Abstract
Background
The aim of this study was to study the feasibility and acceptability of electroacupuncture (EA) for preventing postoperative gastrointestinal complications in patients undergoing thoracoscopic segmentectomy/lobectomy.
Material/Methods
Sixty patients who underwent video-assisted thoracoscopic (VATS) segmentectomy/lobectomy received either EA treatments plus usual care (EA group) or usual care alone (UC group). Patients in the EA group were given 30 minutes of bilateral electroacupuncture on 3 acupoints [Neiguan (PC6), Zusanli (ST36), and Shangjuxu (ST37)] at 3 time points (24 hours before surgery, and 4 hours and 24 hours after surgery). The primary outcomes were recruitment, retention, acceptability of the EA intervention, incidence and severity of abdominal distension (AD), and time to first flatus and defecation. Secondary outcomes included postoperative nausea and vomiting (PONV), pain intensity, and duration of hospital stay.
Results
We recruited 60 participants and 59 were randomized into 2 groups for this study: 30 in the EA group and 29 in the UC group. In total, 57 participants completed the study. With the exception of one participant in the EA group, all participants completed all three sessions of EA. The one exclusion was a case where a paravertebral block was not used during the surgery. Qualitative findings from the acceptability questionnaire indicated that participants viewed the EA treatment as acceptable. After EA treatment, there was a small but statistically significant improvement in participants’ acceptance of EA for alleviating postoperative gastrointestinal discomfort (P=0.001). The EA group showed improved outcomes compared to the UC group in terms of time to first flatus (20.8±4.6 versus 24.1±6.2 hours, P=0.026) and defecation (53.9±6.0 versus 57.5±7.2 hours, P=0.046). No significant differences appeared regarding AD, rescue medication, or duration of hospitalization. PONV and pain intensity were similar in both groups at the recorded time periods.
Conclusions
EA is feasible and acceptable to patients undergoing VATS surgery. Our preliminary findings of EA promoting postoperative recovery of gastrointestinal function warrants large randomized controlled trials.
MeSH Keywords: Electroacupuncture; Gastrointestinal Motility; Thoracic Surgery, Video-Assisted
Background
Video-assisted thoracoscopic surgery (VATS) has emerged as an alternative to thoracotomy for the diagnosis and treatment of pulmonary nodules in the past few years. It is associated with reduced postoperative pain, shorter hospital stays, and better quality of recovery [1]. In addition to pain control, adequate gastrointestinal function plays a crucial role in promoting physiological recovery after surgery [2]. Moreover, Enhanced Recovery after Surgery (ERAS) programs have been proposed that aim to prevent gastrointestinal dysfunction. However, postoperative gastrointestinal dysfunction (PGD) occurs as a common complication in patients undergoing thoracic surgery, and is associated with discomfort experienced by patients, delayed recovery, prolonged hospitalization, and increased financial burden [3]. Delayed motility of the whole gastrointestinal system was observed on the first and third postoperative days after thoracic surgery, which is an undesirable but inevitable consequence of surgery [4,5]. A follow-up of gastrointestinal function in patients who underwent thoracic surgery in our hospital was consistent with recent literature: PGD was experienced by many patients, with a particularly high incidence of postoperative constipation [5–7]. Therefore, maintaining normal gastrointestinal function is considered to be an essential part of ERAS after thoracic surgery.
The factors responsible for disorders of gastrointestinal function include the patient’s age and PGD history, duration of surgery, inadequate pain control, opioid use, gastrointestinal nerve dysfunction, hormone secretion disorder, inflammation, or edema [8,9]. Current treatment for PGD has focused on a multimodal approach including pharmacological interventions and other nonpharmacologic strategies [10,11]. Pharmacological interventions may have potential side effects and high costs, which could reduce acceptability [12]. Other strategies include rapid postoperative rehabilitation measures such as chewing gum, education, limitation of preoperative fasting and early oral feeding, minimal access surgery, and sufficient analgesia [13]. Until now, no specific drug or intervention to prevent PGD has been approved by clinical guidelines or recommendations.
