of the SI Appendix.
Supplementary Material
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science (JSPS) (to K.H., 17H04243), Japan Agency for Medical Research and Development (to K.H., JP19dm0107119), the National Institute of Environmental Health Sciences (NIEHS) River Award R35 ES030443-01 (to B.D.H.), and NIEHS Superfund Program P42 ES004699 (to B.D.H.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1922287117/-/DCSupplemental.
References
- 1.Lai M. C., Lombardo M. V., Baron-Cohen S., Autism. Lancet 383, 896–910 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Lord C., Elsabbagh M., Baird G., Veenstra-Vanderweele J., Autism spectrum disorder. Lancet 392, 508–520 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen S. N., Schendel D. E., Parner E. T., Explaining the increase in the prevalence of autism spectrum disorders: The proportion attributable to changes in reporting practices. JAMA Pediatr. 169, 56–62 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Baio J., et al. , Prevalence of autism spectrum disorder among children aged 8 years–autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 67, 1–23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallmayer J., et al. , Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry 68, 1095–1102 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sealey L. A., et al. , Environmental factors in the development of autism spectrum disorders. Environ. Int. 88, 288–298 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Sagiv S. K., et al. , Prenatal organophosphate pesticide exposure and traits related to autism spectrum disorders in a population living in proximity to agriculture. Environ. Health Perspect. 126, 047012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J. Y., et al. , Environmental risk factors and biomarkers for autism spectrum disorder: An umbrella review of the evidence. Lancet Psychiatry 6, 590–600 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Bradberry S. M., Proudfoot A. T., Vale J. A., Glyphosate poisoning. Toxicol. Rev. 23, 159–167 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Kier L. D., Kirkland D. J., Review of genotoxicity studies of glyphosate and glyphosate-based formulations. Crit. Rev. Toxicol. 43, 283–315 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Samsel A., Seneff S., Glyphosate, pathways to modern diseases II: Celiac sprue and gluten intolerance. Interdiscip. Toxicol. 6, 159–184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson N. L., Leu A., Abrahamson J., Wallet B., Genetically engineered crops, glyphosate and the deterioration of health in the United States of America. J. Org. Syst. 9, 6–37 (2014). [Google Scholar]
- 13.Samsel A., Seneff S., Glyphosate, pathways to modern diseases III: Manganese, neurological diseases, and associated pathologies. Surg. Neurol. Int. 6, 45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Ehrenstein O. S., et al. , Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: Population based case-control study. BMJ 364, l962 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estes M. L., McAllister A. K., Maternal immune activation: Implications for neuropsychiatric disorders. Science 353, 772–777 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H. Y., et al. , Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behav. Immun. 58, 165–172 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Careaga M., Murai T., Bauman M. D., Maternal immune activation and autism spectrum disorder: From rodents to nonhuman and human primates. Biol. Psychiatry 81, 391–401 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zerbo O., et al. , Association between influenza infection and vaccination during pregnancy and risk of autism spectrum disorder. JAMA Pediatr. 171, e163609 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto K., Recent advances in the early intervention in schizophrenia: Future direction from preclinical findings. Curr. Psychiatry Rep. 21, 75 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Brown A. S., Meyer U., Maternal immune activation and neuropsychiatric illness: A translational research perspective. Am. J. Psychiatry 175, 1073–1083 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morisseau C., Hammock B. D., Epoxide hydrolases: Mechanisms, inhibitor designs, and biological roles. Annu. Rev. Pharmacol. Toxicol. 45, 311–333 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Imig J. D., Hammock B. D., Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 8, 794–805 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morisseau C., Hammock B. D., Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 53, 37–58 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto K., Role of soluble epoxide hydrolase in metabolism of PUFAs in psychiatric and neurological disorders. Front. Pharmacol. 