Abstract
Background
Cisplatin (CDDP) remains one of the primary chemotherapeutic agents for gastric cancer patients. However, relapse and metastasis are common because of innate and acquired chemo-resistance. Family with sequence similarity 3 (FAM3) is a novel cytokine-like protein that has an important role in tumor progression, but little is known about the role of FAM3B in human gastric cancer CDDP resistance. In this study, we investigated the role of FAM3B in gastric cancer CDDP resistance and reveal the possible underlying mechanism.
Material/Methods
We firstly developed a CDDP-resistant gastric cell line AGS/CDDP by treating AGS cells to a continuous exposure of CDDP. The FAM3B levels were compared in these 2 cell lines by quantitative real time polymerase chain reaction (qRT-PCR) and western blotting. Cell viability, apoptosis and epithelial-mesenchymal transition (EMT) related changes were detected after ectopic expression or interfering of FAM3B.
Results
We found increased FAM3B expression in AGS/CDDP cells. FAM3B overexpression induced CDDP resistance in AGS cells. Conversely, FAM3B knockdown enhanced CDDP sensitivity of AGS/CDDP cells. Moreover, FAM3B induced EMT in gastric cancer cells by upregulating snail. Inhibition of snail reversed FAM3B-triggered EMT and CDDP resistance.
Conclusions
Upregulation of FAM3B triggered CDDP resistance in gastric cancer cells by inducing EMT in a snail-dependent manner, making FAM3B a promising therapeutic target to reverse gastric cancer chemo-resistance.
MeSH Keywords: Cisplatin, Epithelial-Mesenchymal Transition, Stomach Neoplasms
Background
Gastric cancer is one of the most commonly diagnosed malignancy in men and women, with more than 1 million new cases happening every year. It is also one of the leading causes of cancer-related death in males and females, resulting in over 700 000 deaths in 2012 worldwide [1]. Though incidence and mortality rates of gastric cancer have declined steadily in the majority of more developed countries since the middle of the 20th century, they are still higher in China. Gastric cancer ranks the second most commonly diagnosed and the second most deadly cancer in China [2].
The comprehensive therapy of gastric cancer is based on surgery accompanied with radiation and/or chemotherapy. The combined pre-operative and post-operative chemotherapy has been reported to show promising effects by increasing patient survival, among which cisplatin (CDDP) remains one of the primary chemotherapeutic agents for gastric cancer. The anti-cancer effect of CDDP lies in causing DNA damage which leads to inhibition of DNA replication and transcription, thus results in cell cycle arrest and cell death [3]. Despite that it has a consistent rate of initial responses, innate and acquired chemo-resistance remains the most significant obstacles to effective chemotherapy and better prognosis of gastric cancer patients. Thus, the investigation into the mechanisms underlying CDDP resistance of gastric cancer has clinical significance in preventing and reversing chemo-resistance.
FAM3 is a cytokine-like gene family containing 4 genes: FAM3A, FAM3B, FAM3C, and FAM3D. The 4 members all encode a protein (224–235 amino acids) with a hydrophobic leader sequence [4]. FAM3 has been reported to have important functions in amount of major diseases, such as diabetes, Alzheimer disease, and cancer [5–7]. FAM3B, also named pancreatic-derived factor (PANDER), is the most studied FAM3 family member. FAM3B was originally found in pancreatic α and β cells in endocrine pancreas and was involved in glucose metabolism and lipogenesis [4,8,9]. Besides its function in glycolipid metabolism, FAM3B has also been reported to have an important role in tumor progression. In colon cancer, esophageal squamous cell carcinoma (ESCC) and prostate cancer, FAM3B was reported to be upregulated and was correlated with bad prognosis of the patients. FAM3B inhibits cell death through different mechanisms [10–12]. However, the role of FAM3B in human gastric cancer CDDP resistance has not been reported.
In this research, we aimed to investigate the role of FAM3B in gastric cancer CDDP resistance and reveal the possible underlying mechanism. Our study found that FAM3B was upregulated in CDDP-resistant AGS/CDDP cells. FAM3B overexpression induced CDDP resistance in AGS cells, nevertheless, FAM3B knockdown sensitized AGS/CDDP cells to CDDP. Mechanically, FAM3B triggered CDDP resistance by inducing epithelial-mesenchymal transition (EMT) in a snail-dependent manner. Our study demonstrated that the role FAM3B/EMT regulatory axis in mediating gastric cancer CDDP resistance.
Material and Methods
Cell lines and reagents
The human gastric cancer cell line AGS was purchased from ATCC (Manassas, VA, USA). The CDDP-resistant AGS/CDDP cell line was developed by continuous administration of CDDP starting from 0.1 μg/mL to 5 μg/mL and maintained in 5 μg/mL of CDDP. To perform experiments, AGS/CDDP cells were resuspended in CDDP-free RPMI-1640 medium (Gibco, Gaithersburg, MD, USA) containing 10% fetal bovine serum (FBS; Gibco, Gaithersburg, MD, USA) overnight for attachment onto the culture plate. AGS cells were maintained in the RPMI-1640 medium with 10% FBS and incubated at 37°C with 5% CO2.
CDDP was obtained from Selleck Chemicals (Houston, TX, USA). The rabbit monoclonal antibodies against human E-cadherin (3195), vimentin (5741), snail (3879), P65 (8242), P-P65 (3033), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (5174) were purchased from Cell Signaling Technology (Beverly, MA, USA). Rabbit anti-human FAM3B polyclonal antibody (ab103154) was obtained from Abcam (Cambridge, UK).
