Abstract
Background
Vascular aging is characterized by increasing arterial stiffness as measured by pulse wave velocity. The present study evaluated the factors influencing vascular aging in Chinese healthy older subjects.
Material/Methods
Disease- and treatment-free aged (≥60 years) participants were recruited from 2014 to 2019. Cardiometabolic risk factors and brachial-ankle pulse wave velocity (baPWV) were assessed. We defined healthy vascular aging (HVA) as the lowest 10% and early vascular aging (EVA) as the highest 10% of the baPWV distribution, after adjustment for age and blood pressure (BP). We fitted linear and logistic regression models to assess the determinants.
Results
In all, 794 subjects (mean age 66.5±6.8 years, 71.0% male) were recruited; the 10th and 90th percentiles of baPWV were 1278 cm/s and 1955 cm/s, respectively. Age, BP, heart rate, and triglycerides were all positively associated with baPWV, whereas male subjects and body mass index (BMI) were negatively associated with baPWV. The number of participants diagnosed with either HVA or EVA was 80. Logistic regression models showed that sex, BMI, heart rate, and triglycerides were associated with HVA and EVA after adjustment for age, BP, and other confounding factors.
Conclusions
Male, high BMI, low heart rate, and low triglycerides are protective factors for vascular aging in the healthy aged population. Management of BMI, heart rate, triglycerides in a reasonable range may help to alleviate the vascular aging process.
MeSH Keywords: Aging, Pulse Wave Analysis, Vascular Stiffness
Background
Cardiovascular disease (CVD) has become the leading cause of death in China, and the increasing age of patients has further aggravated the burden of CVD [1]. Aging of the vasculature, resulting in high elastic artery stiffness and endothelial dysfunction, plays a central role in CVD in older people [2]. Clinically, arterial stiffness contributes to the pathophysiological process of hypertension, atherosclerosis, and stroke. Moreover, increased arterial stiffness has been regarded as the fundamental and vital link in the Vascular Aging Continuum [3]. The degree of arterial stiffness reflects the cumulative damaging effects of cardiovascular risk factors on the arterial wall with aging and has been used as an established hallmark of vascular aging [4–9].
Not all individuals undergo the vascular aging process in a uniform way. Like other clinical features, vascular aging also has a polygenic inheritance pattern and a multi-factorial etiology [10,11]. Vascular aging, evaluated by pulse wave velocity (PWV), progresses at different rates. Although there is no standard method for quantifying vascular aging [5–9], the core concept is that healthy vascular aging (HVA) means the lack of the age-related increase in arterial stiffness and can be diagnosed in subjects who present a lower PWV than the expected level for their age and blood pressure (BP). Conversely, early vascular aging (EVA), the opposite of HVA, can be diagnosed in subjects who present a higher PWV for their age and BP.
There is significant heterogeneity in the health trajectories among the elderly population, resulting in varying aging patterns, such as disease-free aging, or aging with increased risk for CVD or diabetes [12]. However, older participants enrolled in epidemiological studies are often affected by a variety of chronic diseases. To reduce the potential bias from disease and maximize the chance to capture the age-related differences, it is reasonable to select healthy older individuals according to strict criteria. Healthy aged subjects with HVA remain protected for a long period of time. We aimed to identify the factors protecting against vascular aging, owing to the high predictive value of vascular aging for cardiovascular events [4–6]. Another goal of our study was to assess the distribution of baPWV in the Chinese healthy aged population.
Material and Methods
This study was conducted at the physical examination center of the Geriatrics Department of Tongji Hospital and was approved by the Medical Ethics Committee. Healthy older subjects (aged ≥60 years), who underwent arterial stiffness assessment between January 2014 and October 2019 were consecutively recruited. We excluded individuals with hypertension (systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg, or use of antihypertensive drugs), diabetes [fasting glucose level of ≥7.0 mmol/L or glycated hemoglobin A1c (HbA1c) ≥6.5%, or use of hypoglycemic medications] [13], previous CVD, stroke, obvious arrhythmia (persistent atrial fibrillation, frequent premature beats, or wearing a pacemaker), cardiomyopathy, valvular heart disease, cancer, lung disease, severe chronic liver/kidney disease, and ankle-brachial index (ABI) less than 0.9 [14]. We also excluded patients receiving any medical treatment (e.g., lipid-lowering drugs, aspirin, antibiotics). Consequently, 794 participants were included in the analysis.
