Abstract
Background:
Former smokers now outnumber current smokers in many developed countries, and current smokers are smoking fewer cigarettes per day. Limited data suggest that lung function decline normalizes with smoking cessation; however, mechanistic studies suggest ongoing risk. We hypothesized that former smokers and low-intensity current smokers have accelerated lung function decline compared with never-smokers, including among those without prevalent lung disease.
Methods:
Longitudinal spirometry measures and self-reported smoking behaviors were harmonized across six US population-based cohorts. FEV1 decline of sustained former smokers and current smokers was compared to that of never-smokers using mixed models adjusted for socio-demographic and anthropometric factors. Differential FEV1 decline was also evaluated according to duration of smoking cessation and cumulative (pack-years) and current (cigarettes-per-day) cigarette consumption.
Findings:
25,352 participants (ages 17—93 years) completed 70,228 valid spirometry exams. Over median 7-year follow-up (interquartile range, 3—20), FEV1 decline at the median age (57 years) was 31·01, 34·97, and 39·92 mL/year in sustained never-smokers, former smokers, and current smokers, respectively. With adjustment, former smokers demonstrated 1·82 mL/year accelerated FEV1 decline (95% CI, 1·24—2·40) compared to never-smokers, which was 20% of the effect estimate for current smokers. Compared to never-smokers, accelerated FEV1 decline was observed for decades after smoking cessation and in smokers with low cumulative cigarette consumption (<10 pack-years). With respect to current cigarette consumption, the effect estimate for FEV1 decline in current smokers of <5 cigarettes-per-day was 68% of those in current smokers of ≥30 cigarettes-per-day, and 5 times greater than in former smokers. Among participants without prevalent lung disease, associations were attenuated but were consistent with the main results.
Interpretation:
Former smokers and low-intensity current smokers have accelerated lung function decline compared to never-smokers. This suggests that all levels of smoking exposure may be associated with lasting and progressive lung damage.
Funding:
National Institutes of Health/National Heart Lung and Blood Institute, US Environmental Protection Agency
INTRODUCTION
In many developed countries, declines in cigarette smoking represent one of the most important public health successes of the last fifty years.1 In the United States (US), the prevalence of current smoking in adults has fallen from 42% to 16% over that time period, and former smokers now constitute 22% of the adult population.1–4 In addition, many current smokers report smoking fewer cigarettes per day, with the average in the US dropping from 21 in to 14 over the last 25 years.4 Yet the number of deaths from chronic obstructive pulmonary disease (COPD) has increased such that COPD is now the third leading cause of death worldwide.5 Hence, it is increasingly important to understand possible ongoing lung function impairment in former smokers, and to define more clearly the respiratory risks among low-intensity current smokers, in order to inform prevention strategies for COPD.
Whereas there is broad agreement that current smoking increases age-related decline in adult lung function,6 which may lead to COPD,7–9 and that smoking cessation slows this decline,10 it is less clear whether the rate of lung function decline in former smokers “normalizes” to that of never-smokers or remains increased. This distinction is important since “normalization” implies a lack of ongoing lung damage after smoking cessation, whereas a persistent increase implies ongoing deterioration that may warrant additional preventative strategies. A recent meta-analysis of 88,887 adults participating in 47 studies (37 general population-based, 6 disease-focused, 4 interventional)11 found non-significantly different rates of FEV1 decline in former smokers. However, additional evidence is warranted, since many of the included studies were small, used variably standardized spirometry, included mostly males of European ancestry, and yielded variable results. Furthermore, most prior studies did not exclude participants with prevalent lung disease, which has been independently associated with accelerated lung function decline.12
In addition, there is mounting evidence from large prospective cohorts that low-intensity current smoking, which is frequently perceived as low risk,13,14 is associated with disproportionately high risks for cardiovascular events and numerous cancers.15–17 To date, no adequately powered cohorts including women and race/ethnic minorities, among whom low-intensity current smoking is more common,18 have established associations between low-intensity current smoking and lung function decline, and prominent COPD cohort studies19,20 have required that participants report at least 10 pack-years of cumulative smoking exposure, limiting the data available on smokers with <10 pack-years.
We hypothesized that former smokers and low-intensity current smokers have accelerated lung function decline compared with never-smokers, including among those without prevalent lung disease. We tested these hypotheses in a large, multi-ethnic, pooled sample of women and men with highly standardized spirometry.
METHODS
Sample
The NHLBI Pooled Cohorts Study harmonized and pooled data from nine US general population-based cohorts.21 All cohorts sampled community-dwelling adults, mostly via random digit dialing, and some but not all included representative sampling. All studies were approved by Institutional Review Boards (IRB) at participating institutions and all participants provided written informed consent. The Columbia University IRB provided approval for the secondary data analyses in this report. For the current work, we restricted the sample to participants in six cohorts with valid spirometry at ≥2 exams (Table S1). Two cohorts recruited younger adults, two recruited middle-aged and older adults, and two recruited only elderly adults; participants in the pooled cohort were 17—93 years old at baseline.
Measures
Highly standardized and often identical protocols were used across cohorts, allowing for systematic quality control (QC), harmonization, and pooling of measures, as previously described.21
Lung function was measured by pre-bronchodilator spirometry following American Thoracic Society (ATS) standards current at time of testing (1983—2014) using standardized protocols, often with the same equipment and acquired by the same investigators.21 We harmonized these data and retrospectively performed QC according to the ATS/European Respiratory Society (ERS) 2005 standards, which define valid exams as ≥2 acceptable curves reproducible within 150 mL.22 The current report was limited to spirometry exams meeting ATS/ERS 2005 standards, since this approach was previously found to reduce between- and with-person variability, outliers, and lung-function trend irregularities.21 Reference equations were used to define percent-predicted and lower-limit-of-normal (LLN).21,23
Smoking exposures, which were self-reported in a similar manner across cohorts (Table S2), were systematically quality-controlled for within-individual consistency over follow-up and harmonized prior to pooling.21 Smoking status was self-reported as “ever” and “current” at each spirometry exam, with biochemical verification in a subset. Former smokers were defined as ever-smokers who denied current smoking. For our primary analysis of smoking status, participants reporting the same smoking status at all spirometry exams were classified as sustained never-smokers, former smokers, or current smokers; all others were classified as having variable smoking status (Table S3). For secondary analyses, “observed quitters” were defined as the subset of participants with variable smoking status who demonstrated a single, sustained transition from current smoking at the first exam to former smoking at subsequent exams. To examine trends with respect to duration of smoking cessation, sustained former smokers were classified by years-since-quit-date at the last spirometry exam, which were categorized at thresholds of <10, 10-20, 20-30, and ≥30 years. For secondary analyses, former smokers were stratified by years-since-quit-date at the baseline exam. Cumulative cigarette exposure was defined among sustained former smokers and current smokers in terms of pack-years, which were calculated at baseline as average-cigarettes-per-day*years-smoked/20, and categorized at thresholds of 1, 10, and 20 pack-years. Current cigarette exposure was defined among sustained current smokers by time-variant cigarettes-per-day, which was categorized in increments of 5, from “low-intensity (<5) to “high-intensity” (≥30). All ranges were inclusive of the lower boundary point and exclusive of the upper boundary point. With respect to other tobacco-related exposures, secondhand smoke was classified as any (>0 hours/week or living with smoker) or none (0 hours/week or not living with smoker) in five cohorts collecting relevant data over follow-up, and cigar and/or pipe use was dichotomized as never or ever (Table S2).
Sex, educational attainment, and birth-year were self-reported at baseline. Time-variant height and weight were measured at each spirometry exam using standard methods.