Cell viability
The cell viability was determined by using Cell Counting Kit-8 (CCK-8) assay. In brief, gastric cancer cells were seeded in 96-well plates and cultured overnight. Then the cells were incubated with CDDP of various concentrations for 48 hours. Thereafter, CCK-8 (Dojindo Laboratories, Japan) was added to the plates and incubated for 2 hours at 37°C. The absorbance at 490 nm was detected by a spectrophotometer (Thermo Fisher Scientific, USA).
Flow cytometry
Gastric cancer cells which were used to determine the apoptotic rates were collected by centrifugation at 1000×g for 5 minutes. After resuspension, the cells were incubated with 5 μL of Annexin V-FITC and 5 μL of propidium iodide (PI) (MultiSciences, Hangzhou, China) and maintained in the dark for 10 minutes, and then cells were subjected to flow cytometric analysis. The apoptotic cell rates were compared.
Western blotting
Whole-cell protein lysates were prepared, and western blotting analysis were performed as previously described [13]. The signals were developed by using enhanced chemiluminescence (ECL) (Millipore, Switzerland) and captured by a Tanon-5200 Chemi-luminescent Imaging System (Tanon, China). The density of western blotting bands was quantified by ImageJ.
Quantitative real time polymerase chain reaction (qRT-PCR)
The quantitative real time polymerase chain reaction (qRT-PCR) was conducted as previously described [13]. The relative mRNA expression levels were quantified by the 2−ΔΔCq method [14]. The sequences of the primers used in our research were listed in Table 1.
Table 1.
The sequences of the primers used in qRT-PCR.
| Gene | Primer sequence (5′-3′) | |
|---|---|---|
| SNAIL | Forward | ACTGCAACAAGGAATACCTCAG |
| Reverse | GCACTGGTACTTCTTGACATCTG | |
| SLUG | Forward | TGTGACAAGGAATATGTGAGCC |
| Reverse | TGAGCCCTCAGATTTGACCTG | |
| ZEB1 | Forward | CAGCTTGATACCTGTGAATGGG |
| Reverse | TATCTGTGGTCGTGTGGGACT | |
| ZEB2 | Forward | GCGATGGTCATGCAGTCAG |
| Reverse | CAGGTGGCAGGTCATTTTCTT | |
| TWIST1 | Forward | GTCCGCAGTCTTACGAGGAG |
| Reverse | CTTGAGGGTCTGAATCTTGCT | |
| GAPDH | Forward | ACAACTTTGGTATCGTGGAAGG |
| Reverse | GCCATCACGCCACAGTTTC |
Ectopic expression of FAM3B
Lentivirus carrying pcDNA3.1 plasmid containing FAM3B cDNA was synthesized by GeneChem Company Ltd. (Shanghai, China). The transfection was conducted following the manufacturer’s instructions.
Transfection of siRNA
The human FAM3B siRNA (sc-91522) and control siRNA (sc-37007) was obtained from Santa Cruz Biotechnology, Inc. (CA, USA) and transfected using Lipofectamine RNAiMAX (Thermo Fisher Scientific, MA, USA). For transfection, 100 pM of siRNA and 5 μM of Lipofectamine RNAiMAX was mixed with 250 μM Opti-MEM (Thermo Fisher Scientific, USA), respectively. Thereafter, they were mixed and incubated at room temperature for 20 minutes, and then added to the 6-well plate to make the final volume of culture medium to be 2 mL.
Statistical analysis
The data were presented as means±standard deviation (SD). Student’s t-test and one-way ANOVA were used to analyze the differences by using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). P value <0.05 was considered significantly different.
Results
FAM3B was upregulated in AGS/CDDP cells
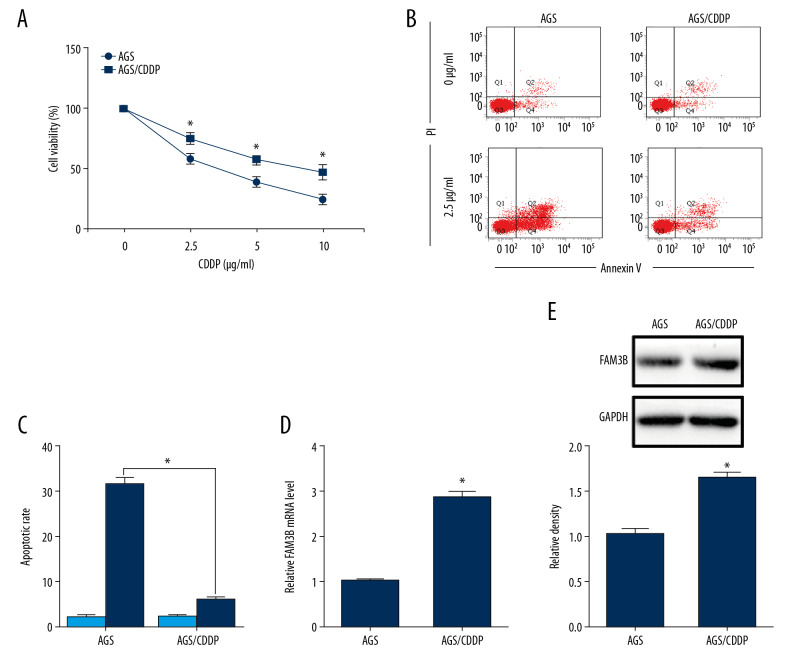
We firstly established a CDDP-resistant gastric cancer cell line by treating AGS cells with continuous exposure to CDDP at the concentration which started at 0.1 μg/mL and increased to 5 μg/mL in a stepwise manner and we named the cells AGS/CDDP. Then we confirmed the CDDP resistance by CCK-8 assay. The results showed that AGS/CDDP cells had higher cell viability than that in AGS after the incubation with CDDP for 48 hours (Figure 1A). The flow cytometry results detected less cell apoptosis in AGS/CDDP cells (Figure 1B, 1C). To reveal whether FAM3B had a role in CDDP resistance of gastric cancer, FAM3B level was detected in AGS/CDDP cells. The mRNA and protein levels of FAM3B were upregulated in AGS/CDDP cells compared to AGS cells (Figure 1D, 1E).