Electroacupuncture (EA) has been widely used for a variety of gastrointestinal problems with minimal adverse events [14,15]. EA can accelerate gastric emptying and colonic motility, and has been found to be safe and effective as an adjunctive therapy for PGD in patients undergoing major surgery, especially abdominal surgery [16,17]. Acupuncture treatments have improved the recovery of postoperative gastrointestinal function, which is characterized by reduced gastrointestinal paralysis, and promotion of flatus and defecation [18]. EA has demonstrated benefit in reducing the duration of postoperative ileus, time to out-of-bed activity, and postoperative analgesic consumption [13]. EA applied before surgery can improve intestinal recovery, alleviate abdominal distention, and shorten discharge time in patients undergoing surgery [19].
Clinical evidence suggests that PGD is also experienced by patients undergoing thoracotomy [20]. Although EA has been shown to be effective for treating PGD following various surgeries, there is little evidence on the use of EA for patients undergoing thoracoscopic lung surgery. We proposed a randomized, open-label trial to investigate the feasibility, acceptability, as well as the initial efficacy of EA on the gastrointestinal function in patients undergoing thoracoscopic surgery.
Material and Methods
Recruitment and ethical considerations
This study was a single-center, randomized and open-label trial, which enrolled a cohort of patients scheduled for lobectomy or segmentectomy by VATS at the Affiliated Hospital of Nanjing University of Traditional Chinese Medicine (TCM). The trial was approved by the Ethics Committee of the Affiliated Hospital of Nanjing University of TCM and was in agreement with the Declaration of Helsinki (Version Fortaleza 2012). The study was registered in the Chinese Clinical Trial Registry (ChiCTR1800014461). All participants received information about the study and were screened in accordance with the eligibility criteria. All participating patients gave signed informed consent before being enrolled in this study.
The inclusion and exclusion criteria
Participants undergoing VATS segmentectomy/lobectomy were enrolled in the study. Inclusion criteria were as follows: patients who were diagnosed with a benign nodule or tumor without metastasis; American Society of Anesthesiologists class I–III physical status, normal lung function, no severe cardiovascular, hepatic, or renal abnormalities; no infection around the sites of acupuncture; no medication taken for constipation prior to enrollment; and willing to sign the informed consent. Exclusion criteria applied to patients with any of the following: poor preoperative pulmonary function tests; pre-existing severe cardiovascular, liver, or kidney system diseases; cognitive dysfunction or psychological disorders who were unable to communicate and cooperate; history of postoperative nausea and vomiting (PONV), opioid addiction, or long-term use of pain medication (non-steroidal anti-inflammatory drugs or opioids) which may interfere with validity of the study; cardiac pacemaker implantation; gastrointestinal disorders; and those with a previous history of EA use.
Randomization and blinding
Group allocation was concealed using a sealed envelope containing the allocation sequence generated by SPSS in a 1: 1 ratio by the recruiter was employed in this study. This was given to the acupuncture provider and was opened immediately before the first treatment. Eligible patients were randomly allocated to either the EA group or usual care (UC) group according to the contents of the envelope. Participants and the acupuncture provider were not blind to the groups because of the specificity of EA treatment. The assessors, anesthetists, and statisticians were unaware of study-group assignments throughout the entire trial.
Intervention
Selection of acupoints was based on previous studies and expert opinion. The acupuncture locations are described in The National Standards for Acupoint Location [21]. Patients in the EA group first received EA 24 hours before surgery. Each session lasted for 30 minutes and was repeated at 4 hours and 24 hours after surgery. EA was performed on patients in a supine position by an accredited acupuncturist with 10 years of experience. Sterile and disposable needles (size 0.30×40 mm, Suzhou Medical Supplies Factory Co., Ltd., China) were inserted to a depth of 3–5 mm at point PC6 (Neiguan), ST36 (Zusanli, positive electrode), and ST37 (Shangjuxu). The needles were manipulated until a de qi sensation was felt by the patient [22]. Then, EA was adjusted at 15 Hz with a 3–5 mA current until the patient reported maximum comfort level by the EA stimulator (XS-998B, Nanjing Xiaosong Medical Instrument Research Institute, Nanjing, China). Patients in the UC group received standard care.