Trained personnel conducted standardized in-person interviews with the patients to collect information regarding their lifestyle, medical history, and medication use. Based on smoking status, the subjects were categorized as non-current smokers or current smokers. Anthropometric measurement (weight and height) and resting heart rate were also recorded. Body mass index (BMI) was computed as the weight in kilograms divided by the square of the height in meters. Fasting venous blood samples were collected and sent to the hospital’s clinical chemistry laboratory to measure total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), glucose, HbA1c, creatinine, and uric acid.
We evaluated arterial stiffness with brachial-ankle pulse wave velocity (baPWV). BaPWV is widely used to screen vascular damages in East Asian populations. BaPWV, BP, and ABI were measured using the Vascular Profiler BP-203RPEIII (Omron, Kyoto, Japan). The examination room was maintained at a standardized temperature. Trained technicians placed 4 pressure cuffs on the subjects (one on the upper part of each arm and one on each ankle). Then, the subjects were examined after 10 minutes of rest in the supine position. The device simultaneously recorded the bilateral systolic and diastolic BP, ABI, and baPWV. BaPWV was calculated as the ratio of the traveled distance (which was automatically estimated from the body height) divided by the transit time of the pulse wave between the brachial and posterior tibial arteries. The higher-side baPWV values were used for analysis [14]. Pulse pressure was calculated as the systolic BP minus the diastolic BP, and the mean arterial pressure was calculated as the diastolic BP plus the pulse pressure divided by 3. We adjusted baPWV by age and mean arterial pressure using a general linear model to calculate the adjusted values with the residual method. For this healthy older sample with normal BP, we defined HVA as the lowest 10% and EVA as the highest 10% of the adjusted baPWV distribution, and the rest of the subjects were categorized as the non-HVA group.
Data were analyzed using R 3.6.1. Continuous variables are presented as means±standard deviation (SD) or median (interquartile range), as appropriate for distribution. Categorical variables are shown as count (percentage). The normal distribution was tested using the probability density function. The difference in baPWV between males and females was assessed by t tests. Correlations between age, mean arterial pressure, and baPWV were assessed by Pearson’s correlation. A linear regression model was fitted to identify the independent determinants of baPWV; total cholesterol was excluded to avoid the effect of multicollinearity. We compared the variables between vascular aging status using ANOVA or chi-squared test and also did post hoc pairwise comparisons. We performed multivariable logistic regression analysis to determine the independent influencing factors for HVA and EVA, resistively. Since the residuals which were used to define vascular aging represented the fraction of baPWV that could not be explained by age and mean arterial pressure, we did not add those 2 variables in the models, and total cholesterol was also excluded. Only variables staying in the final model were presented. Give the sex imbalance, we also tested for interactions with sex by entering corresponding cross-product terms into the models. Two-tailed P values less than 0.05 were considered significant.
Results
Study population and reference values for baPWV
In all, 794 healthy older subjects (mean age 66.5±6.8 years, 71.0% male) were recruited and the characteristics are presented in Table 1. The mean value of baPWV was 1570±281 cm/s, and there was no significant difference between males and females (1570±280 cm/s vs. 1580±284 cm/s, p=0.82). The baPWV value was positively associated with age (r=0.52, p<0.001) and mean arterial pressure (r=0.38, p<0.001). The baPWV distribution stratified for the age of this healthy older sample is shown in Figure 1 and the exact reference values according to age categories are presented in Table 2. The 10th and 90th percentiles of baPWV of the sample were 1278 cm/s and 1955 cm/s, respectively. This could help doctors judge the vascular aging status in the aged population.
Table 1.