Figure 1.
FAM3B was upregulated in CDDP-resistant AGS cells. (A) AGS and AGS/CDDP cells were incubated with CDDP (0, 2.5, 5, and 10 μg/mL) for 48 hours. The cell viability was measured by CCK-8 assay. (B) AGS and AGS/CDDP cells treated with 0 μg/mL or 2.5 μg/mL CDDP for 48 hours were used in flow cytometry analysis. (C) Comparison of apoptotic cell rates. (D) qRT-PCR results of the FAM3B mRNA level in AGS and AGS/CDDP cells. (E) Western blotting results of the FAM3B protein level in AGS and AGS/CDDP cells. Quantification of the relative band density was in the lower panel (* P<0.05). CCDP – cisplatin; AGS – human gastric cancer cell line; CCK-8 – Cell Counting Kit-8; qRT-PCR – quantitative real time polymerase chain reaction.
FAM3B overexpression induced CDDP resistance in AGS cells
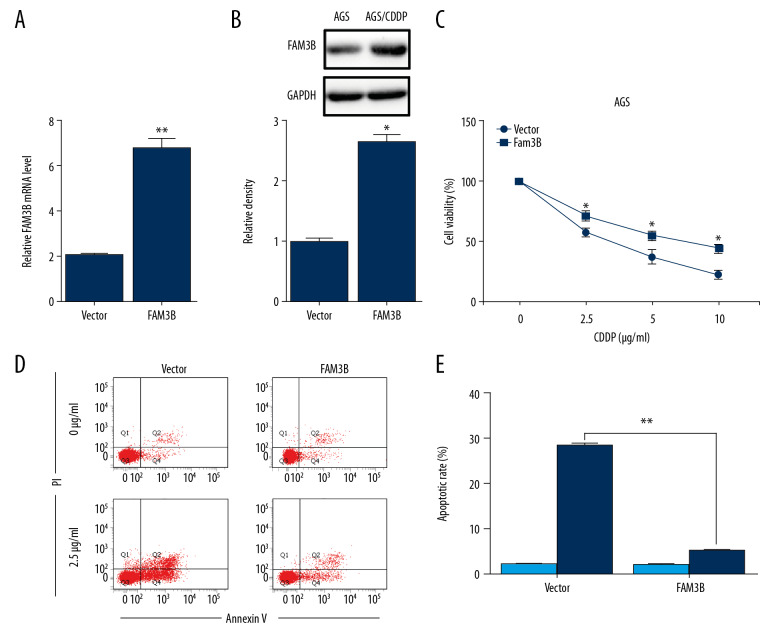
To investigate the effect of upregulated FAM3B on gastric cancer CDDP resistance, FAM3B overexpression vector (FAM3B) or empty vector (Vector) were transfected into AGS cells. We firstly confirmed the overexpression efficiency (Figure 2A, 2B). FAM3B overexpression impaired the sensitivity of AGS cells to CDDP (Figure 2C). The AGS cells with or without FAM3B overexpression were treated with 2.5 μg/mL CDDP for 48 hours and subjected to flow cytometry analysis. As expected, FAM3B overexpression significantly decreased CDDP-induced apoptosis in AGS cells (Figure 2D, 2E). The aforementioned results demonstrated that FAM3B overexpression induced CDDP resistance in AGS cells.
Figure 2.
FAM3B overexpression induced CDDP resistance in AGS cells. (A) FAM3B transfection was confirmed by qRT-PCR in AGS cells. (B) FAM3B transfection was confirmed by western blotting in AGS cells. Quantification of the relative band density was in the lower panel. (C) AGS cells with FAM3B overexpression were treated with CDDP (0, 2.5, 5, and 10 μg/mL) for 48 hours, and the cell viability was measured by CCK-8 assay. (D) AGS cells with FAM3B overexpression treated with 0 μg/mL or 2.5 μg/mL CDDP for 48 hours were used in flow cytometry analysis. (E) Comparison of apoptotic cell rates (* P<0.05; ** P<0.01). CCDP – cisplatin; AGS – human gastric cancer cell line; CCK-8 – Cell Counting Kit-8; qRT-PCR – quantitative real time polymerase chain reaction.