Anesthesia and perioperative procedure
Anesthesia was induced with intravenous midazolam 0.5 mg/kg, 2% lidocaine 1 mg/kg, propofol 2 mg/kg, dexamethasone 5 mg and rocuronium 0.6 mg/kg. We performed intubation using a double lumen tube of appropriate size. Anesthesia was maintained with 1–2% sevoflurane in oxygen and rocuronium was administered as required. Restrictive intravenous fluid therapy strategy was used intraoperatively. If mean blood pressure dropped by ≥30% of baseline, phenylephrine or ephedrine was given intravenously with reference to heart rate. The patients were given intravenous ondansetron 0.1 mg/kg at the end of surgery.
After lateral positioning, thoracic paravertebral block (PVB) was performed in the paravertebral spaces between T4 and T5 with ultrasound guidance, and 20 mL 0.375% ropivacaine was administered. All patients received intravenous patient-controlled analgesia (PCA) containing morphine 1 mg/kg, ketamine, 1 mg/kg and ondansetron 10 mg diluted with normal saline to 100 mL, and routine oral celecoxib 200 mg every 12 hours. The PCA device was set to deliver a 2 mL/hour background infusion and 1 mL on-demand bolus, with a 10-minute lockout time. If the Visual Analogue Scale (VAS) exceeded 3/10, an additional intramuscular injection of bucinnazine 100 mg was administered as a rescue medication.
Outcome measures
The primary outcomes of this feasibility study included recruitment rate, the acceptability of EA, incidence and degree of abdominal distension (AD), time to first flatus, and first defecation. The 4-item acceptability questionnaire uses a 5-point scale to assess patients’ attitude towards EA in terms of their expectations of the experience and confidence in EA (1=not at all, 2=a little, 3=moderately, 4=quite a lot, 5=extremely). It was completed by the patients both before and after the EA treatment. The range of possible scores was 4 to 20. AD was measured by a 5-point Likert-type scale ranging from “0 = I feel no abdominal distension at all” to “4=I feel horrible abdominal distension and need it addressed” [8,14]. Times to first flatus and defecation measured in hours were checked regularly.
Secondary outcomes included PONV, pain scores, time to out-of-bed activity and length of hospital stay. PONV was rated using a validated 4-point rating scale score (from =no nausea or vomiting, to 3=the worst imaginable nausea or vomiting), and pain intensity was graded on a VAS from 0–10 (0=no pain, 10=worst pain). An independent observer assessed PONV and pain intensity at 24, 48, and 72 hours after surgery. EA related adverse events were also recorded.
Statistical analysis
The sample size of 60 was not determined by calculation but was the total number of patients we could recruit during the period available. To determine the feasibility of EA, a sample size of 30 per group was sufficient to assess the participants’ acupuncture experience. Data were analyzed using SPSS 19.0 software. Student’s t-test or Mann–Whitney U test was used for the analysis of continuous data; Pearson’s chi-square test or Fisher’s exact test was used to compare categorical data. All reported P values were 2-sided, and P-value less than 0.05 was considered statistically significant.
Results
The study flow chart is shown in Figure 1. Between December 25, 2017 and June 20, 2018, a total of 60 patients were approached and screened for the study; one of whom refused to receive EA. The remaining 59 participants were randomized into either the EA group (n=30) or the UC group (n=29). Finally, 57 patients completed all treatments and their data was analyzed. During the study process, one patient from the EA group was excluded due to the absence of PVB during surgery, whereas another in the UC group withdrew from the study before surgery without any cause.
Figure 1.