Prevalent lung disease was defined as baseline airflow limitation, defined as ratio of the forced expiratory volume in one second (FEV1) to the forced vital capacity (FVC)<LLN; restrictive spirometry, defined as FEV1/FVC≥LLN with FVC<LLN; or diagnosed clinical lung disease, defined as self-reported physician diagnosis of asthma, COPD, emphysema, or chronic bronchitis, or inhaler use.9,21
Statistical analyses
Linear mixed models with cohort-specific unstructured covariance matrices were used to test associations with repeated measures of the FEV1.24 Due to the large age range included in the data, the age-dependence of lung function,23 and left truncation of data due to delayed time-entry, the time scale for analyses was defined as age-at-exam (hereafter referred to as “age”) rather than time-since-enrollment. Unadjusted mean FEV1 decline was estimated from a model including only age and age-squared as predictors. Unadjusted models were performed separately for each stratum of the primary exposures: smoking status, duration of smoking cessation, and cumulative and current cigarette exposure. Since age distribution differed by stratum, unadjusted FEV1 declines were consistently estimated at 57 years, the overall median age.
Adjusted effect estimates for smoking exposures, relative to never-smoking, were generated using models adjusted for a priori confounders and precision variables (e.g., height) that account for a large proportion of normal FEV1 variance. These covariates were age, age-squared, height, height-squared, sex, and race/ethnicity, which are in standard reference equations for lung function,23 as well as weight. Due to the inclusion of multi-center studies conducted over several decades, models were also adjusted for birth year, site, study, and educational attainment. To account for potential confounding of associations with FEV1 slope, multiplicative interactions with age were modeled for all covariates. To these models was added the smoking exposure of interest and its interaction with age (smoking-exposure*age); the effect estimate for this multiplicative interaction term was interpreted as the association of the smoking exposure with annualized lung function decline.
Fully-adjusted models were also used to plot predicted FEV1 curves. To reflect differences in age distributions across strata of smoking exposures, predictions at the extremes of each stratum-specific age distribution (<5th or >95th percentile) were not shown.
Several sensitivity analyses were performed. Since accelerated lung function decline has been previously demonstrated in adults with airway disease,19 effect modification by prevalent lung disease was assessed by multiplicative interaction terms (smoking-exposure*age*prevalent-lung-disease) and in disease-stratified models. Effect modification by secondhand smoke, cigar/pipe exposure, and the covariates was tested via multiplicative interaction terms and stratification. Analyses were repeated in observed quitters; using baseline rather than time-variant categorization of cigarettes-per-day; and, excluding all observations obtained at less than 30 years old. In addition, associations with FEV1/FVC decline were tested.
Analyses were completed in SAS, v9.4. Statistical significance was defined as a two-tailed P<0·05. Because of the potential for type 1 error with multiple comparisons, findings for secondary analyses should be interpreted as exploratory. Since missing covariate data were infrequent (2%), only complete cases were analyzed.
Role of the funding source
NHLBI staff routinely monitored the performance of component studies but neither NHLBI nor the US Environmental Protection Agency were involved in data collection, analysis, interpretation, or writing of this report, nor the decision to submit the paper for publication. The corresponding author (ECO) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
25,352 adults contributed 70,228 spirometry exams over median 7 (interquartile range [IQR], 3—20) years of follow-up (Figure 1). There were 10,087 never-smokers, 6,989 former smokers, 2,462 current smokers, and 5,814 participants reporting variable smoking status over follow-up, which included 2,599 observed quitters (Table S3). Among former smokers, median pack-years was 16 (IQR, 6—33) and 20% (1,369/6,989) quit ≥30 years prior to baseline. Current smokers had greater pack-years at baseline (27, IQR, 10—42), when 5% (118/2,462) were low-intensity smokers. Compared to former smokers, current smokers were younger, had more prevalent lung disease, and had shorter spirometry follow-up (Table 1).
Figure 1. CONSORT diagram.

HCHS/SOL = Hispanic Community Health Study/Study of Latinos; JHS = Jackson Heart Study; MESA = Multi-Ethnic Study of Atherosclerosis; NHLBI = National Heart, Lung, Blood Institute; SHS = Strong Heart Study.
Table 1.
Baseline characteristics, according to smoking status.
| Total N = 25,352 | Never-smokers (n = 10,087; 39·8%) |
Former smokers (n= 6,989; 27·6%) |
Current smokers (n = 2,462; 9·7%) |
Variable smoking status (n = 5,814; 22·9%) |
|
|---|---|---|---|---|---|
| Age, mean (SD), years | 52·0 (17·1) | 59·7 (12·5) | 47·6 (14·9) | 49·2 (15·5) | |
| Birth year, median (Q1, Q3) | 1936 (1927, 1951) | 1930 (1924, 1938) | 1938 (1931, 1956) | 1939 (1930, 1951) | |
| Sex – no. (%) | |||||
| Male (n=11,241; 44·3%) | 3,353 (33·2) | 4,032 (57·7) | 1,153 (46·8) | 2,703 (46·5) | |
| Female (n=14,111; 55·7%) | 6,734 (66·8) | 2,957 (42·3) | 1,309 (53·2) | 3,111 (53·5) | |
| Body mass index, mean (SD), kg/m2 | 26·9 (5·3) | 27·5 (4·8) | 25·6 (4·9) | 26·6 (5·1) | |
| Height, mean (SD), cm | 166·3 (9·5) | 169·6 (9·4) | 168·8 (9·2) | 168·7 (9·4) | |
| Race/ethnicity, no. (%) | |||||
| European-American (n=18,551; 73·2%) | 7,011 (69·5) | 5,658 (81·0) | 1,576 (64·0) | 4,306 (74·1) | |
| African-American (n=6,020; 23·8%) | 2,629 (26·1) | 1,144 (16·4) | 868 (35·3) | 1,379 (23·7) | |
| Other (n=781; 3·1%) | 447 (4·4) | 187 (2·7) | 18 (0·7) | 129 (2·2) | |
| Education status, no. (%) | |||||
| Less than high school (n=2,571; 10·1%) | 797 (7·9) | 700 (10·0) | 442 (18·0) | 632 (10·9) | |