Characteristics of the study population by vascular aging status.
| Variables | All (n=794) | HVA (n=80) | Non-HVA (n=634) | EVA (n=80) | p overall | p for interaction |
|---|---|---|---|---|---|---|
| Age, years | 66.5±6.8 | 68.6±7.6 | 66.0±6.5* | 68.0±7.7# | <0.001 | 0.76 |
| Male, % | 564 (71.0%) | 61 (76.2%) | 454 (71.6%) | 49 (61.2%) | 0.09 | – |
| Smoker, % | 154 (19.4%) | 14 (17.5%) | 131 (20.7%) | 9 (11.2%) | 0.12 | – |
| BMI, kg/m2 | 23.6±2.8 | 24.9±3.2 | 23.7±3.6* | 23.1±3.9* | <0.001 | 0.20 |
| Systolic BP, mmHg | 123±10 | 124±9 | 123±10 | 126±10# | 0.03 | 0.15 |
| Diastolic BP, mmHg | 72±8 | 74±7 | 72±8 | 73±8 | 0.04 | 0.55 |
| Pulse pressure, mmHg | 51±8 | 50±8 | 51±8 | 53±9 | 0.06 | 0.04 |
| Mean arterial pressure, mmHg | 89±8 | 91±7 | 89±8 | 91±8 | 0.03 | 0.57 |
| Heart rate, beats/min | 67±10 | 63±9 | 67±10* | 75±10*,# | <0.001 | 0.08 |
| Total cholesterol, mmol/L | 4.59±0.87 | 4.54±0.92 | 4.59±0.86 | 4.60±0.90 | 0.86 | 0.15 |
| Triglycerides, mmol/L | 1.35±0.77 | 1.17±0.58 | 1.34±0.75 | 1.57±1.04*,# | 0.004 | 0.93 |
| HDL-C, mmol/L | 1.25±0.32 | 1.23±0.30 | 1.26±0.31 | 1.26±0.42 | 0.82 | 0.86 |
| LDL-C, mmol/L | 2.85±0.75 | 2.87±0.84 | 2.86±0.74 | 2.72±0.74 | 0.26 | 0.06 |
| Glucose, mmol/L | 5.06±0.51 | 5.00±0.54 | 5.07±0.52 | 5.06±0.45 | 0.44 | 0.97 |
| HbA1c, % | 5.68±0.30 | 5.66±0.31 | 5.67±0.30 | 5.71±0.33 | 0.45 | 0.40 |
| Uric acid, umol/L | 347±83 | 355±80 | 346±83 | 342±86 | 0.56 | 0.43 |
| Creatinine, umol/L | 78.0±15.5 | 80.2±15.7 | 78.0±15.3 | 75.5±16.0 | 0.16 | 0.37 |
| ABI | 1.13±0.07 | 1.11±0.07 | 1.13±0.07* | 1.13±0.09* | 0.02 | 0.52 |
| BaPWV, cm/s | 1570±281 | 1320±155 | 1540±215* | 2060±290*,# | <0.001 | 0.07 |
p<0.05 vs. HVA;
p<0.05 vs. Non-HVA.
Figure 1.
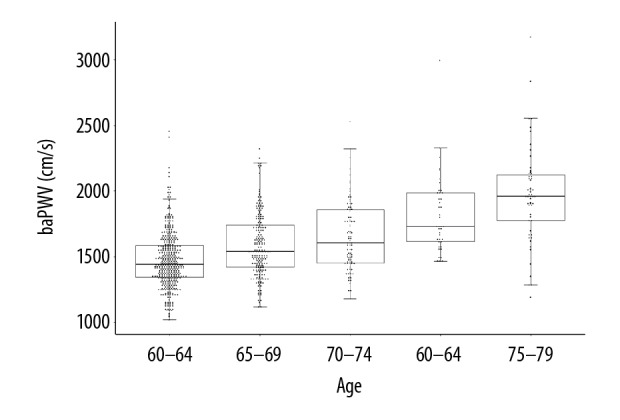
The distribution of baPWV by different age groups in healthy older subjects.