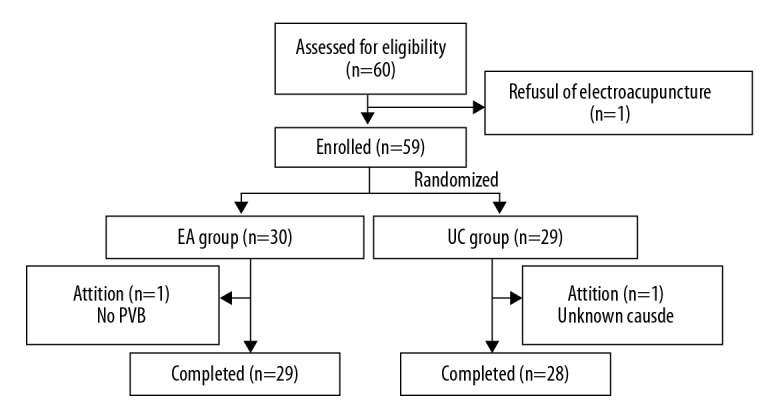
Flow chart of participants through the study period. EA – electroacupuncture; UC – usual care; PVB – paravertebral block.
No differences were observed between groups with regard to patient characteristics, diagnosis, type of operation, procedure duration, or blood loss (Table 1). Patient compliance with the protocol was good. The majority of the participants tolerated the EA well in terms of its intensity, duration, and frequency. All patients except one allocated to the EA group completed all 3 sessions of EA. The excluded participant received only the preoperative session of EA.
Table 1.
Demographic data and surgical details.
| EA group | UC group | P | |
|---|---|---|---|
| Age (years)* | 58.93±11.77 | 56.39±9.34 | 0.372 |
| Weight (kg)* | 62.14±7.67 | 65.5±7.74 | 0.098 |
| Gender (n [%])** | 0.140 | ||
| Male | 13 (44.83) | 18 (64.29) | |
| Female | 16 (55.17) | 10 (35.71) | |
| Type of surgical procedure lobectomy (n [%])** | 16 (55.17) | 14 (50.00) | 0.696 |
| Segmentectomy (n [%])** | 13 (44.83) | 14 (50) | |
| Surgery duration (min)# | 100 (80–150) | 97.5 (70–148.75) | 0.486 |
| Single lung ventilation time (min)# | 60 (48.25–97.50) | 62.5 (41.25–100.00) | 0.502 |
| IV fluid (mL)v | 1000 (1000–1000) | 1000 (1000–1400) | 0.293 |
| Median blood loss (mL)v | 30 (20–60) | 20 (20–60) | 0.362 |
| Duration of chest tube (d)# | 4 (3–5) | 4 (3–5) | 0.233 |
EA – electroacupuncture; UC – usual care; IV – intravenous; min – minute; d – day. Data were expressed as mean±SD*, N(%)** or median (quartile range)#.
As shown in Table 2, there were significant differences in the 4-item acceptability questionnaires before and after EA. Initially, the patients had few expectations regarding EA. After EA treatment, there was a small but statistically significant improvement in their acceptance of EA for postoperative gastrointestinal discomfort (P=0.001).
Table 2.
The acceptability questionnaires from the EA group (n=29).
| Before EA | After EA | P | |
|---|---|---|---|
| How confident do you feel that this treatment can alleviate your complaint? | 1.66±0.61 | 2.21±0.68 | 0.001 |
| How logical does this treatment seem to you? | 1.72±0.59 | 2.17±0.71 | 0.005 |
| How confident would you be in recommending this treatment to a friend? | 1.83±0.76 | 2.38±0.73 | 0.005 |
| How successful do you think this treatment would be in alleviating other complaints? | 1.86±0.95 | 2.21±0.77 | 0.086 |
| Total | 7.00±2.45 | 9.10±2.54 | 0.001 |
EA – electroacupuncture. Data were expressed as mean±SD.
As shown in Table 3, the time to first flatus and defecation were significantly shorter in the EA group than in the UC group (P=0.026 and 0.046, respectively). However, there were no differences between groups in incidence and severity of AD. The proportion of participants who could endure the distension and did not require any intervention was 75% in the UC group and 79.3% in the EA group. Additionally, the frequency of use of rescue antiemetic medicine was comparable in both groups (P=0.473).