FAM3B knockdown overcame CDDP resistance in AGS/CDDP cells
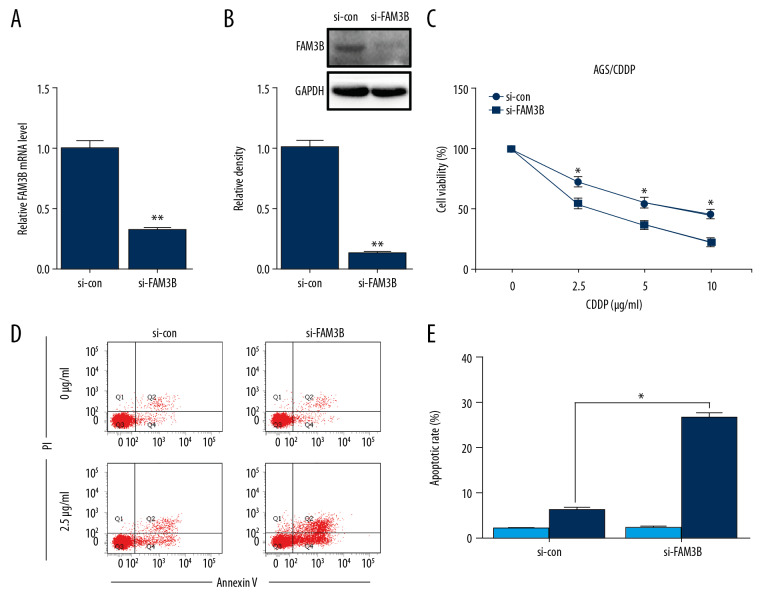
To further reveal whether the upregulated FAM3B was responsible for CDDP resistance in AGS/CDDP cells, we adopted FAM3B siRNA to inhibit the expression of FAM3B in AGS/CDDP cells. The knockdown efficiency was confirmed by qRT-PCR and western blotting (Figure 3A, 3B). The CCK-8 assay showed that FAM3B inhibition reduced the cell viability after CDDP incubation in AGS/CDDP (Figure 3C). To further determine the effect of FAM3B downregulation on CDDP-induced apoptosis, flow cytometry analysis was conducted in AGS/CDDP cells with or without FAM3B knockdown after the exposure to 2.5 μg/mL CDDP for 48 hours. FAM3B siRNA transfection significantly increased CDDP-induced apoptosis (Figure 3D, 3E). Collectively, FAM3B knockdown reversed CDDP resistance in AGS/CDDP cells.
Figure 3.
FAM3B knockdown overcame CDDP resistance in AGS/CDDP cells. (A) The inhibiting efficiency of FAM3B siRNA was confirmed by qRT-PCR in AGS/CDDP cells. (B) The knockdown efficiency of FAM3B siRNA was confirmed by western blotting in AGS/CDDP cells. Quantification of the relative band density was in the lower panel. (C) AGS/CDDP cells with FAM3B inhibition and control cells were treated with CDDP for 48 hours, and the cell viability was measured by CCK-8 assay. (D) AGS/CDDP cells with FAM3B inhibition treated with 0 μg/mL or 2.5 μg/mL CDDP for 48 hours were used in flow cytometry analysis. (E) Comparison of apoptotic cell rates (* P<0.05). CCDP – cisplatin; AGS – human gastric cancer cell line; CCK-8 – Cell Counting Kit-8; qRT-PCR – quantitative real time polymerase chain reaction.
Cell migration and invasion was enhanced in AGS/CDDP cells
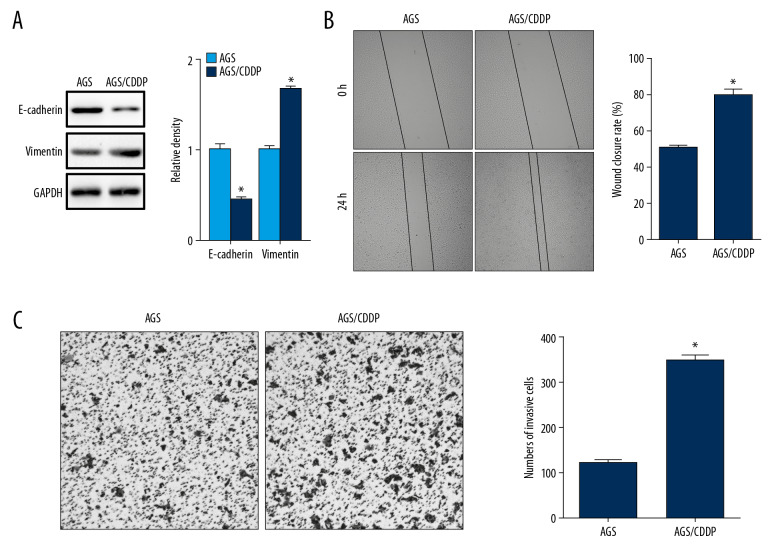
To reveal the mechanism underlying FAM3B-mediated CDDP resistance in gastric cancer, we focused on EMT which was reported to have an important function in therapy resistance of cancers [15]. We first examined the protein level the epithelial marker E-cadherin and the mesenchymal marker vimentin in AGS/CDDP cells. We found decreased E-cadherin and increased vimentin in AGS/CDDP cells (Figure 4A). Then we compared the cell migration in AGS and AGS/CDDP cells by wound healing assay which showed enhanced migration capacity in AGS/CDDP cells (Figure 4B). The Transwell assay also demonstrated enhanced cell invasion in AGS/CDDP cells (Figure 4C). Together, AGS/CDDP cells might undergo EMT while acquiring CDDP resistance.