| High school (n=7,468; 29·5%) | 2,925 (29·0) | 1,847 (26·4) | 873 (35·5) | 1,823 (31·4) | |
| Some college (n=4,502; 17·7%) | 1,906 (18·9) | 1,063 (15·2) | 403 (16·4) | 1,130 (19·4) | |
| College or more (n=10,811; 42·6%) | 4,459 (44·2) | 3,379 (48·4) | 744 (30·2) | 2,229 (38·3) | |
| Cohort, no· (%) | |||||
| ARIC (n=11,566; 45·6%) | 4,123 (40·9) | 3,510 (50·2) | 1,431 (58·1) | 2,502 (43·0) | |
| CARDIA (n=4,575; 18·0%) | 2,247 (22·3) | 368 (5·3) | 647 (26·3) | 1,313 (22·6) | |
| CHS (n=2,903; 10·8%) | 1,128 (11·2) | 1,216 (17·4) | 116 (4·7) | 443 (7·6) | |
| FHS Offspring (n=2,725; 10·8%) | 956 (9·5) | 461 (6·6) | 145 (5·9) | 1,163 (20·0) | |
| Health ABC (n=1,665; 6·6%) | 736 (7·3) | 777 (11·0) | 41 (1·7) | 111 (1·9) | |
| MESA (n=1,918; 7·6%) | 897 (8·9) | 657 (9·4) | 82 (3·3) | 282 (4·9) | |
| Diagnosed clinical lung disease at baseline, no (%) | |||||
| COPD (n=1,637; 6·5%) | 416 (4·1) | 542 (7·8) | 295 (12·0) | 384 (6·6) | |
| Asthma (n=1,440; 5·7%) | 570 (5·7) | 410 (5·9) | 141 (5·7) | 319 (5·5) | |
| Smoking behavior at baseline, median (Q1, Q3) | |||||
| Pack-years | 16 (6, 33) | 27 (10, 42) | 7 (0, 29) | ||
| Cigarettes per day | 0 | 20 (10, 20) | 10 (0, 20) | ||
| Age at smoking initiation, years | 18 (16, 20) | 17 (15, 20) | 21 (17, 35) | ||
| Age at smoking cessation, years | 40 (30, 50) | 55 (45, 65) | |||
| Lung function at baseline | |||||
| FEV1, mean (SD), L | 2·83 (0·86) | 2·81 (0·85) | 2·76 (0·84) | 2·93 (0·85) | |
| FVC, mean (SD), L | 3·63 (1·05) | 3·81 (1·05) | 3·74 (1·00) | 3·87 (1·02) | |
| FEV1 percent-predicted, mean (SD), % | 97·8 (14·6) | 94·1 (17·5) | 87·9 (17·0) | 93·1 (16·4) | |
| FVC percent-predicted, mean (SD), % | 99·4 (13·6) | 97·9 (14·7) | 95·1 (14·4) | 97·8 (14·1) | |
| FEV1/FVC, mean (SD), percent | 77·8 (7·0) | 73·7 (8·3) | 73·7 (9·8) | 75·4 (8·6) | |
| Airflow limitation, no. (%) (n=3,603; 14·2%) | 808 (8·0) | 1,072 (15·3) | 690 (28·0) | 1,033 (17·8) | |
| Restrictive pattern, no· (%) (n=1,401; 5·5%) | 489 (4·9) | 407 (5·8) | 170 (6·9) | 335 (5·8) | |
| No. spirometry exams, median (Q1, Q3) | 3 (2, 4) | 2 (2, 3) | 2 (2, 3) | 3 (2, 4) | |
| 2 (n=13,589; 53·6%) | 4,969 (49·3) | 4,220 (60·4) | 1,693 (68·8) | 2,695 (45·6) | |
| 3 (n=6,459; 25·5%) | 2,573 (25·5) | 1,921 (27·5) | 405 (16·5) | 1,560 (26·8) | |
| 4 (n=3,069; 12·1%) | 1,454 (14·4) | 562 (8·0) | 226 (9·2) | 827 (14·2) | |
| ≥5 (n=2,235; 8·8%) | 1,091 (10·8) | 286 (4·1) | 138 (5·6) | 720 (12·4) | |
| Spirometry observations, no. | 29,015 | 17,915 | 6,203 | 17,095 | |
| Years of follow-up, median (Q1, Q3) | 8 (3, 20) | 5 (3, 16) | 3 (3, 10) | 10 (3, 20) | |
ARIC = Atherosclerosis Risk in Communities; CARDIA = Coronary Artery Risk Development in Young Adults; CHS = Cardiovascular Health Study; FHS-O = Framingham Offpsring Study; HABC = Health ABC; MESA = Multi-Ethnic Study of Atherosclerosis; Q1 = first quartile; Q3 = third quartile.
Column percents reported.
Former smoking
Former smokers demonstrated accelerated FEV1 decline compared to never-smokers. The unadjusted FEV1 decline in former smokers was 34·97 mL/year (95% CI, 34·36—35·57), compared to 31·01 mL/year (95% CI, 30·66—31·37) in never-smokers, and 39·92 mL/year (95% CI, 38·92—40·92) in current smokers (Table 2). In adjusted models, former smokers demonstrated 1·82 mL/year accelerated FEV1 decline (95% CI, 1·24—2·40) compared to never-smokers; this estimate was 20% of the magnitude of the effect estimate for current smokers (9·21 mL/year; 95% CI, 8·35—10·08) (Table 2, Figure 2). Effect estimates were intermediate in participants with variable smoking status (2·56 mL/year; 95% CI, 2·03—3·08), including among 2,599 observed quitters (3·00 mL/year, 95% CI, 2·29—3·71) (Table S4).
Table 2.
Associations between smoking status, duration of smoking cessation, cumulative and current cigarette consumption, and FEV1 decline (mL/year).
| Total N = 25,352 | No. Participants* (Observations) | Unadjusted FEV1 decline in mL/year† (95% CI) | Adjusted difference in FEV1 decline in mL/year‡ (95% CI) | P-value | |
|---|---|---|---|---|---|
| Smoking status | |||||
| Never-smokers | 10,087 (29,015) | 31·01 (30·66, 31·37) | Referent | ||
| Former smokers | 6,989 (17,915) | 34·97 (34·36, 35·57) | 1·82 (1·24, 2·40) | <0·0001 | |
| Current smokers | 2,462 (6,203) | 39·92 (38·92, 40·92) | 9·21 (8·35, 10·08) | <0·0001 | |
| Variable smoking status | 5,814 (17,095) | 32·90 (32·37, 33·43) | 2·56 (2·03, 3·08) | <0·0001 | |
| Duration of smoking cessation | |||||
| Never-smokers | 10,087 (29,015) | 31·01 (30·66, 31·37) | Referent | ||
| Former smokers, by duration of cessation | |||||
| 30+ years | 3,072 (8,667) | 35·17 (34·21, 36·14) | 0·93 (0·22, 1·64) | 0·0104 | |
| 20 to <30 years | 1,841 (4,744) | 35·23 (34·03, 36·44) | 2·50 (1·61, 3·40) | <0·0001 | |
| 10 to <20 years | 1,252 (2,800) | 38·16 (35·51, 40·80) | 5·67 (4·15, 7·19) | <0·0001 | |
| < 10 years | 814 (1,682) | 46·33 (42·58, 50·08) | 6·75 (4·48, 9·02) | <0·0001 | |
| Current smokers | 2,462 (6,203) | 39·92 (38·92, 40·92) | 9·42 (8·56, 10·28) | <0·0001 | |
| Cumulative cigarette consumption | |||||
| Never-smokers | 10,087 (29,015) | 31·01 (30·66, 31·37) | Referent | ||
| Ever smokers, by baseline pack-years | |||||
| <1 pack-year | 546 (1,652) | 31·29 (29·82, 32·76) | −0·08 (−1·35, 1·19) | 0·90 | |
| 1 to <10 pack-years | 2,475 (6,884) | 31·38 (30·72, 32·05) | 0·87 (0·17, 1·57) | 0·0153 | |
| 10 to <20 pack-years | 1,779 (4,601) | 33·28 (32·37, 34·18) | 1·76 (0·85, 2·68) | 0·0002 | |
| 20+ pack-years | 4,476 (10,556) | 38·50 (37·48, 39·52) | 1·53 (0·74, 2·32) | 0·0002 | |
| Current cigarette consumption | |||||
| Never-smokers | 10,087 (29,015) | 31·01 (30·66, 31·37) | |||
| Former smokers | 6,989 (17,915) | 34·97 (34·36, 35·58) | 1·57 (1·00, 2·14) | <0·0001 | |
| Current smokers, by cigarettes/day | |||||
| <5 cigarettes/day | 118 (426) | 33·21 (30·26, 36·17) | 7·65 (6·21, 9·09) | <0·0001 | |
| 5 to <20 cigarettes/day | 989 (2,498) | 36·72 (35·22, 38·22) | 8·93 (7·98, 9·89) | <0·0001 | |
| 20 to <30 cigarettes/day | 975 (2,223) | 40·96 (39·28, 42·64) | 9·98 (8·95, 11·00) | <0·0001 | |
| 30+ cigarettes/day | 452 (1,035) | 43·98 (41·36, 46·60) | 11·24 (9·86, 12·62) | <0·0001 | |
CI = confidence interval.