Table 3.
Clinical outcomes on gastrointestinal function.
| Variables | EA group | UC group | P |
|---|---|---|---|
| Time to first flatus (h)* | 20.8±4.6 | 24.1±6.2 | 0.026 |
| Time to first defecation (h)* | 53.9±6.0 | 57.5±7.2 | 0.046 |
| Rescue medication given (n [%])** | 5 (17.24) | 7 (25.00) | 0.473 |
| AD’s incidence (n [%])** | 9 (31.03) | 11 (39.28) | 0.514 |
| AD’s degree | 0.290 | ||
| 0 | 20 (70) | 17 (60.7) | |
| 1 | 1 (3.4) | 2 (7.1) | |
| 2 | 2 (6.9) | 2 (7.1) | |
| 3 | 6 (20.7) | 7 (25.0) | |
| 4 | 0 (0) | 0 (0) |
EA – electroacupuncture; UC – usual care; AD – abdominal distension; h – hour. Data presented as mean±SD* or n [%]**.
As shown in Table 4, the 2 groups did not differ on other measures, including the incidence and severity of PONV, postoperative static and dynamic pain VAS scores, time to out-of-bed activity and duration of hospitalization.
Table 4.
Other clinical outcomes.
| Variables | EA group | UC group | P |
|---|---|---|---|
| 0–24 h after surgery | |||
| Nausea score* | 0 (0–1) | 0 (0–1) | 1.000 |
| Vomiting score* | 0 (0–0) | 0 (0–0.75) | 0.486 |
| Nausea [incidence; n (%)]** | 8 (27.59) | 8 (28.57) | 0.934 |
| Vomiting [incidence; n (%)]** | 5 (17.24) | 7 (25.00) | 0.473 |
| Static Pain VAS* | 2 (2–2) | 2 (2–2) | 0.651 |
| Dynamic Pain VAS* | 3 (3–3) | 3 (3–3) | 0.153 |
| 24–48 h after surgery | |||
| Nausea score* | 0 (0–0) | 0 (0–0) | 0.726 |
| Vomiting score* | 0 | 0 | |
| Nausea [incidence; n (%)]** | 4 (13.79) | 3 (10.71) | 1.000 |
| Vomiting [incidence; n (%)]** | 0 (0) | 0 (0) | – |
| Static pain VAS* | 2 (2–3) | 2 (2–3) | 0.930 |
| Dynamic pain VAS* | 3 (3–3) | 3 (3–3) | 0.401 |
| 48–72 h after surgery | |||
| Nausea score* | 0 | 0 | – |
| Vomiting score* | 0 | 0 | – |
| Nausea [incidence; n (%)]** | 0 (0) | 0 (0) | – |
| Vomiting [incidence; n (%)]** | 0 (0) | 0 (0) | – |
| Static pain VAS* | 3 (2–3) | 3 (2–3) | 0.703 |
| Dynamic pain VAS* | 3 (3–3) | 3 (3–3) | 0.079 |
| Time to out-of-bed activity (h)# | 11.2±1.0 | 13±3.5 | 0.5 |
| Hospital stay (d)# | 6.1±2.15 | 6.5±2.05 | 0.442 |
EA – electroacupuncture; UC – usual care; VAS – Visual Analogue Scale; h – hour; d – day. Data were expressed as median (quartile range)*, N (%)**, or mean±SD#.
There were no serious EA-related adverse events reported throughout the study. The most commonly reported adverse events were hematoma, sleeplessness, and mild or transient sharp pain.
Discussion
In this feasibility study, we found that Chinese patients undergoing segmentectomy or lobectomy by VATS could accept EA treatment delivered before and after surgery, and that compliance was high. Our standard EA treatment was safe and could attenuate gastrointestinal dysfunction in these patients but had no effect on PONV or postoperative pain. Our results show that EA was feasible and acceptable to those participants.