Table 2.
Reference values of baPWV (cm/s) by different age groups of the healthy older sample.
| Age groups | Min–max | Means±SD | Percentiles | ||
|---|---|---|---|---|---|
| 10% | 50% | 90% | |||
| 60–64 (n=412) | 1020–2460 | 1470±210 | 1244 | 1441 | 1731 |
| 65–69 (n=193) | 1120–2320 | 1590±242 | 1311 | 1538 | 1903 |
| 70–79 (n=134) | 1180–3000 | 1710±285 | 1387 | 1662 | 2052 |
| ≥80 (n=55) | 1190–3180 | 1970±367 | 1562 | 1960 | 2417 |
| All (n=794) | 1020–3180 | 1570±281 | 1278 | 1518 | 1955 |
Linear regression analysis of determinants of baPWV
Using baPWV as the dependent continuous variable in multiple linear regression, age, BP, heart rate, and triglycerides were all positively associated with baPWV, whereas male subjects and BMI were both negatively associated with baPWV after adjusting for confounding factors, as illustrated in Table 3.
Table 3.
Linear regression analysis of determinants of baPWV.
| Variables | β | SE | p |
|---|---|---|---|
| Age, years | 18.2 | 1.2 | <0.001 |
| Male, % | −48.9 | 15.2 | 0.001 |
| BMI, kg/m2 | −15.6 | 3.0 | <0.001 |
| Mean arterial pressure, mmHg | 13.0 | 1.0 | <0.001 |
| Heart rate, beats/min | 6.7 | 0.8 | <0.001 |
| Triglycerides, mmol/L | 26.6 | 10.7 | 0.01 |
| Adjusted R2 | 0.48 |
Factors associated with HVA and EVA
Eighty participants were diagnosed with HVA and the other 80 participants were diagnosed with EVA. Characteristics of the study population by vascular aging status are also presented in Table 1. The unadjusted baPWV values were significantly different among the HVA, the non-HVA, and the EVA groups. In contrast with those in the HVA group, individuals without HVA had significantly lower BMI and younger age, and higher heart rate and ABI; while subjects with EVA had lower BMI and higher heart rate, triglycerides, and ABI. No statistically significant interactions were observed between sex and any of the variables except for blood pressure.
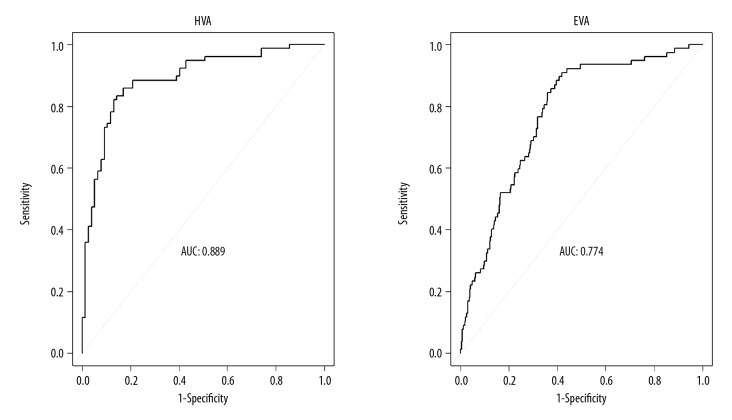
In the logistic regression models (Table 4), we observed that male [OR (95% CI) 2.36 (1.24–4.71), p=0.01], higher BMI [OR (95% CI) 1.22 (1.05–1.42), p=0.01], lower heart rate [OR (95% CI) 0.85 (0.80–0.90), p<0.001], and lower triglycerides [OR (95% CI) 0.38 (0.17–0.71), p=0.01] were all associated with a higher likelihood of having HVA. The area under the receiver operating characteristic curve (AUC) for the multivariable logistic models in predicting the presence of HVA was 0.89 is illustrated in Figure 2. Female sex [male OR (95% CI) 0.65 (0.44–0.96), p=0.03], lower BMI [OR (95% CI) 0.91 (0.82–0.99), p=0.04], higher heart rate [OR (95% CI) 1.08 (1.06–1.11), p<0.001], and higher triglycerides [OR (95% CI) 1.41 (1.07–1.84), p=0.01] added excessive risk to EVA; and the AUC for the model was 0.77 in predicting the presence of EVA. Similarly, there was no significant interaction between sex and any of the significant correlates of HVA and EVA in the multivariable models.