Linear mixed models with cohort-specific unstructured covariance matrices were used to test associations with repeated measures of the FEV1. Participants with variable smoking status were excluded from analyses of duration of smoking cessation and of cumulative and current cigarette consumption.
Number of participants in group at baseline. Time-variant cigarettes per day was used to classify sustained current smokers, such that certain current smokers changed cigarette-per-day groups over follow-up.
Unadjusted mean FEV1 decline was estimated from a model including only age and age2 as predictors. Unadjusted models were performed separately for each stratum of the primary exposures. Since age distribution differed by stratum, unadjusted FEV1 declines were consistently estimated at 57 years, the overall median age at exam.
Adjusted effect estimates for smoking exposures, relative to never-smoking, were generated using models adjusted for the smoking parameter, age, age2, height, height2, sex, weight, race/ethnicity, birth year, site, study, and educational attainment. Multiplicative interactions with age were modeled for all covariates. The effect estimate for (smoking-exposure*age) was interpreted as the association of the smoking exposure with annualized lung function decline.
Figure 2. Predicted FEV1 curves according to (A) smoking status, (B) duration of smoking cessation, (C) cumulative cigarette consumption, and (D) current cigarette consumption.

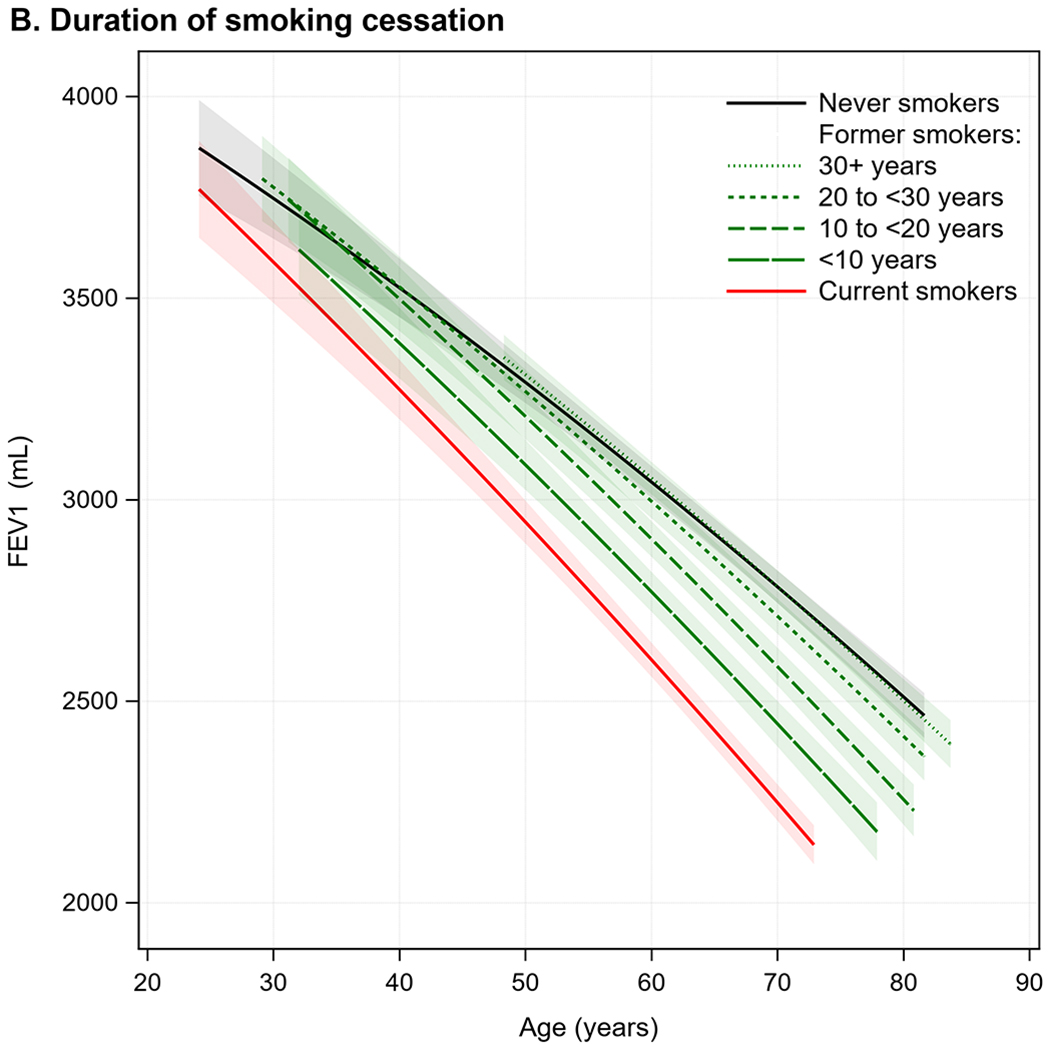


Predicted FEV1 curves were generated from linear mixed models with cohort-specific unstructured covariance matrices adjusted for age, age2, height, height2, sex, race/ethnicity, weight, birth year, site, study, educational attainment, and the smoking parameter of interest. Multiplicative interactions with age were modeled for all covariates. 95% confidence intervals are indicated by shading. To reflect differences in age distributions across strata of smoking exposures, predictions at the extremes of each stratum-specific age distribution (<5th or >95th percentile) were not shown. Participants with variable smoking status were excluded from analyses of duration of smoking cessation and of cumulative and current cigarette consumption. With respect to categorizations of duration of smoking cessation and cumulative and current cigarette consumption, all ranges were inclusive of the lower boundary point and exclusive of the upper boundary point.
Although there was statistical evidence of effect modification by secondhand smoke exposure (P=0·0229) and cigar/pipe use (P=0·0105), effect estimates for former smokers were comparable in persons both with and without these exposures (Figure S1). Effect sizes did not change considerably, despite significant multiplicative interaction terms, across strata of sex, race/ethnicity, age, and cohort, with the exception of participants ≥70 years old at baseline and a cohort limited to elderly participants (Figures S2—S3).
Duration of smoking cessation
Shorter durations of smoking cessation were associated with accelerated FEV1 decline compared to longer durations (Figure 2); nonetheless, accelerated FEV1 decline was observed for decades after cessation. In adjusted models, compared to never-smokers, FEV1 decline was accelerated by 2·50 mL/year (95% CI, 1·61—3·40) in former smokers with 20—30 years of cessation, and by 0·93 mL/year (95% CI, 0·22—1·64) among those with ≥30 years of cessation (Table 2). Accelerated FEV1 decline compared to never-smokers was also observed in former smokers who quit 20—30 and ≥30 years prior to the baseline exam (Table S5). Among observed quitters with ≥20 years since cessation, of whom there were fewer (N=499), FEV1 decline was similar to never-smokers (Table S6).
Low cumulative cigarette exposure
Compared to never-smokers, participants with 1—10 pack-years demonstrated accelerated FEV1 decline (0·87 mL/year; 95% CI, 0·17—1·57) (Table 2, Figure 2). Stratifying by smoking status, effect estimates increased monotonically with greater pack-years in current smokers; in former smokers, unadjusted mean FEV1 decline increased with greater pack-years, but the adjusted effect estimate for ≥20 pack-years was attenuated (0·80 mL/year; 95% CI, −0·11—1·71) (Table S7).