Figure 4.
Cell migration and invasion was enhanced in AGS/CDDP cells. (A) Western blotting results of E-cadherin and vimentin in AGS and AGS/CDDP cells. Quantification of the relative band density was in the right panel. (B) The cell migration of AGS and AGS/CDDP cells was evaluated by wound healing assay. Comparison of wound closure rate was in the right panel. (C) The cell invasion of AGS and AGS/CDDP cells was evaluated by Transwell assay. Comparison of invasive cell counts was in the right panel (* P<0.05). CCDP – cisplatin; AGS – human gastric cancer cell line.
FAM3B overexpression induced EMT
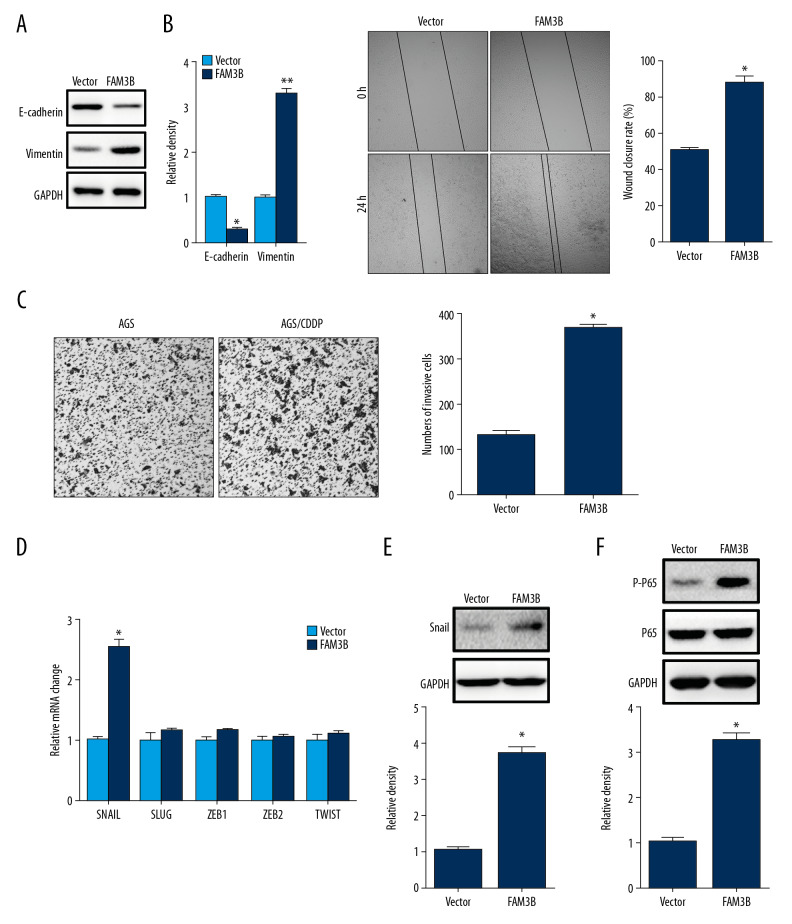
To find out whether FAM3B overexpression induced EMT in AGS cells, we detected E-cadherin and vimentin protein levels in AGS cells with FAM3B overexpression. The results showed that E-cadherin decreased while vimentin increased after FAM3B overexpression (Figure 5A). The cell migration and invasion were also evaluated. We found enhanced cell migration and invasion after ectopic overexpression of FAM3B (Figure 5B, 5C). Then we detected the mRNA changes of EMT transcription factors SNAIL, SLUG, ZEB1, ZEB2, and TWIST1 by qRT-PCR which showed that SNAIL was upregulated in AGS cells with FAM3B overexpression (Figure 5D). Increased snail protein level was also detected (Figure 5E). As it has been reported that loss of E-cadherin can be mediated through NF-κB-induced snail upregulation in gastric cancer [16], we also detected P-P65 in AGS cells after FAM3B overexpression and found increased P65 activation (Figure 5F). These results demonstrated that FAM3B induced EMT in AGS cells.
Figure 5.
FAM3B overexpression induced EMT. (A) Western blotting results of E-cadherin and vimentin in AGS cells with FAM3B overexpression. Quantification of the relative band density was in the right panel. (B) The cell migration of AGS cells with or without FAM3B overexpression was evaluated by wound healing assay. Comparison of wound closure rate was in the right panel. (C) The cell invasion of AGS cells with or without FAM3B overexpression was evaluated by Transwell assay. Comparison of invasive cell counts was in the right panel. (D) qRT-PCR results of the SNAIL, SLUG, ZEB1, ZEB2, and TWIST1 mRNA levels in AGS cells with ectopic FAM3B overexpression. (E) Western blotting results of snail in AGS cells with ectopic FAM3B overexpression. Quantification of the relative band density was in the lower panel. (F) Western blotting results of P-P65 in AGS cells with ectopic FAM3B overexpression. Quantification of the relative band density was in the lower panel (* P<0.05). EMT – epithelial-mesenchymal transition; AGS – human gastric cancer cell line; qRT-PCR – quantitative real time polymerase chain reaction.