A majority of patients were willing to receive acupuncture. We approached 60 patients within 6 months and only one refused to take part in the study, resulting in a participation rate of 95%, which supports the feasibility of EA among these patients. Patients’ acceptance of EA was improved from little confidence to moderate after EA treatment. Their willingness to recommend EA to their friends also increased. Therefore, our study confirmed that EA has good tolerability and safety.
Significant improvements involving time to first defecation and flatus were observed after EA intervention. Our results are consistent with those reported by Chao et al. [23]. Because patients were unblinded to the treatment, we cannot rule out the possibility that the clinical improvement in gastrointestinal discomfort was due to expectation or placebo effects [24].
Studies in the literature have used various endpoints, such as bowel sounds, flatus, and bowel movements to evaluate gastrointestinal function [25,26]. However, due to the variation in individual capacity to report different types and amounts of food intake, those outcome measures may be biased. Radionuclide markers reported in the literature not only cause trauma, but also increase the cost [27]. Recording the time to presence of bowel sounds could not be easily carried out by the attending clinicians due to a lack of time [28]. Time to first flatus and time to defecation may still be essential tools in assessing postoperative gastrointestinal function. Future studies may benefit from adopting more appropriate instruments to capture earlier changes in symptoms.
Various benefits of EA, for example, decreasing postoperative pain, reducing PONV, and improving postoperative well-being have been reported in the literature [26,29]. The therapeutic effects of EA are influenced by many factors, such as the number of sessions, the optimal timing, the selection of acupoints, and concomitant environment [8,23]. In our study, however, there were no differences in PONV between the 2 groups. This may be due to the following reasons. Firstly, prophylactic ondansetron and dexamethasone were routinely administered, and the combination is significantly more effective than either alone in preventing PONV [30]. Secondly, there were fewer factors predisposing towards PONV in VATS surgery. Thirdly, insufficient sample size may underestimate the efficacy of EA. Furthermore, sufficient pain control could decrease opioid use, thus decreasing the incidence of PONV. There were no differences in pain scores at the recorded time points between the groups in our study. Paravertebral block (PVB) alone has been shown to be effective for analgesia after video-assisted thoracoscopic surgery [31]. Dexamethasone demonstrated an ability to lower postoperative pain scores, and to reduce the amount of opioids required to achieve adequate pain scores. Minimally invasive techniques combined with a multimodal analgesic regimen, including lower opioid dose and PVB application, may produce sufficient postoperative pain relief, leading to little scope for improvement with EA.
Acupuncture is a component of oriental medicine and is now used in many Western countries. The current study was, however, conducted among Chinese patients, and the acceptability data may not be transferable to patients in the Western countries. However, 2 recent studies demonstrate that patients in the West may have a higher acceptance of acupuncture than the Chinese patients do. A similar survey was delivered to patients in Australia and in China. When patients were asked if they would like to use acupuncture to reduce PONV, 87% of Australians said yes [32], in comparison to 40% in China [33].
This feasibility study has several limitations. Firstly, thoracic PVB can improve early gastrointestinal function by modulating both the sympathetic and vagal neural pathways, which may interfere with the validity of the results. Secondly, as this feasibility study did not include a sham EA group, a placebo effect of EA could not be ruled out. Nevertheless, our primary aim was to gain an understanding of whether the EA might be a clinically relevant supplement to standard care, and we have achieved this aim.
Conclusions
In conclusion, our feasibility study demonstrated that EA treatment may serve as a safe and acceptable adjuvant for promoting recovery of gastrointestinal function after VATS surgery. An appropriate powered trial with a larger sample size to evaluate the effect of EA on PGD is needed.