Low current cigarette exposure
At all levels of current smoking intensity, current smokers had accelerated FEV1 decline compared to never-smokers (Table 2, Figure 2). The adjusted effect estimate for <5 cigarettes-per-day (7·65 mL/year; 95% CI, 6·21—9·09) was 4·87 times greater than for former smokers (1·57 mL/year; 95% CI, 1·00—2·14) and equivalent to 68% of the effect estimate for ≥30 cigarettes-per-day.
Prevalent lung disease
Prevalent lung disease was an effect modifier for associations between smoking exposures and FEV1 decline (P<0·0001 for all). In adjusted models, effect estimates were greater in the sub-group with prevalent disease (Table S8) and attenuated in the sub-group without prevalent disease (Table 3), but trends were similar. Compared to never-smokers, the effect estimates for former smokers were 3·40 mL/year (95%CI: 2·00—4·80) and 1·10 mL/year (95%CI: 0·52—1·68) in those with and without prevalent lung disease, respectively. In the absence of prevalent lung disease, the rate of FEV1 decline in former smokers with ≥30 years of cessation approached that of never-smokers (0·53 mL/year; 95% CI, −0·18—1·25), while effect estimates for low-intensity current smokers remained relatively large versus never-smokers (4·72 mL/year; 95% CI, 3·10—6·33; Table 3).
Table 3.
Associations between smoking status, duration of smoking cessation, cumulative and current cigarette consumption, and FEV1 decline (mL/year), among persons without prevalent lung disease.
| Total N = 18,672 | No. Participants* (Observations) | Unadjusted FEV1 decline in mL/year† (95% CI) | Adjusted difference in FEV1 decline in mL/year‡ (95% CI) | P-value | |
|---|---|---|---|---|---|
| Smoking status | |||||
| Never-smokers | 8,154 (23,617) | 31·60 (31·21, 31·98) | Referent | ||
| Former smokers | 5,016 (13,063) | 34·88 (34·21, 35·54) | 1·10 (0·52, 1·68) | 0·0002 | |
| Current smokers | 1,412 (3,738) | 38·23 (36·92, 39·55) | 6·07 (5·14, 7·01) | <0·0001 | |
| Variable smoking status | 4,090 (12,350) | 32·86 (32·26, 33·46) | 1·38 (0·84, 1·91) | <0·0001 | |
| Duration of smoking cessation | |||||
| Never-smokers | 8,154 (23,617) | 31·60 (31·21, 31·98) | Referent | ||
| Former smokers, by duration of cessation | |||||
| 30+ years | 2,388 (6,785) | 34·43 (33·41, 35·45) | 0·53 (−0·18, 1·25) | 0·1457 | |
| 20 to <30 years | 1,314 (3,419) | 33·82 (32·46, 35·17) | 1·34 (0·42, 2·27) | 0·0043 | |
| 10 to <20 years | 835 (1,864) | 38·22 (35·12, 41·33) | 3·49 (1·93, 5·05) | <0·0001 | |
| < 10 years | 474 (983) | 43·59 (39·42, 47·77) | 2·69 (0·35, 5·02) | 0·0241 | |
| Current smokers | 1,412 (3,738) | 38·23 (36·92, 39·55) | 6·30 (5·37, 7·24) | <0·0001 | |
| Cumulative cigarette consumption | |||||
| Never-smokers | 8,154 (23,617) | 31·60 (31·21, 31·98) | Referent | ||
| Ever smokers, by baseline pack-years | |||||
| <1 pack-year | 430 (1,305) | 30·71 (29·04, 32·37) | −0·34 (−1·64, 0·96) | 0·61 | |
| 1 to <10 pack-years | 1,958 (5,479) | 31·78 (31·04, 32·53) | 0·80 (0·09, 1·51) | 0·0264 | |
| 10 to <20 pack-years | 1,331 (3,486) | 32·96 (31·93, 34·00) | 1·07 (0·13, 2·01) | 0·0253 | |
| 20+ pack-years | 2,590 (6,240) | 38·79 (37·58, 40·00) | 0·91 (0·05, 1·77) | 0·0392 | |
| Current cigarette consumption | |||||
| Never-smokers | 8,154 (23,617) | 31·60 (31·21, 31·98) | |||
| Former smokers | 5,016 (13,063) | 34·88 (34·21, 35·54) | 0·96 (0·38, 1·53) | 0·0012 | |
| Current smokers, by cigarettes/day | |||||
| <5 cigarettes/day | 80 (307) | 32·59 (28·90, 36·28) | 4·72 (3·10, 6·33) | <0·0001 | |
| 5 to <20 cigarettes/day | 602 (1,673) | 34·50 (32·59, 36·41) | 5·97 (4·93, 7·01) | <0·0001 | |
| 20 to <30 cigarettes/day | 528 (1,267) | 38·53 (36·17, 40·88) | 6·38 (5·22, 7·54) | <0·0001 | |
| 30+ cigarettes/day | 189 (476) | 44·00 (40·14, 47·87) | 9·09 (7·41, 10·77) | <0·0001 | |
CI = confidence interval.
Prevalent lung disease defined as baseline airflow limitation, baseline restrictive pattern, and/or clinically diagnosed lung disease at baseline. Participants with variable smoking status were excluded from analyses of duration of smoking cessation and of cumulative and current cigarette consumption.
Number of participants in group at baseline. Time-variant cigarettes per day was used to classify sustained current smokers, such that certain current smokers changed cigarette-per-day groups over follow-up.
Unadjusted mean FEV1 decline was estimated from a model including only age and age2 as predictors. Unadjusted models were performed separately for each stratum of the primary exposures. Since age distribution differed by stratum, unadjusted FEV1 declines were consistently estimated at 57 years, the overall median age at exam.
Adjusted effect estimates for smoking exposures, relative to never-smoking, were generated using models adjusted for the smoking parameter, age, age2, height, height2, sex, weight, race/ethnicity, birth year, site, study, and educational attainment. Multiplicative interactions with age were modeled for all covariates. The effect estimate for (smoking-exposure*age) was interpreted as the association of the smoking exposure with annualized lung function decline.
Additional sensitivity analyses
Results were similar using baseline rather than time-variant cigarettes-per-day (Table S9) and excluding observations at <30 years (Table S10). Similar to FEV1 decline, smoking exposures were associated with accelerated FEV1/FVC decline (Table S11). There was no evidence of bias related to regression to the mean (Figure S4).
DISCUSSION
In a large, US population-based sample, former smokers and low-intensity current smokers had accelerated lung function decline compared to never-smokers. Accelerated lung function decline persisted for decades after smoking cessation; was present in smokers with <10 pack-years; and was evident in current smokers reporting <5 cigarettes-per-day. These findings persisted in adults without prevalent lung disease. Our results thereby reinforce that there is no safe level of tobacco smoke exposure and that smoking cessation is the most effective means of harm reduction.
Accelerated lung function decline in former smokers is consistent with sustained pathophysiologic abnormalities of the lung after smoking cessation. Indeed, sustained dysregulation has been observed along pathways relevant to smoking-related lung function decline, including abnormalities in inflammation,25–27 infection and immunity,27–29 epigenetic alterations,30–32 airway hyper-responsiveness,29,33 mucous hypersecretion,34 and altered airway dimensions.35–37 There is also genetic evidence that supports a causal relationship between smoking and increased white and red blood cells38 and systemic inflammation.39 In addition, smoking-related emphysema, which does not reverse after smoking cessation, has been associated with lung function decline,40 potentially due to mechanical stress placed on adjacent lung.41 Meanwhile, our results regarding the adverse effects of low cumulative and current cigarette use are consistent with prior studies in which a single cigarette exposure has been linked to neutrophil retention in the lungs, increased endothelial permeability, increased oxidative stress, and dysregulated repair, which persist for hours to days.42,43 Contrary to perceptions among “social” smokers,13,14 animal models have suggested that intermittent cigarette exposure may be more mutagenic and cause more emphysema than constant cigarette exposure.44 Hence, the current understanding of the pathophysiology of lung function decline is congruent with our results.