Inhibition of snail reversed CDDP resistance in AGS/CDDP cells
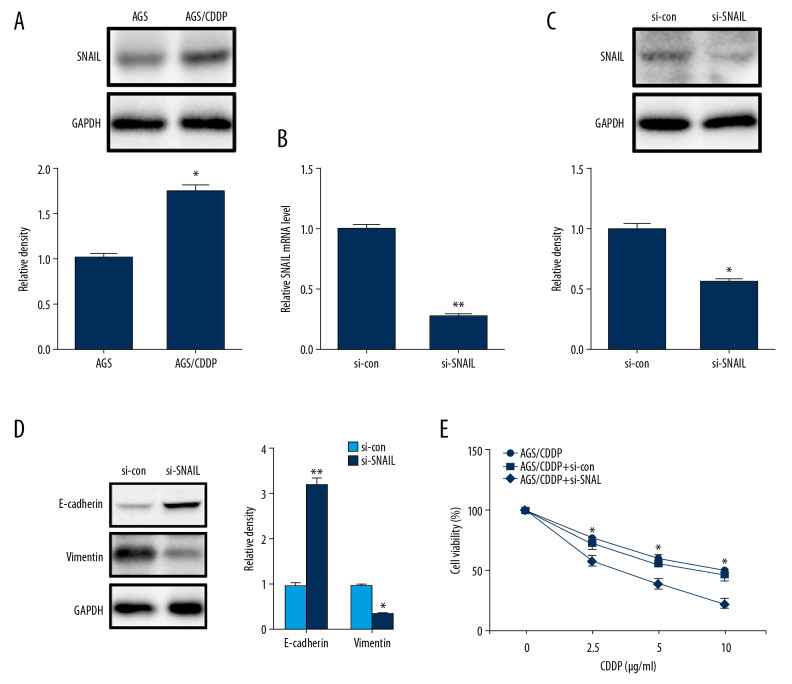
To further confirm whether FAM3B induced gastric cancer cell CDDP resistance through inducing EMT, we also evaluated the snail protein level in AGS/CDDP cells and found increased snail by western blotting (Figure 6A). Then we used SNAIL siRNA to inhibit its expression in AGS/CDDP cells. The inhibition efficiency was firstly confirmed (Figure 6B, 6C). Inhibition of SNAIL reversed the E-cadherin decrease and vimentin increase in AGS/CDDP cells (Figure 6D). After the inhibition of SNAIL, CDDP resistance was reversed as it was evident by the decrease of cell viability in AGS/CDDP cells after CDDP incubation (Figure 6E). Taken together, the inhibition of EMT reversed CDDP resistance in AGS/CDDP cells.
Figure 6.
Inhibition of snail reversed CDDP resistance in AGS/CDDP cells. (A) Western blotting results of snail in AGS and AGS/CDDP cells. Quantification of the relative band density was in the lower panel. (B) The knockdown efficiency of SNAIL siRNA was accessed by qRT-PCR in AGS/CDDP cells. (C) The knockdown efficiency of SNAIL siRNA was accessed by western blotting in AGS/CDDP cells. Quantification of the relative band density was in the lower panel. (D) Western blotting results of E-cadherin and vimentin in AGS/CDDP cells with SNAIL inhibition and control cells. Quantification of the relative band density was in the right panel. (E) AGS/CDDP cells with SNAIL inhibition and control cells were treated with CDDP (0, 2.5, 5, and 10 μg/mL) for 48 hours, and the cell viability was measured by CCK-8 assay (* P<0.05; ** P<0.01). CCDP – cisplatin; AGS – human gastric cancer cell line; CCK-8 – Cell Counting Kit-8; qRT-PCR – quantitative real time polymerase chain reaction.
Discussion
CDDP-based chemotherapy is the primary strategy for adjuvant chemotherapy in early-stage gastric cancer patients and systemic chemotherapy in late-stage gastric cancer patients. Unfortunately, CDDP resistance is a major problem, strongly limiting the efficiency [17]. Thus, elucidating the molecular mechanism underlying CDDP resistance will help develop reasonable and effective therapeutic strategies to overcome CDDP resistance. To date, many researches have revealed different mechanisms in gastric cancer CDDP resistance. Duan et al. found that taxol resistance gene 1 (TXR1) triggered CDDP resistance in vivo and in vitro [18]. Zou et al. found that inhibition of glutathione S-transferase pi 1 (GSTP1) sensitized CDDP-resistant gastric cancer cells to CDDP treatment [19]. Jiang et al. found that phosphoprotein enriched in astrocytes 15 (PEA-15) was involved in AKT-regulated cisplatin resistance [20]. The research by Li et al. found that psoriasin decreased the sensitivity of gastric cancer cells to CDDP by activating the extracellular signal-regulated kinase (ERK) signaling pathway [21]. Besides, some long non-coding RNA (like PVT1) and microRNAs (like miR-876-3p) have also been demonstrated to function in CDDP resistance of gastric cancer [22,23]. In this study, we found that FAM3B was increased in CDDP-resistant gastric cancer cells. Additionally, FAM3B overexpression could induce CDDP resistance of gastric cancer cells by reducing CDPP-induced apoptosis, while FAM3B knockdown sensitized gastric cancer cells to CDDP treatment. More importantly, FAM3B induced EMT by upregulating snail. Inhibition of EMT by silencing SNAIL reversed FAM3B induced CDDP resistance.