Table 4.
Determinants of HVA and EVA as compared with the control group by multivariable logistic regression analysis.
| Variables | OR | 95% CI | p |
|---|---|---|---|
| HVA | |||
| Male | 2.36 | 1.24–4.71 | 0.01 |
| BMI, per kg/m2 | 1.22 | 1.05–1.42 | 0.01 |
| Heart rate, per beats/min | 0.85 | 0.80–0.90 | <0.001 |
| Triglycerides, per mmol/L | 0.38 | 0.17–0.71 | 0.01 |
| EVA | |||
| Male | 0.65 | 0.44–0.96 | 0.03 |
| BMI, per kg/m2 | 0.91 | 0.82–0.99 | 0.04 |
| Heart rate, per beats/min | 1.08 | 1.06–1.11 | <0.001 |
| Triglycerides, per mmol/L | 1.41 | 1.07–1.84 | 0.01 |
Figure 2.
Receiver operating characteristic curves for the multivariable logistic models in predicting the presence of HVA or EVA. The independent variables of the 2 models included sex, BMI, heart rate, and triglycerides.
Discussion
Arterial stiffness, as measured by baPWV, is one of the major age-related arterial phenotypes, and is an independent predictor of future CVD in older adults [15]. While baPWV has been widely applied in clinical work, investigations of the reference values of baPWV are limited, especially for healthy aged individuals, and the absence of reference values hampers the understanding of its implications. At present, individuals with a baPWV level greater than 1400 cm/s are considered to have arterial stiffness [16–18]; however, this is unreasonable, since the baPWV values are greatly influenced by age and BP. Just like carotid-femoral PWV [19], practical reference values of baPWV should be established according to age, BP, and other cardiovascular risk factors. Previous similar studies have been carried out in other areas of China [20–22], but the numbers of the healthy aged people included were small. Our study only focused on healthy older subjects with normal BP, and the sample size was relatively large. We provided reliable reference values for the healthy aged population of central China.
There are still no generally accepted methods for quantifying vascular aging. While PWV was used in most studies, other structural and functional parameters of arteries also provide information about vascular aging, such as augmentation index, artery intima-media thickness, or coronary artery calcium score [23,24]. Understanding of vascular aging is far from sufficient. A variety of factors (e.g., arterial hypertension, cardiometabolic factors, adiposity, unhealthy lifestyle) have all been linked to vascular aging in previous studies [25,26]. We tried to determine the modifiable determinants of vascular aging for the healthy aged population, especially those with HVA. After adjustment for age and BP, we found that sex, BMI, heart rate, and triglycerides were all independently associated with both HVA and EVA.
In the healthy aged subjects, high BMI was found to be a protective factor for vascular aging, even when adjusted for age, BP, and other confounding factors. Our findings are quite similar to the research by Yang et al., which also found a negative correlation between BMI and baPWV in 414 elderly subjects [27]. Moreover, a similar relationship also existed in other demographic groups, such as youth [28] and individuals with hypertension [29]. Increased blood volume and lager vessel inner diameter of the subjects with higher weight might be a potential mechanism. Numerous studies reported that obese individuals often had a lower CVD and mortality risk than normal-weight individuals, a phenomenon termed the “obesity paradox”; and this was also elucidated in the elderly population [30–32]. The specific mechanisms are un clear, and it may be related to the greater muscle mass and muscular strength, earlier presentation of heavier patients, greater likelihood of receiving optimal medical therapy, and so on [30,31]. We demonstrated that those with higher BMI had lower baPWV (i.e., younger arteries with greater elasticity) than those with normal BMI, and this might be a vascular mechanism that contributes to the “obesity paradox”.