Our findings that cigarette-related lung damage may be incompletely reversible and non-linear with respect to smoking intensity, even in healthy adults, build upon an extensive prior epidemiologic literature. A large number of observational studies and clinical trials have established the benefits of smoking cessation with respect to decelerating FEV1 decline.6,11 Nonetheless, whether FEV1 decline in former smokers decelerates or “normalizes” to the rate among never-smokers has remained uncertain. In fact, somewhat counter to the biological literature described above, recent meta-analysis of 88,887 adults included in 47 studies, one third of which were conducted prior to 1970, estimated that former smokers have non-significantly decelerated FEV1 decline compared to never-smokers.11
The contrast between the effect estimate for former versus never smoking from this recent meta-analysis11 (−1·6 mL/year, P≥0·05) and our result (+1·82 mL/year, 95%CI: +1·24—+2·40; P<0·0001) may be explained by several factors. First, whereas our study used only spirometry measures that were systematically validated by contemporary quality standards, the meta-analysis included many studies conducted before spirometry standards were first established in 1979; this would be expected to increase measurement error, which leads to wider confidence intervals, exacerbates problems of regression to the mean (not observed in our data), and biases results towards the null. Second, FEV1 decline in former smokers may be underestimated by observations during the immediate post-cessation period, when FEV1 may even improve10; by contrast, the majority of former smokers in our study were remote quitters, allowing us to confirm accelerated lung function decline decades after cessation. Third, since smoking increases risks of disability and death, it influences probability of recruitment into, and follow-up by, epidemiologic studies. Moreover, smokers sampled by studies of healthy adults would be more likely to have a reduced susceptibility to tobacco smoke.45 The resulting survivor bias and “healthy-smoker effect” are expected to result in underestimation of associations between smoking behaviors and FEV1 decline. This may have been particularly problematic in earlier studies included in the meta-analysis, since cardiovascular mortality was much greater in the mid-20th Century.
There was nonetheless evidence of survivor bias in our study, suggesting that our results may be underestimated. Current smoking was relatively rare in the two cohorts designed to study elderly adults, contributing to a younger average baseline age among current smokers in this study, and follow-up of current smokers was shorter. Thus, survivor bias may contribute to the absence of significant effects in the elderly. A combination of survival, “healthy-smoker,” and recall bias regarding details of remote smoking history may contribute to the lack of significantly accelerated FEV1 decline in former smokers with ≥20 pack-years, as this was contrary to monotonically increasing rates of FEV1 decline in current smokers with greater pack-years. The extent to which decelerations in FEV1 decline with greater durations of smoking cessation may be due to physiologic recovery versus the increasing importance of survivor biases remains unclear. At ≥30 years of smoking cessation, FEV1 declines approximated those of never-smokers among observed quitters and former smokers without prevalent lung disease; by contrast, accelerated FEV1 decline was observed in former smokers who quit ≥30 years prior to baseline. The cautious interpretation of these sub-group analyses is the same as for the primary results: that persistent acceleration in FEV1 decline may be observed for decades after smoking cessation.
Contrary to survivor bias, it is interesting to consider the possibility that “susceptible smokers” could be over-represented, via self-selection, among former smokers and those with lower cumulative and/or current cigarette exposure, which could lead to overestimation of our associations. Potentially consistent with this, former smokers with <10 years of smoking cessation had greater rates of FEV1 decline than current smokers in unadjusted analyses; however, the expected pattern was found after adjustment. In our analyses excluding “susceptible” participants with prevalent lung disease, associations were attenuated, but they remained consistent with our main conclusions.
With respect to the significance of low cumulative tobacco exposure and low-intensity current smoking, greater pack-years have been previously associated with accelerated lung function decline,46 yet prominent COPD cohort studies have required that participants report ≥10 pack-years.19,20 Our findings confirm a gradient of harm with respect to accelerated lung function decline that extends below 10 pack-years, suggesting ongoing lung deterioration in persons who do not meet heretofore standard criteria for elevated COPD risk.9 Our finding that current smoking of <5 cigarettes-per-day was associated with accelerated lung function decline is compatible with recent literature showing markedly increased risks of cardiovascular events, lung cancers, and all-cause mortality, even among low-intensity current smokers.15–17
Strengths of the current work include the large, highly-characterized sample with individual-level, longitudinal data over decades of follow-up, and rigorously quality-controlled spirometry harmonized to contemporary ATS/ERS standards.21 There are nonetheless limitations.
Smoking status was classified by self-report and was therefore subject to reporting biases. There may have been misclassification of former smokers as never-smokers and vice-versa, which would bias are results towards the null, as well as misclassification of current smokers as former smokers and of high-intensity current smokers as low-intensity current smokers, which could bias our results away from the null.47 This adds uncertainty to our findings. Nonetheless, we believe that misclassification was unlikely to explain our main results for several reasons. First, in two cohorts with cotinine measures, only 2—4% of participants who denied current smoking were reclassified as current smokers by cotinine measures, suggesting any such bias is small.48,49 Second, our findings were of consistent magnitude across socio-demographic groups that are reported to have differential smoking misclassification.49 Third, longitudinal smoking data were used to identify changes in self-reported smoking status over follow-up, and participants with variable smoking status over follow-up were treated separately. Finally, contrary to some prior work,50 we observed differences for former smokers reporting remote quit dates, among whom relapse is relatively uncommon51 and survival biases are of greatest concern.
The possibility of additional unmeasured confounding due to socioeconomic, secular, or other factors cannot be excluded. Notably, we lacked data on cigarette type or composition (e.g., menthol), which have been previously hypothesized to contribute to increasing tobacco-related mortality in recent birth cohorts,52 yet our results were independent of birth year. Information on alternative tobacco products, such as cigars and pipes, was also limited, as was measurement of secondhand smoke exposure; nonetheless, excluding participants with these exposures did not substantially alter the findings. Although we believe results are generalizable to the US population, the applicability of our results to non-US settings, including cigarette products not sold in the US, is anticipated but cannot be definitively addressed by this study. We also were unable to test to what extent the relatively similar rates of lung function decline among low-intensity, modest, and high-intensity current smokers could be due to deeper or prolonged inhalations among lower-intensity smokers, thus yielding higher respiratory toxicity per cigarette.53
Post-bronchodilator spirometry, which is required for diagnosis of COPD,9 was not available. Nonetheless, pre-bronchodilator spirometry has been shown to be highly prognostic in general population settings54 and contemporary spirometry quality standards were systematically applied.21
The magnitude of some estimated effects was small. Nevertheless, a 1·55 mL/year increase in the rate of FEV1 decline was equivalent to 5% of the average rate in never-smokers, and the majority of our effect estimates met this standard for clinical significance.55 Regardless, our results highlight the importance of smoking prevention and cessation, including with respect to “social smoking.” Our findings regarding the disproportionate harms of even “low intensity” current smoking also raise concerns regarding novel tobacco products marketed as “low dose.”