FAM3B is also named PANDER due to its robust expression in the endocrine pancreas [5]. It has been shown to regulate glucose homeostasis, α and β cell function [24]. In recent years, FAM3B has been found to be involved in tumor progression. In colon cancer, a non-secretory form of FAM3B (FAM3B-258) was found abundant in cancer cells and tissues. FAM3B-258 promotes colon cancer cell invasion and metastasis through slug upregulation [10]. In ESCC, FAM3B was also detected higher than adjacent normal tissues. High FAM3B expression was correlated with high TNM stage and predicted poor prognosis of ESCC patients. Mechanically FAM3B could regulate the AKT-MDM2.p53 pathway and EMT which inhibited cell death and promoted tumor growth [11]. In prostate cancer, FAM3B expression was found increased compared to normal tissues. Overexpression of FAM3B contributed to increased resistance to cell death and tumor growth through upregulating gene expression of anti-apoptotic Bcl-2 and Bcl-XL and downregulating expression of pro-apoptotic Bax [12]. We also found increased FAM3B in CDDP-resistant gastric cancer cells, while inhibition of FAM3B reversed CDDP resistance.
EMT is reported to have a critical role in mediating cancer progression, recurrence, metastasis and drug resistance [25]. The role of EMT in cancer drug resistance has been increasingly recognized [15,26]. EMT accompanies the development of chemo-resistance in a number of cancer types such as ovarian cancer, hepatocellular carcinoma, neuroblastoma, and gastric cancer [13,15,27,28]. EMT also plays a significant role in resistance to CDDP. CDDP-resistant cells were significantly enriched for a mesenchymal gene expression signature, while highly proliferative non-EMT cancer cells were sensitive to CDDP [29]. In urothelial cancer, CDDP-resistant cells acquired morphological changes characteristic of EMT with increased invasiveness [30]. Furthermore, EMT transcription factors are involved in CDDP resistance through different molecular mechanisms. Inhibition of TWIST1 sensitized human lung cancer cells to CDDP by activation of JNK/mitochondrial pathway [31]. TWIST2 is associated with the cervical malignant conversion, cervical cancer metastasis, and CDDP resistance [32]. ZEB1 was found important in CDDP sensitivity in pancreatic cancer cell lines [33]. Hsu et al. found that SNAIL induced EMT and CDDP resistance by direct regulation of excision repair cross-complementation croup 1 (ERCC1) in human head and neck squamous cell carcinoma [34]. Our study revealed that CDDP-resistant gastric cancer cells acquired EMT phenotype with decreased E-cadherin and increased vimentin. Notably, AGS/CDDP cells exhibited enhanced activities of migration and invasion. The phenotypic changes were attributed to snail upregulation induced by P-P65 activation. These findings confirmed that gastric cancer cell CDDP resistance is associated with EMT.
Unlike FAM3C which is revealed to be involved in EMT by extensive researches [35], the relationship between FAM3B and EMT is seldom reported. Li et al. found that FAM3B-258 promoted human colon cancer cell invasion and metastasis by upregulating slug. He et al. demonstrated that FAM3B induced EMT in ESCC cells through increasing snail. In our research, we also found increased snail after FAM3B overexpression. FAM3B promoted EMT via snail upregulation in AGS cells.
Conclusions
Upregulation of FAM3B induced CDDP resistance in gastric cancer cells by regulating EMT in a snail-dependent manner, making FAM3B a novel therapeutic target for gastric cancer chemo-resistance.
Footnotes
Source of support: Departmental sources
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Siddik ZH. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Xu G, Patel A, et al. Cloning, expression, and initial characterization of a novel cytokine-like gene family. Genomics. 2002;80:144–50. doi: 10.1006/geno.2002.6816. [DOI] [PubMed] [Google Scholar]
- 5.Cao X, Gao Z, Robert CE, et al. Pancreatic-derived factor (FAM3B), a novel islet cytokine, induces apoptosis of insulin-secreting beta-cells. Diabetes. 2003;52:2296–303. doi: 10.2337/diabetes.52.9.2296. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa H, Liu L, Tooyama I, et al. The FAM3 superfamily member ILEI ameliorates Alzheimer’s disease-like pathology by destabilizing the penultimate amyloid-beta precursor. Nat Commun. 2014;5:3917. doi: 10.1038/ncomms4917. [DOI] [PubMed] [Google Scholar]
- 7.Woosley AN, Dalton AC, Hussey GS, et al. TGFbeta promotes breast cancer stem cell self-renewal through an ILEI/LIFR signaling axis. Oncogene. 2019;28:3794–811. doi: 10.1038/s41388-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Wang C, Li J, et al. PANDER binds to the liver cell membrane and inhibits insulin signaling in HepG2 cells. FEBS Lett. 2009;583:3009–15. doi: 10.1016/j.febslet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Robert CE, Burkhardt BR, et al. Mechanisms of glucose-induced secretion of pancreatic-derived factor (PANDER or FAM3B) in pancreatic beta-cells. Diabetes. 2005;54:3217–28. doi: 10.2337/diabetes.54.11.3217. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Mou H, Wang T, et al. A non-secretory form of FAM3B promotes invasion and metastasis of human colon cancer cells by upregulating Slug expression. Cancer Lett. 2013;328:278–84. doi: 10.1016/j.canlet.