Acknowledgements
The authors wish to thank Ms. Jenny Layton for English language editing.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by a research grant from the Affiliated Hospital of Nanjing University of Chinese Medicine (Y18021)
References
- 1.Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol. 2016;17:836–44. doi: 10.1016/S1470-2045(16)00173-X. [DOI] [PubMed] [Google Scholar]
- 2.Vather R, Trivedi S, Bissett I. Defining postoperative ileus: Results of a systematic review and global survey. J Gastrointestinal Surg. 2013;17:962–72. doi: 10.1007/s11605-013-2148-y. [DOI] [PubMed] [Google Scholar]
- 3.Venere A, Neunlist M, Slim K, et al. Postoperative ileus: Pathophysiology, incidence, and prevention. J Visc Surg. 2016;153:439–46. doi: 10.1016/j.jviscsurg.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Guha A, Scawn ND, Rogers SA, et al. Gastric emptying in post-thoracotomy patients receiving a thoracic fentanyl bupivacaine epidural infusion. Eur J Anaesthesiol. 2002;19:652–57. doi: 10.1017/s0265021502001072. [DOI] [PubMed] [Google Scholar]
- 5.Zoumprouli A, Chatzimichali A, Papadimitriou S, et al. Gastrointestinal motility following thoracic surgery: The effect of thoracic epidural analgesia. A randomised controlled trial. BMC Anesthesiol. 2017;17:139. doi: 10.1186/s12871-017-0427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen LS, Pedersen PU. Constipation and defecation pattern the first 30 days after thoracic surgery. Scand J Caring Sci. 2010;24:244–50. doi: 10.1111/j.1471-6712.2009.00713.x. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Qi D, Gong L, et al. Effect of auricular points treatment combined with acupoints application in patients with constipation after lung cancer surgery. J Cancer Res Ther. 2017;13:844–48. doi: 10.4103/jcrt.JCRT_709_17. [DOI] [PubMed] [Google Scholar]
- 8.Bragg D, Elsharkawy AM, Psaltis E, et al. Postoperative ileus: Recent developments in pathophysiology and management. Clin Nutr. 2015;34:367–76. doi: 10.1016/j.clnu.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Artinyan A, Nunoo-Mensah JW, Balasubramaniam S, et al. Prolonged postoperative ileus – definition, risk factors, and predictors after surgery. World J Surg. 2008;32:1495–500. doi: 10.1007/s00268-008-9491-2. [DOI] [PubMed] [Google Scholar]
- 10.Asgeirsson T, EI-Badawi KI, Mahmood A, et al. Postoperative ileus: It costs more than you expect. J Am Coll Surg. 2012;210:228–31. doi: 10.1016/j.jamcollsurg.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Kehlet H. Postoperative ileus – an update on preventive techniques. Nat Clin Pract Gastroenterol Hepatol. 2008;5:552–58. doi: 10.1038/ncpgasthep1230. [DOI] [PubMed] [Google Scholar]
- 12.Van Bree SH, Nemethova A, Cailotto C, et al. New therapeutic strategies for postoperative ileus. Nat Rev Gastroenterol Hepatol. 2012;9:675–83. doi: 10.1038/nrgastro.2012.134. [DOI] [PubMed] [Google Scholar]
- 13.Kronberg U, Kiran RP, Soliman MS, et al. A characterization of factors determining postoperative ileus after laparoscopic colectomy enables the generation of a novel predictive score. Ann Surg. 2011;253:78–81. doi: 10.1097/SLA.0b013e3181fcb83e. [DOI] [PubMed] [Google Scholar]
- 14.Ng SS, Leung WW, Mak TW, et al. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology. 2013;144:307–13. doi: 10.1053/j.gastro.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Yan S, Wu J, et al. Acupuncture for chronic severe functional constipation: A randomized trial. Ann Intern Med. 2016;165:761–69. doi: 10.7326/M15-3118. [DOI] [PubMed] [Google Scholar]
- 16.Xu S, Hou X, Zha H, et al. Electroacupuncture accelerates solid gastric emptying and improves dyspeptic symptoms in patients with functional dyspepsia. Dig Dis Sci. 2006;51:2154–59. doi: 10.1007/s10620-006-9412-x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Wang C, Li Q, et al. Electroacupuncture at ST36 accelerates the recovery of gastrointestinal motility after colorectal surgery: A randomised controlled trial. Acupunct Med. 2014;32:223–26. doi: 10.1136/acupmed-2013-010490. [DOI] [PubMed] [Google Scholar]