We conclude that former smokers and low-intensity current smokers have accelerated lung function decline compared to never-smokers. These findings reinforce the US Surgeon General’s position that there is no safe level of tobacco smoke exposure and that smoking cessation is the most effective means of harm reduction.1 They furthermore justify investigation into potential preventive interventions in the rising ranks of former smokers, who remain at increased risk for COPD due to ongoing lung function deterioration.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before the study
Over 40 years ago, Fletcher and Peto published their landmark study on the effect of smoking on age-related lung function decline in British working men. The Lung Health Study, a randomized controlled trial of smoking cessation, subsequently confirmed Fletcher and Peto’s observation that adults who successfully quit smoking have slower lung function decline than continuing smokers. However, Fletcher and Peto’s conclusion that “further damage to FEV [forced expiratory volume] due to smoking ceases within at most a few years of stopping” remains controversial. A recent meta-analysis of 88,887 adults included in 47 studies (37 general population-based, 6 disease-focused, 4 interventional), one third of which were conducted prior to 1970, found that former smokers had a non-significantly slower rate of FEV1 decline (27·6 mL/year, 95% CI 25·9—29·4) compared to never-smokers (29·2 mL/year; 95% CI 28·1—30·4). Additional evidence is warranted, since many of the included studies were small, used variably standardized spirometry, included mostly males of European ancestry, and yielded variable results. Moreover, from a pathophysiologic standpoint, a large number of mechanistic studies, both in vitro and in vivo, have suggested ongoing smoking-related harm to the lungs after smoking cessation.
Added value of this study
In a large, US general-population based sample, we demonstrate that former smokers and low-intensity current smokers have accelerated lung function decline compared to never-smokers. Accelerated lung function decline was observed after ≥30 years of smoking cessation and in smokers with relatively low cumulative cigarette consumption (<10 pack-years). Even at less than 5 cigarettes-per-day, current smoking was associated with substantially accelerated lung function decline compared to former smokers. Associations were attenuated but were consistent among participants without prevalent lung disease.
Implications of all the available evidence
Our findings reinforce that there is no safe level of tobacco smoke exposure and that smoking cessation is the most effective means of harm reduction. They furthermore justify investigation into potential preventive interventions in the rising ranks of former smokers, who remain at increased risk for chronic lung diseases due to ongoing lung function deterioration.
Acknowledgments
SOURCES OF FUNDING
NHLBI Pooled Cohorts Study: NIH/NHLBI R21-HL121457, R21-HL-129924, K23-HL-130627.
Atherosclerosis Risk In Communities (ARIC) Study: The authors thank the staff and participants of the ARIC study for their important contributions The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I, HHSN2682017000021).
The Jackson Heart Study has been funded by National Heart, Lung, and Blood Institute and National Institute for Minority Health and Health Disparities (contracts HHSN268201300049C and HHSN268201300050C to Jackson State University, HHSN268201300048C to Tougaloo College, and HHSN268201300046C and HHSN268201300047C to the University of Mississippi Medical Center).
Coronary Artery Risk Development in Young Adults (CARDIA) Study: CARDIA is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005), as well as an NHLBI grant (to Dr. Kalhan) R01 HL122477. This manuscript has been reviewed by CARDIA for scientific content.
Cardiovascular Health Study (CHS): This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Framingham Heart Study (FHS): From the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195; HHSN268201500001I).
Health Aging and Body Composition (Health ABC) Study: This research was supported by National Institute on Aging (NIA) contracts #N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050; NINR grant R01-NR012459.
Multi-Ethnic Study of Atherosclerosis (MESA): NIH/NHLBI R01-HL-077612, R01-HL-093081, RC1-HL-100543, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169. This publication was also developed under a STAR research assistance agreement, No. RD831697 (MESA Air), awarded by the U.S Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication.
DECLARATION OF INTERESTS
In addition to grants from the NIH, the authors declare the following interests. Dr. Bhatt reports personal fees from Sunovion. Dr Kalhan reports grants and personal fees from Boehringer Ingelheim, grants from PneumRx (BTG), grants from Spiration, grants and personal fees from AstraZeneca, personal fees from CVS Caremark, personal fees from Aptus Health, grants and personal fees from GlaxoSmithKline, personal fees from Boston Scientific, personal fees from Boston Consulting Group, outside the submitted work. Dr. Yende reports consultancy feeds from Atox Bio Inc and grants from Roche and Bristol-Myers-Squibb.
Contributor Information
Elizabeth C. Oelsner, Columbia University, New York, NY, USA.
Pallavi P. Balte, Columbia University, New York, NY, USA.
Surya P. Bhatt, University of Alabama at Birmingham, Birmingham, AL, USA.
Patricia A. Cassano, Cornell University, Ithaca, NY, USA.
David Couper, University of North Carolina, Chapel Hill, NC, USA.
Aaron R. Folsom, University of Minnesota, Minneapolis, MN, USA.
Neal D. Freedman, Division of Cancer Epidemiology & Genetics, National Cancer Institute.
David R. Jacobs, Jr, University of Minnesota, Minneapolis, MN, USA.
Ravi Kalhan, Northwestern University, Chicago, IL, USA.
Amanda R. Mathew, Rush University Medical Center, Chicago, IL, USA.
Richard A. Kronmal, University of Washington, Seattle, WA, USA.
Laura R. Loehr, University of North Carolina, Chapel Hill, NC, USA.
Stephanie J. London, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, Research Triangle Park, NC, USA.
Anne B. Newman, University of Pittsburgh, Pittsburgh, PA, USA.
George T. O’Connor, Boston University, Boston, MA, USA.
Joseph E. Schwartz, Columbia University, New York, NY, 10032.
Lewis J. Smith, Northwestern University, Chicago, IL, USA.
Wendy B. White, Tougaloo College, Tougaloo, MS, USA.
Sachin Yende, University of Pittsburgh, Pittsburgh, PA, USA.
REFERENCES
- 1.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 2.Clarke TC, Norris T, Schiller JS. Early Release of Selected Estimates Based on Data from the 2016 National Health Interview Survey., 2017.