The sex differences in the time course of aging-related arterial stiffness have been shown in previous studies, and the rate of change in PWV was higher in females [33,34]. We further confirmed that aged males were more likely to have HVA and less likely to have EVA compared with aged females. Low heart rate and triglycerides also played a protective role in vascular aging of the healthy aged population. The value of triglycerides in predicting arterial stiffness was also found in other studies [35–37]. However, ApoA5-1131T>C polymorphism was not associated with increased baPWV, and this suggests that the association between circulating triglyceride level and baPWV may reflect confounding and reverse causation [38]. Longitudinal studies are needed to confirm the causal effect. The independent influence of resting heart rate on arterial stiffness remained controversial since results were confounded by BP and other confounding factors [39,40]. In our study, one beat per minute increase in heart rate led to a 15% decrease in the likelihood of having HVA and an 8% increase in the likelihood of having EVA independently. Moreover, long-term resting heart rate pattern was also proven to predict the risk of having arterial stiffness [17].
We have some limitations to consider. First, all the findings were based on analyses of healthy aged people and may not apply to younger individuals or to those at high risk for CVD. Second, this study was conducted at a single center, resulting in a sample that was comprised of more males than females. Third, subjects with untreated dyslipidemia were not excluded since they accounted for 54% of the sample [41]. In addition, a study of patients with heart failure demonstrated that patients with the lowest baPWV values had the highest frequency of heart failure-related events [42]. However, its study population was quite different from the healthy elderly. The follow-up of the individuals with very low baPWV value in our study has important value.
Conclusions
In conclusion, in this study, we describe the distribution of baPWV in a Chinese healthy older sample. We also show that after adjustment for age and BP, vascular aging is associated with several traditional cardiovascular risk factors. Male sex, high BMI, low heart rate, and low triglycerides are the most significant protective factors. Management of BMI, heart rate, and triglycerides in a reasonable range may help to alleviate the vascular aging process of healthy older adults.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the Major Technology Innovation of Hubei Province (Program No. 2019ACA141)
References
- 1.Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: Current features and implications. Nat Revi Cardiol. 2019;16(4):203–12. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 2.Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. 2018;123(7):825–48. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Rourke MF, Safar ME, Dzau V. The Cardiovascular Continuum extended: Aging effects on the aorta and microvasculature. Vasc Med. 2010;15(6):461–68. doi: 10.1177/1358863X10382946. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Boutouyrie P, Cunha PG, et al. Concept of extremes in vascular aging. Hypertension. 2019;74(2):218–28. doi: 10.1161/HYPERTENSIONAHA.119.12655. [DOI] [PubMed] [Google Scholar]
- 5.Niiranen TJ, Lyass A, Larson MG, et al. Prevalence, correlates, and prognosis of healthy vascular aging in a Western community-dwelling cohort: The Framingham heart study. Hypertension. 2017;70(2):267–74. doi: 10.1161/HYPERTENSIONAHA.117.09026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Wang A, Yuan X, et al. Association between healthy vascular aging and the risk of the first stroke in a community-based Chinese cohort. Aging (Albany NY) 2019;11(15):5807–16. doi: 10.18632/aging.102170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsson PM, Laurent S, Cunha PG, et al. Characteristics of healthy vascular ageing in pooled population-based cohort studies: The global Metabolic syndrome and Artery REsearch Consortium. J Hypertens. 2018;36(12):2340–49. doi: 10.1097/HJH.0000000000001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha PG, Cotter J, Oliveira P, et al. Pulse wave velocity distribution in a cohort study: From arterial stiffness to early vascular aging. J Hypertens. 2015;33(7):1438–45. doi: 10.1097/HJH.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 9.Ji H, Teliewubai J, Lu Y, et al. Vascular aging and preclinical target organ damage in community-dwelling elderly: The Northern Shanghai Study. J Hypertens. 2018;36(6):1391–98. doi: 10.1097/HJH.0000000000001692. [DOI] [PubMed] [Google Scholar]