- 18.Liu YH, Dong GT, Ye Y, et al. Effectiveness of acupuncture for early recovery of bowel function in cancer: A systematic review and meta-analysis. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/2504021. 2504021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mai S, Meng J, Wang W, Lang S. [Influence of electroacupuncture pretreatment on intestinal function in the patients of colorectal cancer surgery]. Zhongguo Zhen Jiu. 2017;37:483–87. doi: 10.13703/j.0255-2930.2017.05.008. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 20.Kampe S, Weinreich G, Darr C, et al. The impact of epidural analgesia compared to systemic opioid-based analgesia with regard to length of hospital stay and recovery of bowel function: Retrospective evaluation of 1555 patients undergoing thoracotomy. J Cardiothoracic Surg. 2014;9:175. doi: 10.1186/s13019-014-0175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou K, Fang J, Wang X, et al. Characterization of de qi with electroacupuncture at acupoints with different properties. J Altern Complement Med. 2011;17:1007–13. doi: 10.1089/acm.2010.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.General administration of quality supervision, inspection and quarantine of the People’s Republic of China, standardization administration of the People’s Republic of China. Nomenclature and location of acupuncture points. (GB/T 12346-2006)
- 23.Chao HL, Miao SJ, Liu PF, et al. The beneficial effect of ST-36 (Zusanli) acupressure on postoperative gastrointestinal function in patients with colorectal cancer. Onco Nurs Forum. 2013;40:E61–68. doi: 10.1188/13.ONF.E61-E68. [DOI] [PubMed] [Google Scholar]
- 24.Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;20:CD001977. doi: 10.1002/14651858.CD001977.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read TE, Brozovich M, Andujar JE, et al. Bowel sounds are not associated with flatus, bowel movement, or tolerance of oral intake in patients after major abdominal surgery. Dis Colon Rectum. 2017;60:608–13. doi: 10.1097/DCR.0000000000000829. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, May BH, Zhang AL, et al. Acupuncture and related therapies for treatment of postoperative ileus in colorectal cancer: A systematic review and meta-analysis of randomizd controlled trials. Evid Based Complement Alternat Med. 2018;2018 doi: 10.1155/2018/3178472. 3178472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung SY, Chae HD, Kang AR, et al. Effect of acupuncture on postoperative ileus after distal gastrectomy for gastric cancer. J Gastric Cancer. 2017;17:11–20. doi: 10.5230/jgc.2017.17.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Gao YH, Xu J, et al. Zusanli (ST36) acupoint injection for preventing postoperative ileus: A systematic review and meta-analysis of randomized clinical trials. Complement Ther Med. 2015;23:469–83. doi: 10.1016/j.ctim.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheong KB, Zhang JP, Huang Y. The effectiveness of acupuncture in postoperative gastroparesis syndrome – a systematic review and meta-analysis. Complement Ther Med. 2014;22:767–86. doi: 10.1016/j.ctim.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Culy CR, Bhana N, Plosker GL. Ondansetron: A review of its use as an antiemetic in children. Paediatr Drugs. 2001;3:441–79. doi: 10.2165/00128072-200103060-00007. [DOI] [PubMed] [Google Scholar]
- 31.Komatsu T, Sowa T, Takahashi K, Fujinaga T. Paravertebral block as a promising analgesic modality for managing post-thoracotomy pain. Ann Thorac Cardiovasc Surg. 2014;20:113–16. doi: 10.5761/atcs.oa.12.01999. [DOI] [PubMed] [Google Scholar]
- 32.Weeks EM, Trinca J, Zheng Z. Knowledge of and willingness to try acupuncture for postoperative nausea and vomiting: An Australian survey of surgical patients. Acupunct Med. 2017;35:345–51. doi: 10.1136/acupmed-2016-011191. [DOI] [PubMed] [Google Scholar]
- 33.Wang F, Zheng M, Zhu J, et al. Patients’ attitudes to the perioperative application of acupuncture: A Chinese survey. Eur J Integr Med. 2017;9:131–40. [Google Scholar]