- 3.Jamal A, Phillips E, Gentzke AS, et al. Current Cigarette Smoking Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep 2018; 67(2): 53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns DM, Major JM, Shanks TG. Changes in number of cigarettes smoked per day: cross-sectional and birth cohort analyses using NHIS In: Those who continue to smoke: is achieving abstinence harder and do we need to change our interventions? Smoking and tobacco control monograph no. 15. Bethesda, MD: National Cancer Institute, 2003:83–99. (NIH publication no. 03-5370.). [Google Scholar]
- 5.Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000-2016. Geneva, World Health Organization; 2018. [Google Scholar]
- 6.Samet JM, Lange P. Longitudinal studies of active and passive smoking. Am J Respir Crit Care Med 1996; 154(6 Pt 2): S257–65. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977; 1(6077): 1645–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange P, Celli B, Agusti A, Boje Jensen G, Divo M, Faner R, Guerra S, Marott JL, Martinez FD, Martinez-Camblor P, Meek P, Owen CA, Petersen H, Pinto-Plata V, Schnohr P, Sood A, Soriano JB, Tesfaigzi Y, Vestbo J. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med 2015;373:111–122. [DOI] [PubMed] [Google Scholar]
- 9.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017; 195(5):557–82. [DOI] [PubMed] [Google Scholar]
- 10.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994; 272(19): 1497–505. [PubMed] [Google Scholar]
- 11.Lee PN, Fry JS. Systematic review of the evidence relating FEV1 decline to giving up smoking. BMC Med 2010; 8: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lange P, Colak Y, Ingebrigtsen TS, Vestbo J, Marott JL. Long-term prognosis of asthma, chronic obstructive pulmonary disease, and asthma-chronic obstructive pulmonary disease overlap in the Copenhagen City Heart study: a prospective population-based analysis. Lancet Respir Med 2016; 4(6): 454–62. [DOI] [PubMed] [Google Scholar]
- 13.Denlinger-Apte RL, Joel DL, Strasser AA, Donny EC. Low Nicotine Content Descriptors Reduce Perceived Health Risks and Positive Cigarette Ratings in Participants Using Very Low Nicotine Content Cigarettes. Nicotine Tob Res 2017; 19(10): 1149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amrock SM, Weitzman M. Adolescents’ perceptions of light and intermittent smoking in the United States. Pediatrics 2015; 135(2): 246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackshaw A, Morris JK, Boniface S, Tang JL, Milenkovic D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ 2018; 360: j5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue-Choi M, Hartge P, Liao LM, Caporaso N, Freedman ND. Association between long-term low-intensity cigarette smoking and incidence of smoking-related cancer in the national institutes of health-AARP cohort. Int J Cancer 2018; 142(2): 271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue-Choi M, Liao LM, Reyes-Guzman C, Hartge P, Caporaso N, Freedman ND. Association of Long-term, Low-Intensity Smoking With All-Cause and Cause-Specific Mortality in the National Institutes of Health-AARP Diet and Health Study. JAMA Intern Med 2017; 177(1): 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes-Guzman CM, Pfeiffer RM, Lubin J, et al. Determinants of Light and Intermittent Smoking in the United States: Results from Three Pooled National Health Surveys. Cancer Epidemiol Biomarkers Prev 2017; 26(2): 228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010; 7(1): 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Couper D, LaVange LM, Han M, et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax 2014; 69(5): 491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oelsner EC, Balte PP, Cassano P, et al. Harmonization of Respiratory Data From Nine US Population-Based Cohorts: The NHLBI Pooled Cohorts Study. Am J Epidemiol 2018; 187(11):2265–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26(2): 319–38. [DOI] [PubMed] [Google Scholar]
- 23.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159(1): 179–87. [DOI] [PubMed] [Google Scholar]
- 24.Oelsner EC, Balte PP, Grams ME, et al. Albuminuria, Lung Function Decline, and Risk of Incident COPD: The NHLBI Pooled Cohorts Study. Am J Respir Crit Care Med 2019; 199(3):321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogg JC. Why does airway inflammation persist after the smoking stops? Thorax 2006; 61(2): 96–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rutgers SR, Postma DS, ten Hacken NH, et al. Ongoing airway inflammation in patients with COPD who do not currently smoke. Thorax 2000; 55(1): 12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiels MS, Katki HA, Freedman ND, et al. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst 2014; 106(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 167(8): 1090–5. [DOI] [PubMed] [Google Scholar]
- 29.Lapperre TS, Postma DS, Gosman MM, et al. Relation between duration of smoking cessation and bronchial inflammation in COPD. Thorax 2006; 61(2): 115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belinsky SA, Palmisano WA, Gilliland FD, et al. Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer Res 2002; 62(8): 2370–7. [PubMed] [Google Scholar]
- 31.Soria JC, Rodriguez M, Liu DD, Lee JJ, Hong WK, Mao L. Aberrant promoter methylation of multiple genes in bronchial brush samples from former cigarette smokers. Cancer Res 2002; 62(2): 351–5. [PubMed] [Google Scholar]
- 32.Wang G, Wang R, Strulovici-Barel Y, et al. Persistence of smoking-induced dysregulation of miRNA expression in the small airway epithelium despite smoking cessation. PLoS One 2015; 10(4): e0120824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339(17): 1194–200. [DOI] [PubMed] [Google Scholar]
- 34.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 1996; 153(5): 1530–5. [DOI] [PubMed] [Google Scholar]
- 35.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(26): 2645–53. [DOI] [PubMed] [Google Scholar]
- 36.Oelsner EC, Smith BM, Hoffman EA, et al. Prognostic Significance of Large Airway Dimensions on Computed Tomography in the General Population: The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Ann Am Thorac Soc 2018; 15(6):718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verbanck S, Schuermans D, Paiva M, Meysman M, Vincken W. Small airway function improvement after smoking cessation in smokers without airway obstruction. Am J Respir Crit Care Med 2006; 174(8): 853–7. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen KM, Colak Y, Ellervik C, Hasselbalch HC, Bojesen SE, Nordestgaard BG. Smoking and Increased White and Red Blood Cells. Aterioscler Thromb Vasc Biol 2019; 39(5):965–977. [DOI] [PubMed] [Google Scholar]
- 39.Colak Y, Afzal S, Lange P, Nordestgaard BG. Smoking, Systemic Inflammation, and Airflow Limitation: A Mendelian Randomization Analysis of 98 085 Individuals from the General Population. Nicotine Tob Res 2018; doi: 10.1093/ntr/nty077 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40.Oelsner EC, Smith BM, Hoffman EA, et al. Associations between emphysema-like lung on CT and incident airflow limitation: a general population-based cohort study. Thorax 2018; 73(5):486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatt SP, Bodduluri S, Hoffman EA, et al. Computed Tomography Measure of Lung at Risk and Lung Function Decline in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2017; 196(5): 569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacNee W, Wiggs B, Belzberg AS, Hogg JC. The effect of cigarette smoking on neutrophil kinetics in human lungs. N Engl J Med 1989; 321(14): 924–8. [DOI] [PubMed] [Google Scholar]
- 43.Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med 1999; 159(2): 473–9. [DOI] [PubMed] [Google Scholar]
- 44.Kameyama N, Chubachi S, Hegab AE, et al. Intermittent Exposure to Cigarette Smoke Increases Lung Tumors and the Severity of Emphysema More Than Continuous Exposure. Am J Respir Cell Mol Biol 2018; 59(2):179–188. [DOI] [PubMed] [Google Scholar]
- 45.Kerstjens HA, Rijcken B, Schouten JP, Postma DS. Decline of FEV1 by age and smoking status: facts, figures, and fallacies. Thorax 1997; 52(9): 820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. Combined Impact of Smoking and Early-Life Exposures on Adult Lung Function Trajectories. Am J Respir Crit Care Med 2017; 196(8): 1021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health 1994; 84(7): 1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med 2010; 152(4): 201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagenknecht LE, Burke GL, Perkins LL, Haley NJ, Friedman GD. Misclassification of smoking status in the CARDIA study: a comparison of self-report with serum cotinine levels. Am J Public Health 1992; 82(1): 33–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burchfiel CM, Marcus EB, Curb JD, et al. Effects of smoking and smoking cessation on longitudinal decline in pulmonary function. Am J Respir Crit Care Med 1995; 151(6): 1778–85. [DOI] [PubMed] [Google Scholar]
- 51.Garcia-Rodriguez O, Secades-Villa R, Florez-Salamanca L, Okuda M, Liu SM, Blanco C. Probability and predictors of relapse to smoking: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend 2013; 132(3): 479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med 2013; 368(4): 351–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krebs NM, Chen A, Zhu J, et al. Comparison of Puff Volume With Cigarettes per Day in Predicting Nicotine Uptake Among Daily Smokers. Am J Epidemiol 2016; 184(1): 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mannino DM, Diaz-Guzman E, Buist S. Pre- and post-bronchodilator lung function as predictors of mortality in the Lung Health Study. Respir Res 2011; 12: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones PW, Bee KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal Clinically Important Differences in Pharmacological Trials. Am J Respir Crit Care Med 2013; 189(3):250–255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