10, 36 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Q., et al. , Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc. Natl. Acad. Sci. U.S.A. 113, E1944–E1952 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto K., Soluble epoxide hydrolase: A new therapeutic target for depression. Expert Opin. Ther. Targets 20, 1149–1151 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Wagner K. M., McReynolds C. B., Schmidt W. K., Hammock B. D., Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol. Ther. 180, 62–76 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swardfager W., et al. , Metabolic/inflammatory/vascular comorbidity in psychiatric disorders; soluble epoxide hydrolase (sEH) as a possible new target. Neurosci. Biobehav. Rev. 87, 56–66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren Q., et al. , Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 115, E5815–E5823 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma M., et al. , Key role of soluble epoxide hydrolase in the neurodevelopmental disorders of offspring after maternal immune activation. Proc. Natl. Acad. Sci. U.S.A. 116, 7083–7088 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto K., Understanding the link between maternal infections and neurodevelopmental disorders in offspring: The role of abnormalities in metabolism of polyunsaturated fatty acids. Brain Behav. Immun. 81, 4–5 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Atone J., Wagner K., Hashimoto K., Hammock B. D., Cytochrome P450 derived epoxidized fatty acids as a therapeutic tool against neuroinflammatory diseases. Prostaglandins Other Lipid Mediat. 147, 106385 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinohe A., et al. , Increased serum levels of glutamate in adult patients with autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 1472–1477 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Shimmura C., et al. , Alteration of plasma glutamate and glutamine levels in children with high-functioning autism. PLoS One 6, e25340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Z., Zhu T., Qu Y., Mu D., Blood glutamate levels in autism spectrum disorder: A systematic review and meta-analysis. PLoS One 11, e0158688 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M., et al. , Alterations in gut glutamate metabolism associated with changes in gut microbiota composition in children with autism spectrum disorder. mSystems 4, e00321-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomova A., et al. , Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 138, 179–187 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Vuong H. E., Hsiao E. Y., Emerging roles for the gut microbiome in autism spectrum disorder. Biol. Psychiatry 81, 411–423 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu F., et al. , Altered composition and function of intestinal microbiota in autism spectrum disorders: A systematic review. Transl. Psychiatry 9, 43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu M., Xu X., Li J., Li F., Association between gut microbiota and autism spectrum disorder: A systematic review and meta-analysis. Front. Psychiatry 10, 473 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose T. E., et al. , 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: Structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J. Med. Chem. 53, 7067–7075 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ostermann A. I., et al. , Oral treatment of rodents with soluble epoxide hydrolase inhibitor 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)urea (TPPU): Bioavailability, resulting drug levels and modulation of oxylipin pattern. Prostaglandins Other Lipid Mediat. 121, 131–137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji H., Xu L., Wang Z., Fan X., Wu L., Differential microRNA expression in the prefrontal cortex of mouse offspring induced by glyphosate exposure during pregnancy and lactation. Exp. Ther. Med. 15, 2457–2467 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu N., et al. , Circular RNA expression profiles in hippocampus from mice with perinatal glyphosate exposure. Biochem. Biophys. Res. Commun. 501, 838–845 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Williams G. M., Kroes R., Munro I. C., Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. 31, 117–165 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Herrmann K. M., Weaver L. M., The shikimate pathway. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 473–503 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Maeda H., Dudareva N., The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 63, 73–105 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Samsel A., Seneff S., Glyphosate’s suppression of cytochrome P450 enzymes and amino acid biosynthesis by the gut microbiome: Pathways to modern diseases. Entropy 15, 1416–1463 (2013). [Google Scholar]
- 49.Rueda-Ruzafa L., Cruz F., Roman P., Cardona D., Gut microbiota and neurological effects of glyphosate. Neurotoxicology 75, 1–8 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Argou-Cardozo I., Zeidán-Chuliá F., Clostridium bacteria and autism spectrum conditions: A systematic review and hypothetical contribution of environmental glyphosate levels. Med. Sci. 6, 29 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L., et al. , Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 57, 2096–2102 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Cryan J. F., et al. , The micribiota-gut-brain axis. Physiol. Rev. 99, 1877–2013 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Sherwin E., Bordenstein S. R., Quinn J. L., Dinan T. G., Cryan J. F., Microbiota and the social brain. Science 366, eaar2016 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Solomon K. R., Estimated exposure to glyphosate in humans via environmental, occupational, and dietary pathways: An updated review of the scientific literature. Pest Manag. Sci., 10.1002/ps.5717 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in the paper are included in the dataset of the SI Appendix.