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He SL, Wang WP, Yang YS, et al. FAM3B promotes progression of oesophageal carcinoma via regulating the AKT-MDM2-p53 signalling axis and the epithelial-mesenchymal transition. J Cell Mol Med. 2019;23:1375–85. doi: 10.1111/jcmm.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maciel-Silva P, Caldeira I, de Assis Santos I, et al. FAM3B/PANDER inhibits cell death and increases prostate tumor growth by modulating the expression of Bcl-2 and Bcl-XL cell survival genes. BMC Cancer. 2018;18:90. doi: 10.1186/s12885-017-3950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Yang J, Zhang Y, et al. Regorafenib reverses HGF-induced sorafenib resistance by inhibiting epithelial-mesenchymal transition in hepatocellular carcinoma. FEBS Open Bio. 2019;9:335–47. doi: 10.1002/2211-5463.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–30. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Z, Liu X, Tang Z, et al. Possible regulatory role of Snail in NF-kappaB-mediated changes in E-cadherin in gastric cancer. Oncol Rep. 2013;29:993–1000. doi: 10.3892/or.2012.2200. [DOI] [PubMed] [Google Scholar]
- 17.Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. 2016;22:2403–14. doi: 10.3748/wjg.v22.i8.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan S, Yin J, Bai Z, Zhang Z. Effects of taxol resistance gene 1 on the cisplatin response in gastric cancer. Oncol Lett. 2018;15:8287–94. doi: 10.3892/ol.2018.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou M, Hu X, Xu B, et al. Glutathione Stransferase isozyme alpha 1 is predominantly involved in the cisplatin resistance of common types of solid cancer. Oncol Rep. 2019;41:989–98. doi: 10.3892/or.2018.6861. [DOI] [PubMed] [Google Scholar]
- 20.Jiang X, Zhang C, Li W, et al. PEA15 contributes to the clinicopathology and AKTregulated cisplatin resistance in gastric cancer. Oncol Rep. 2019;41:1949–59. doi: 10.3892/or.2018.6934. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Cui Y, Ye L, et al. Psoriasin overexpression confers drug resistance to cisplatin by activating ERK in gastric cancer. Int J Oncol. 2018;53:1171–82. doi: 10.3892/ijo.2018.4455. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XW, Liu L, Zhang XZ, Bo P. Kanglaite inhibits the expression of drug resistance genes through suppressing PVT1 in cisplatin-resistant gastric cancer cells. Exp Ther Med. 2017;14:1789–94. doi: 10.3892/etm.2017.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng C, Huang K, Liu G, et al. MiR-876-3p regulates cisplatin resistance and stem cell-like properties of gastric cancer cells by targeting TMED3. J Gastroenterol Hepatol. 2019;34:1711–19. doi: 10.1111/jgh.14649. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Burkhardt BR, Guan Y, Yang J. Role of pancreatic-derived factor in type 2 diabetes: Evidence from pancreatic beta cells and liver. Nutr Rev. 2012;70:100–6. doi: 10.1111/j.1753-4887.2011.00457.x. [DOI] [PubMed] [Google Scholar]
- 25.Du B, Shim JS. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. 2016;21 doi: 10.3390/molecules21070965. pii: E965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer KR, Durrans A, Lee S, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–76. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haslehurst AM, Koti M, Dharsee M, et al. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer. 2012;12:91. doi: 10.1186/1471-2407-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang D, Duan H, Huang H, et al. Cisplatin resistance in gastric cancer cells is associated with HER2 upregulation-induced epithelial-mesenchymal transition. Sci Rep. 2016;6:20502. doi: 10.1038/srep20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowanadisai W, Messerli SM, Miller DH, et al. Cisplatin resistant spheroids model clinically relevant survival mechanisms in ovarian tumors. PLoS One. 2016;11:e0151089. doi: 10.1371/journal.pone.0151089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hohn A, Kruger K, Skowron MA, et al. Distinct mechanisms contribute to acquired cisplatin resistance of urothelial carcinoma cells. Oncotarget. 2016;7:41320–35. doi: 10.18632/oncotarget.9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhuo W, Wang Y, Zhuo X, et al. Knockdown of Snail, a novel zinc finger transcription factor, via RNA interference increases A549 cell sensitivity to cisplatin via JNK/mitochondrial pathway. Lung Cancer. 2008;62:8–14. doi: 10.1016/j.lungcan.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Wang W, Yang R, et al. Correlation of TWIST2 up-regulation and epithelial-mesenchymal transition during tumorigenesis and progression of cervical carcinoma. Gynecol Oncol. 2012;124:112–18. doi: 10.1016/j.ygyno.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Tillo E, Siles L, de Barrios O, et al. Expanding roles of ZEB factors in tumorigenesis and tumor progression. Am J Cancer Res. 2011;1:897–912. [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu DS, Lan HY, Huang CH, et al. Regulation of excision repair cross-complementation group 1 by Snail contributes to cisplatin resistance in head and neck cancer. Clin Cancer Res. 2010;16:4561–71. doi: 10.1158/1078-0432.CCR-10-0593. [DOI] [PubMed] [Google Scholar]
- 35.Waerner T, Alacakaptan M, Tamir I, et al. ILEI: A cytokine essential for EMT, tumor formation, and late events in metastasis in epithelial cells. Cancer Cell. 2006;10:227–39. doi: 10.1016/j.ccr.2006.07.020. [DOI] [PubMed] [Google Scholar]