- 10.Ungvari Z, Tarantini S, Donato AJ, et al. Mechanisms of vascular aging. Circ Res. 2018;123(7):849–67. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nilsson PM, Boutouyrie P, Cunha P, et al. Early vascular ageing in translation: From laboratory investigations to clinical applications in cardiovascular prevention. J Hypertens. 2013;31(8):1517–26. doi: 10.1097/HJH.0b013e328361e4bd. [DOI] [PubMed] [Google Scholar]
- 12.Sebastiani P, Thyagarajan B, Sun F, et al. Biomarker signatures of aging. Aging Cell. 2017;16(2):329–38. doi: 10.1111/acel.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes – 2019. Diabetes Care. 2019;42(Suppl 1):S13–28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 14.Ato D. Pitfalls in the ankle-brachial index and brachial-ankle pulse wave velocity. Vasc Health Risk Manag. 2018;14:41–62. doi: 10.2147/VHRM.S159437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ato D. Brachial-ankle pulse wave velocity, cardio-ankle vascular index, and prognosis. Vasc Health Risk Manag. 2018;14:321–48. doi: 10.2147/VHRM.S179366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Yang Y, Wang A, et al. Association of long-term blood pressure variability and brachial-ankle pulse wave velocity: A retrospective study from the APAC cohort. Sci Rep. 2016;6:21303. doi: 10.1038/srep21303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Li W, Jin C, et al. Resting heart rate trajectory pattern predicts arterial stiffness in a community-based chinese cohort. Arterioscler Thromb Vasc Biol. 2017;37(2):359–64. doi: 10.1161/ATVBAHA.116.308674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Fan F, Kou M, et al. Brachial-ankle pulse wave velocity is associated with the risk of new carotid plaque formation: Data from a Chinese community-based cohort. Sci Rep. 2018;8(1):7037. doi: 10.1038/s41598-018-25579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur Heart J. 2010;31(19):2338–50. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Xie J, Zhang LJ, et al. Reference values of brachial-ankle pulse wave velocity for Northern Chinese. Chin Med J (Engl) 2009;122(18):2103–6. [PubMed] [Google Scholar]
- 21.Ai ZS, Li J, Liu ZM, et al. Reference value of brachial-ankle pulse wave velocity for the eastern Chinese population and potential influencing factors. Braz J Med Biol Res. 2011;44(10):1000–5. doi: 10.1590/s0100-879x2011007500108. [DOI] [PubMed] [Google Scholar]
- 22.Yiming G, Zhou X, Lv W, et al. Reference values of brachial-ankle pulse wave velocity according to age and blood pressure in a central Asia population. PLoS One. 2017;12(4):e0171737. doi: 10.1371/journal.pone.0171737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terentes-Printzios D, Vlachopoulos C, Xaplanteris P, et al. Cardiovascular risk factors accelerate progression of vascular aging in the general population: Results from the CRAVE study (Cardiovascular Risk Factors Affecting Vascular Age) Hypertension. 2017;70(5):1057–64. doi: 10.1161/HYPERTENSIONAHA.117.09633. [DOI] [PubMed] [Google Scholar]
- 24.Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: Coronary artery calcium nondevelopment in the MESA study. JACC Cardiovasc Imaging. 2015;8(12):1393–400. doi: 10.1016/j.jcmg.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Schellinger IN, Mattern K, Raaz U. The hardest part. Arterioscler Thromb Vasc Biol. 2019;39(7):1301–6. doi: 10.1161/ATVBAHA.118.311578. [DOI] [PubMed] [Google Scholar]
- 26.Kucharska-Newton AM, Stoner L, Meyer ML. Determinants of vascular age: An epidemiological perspective. Clin Chem. 2019;65(1):108–18. doi: 10.1373/clinchem.2018.287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, Zhao J, Deng X, et al. Pulse wave velocity is decreased with obesity in an elderly Chinese population. J Clin Hypertens (Greenwich) 2019;21(9):1379–85. doi: 10.1111/jch.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lurbe E, Torro I, Garcia-Vicent C, et al. Blood pressure and obesity exert independent influences on pulse wave velocity in youth. Hypertension. 2012;60(2):550–55. doi: 10.1161/HYPERTENSIONAHA.112.194746. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Chen Z, Yuan J, et al. Association between body mass index (BMI) and brachial-ankle pulse wave velocity (baPWV) in males with hypertension: A community-based cross-section study in North China. Med Sci Monit. 2019;25:5241–57. doi: 10.12659/MSM.914881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy RA, Reinders I, Garcia ME, et al. Adipose tissue, muscle, and function: potential mediators of associations between body weight and mortality in older adults with type 2 diabetes. Diabetes Care. 2014;37(12):3213–19. doi: 10.2337/dc14-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jahangir E, De Schutter A, Lavie CJ. Low weight and overweightness in older adults: Risk and clinical management. Prog Cardiovasc Dis. 2014;57(2):127–33. doi: 10.1016/j.pcad.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Leistner DM, Bazara S, Munch C, et al. Association of the body mass index with outcomes in elderly patients (>/=80years) undergoing percutaneous coronary intervention. Int J Cardiol. 2019;292:73–77. doi: 10.1016/j.ijcard.2019.06.044. [DOI] [PubMed] [Google Scholar]
- 33.DuPont JJ, Kenney RM, Patel AR, Jaffe IZ. Sex differences in mechanisms of arterial stiffness. Br J Pharmacol. 2019;176(21):4208–25. doi: 10.1111/bph.14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scuteri A, Morrell CH, Orru M, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension. 2014;64(6):1219–27. doi: 10.1161/HYPERTENSIONAHA.114.04127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen J, Huang Y, Lu Y, Yuan H. Associations of non-high-density lipoprotein cholesterol, triglycerides and the total cholesterol/HDL-c ratio with arterial stiffness independent of low-density lipoprotein cholesterol in a Chinese population. Hypertens Res. 2019;42(8):1223–30. doi: 10.1038/s41440-019-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen J, Zhong Y, Kuang C, et al. Lipoprotein ratios are better than conventional lipid parameters in predicting arterial stiffness in young men. J Clin Hypertens (Greenwich) 2017;19(8):771–76. doi: 10.1111/jch.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azhar W, Buczkowski B. Impact of circulating triglycerides concentration on atherosclerotic disease status in middle-aged Saudi Arabian dwellers. Nutrients. 2018;10(11) doi: 10.3390/nu10111642. pii: E1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao WM, Zhang HF, Zhu ZY, et al. Genetically elevated levels of circulating triglycerides and brachial-ankle pulse wave velocity in a Chinese population. J Hum Hypertens. 2013;27(4):265–70. doi: 10.1038/jhh.2012.23. [DOI] [PubMed] [Google Scholar]
- 39.Tan I, Butlin M, Spronck B, et al. Effect of heart rate on arterial stiffness as assessed by pulse wave velocity. Curr Hypertens Rev. 2018;14(2):107–22. doi: 10.2174/1573402113666170724100418. [DOI] [PubMed] [Google Scholar]
- 40.Papaioannou TG, Oikonomou E, Lazaros G, et al. The influence of resting heart rate on pulse wave velocity measurement is mediated by blood pressure and depends on aortic stiffness levels: Insights from the Corinthia study. Physiol Meas. 2019;40(5):055005. doi: 10.1088/1361-6579/ab165f. [DOI] [PubMed] [Google Scholar]
- 41.Joint Committee for Guideline Revision. 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15(1):1–29. doi: 10.11909/j.issn.1671-5411.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tokitsu T, Yamamoto E, Oike F, et al. Clinical significance of brachial-ankle pulse-wave velocity in patients with heart failure with preserved left ventricular ejection fraction. J Hypertens. 2018;36(3):560–68. doi: 10.1097/HJH.0000000000001589. [DOI] [PubMed] [Google Scholar]